Abstract
Death signaling provided by tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) can induce death in cancer cells with little cytotoxicity to normal cells; this cell death has been thought to involve caspase-dependent apoptosis. Reactive oxygen species (ROS) are also mediators that induce cell death, but their roles in TRAIL-induced apoptosis have not been elucidated fully. In the current study, we investigated ROS and caspases in human pancreatic cancer cells undergoing two different types of TRAIL-induced cell death, apoptosis and necroptosis. TRAIL treatment increased ROS in two TRAIL-sensitive pancreatic cancer cell lines, MiaPaCa-2 and BxPC-3, but ROS were involved in TRAIL-induced apoptosis only in MiaPaCa-2 cells. Unexpectedly, inhibition of ROS by either N-acetyl-L-cysteine (NAC), a peroxide inhibitor, or Tempol, a superoxide inhibitor, increased the annexin V-/propidium iodide (PI)+ early necrotic population in TRAIL-treated cells. Additionally, both necrostatin-1, an inhibitor of receptor-interacting protein kinase 1 (RIP1), and siRNA-mediated knockdown of RIP3 decreased the annexin V-/PI+ early necrotic population after TRAIL treatment. Furthermore, an increase in early apoptosis was induced in TRAIL-treated cancer cells under inhibition of either caspase-2 or -9. Caspase-2 worked upstream of caspase-9, and no crosstalk was observed between ROS and caspase-2/-9 in TRAIL-treated cells. Together, these results indicate that ROS contribute to TRAIL-induced apoptosis in MiaPaCa-2 cells, and that ROS play an inhibitory role in TRAIL-induced necroptosis of MiaPaCa-2 and BxPC-3 cells, with caspase-2 and -9 playing regulatory roles in this process.
Introduction
Members of the tumor necrosis factor (TNF) cytokine family, such as TNFα and Fas ligand (FasL), play important roles in inflammation and immunity [1]. Although TNF-related apoptosis-inducing ligand (TRAIL) is a member of this family, this molecule can induce cancer cell death while causing almost no cytotoxicity to normal cells [2]. There are positive and negative receptors; death receptor (DR)4 and DR5 provide pro-apoptotic signaling, whereas decoy receptor (DcR)1 and DcR2 inhibit apoptotic signaling [3]. Normal cells are reported to show TRAIL-resistance with their preferential expression of DcRs [4]. Therefore, TRAIL and its DR are expected to be useful anti-cancer molecules and targets and have been used and targeted in several clinical trials [5, 6]. Binding of TRAIL to DR on cancer cells provides caspase-8-dependent death signaling and triggers the ‘extrinsic’ apoptotic pathway [7]. Activation of caspase-8 transforms Bid to tBid, thereby promoting the mitochondria-mediated caspase-9-dependent ‘intrinsic’ apoptotic pathway [8]. Alternatively, ROS (reactive oxygen species) also play important roles in cell death and signaling [9]. ROS induce intrinsic apoptosis by triggering DNA damage [10]. Conversely, DNA damage induces ROS production [11]. DNA damage and/or ROS production can trigger caspase-9-dependent apoptosis. Additionally, some reports suggested that ROS is involved in apoptosis in TRAIL-treated human cancer cells [12–14]. However, the roles of ROS in TRAIL-induced cancer cell death have yet to be investigated fully.
In addition to apoptosis, necroptosis is now recognized as another form of programmed cell death [15, 16]. Necroptosis is programmed necrosis that can be activated upon stimulation by TNFα, FasL, or TRAIL. The roles and mechanisms of apoptosis and necrosis have been well established, whereas those of necroptosis have been the focus of recent investigations [16–18]. Necroptosis has received much attention as a type of cell death that induces inflammation [17]. Under caspase-8 inhibition, receptor-interacting protein kinase 1 (RIP1) and RIP3 form a complex and trigger necroptosis [19, 20]; additional recent reports have revealed that RIP3 plays a central role in the process [18, 21]. Although some reports suggest that TRAIL can induce necroptosis in cancer cells [22–24], the mechanisms have not been elucidated fully.
This study investigated the roles of ROS and caspases in TRAIL-induced apoptosis and necroptosis of human pancreatic cancer cells. Among four human pancreatic cancer cell lines, ROS levels were elevated in only two TRAIL-sensitive lines: MiaPaCa-2 and BxPC-3. However, ROS played a pro-apoptotic role only in MiaPaCa-2 cells, but not in BxPC-3 cells. In these experiments, we found that TRAIL treatment under ROS inhibition increased the population of annexin V-/propidium iodide (PI)+ early necrotic cells in MiaPaCa-2 and BxPC-3 cells, suggesting that ROS played an inhibitory role in TRAIL-induced necroptosis in both cell lines. In addition, necrostatin-1 (a RIP1 inhibitor) decreased the annexin V-/PI+ population in these TRAIL-treated cells, and siRNA-mediated knockdown of RIP3 showed similar results in BxPC-3 cells, implying that the cell death observed was caused by necroptosis. Finally, necroptosis was promoted by TRAIL treatment under the inhibition of either caspase-9 or -2, with the latter considered an ‘orphan’ caspase whose roles in cell death have not been elucidated fully [25, 26]. Together, these findings indicate that ROS are increased in TRAIL-sensitive MiaPaCa-2 and BxPC-3 cells, but that ROS are involved in apoptosis only in TRAIL-treated MiaPaCa-2 cells. Our findings also showed that ROS and caspase-9/-2 play regulatory roles in the TRAIL-induced necroptosis of human pancreatic cancer cells.
Materials and Methods
Cell lines
Two human pancreatic cancer cell lines (AsPC-1 and BxPC-3) were purchased from the American Type Culture Collection (Manassas, VA, USA). Two other human pancreatic cancer cell lines (MiaPaCa-2 and Panc-1) were kindly provided by Dr. K. Takenaga (Shimane University Faculty of Medicine) [27]. These cell lines were maintained in DMEM (Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% fetal calf serum (Invitrogen, Grand Island, NY, USA) and 20 μg/ml gentamicin (Sigma-Aldrich). PrEC is a normal prostate epithelial cell line purchased from Lonza (Walkersville, MD, USA) and was maintained in PrEBM (Lonza).
Cell viability assay
Cell viability was analyzed using the WST-8 assay (Nacalai Tesque, Kyoto, Japan). At the end of the incubation period, 10 μl WST-8 solution was added to each well, and the plates were incubated for an additional 3 h. Absorbance in each well was measured at 560 nm using a microplate reader (Beckman Coulter, Brea, CA, USA).
Reagents
For inhibition assays, the following inhibitors were added 1 h before the addition of TRAIL: pan-caspase inhibitor Z-VAD-FMK (Enzo Life Sciences, Farmingdale, NY, USA), caspase-8 inhibitor Z-IETD-FMK (R&D Systems, Minneapolis, MN, USA), caspase-9 inhibitor Z-LEHD-FMK (R&D Systems), and caspase-2 inhibitor Z-VDVAD-FMK (R&D Systems). N-acetyl-l-cysteine (NAC) was purchased from Nacalai Tesque. Tempol and necrostatin-1 were purchased from Santa Cruz Biotechnology, Santa Cruz, CA, USA.
