Abstract
Background
This review is an update of the first Cochrane publication on selenium for preventing cancer (Dennert 2011).
Selenium is a metalloid with both nutritional and toxicological properties. Higher selenium exposure and selenium supplements have been suggested to protect against several types of cancers.
Objectives
Two research questions were addressed in this review: What is the evidence for:
1. an aetiological relation between selenium exposure and cancer risk in humans? and 2. the efficacy of selenium supplementation for cancer prevention in humans?
Search methods
We conducted electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL, 2013, Issue 1), MEDLINE (Ovid, 1966 to February 2013 week 1), EMBASE (1980 to 2013 week 6), CancerLit (February 2004) and CCMed (February 2011). As MEDLINE now includes the journals indexed in CancerLit, no further searches were conducted in this database after 2004.
Selection criteria
We included prospective observational studies (cohort studies including sub‐cohort controlled studies and nested case‐control studies) and randomised controlled trials (RCTs) with healthy adult participants (18 years of age and older).
Data collection and analysis
For observational studies, we conducted random effects meta‐analyses when five or more studies were retrieved for a specific outcome. For RCTs, we performed random effects meta‐analyses when two or more studies were available. The risk of bias in observational studies was assessed using forms adapted from the Newcastle‐Ottawa Quality Assessment Scale for cohort and case‐control studies; the criteria specified in the Cochrane Handbook for Systematic Reviews of Interventions were used to evaluate the risk of bias in RCTs.
Main results
We included 55 prospective observational studies (including more than 1,100,000 participants) and eight RCTs (with a total of 44,743 participants). For the observational studies, we found lower cancer incidence (summary odds ratio (OR) 0.69, 95% confidence interval (CI) 0.53 to 0.91, N = 8) and cancer mortality (OR 0.60, 95% CI 0.39 to 0.93, N = 6) associated with higher selenium exposure. Gender‐specific subgroup analysis provided no clear evidence of different effects in men and women (P value 0.47), although cancer incidence was lower in men (OR 0.66, 95% CI 0.42 to 1.05, N = 6) than in women (OR 0.90, 95% CI 0.45 to 1.77, N = 2). The most pronounced decreases in risk of site‐specific cancers were seen for stomach, bladder and prostate cancers. However, these findings have limitations due to study design, quality and heterogeneity that complicate interpretation of the summary statistics. Some studies suggested that genetic factors may modify the relation between selenium and cancer risk—a hypothesis that deserves further investigation.
In RCTs, we found no clear evidence that selenium supplementation reduced the risk of any cancer (risk ratio (RR) 0.90, 95% CI 0.70 to 1.17, two studies, N = 4765) or cancer‐related mortality (RR 0.81, 95% CI 0.49 to 1.32, two studies, N = 18,698), and this finding was confirmed when the analysis was restricted to studies with low risk of bias. The effect on prostate cancer was imprecise (RR 0.90, 95% CI 0.71 to 1.14, four studies, N = 19,110), and when the analysis was limited to trials with low risk of bias, the interventions showed no effect (RR 1.02, 95% CI 0.90 to 1.14, three studies, N = 18,183). The risk of non‐melanoma skin cancer was increased (RR 1.44, 95% CI 0.95 to 1.17, three studies, N = 1900). Results of two trials—the Nutritional Prevention of Cancer Trial (NPCT) and the Selenium and Vitamin E Cancer Trial (SELECT)—also raised concerns about possible increased risk of type 2 diabetes, alopecia and dermatitis due to selenium supplements. An early hypothesis generated by NPCT that individuals with the lowest blood selenium levels at baseline could reduce their risk of cancer, particularly of prostate cancer, by increasing selenium intake has not been confirmed by subsequent trials. As the RCT participants were overwhelmingly male (94%), gender differences could not be systematically assessed.
Authors' conclusions
Although an inverse association between selenium exposure and the risk of some types of cancer was found in some observational studies, this cannot be taken as evidence of a causal relation, and these results should be interpreted with caution. These studies have many limitations, including issues with assessment of exposure to selenium and to its various chemical forms, heterogeneity, confounding and other biases. Conflicting results including inverse, null and direct associations have been reported for some cancer types.
RCTs assessing the effects of selenium supplementation on cancer risk have yielded inconsistent results, although the most recent studies, characterised by a low risk of bias, found no beneficial effect on cancer risk, more specifically on risk of prostate cancer, as well as little evidence of any influence of baseline selenium status. Rather, some trials suggest harmful effects of selenium exposure. To date, no convincing evidence suggests that selenium supplements can prevent cancer in humans.
Keywords: Female, Humans, Male, Case‐Control Studies, Neoplasms, Neoplasms/prevention & control, Observational Studies as Topic, Odds Ratio, Randomized Controlled Trials as Topic, Selenium, Selenium/administration & dosage, Selenium/adverse effects, Sex Factors, Trace Elements, Trace Elements/administration & dosage, Trace Elements/adverse effects
Selenium for preventing cancer
Review question
We reviewed the evidence suggesting that selenium can help to prevent cancer. This review updates the first Cochrane review on this topic (Dennert 2011).
Background
Selenium is a naturally occurring element found in crops, animal products and water. Small amounts of selenium are needed for proper human nutrition. Starting in the 1960s, numerous studies reported that people with high levels of selenium in their diet or in their body tissues had lower rates of cancer. Some laboratory studies also suggested that selenium could inhibit the growth of cancer cells. This led to widespread interest and claims that taking selenium supplements could prevent cancer. Over the next decades, many more studies were conducted to compare cancer rates among individuals with high and low selenium levels, and several trials were conducted in which individuals were randomly assigned to receive selenium supplements or placebo and then were followed so their cancer rates could be determined. Particular interest focused on whether selenium could prevent prostate, skin or other specific types of cancer.
Study characteristics
This review includes 55 studies in which adults observed to have high or low selenium levels were followed over time to determine whether they developed cancer, along with eight trials in which adults were randomly assigned to receive selenium supplements or placebo. The evidence is current to February 2013.
Key results
We found limited evidence suggesting that individuals observed to have higher selenium levels have a lower incidence of cancer. However, it is not possible to conclude from these studies that selenium was the reason for the lower cancer risk, because a high selenium level might be associated with other factors that reduce cancer risk, such as a healthier diet or lifestyle. Also, selenium comes in many different chemical forms that have different biological activity, and these studies did not identify which chemical forms were being measured. Selenium levels in body tissues in which people might develop cancer (e.g. the prostate) also were not examined.
The randomised controlled trials that assessed whether taking selenium supplements might prevent cancer differed considerably in methodological quality and are not equally reliable. Several studies reported that individuals receiving selenium supplements decreased their liver cancer risk, but these studies reported insufficient details about their randomisation process and participant follow‐up to be convincing. Recent trials that were judged to be well conducted and reliable have found no effects of selenium on reducing the overall risk of cancer or on reducing the risk of particular cancers, including prostate cancer. In contrast, some trials suggest that selenium may increase the risk of non‐melanoma skin cancer, as well as of type 2 diabetes, raising concern about the safety of selenium supplements.
Overall, no convincing evidence suggests that selenium supplements can prevent cancer. However, for a full understanding of the role of this metalloid in cancer development, more research is needed on how selenium may act differently in individuals with different genetic backgrounds or nutritional status, and on the different biological activities of the various selenium compounds, which are still largely unknown.
Background
This review is an update of the first Cochrane publication on selenium for preventing cancer (Dennert 2011).
Description of the condition
Cancer is a leading cause of death worldwide (WHO 2008). According to World Health Organization (WHO) estimates, 14.1 million people developed and 8.2 million died of cancer in 2012, with more than half of all new cases occurring in less developed regions of the world (IARC 2012).
The role of diet and nutrition in carcinogenesis and cancer prevention has been an area of active research for decades. A holy grail has been the identification of nutritional supplements with cancer preventive properties. Such dietary factors would clearly have major public health implications, but unfortunately, investigations into supplementation of various vitamins, trace elements and other dietary constituents have generally yielded disappointing and even troubling results (Ashar 2010; Bjelakovic 2012; Driscoll 2010; Fortmann 2013; Guallar 2013; Jerome‐Morais 2011; Marik 2012; Martinez 2012; Mayne 2012; Rocourt 2013).
Description of the intervention
The metalloid selenium is one of the dietary elements that has received considerable attention as a potential cancer preventive agent. Selenium is nutritionally essential for humans but is toxic at higher levels, with a narrow safe range of intake (Rayman 2012; Vinceti 2013a; Vinceti 2013b). Whether selenium provides various health benefits (including a cancer preventive effect) beyond its essential nutritional role is a matter of ongoing debate (Bodnar 2012; Fortmann 2013; Karp 2013; Lippman 2009, in: SELECT 2009; Rayman 2012; Stranges 2010;Vinceti 2013a; Vinceti 2013b; Vinceti 2013d). Humans usually ingest this trace element with crop and animal products and sometimes in functional foods or supplements (Hurst 2013; Vinceti 2000a). Chemical forms and concentrations of selenium in environmental matrices, foods, drinking water and other sources of exposure vary considerably, depending on factors such as plant and animal metabolism and growth conditions or animal nutrition (Rayman 2008a; Rayman 2008b).
Selenium species can be classified into organically bound selenium forms (e.g. selenomethionine, selenocysteine) and inorganic forms (e.g. selenate, selenite) (Gammelgaard 2011; Weekley 2013). Selenium yeast refers to a selenium‐enriched yeast medium that usually contains nearly entirely organically bound selenium with a high proportion of selenomethionine (Block 2004; Rayman 2004).
The recommended intake of selenium differs between regulatory agencies (Hurst 2013; Vinceti 2009; Vinceti 2013a). For example, the US Institute of Medicine recommends a daily intake of 55 µg/d for adults (Institute of Medicine 2009), whereas the WHO recommends values ranging from 25 to 34 µg/d, depending on age and sex (WHO 2004). These various standards do not take into account the chemical forms of selenium, despite growing evidence of the importance of selenium speciation (Vinceti 2013a; Vinceti 2013c; Weekley 2013).
To prevent adverse effects due to excessive selenium intake, the US Institute of Medicine has set the tolerable upper intake level to 400 µg/d for adults (Office of Dietary Supplements 2009); however, recent epidemiological studies suggest toxicity at lower intake levels (Lippman 2009, in: SELECT 2009; Stranges 2007; Vinceti 2013a). In addition to the acute and chronic toxicity of high selenium exposure, possible harmful effects of long‐term intake of lower dosages have been a matter of concern. However, such effects are still inadequately investigated (Vinceti 2001; Vinceti 2009). Furthermore, strong evidence shows different biological activities of the various organic and inorganic forms of selenium (Hazane‐Puch 2013; Rayman 2008a; Vinceti 2009; Vinceti 2013c;Weekley 2013), suggesting the opportunity to better characterise the specific toxicological and nutritional properties of each selenium species in humans, in animals and in the environment. Recent publications have questioned the adequacy of the current upper 'safe' limit of intake (Jerome‐Morais 2011;Morris 2013; Moyad 2012;Rocourt 2013;Sacco 2013;Vinceti 2009; Vinceti 2013b) and have espoused the need to set different limits for the many different sources of organic and inorganic selenium.
Accurate estimation of selenium exposure in epidemiological studies presents several challenges. Individual exposure is typically assessed by using peripheral biomarkers of exposure, such as blood (generally plasma or serum) or nail concentrations, or by estimating dietary intake (Ashton 2009). All of these methods have strengths and limitations, and their validity has been questioned (Ashton 2009;Haldimann 1996;Vinceti 2013b). However, levels of selenium in peripheral biomarkers such as blood, toenail and hair have been found to correlate to a moderate degree with dietary intake as assessed through self reported consumption of supplements, food frequency questionnaires and dietary records (Hurst 2013; Longnecker 1996; Ovaskainen 1993; Pestitschek 2013; van den Brandt 1993a). )Stronger correlation has been seen at high intake levels (Morris 2013), although results of other studies were not consistent (Hunter 1990; Karita 2003; Satia 2006; Vinceti 2012). Assessment of selenium levels in highly specific body tissues, is extremely complex, as these levels are not necessarily homogeneously reflected by all biomarkers because overall selenium exposure, as well as its chemical forms and other factors, influences distribution of the metalloid into various body compartments (Behne 1996; Behne 2010; Panter 1996; Vinceti 2000a; Vinceti 2013c). For example, circulating levels of some selenium species and of total selenium did not correlate with selenium content in the central nervous system as assessed by cerebrospinal fluid concentrations (Solovyev 2013; Vinceti 2013c), indicating not only the tissue‐specific significance of biomarkers but also the importance of selenium speciation when the distribution of selenium in different body compartments is assessed, representing target organs for different diseases.
Selenium levels found in human specimens (Rayman 2008b), as well as the estimated intake of selenium (Fairweather‐Tait 2011; Haldimann 1996; Jablonska 2013), show high global variability due to factors such as dietary habits, ethnicity, gender, age, individual metabolism, occupational exposure, exposure to coal and other sources of combustion and smoking. It is interesting to note that smoking tends to lower selenium biomarker concentrations, although it is a source of selenium exposure (Jossa 1991;Kafai 2003)—a phenomenon that might be related to altered metabolism of the metalloid due to an interaction with cadmium. Globally, inconsistencies have been noted as to how these factors are associated with selenium levels (Haldimann 1996; Vinceti 2000a). For example, selenium levels increased with age in women, but not in men, in the French SU.VI.M.AX cohort study (Arnaud 2007) and decreased with age in a female population in Ohio (Smith 2000); however, two studies in Switzerland and Austria could not find an association between age and selenium status in either gender (Burri 2008; Gundacker 2006). Gender‐specific nutritional and health behaviours, as well as gender‐specific differences in selenium metabolism, may contribute to observed discrepancies in selenium levels between males and females (Combs 2012; Rodriguez 1995). Gender might more generally influence the ability of selenium to induce adverse metabolic effects, as suggested by the recent observation of a direct association between metabolic syndrome and selenium in females but not in males in a European case‐control study (Arnaud 2012).
How the intervention might work
The ability of selenium to counteract cancer cell growth, as has been observed in a large number of laboratory studies, may be due to its effects on DNA stability, cell proliferation, necrotic and apoptotic cell death in healthy and malignant cells, regulation of oxidative stress and the immune system (for reviews, see: Davis 2012; Jackson 2008; Steinbrenner 2013; Weekley 2013). These features have also suggested the possibility of using selenium compounds in cancer therapy—a hypothesis that has been under investigation (Chintala 2012; Fan 2013; Kim 2012; Sonaa 2013). Selenium may be involved in these processes through several mechanisms as a source of selenometabolites and as a component of selenium‐containing enzymes (Davis 2012; Hatfield 2009; Jackson 2008; Steinbrenner 2013; Weekley 2013). The optimum level for the retardation of carcinogenesis in human cells has been debated and is thought to be higher than the level commonly achieved through dietary changes (Whanger 2004). However, in laboratory studies, selenium has been shown to promote malignant cell transformation and progression (Chen 2000; Kandas 2009; National Toxicology Program 2011; Novoselov 2005; Rose 2014; Su 2005), thus confirming a ‘dual personality’ of this Janus‐faced element and of selenoproteins in both preventing and promoting cancer (Hatfield 2014).
Numerous epidemiological studies have reported an inverse association between selenium exposure and cancer risk. The first such studies had ecological study designs (Schrauzer 1977; Shamberger 1969). These were followed by case‐control and cohort observational studies and randomised trials, some of which received substantial attention from both the general population and the scientific community (Brinkman 2006; Fortmann 2013; Steinbrenner 2013; Vinceti 2013b). Gender‐related differences regarding the effects of selenium on cancer risk have also been suggested by some observational and experimental human studies, and differences in selenium tissue distribution, tumour biology and other factors have been suggested to explain a possible greater beneficial effect in males than in females (NPCT 2002; Waters 2004).
Why it is important to do this review
Selenium has been suggested to be involved in central anticarcinogenic processes. This has led to wide marketing of selenium supplements with associated health claims, particularly the prevention of both cancer (Dennert 2011; Vinceti 2013b) and cardiovascular disease (Rees 2013). In recent decades, worldwide debate has continued about the association between selenium exposure and cancer risk, including whether selenium supplements are effective in decreasing the incidence of or mortality from cancer. Epidemiological and other data have yielded conflicting results, sometimes suggesting different effects in men and women, and it has been suggested that selenium supplements might even have harmful effects. This review is timely and important, as several meta‐analyses and systematic reviews have been published, but an updated comprehensive summary synthesising evidence from both observational studies and intervention trials that include all types of cancer and look for gender‐related differences has not been conducted since the
time of the first Cochrane publication on the use of selenium for preventing cancer (Dennert 2011).
Objectives
Two research questions were addressed in this review: What is the evidence for:
an aetiological relation between selenium exposure and cancer risk in humans? and
the efficacy of selenium supplementation for cancer prevention in humans?
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and prospective observational studies (cohort studies and nested case‐control studies) were included, irrespective of publication year, publication status or language, provided they were published in extenso. We did not include conference abstracts in this review.
Types of participants
All adult participants (18 years of age and older).
Types of interventions
We considered prospective observational studies (cohort studies and cohort‐nested and nested case‐control studies) for inclusion if they assessed baseline exposure to selenium in apparently cancer‐free individuals either as biochemical selenium status or as estimated selenium intake at study entry.
We considered RCTs for inclusion if they used selenium supplementation at any dose or route of administration for a minimum of four weeks versus placebo or no intervention. We excluded trials using selenium supplementation as part of a multi‐component preparation without a study arm using selenium monotherapy supplementation.
Types of outcome measures
We analysed primary and secondary outcomes.
Primary outcomes
Incidence of any cancer and of site‐specific cancers, assessed as the proportion of participants developing cancers during the study period.
Mortality from any cancer and from site‐specific cancer, assessed as the proportion of participants dying from cancers during the study period.
Secondary outcomes
Incidence of selected adverse effects, assessed as the proportion of participants developing adverse health conditions. These outcomes were assessed in RCTs only.
Search methods for identification of studies
We conducted electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL, 2013, Issue 1), MEDLINE (Ovid, 1966 to February 2013 week 1), EMBASE (1980 to 2013 week 6), CancerLit (February 2004) and CCMed (February 2011). We conducted the initial search in 2004 and updates in July 2007, January 2009, October 2009, February 2011 and February 2013. As MEDLINE now includes the journals indexed in CancerLit, no further searches were conducted in this database after 2004.
We also searched the following online clinical trials databases in the previous review (Dennert 2011).
Clinical Trials of the American Cancer Society (http://www.cancer.gov, February 2011).
The metaRegister of Controlled Trials (mRCT, http://www.controlled‐trials.com, February 2011).
The German Cancer Study Register (http://www.studien.de, February 2011).
The System for Information on Grey Literature in Europe (SIGLE) (February 2004, discontinued in 2005).
The search strategies are provided in Appendix 1.
Data collection and analysis
Selection of studies
Two review authors independently checked all electronic search results for eligibility. When search results could not be rejected with certainty on the basis of title, abstract or both, we obtained full‐text material.
We scanned bibliographies of papers retrieved using the described search strategy to identify additional studies. If additional information was needed, we contacted the correspondent authors of the included studies; we also asked investigators for information about unpublished trials.
Two review authors (MV and MH) independently applied the inclusion and exclusion criteria, if necessary with the assistance of a translator. We resolved disagreements by discussion and with the involvement of a third review author.
Data extraction and management
We used piloted extraction forms for epidemiological studies and RCTs to document data from the original material and to assess the quality of studies. One review author (CDG) extracted data, and a second review author (MV) checked extracted data for discrepancies, which were discussed between the two review authors (CDG and MV). In a small number of cases, we sought the opinion of a third review author (GD or CMC) to reach a consensus. If several reports from the same study were available, we considered as primary publications studies reporting the entire period of follow‐up with active selenium supplementation, when available, but study details available from other publications were also extracted if not reported in the primary study reference.
For comparison of selenium exposure measured in serum and plasma specimens, we converted all data into the unit µg/L. Results provided as ppm (parts per million) or µg/g were converted using the factor 1.026 g/mL (density of blood plasma), and data provided as µmol/L were converted using the factor 78.96 (molecular weight of selenium).
To be included, prospective observational studies had to report estimates of risk ratio (RR), for example, odds ratio (OR), for various selenium exposure levels. Studies reporting only the RR for a one‐unit increase in selenium exposure were not included in the analysis.