Detection of DR and decoy receptor (DcR) expression on cells
To examine the expression of DR4 (CD261) and DR5 (CD262), cells were incubated with either anti-DR4 (eBioscience, San Diego, CA, USA) or anti-DR5 (eBioscience), followed by staining with FITC-conjugated goat anti-mouse IgG (H+L) (KPL, Gaithersburg, MD, USA). To examine the expression of DcR1 (CD263) and DcR2 (CD264), cells were stained with either FITC-conjugated anti-DcR1 (CD263) (GeneTex, Irvine, CA, USA), or FITC-conjugated anti-DcR2 (CD264) (GeneTex). For these incubations, isotype-matched FITC-conjugated mouse IgG1was used as a control. Analysis was performed using a FACSCalibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA).
Apoptosis assay
Cell death was assessed using the Annexin V-FITC Apoptosis Detection Kit (BioVision, Mountain View, CA, USA) and PI. Each caspase inhibitor (20 μM), or the same volume of DMSO as a vehicle control, was added 1 h before the addition of TRAIL. To examine effects of NAC and Tempol on TRAIL-induced apoptosis, cells were cultured with TRAIL (50 ng/mL) with or without NAC (10 mM) or Tempol (1 mM) for 24 h. To examine effects of necrostatin-1, necrostatin-1 (20 μM) was added at the initiation of culture. After staining with annexin V-FITC/PI, flow cytometric analysis was performed. Analysis was performed using a FACSCalibur flow cytometer.
ROS measurement
Intracellular ROS were measured using carboxy-H2DCFDA (Molecular Probes, Carlsbad, CA, USA). Cells were cultured with TRAIL (50 ng/mL). After 6 h for MiaPaCa-2 and 12 h for the other lines, carboxy-H2DCFDA (50 μM) was added and cultured additionally for 30 min. Collected cells were analyzed by flow cytometry. Each caspase inhibitor (20 μM) was added 1 h before the addition of TRAIL.
Immunoblotting
Cells were lysed with a mammalian protein extraction reagent (M-PER; Thermo Scientific, Rockford, IL, USA) containing a protease-inhibitor cocktail (Nacalai Tesque). Equal amounts of protein were resolved on 4–12% gradient or 12% SDS-PAGE gels and transferred to polyvinylidene fluoride membranes. The membranes were blocked and the blots incubated with the following primary antibodies: anti-RIP3 (13526; Cell Signaling Technology, Danvers, MA, USA), anti-caspase-3 (9668; Cell Signaling Technology), anti-caspase-8 (M032-3; Medical and Biological Laboratories, Nagoya, Japan), anti-caspase-9 (9508; Cell Signaling Technology), anti-caspase-2 (2224; Cell Signaling Technology), anti-β-actin (BioLegend, San Diego, CA, USA), or anti-α-tubulin (Santa Cruz Biotechnology). After washing, room temperature incubation of membranes for 30 min with either goat anti-rabbit or goat anti-mouse alkaline phosphatase-conjugated secondary antibodies (Invitrogen) was used to detect the primary antibodies. Protein bands were visualized using CDP-star chemiluminescence and imaged using an ImageQuant LAS-4000 system (FujiFilm, Tokyo, Japan).
Transfection of small interfering RNA (siRNA)
Transfection of siRNA was performed using Lipofectamine RNAiMAX (Invitrogen), according to the manufacturer’s instructions. RIP3 siRNA (sc-61482) was purchased from Santa Cruz Biotechnology. The control siRNA (6568) was purchased from Cell Signaling Technology. Three days after siRNA transfection, the cells were used for subsequent experiments.
Statistical analyses
Data were evaluated statistically using unpaired two-tailed Student’s t-tests. A P value of less than 0.05 was considered to indicate statistical significance.
Results
Production of ROS in TRAIL-sensitive pancreatic cancer cells
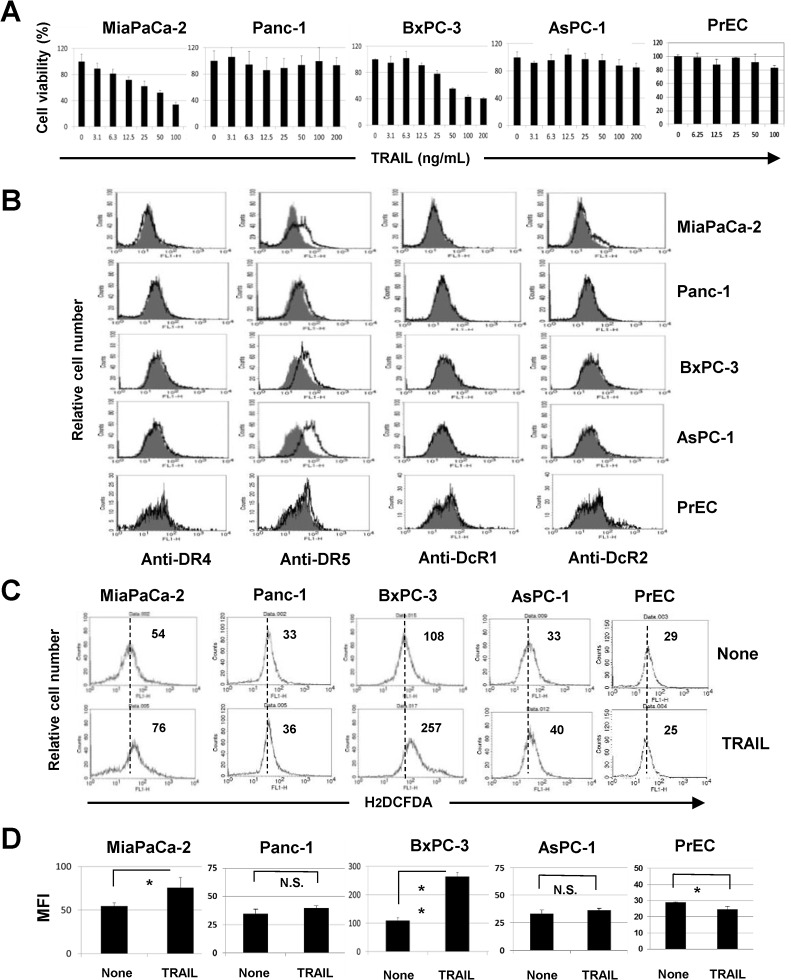
We first examined the sensitivity of four human pancreatic cancer cell lines and a normal prostate epithelial cell line PrEC to TRAIL treatment. We selected these cancer cell lines because they have been well-characterized for their mutations in K-ras and p53 [28] and because we previously examined their TRAIL sensitivity [29]. As a result, the viability of both MiaPaCa-2 and BxPC-3 cells decreased in the presence of TRAIL in a dose-dependent manner, whereas the other three lines (Panc-1, AsPC-1, and PrEC) showed no clear sensitivity toward TRAIL (Fig 1A). We next examined the expression of TRAIL receptors on these cells (Fig 1B). The expression of DR4 was almost undetectable in all cell lines. Although DR5 expression on Panc-1 and PrEC was low, all cell lines were positive for DR5. In terms of decoy receptors, MiaPaCa-2 and PrEC cells were partially positive for DcR2. We next determined whether ROS were produced in these cell lines and found that TRAIL treatment significantly increased ROS levels only in MiaPaCa-2 and BxPC-3 cells (P<0.05 for MiaPaCa-2, P<0.01 for BxPC-3) (Fig 1C and 1D). PrEC cells reduced the level of ROS after TRAIL treatment. These results indicated that ROS are produced only in TRAIL-sensitive pancreatic cancer cell lines (MiaPaCa-2 and BxPC-3).