Assessment of risk of bias in included studies
Observational studies
The risk of bias in observational studies was assessed using assessment forms adapted from the Newcastle‐Ottawa Quality Assessment Scale (NOS) for cohort and case‐control studies (Wells 2004). The NOS form for cohort studies was used for all included observational studies, and the NOS case‐control form was used for nested case‐control studies. Both forms must be adapted a priori for use in a systematic review according to the research question and the review topic. The NOS uses a star system in which studies are judged on key domains pertaining to the selection and comparability of study groups, the ascertainment of exposure and outcome, and the duration of follow‐up. For each domain, either a 'star' or 'no star' is assigned, with a 'star' indicating that that study design element was considered adequate and less likely to introduce bias. A study could receive a maximum of nine stars in the cohort assessment (Appendix 2) and nine stars in the assessment of the case‐control portion (Appendix 3).
The risk of bias assessment was based on data provided in the included publications. We did not check other publications for details if they were not included in the review. If an included study encompassed more than one publication with divergent ratings in the NOS, we used the publication with the highest score.
Randomised controlled trials
We categorised generation of allocation sequence, allocation concealment, blinding and completeness of outcome data as adequate (low risk of bias), inadequate (high risk of bias) or unclear, according to the criteria specified in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a) and suggested by Higgins et al. (Higgins 2011b). We considered these four items to be key domains for risk of bias assessment. Studies that were categorised as "adequate" in all four domains were considered to have a low risk of bias; studies with inadequate procedures in one or more key domains were considered to have a high risk of bias. Studies with unclear procedures in one or more key domains were considered to have an unclear risk of bias.
We assessed the fulfilment of ethical standards as follows.
Was informed consent obtained from participants? (yes/no/unclear).
Was approval obtained from an ethics board? (yes/no/unclear).
Measures of treatment effect
This review includes only the binary outcome of cancer diagnosis (i.e. cancer incidence) or death from cancer (i.e. cancer mortality), or a combination of both. The term 'cancer risk' is used in this paper as a generic term and refers generally to cancer incidence, cancer mortality and combined incidence/mortality data.
For observational studies, we used the odds ratio (OR) or the risk ratio (RR) and its 95% confidence interval (95% CI) as measures of the association between cancer risk and selenium exposure. When adjusted ORs were reported, we used the OR with the most extensive covariate adjustment reported in the publication.
For RCTs, we used RRs and their 95% CIs. When hazard ratios (HRs) rather than RRs were reported in the original study, we reported the individual study results as HRs with their 95% CIs; however, when data from such studies were included in meta‐analyses, we entered the RRs, and only RRs were pooled.
Dealing with missing data
When data were missing or when discrepancies in study publications were found, we tried to contact the study investigators to request further information. Contacting study authors helped to clarify discrepancies in several publications (e.g. differing data in text and tables within the same report); however, we retrieved no missing data or study details.
Assessment of heterogeneity
We performed a Chi2 test for heterogeneity of study results. Additionally, we used I2 statistics (Higgins 2003) to quantify inconsistency.
Assessment of reporting biases
The possibility of reporting bias was evaluated by using funnel plots.
Data synthesis
We performed data synthesis and analysis separately for RCTs and observational studies.
For observational studies, we conducted random effects meta‐analyses for all cancers or for site‐specific cancers for which at least five studies were available. We applied this restriction for two reasons. The first was practical: to limit the number of analyses to be performed. The second was that we expected results to be heterogeneous, but heterogeneity cannot be described and quantified well if too few studies are available (Higgins 2009). Although the cutoff at five studies is somewhat arbitrary, this decision was made very early in the review process; it was declared in the protocol and confirmed in its update. RCTs were less numerous, but given their fundamental importance in epidemiological research, we decided in the current review update to perform meta‐analyses for all cancers or site‐specific cancers when data from two or more trials were available.
Observational studies
We conducted random effects meta‐analyses of summary statistics from observational studies if data were available from at least five studies for all cancers or specific types of cancer. We used the OR or RR comparing the highest and lowest selenium exposure categories. Effect estimates were entered as the natural logarithm of the OR or RR, and the squared standard error of the natural logarithm of the OR or RR was used as a weight. The latter was calculated from the reported upper and lower boundaries of the 95% CI of the OR or RR. If a 95% CI was not reported, we used the total number of cases and the total number of controls, as well as the number of categories of selenium exposure, to estimate the numbers of cases and controls per exposure category. We then used the standard normal approximation formula to calculate the standard error of the OR (comparing the highest versus the lowest exposure category (lnOR = (1/a + 1/b + 1/c + 1/d), where a, b, c and d are the four counts needed to calculate the OR via (a*d)/(b*c)).
Meta‐analyses were conducted by using STATA (version 10 to 12) statistical software. We repeated meta‐analyses that were included in this review publication using the Review Manager 5 statistical tool; for this, logarithmic data for the OR and the standard error were copied from STATA into Review Manager 5, and results were double‐checked for errors.
Randomised controlled trials
We performed random effects meta‐analyses of summary statistics using RCT data if data were available from at least two studies for all cancers or specific types of cancer. When more than one publication from the same trial was available and reported different periods of follow‐up for the same cancer site, we included in the meta‐analysis only the longest period of follow‐up, provided that the experimental protocol was still ongoing at the time of follow‐up (i.e. that selenium supplementation was still actively supplied).
RRs and 95% CIs were calculated on the basis of the numbers of participants and cases when these were provided in the publication, using the meta‐analysis tool provided by Review Manager 5; otherwise, we used the RRs reported in the original publication. When an adjusted measure was also reported, we reported both the crude RR and the adjusted RR. We also calculated the RR of adverse outcomes and 95% CIs if sufficient data were available.
Subgroup analysis and investigation of heterogeneity
For observational studies, we used gender‐disaggregated data from mixed‐gender studies, together with data from single‐gender cohorts, for subgroup analyses by gender. We conducted the latter subgroup analyses to account for potential gender differences in selenium health effects (see Background).
Sensitivity analysis
For RCTs, we repeated analyses confining the included studies versus those with low risk of bias. For observational studies, we conducted sensitivity analyses to assess the effects of the different methods used to assess selenium status/intake.
Results
Description of studies
Citation style: Please note that we reference the sources of relevant information in a certain way to enhance traceability of our results for interested readers. When the source of information is not the primary publication of an included study, the specific publication of interest is also referenced. For example "Hakama 1990, in: Knekt 1990" indicates that the cited paper is "Hakama 1990" as part of the mentioned study.
Three full‐text theses published in the US could not be accessed (Coates 1987, in: Coates 1988; Menkes 1986a, in: Menkes 1986; Schober 1986, in: Menkes 1986). However, later journal publications were available and were included in this review as main study publications (Coates 1988, in: Coates 1988; Menkes 1986b, in: Menkes 1986; Schober 1987, in: Menkes 1986). Thus retrieval of the full‐text theses was considered to be unnecessary.
Results of the search
In the previous Cochrane review, of 4082 hits of potential relevance, 268 publications were retrieved in full text. Of these, 137 papers were considered as relevant (see the flow chart of the literature search in Dennert 2011).
In our updated search, after internal duplicates and duplicates against the database of the literature search conducted in January 2011 were excluded, 766 hits were retrieved. Of these, we excluded 744 references as being clearly irrelevant on the basis of title and abstract (flow chart of literature search: Figure 1). The reasons for exclusion were as follows.
Figure 1.
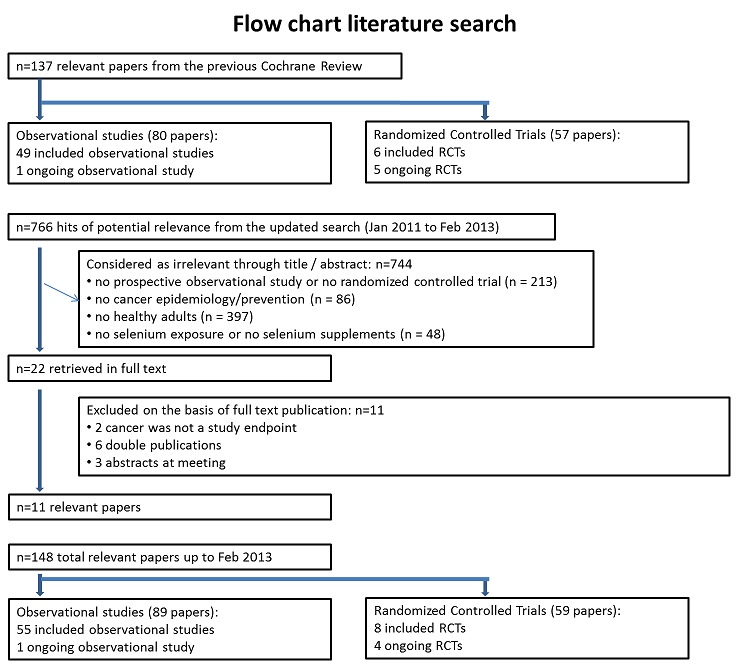
Flow chart.
Type of study: no prospective observational study or no randomised controlled trial (n = 213).
Type of outcome measure: no cancer epidemiology/prevention (n = 86).
Types of participants: no healthy adults (n = 397).
Type of exposure/intervention: no selenium exposure or no selenium supplements (n = 48).
The remaining 22 publications were considered of possible relevance and were reevaluated and retrieved in full text from this updated search (268 were retrieved in full text from the previous review). Upon further review, 11 of these publications were deemed relevant.
Included studies
In total, from the previous Cochrane review and from our update, 148 papers were identified for inclusion in this review: 89 papers referred to one ongoing and 55 completed observational studies, and 59 papers referred to four ongoing and eight completed RCTs.
A detailed description of the studies included is given in the table Characteristics of included studies.
1. Observational studies
Fifty‐five completed observational studies were included in this review. Forty‐one studies were nested case‐control studies, the others were subcohort controlled or cohort studies, and one study used a cohort together with a nested case‐control design. Subcohort controlled studies used (random) samples of the cohort as controls. The original papers were published between 1983 and 2013. Six studies were conducted in Asia (China, Japan and Taiwan), one in Australia, 22 in Europe (including data from Belgium, Denmark, Germany, Greece, Italy, Netherlands, Norway, Spain, Sweden, Channel Islands, Finland, France and UK) and 25 in the US. Overall, the studies included more than 1,100,000 participants. European study populations made up 45%, US 45%, Asia 9.4% and Australia 0.2% of all study participants. The median size of the study populations was 8801. Twenty‐eight studies included men and women, one did not report gender, 21 included only men and five only women. For a substantial proportion of the study populations (38%), gender was not reported. Forty‐three percent of participants were men, and 23% were women. Six studies with mixed‐gender populations reported results stratified by gender. The study populations were derived from 48 different cohorts. Twenty‐three cohorts were non‐randomly recruited (e.g. included volunteers), and 20 cohorts consisted of a random (or total) sample of the population of interest, which was either a specifically exposed population such as male tin‐miners in China or the general population.
Forty‐three studies specified the age range of their included participants; most included adults older than 40 years of age.
Seven studies investigated nutritional and/or supplemental selenium intake by using food frequency questionnaires or interviews. Forty‐eight studies assessed biochemical selenium status where:
8 used toenail specimens,
12 plasma specimens,
27 serum specimens,
and one used both serum and plasma specimens.
One study measured both serum selenium levels and intake.
The mean follow‐up period was up to three years in five studies and longer than three years in the remaining studies. Generally, study authors grouped the cases according to the International Classification of Diseases (ICD) classification that was up‐to‐date at the inception of the cohort observation. The level of disaggregation of data varied markedly between the studies. Although some studies reported cancer risk according to organ system (e.g. urinary tract, respiratory tract), others reported cancer risk for one or two organs (e.g. female breast, urinary bladder). Only in the case of skin cancer did studies also differentiate according to histological type (e.g. melanoma, basal cell carcinoma).
For the following outcomes, five or more studies were included in the review, and observational data were meta‐analysed.
Any cancer (16 studies).
Female breast cancer (7 studies).
Urinary bladder cancer (6 studies).
Lung cancer (14 studies).
Prostate cancer (17 studies).
Stomach cancer (5 studies).
Colon/colorectal cancer (5 studies).
Bates 2011 was not included in the meta‐analysis for any cancer, as it provided only the HR associated with an increase of one standard deviation of selenium exposure.
Table 3 provides an overview of the studies for each outcome. Five studies gave data for the group of “other” cancers, which encompassed any type of cancer not reported separately in the study publications. The definition of “other” cancers varied between studies, including predominantly rare cancers but also cancers of unknown origin. The results of the studies within the category "other cancers" are mentioned for the sake of completeness; however, because of the diversity of outcomes, the results were not included in further analysis or discussion of this review.
Table 1.
Included observational studies by outcome
| Organ system | Outcome | Number of studies/case definitions | Meta‐ analysis | Countries | Number of participants | Number of cases | Selenium assessment | Reporting study |
| Any cancer | Any cancer | total: 16 incidence: 8 mortality: 6 incidence and mortality combined: 1 |
✓ yes | US Finland Netherlands Sweden Norway Belgium France Japan |
total: ˜ 152,000 | total: 3010 male: 1700 female: 736 |
serum: 12 plasma: 2 serum + plasma: 1 plasma selenium P: 1 |
Knekt 1990
Coates 1988
Kok 1987a
Salonen 1984
Nomura 1987
Virtamo 1987
Willett 1983
Fex 1987
Ringstad 1988
Persson 2000
Salonen 1985
Peleg 1985,Kornitzer 2004
Akbaraly 2005
Bleys 2008 Fujishima 2011 |
| Gynaecological cancer | Female breast cancer | total: 7 incidence: 7 incidence and mortality combined: 0 |
✓ yes | US Finland Netherlands Channel Islands | total/female: > 155,000 (one study did not report cohort size) |
total/female: 1078 | serum: 2 plasma: 1 serum + plasma: 1 toenail: 3 |
Dorgan 1998 van den Brandt 1993a Coates 1988 Overvad 1991 Knekt 1990 Garland 1995 van Noord 1987 |
| Cervical cancer | total: 2 incidence: 2 mortality: 0 incidence and mortality combined: 0 |
✗ no | US | total/female: > 15,161 (one study did not report cohort size) |
total/female: 62 | serum: 2 | Menkes 1986 Coates 1988 | |
| Uterine cancer | total: 1 incidence: 1 mortality: 0 incidence and mortality combined: 0 |
✗ no | US | total/female: 62,641 | total/female: 91 | toenail: 1 | Garland 1995 | |
| Ovarian cancer | total: 4 incidence: 4 mortality: 0 incidence and mortality combined: 0 |
✗ no | US Finland | total/female: ˜ 214,000 | total/female: 568 | serum: 2 toenail: 1 supplemental intake: 1 |
Knekt 1990 Garland 1995 Menkes 1986 Thomson 2008 | |
| Gynaecological cancer (without breast cancer) | total: 1 incidence: 1 mortality: 0 incidence and mortality combined: 0 |
✗ no | Finland | total/female: ˜ 18,000 | total/female: 86 | serum: 1 | Knekt 1990 | |
| Urological cancers | Urinary bladder cancer | total: 6 incidence: 6 mortality: 0 incidence & mortality combined: 0 |
✓ yes | US/Hawaii Finland Netherlands | total: 356,150 female: 130,786 male: 128,009 |
total: 1295 female: 175 male 755 |
serum: 3 toenail: 3 |
Menkes 1986
Nomura 1987
Michaud 2002
van den Brandt 1993a
Michaud 2005 Hotaling 2011 |
| Urinary tract cancer | total: 2 incidence: 2 mortality: 0 incidence & mortality combined: 0 |
✗ no | Netherlands Finland | total: 48,000 | total: 104 male: 91 female: 13 |
serum: 1 plasma: 1 |
Knekt 1990 Persson 2000 | |
| Respiratory tract cancers | Lung cancer | total: 14 incidence: 12 mortality: 2 incidence and mortality combined: 0 |
✓ yes | China Japan US Finland Netherlands Denmark |
total: ˜ 336,000 male: 125,341 female: 181,895 |
total: 2002 male: 1256 female: 333 |
serum: 9 serum + plasma: 2 toenail: 2 dietary intake: 1 (one study reported both serum levels and food intake) |
Knekt 1990
Knekt 1998
Garland 1995
Coates 1988
Nomura 1987
van den Brandt 1993a
Kabuto 1994
Menkes 1986
Goodman 2001
Comstock 1997
Kromhout 1987
Ratnasinghe 2000
Epplein 2009 Suadicani 2012 |
| Oral/pharyngeal cancer | total: 1 incidence: 1 mortality: 0 incidence and mortality combined: 0 |
✗ no | US | total: 25,804 | total: 28 | serum: 1 | Menkes 1986 | |
| Any cancer of the respiratory tract | total: 1 incidence: 1 mortality: 0 incidence and mortality combined: 0 |
✗ no | Sweden | total/male: ˜ 9500 | total/male: 69 | plasma selenium P: 1 | Persson 2000 | |
| Andrological cancers | Prostate cancer | total: 17 incidence: 17 mortality: 0 incidence and mortality combined: 0 |
✓ yes | US Europe | total/male: > 421,000 (one study did not report cohort size) |
total/male: 6366 | serum: 8 plasma: 3 toenail: 3 dietary intake: 3 |
Hartman 1998,Helzlsouer 2000 Coates 1988 Brooks 2001 van den Brandt 1993a Nomura 2000 Goodman 2001 Yoshizawa 1998 Li 2004a Peters 2007 Peters 2008 Allen 2008 Epplein 2009 |
| Gastrointestinal cancers | Oesophageal cancer | total: 2 incidence: 2 mortality: 1 incidence and mortality combined: 0 |
✗ no | China US | total: 29,923 | total: > 959 | serum: 1 supplemental intake: 1 |
Wei 2004 Dong 2008 |
| Oesophageal squamous cell carcinoma | total:1 incidence: 1 mortality:0 incidence and mortality combined: 0 |
✗ no | Netherlands | total: 120,852 | total: 64 | toenail: 1 | Steinbrecher 2010 | |
| Oesophageal adenocarcinoma | total:1 incidence:1 mortality:0 incidence and mortality combined: 0 |
✗ no | Netherlands | total: 120,852 | total: 112 | toenail: 1 | Steinbrecher 2010 | |
| Oesophageal/stomach cancer | total: 1 incidence: 1 mortality: 0 incidence and mortality combined: 0 |
✗ no | Netherlands | total: 36,265 | total: 86 male: 51 female: 35 |
serum: 1 | Knekt 1998 | |
| Gastric cardia adenocarcinoma | total:1 incidence:1 mortality:0 incidence and mortality combined: 0 |
✗ no | Netherlands | total: 120,852 | total:114 | toenail: 1 | Steinbrecher 2010 | |
| Stomach cancer | total: 5 incidence: 5 mortality: 1 incidence and mortality combined: 0 |
✓ yes | China Japan US/Hawaii Finland Netherlands | total: ˜ 197,000 male: 86,311 female: 80,669 |
total: 955 male: 626 female: 329 |
serum: 4 toenail: 1 |
Knekt 1990 van den Brandt 1993a Nomura 1987 Kabuto 1994 Wei 2004 | |
| Primary liver cancer | total: 2 incidence: 1 mortality: 1 incidence and mortality combined: 0 |
✗ no | Taiwan | total: 46,404 | total: 235 male: 223 female: 12 |
plasma: 1 toenail: 1 |
Yu 1999 Sakoda 2005 | |
| Pancreatic cancer | total: 2 incidence: 2 mortality: 0 incidence and mortality combined: 0 |
✗ no | US Finland | total: 65,072 | total: 67 male: 31 female: 36 |
serum: 2 | Menkes 1986 Knekt 1990). | |
| Colon/colorectal cancer | total: colon 2, colorectum 3 incidence: 5 mortality: 0 incidence and mortality combined: 0 |
✓ yes | US Netherlands Finland | total: 255,425 male: 86,311 female: 143,310 |
total: 617 male: 285 female: 332 |
serum: 3 toenail: 2 |
van den Brandt 1993a Nomura 1987 Menkes 1986 Garland 1995 Knekt 1990 | |
| Rectal cancer | total: 2 incidence: 2 mortality: 0 incidence and mortality combined: 0 |
✗ no | US/Hawaii Netherlands | total: 127,712 | total: 145 male: 109 female: 36 |
serum: 1 toenail: 1 |
van den Brandt 1993a Nomura 1987 | |
| All gastrointestinal cancers | total: 2 incidence: 2 mortality: 0 incidence and mortality combined: 0 |
✗ no | US Sweden | total: > 9500 (one study did not report cohort size) |
total: 143 | plasma + serum: 1 plasma selenium P: 1 |
Coates 1988 Persson 2000 | |
| Skin cancer | Melanoma | total: 3 incidence: 3 mortality: 0 incidence and mortality combined: 0 |
✗ no | US | total: ˜ 158,000 | total: 547 | serum: 1 toenail: 1 supplemental intake: 1 |
Garland 1995,Menkes 1986,Peters 2008 |
| Basal cell carcinoma | total: 3 incidence: 3 mortality: 0 incidence and mortality combined: 0 |
✗ no | Australia US Finland | total: > 66,000 | total: 292 | serum: 3 dietary intake: 1 |
Knekt 1990 Menkes 1986 McNaughton 2005 | |
| Squamous cell carcinoma | total: 4 incidence: 4 mortality: 0 incidence and mortality combined: 0 |
✗ no | Australia US | total: ˜ 30,000 | total: 488 | serum: 2 plasma: 1 dietary intake: 1 |
Combs 1993 Karagas 1997 Menkes 1986 McNaughton 2005 | |
| Total non‐melanoma skin cancer | total: 1 incidence: 1 mortality: 0 incidence and mortality combined: 0 |
✗ no | US | total: 117 | total: 19 | plasma: 1 | Clark 1985 | |
| Rare and other cancers | Haematological cancers | total: 1 incidence: 1 mortality: 0 incidence and mortality combined: 0 |
✗ no | US | total: ˜ 6200 | total: 12 | serum + plasma: 12 | Coates 1988 |
| Thyroid cancer | total: 1 incidence: 1 mortality: 0 incidence and mortality combined: 0 |
✗ no | Norway | total: 100,000 | total: 43 male: 12 female: 31 |
serum: 1 | Glattre 1989 | |
| Other cancers | total: 5 incidence:4 mortality:1 incidence and mortality combined: 0 |
✗ no | China US Finland Sweden | total: 573 male: 230 female: 285 |
Garland 1995 Coates 1988 Knekt 1990 Wei 2004 Persson 2000 |
Some studies did not report the gender of participants or cancer cases; consequently, figures for women and men do not always sum up to the total number of participants or cancer cases.
2. Randomised controlled trials
Eight randomised controlled trials with a total of 44,743 participants (94% men) were included in this review. All used parallel‐group designs with two arms (Dreno 2007; Li 2000; Marshall 2011; NPCT 2002; Reid 2008; Yu 1991; Yu 1997), three arms (Algotar 2013) or four arms (SELECT 2009). Three were conducted in China (Li 2000; Yu 1991; Yu 1997), three in the US (Marshall 2011; NPCT 2002; Reid 2008), one in the US/New Zealand (Algotar 2013) and one in the US/Canada/Puerto Rico (SELECT 2009).
Selenium supplements and placebos were administered daily. As an active intervention, trials used 200 µg/d (Dreno 2007; Marshall 2011; NPCT 2002; Yu 1991; Yu 1997) or 400 µg/d (Reid 2008) selenium in the form of selenised yeast tablets, composed nearly entirely of organic selenium and particularly of selenomethionine (Block 2004). Algotar 2013 used 200 µg and 400 µg as different arms. Li 2000 used 500 µg sodium selenite, and SELECT 2009 used 200 µg/L selenomethionine.
Three Chinese trials investigated the preventive efficacy of selenium supplementation against primary liver cancer in different high‐risk populations. Participants were carriers of the hepatitis B surface antigen (HBs‐Ag) with normal liver function or first‐degree relatives of liver cancer patients. Two trials used selenised yeast (Yu 1991; Yu 1997), and one used sodium selenite (Li 2000).
The Nutritional Prevention of Cancer Trial (NPCT) investigated the influence of selenium on the development of non‐melanoma skin cancer (basal and squamous cell carcinoma) in a population considered at high risk of the disease, namely, patients with a history of non‐melanoma skin cancer (NPCT 2002). Participants were 1312 men and women from the eastern US 18 to 80 years of age, with a history of two or more basal cell carcinomas or of one squamous cell carcinoma. RR estimates for basal cell carcinoma, squamous cell carcinoma and overall non‐melanoma skin cancer were reported for two periods of follow‐up: an intermediate study period (from 15 September 1983 to 31 December 1993: Clark 1996, in: NPCT 2002) and the entire blinded intervention period (from 15 September 1983 to 31 January 1996: Duffield‐Lillico 2002 for the secondary outcomes; Duffield‐Lillico 2003 for the primary outcome, i.e. non‐melanoma skin cancer; and Duffield‐Lillico 2003 for an in‐depth analysis of prostate cancer risk; see NPCT 2002). In the present analysis, only the final reports concerning the entire period of blinded follow‐up, also characterised by active administration of selenium supplements, were used.
In 1990, additional secondary endpoints were identified post hoc in NPCT 2002 (total cancer mortality, total cancer incidence, incidence of lung, prostate and colorectal cancers). The incidences of female breast cancer, bladder cancer, oesophageal cancer, melanoma, haematological cancer and cancers of the head and neck were also reported in trial publications (NPCT 2002).
A substudy of the NPCT (Reid 2008) investigated the efficacy of a higher selenium dose, supplied as selenised yeast orally, in the prevention of non‐melanoma skin cancer at one of the NPCT study sites. Study design was similar to the NPCT study, except that 423 participants at this site were randomly assigned to placebo or intervention with higher selenium content. Reid 2008 also reported the incidence of internal cancers.
The incidence of skin cancer was evaluated as a secondary outcome by Dreno 2007 in a group of 184 organ transplant recipients who received 200 µg/d of selenium for three years and then were followed up for an additional two years. In this multi‐centre, randomised, placebo‐controlled trial, 91 selenium‐supplemented participants and 93 non‐supplemented participants were monitored for the development of both non‐malignant (warts and various keratoses) and malignant skin lesions.
The Selenium and Vitamin E Cancer Prevention Trial (SELECT 2009) investigated the effect of selenium as L‐selenomethionine and/or vitamin E supplementation in men of diverse ethnic backgrounds against the development of prostate cancer and other 'secondary' outcomes (i.e. the risk of all cancers, lung cancer, colorectal cancer, and bladder cancer). This study was a very large phase 3 randomised, placebo‐controlled trial, activated in June 2001 and originally designed for a seven‐ to 12‐year period of follow‐up, carried out at 427 sites in the US, Canada and Puerto Rico. However, the independent Data and Safety Monitoring Commitee recommended in September 15, 2008, the discontinuation of study supplements based on the absence of benefit from vitamin E or selenium and no possibility of a benefit to the planned degree with additional follow‐up (SELECT 2009). The committee also expressed concern about increased prostate cancer risk among vitamin E–treated participants and increased diabetes risk among selenium‐supplemented participants (SELECT 2009). Administration of these supplements was therefore discontinued on October 23, 2008, in spite of the planned supplementation period of 12 years. The results of SELECT are based on the follow‐up provided at the end of the blinded supplementation period, which included 117,660 person‐years of follow‐up, and not on an extended period of follow‐up, which encompassed an additional 32 months of surveillance (144,846 person‐years in total) after the end of the supplementation period. The endpoints were prostate cancer (the 'primary' endpoint) and colorectal cancer, lung cancer, all the other cancers and all cancers overall. A subsequent study from SELECT also evaluated the risk of bladder cancer, adding to the standard follow‐up an additional post supplementation period of 32 months (SELECT 2009).
The effect of selenium supplementation on prostate cancer was also evaluated in two phase 3 trials published in 2011 (Marshall 2011) and in 2013 (Algotar 2013). In Marshall 2011, 423 men with high‐grade prostatic intraepithelial neoplasia, and therefore considered to be at very high risk of prostate cancer, were randomly assigned to selenium (200 µg/d as selenomethionine) or placebo. Algotar 2013 evaluated whether supplementation with 200 or 400 µg/d of selenium as selenised yeast reduced the risk of prostate cancer among men at high risk of the disease, based on a prostate‐specific antigen (PSA) level exceeding 4 ng/L, suspicious digital rectal examination and PSA velocity greater than 0.75 ng/mL/y. The trial, called 'The Negative Biopsy Trial', followed the study participants for five years in the US (where both supplementation and follow‐up were complete for such period) and for no longer than three years in New Zealand, and was discontinued after a recommendation to stop the trial was issued by an external Data and Safety Monitoring Committee.
Excluded studies
Of 22 potentially relevant papers retrieved in the updated search, 11 papers did not fulfil the inclusion criteria. Nine of these publications were rejected as including duplication of data from already included studies or posters/abstracts at meetings; two papers were excluded because cancer was not a study endpoint. The table Characteristics of excluded studies describes the reasons for exclusion from the previous Cochrane review (see Dennert 2011 for the main reasons for exclusion) and from this update.
Risk of bias in included studies
Observational studies
A summary of study ratings according to the Newcastle‐Ottawa Scale (NOS) is presented in Table 4. The median number of assigned stars was eight for the (nested) case‐control study assessments and seven for the cohort study assessments, out of a maximum of nine stars each (Figure 2 and Figure 3).
Table 2.
Risk of bias: observational studies
| Study | Publication | Newcastle Ottawa Scale (cohort) | Newcastle Ottawa Scale (case‐control) | ||||||
| Selection | Comparability | Outcome | Total | Selection | Comparability | Exposure | Total | ||
| Kabuto 1994 | Kabuto 1994 | 0‐1‐1‐1 | 2 | 1‐1‐0 | 7 | 0‐1‐1‐1 | 2 | 1‐1‐1 | 8 |
| Ratnasinghe 2000 | Ratnasinghe 2000 | 1‐1‐1‐1 | 2 | 1‐0‐0 | 7 | 0‐0‐1‐1 | 2 | 1‐1‐1 | 7 |
| Sakoda 2005 | Sakoda 2005 | 0‐1‐1‐0 | 1 | 1‐1‐0 | 5 | 1‐1‐1‐1 | 1 | 1‐1‐1 | 8 |
| Wei 2004 | Wei 2004 | 1‐1‐1‐1 | 1 | 1‐1‐1 | 8 | .‐.‐.‐. | . | .‐.‐. | . |
| Mark 2000 | 1‐1‐1‐1 | 1 | 1‐1‐1 | 8 | .‐.‐.‐. | . | .‐.‐. | . | |
| Yu 1999 | Yu 1999 | 0‐1‐1‐1 | 2 | 1‐1‐0 | 7 | 1‐1‐1‐1 | 2 | 1‐1‐1 | 9 |
| McNaughton 2005 | McNaughton 2005 | 1‐1‐1‐1 | 1 | 1‐1‐0 | 7 | 1‐1‐1‐1 | 1 | 1‐1‐1 | 8 |
| Heinen 2007 | 1‐1‐1‐1 | 2 | 1‐1‐1 | 9 | .‐.‐.‐. | . | .‐.‐. | . | |
| van der Pols 2009 | 1‐1‐1‐1 | 2 | 1‐1‐0 | 8 | .‐.‐.‐. | . | .‐.‐. | . | |
| Akbaraly 2005 | Akbaraly 2005 | 0‐1‐1‐1 | 2 | 0‐1‐0 | 6 | .‐.‐.‐. | . | .‐.‐. | . |
| Allen 2008 | Allen 2008 | 1‐1‐1‐1 | 2 | 1‐1‐0 | 8 | 1‐1‐1‐1 | 2 | 1‐1‐1 | 9 |
| Fex 1987 | Fex 1987 | 1‐1‐1‐0 | 2 | 1‐1‐1 | 8 | 1‐0‐1‐1 | 2 | 1‐1‐1 | 8 |
| Glattre 1989 | Glattre 1989 | 0‐1‐1‐0 | 1 | 1‐1‐1 | 6 | 1‐1‐1‐1 | 1 | 1‐1‐1 | 8 |
| Hartman 1998 | Hartman 1998 | 1‐1‐0‐1 | 2 | 1‐1‐0 | 7 | .‐.‐.‐. | . | .‐.‐. | . |
| Knekt 1990 | Knekt 1990 | 1‐1‐1‐1 | 2 | 1‐1‐1 | 9 | 0‐1‐1‐1 | 2 | 1‐1‐1 | 8 |
| Hakama 1990 | 1‐1‐1‐1 | 2 | 1‐1‐1 | 9 | 0‐1‐1‐1 | 2 | 1‐1‐1 | 8 | |
| Knekt 1988 | 1‐1‐1‐1 | 2 | 1‐1‐1 | 9 | 0‐0‐1‐1 | 2 | 1‐1‐1 | 7 | |
| Knekt 1996 | 1‐1‐1‐1 | 1 | 1‐1‐1 | 8 | 0‐1‐1‐1 | 1 | 1‐1‐1 | 7 | |
| Knekt 1991 | 1‐1‐1‐1 | 2 | 1‐1‐1 | 9 | 0‐1‐1‐1 | 2 | 1‐1‐1 | 8 | |
| Knekt 1998 | Knekt 1998 | 1‐1‐1‐1 | 2 | 1‐1‐1 | 9 | 0‐1‐1‐1 | 2 | 1‐1‐1 | 8 |
| Kok 1987a | Kok 1987b | 1‐1‐1‐1 | 2 | 1‐1‐1 | 9 | 1‐0‐1‐1 | 2 | 1‐1‐1 | 8 |
| Kok 1987a | .‐.‐.‐. | . | .‐.‐. | . | .‐.‐.‐. | . | .‐.‐. | . | |
| Kornitzer 2004 | Kornitzer 2004 | 1‐1‐1‐0 | 1 | 1‐1‐1 | 7 | 1‐1‐1‐1 | 1 | 1‐1‐1 | 8 |
| Kromhout 1987 | Kromhout 1987 | 1‐1‐1‐0 | 2 | 1‐1‐1 | 8 | .‐.‐.‐. | . | .‐.‐. | . |
| Michaud 2002 | Michaud 2002 | 1‐1‐1‐1 | 2 | 1‐1‐0 | 8 | 0‐1‐1‐1 | 2 | 1‐1‐1 | 8 |
| Overvad 1991 | Overvad 1991 | 1‐1‐1‐0 | 1 | 1‐1‐0 | 6 | .‐.‐.‐. | . | .‐.‐. | . |
| Persson 2000 | Persson‐Moschos 2000 | 1‐1‐1‐0 | 2 | 1‐1‐1 | 8 | 1‐0‐1‐1 | 2 | 1‐1‐1 | 8 |
| Ringstad 1988 | Ringstad 1988 | 1‐1‐1‐1 | 2 | 1‐1‐0 | 8 | 1‐1‐1‐1 | 2 | 1‐1‐1 | 9 |
| Salonen 1984 | Salonen 1984 | 1‐1‐1‐1 | 2 | 1‐1‐1 | 9 | 0‐1‐1‐1 | 2 | 1‐1‐1 | 8 |
| Salonen 1985 | Salonen 1985 | 1‐1‐1‐1 | 2 | 1‐1‐1 | 9 | 1‐1‐1‐1 | 2 | 1‐1‐1 | 9 |
| van Noord 1987 | van Noord 1987 | 1‐1‐1‐0 | 1 | 1‐0‐1 | 6 | 1‐1‐1‐0 | 1 | 1‐1‐1 | 7 |
| van den Brandt 1993a | van den Brandt 1993 | 1‐1‐1‐1 | 2 | 1‐1‐1 | 9 | .‐.‐.‐. | . | .‐.‐. | . |
| van den Brandt 1994 | 1‐1‐1‐1 | 2 | 1‐1‐1 | 9 | .‐.‐.‐. | . | .‐.‐. | . | |
| van den Brandt 1993 | 1‐1‐1‐1 | 2 | 1‐1‐1 | 9 | .‐.‐.‐. | . | .‐.‐. | . | |
| van den Brandt 2003 | 1‐1‐1‐1 | 2 | 1‐1‐1 | 9 | .‐.‐.‐. | . | .‐.‐. | . | |
| Zeegers 2002 | 1‐1‐1‐1 | 2 | 1‐1‐1 | 9 | .‐.‐.‐. | . | .‐.‐. | . | |
| Steevens 2010 | 1‐1‐1‐1 | 2 | 1‐1‐1 | 9 | 0‐1‐1‐1 | 2 | 1‐0 | 6 | |
| Virtamo 1987 | Virtamo 1987 | 0‐1‐1‐1 | 2 | 1‐1‐1 | 8 | .‐.‐.‐. | . | .‐.‐. | . |
| Bleys 2008 | Bleys 2008 | 1‐1‐1‐1 | 2 | 1‐1‐1 | 9 | .‐.‐.‐. | . | .‐.‐. | . |
| Brooks 2001 | Brooks 2001 | 0‐1‐1‐0 | 2 | 1‐0‐0 | 5 | 1‐0‐1‐1 | 2 | 1‐1‐0 | 7 |
| Clark 1985 | Clark 1985 | 0‐1‐1‐0 | 0 | 0‐0‐0 | 2 | .‐.‐.‐. | . | .‐.‐. | . |
| Coates 1988 | Coates 1988 | 0‐1‐1‐0 | 1 | 1‐1‐0 | 5 | 1‐0‐1‐0 | 1 | 1‐1‐1 | 6 |
| Coates 1987 | .‐.‐.‐. | . | .‐.‐. | . | .‐.‐.‐. | . | .‐.‐. | . | |
| Combs 1993 | Combs Jr 1993 | 0‐1‐1‐0 | 2 | 1‐0‐0 | 5 | .‐.‐.‐. | . | .‐.‐. | . |
| Comstock 1997 | Comstock 1997 | 0‐1‐1‐0 | 2 | 1‐1‐0 | 6 | 1‐1‐1‐1 | 2 | 1‐1‐1 | 9 |
| Dong 2008 | Dong 2008 | 1‐1‐1‐1 | 2 | 1‐1‐1 | 9 | .‐.‐.‐. | . | .‐.‐. | . |
| Dorgan 1998 | Dorgan 1998 | 0‐1‐1‐1 | 2 | 0‐1‐0 | 6 | 1‐1‐1‐1 | 2 | 1‐1‐1 | 9 |
| Epplein 2009 | Epplein 2009 | 0‐1‐1‐1 | 2 | 1‐1‐0 | 7 | 0‐1‐1‐1 | 2 | 1‐1‐1 | 8 |
| Gill 2009 | 0‐1‐1‐1 | 1 | 1‐1‐0 | 6 | 0‐1‐1‐1 | 1 | 1‐1‐1 | 7 | |
| Garland 1995 | Garland 1995 | 0‐1‐1‐1 | 2 | 1‐1‐1 | 8 | 1‐1‐1‐1 | 2 | 1‐1‐1 | 9 |
| Hunter 1990 | 0‐1‐1‐1 | 2 | 1‐1‐1 | 8 | 1‐1‐1‐1 | 2 | 1‐1‐1 | 9 | |
| Goodman 2001 | Goodman 2001 | 0‐1‐1‐0 | 2 | 1‐1‐0 | 6 | 1‐1‐1‐1 | 2 | 1‐1‐1 | 9 |
| Helzlsouer 2000 | Helzlsouer 2000 | 0‐1‐1‐1 | 1 | 1‐1‐0 | 6 | 1‐1‐1‐1 | 1 | 1‐1‐1 | 8 |
| Karagas 1997 | Karagas 1997 | 0‐1‐1‐1 | 2 | 1‐1‐1 | 8 | 1‐1‐1‐1 | 2 | 1‐1‐1 | 9 |
| Li 2004a | Li 2004 | 0‐1‐1‐1 | 2 | 0‐1‐1 | 7 | 1‐1‐1‐1 | 2 | 1‐1‐1 | 9 |
| Menkes 1986 | Menkes 1986 | 0‐1‐1‐1 | 2 | 1‐1‐0 | 7 | 1‐1‐1‐1 | 2 | 1‐1‐1 | 9 |
| Batieha 1993 | 0‐1‐1‐1 | 2 | 1‐1‐0 | 7 | 1‐1‐1‐1 | 2 | 1‐1‐1 | 9 | |
| Breslow 1995 | 0‐1‐1‐1 | 2 | 1‐1‐0 | 7 | 1‐0‐1‐1 | 2 | 1‐1‐1 | 8 | |
| Burney 1989 | 0‐1‐1‐1 | 2 | 1‐1‐0 | 7 | 0‐1‐1‐1 | 2 | 1‐1‐1 | 8 | |
| Helzlsouer 1996 | 0‐1‐1‐1 | 2 | 1‐1‐0 | 7 | 0‐1‐1‐1 | 2 | 1‐1‐1 | 8 | |
| Helzlsouer 1989 | 0‐1‐1‐1 | 2 | 1‐1‐0 | 7 | 1‐1‐1‐1 | 2 | 1‐1‐1 | 9 | |
| Ko 1994 | 0‐1‐1‐0 | 2 | 1‐1‐0 | 6 | 1‐1‐1‐1 | 2 | 1‐1‐1 | 9 | |
| Menkes 1986 | .‐.‐.‐. | . | .‐.‐. | . | .‐.‐.‐. | . | .‐.‐. | . | |
| Schober 1987 | 0‐1‐1‐1 | 1 | 1‐1‐0 | 6 | 0‐1‐1‐1 | 1 | 1‐1‐1 | 7 | |
| Schober 1986 | .‐.‐.‐. | . | .‐.‐. | . | .‐.‐.‐. | . | .‐.‐. | . | |
| Zheng 1993 | 0‐1‐1‐1 | 2 | 1‐1‐0 | 7 | 0‐1‐1‐1 | 2 | 1‐1‐1 | 8 | |
| Michaud 2005 | Michaud 2005 | 0‐1‐1‐1 | 2 | 0‐1‐0 | 6 | 1‐1‐1‐1 | 2 | 1‐1‐1 | 9 |
| Nomura 1987 | Nomura 1987 | 1‐1‐1‐1 | 2 | 1‐1‐1 | 9 | 1‐1‐1‐1 | 2 | 1‐1‐1 | 9 |
| Nomura 2000 | Nomura 2000 | 1‐1‐1‐1 | 2 | 1‐1‐1 | 9 | 1‐1‐1‐1 | 2 | 1‐1‐1 | 9 |
| Peleg 1985 | Peleg 1985 | 1‐1‐1‐1 | 1 | 1‐1‐0 | 7 | 1‐1‐1‐1 | 1 | 1‐1‐1 | 8 |
| Peters 2007 | Peters 2007 | 0‐1‐1‐1 | 2 | 1‐1‐0 | 7 | 1‐1‐1‐1 | 2 | 1‐1‐1 | 9 |
| Peters 2008 | Peters 2008 | 0‐1‐1‐1 | 1 | 1‐1‐1 | 7 | .‐.‐.‐. | . | .‐.‐. | . |
| Asgari 2009 | 0‐1‐1‐1 | 1 | 1‐1‐0 | 6 | .‐.‐.‐. | . | .‐.‐. | . | |
| Hotaling 2011 | 0‐1‐0‐1 | 0 | 1‐1‐1 | 5 | .‐.‐.‐. | . | .‐.‐. | . | |
| Walter 2011 | 0‐1‐0‐1 | 2 | 1‐1‐1 | 7 | .‐.‐.‐. | . | .‐.‐. | . | |
| Thomson 2008 | Thomson 2008 | 0‐1‐1‐1 | 2 | 0‐1‐0 | 6 | .‐.‐.‐. | . | .‐.‐. | . |
| Willett 1983 | Willett 1983 | 1‐1‐1‐0 | 2 | 1‐1‐0 | 7 | 1‐1‐1‐1 | 2 | 1‐1‐1 | 9 |
| Yoshizawa 1998 | Yoshizawa 1998 | 0‐1‐1‐1 | 2 | 1‐1‐1 | 8 | 1‐0‐1‐1 | 2 | 1‐1‐1 | 8 |
| Fujishima 2011 | Fujishima 2011 | 1‐1‐1‐1 | 2 | 1‐1‐1 | 9 | .‐.‐.‐. | . | .‐.‐. | . |
| Grundmark 2011 | Grundmark 2011 | 1‐1‐1‐1 | 2 | 1‐1‐1 | 9 | .‐.‐.‐. | . | .‐.‐. | . |
| Bates 2011 | Bates 2011 | 1‐1‐1‐1 | 1 | 1‐1‐1 | 8 | .‐.‐.‐. | . | .‐.‐. | . |
| Suadicani 2012 | Suadicani 2012 | 0‐1‐1‐1 | 2 | 1‐1‐1 | 8 | .‐.‐.‐. | . | .‐.‐. | . |
| Agalliu 2011 | Agalliu 2011 | 0‐1‐0‐1 | 1 | 1‐1‐0 | 5 | 0‐1‐0‐1 | 1 | 1‐1‐0 | 5 |
| Steinbrecher 2010 | Steinbrecher 2010 | 1‐1‐1‐1 | 2 | 0‐1‐0 | 7 | 1‐1‐1‐1 | 2 | 0‐1‐1 | 8 |
Figure 2.