Fig 1. ROS production in TRAIL-sensitive human pancreatic cancer cell lines.
(A) Four human pancreatic cancer lines and PrEC cells were cultured in the presence of TRAIL. After 48 h, cell viability was determined by the WST-8 assay. The data shown represent the mean of three wells. (B) The expression of DR4, DR5, DcR1, and DcR2 in five cell lines was examined by flow cytometry. The line represents staining with mAb specific to either DR4 or DR5, followed by a FITC-conjugated secondary antibody. Solid gray represents staining with FITC-conjugated anti-mouse IgG alone. Regarding the expression of DcR1 and DcR2, the line represents staining with mAb specific to DcR1 and DcR2; solid gray represents staining with isotype-matched FITC-conjugated anti-mouse IgG. (C) Five cell lines were cultured with TRAIL (50 ng/mL). After 6 h for MiaPaCa-2 and 12 h for the other four lines, these cells were cultured with carboxy-H2DCFDA (50 μM) for 30 min and examined for their ROS levels by flow cytometry. The number represents the mean fluorescence intensity. (D) The data shown represent the mean of three wells. MFI: mean fluorescence intensity. *P<0.05, **P<0.01, N.S., not significant.
Inhibition of ROS decreased TRAIL-induced apoptosis only in MiaPaCa-2 cells
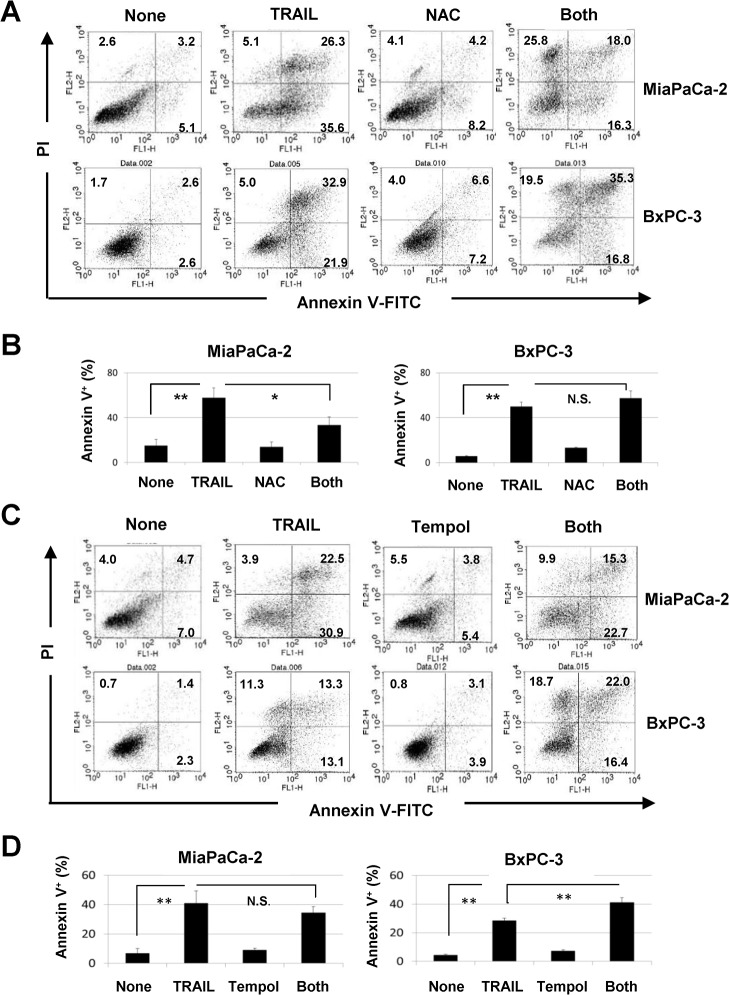
We next examined the effects of two ROS inhibitors, NAC and Tempol [30], on TRAIL-induced apoptosis of TRAIL-sensitive MiaPaCa-2 and BxPC-3 cells. TRAIL significantly increased the percentages of annexin V+ cells among both cell lines (P<0.01). The addition of NAC, a peroxide inhibitor, significantly decreased the percentage of annexin V+ TRAIL-treated MiaPaCa-2 cells (P<0.05) but failed to decrease apoptosis in TRAIL-treated BxPC-3 cells (Fig 2A and 2B). Alternatively, the addition of Tempol, a superoxide inhibitor, had no effect on TRAIL-induced apoptosis of MiaPaCa-2 cells but increased it in TRAIL-treated BxPC-3 cells (P<0.01) (Fig 2C and 2D). These results indicate that ROS, peroxide and superoxide, exert opposite effects on the two TRAIL-sensitive cell lines; peroxide plays a pro-apoptotic role in TRAIL-treated MiaPaCa-2 cells, but superoxide is anti-apoptotic in TRAIL-treated BxPC-3 cells.
Fig 2. ROS-dependent apoptosis in TRAIL-treated MiaPaCa-2 cells.
(A) MiaPaCa-2 and BxPC-3 cells were cultured with TRAIL (50 ng/mL) with or without NAC (10 mM) for 24 h. After staining with annexin V-FITC/PI, flow cytometric analysis was performed. The numbers represent the proportions of each subset. (B) The percentages of annexin V+ cells are shown. (C) Both cell lines were cultured with TRAIL (50 ng/mL) with or without Tempol (1 mM) for 24 h and analyzed by flow cytometry. (D) The percentages of annexin V+ cells are shown. All data points shown represent the mean of three culture wells. *P<0.05, **P<0.01, N.S., not significant.