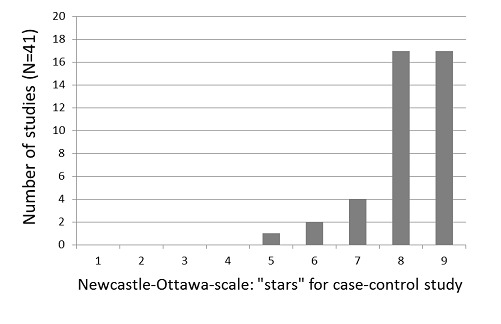
Newcastle‐Ottawa Scale: number of studies by number of "stars" assigned in the case‐control portion of studies.
Figure 3.
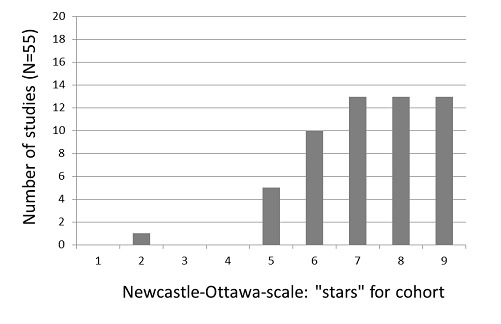
Newcastle‐Ottawa Scale: number of studies by number of "stars" assigned in the cohort portion of studies.
All but one cohort study received five to nine stars on the NOS. The exception (two stars) was an early investigation, which was available only in abstract form for assessment (Clark 1985). For three items on the NOS cohort assessment, 85% of the included studies were considered adequate: representativeness of the cohort for the target population (58% of the studies received a star), demonstration that cancer was not present at study commencement (85%) and completeness of follow‐up data (58%).
The representativeness of the cohort for the target population is a matter of external validity and generalisability of study results, but a systematic deviation of participants from the target population might also introduce bias into study results. The target population of included studies depended on the study objectives and could have been the general population, as well as special occupational groups. Studies that did not identify their target population or recruited volunteers were not assigned a star for this question. Differential selection of study participants (e.g. volunteers) from the target population can lead to confounding by factors associated with selenium status and cancer incidence (e.g. nutritional behaviour, socioeconomic position). All included studies chose comparison groups (cases/controls or exposed/non‐exposed) from the same study population. This approach enhanced comparability between groups.
Follow‐up data were considered as complete or as missing data unlikely to introduce bias to study results in 45% of included observational studies. In the other cohorts, losses to follow‐up were greater than 5% and a description of losses to follow‐up was not provided. A high attrition rate may alter the characteristics of the population under investigation and may impede the generalisability of study results to the intended target population (external validity). The presence of attrition does not necessarily mean that the study results are biased. However, given the possibility that selenium status may be linked to sociodemographic variables and socioeconomic position, which may also influence participation in follow‐up procedures, a differential effect of attrition may introduce bias towards underestimation or overestimation of the true exposure effect.
Forty‐one included observational studies were nested case‐control studies and therefore were assessed using the NOS case‐control form. The number of stars in the NOS assessment of the case‐control studies ranged from five to nine, with 89% receiving eight or nine stars. Although the included prospective case‐control studies were generally assessed as having a low risk of bias, in some studies concern arose regarding case definition and the question of representativeness of the cases.
The definition of cases was considered inadequate in 44% of the nested case‐control studies, as cases were identified by self reporting; linkage to databases with unclear validity or procedures was not described. The magnitude and direction of bias that might have been introduced to the study results remain unclear.
In 22% of studies, not all identified cases (or an appropriate sample of them) were included in the trial analyses, or selection procedures for analysed cases were not reported. In some studies, blood specimens were lost as the result of technical problems (e.g. cooler breakdown at one study centre); in other studies, material available for analysis was insufficient; and in others, cases for analysis were selected in a non‐random manner. This might bias the estimates of association in either direction.
No obvious asymmetry (as an indicator of publication bias) was noted in the funnel plots of the studies on total and prostate cancer risk (Figure 4 and Figure 5).
Figure 4.
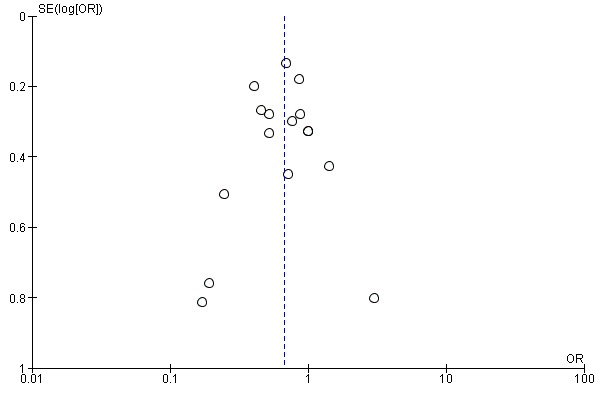
Funnel plot of comparison: 1 Highest versus lowest selenium exposure, outcome: 1.17 Total cancer incidence and mortality.
Figure 5.
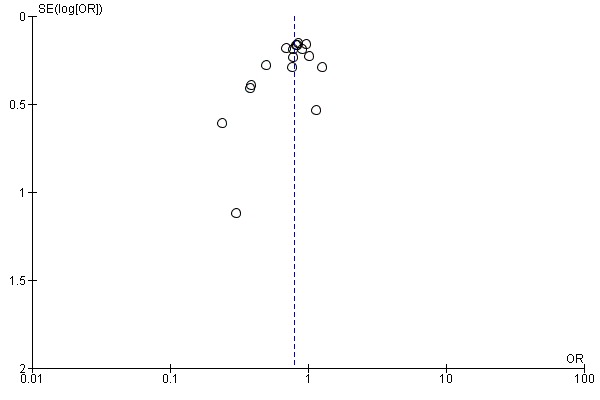
Funnel plot of comparison: 1 Highest versus lowest selenium exposure, outcome: 1.7 Prostate cancer risk.
Randomised controlled trials
An overview of the risk of bias in the included randomised controlled trials, performed according to Cochrane criteria for bias assessment (Higgins 2011a; Higgins 2011b), is presented in Table 5.
Table 3.
Risk of bias: randomised controlled trials
| Study | Sequence generation | Allocation concealment | Blinding | Completeness of outcome data | Risk of bias |
| NPCT 2002 | adequate | adequate | unclear | adequate | unclear |
| Li 2000 | unclear | unclear | adequate | adequate | unclear |
| Yu 1997 | unclear | unclear | adequate | unclear | unclear |
| Yu 1991 | unclear | unclear | adequate | unclear | unclear |
| SELECT 2009 | adequate | adequate | adequate | adequate | low |
| Algotar 2013 | adequate | adequate | adequate | adequate | low |
| Marshall 2011 | adequate | adequate | adequate | adequate | low |
| Dreno 2007 | adequate | adequate | unclear | adequate | unclear |
The final results of the NPCT study, encompassing the whole period of follow‐up (blinded and with active selenium administration), were reported in the three Duffield‐Lillico et al. papers published in 2002, 2003 and 2003, and a preliminary report of that trial based on a shorter period of follow‐up was published by Clark et al. in 1996.
All three trials on liver cancer risk (Li 2000; Yu 1991; Yu 1997) were considered to have an unclear risk of bias. In these trials, generation of allocation sequence and allocation concealment were not reported. One study mentioned that the dropout rate was similar in the intervention and control groups; the remaining two studies did not report the completeness of outcome data. Blinding was judged as adequate in all three studies, as the use of placebo supplements was reported. We inferred from this procedure that at least the study participants and the physicians directly involved were blinded towards treatment status.
It is unclear whether Li 2000 was an individually randomised controlled trial. Study investigators used the phrase 'randomisation based on the residence area' and did not describe the randomisation procedure any further. As participants were recruited from 17 villages, the villages, not the individual participants, may have been randomly assigned to the intervention and control groups. However, we could not make contact with the study investigators to clarify these questions. Randomisation of villages instead of individuals could have introduced bias to the study results, as the incidence of liver cancer is known to differ between areas as a result of environmental factors.
RCTs with inadequate or unclear allocation concealment have been found to overestimate the benefit of interventions, especially trials with subjective outcomes (Pildal 2007; Wood 2008). In all three liver cancer RCTs, follow‐up and case detection procedures were not reported, so the influence of subjective factors on case detection, such as interpretation of bodily symptoms as triggers of further diagnostic tests, is unknown. Although we judged blinding as 'adequate' in all three liver cancer trials, we do not know whether it was successful in practice for participants, healthcare providers and outcome assessors.
These uncertainties about study methods seriously weaken our confidence in reported RCT results on liver cancer risk.
SELECT 2009, Algotar 2013 and Marshall 2011 were considered to have a low risk of bias because they reported adequate generation of allocation sequence, allocation concealment, blinding and completeness of outcome data.
Dreno 2007 and Duffield‐Lillico 2002 to 2003, in: NPCT 2002 were judged to have unclear risk of bias. Dreno 2007 provided unclear generation of allocation sequence, allocation concealment and blinding; only completeness of outcome data was adequate. NPCT was considered to be at unclear risk of bias because of exposure‐related detection bias for its primary outcome, as the percentage of study participants with an abnormal PSA (> 4 ng/mL) who underwent biopsy varied according to selenium treatment group, with 35% in the placebo group and 14% in the selenium‐treated group (Duffield‐Lillico 2003, in: NPCT 2002;Marshall 2011). In analyses stratified by baseline selenium concentration, the difference was greatest among participants in the lowest tertile, in whom the inverse association between selenium administration and prostate cancer risk was strongest. The difference in biopsy rates could not be accounted for by factors such as PSA concentration, age at which abnormal PSA was detected and alternative diagnostic procedures. Although a difference this large could have occurred by chance, this finding raises concerns about possible disruption of blinding. No information was provided as to the prostate biopsy rate among participants with lower PSA levels or biopsy rates for the primary outcome of non‐melanoma skin cancer, which also requires pathological confirmation, nor for the other secondary outcomes examined in this trial.
Ethical criteria
Informed consent and ethics board approval were fulfilled by all trials (Algotar 2013; Dreno 2007; Marshall 2011; NPCT 2002; Reid 2008; SELECT 2009), except for Li 2000, Yu 1997, and Yu 1991, in which they were not mentioned.
Effects of interventions
1. Observational studies
When the risk of cancer for higher and lower levels of selenium exposure is compared, a summary risk estimate of one suggests that there is no association between selenium exposure and cancer, a summary risk estimate below one suggests a possible protective effect of higher selenium exposure and a summary risk estimate above one suggests a possible harmful effect of higher selenium exposure.
1.1. Aetiological association: results from meta‐analyses
1.1.1. Any cancer
Results of 16 prospective observational studies on total cancer risk, including data on more than 144,000 participants, were meta‐analysed. The cohorts of Salonen 1984 and Salonen 1985 overlapped. Hence, only data from Salonen 1985 were included in the meta‐analysis. Fex 1987 had to be omitted, as the CI value was not reported and could not be calculated from the available data.
For participants in the highest category of prediagnostic selenium exposure, the summary risk estimate was OR 0.69 (95% CI 0.53 to 0.91) for cancer incidence and OR 0.60 (95% CI 0.39 to 0.93) for cancer mortality for both genders combined (Analysis 1.1) when compared with participants in the lowest exposure category. Heterogeneity was observed for both incidence (I² = 49%) and mortality (I² = 62%).
Analysis 1.1.
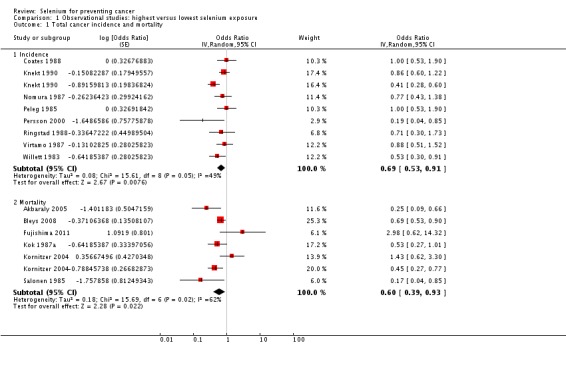
Comparison 1 Observational studies: highest versus lowest selenium exposure, Outcome 1 Total cancer incidence and mortality.
Analyses by gender found lower point estimates for men (incidence: OR 0.66, 95% CI 0.42 to 1.05; mortality: OR 0.56, 95% CI 0.38 to 0.81) (Analysis 1.2) than for women (incidence: OR 0.90, 95% CI 0.45 to 1.77; mortality: OR 0.92, 95% CI 0.79 to 1.07) (Analysis 1.3), However, a test for subgroup differences found no clear evidence of different effects in men and women (P value 0.47).
Analysis 1.2.
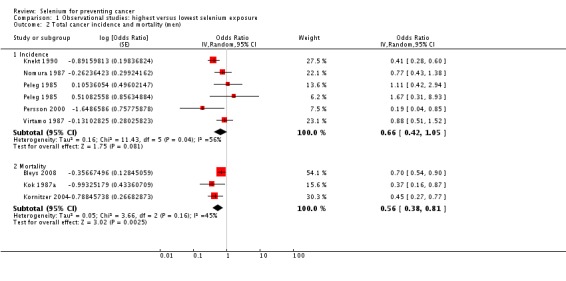
Comparison 1 Observational studies: highest versus lowest selenium exposure, Outcome 2 Total cancer incidence and mortality (men).
Analysis 1.3.
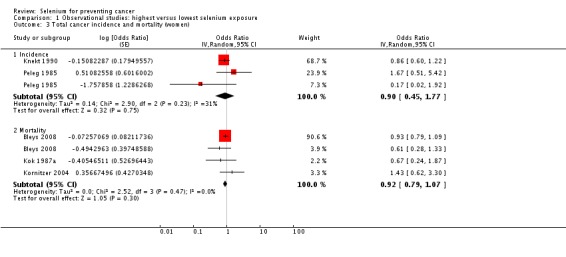
Comparison 1 Observational studies: highest versus lowest selenium exposure, Outcome 3 Total cancer incidence and mortality (women).
All studies used either serum or serum and plasma biomarker levels for assessment of selenium status. Analysis 1.4 shows the results in ascending order of baseline exposure for those studies that reported category borders. The graph does not reveal a clear pattern of a relation between baseline biomarker level and cancer risk.
Analysis 1.4.
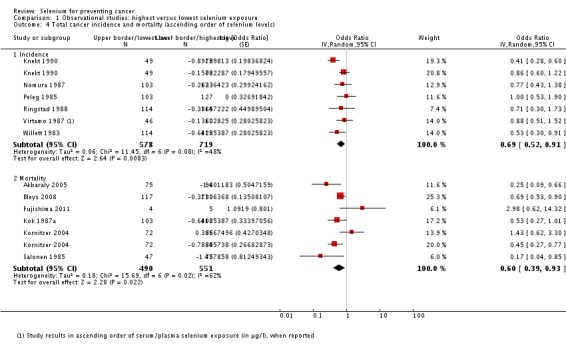
Comparison 1 Observational studies: highest versus lowest selenium exposure, Outcome 4 Total cancer incidence and mortality (ascending order of selenium levels).
1.1.2. Female breast cancer
Eight studies were included in the meta‐analysis. No association was seen between baseline selenium levels and breast cancer risk, with overall OR 0.91 (95% CI 0.69 to 1.20) (Analysis 1.5). The heterogeneity of results (I² = 38%) was low.
Analysis 1.5.
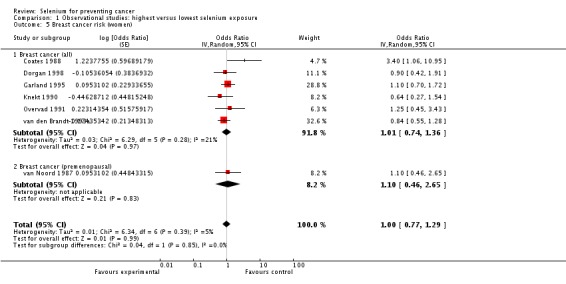
Comparison 1 Observational studies: highest versus lowest selenium exposure, Outcome 5 Breast cancer risk (women).