RIP1- and RIP3-dependent necroptosis in TRAIL-treated pancreatic cancer cells under ROS inhibition
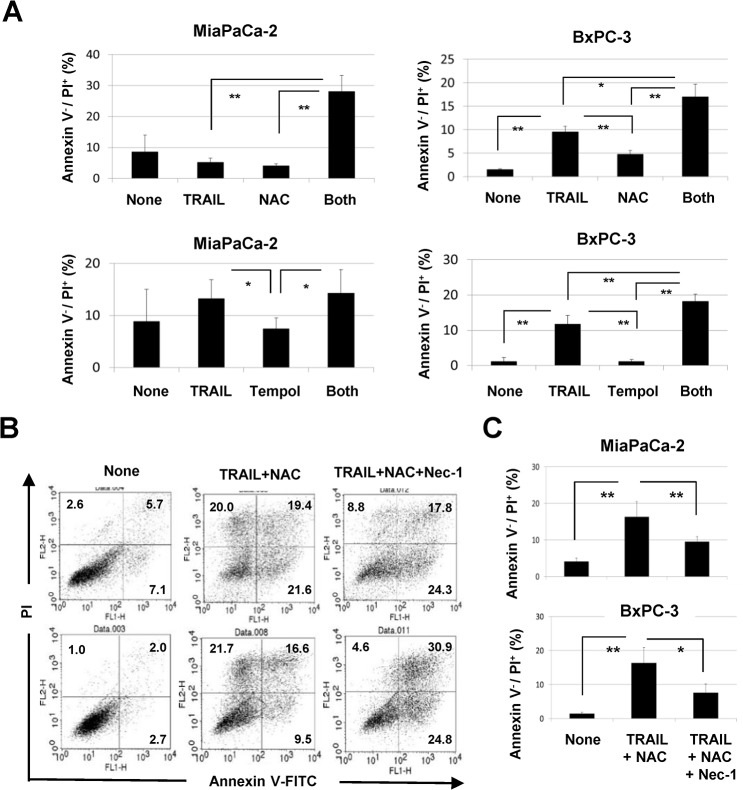
During examination of the effects of ROS inhibition on TRAIL-induced apoptosis of MiaPaCa-2 and BxPC-3 cells, we found that the percentages of annexin V-/PI+ early necrotic cells were increased by TRAIL treatment under ROS inhibition (Fig 2A and 2C). The results of annexin V-/PI+ early necrotic cells are calculated and presented in Fig 3A. The addition of NAC significantly increased the percentages of annexin V-/PI+ TRAIL-treated cells (P<0.01 for MiaPaCa-2, P<0.05 for BxPC-3). A similar result was observed when Tempol was added in TRAIL-treated BxPC-3 cells (P<0.01 for BxPC-3), but not in MiaPaCa-2 cells. Since annexin V-/PI+ cells represent early necrotic cells, these results suggest that TRAIL induced programmed necrosis (necroptosis) under ROS inhibition, especially peroxide inhibition. We next asked whether the addition of necrostatin-1, an inhibitor of RIP1 and of necrosis [31], could decrease these early necrotic cells. As shown in Fig 3B and 3C, the combination of TRAIL and NAC significantly increased the proportions of annexin V-/PI+ MiaPaCa-2 and BxPC-3 cells (P<0.01 for MiaPaCa-2 and BxPC-3), whereas the addition of necrostatin-1 significantly decreased them (P<0.01 for MiaPaCa-2, P<0.05 for BxPC-3).
Fig 3. TRAIL-induced necroptosis in human pancreatic cancer cells under ROS inhibition.
(A) The percentages of annexin V-/PI+ cells were calculated based on the results of Fig 2. (B) Both cell lines were cultured with TRAIL (50 ng/mL) and NAC (10 mM), with or without necrostatin-1 (20 μM) for 24 h. After staining with annexin V-FITC/PI, flow cytometric analysis was performed. The numbers represent the proportions of each subset. (C) The percentages of annexin V-/PI+ cells were calculated. All data points shown represent the mean of three culture wells. *P<0.05, **P<0.01.
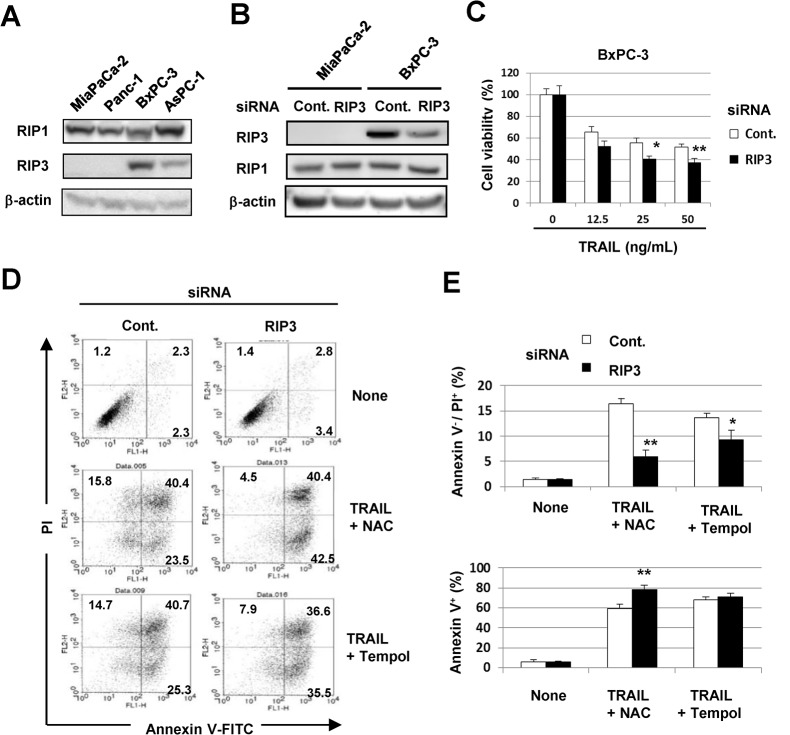
RIP1 and RIP3 are critical molecules for necroptosis [18–21]. Therefore, we examined the expression of RIP1 and RIP3 in four pancreatic cancer cell lines and found that all were positive for RIP1, while MiaPaCa-2 and Panc-1 were negative for RIP3 (Fig 4A). We further examined the effect of siRNA-mediated knockdown of RIP3 on necroptosis induced by coincubation with TRAIL and NAC. Transfection of RIP3 siRNA decreased the RIP3 protein expression in BxPC-3 cells, but showed no effect on the expression of RIP1 (Fig 4B). Knockdown of RIP3 slightly, but significantly, decreased the cell viability in response to TRAIL (P<0.05 at 25 ng/mL, P<0.01 at 50 ng/mL) (Fig 4C). As shown in Fig 4D and 4E, selective knockdown of RIP3 significantly decreased the proportions of annexin V-/PI+ cells after TRAIL treatment of BxPC-3 cells cultured with either NAC or Tempol (P<0.01 for NAC, P<0.05 for Tempol). Interestingly, inhibition of peroxide by NAC significantly increased the proportion of annexin V+ apoptotic cells in RIP3 siRNA-transfected and TRAIL-treated BxPC-3 cells (P<0.01). These results suggested that RIP1-dependent necroptosis is promoted in TRAIL-treated MiaPaCa-2 and BxPC-3 cells under inhibition of peroxide, and that TRAIL treatment under inhibition of ROS (peroxide and superoxide) promotes RIP3-dependent necroptosis in BxPC-3 cells.
Fig 4. RIP3-dependent necroptosis in TRAIL-treated BxPC-3 cells under ROS inhibition.
(A) The expression of RIP3 and RIP1 protein was examined in four cancer cell lines. β-Actin was used as a control. (B) MiaPaCa-2 and BxPC-3 cells were transfected with control siRNA or RIP3 siRNA. Three days after transfection, the cells were harvested and examined for RIP3 and RIP1 protein expression. (C) BxPC-3 cells transfected with either control siRNA or RIP3 siRNA 3 days prior were cultured with TRAIL. After 48 h, cell viability was determined by the WST-8 assay. (D) BxPC-3 cells transfected with either control siRNA or RIP3 siRNA 3 days prior were cultured with TRAIL and with either NAC (10 mM) or Tempol (1 mM) for 24 h. After staining with annexin V-FITC/PI, flow cytometric analysis was performed. The numbers represent the proportions of each subset. (E) The percentages of annexin V-/PI+ cells and annexin V+ cells were calculated. All data points shown represent the mean of three culture wells. *P<0.05, **P<0.01.
The roles of caspases in TRAIL-induced apoptosis and necroptosis
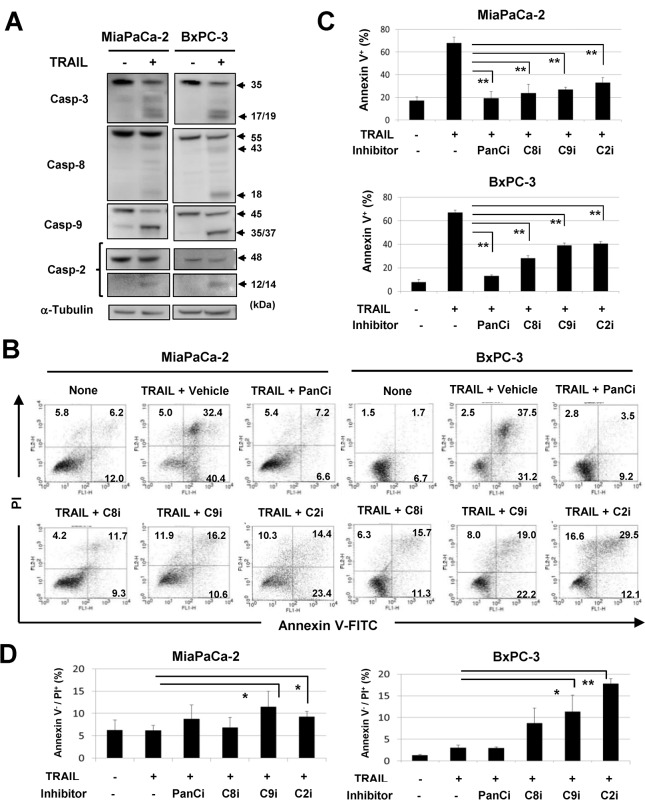
We next examined the roles of caspases in apoptosis and necroptosis in TRAIL-treated MiaPaCa-2 and BxPC-3 cells. TRAIL treatment activated caspase-3, -8, and -9 in both cell lines (Fig 5A). Since the role of caspase-2 in cell death has not been established fully [25, 32], we monitored this caspase and found that TRAIL treatment also activated caspase-2. We further examined the effects of a panel of caspase inhibitors on TRAIL-induced apoptosis (Fig 5B and 5C). The addition of the pan-caspase inhibitor Z-VAD profoundly inhibited TRAIL-induced apoptosis in both cell lines, and inhibitors of caspase-8, -9, or -2 also significantly inhibited TRAIL-induced apoptosis (P<0.01). We examined the effect of these inhibitors on annexin V-/PI+ early apoptotic cells (Fig 5D). Interestingly, inhibitors of either caspase-9 or -2 significantly increased the proportions of early apoptotic cells in TRAIL-treated MiaPaCa-2 and BxPC-3 cultures (P<0.05 or P<0.01). These results indicate that a panel of caspases participate in TRAIL-induced apoptosis in these lines and suggest that caspase-9 and -2 play an inhibitory role in TRAIL-induced necroptosis.
Fig 5. The roles of caspases in apoptosis and necroptosis of TRAIL-treated cells.
(A) MiaPaCa-2 and BxPC-3 cells were cultured with TRAIL (50 ng/mL) for 12 h, and protein expression levels of caspase-3, caspase-8, caspase-9, and caspase-2 were evaluated by immunoblot. α-Tubulin was used as the control. (B) MiaPaCa-2 and BxPC-3 cells were cultured with TRAIL (50 ng/mL) in the presence of a panel of caspase inhibitors (20 μM) for 24 h. After staining with annexin V-FITC/PI, flow cytometric analysis was performed. The numbers represent the proportions of each subset. The percentages of annexin V+ cells (C) and annexin V-/PI+ cells (D) were determined by flow cytometry. All data points shown represent the mean of three culture wells. *P<0.05, **P<0.01. panCi, pan-caspase inhibitor; C9i, caspase-9 inhibitor; C8i, caspase-8 inhibitor; C2i, caspase-2 inhibitor. Vehicle controls received an equal volume of DMSO.
No crosstalk between caspase-2/-9 and ROS in TRAIL-treated cancer cells
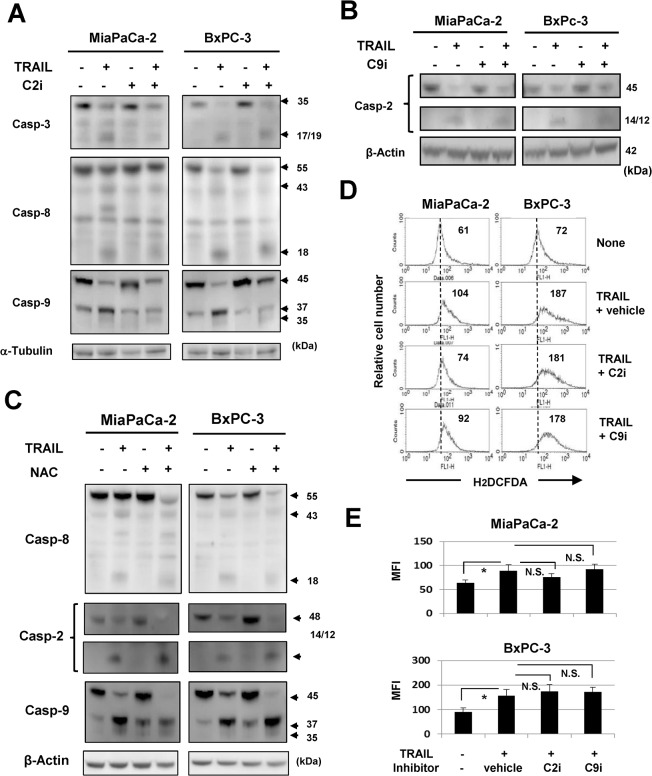
We next examined the relationship between caspase-9, -2, and ROS in TRAIL-treated pancreatic cancer cells. The addition of a caspase-2 inhibitor inhibited TRAIL-induced caspase-9 cleavage in two cell lines but had no apparent suppressive effect on caspase-8 activation (Fig 6A). A caspase-9 inhibitor did not suppress caspase-2 activation (Fig 6B). The addition of NAC failed to suppress TRAIL-induced activation of caspase-9 and -2 (Fig 6C). Finally, we examined the effects of caspase-2 or -9 inhibition on ROS production by TRAIL-treated cells. No clear suppression of ROS production was noted (Fig 6D and 6E). These results indicate that caspase-2 is upstream of caspase-9, and that there is no crosstalk between these caspases and ROS in TRAIL-treated MiaPaCa-2 and BxPC-3 cells.
Fig 6. No crosstalk among caspase-2/-9 and ROS in TRAIL-treated cancer cells.
(A) MiaPaCa-2 and BxPC-3 cells were cultured with TRAIL (50 ng/mL) with or without caspase-2 inhibitor (20 μM) for 12 h, and the protein expression levels of caspase-3, -8, and -9 were evaluated by immunoblot. α-Tubulin was used as the control. (B) MiaPaCa-2 and BxPC-3 cells were cultured with TRAIL (50 ng/mL) with or without caspase-9 inhibitor (20 μM) for 12 h, and the protein expression of caspase-2 was evaluated by immunoblot. β-Actin was used as the control. (C) MiaPaCa-2 and BxPC-3 cells were cultured with TRAIL (50 ng/mL) with or without NAC (10 mM) for 12 h, and the protein expression of caspase-8, -2, and -9 was evaluated by immunoblot. β-Actin was used as the control. (D) MiaPaCa-2 and BxPC-3 cells were cultured with TRAIL (50 ng/mL) with the indicated inhibitors (20 μM). As a vehicle control, an equal volume of DMSO was added. After 6 h for MiaPaCa-2 and 12 h for BxPC-3, these cells were cultured with carboxy-H2DCFDA for 30 min and examined for ROS levels by flow cytometry. The number represents the mean fluorescence intensity. (E) Data represent the mean of three culture wells. MFI: mean fluorescence intensity. *P<0.05, N.S., not significant.