1.1.3. Bladder cancer
Meta‐analysis of bladder cancer incidence in five observational studies found an inverse association, with an overall risk estimate of 0.67 (95% CI 0.46 to 0.97), suggesting a protective effect of higher selenium levels against bladder cancer (Analysis 1.6) (overall heterogeneity: I² = 30%).
Analysis 1.6.
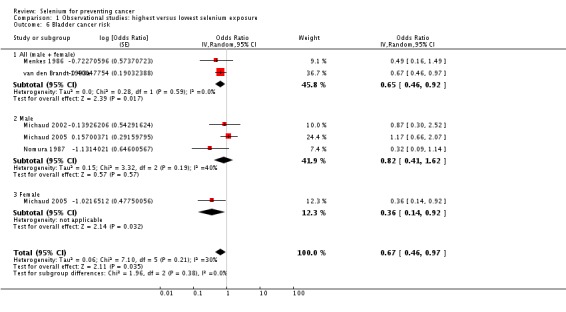
Comparison 1 Observational studies: highest versus lowest selenium exposure, Outcome 6 Bladder cancer risk.
Gender‐disaggregated data were available only from Michaud 2005, indicating a protective effect in women, but not in men in this study. However, two studies (Michaud 2002; Nomura 1987) included only male participants, and both found a reduced but statistically very imprecise bladder cancer risk for higher selenium exposure (Analysis 1.6). Heterogeneity was not reduced by gender stratification (I² = 40% in study results for men).
1.1.4. Lung cancer
Twelve studies were included in this meta‐analysis. Data from Menkes 1986 and Knekt 1990 were not meta‐analysed, as the study population of the former overlapped with that of another meta‐analysed study (Comstock 1997) and results of the latter were presented in insufficient detail.
The summary risk estimate for lung cancer incidence for both genders combined was 0.75 (95% CI 0.54 to 1.03) (Analysis 1.7). Moderate heterogeneity was seen between study results (I² = 54%).
Analysis 1.7.
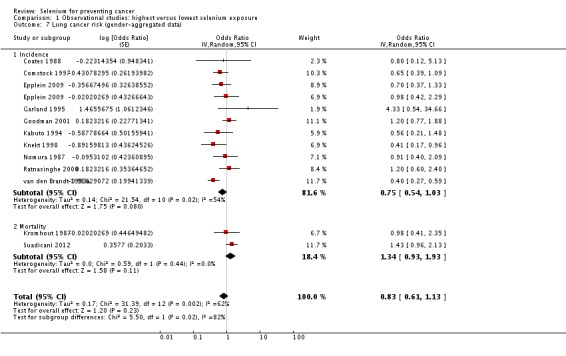
Comparison 1 Observational studies: highest versus lowest selenium exposure, Outcome 7 Lung cancer risk (gender‐aggregated data).
In the meta‐analysis according to gender using gender‐stratified study results (Analysis 1.8), the summary risk estimate for women was OR 0.83 (95% CI 0.43 to 1.61) and for men OR 0.98 (95% CI 0.68 to 1.39). Heterogeneity among study results was not reduced by stratification. However, we expected the results for gender‐combined data to be more or less a combination of the separate results for women and men. This was not the case here, with 'gender‐neutral' data suggesting a greater protective effect than was seen with gender‐stratified data. This discrepancy might be related to differences in study design or in study populations. In Knekt 1998, 95% of lung cancer cases occurred in men. We repeated the meta‐analysis of gender‐disaggregated data categorising Knekt 1998 as a 'men‐only' study and found a slightly changed summary relative risk estimate for men (OR 0.81, 95% CI 0.56 to 1.18).
Analysis 1.8.
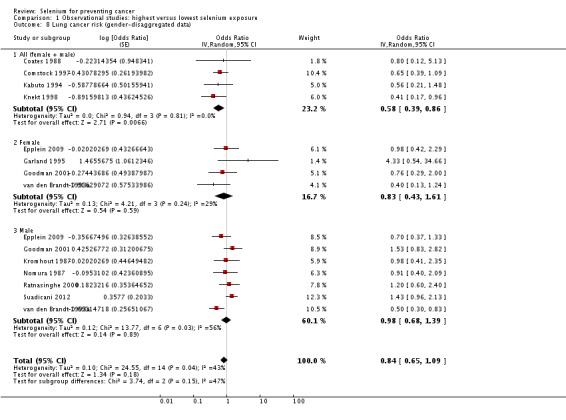
Comparison 1 Observational studies: highest versus lowest selenium exposure, Outcome 8 Lung cancer risk (gender‐disaggregated data).
The only study that used nutritional intake assessment for exposure classification (Kromhout 1987) found no association with lung cancer risk (Analysis 1.9). Two studies measured selenium content in toenails, with inconsistent results: participants (all women) in the Nurses' Health Study (Garland 1995) showed increased lung cancer risk with higher selenium toenail levels, although an inverse association was observed in the Netherlands cohort study (van den Brandt 1993a). The remaining nine studies used serum or plasma selenium levels. The summary OR was 0.91 (95% CI 0.70 to 1.018) with low heterogeneity (I² = 33%).
Analysis 1.9.
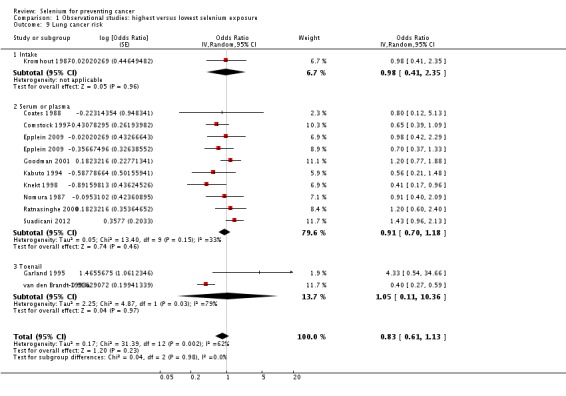
Comparison 1 Observational studies: highest versus lowest selenium exposure, Outcome 9 Lung cancer risk.
We plotted the studies using serum/plasma in ascending order of baseline exposure level (Analysis 1.10). No clear pattern of a relation between baseline exposure levels and lung cancer risk could be seen on this graph. The two studies suggesting the greatest protective effect of higher selenium levels were Knekt 1998 and Kabuto 1994. However, two other studies with similar biomarker levels reported discrepant results (Nomura 1987; Ratnasinghe 2000). A recent Danish study also found a direct association between baseline selenium exposure and subsequent lung cancer incidence, which was considerably enhanced in smokers characterised by high serum cotinine levels (Suadicani 2012),
Analysis 1.10.
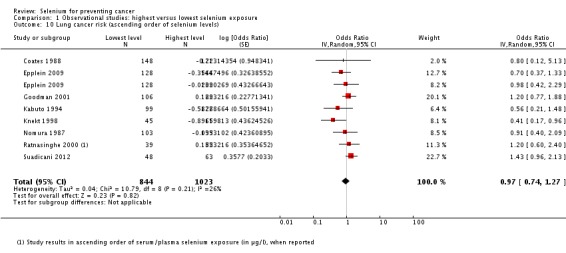
Comparison 1 Observational studies: highest versus lowest selenium exposure, Outcome 10 Lung cancer risk (ascending order of selenium levels).
1.1.5. Prostate cancer
Seventeen epidemiological studies on prostate cancer incidence were included in the meta‐analysis. The summary risk estimate for higher selenium exposure was OR 0.79 (95% CI 0.69 to 0.90) (heterogeneity: I² = 23%) (Analysis 1.11).
Analysis 1.11.
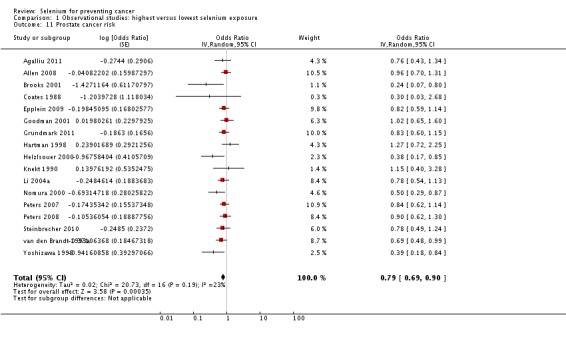
Comparison 1 Observational studies: highest versus lowest selenium exposure, Outcome 11 Prostate cancer risk.
Stratification by method of selenium assessment showed a reduction in prostate cancer risk for higher baseline biochemical markers (OR 0.76, 95% CI 0.67 to 0.88) but not for higher estimated selenium intake (OR 1.00, 95% CI 0.73 to 1.36) (Analysis 1.12). The inverse association between selenium biomarkers and prostate cancer incidence was stronger for toenail levels (OR 0.53, 95% CI 0.35 to 0.81) than for blood levels (OR 0.82, 95% CI 0.72 to 0.93) (Analysis 1.13). Heterogeneity among study results was slightly reduced by these stratifications.
Analysis 1.12.
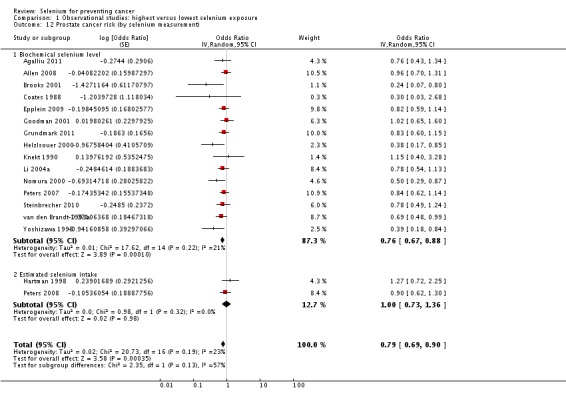
Comparison 1 Observational studies: highest versus lowest selenium exposure, Outcome 12 Prostate cancer risk (by selenium measurement).
Analysis 1.13.
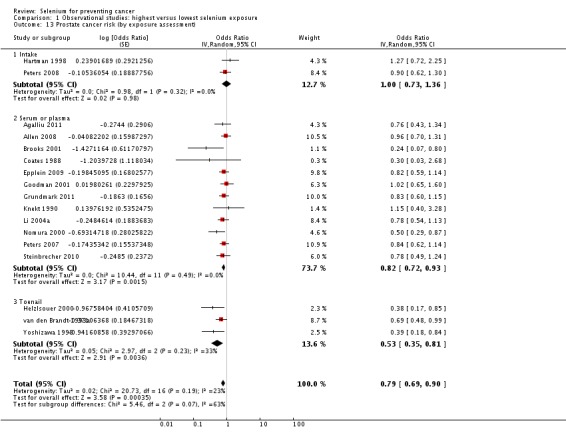
Comparison 1 Observational studies: highest versus lowest selenium exposure, Outcome 13 Prostate cancer risk (by exposure assessment).
Stratification by country and by continent found the risk reduction more pronounced in the US than in Europe (Analysis 1.14; Analysis 1.15).
Analysis 1.14.
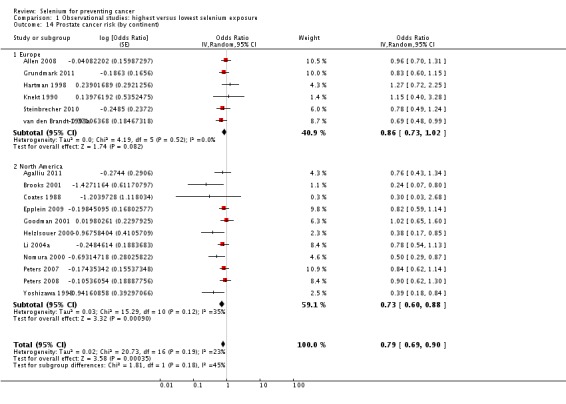
Comparison 1 Observational studies: highest versus lowest selenium exposure, Outcome 14 Prostate cancer risk (by continent).
Analysis 1.15.
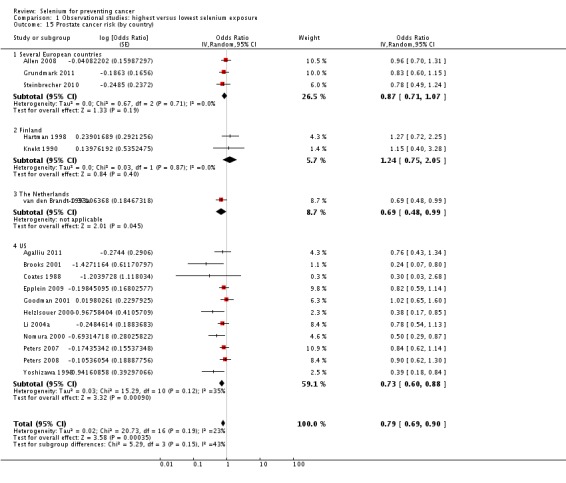
Comparison 1 Observational studies: highest versus lowest selenium exposure, Outcome 15 Prostate cancer risk (by country).
Overall, the strongest inverse associations were seen in studies from the US published before 2001. These findings cannot be explained by differences in baseline selenium levels alone. Analysis 1.16 shows the results of studies using serum or plasma measurements in ascending order of selenium levels. For similar categories of selenium concentration, studies indicated different effects (Goodman 2001 versus Clark 1985; Nomura 2000 versus Peters 2007 and Gill 2009, see Epplein 2009).
Analysis 1.16.
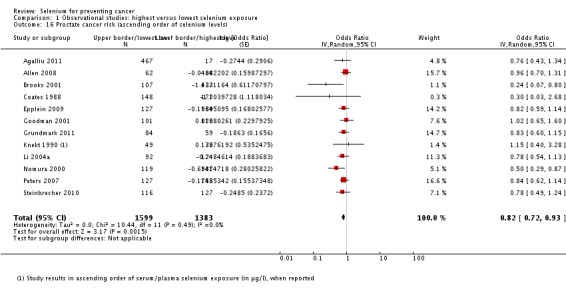
Comparison 1 Observational studies: highest versus lowest selenium exposure, Outcome 16 Prostate cancer risk (ascending order of selenium levels).
1.1.6. Stomach cancer
Five observational studies were included in the meta‐analysis of gastric cancer incidence. The summary risk estimate for both genders combined was OR 0.66 (95% CI 0.43 to 1.01) in the highest exposure category when compared with the lowest (I² = 51%) (Analysis 1.17). However, in this meta‐analysis, one cohort (Mark 2000, in: Wei 2004) is included twice because the results were reported stratified according to cardia and non‐cardia gastric cancer.
Analysis 1.17.
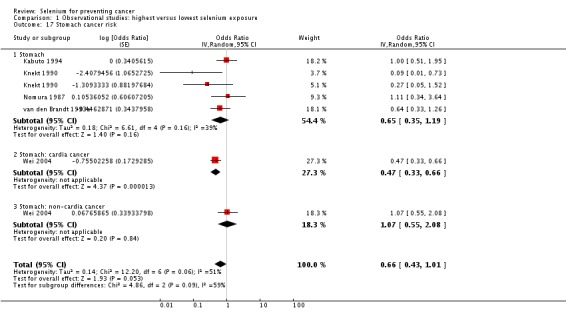
Comparison 1 Observational studies: highest versus lowest selenium exposure, Outcome 17 Stomach cancer risk.
We repeated the meta‐analyses and included the results of Mark 2000 (see: Wei 2004) for cardia and non‐cardia gastric cancer separately. The summary OR was 0.75 (95% CI 0.47 to 1.21) when data for non‐cardia cancer were included and OR 0.59 (95% CI 0.38 to 0.93) when data for cardia cancer were included.
Using the available gender‐stratified results for meta‐analysis, the risk estimate for men was OR 0.43 (95% CI 0.14 to 1.32) (I² = 56%) and for women OR 0.73 (95% CI 0.12 to 4.35) (I² = 62%) (Analysis 1.18).
Analysis 1.18.
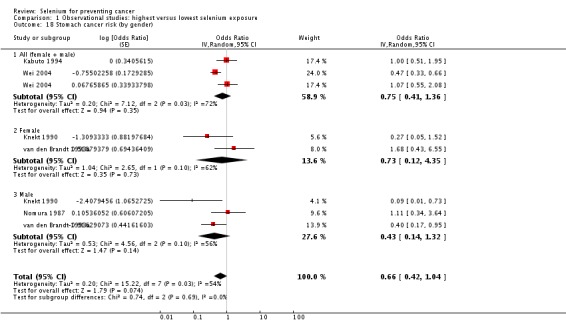
Comparison 1 Observational studies: highest versus lowest selenium exposure, Outcome 18 Stomach cancer risk (by gender).
1.1.7. Colon/colorectal cancer
Five observational studies reported data on colon or colorectal cancer incidence. The summary risk estimate was OR 0.89 (95% CI 0.65 to 1.23) for both genders combined (I² = 3.8%) (Analysis 1.19), OR 0.69 (95% CI 0.42 to 1.12) for men and OR 1.06 (95% CI 0.57 to 2.00) for women (Analysis 1.20).
Analysis 1.19.
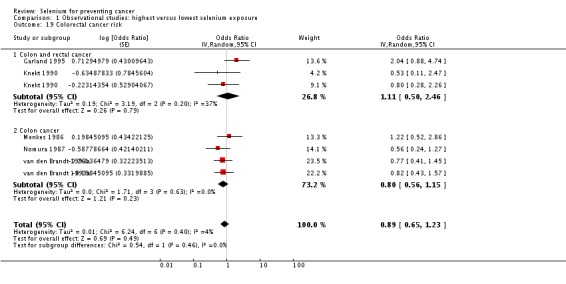
Comparison 1 Observational studies: highest versus lowest selenium exposure, Outcome 19 Colorectal cancer risk.
Analysis 1.20.
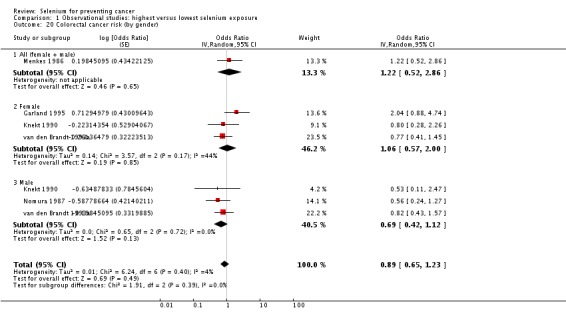
Comparison 1 Observational studies: highest versus lowest selenium exposure, Outcome 20 Colorectal cancer risk (by gender).
1.2. Aetiological association: other results
For all other types of cancer, data were available from fewer than five epidemiological studies; thus results were not meta‐analysed. Results of observational studies not included in meta‐analyses are reported in Table 6. None of the study results supported an association between selenium exposure and gynaecological cancer risk, and results for cancers of the gastrointestinal, respiratory or urological tract were inconsistent. For respiratory and urological cancers, studies reported either no association or increased risk for participants with a higher selenium exposure. For gastrointestinal cancers, studies found either no association or reduced risk with a higher selenium exposure.
Table 4.