Discussion
Since pancreatic cancer is highly resistant to conventional therapies and is associated with a poor prognosis [33], new treatment modalities are required. TRAIL may be useful therapeutically, because this molecule can induce cell death in many types of cancers while causing almost no cytotoxicity to normal cells [2]. In this study, we first investigated the roles of ROS in TRAIL-induced apoptosis of human pancreatic cancer cell lines. During these experiments, we unexpectedly found that inhibition of ROS promoted TRAIL-induced necrosis of cancer cells. We then determined that this cell death was necroptosis.
ROS play diverse roles in many types of cells. Low physiological levels of ROS function as second messengers in intracellular signaling and are required for normal cell functions, whereas excessive ROS impair cell functions and promote cell death [34]. TRAIL has been shown to induce ROS generation in cancer cells [35], and antioxidants block DR signaling-mediated apoptosis [36, 37], suggesting that ROS are mediators of death ligand-induced apoptosis. Here, we found that ROS were produced only in TRAIL-sensitive pancreatic cancer cell lines, and that peroxide contributes, at least partially, to TRAIL-induced apoptosis in MiaPaCa-2 cells (Fig 2B). Alternatively, the inhibitor of superoxide Tempol unexpectedly increased apoptosis in TRAIL-treated BxPC-3 cells (Fig 2D), suggesting that superoxide acts as an anti-apoptotic mediator under these conditions. What was the mechanism of the inhibitory effect of superoxide on apoptosis in TRAIL-treated BxPC-3 cells? ROS, especially superoxide, can induce autophagy [26, 38, 39], and autophagy can function cytoprotectively [40]. We recently reported that autophagy plays an anti-apoptotic role in TRAIL-treated pancreatic cancer cells [29]. Therefore, we explored whether autophagy was induced in BxPC-3 cells and the superoxide inhibitor Tempol suppressed ROS-induced autophagy, resulting in the promotion of apoptosis. We evaluated the level of autophagy in cancer cells by examining the expression of LC3. As a result, autophagy was strongly induced in BxPC-3 cells compared with the other pancreatic cancer cell lines, while Tempol failed to inhibit the expression of LC3-type II, as a marker of autophagy [41], in BxPC-3 cells “S1 Fig”. Autophagy does not contribute to ROS-mediated protection in TRAIL-treated BxPC-3 cells. Further studies are required to elucidate the precise mechanism.
Some ligands, including TNFα, FasL, and TRAIL, can induce both apoptosis and necroptosis [1]. The mechanisms that dictate the cellular decision to undergo apoptosis or necroptosis have been investigated intensively. In apoptosis, caspases are the executioners of apoptosis; however, these proteases have no positive role in necroptosis [15]. ROS are proposed to be executioners of necroptosis [42], and some cell types, such as mouse L929 and embryonic fibroblasts, produce ROS in response to TNFα and show necroptosis [43, 44]. Additionally, treatment with antioxidants inhibits necroptosis in some cell types [44]. However, ROS are not required for necroptosis in all cell types and antioxidants are unable to protect some cell lines from this type of cell death [45, 46]. These lines of evidence suggest that ROS-mediated necroptosis is likely a cell type-dependent phenomenon. In this study, we showed that the addition of NAC to inhibit peroxide promoted necroptosis upon TRAIL stimulation in two human pancreatic cancer cell lines (Fig 3A). This increase was decreased by necrostatin-1, a RIP1 inhibitor [30] (Fig 3B and 3C), and siRNA-mediated RIP3 knockdown decreased necroptosis in TRAIL-treated BxPC-3 cells under inhibition of ROS (peroxide and superoxide) (Fig 4D and 4E). These results indicated that ROS play an inhibitory role in TRAIL-induced necroptosis in BxPC-3 cells. Alternatively, MiaPaCa-2 cells were negative for RIP3 (Fig 4), suggesting that RIP3 was dispensable for necroptosis in TRAIL-treated MiaPaCa-2 cells.
Our results suggest that no obvious crosstalk between ROS and caspase-2/caspase-9 exists in the experimental system. It remains unresolved how an early necrotic population was induced by TRAIL treatment under inhibition of caspase-2 or -9. Inhibition of caspase-8 promotes RIP1/RIP3 complex formation, resulting in death signaling-induced necroptosis [19, 20]. However, this did not seem to be the case in these experiments, where inhibition of caspase-8 had less of an effect than did inhibition of caspase-2 or -9 (Fig 5B and 5D). Furthermore, the pan-caspase inhibitor Z-VAD had no effect on TRAIL-induced necroptosis of pancreatic cancer cells.
Caspase-2 has been referred to as an “orphan” caspase [25], and its role in TRAIL-induced apoptosis of cancer cells has not been established fully. It has been reported that caspase-2 is necessary for optimal TRAIL-mediated cleavage of Bid in human colon cancer cells [47]. In addition, caspase-2 primes cancer cells for TRAIL-induced apoptosis by processing procaspase-8 [48]. In this study, we showed that inhibition of caspase-2 suppressed apoptosis in TRAIL-treated cancer cells. Alternatively, ROS activate caspase-2, and DNA damage also induces its cleavage [49]. We recently reported that ROS trigger DNA damage, thereby leading to activation of caspase-2 in renal cell carcinoma lines [50]. Caspase-2 activation in response to DNA damage provides an important link between such damage and engagement of the apoptotic pathway [50, 51]. Additionally, ROS triggered caspase-2 activation and induced apoptosis in a human leukemic T cell line [51]. However, in this study, we found no crosstalk between ROS and caspases (Fig 6).
The expression of either DR4 or DR5 is prerequisite for TRAIL-induced apoptosis. The five cell lines studies were positive for DR5, but not for DR4 (Fig 1B). Therefore, the TRAIL-induced apoptosis of MiaPaCa-2 and BxPC-3 cells must act through DR5. By contrast, the other two pancreatic cancer cell lines and PrEC cells were relatively resistant to TRAIL, although they expressed DR5. Although DcRs are thought to prevent TRAIL-induced apoptosis in normal cells [4], normal epithelial PrEC cells partially expressed DcR2 to the same level as TRAIL-sensitive MiaPaCa-2 cells. Therefore, the expression of DcRs cannot account for TRAIL-resistance of Panc-1 and AsPC-1 cells, as well as that of PrEC cells. However, TRAIL-mediated apoptosis is regulated by several other mechanisms [52]: dysfunction or mutations in DR4/DR5; defects in the death-inducing signaling complex; a defect in effector caspases; and changes in pro-apoptotic or anti-apoptotic proteins. At this time, the differences in TRAIL-sensitivity among the five cell lines used in this study remain unclear.
In conclusion, we show that peroxide is involved in TRAIL-induced apoptosis in human pancreatic cancer MiaPaCa-2 cells, and that both peroxide and superoxide play regulatory roles in necroptosis of TRAIL-treated cells. These results show that TRAIL can induce two types of cell death, i.e., apoptosis and necroptosis, in human pancreatic cancer cells under specific conditions. Necroptosis can induce inflammation more vigorously than apoptosis [17, 18], but ROS play regulatory roles in cell death-associated inflammation via preferential inhibition of necroptosis compared to apoptosis. Dying cancer cells might produce ROS to inhibit cell death-associated inflammation. Given that pancreatic cancer is accompanied by inflammation [53], this study of necroptosis should increase our understanding of the pathophysiology of pancreatic cancer.
Supporting Information
(A) Four pancreatic cancer cell lines were examined for expression of LC3 by immunoblot. (B) LC3 expression in BxPC-3 cells treated with Tempol (1 mM) for 24 h was examined by immunoblot. β-Actin was used as the control.
(TIF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported in part by grants from the Ministry of Education, Science, Sport, Culture, and Technology of Japan (no. 25430150 to N. H., and no. 24501331 to M. H.) and from the Shimane University “SUIGANN” Project. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Ashkenazi A, Dixit VM (1999) Apoptosis control by death and decoy receptors. Curr Opin Cell Biol 11: 255–60. [DOI] [PubMed] [Google Scholar]
- 2. Almasan A, Ashkenazi A (2003) Cytokine & Growth Factor Reviews 14: 337–48. [DOI] [PubMed] [Google Scholar]
- 3. Almasan A, Ashkenazi A (2003) Apo2L/TRAIL: apoptosis signaling, biology, and potential for cancer therapy. Cytokine Growth Factor Rev 14:337–348. [DOI] [PubMed] [Google Scholar]
- 4. Pan G, Ni J, Wei YF, Yu G, Gentz R, Dixit VM (1997) An antagonist decpy receptor and a death domain-containing receptor for TRAIL. Science 277:815–818. [DOI] [PubMed] [Google Scholar]
- 5. Herbst RS, Eckhardt G, Kurzrock R, Ebbinghaus S, O’Dwyer PJ, Gordon MS (2010) Phase I dose-escalation study of recombinant human Apo2L/TRAIL, a dual proapoptotic receptor agonist, in patients with advanced cancer. J Clin Oncol 28: 2839–2846. 10.1200/JCO.2009.25.1991 [DOI] [PubMed] [Google Scholar]
- 6. Soria JC, Márk Z, Zatloukal P, Szima B, Albert I, Juháse E, (2011) Randomized phase II study of dulanermin in comination with paclitaxel, carboplatin, and bevacizuan in advanced non-small-cell lung cancer. J Clin Oncol 29: 4442–4451. 10.1200/JCO.2011.37.2623 [DOI] [PubMed] [Google Scholar]
- 7. Konopleva M, Zhao S, Xie Z, Segall H, Younes A, Claxton DF, et al. (1999) Apoptosis. molecules and mechanisms. Adv Exp Med Biol 457: 217–236. [PubMed] [Google Scholar]
- 8. Li H, Zhu H, Xu CJ, Yuan J (1998) Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94: 491–501. [DOI] [PubMed] [Google Scholar]
- 9. Thannickal VJ, Fanburg BL (2000) Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol 279: L1005–L1028. [DOI] [PubMed] [Google Scholar]
- 10. Singh KK. (2006) Mitochondria damage checkpoint, aging and cancer. Ann N Y Acad Sci 1067: 182–190. [DOI] [PubMed] [Google Scholar]
- 11. Kim BM, Rode AB, Han EJ, Hong IS, Hong SH (2012) 5-Phenylselenyl- and 5-methylselenyl-methyl-2’-deoxyuridine induce oxidative stress, DNA damage, and caspase-dependent apoptosis in cancer cells. Apoptosis 17: 200–216. 10.1007/s10495-011-0665-2 [DOI] [PubMed] [Google Scholar]
- 12. Suzuki-Karasaki Y, Suzuki-Karasaki M, Uchida M, Ochiai T (2014) Depolarization controls TRAIL-sensitization and tumor-selective killing of cancer cells: crosstalk with ROS. Frontiers in Oncol 4: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yi L, Zongyuan Y, Cheng G, Lingyun Z, Guilian Y, Wei G (2014) Quercetin enhances apoptotic effect of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in ovarian cancer cells through reactive oxygen species (ROS) mediated CCAAT enhancer receptor 5 pathway. Cancer Sci 105: 520–527. 10.1111/cas.12395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Park KJ, Lee CH, Kim A, Jeong KJ, Kim CH, Kim YS (2012) Death Receptors 4 and 5 Activate Nox 1 NADPH Oxidase through Riboflavin Kinase to Induse Reactive Oxygen Species-mediated Apoptotic Cell Death. J Biol Cehm 287: 3313–3325. 10.1074/jbc.M111.309021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Christofferson DE, Yuan J (2010) Necroptosis as an alternative form of programmed cell death. Current Opi Cell Biol 22: 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Linkermann A, Green DR (2014) Necroptosis. N Engl J Med 370: 455–465. 10.1056/NEJMra1310050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kaczmarek A, Vandenabeele P, Krysko DV (2013) Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity 38: 209–223. 10.1016/j.immuni.2013.02.003 [DOI] [PubMed] [Google Scholar]
- 18. Moriwaki K, Chan F K-M (2013) RIP3: a molecular switch for necrosis and inflammation. Genes Develop 27: 1640–1649. 10.1101/gad.223321.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dillon CP, Weinlich R, Rodriguez DA, Cripps JG, Quarato G, Gurung P, et al. (2014) RIPK1 Blocks Early Postnatal Lethality Mediated by Caspase-8 and RIP3. Cell 157: 1189–1202. 10.1016/j.cell.2014.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Green DR, Oberst A, Dillon CP, Weinlich R, Salvesen GS (2011) RIP-dependent necrosis and regulation by caspase: A mystery in five acts. Mol Cell 44: 9–16. 10.1016/j.molcel.2011.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, et al. (2009) RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 325: 332–336. 10.1126/science.1172308 [DOI] [PubMed] [Google Scholar]
- 22. Jouan-Lanhouet S, Arshad MI, Piquiet-Pellorce C, Martin-Chouly C, Moigne-Muller GL, Van Herreweghe F, et al. (2012) TRAIL induces necroptosis involving RIPK1/RIPK3-dependent PARP-1 activation. Cell Death Differ 19: 2003–2014. 10.1038/cdd.2012.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Meurette O, Rebillard A, Huc L, Moigne GL, Merino D, Micheau O, et al. , (2007) TRAIL induces receptor-interacting protein 1-dependent and caspase-dependent necrosis-like cell dearth under acidic extracellular conditions. Cancer Res 67: 218–226. [DOI] [PubMed] [Google Scholar]
- 24. Karl I, Jossberger-Werner M, Schmidt N, Horn S, Goebeler M, Leverkus M, et al. (2014) TRAF2 inhibits TRAIL- and CD95L-induced apoptosis and necroptosis. Cell Death Dis 5: e1444 10.1038/cddis.2014.404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhivotovsly B, Orrenius S (2005) Caspase-2 function in response to DNA damage. Biochem Biophys Res Commun 331: 859–867. [DOI] [PubMed] [Google Scholar]