Results of observational studies not included in meta‐analysis
| Organ system | Cancer | Case definition | Relative risk estimate (highest vs lowest exposure category) | 95% CI | Selenium marker | Gender | Study |
| Gynaecological | Cervix | incidence | 0.89 | 0.40 to 2.00 | serum | women | Menkes 1986 (Batieha 1993) |
| 1.10 | n.r. | serum | Coates 1988 | ||||
| Gynaecological (without breast) | incidence | 0.96 | n.r. | serum | Knekt 1990 | ||
| Ovary | incidence | 0.87 | 0.25 to 5.26 | serum | Knekt 1990 (Knekt 1996) | ||
| 1.22 | 0.44 to 3.38 | toenail | Garland 1995 | ||||
| 0.58 | 0.2 to 1.7 | serum | Menkes 1986 (Helzlsour 1996) | ||||
| 1.00 (HR) | 0.73 to 1.37 | suppl. intake | Thomson 2008 | ||||
| Uterus | incidence | 1.38 | 0.62 to 3.08 | toenail | Garland 1995 | ||
| Gastrointestinal | Gastrointestinal tract (all) | incidence | 1.00 | n.r. | serum/plasma | both | Coates 1988 |
| 0.29 | 0.10 to 0.91 | plasma | men | Persson 2000 | |||
| Oesophageal squamous cell carcinoma | incidence | 0.37 | 0.16 to 0.86 | toenail | both | Steevens 2010 | |
| Oesophageal adenocarcinoma | incidence | 0.76 | 0.41 to 1.40 | toenail | both | Steevens 2010 | |
| Oesophagus | incidence | 0.56 | 0.44 to 0.71 | serum | both | Wei 2004 (Mark 2000) | |
| mortality | 0.62 | 0.44 to 0.89 | serum | ||||
| mortality | 0.35 | 0.16 to 0.81 | serum | both | Wei 2004 (Wei 2004) | ||
| incidence | 0.27 | 0.03 to 2.21 | suppl. intake | both | Dong 2008 | ||
| Gastric cardio adenocarcinoma | incidence | 0.52 | 0.27 to 1.02 | toenail | both | Steevens 2010 | |
| Oesophagus and stomach | incidence | 0.45 | n.r. | serum | men | Knekt 1990 (Knekt 1988) | |
| incidence | 0.67 | n.r. | serum | women | |||
| Liver | incidence | 0.62 | 0.21 to 1.86 | plasma | men | Yu 1999 | |
| incidence | 0.50 | 0.28 to 0.90 | toenail | both | Sakoda 2005 | ||
| mortality | 0.50 | 0.28 to 0.90 | both | ||||
| 0.57 | 0.31 to 1.05 | men | |||||
| 0.18 | 0.03 to 1.13 | women | |||||
| Pancreas | incidence | 0.08 | 0.01 to 0.56) | serum | men | Menkes 1986 (Burney 1989) | |
| 0.83 | 0.4 to 1.67 | women | |||||
| 0.58 | n.r. | serum | men | Knekt 1990 | |||
| 3.49 | n.r. | women | |||||
| Rectum | incidence | 0.625 | n.r. | serum | men | Nomura 1987 | |
| 1.05 | 0.54 to 2.03 | toenail | both |
van den Brandt 1993a |
|||
| 0.91 | 0.41 to 2.00 | men | |||||
| 1.58 | 0.59 to 4.22 | women | |||||
| Urinary tract | Urinary tract (all) | incidence | 0.97 | 0.72 to 1.31 | serum | both | Hotaling 2011 |
| 5.0 | 0.71 to ∞ | plasma | men | Persson 2000 | |||
| 0.81 | n.r. | serum | men | Knekt 1990 | |||
| 4.12 | n.r. | women | |||||
| Respiratory tract | Cavum oris/pharynx | incidence | 5.43 | n.r. | serum | both | Menkes 1986 (Zheng 1993) |
| Respiratory tract (all) | incidence | 6.0 | 1.5 to 24.2 | plasma | men | Persson 2000 | |
| Skin | Melanoma | incidence | 1.66 | 0.71 to 3.85 | toenail | women | Garland 1995 |
| 0.90 | 0.30 to 2.50 | serum | both | Menkes 1986 (Breslow 1995) | |||
| 0.98 | 0.69 to 1.41 | suppl. intake | both | Peters 2008 (Asgari 2009) | |||
| Any non‐melanoma cancer | incidence | 0.77 | n.r. | plasma | both | Clark 1985 | |
| Basal cell carcinoma | incidence | 0.54 | n.r. | serum | men | Knekt 1990 | |
| 1.55 | n.r. | women | |||||
| 0.80 | 0.10 to 4.5 | serum | both | Menkes 1986 (Breslow 1995) | |||
| 0.86 | 0.38 to 1.96 | serum | both | McNaughton 2005 | |||
| 0.95 | 0.59 to 1.50 | nutritional intake | |||||
| Squamous cell carcinoma | incidence | 0.69 | 0.51 to 0.92 | plasma | both | Combs 1993 | |
| 0.60 | 0.20 to 1.50 | serum | both | Menkes 1986 (Breslow 1995) | |||
| 0.86 | 0.47 to 1.58 | plasma | both | Karagas 1997 | |||
| 1.30 | 0.77 to 2.3 | nutritional intake | both | McNaughton 2005 | |||
| 0.49 | 0.24 to 0.99 | serum | |||||
| Other | Haematological | incidence | 0.60 | n.r. | serum/plasma | both | Coates 1988 |
| incidence | 0.95 | 0.75 to 1.20 | suppl. intake | both | Walter 2011 | ||
| Thyroid | incidence | 0.15 | 0.0 to 5.0 | serum | men | Glattre 1989 | |
| 0.12 | 0.01 to 1.11 | women | |||||
| 0.13 | 0.02 to 0.77 | both | |||||
| Not defined | mortality | 0.72 (HR) | 0.58 to 0.89 | plasma | both | Bates 2011 |
n.r. = not reported
2. Randomised controlled trials
We report results from Duffield‐Lillico 2002 for all evaluated outcomes in the NPCT study (NPCT 2002) (prostate, lung, bladder, colorectal and breast cancer; any cancer; and death from cancer), except for prostate cancer, for which we also used Duffield‐Lillico 2003 BJU, and for the primary outcome, non‐melanoma skin cancer, whose results were reported in Duffield‐Lillico 2003 JNCI. For the SELECT study (SELECT 2009), we included only the results from Lippman 2009, which reported on the blinded period of follow‐up with continuing selenium supplementation, not from Klein 2011, which reported a longer period of follow‐up, including a subsequent period without selenium supplementation, discontinued in 2008 in compliance with the recommendation of the trial's independent Data and Safety Monitoring Committee. This second report by Klein et al. included an additional period of 32 months (23% person‐time increase) along with the first follow‐up period, and results were essentially similar to those of Lippman et al. 2009. For bladder cancer risk in SELECT, we used data from Lotan 2012, which encompassed the same extended period of follow‐up as Klein 2011 but was the only available report from the SELECT trial on this cancer type.
2.1. Preventive efficacy outcomes
2.1.1. Any cancer incidence and mortality
The outcomes of any cancer incidence and any cancer mortality were evaluated by pooling the data from two studies—NPCT 2002 and SELECT 2009. For RCTs, we repeated analyses confined to trials with low risk of bias; for any cancer incidence and mortality outcomes, analysis was limited to SELECT alone. We observed no evidence of reduced incident cancer risk (RR 0.90, 95% CI 0.70 to 1.17) (Analysis 2.1) or cancer mortality (RR 0.81, 95% CI 0.49 to 1.32) (Analysis 2.2) in the selenium group compared with the placebo group. When analysis was limited to SELECT, no evidence was found of an effect on all cancers (RR 1.01, 95% CI 0.92 to 1.11) or on death from cancer (RR 1.02, 95% CI 0.80 to 1.30).
Analysis 2.1.
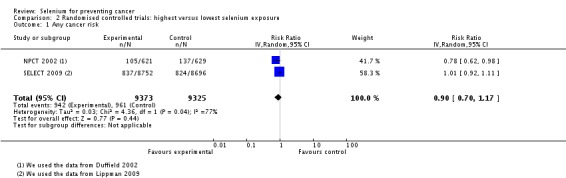
Comparison 2 Randomised controlled trials: highest versus lowest selenium exposure, Outcome 1 Any cancer risk.
Analysis 2.2.
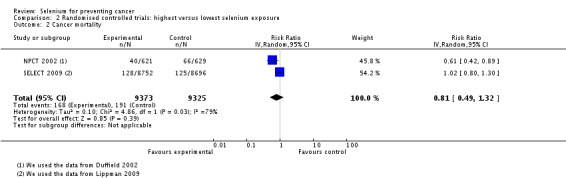
Comparison 2 Randomised controlled trials: highest versus lowest selenium exposure, Outcome 2 Cancer mortality.
2.1.2. Primary liver cancer
Three RCTs investigated the efficacy of selenium supplementation for liver cancer prevention. All three were conducted in China with participants of different high‐risk groups in Qidong province.
Yu 1991 reported on a trial with 2474 male and female first‐degree relatives of liver cancer patients. During the study period of two years, 10 participants in the selenium group, who received 200 µg selenium yeast/d, and 13 participants in the placebo group were observed (RR 0.55, 95% CI 0.24 to 1.25).
Yu 1997 investigated a four‐year supplementation period with 200 µg selenium yeast/d in 226 male and female hepatitis B‐surface antigen (HBs‐Ag) carriers. Eleven cases (person‐time incidence rate: 1573.03/100,000) were detected in the placebo group and four cases in the selenium group (RR 0.36, 95% CI 0.12 to 1.11) during the eight‐year follow‐up period. The mean blood selenium level during the intervention period was 152 ng/mL in the intervention group and 107 ng/mL in the control group.
Li 2000 randomly assigned 2065 male HBs‐Ag carriers to receive 0.5 mg sodium selenite or placebo daily for three years. Thirty‐four cases of liver cancer occurred among 1112 participants receiving selenium and 57 cases among 953 placebo participants (RR 0.51, 95% CI 0.34 to 0.77).
The pooled risk ratio of the three studies was 0.50, with 95% CI 0.35 to 0.77, corresponding to a strong reduction in the incidence of liver cancer in participants assigned to selenium compared with those assigned to placebo (Analysis 2.3). However, all three trials were considered to have an unclear risk of bias, caused by lack of clear reporting of generation of allocation sequence, allocation concealment and/or completeness of outcome data.
Analysis 2.3.
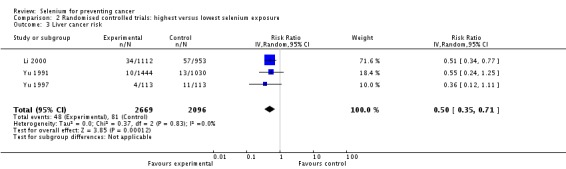
Comparison 2 Randomised controlled trials: highest versus lowest selenium exposure, Outcome 3 Liver cancer risk.
2.1.3. Non‐melanoma skin cancer
2.1.3.1. Total non‐melanoma skin cancer
Higher risk for non‐melanoma skin cancer was seen in the selenium supplementation group (200 µg/d) of the NPCT compared with the placebo group (unadjusted RR 1.27, 95% CI 1.11 to 1.45) (Duffield‐Lillico 2003a, in: NPCT 2002). This increase was confirmed by multivariate analysis after adjustment for confounders (HR 1.17, 95% CI 1.02 to 1.34) and was concentrated among participants in the highest two tertiles of baseline plasma selenium (≥ 105.6 ng/mL). No variation in this effect appeared to be induced by age, gender or smoking habits. Eliminating cases that occurred during the first period of selenium supplementation (one to two years) induced a slight decline in RRs. The mean selenium plasma concentration of participants was 114 ng/mL at the time of randomisation. Increased risk for total non‐melanoma skin cancer was seen in all tertiles of baseline plasma selenium levels (Reid 2008).
In this NPCT substudy carried out in Macon, which included both 200 and 400 µg/d selenium supplementation (Reid 2008), after adjustment for age, gender and smoking, non‐melanoma skin cancer risk increased in the 200 µg/d arm (unadjusted RR 1.49, 95% CI 1.10 to 2.03; adjusted HR 1.5, 95% CI 1.13 to 2.04) but not in the 400 µg/d arm (unadjusted RR 0.88, 95% CI 0.66 to 1.16; adjusted HR 0.9, 95% CI 0.7 to 1.2). At the remaining sites, where only 200 µg/d of supplemental selenium was used, the RR was 1.24 (95% CI 1.07 to 1.45) and the HR was 1.2 (95% CI 1.0 to 1.4). Distribution of baseline plasma selenium levels was similar in this substudy to that in the NPCT main study, and no evidence of effect modification according to baseline selenium exposure emerged.
Overall, NPCT did not support preventive efficacy of selenium yeast supplementation against non‐melanoma skin cancer in these populations; on the contrary, it indicated a cancer‐promoting effect of selenium on this cancer type, which was the primary trial endpoint, raising concern about potential harmful effects of such selenium supplementation.
Unfortunately, non‐melanoma skin cancer incidence thus far has not been investigated in SELECT, which is the largest selenium supplementation trial conducted to date (Lippman 2009, Klein 2011, in: SELECT 2009). This endpoint was investigated in a small trial in a French population of 184 organ graft recipients who were considered to be at high risk of pre‐malignant and malignant epithelial lesions (Dreno 2007). This trial detected a higher incidence of skin cancer in 91 selenium‐supplemented participants (six cases; 6.6%) compared with 93 placebo‐supplemented participants (two cases; 2.2%; P value 0.15) during a five‐year follow‐up, which comprised in its first three years daily supplementation with selenised yeast containing 200 µg selenium.
A small trial among participants at high risk for prostate cancer also investigated the effect on risk of non‐melanoma skin cancer of using selenium supplements of 200 and 400 µg/d, with a median follow‐up of three years (Algotar 2013). Results for non‐melanoma skin cancer from this study showed an occurrence of three cases among 232 placebo‐treated participants and 11 cases among 467 selenium‐supplemented participants (eight cases among 234 individuals receiving 200 µg/d of selenium, and three cases among 233 receiving 400 µg/d), with increased risk after overall selenium supplementation (incidence rate ratio from our calculation 1.8, 95% CI 0.5 to 10.2) but no evidence of a dose‐response relation.
We computed a summary RR for non‐melanoma skin cancer in selenium‐supplemented participants by pooling the RRs from the above three trials (Algotar 2013; Dreno 2007; NPCT 2002; N = 1900), rather than by using numbers of participants and cases, because the number of skin cancer cases diagnosed in the NPCT was not reported in the relevant publication (Duffield‐Lillico 2003). The estimated risk ratio (Analysis 2.4) indicated an increased risk of non‐melanoma skin cancer associated with selenium supplementation of 200 µg/d (RR 1.44, 95% CI 0.95 to 2.17). When the analysis for non‐melanoma skin cancer was limited to Algotar 2013—the only study with low risk of bias—the risk ratio was still well over unity but was statistically very unstable as the result of the very low number of cases (RR 2.64, 95% CI 0.71 to 9.84).
Analysis 2.4.
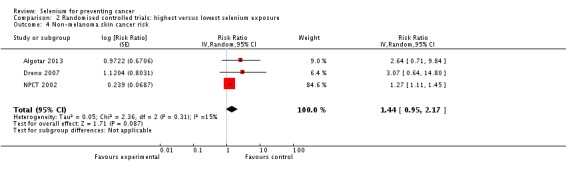
Comparison 2 Randomised controlled trials: highest versus lowest selenium exposure, Outcome 4 Non‐melanoma skin cancer risk.
2.1.3.2. Basal cell carcinoma (BCC)
At the end of the blinded treatment period in NPCT 2002, the unadjusted RR for basal cell carcinoma in the 200 µg/d selenium group was 1.17 (95% CI 1.02 to 1.35). Computation of the adjusted HR in multivariate analysis yielded a value of 1.09 (95% CI 0.94 to 1.26). Eliminating cases that occurred within the first two years of supplementation had no further effect on the RR. Variables such as age, gender and smoking status had little effect on this estimate. In another, much smaller trial in which investigators administered 200 µg/d selenium and no RR estimates were reported (Dreno 2007), three cases of BCC occurred among 91 selenium‐supplemented participants, along with one case among 93 placebo‐receiving participants.
Reid 2008 found a crude RR of 0.90 (95% CI 0.65 to 1.24) and an adjusted HR of 0.95 (95% CI 0.69 to 1.29) for this cancer type in the 400 µg/d selenium substudy.
2.1.3.3. Squamous cell carcinoma (SCC)
In NPCT 2002, selenium supplementation increased the risk of squamous cell carcinoma, both in the unadjusted analysis (RR 1.32, 95% CI 1.09 to 1.60) and in the adjusted one (HR 1.25, 95% CI 1.03 to 1.51). After exclusion of cases that occurred within the first two years, a slight decline in the effect of selenium supplementation was seen. Little influence on the point estimates of age, gender and smoking status was noted. The adverse effects of selenium supplementation on SCC risk appeared to increase with increasing plasma selenium levels at baseline. A higher risk of non‐melanoma skin cancer incidence was seen only in participants with baseline plasma levels in the highest two tertiles of baseline levels (≥ 105.6 ng/mL), suggesting an interaction between supplementation and baseline exposure.
In the 400 µg/d selenium substudy (Reid 2008), no alteration of SCC risk by selenium supplementation was reported (crude RR 1.20, 95% CI 0.85 to 1.68; adjusted HR 1.05, 95% CI 0.71 to 1.56). The smaller trial by Dreno et al. (Dreno 2007) reported that two among 91 selenium‐supplemented individuals were diagnosed with SCC, whereas no cases were described among placebo participants.
2.1.4. Prostate cancer
The meta‐analysis for prostate cancer, which is provided in Analysis 2.5, found an RR of 0.90 for participants supplemented with selenium compared with placebo (95% CI 0.71 to 1.14). When the analysis was limited to low‐bias trials, no evidence of any beneficial effect of selenium supplementation emerged (Analysis 2.6).
Analysis 2.5.
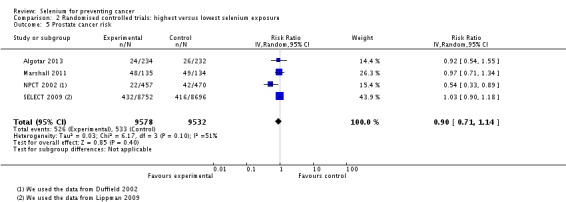
Comparison 2 Randomised controlled trials: highest versus lowest selenium exposure, Outcome 5 Prostate cancer risk.
Analysis 2.6.
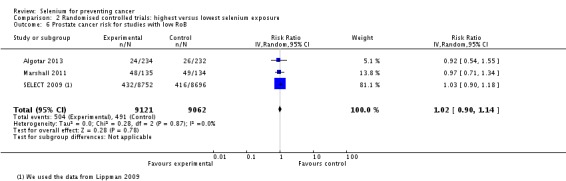
Comparison 2 Randomised controlled trials: highest versus lowest selenium exposure, Outcome 6 Prostate cancer risk for studies with low RoB.
The trial that first investigated the relation between selenium exposure and prostate cancer risk—NPCT 2002 (see Duffield‐Lillico 2002 and Duffield‐Lillico 2003)—reported a reduction in prostate cancer incidence in the selenium‐treated group, which was particularly strong in a first period of follow‐up (1983 to 1993; adjusted HR 0.35, 95% CI 0.16 to 0.65) and was slightly higher but still much lower than unity during the entire period of follow‐up (1983 to 1996; HR 0.48, 95% CI 0.28 to 0.80). Analyses stratified by baseline plasma selenium category showed a greatly reduced risk associated with active treatment in participants with plasma selenium ≤ 106.4 µg/L (HR 0.14, 95% CI 0.03 to 0.61), but in the intermediate category (106.8 to 123.2 µg/L) and in the upper category (> 123.2 µg/L), HRs were 0.33 (95% CI 0.13 to 0.82) and 1.14 (95% CI 0.51 to 2.59), respectively. Selenium supplementation in participants with baseline PSA ≤ 4 ng/mL was associated with considerably reduced risk (HR 0.33, 95% CI 0.14 to ‐0.79) compared with risk in individuals with PSA > 4 ng/mL (HR 0.95, 95% CI 0.42 to ‐2.14).
Interpretation of NPCT findings is complicated by a potentially severe source of bias. As reported by the study authors, a considerably higher percentage of participants with elevated PSA levels underwent prostatic biopsy in the placebo group as compared with the selenium group (35% vs 14%; P < 0.05; NPCT 2002, see Duffield‐Lillico 2003). Differences in biopsy rates were greatest among participants with the lowest baseline selenium concentrations, which was the subgroup that appeared to derive the greatest beneficial effects of selenium administration. This may have contributed to an overestimation in the NPCT of the effects of selenium supplementation.
The SELECT trial found no evidence of benefit derived from selenium supplementation (compared with placebo) over a median of 5.5 years in terms of prostate cancer incidence (HR 1.03, 95% CI 0.90 to 1.18, 99% CI 0.87 to 1.24) (SELECT 2009). The adjusted HR for prostate cancer in the selenium plus vitamin E group compared with the placebo group was 1.05 (95% CI 0.91 to 1.20, 99% CI 0.88 to 1.25). No specific RR estimate according to disease severity was reported in the original report of the trial, but during an extended follow‐up of this cohort after selenium supplementation had ceased (Klein 2011), an increased risk of Gleason 7 or greater disease was found (HR 1.21, 99% CI 0.90 to 1.63). It is interesting to note that the SELECT trial included only participants with PSA ≤ 4 ng/mL—the group that showed the greatest apparent benefit in the NPCT.
The SELECT trial was discontinued in 2008 in compliance with the recommendation of the Data and Safety Monitoring Committee, which expressed some concern regarding an increase in prostate cancer in the vitamin E–alone group (HR 1.13, 99% CI 0.95 to 1.35) and an increase in type 2 diabetes in the selenium group (RR 1.07, 99% CI 0.94 to ‐1.22).