- 26. Chen Y, Azad MB, Gibson SB (2009) Superoxide is the major reactive oxygen specieis regulating autophagy. Cell Death Differ 16: 1040–1052. 10.1038/cdd.2009.49 [DOI] [PubMed] [Google Scholar]
- 27. Shimojo Y, Akimoto M, Hisanaga T, Tanala T, Tajima Y, Honma Y, et al. (2013) Attenuation of reactive oxygen species by antioxidants suppress hypoxia-induced epithelila-mesenchymal transition and metastasis of pancreatic cancer cells. Clin Exp Metastasis 30:143–154. 10.1007/s10585-012-9519-8 [DOI] [PubMed] [Google Scholar]
- 28. Nagaraj NS, Washington MK, Merchant NB (2011) Combined blockade of Src kinase and epidermal growth factor receptor with gemcitabine overcomes STAT3-mediated resistance of inhibition of pancreatic tumor growth. Clin Cancer Res 17:483–493. 10.1158/1078-0432.CCR-10-1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Monma H, Harashima N, Inao T, Okano S, Tajima Y, Harada M (2013) The HSP70 and autophagy inhibitor pifithrin-μ enhances the antitumor effects of TRAIL on human pancreatic cancer. Mol Cancer Ther 12: 341–351. 10.1158/1535-7163.MCT-12-0954 [DOI] [PubMed] [Google Scholar]
- 30. Tiwari M, Sharma LK, Vanegas D, Callaway DA, Bai Y, Lechleiter JD (2014) A nonapoptotic role for CASP2/caspase 2. Autophagy 10: 1054–1070. 10.4161/auto.28528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Degterev A, Hitomi J, Germscheid M, Ch’en IL, Korkina O, Teng X, et al. (2008) Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol 4: 313–321. 10.1038/nchembio.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Puccini J, Dorstyn L, Kumar S (2013) Caspase-2 as a tumor suppressor. Cell Death Differ 20: 1133–1139. 10.1038/cdd.2013.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ (2009) Cancer statistics 2009. CA J Clin 59: 225–49. 10.3322/caac.20006 [DOI] [PubMed] [Google Scholar]
- 34. Circu ML, Aw TY (2010) Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med 48: 749–762. 10.1016/j.freeradbiomed.2009.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mellier G. Pervaiz S (2012) The three Rs along the TRAIL: resistance, re-sensitization and reactive oxygen species (ROS). Free Radic Res 46: 996–1003. 10.3109/10715762.2012.690514 [DOI] [PubMed] [Google Scholar]
- 36. Delhalle S, Deregowski V, Benoit V, Merville MP, Bours V (2002) NF-kappaB-dependent MnSOD expression protects adenocarcinoma cells from TNF-alpha-induced apoptosis. Oncogene 21: 3917–3924. [DOI] [PubMed] [Google Scholar]
- 37. Perez-Cruz I, Carcamo JM, Golde DW (2003) Vitamin C inhibits FAS-induced apoptosis in monocytes and U937 cells. Blood 102: 336–343. [DOI] [PubMed] [Google Scholar]
- 38. Wang Y, Nartiss Y, Steipe B, McQuibban GA, Kim PK (2012) ROS-induced mitochondrial depolarization initiates PARK2/PARKIN-dependent mitochondrial degradation by autophagy. Autophagy 8: 1462–1476. 10.4161/auto.21211 [DOI] [PubMed] [Google Scholar]
- 39. Gewirtz DA (2014) The four faces of autophagy: implication for cancer therapy. Cancer Res 74: 647–651. 10.1158/0008-5472.CAN-13-2966 [DOI] [PubMed] [Google Scholar]
- 40. Han J, Hou W, Goldstein LA, Lu C, Stolz DB, Yin XM, et al. (2008) Involvement of protective autophagy in TRAIL resistance of apoptosis-defective tumor cells. J Bio Chem 283: 19665–19677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T (2000) LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 19: 5720–5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vanlangenakker N, Berghe TV, Bogaert P, Laukens B, Zobel K, Deshayes K, et al. (2011) clAP1 and TAK1 protect cells from TNF-induced necrosis by preventing RIP1/RIP3-dependent reactive oxygen species production. Cell Death Differ 18: 656–665. 10.1038/cdd.2010.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Goossens V, De Vos K, Vercammen D, Steemans M, Vancompemolle K, Fiers W, et al. (1999) Redox regulation of TNF signaling. Biofactors 10: 145–156. [DOI] [PubMed] [Google Scholar]
- 44. Lin Y, Choksi S, Shen HM, Yang QF, Hur GM, Kim YS, et al. (2004) Tumor necrosis factor-induced nonapoptotic cell death requires receptor-interacting protein-mediated cellular reactive oxygen species accumulation. J Biol Chem 279: 10822–10828. [DOI] [PubMed] [Google Scholar]
- 45. He S, Wang L, Miao L, Wang T, Du F, Zhao L, et al. (2009) Receptor interacting protein kinase-3 determine cellular necrotic response to TNF-alpha. Cell 137: 1100–1111. 10.1016/j.cell.2009.05.021 [DOI] [PubMed] [Google Scholar]
- 46. Temkin V, Huang Q, Liu H, Osada H, Pope RM (2006) Inhibition of ADP/ATP exchange in receptor-interacting protein-mediated necrosis. Mol Cell Biol 26: 22125–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wagner KW, Engels IH, Deveraux QL (2004) Caspase-2 Can Function Upstream of Bid Cleavage in the TRAIL Apoptosis Pathway. J Biol Chem 279: 35047–35052. [DOI] [PubMed] [Google Scholar]
- 48. Shin S, Lee Y, Kim W, Ko H, Choi H, Kim K (2005) Caspase-2 primes cancer cells for TRAIL-mediated apoptosis by processing procaspase-8. EMBO J 24: 3532–3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Robertson JD, Enoksson M, Suomela M, Zhivotovsky B, Orrenius S (2002) Caspase-2 acts upstream of mitochondria to promote cytochrome c release during etoposide-induced apoptosis. J Biol Chem 277: 29803–29809. [DOI] [PubMed] [Google Scholar]
- 50. Harashima N, Minami T, Uemura H, Harada H (2014) Transfection of poly(I:C) can induce reactive oxygen species-triggered apoptosis and interferon-β-mediated growth arrest in human renal cell carcinoma cells via innate adjuvant receptors and the 2-5A system. Mol Cancer 13: 217 10.1186/1476-4598-13-217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Prasad V, Chandele A, Jagtap JC, Kumar PS, Shastry P (2006) ROS-triggered caspase 2 activation and feed back amplification loop in β-carotene-induced apoptosis. Free Rad Biol Med 41: 431–442. [DOI] [PubMed] [Google Scholar]
- 52. Zhang L, Fang B (2005) Mechanisms of resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Ther 12:228–237. [DOI] [PubMed] [Google Scholar]
- 53. Ebrahimi B, Tucker SL, Li D, Abbruzzese JL, Kurzrock R (2004) Cytokines in pancreatic cancrcinoma: correlation with phanotypic characteristics and prognosis. Cancer 101: 2727–2736. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Four pancreatic cancer cell lines were examined for expression of LC3 by immunoblot. (B) LC3 expression in BxPC-3 cells treated with Tempol (1 mM) for 24 h was examined by immunoblot. β-Actin was used as the control.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.