In Marshall 2011, the prostate cancer incidence was 35.6% versus 36.6% in selenium‐supplemented compared with placebo‐treated participants after three years of follow‐up, respectively. The overall RR was 0.91, with a 95% CI of 0.55 to 1.52 (courtesy of James Marshall, unpublished data). Analysis of RRs according to baseline plasma selenium levels showed no dose‐response effect, with point estimates of 0.82 (0.40 to 1.69), 1.38 (0.68 to 2.78), 0.98 (0.58 to ‐1.68) and 0.91 (0.45 to 1.84), by increasing the quartile of selenium status at baseline (Marshall 2011).
Algotar 2013 reported an HR of prostate cancer of 0.94 (95% CI 0.52 to ‐1.7) for participants receiving the 200 µg/d dose and 0.90 (0.48 to ‐1.7) for those receiving 400 µg/d, compared with placebo. Although average baseline selenium status, as assessed through plasma selenium, was higher than in the NPCT (median value 126.1 versus 115.0 µg/L), the lowest tertile of plasma selenium levels had a median value (101.1. µg/L) well below the apparent threshold of 120 µg/L, at which a beneficial effect of selenium seemed to occur in the NPCT. Furthermore, as noted by the study authors, 45% of participants enrolled in this study had baseline plasma selenium levels < 123 µg/L, which is the upper threshold for a protective effect of selenium supplementation according to the results of the NPCT. Moreover, the trial authors stated in the paper that 'None of the baseline variables modified the effect of selenium on the primary endpoint';these variables were age, plasma selenium concentration and serum PSA at baseline (Algotar 2013).
We also investigated the risk of prostate cancer associated with selenium supplementation after limiting the analysis to the three trials at low risk of bias (Algotar 2013; Marshall 2011; SELECT 2009). This restriction had limited effects on the statistical precision of the estimates and yielded an overall RR of 1.02 (95% CI 0.90 to 1.14), indicating no effect of intervention (supplementation of organic selenium at 200 µg/d) on prostate cancer risk. These three studies were generally characterised by higher mean baseline selenium values than were seen in the excluded NPCT, but such differences were generally limited; also, analyses stratified according to baseline selenium exposure offered little evidence of a beneficial effect of supplementation even at lower exposure (Algotar 2013; Marshall 2011) (Analysis 2.6).
2.1.5. Lung, bladder and colorectal cancer
Lung, bladder and colorectal cancer outcomes were evaluated by pooling the data from NPCT 2002 and SELECT 2009.
Slight to moderate RR departures from unity, which statistically were very unstable, were observed in the selenium group compared with the placebo group for lung cancer (RR 0.94, 95% CI 0.62 to 1.42) (Analysis 2.7), bladder cancer (RR 1.14, 95% CI 0.81 to 1.61) (Analysis 2.8) and colorectal cancer (RR 0.77, 95% CI 0.37 to 1.62) (Analysis 2.9). When analysis was limited to the trial with low risk of bias (SELECT), evidence showed no effect on risk for colorectal cancer (RR 1.04, 95% CI 0.73 to 1.98) or for lung cancer (RR 1.11, 95% CI 0.80 to ‐1.54).
Analysis 2.7.
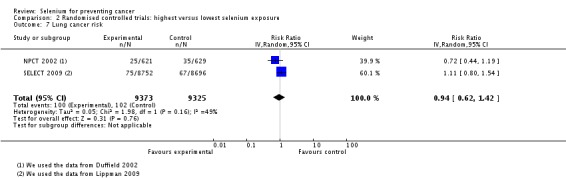
Comparison 2 Randomised controlled trials: highest versus lowest selenium exposure, Outcome 7 Lung cancer risk.
Analysis 2.8.
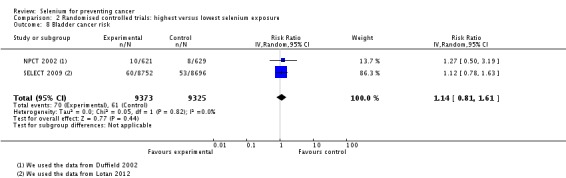
Comparison 2 Randomised controlled trials: highest versus lowest selenium exposure, Outcome 8 Bladder cancer risk.
Analysis 2.9.
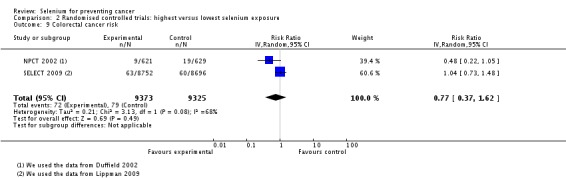
Comparison 2 Randomised controlled trials: highest versus lowest selenium exposure, Outcome 9 Colorectal cancer risk.
2.2. Adverse effects
In NPCT 2002 and SELECT 2009, adverse effects associated with selenium supplements were unexpectedly observed. In NPCT, 35 participants withdrew from the study because of adverse effects, mainly gastrointestinal upset. The RR for adverse events in the selenium group was 1.51 (95% CI 0.74 to 3.11) (our calculation, based on the number of randomly assigned participants). Increased risk of glaucoma was also reported (Marshall 2011; NPCT 2002 ), which prompted additional studies on this issue (Bruhn 2009) and likely led to the inclusion of cataract and glaucoma among the several potential adverse events monitored in subsequent trials in which selenium was administered (Algotar 2013).
A secondary analysis of participants who did not have diabetes at the start of the study revealed an excess risk of type 2 diabetes mellitus in the selenium group (adjusted HR 1.55, 95% CI 1.03 to 2.33) (Stranges 2007). In that study, increased risk of developing type 2 diabetes associated with selenium supplementation was found across all tertiles of baseline plasma selenium levels, although the excess was much greater for the upper category of > 121.6 ng/mL (RR 2.70, 95% CI 1.30 to 5.61) than for the lower (RR 1.13, 95% CI 0.58 to 2.18) and intermediate (RR 1.36, 95% CI 0.60 to 3.09) subgroups. The increased risk of diabetes associated with selenium supplementation was independent of baseline age, sex, smoking status and body mass index (BMI), with the exception of participants in the top tertile of BMI. In SELECT, men in the selenium group had an increased risk of alopecia (RR 1.28, 99% CI 1.01 to 1.62), dermatitis (grade 1 to 2, RR 1.17, 99% CI 1.00 to 1.35; grade 3 to 4, RR 1.74, 99% CI 0.56 to 5.44) and halitosis (RR 1.17, 99% CI 0.99 to 1.38). An increase in diabetes mellitus type 2 was seen in the selenium‐alone group (RR 1.07, 99% CI 0.94 to 1.22). Such excess risk decreased over time after selenium supplementation ceased, as shown by the results of the Klein study, which expanded by 32 months the follow‐up of SELECT participants in the absence of further supplementation (Klein 2011, in: SELECT 2009). In this study, the RR of diabetes was 1.04 (99% CI 0.93 to ‐1.17), thus supporting a short‐term effect of selenium supplementation on diabetes risk. Thus, both NPCT and SELECT results suggest that supplementation with selenium may increase the risk for type 2 diabetes.
The three trials on liver cancer and the Reid 2008 study did not mention the occurrence of adverse effects. One paper stated that no case of selenosis had been observed during the trial. Two recent phase 3 trials have investigated the occurrence of diabetes after selenium supplementation for cancer prevention. During five years of follow‐up of 699 participants at high risk for prostate cancer supplemented with 200 or 400 µg/d of selenium or placebo, Algotar 2013 reported the occurrence of diabetes in 12, 12 and seven subjects, respectively. This allowed us to compute an incidence rate ratio of 1.70 (95% CI 0.62 to ‐5.10) and 1.71 (0.62 to ‐5.12) among the 200 and 400 µg/d selenium‐supplemented participants, respectively, compared with those given placebo. No assessment of diabetes incidence was reported for the Dreno 2007 or the Marshall 2011 trial.
In a recent phase 3 trial carried out in 1561 participants with resected stage I non–small‐cell lung cancer, which was discontinued for futility in compliance with the recommendation of the Data and Safety Monitoring Committee, and which showed a slightly higher risk of lung second primary tumors and overall second primary tumors among selenium‐supplemented participants (Karp 2013), the RR of diabetes during follow‐up was not reported by the trial authors. However, occurrence during four years of follow‐up (2007 to 2011) was stated as 26 new diagnoses of diabetes in the selenium arm (1040 participants at baseline, of whom 865 underwent toxicity assessment) and 12 new diagnoses among placebo‐treated participants (521/477). These numbers allowed us to compute an RR of 1.09 (95% CI 0.53 to 2.36) or, in participants with toxicity assessment, 1.19 (95% CI 0.58 to 2.60)—figures similar to the HRs observed in SELECT (SELECT 2009).
Discussion
Summary of main results
The aims of this review were to examine the efficacy of selenium supplements in preventing cancer and the association between selenium exposure and risk of cancer incidence and mortality, overall and separately, in men and women.
Observational studies and aetiological association
From our meta‐analyses of 16 prospective observational studies on total cancer risk, we found reduced cancer incidence and mortality with higher selenium exposure. The risk of cancer was 31% (95% CI 9% to 47%) lower in the highest category of selenium exposure compared with the lowest; the risk of death from cancer was 36% (95% CI 13% to 54%) lower. Subgroup analyses by gender, however, yielded no convincing evidence of different effects of selenium exposure in men versus women.
The risk of developing bladder cancer was reduced by 33% (95% CI 3% to 54%) and that of prostate cancer by 21% (95% CI 10% to 31%). The risk of lung, gastric or colorectal cancer was also found to be reduced with higher selenium exposure; however, the confidence intervals of the summary risk estimates overlapped unity. No association was seen between selenium and risk of breast cancer.
As is the case with all meta‐analyses of epidemiological data, our findings have potential limitations resulting from study design, as well as from quality and heterogeneity of the data. These limitations complicate interpretation of the summary statistics.
RCTs and preventive efficacy
We identified eight RCTs that investigated mono‐selenium supplements in prevention of non‐melanoma skin cancer, liver cancer and prostate cancer, as well as many secondary outcomes, including incidence and mortality of overall cancer and other site‐specific cancers. Overall, no convincing evidence suggests that selenium supplementation prevented the primary outcomes (non‐melanoma skin cancer, liver cancer and prostate cancer) or the secondary outcomes. The results of two trials—NPCT and SELECT—also raised concerns about possible harmful effects of selenium supplements, including increased incidence of non‐melanoma skin cancer, type 2 diabetes and dermatological effects.
Of the three liver cancer prevention trials, one reported a strongly reduced risk of liver cancer for male carriers of the HBs‐Ag taking inorganic selenium supplements (sodium selenite) for three years, while the other two studies reported little effect of organic selenium supplements (selenium yeast) for the same cancer site. As the result of several methodological concerns related to randomisation and completeness of outcome data, the risk of bias was judged as unclear for all three of these RCTs. Therefore, we could not conclude that there is strong support for selenium supplements as agents for the prevention of liver cancer. Unfortunately, liver cancer was not included among the secondary outcomes in the other trials.
The NPCT (NPCT 2002), which was considered to have an unclear risk of bias related to different prostate biopsy rates in the two arms, found an increase in the incidence of non‐melanoma skin cancer in selenium‐supplemented participants, and analysis of secondary outcomes indicated lower total cancer incidence and mortality in the selenium group in men but not in women. Analyses stratified according to cancer type found a strongly reduced risk for prostate cancer, as well as oesophageal, colorectal and lung cancers, while some increase in other cancers such as breast cancer emerged. When participants were categorised into three tertiles according to baseline serum selenium, HR for all cancers increased from 0.51 (95% CI 0.32 to 0.81) in the bottom category to 0.70 (95% CI 0.44 to 1.09) and 1.20 (95% CI 0.77 to 1.86) in the two upper categories, respectively.
The SELECT trial (SELECT 2009) was a low‐bias and powerful prostate cancer prevention trial carried out in the male general population of North America not at high risk of prostate cancer (≤ 4 ng/mL of serum PSA and a digital rectal examination not suspicious for cancer). This trial found no difference in prostate cancer incidence for L‐selenomethionine–supplemented participants as compared with placebo participants after a median follow‐up of 5.5 years (HR 1.04, 95% CI 0.90 to 1.18), and analysis of secondary outcomes showed no effect of selenium on risk of overall cancers or on risk of other cancers. Median selenium at baseline (135 µg/L in serum in the selenium arm vs 137.6 µg/L in the placebo arm) was higher than in the NPC trial (average plasma selenium 114 µg/L); unfortunately, no analysis stratified by baseline selenium status has so far been reported in SELECT, nor was non‐melanoma skin cancer among the secondary outcomes investigated. This trial used an intervention different from that used in NPCT (selenomethionine in SELECT and selenised yeast in the former), although this is unlikely to have been responsible for the observed differences (Waters 2013), and in both cases, the intervention comprised organic selenium species (Block 2004).
In a small study of organ transplant recipients (Dreno 2007), an unexpected increase in non‐melanoma skin cancer incidence emerged, which was of concern in the light of results of the NPCT. In two recent well‐conducted phase 3 trials in participants at high risk for prostate cancer, 200 µg/d of selenium (as selenomethionine in one study (Marshall 2011) and as selenised yeast in the other (Algotar 2013)) did not decrease subsequent cancer incidence compared with placebo. In these latter studies, selenium exposure at baseline did not modify the effects of selenium supplementation (i.e. no evidence indicated that a lower baseline selenium status as reflected by plasma selenium levels was associated with a more beneficial effect of subsequent selenium treatment).
Overall completeness and applicability of evidence
Observational studies and aetiological association
We reviewed data from prospective observational studies in which selenium exposure was measured in populations without evidence of cancer, who were then followed up for a specified period of time. This approach minimised the risk of reverse causality.
The included studies differed in terms of selenium exposure measurement, types of outcomes, study designs and study populations. The low number of studies for most of the meta‐analysed types of cancers prevented a thorough investigation of sources of heterogeneity between study results. In particular, we could not explore the influence of specific sources of bias or the methodological quality of epidemiological studies on heterogeneity.
The investigations included more than 1,100,000 individuals from diverse study populations, predominantly from Europe and the US, and to a lesser extent, Asia and Australia (also see: Dennert 2008). No prospective observational study on selenium and cancer risk could be identified from Africa or South America. This regional distribution reflects the underrepresentation of non‐Western and resource‐poor countries in epidemiological research (Pearce 2004). Differential regional representation in epidemiological studies is of special interest for this review, as selenium levels in humans around the world vary significantly. Selenium levels measured in the included cohorts reflect a broad range of naturally occurring selenium exposure, as documented by several epidemiological studies worldwide. However, some of the lowest and the highest selenium levels in humans were reported in populations in South America (Jaffé 1992)—a region not investigated in any of the reviewed observational studies.
More than half of the studies included mixed‐gender populations, but most reported no gender‐disaggregated results. In the available gender‐specific results, men are overrepresented—a fact that could hamper the potential assessment of the relation between selenium exposure and cancer risk in females.
RCTs and preventive efficacy
This review investigated a diverse range of cancers, but cancer is not a uniform condition, and malignant neoplasms show great differences in tumour biology. Only non‐melanoma skin cancer, liver cancer and prostate cancer were investigated as primary outcomes in the included prevention trials, and regarding these main outcomes, specific characteristics of the study populations may also limit the generalisability of results. Participants in the included RCTs on skin and liver cancer belonged to populations at high risk for the outcome under investigation, and participants in the three prostate cancer trials were at average risk (SELECT 2009) or at high risk (Algotar 2013; Marshall 2011) for this disease. Most participants in the NPCT were older and white, predominantly male inhabitants of the US, and the most recent trials were limited to the US male population. Average baseline selenium exposure in the NPCT was lower than that characterising subsequent trials carried out in the US, in which selenium intake was generally higher that that characterising most European populations. Although the NPCT suggested that selenium supplementation was highly beneficial only in the lowest range of baseline selenium exposure, the most recent studies, carried out in populations generally characterised by higher average selenium exposure, did not suggest such an interaction. An indication of strong effect modification was also found for gender in the NPCT study, as demonstrated, for example, by the HR for all cancers associated with selenium supplementation, which was 0.67 (95%CI 0.50 to 0.89) in males and 1.20 (95% CI 0.66 to 2.20) in females (NPCT 2002).
Participants in the SELECT study on prostate cancer prevention were apparently healthy men over 50 years of age from the general population of North America (SELECT 2009). The large sample size and the inclusion of non‐white participants from different socioeconomic backgrounds supported the generalisability of study findings to other adequately nourished populations.
Selenium supplements contain either organic or inorganic species of selenium or a mixture of both (e.g. in the form of selenised yeast). Different species of selenium may exhibit differential effects on human health. RCTs using selenised yeast supplements, nearly entirely comprising organic selenium forms (Block 2004; Waters 2013), found either a harmful effect or no effect of supplementation on the main study outcome. The SELECT trial used supplements of L‐selenomethionine, which is the major component of selenised yeast, and also found no preventive efficacy. The only RCT investigating sodium selenite supplements found a protective effect against liver cancer but was considered to have an unclear risk of bias. It is also unclear how applicable these results are in other settings and in populations with a different nutritional status. Interpretation of the results of clinical trials using selenium supplements should consider the different chemical forms of selenium, as well as their potentially different health effects when used as supplements (Weekley 2013). In most studies, possibly for safety reasons, organic selenium as selenised yeast (Algotar 2013; NPCT 2002) or selenomethionine (Marshall 2011; SELECT 2009) was used. However, the chemical form used is unlikely to explain the different results between NPCT and the other trials (Waters 2013). With reference to this issue, of interest also are the results of a 'natural experiment' that occurred in Northern Italy, wherein a small population unintentionally consumed for several years drinking water with unusually high content of selenium in its inorganic hexavalent form, selenate. Follow‐up of that population revealed a slightly increased risk of cancer, mainly due to an excess risk of melanoma, kidney cancer and lymphoid malignancies (Vinceti 1998; Vinceti 2000b); the latter observation was of particular interest in view of the recently reported association between exposure to atmospheric selenium and risk of childhood acute lymphoblastic leukaemia in California (Heck 2014).
An important unresolved issue is the possibility that participants with a 'low' baseline selenium status may experience an inverse association between selenium exposure and cancer risk. NPCT found a strong beneficial effect of selenium supplementation among participants in the lowest tertiles of baseline selenium levels; however, the risk of cancer changed abruptly from an apparently protective effect in the two lower tertiles (HR 0.51 and 0.70) to an excess risk (HR 1.20, 95% CI 0.77 to 1.86) in the highest tertile of plasma selenium, despite a difference of only 16.4 µg/L between the lower and upper tertiles. This would imply that a change in dietary intake of around 10 µg would change a strongly protective effect of selenium on cancer risk into a possibly detrimental effect—an implausible scenario given the wide range of selenium intake(from about 20 to several hundred micrograms)characterising Western populations. Moreover, the intermediate tertile of baseline plasma selenium in the NPCT (105.6 to 122.0 µg/L) appeared to be associated not only with reduced overall cancer risk but also with an excess risk of squamous cell skin carcinoma (HR 1.49, 95% CI 1.05 to 2.12) and overall non‐melanoma skin cancer (NPCT 2002), as well as diabetes (RR 1.36, 95% CI 0.60 to 3.09) (Stranges 2007); this occurrence of both adverse and beneficial effects is unlikely if the selenium supplementation was serving to remedy a selenium deficiency. In addition, the strongest effect of selenium on overall cancer risk at the lower levels of baseline selenium status was due to a considerable decrease in prostate cancer, but this finding was subject to detection bias because of a decreased biopsy rate in selenium‐supplemented participants, particularly in those with the lowest baseline selenium status, as recognised by the authors of the NPCT (NPCT 2002). Little evidence of a beneficial effect of selenium supplementation was noted among participants with the lowest baseline selenium exposure (plasma selenium < 106 µg/L) in either the prostate cancer trial of Marshall et al. (Marshall 2011) or the prostate cancer trial of Algotar et al. (Algotar 2013), despite the fact that 45% of the participants in that study had baseline plasma selenium levels < 123 µg/L—the suggested threshold for beneficial effects of selenium supplementation according to the NPCT (NPCT 2002). SELECT was unable to find any beneficial effect of selenium despite its large size and therefore the almost certain inclusion of participants with low baseline selenium levels. However, analyses stratified by baseline selenium status are not available for SELECT: Such analyses would greatly help to elucidate this issue. It is hoped that future work on SELECT will include follow‐up for non‐melanoma skin cancer, whose risk increased after selenium supplementation in three trials (Algotar 2013; Dreno 2007; NPCT 2002); this represents one of the most troublesome effects of selenium supplementation so far identified (Vinceti 2013b).
Quality of the evidence
Observational studies and aetiological association
The 55 observational studies were heterogenous, not only in methodology, but also in the quality and level of detail of reporting.
Confounding and other biases
Selenium measurement and categorical exposure classification
Six observational studies measured nutritional or supplemental selenium intake using questionnaires or interviews. Most studies, however, relied on selenium biomarkers such as toenail, serum or plasma selenium levels. Percentile borders, for example, quartiles or quintiles, were usually applied as cut points for exposure categories. Our analyses were based on the comparison of highest versus lowest baseline exposure category. In our meta‐analyses, different methods of selenium measurement and different numbers of exposure categories covering different absolute selenium levels were combined.
Adequate assessment of total selenium intake with food frequency questionnaires (FFQs) or interviews may be hampered by lack of adequate food composition data reflecting regional and seasonal variations in selenium concentration. The Duffield 1999 trial compared duplicate diet collections, dietary logs, FFQs and biomarkers as measurements for selenium intake and status among New Zealand men and women. The FFQ overestimated the mean selenium intake in study participants when compared with laboratory analyses of duplicate meals. Correlation between dietary measurements and selenium biomarkers (whole blood and plasma) were modest (r = 0.1 to 0.4) at best. Karita 2003 did not find a correlation between estimates of dietary intake and biomarker levels of selenium in a Japanese population, as was observed by other investigators (Hunter 1990; Satia 2006; Vinceti 2012). On the other hand, other studies have found a clear correlation between dietary intake of selenium, assessed through different methodologies and questionnaires, and blood or toenail selenium levels, indicating the adequacy of both approaches for assessing selenium exposure in different contexts characterised by low or high selenium intake (Haldimann 1996; Longnecker 1996; Pestitschek 2013; Swanson 1990; van den Brandt 1993b). Validity problems, possibly leading to exposure misclassification, have been generally reported when questionnaires were used to assess supplement use (Murphy 2002).
Regarding biomarkers for selenium measurement, Ashton 2009 showed in a systematic review that plasma and whole‐blood selenium concentrations increased with higher selenium intake in supplementation studies. Although Ashton 2009 could not identify serum studies for this systematic review, plasma, whole‐blood and presumably serum selenium levels were considered by the authors to adequately reflect a short‐term increase in supplemental selenium intake in healthy adults. However, the review authors also found unexplained heterogeneity in the reaction of participants' plasma selenium levels to selenium supplementation.
Regarding the estimation of long‐term nutritional intake with biomarkers, Longnecker 1996 demonstrated a high correlation between long‐term selenium intake as estimated from duplicate food portions and single measurements from whole blood, serum and toenail specimens.
These findings support the concern that ranking of selenium exposure differs according to the instruments used to assess intake and differences between intake assessment and biomarkers. Exposure misclassification may have biased the results of individual studies, and a meta‐analysis of observational data is likely to reflect these biases. Non‐differential exposure misclassification might have occurred in all included studies as the result of measurement errors or of the gap between the theoretical definition of selenium exposure and the measurement thereof, which served as a proxy. Non‐differential misclassification might lead to an underestimation as well as an overestimation of an effect in the presence of more than two exposure categories. Our approach of performing a meta‐analysis that covered different methods of selenium assessment might have introduced additional heterogeneity into our review results.
Exposure misclassification may have occurred in the great majority of observational studies because of failure to take into consideration the different selenium compounds, each of which has distinctive biochemical properties and toxicological and nutritional activities (Weekley 2013). This failure is likely due to the fact that speciation of different selenium compounds is very complex and expensive and requires sophisticated professional expertise and analytical equipment. The possibility of major biases associated with misclassification of selenium exposure due to different concentrations of inorganic and selenium species is demonstrated by a recent study in amyotrophic lateral sclerosis, which showed a very different distribution of the various forms in the cerebrospinal fluid of participants newly diagnosed with amyotrophic lateral sclerosis compared with controls (Vinceti 2013c). In that selenium speciation study, relative risk calculations carried out on the different selenium species yielded markedly different results, which were even opposite in some cases (e.g. for organic selenium vs selenite). This and other investigations indicate an asymmetrical distribution of the various chemical species of selenium in different body compartments (Behne 1996; Behne 2010; Panter 1996; Solovyev 2013; Vinceti 2013c), suggesting another major source of exposure misclassification (i.e. differential storage of selenium compounds in various body tissues, including target ones for the diseases under investigation). These studies thus indicate the potential for exposure misclassification in observational studies and the pitfalls associated with an approach based on assessment of total selenium content in peripheral biomarkers.
One concern, which we cannot clarify to date, is that biomarkers differentially reflect intake of organic and inorganic selenium species. Animal studies indicate that selenium from inorganic sources is not retained so well in the body as organic selenium. Selenium from organic sources led to higher blood selenium levels and higher activity of glutathione peroxidase than equal doses of inorganic supplements in veterinary studies (Slavik 2008; Steen 2008). However, symptoms of acute toxicity were observed in animals with lower intake of inorganic than organic selenium species (Kim 2001; Tiwary 2006). Panter et al. administered equivalent amounts of selenium to swine in organic and inorganic forms and found higher toxicity despite lower body selenium levels after administration of inorganic forms (Panter 1996). Hall 2008 found an increased genotoxic effect in human cell lines of sodium selenite in comparison with organic selenium. When the possibly differential effects of selenium species on human health are considered, adequate interpretation of the biomarkers representing selenium exposure would require knowledge of the selenium compounds to which the individual was exposed.
In our review we found that in observational studies, cancer risks frequently showed an inverse association with biomarker levels but not with nutritional intake. This might be a consequence of an invalid measurement of nutritional intake, thus biasing results towards the null, but it might likewise reflect that there truly was no association, and that findings from the biomarker studies were the result of inadequate exposure assessment. In some instances, measurements of nutritional intake might provide better exposure estimates than do biomarkers, which may considerably mis‐classify the exposure to inorganic and organic selenium sources.
Furthermore, it must be outlined that comparison of risks between the highest and the lowest exposure categories is most suitable for identifying an effect when a consistent decrease or increase is seen across absolute exposure levels. Other associations (e.g. threshold effects, U‐shaped relations) may have been missed by this method of meta‐analysis, or the true effect might have been diminished.
Comparability of cases and controls and detection of cancer
All included studies recruited participants pre‐diagnostically, and cases and control participants were drawn from the same population. This approach decreased potential differences between comparison groups, which could have influenced cancer disease or death due to factors other than selenium exposure. We included the results from each study in meta‐analyses, which were adjusted for the highest numbers of additional variables.
Any cancer
All studies on total cancer risk identified cases by using registry links or a combination of several methods, and losses to follow‐up were generally very low. Two studies on cancer incidence and two studies on cancer mortality analysed less than 80% of all identified cases (incidence: Coates 1988: 79%; mortality: Kok 1987a: 71%; Kornitzer 2004: 57%; Persson 2000: 76%). The main reason for this loss of sample was missing selenium measurements. Not all studies that assessed mortality as a measure of cancer risk excluded participants with cancer disease at study inception. This might have led to overestimation of a protective effect if selenium levels were lowered by the presence of cancer. We therefore consider the results for cancer incidence to be more valid than the cancer mortality results.
Prostate cancer
All but two of the studies on prostate cancer risk identified cases by using links to cancer registries or a combination of personal follow‐up interviews with PSA screening. Two studies with health professionals used self reporting for case identification, followed by confirmation through medical records. The number of people lost to follow‐up was low in all included studies. Two studies, however, included less than 80% of all identified cases in their analyses (Brooks 2001: 39%; van den Brandt 2003, in: van den Brandt 1993a: 77%) because samples were not available for selenium measurement or diagnosis was not confirmed. In Brooks 2001, bias might have been introduced to the results to some extent, as the demographic variables differed between identified and analysed cases.
Bladder cancer
Losses to follow‐up were low in three studies (Michaud 2002; Nomura 1987; Zeegers 2002 in: van den Brandt 1993a) and unclear in two studies on bladder cancer risk (Helzlsouer 1986, in: Menkes 1986; Michaud 2005). Endpoints were ascertained in elaborate ways in four studies that included linkages to registries and regional and national databases; one study relied on self reporting of study participants (Michaud 2005). The latter investigation compared bladder cancer in the Nurses' Health Study (women) versus the Health Professionals Follow‐Up Study (men) and was the only study to report gender‐disaggregated data. A gender‐differential association between selenium exposure and bladder cancer risk was found, but the role of potential biases due to possibly different self reporting behaviour in these two distinct cohorts remained unclear.
The second study, which found an inverse association between selenium exposure and bladder cancer risk, was Zeegers 2002 (van den Brandt 1993a), which could analyse only 70% of identified bladder cancer cases, as specimens for selenium measurement were not available for the remainder.
Residual confounding and effect modification
Most of the studies included controls for smoking and age by matching or by using multivariate techniques. However, only a few considered the potential effects of other factors. Possible confounding factors could be another food nutrient or a certain behaviour that exhibits cancer protective effects and is associated with higher intake of selenium‐rich foods. Furthermore, intake of heavy metals and other dietary factors may modify selenium health effects or the relations between selenium exposure and biomarkers (overview, in: Vinceti 2000a). Metabolic interactions, for example, are known for arsenic, cadmium and other elements (Zeng 2005; Zwolak 2012).
Even in studies that considered the influence of a specific factor, the validity of assessment of the potential confounder can be challenging and is not commonly reported. For example, control for smoke exposure as a known risk factor for several types of cancer is an important issue in epidemiological studies on cancer risk. Cigarette smokers tend to have lower selenium biomarker levels, although cigarette smoking is a source of selenium exposure itself. Therefore, an inverse association between selenium and lung cancer risk might be the result of residual confounding and effect modification by smoking. Exposure to environmental and household smoking, which has been shown to be associated with increased risk of cancer (Gorlova 2006; Nishino 2001), might also be associated with selenium status due to differential nutritional behaviours or other mechanisms. We are not aware of any study that investigated this issue.
Some potential confounders cluster in population groups according to socioeconomic position (SEP). Only a few studies attempted to control for indicators of adult SEP as potential confounders (e.g. education, occupation, income). None used a composite index of indicators or considered childhood SEP. Some studies restricted their cohorts to certain subgroups of a population, such as occupational groups, and were likely to include only people of a similar adult socioeconomic background. It has been claimed that associations between vitamins and diseases are the result of confounding by social and behavioural factors acting over the course of a lifetime (Lawlor 2004). Lawlor 2004 argued that divergent results from epidemiological and randomised controlled studies on the prevention of cardiovascular diseases can be explained by unmeasured confounding due to SEP. Risk of most cancers—like cardiovascular morbidity—isknown to decrease with higher SEP. Research also indicated a positive association between higher SEP and selenium biomarkers (Barany 2002; Niskar 2003). However, other investigations have not confirmed these findings: Kant 2007, for example, did not find an association between a measure of household poverty and selenium status. The hypothesis of possible confounding due to SEP leading to an indirect association between selenium and cancer would be consistent with the results for all types of cancers in this review for the observational studies—including the null association with breast cancer—with the exception of prostate cancer findings. Prostate cancer has been found to be diagnosed more often in men of a higher SEP (Dalton 2008), although we saw a protective association with higher selenium exposure. It remains unclear whether the more frequent diagnosis of prostate cancer in men with a higher SEP reflects an excess of prostate cancer incidence in this population. It might also result from differential health and screening behaviours leading to detection of otherwise symptom‐free cases, while men with a lower SEP tend to be overrepresented in diagnoses of advanced stages of the disease (Rapiti 2009). More information on screening and diagnostic behaviours of male cohort participants would be necessary to further elucidate these findings.
For prostate cancer, studies published before 2000, especially those from the US, found a greater protective effect with higher selenium levels than did later studies. We consistently observed this in the studies on lung cancer. This might be attributable to differences in study design or populations (with later studies being the larger studies including the general population) or to changing health and screening behaviours over time in the case of prostate cancer studies. It could also reflect publication bias in earlier years favouring positive results. An alternative explanation could be a 'threshold' effect for a possible protective effect of selenium against prostate cancer, which has been diminishing because of increasing use of selenium supplements in the US. Brooks 2001 reportedly observed results consistent with a threshold effect at a level of 108 µg/L serum selenium. Conversely, a threshold effect was not seen in another study with almost the same percentile limits (Goodman 2001) in a population of asbestos workers, who may have had other sources of selenium exposure than were noted in the participants in Brooks 2001 from the general population. It has been frequently suggested that an increase in selenium intake might be beneficial only for men with lower selenium levels, as glutathione peroxidase activity reaches a plateau above approximately 95 (range 89 to 114) µg/L (Rayman 2000). We found no clear indication of a threshold effect in lung or prostate cancer in the overview of study results. Heterogeneity between studies therefore might reflect not a consistent biological threshold effect of baseline selenium exposure levels, but rather a cluster of known and unknown influences of factors related to study design, study population and potential biases.
Another consideration is the role of genetic factors. Some recent observational studies examining selenoprotein‐related single‐nucleotide polymorphisms have suggested a role for genetic variants in genes coding for selenoproteins in modifying cancer risk, or the relation between selenium exposure and subsequent cancer risk, although not all results have been consistent (Geybels 2013; Meplan PLOSone 2012; Penney 2010; Penney 2013; Slattery 2012; Takata CEBP 2011). Moreover, the null results of the most recent low‐bias RCTs (Algotar 2013; Marshall 2011; SELECT 2009) do not suggest that at least the most frequent genotypes may strongly influence the selenium and cancer relation, although such hypotheses cannot be ruled out for more rare genetic variants of selenoprotein or other proteins. In addition, and entirely hypothetically, different genetic factors might both increase and decrease the risk of cancer associated with selenium exposure; therefore opposite effects with final null results in the overall general population might occur. Additional data from SELECT based on genotyping of study participants, if available, might be extremely useful for assessing hypotheses regarding genetic variants of selenoenzymes and their interaction with selenium status.
The role of chance
Large epidemiological studies often investigate a large number of possible associations. In general, when a multiplicity of comparisons is performed, some associations can occur by chance. Thus the possibility that some associations between selenium exposure and cancer endpoints occurred by chance cannot be ruled out.
Summary
Factors that seemed to account in part for interstudy heterogeneity were type of outcome measure (incidence or mortality), assessment of exposure and gender.
Given the possible influences of bias, particularly residual confounding and exposure misclassification, and of modifying factors on the selenium‐cancer relation, the summary estimates from our meta‐analysis and more generally from all meta‐analyses of observational studies on the selenium and cancer relation must be interpreted with caution. Meta‐analyses of spurious findings in observational studies enhance the precision of a summary risk estimate, which does not itself get nearer to the true value and may suggest a non‐existent association (Egger 1998).
RCTs and preventive efficacy
SELECT (SELECT 2009), Marshall (Marshall 2011) and Algotar (Algotar 2013) were the only trials considered to have a low risk of bias with adequate sequence generation, allocation concealment, blinding and reporting of findings, and the consistency of their findings for prostate cancer as well as the strong statistical power of the only study investigating other cancer types—SELECT—makes their results highly reliable.
In the three trials on liver cancer prevention, quality of reporting was an issue, and these trials were considered to have an unknown risk of bias. The individual trials were reported—in some cases, discrepantly—in several papers, and essential questions regarding sequence generation, allocation concealment, handling of dropouts and withdrawals and detection of outcomes remain unanswered. This might be due to inadequate reporting but might also hint of flaws in trial design and implementation. We were uncertain whether the only trial that reported positive results for selenium supplements in liver cancer prevention randomly assigned participants individually. A cluster randomisation of participants who lived in the same area/village, which may have been the procedure in this investigation, might have introduced additional bias to the study results (e.g. as the result of different environmental factors contributing to liver cancer development or detection) and might have led to an overestimation of the protective efficacy of selenium. Duplication of results with a rigorous study design would be necessary to assess the effects of sodium selenite on liver cancer incidence. With regard to the NPCT (NPCT 2002) and the Dreno et al. trial (Dreno 2007), indications of a potentially serious detection bias for the US study and of unclear methodological details (such as blinding) for the French investigation led us to consider those experimental studies to be at unclear risk of bias, as discussed in greater detail elsewhere in this review.
Potential biases in the review process
RCTs and preventive efficacy and observational studies and aetiological association
The literature search included the major international databases in the English and German languages, and we applied a broad search strategy supplemented by handsearching for references. We assume that we identified all randomised controlled studies and prospective observational studies relevant to our review questions. As we did not search databases in other languages (e.g. Chinese, Russian), we cannot rule out that we missed smaller studies that were not published in international journals. We also might have missed observational studies whose results on selenium exposure and cancer were reported in the body of a paper but were not mentioned in the paper's title or abstract, even if the paper is indexed in the searched databases.
We contacted all investigators to ask for missing or additional data on their studies. Sometimes we were unable to obtain answers to questions we had regarding methodology or outcomes, and sometimes investigators gave us the information we needed. We were unable to obtain answers particularly for earlier epidemiological studies, for which primary investigators may have relocated or died, or for which data were not available in a current electronic format. Similarly, we could not make contact with primary investigators of Chinese RCTs.
The risk of bias assessment was based on the included publications. The risk of bias of studies that did not adequately describe the study design in the included publication but gave a reference to another paper might therefore have been overestimated in this review.
Another concern, especially with the epidemiological studies, is publication bias. Cohort and nested case‐control studies often are not exclusively designed to test for a specific exposure‐outcome association but enable researchers to investigate a range of questions. It is conceivable that unfavourable results were less likely to be published.
We decided a priori to conduct meta‐analyses for observational studies only when five or more studies were available for a study outcome. As a result of this cutoff, we did not conduct meta‐analyses for a number of observational study outcomes with two to four studies available (Table 3). Our primary intention was to facilitate the investigation of heterogeneity between studies that were included in meta‐analyses, to avoid producing more precise, but still unexplainably biased, results. On the converse, the choice of reporting meta‐analysis of RCTs when at least two studies were available and of emphasising the analysis conducted for RCTs at low risk of bias was made to highlight the most reliable and recent evidence on selenium and cancer relation, which comes from well‐designed experimental studies.
The authors of this review came from different disciplines and have different focuses (e.g. epidemiology, biostatistics, clinical medicine, nutrition). We consider this internal variety of expertise to be a strength of this review and made use of it by applying double‐checking procedures during the entire review process when possible.
Agreements and disagreements with other studies or reviews
The idea of selenium supplementation for cancer prevention received broad support after the first report was received from the NPCT and after publication of several observational studies that supported the hypothesis of an aetiological relation between low selenium status and cancer development. Combs 2005 stated that "the hypothesis that selenium can affect cancer risk is supported by a remarkably consistent body of scientific evidence" (Combs 2005). These ideas stimulated the largest ever cancer prevention trial, SELECT, which failed to provide support for this hypothesis, and two additional prostate cancer trials (Algotar 2013; Marshall 2011), whose results were in line with the SELECT findings in failing to find a beneficial effect of selenium. Disagreement between results of this systematic review and those of other publications may be explained in part by the differentiation between aetiology and efficacy in the research questions of this review, and by the possibility in the present study of reporting the most recent and sound evidence coming from experimental studies. An additional relevant RCT, which could not be meta‐analysed in this review, since it was released in PubMed in September 2013, also appears to confirm our conclusions (Karp 2013).
Observational studies and aetiological association
A number of systematic reviews on selenium and the risk of different types of cancer have been conducted with and without meta‐analyses. Overall, our combined risk estimates are consistent with these results, and slight discrepancies in numbers are attributable at least in part to different inclusion criteria. However, some of the previous publications arrived at more favourable conclusions regarding a possible protective association of higher selenium exposure against cancer.
Our meta‐analyses of observational studies suggest an inverse association between selenium exposure and risk of several cancers in men, which was reflected in reduced overall cancer incidence and mortality. Associations with toenail selenium levels tended to be greater than with serum or plasma levels, and in general no associations with selenium intake were noted. These findings were consistent with secondary outcomes of the NPCT, particularly in its first report (Clark 1996, in: NPCT 2002), which suggested preventive efficacy of selenium supplements against several types of cancer in men, the strongest of which was prostate cancer. However, the large‐scale SELECT trial and two subsequent RCTs failed to confirm any beneficial effects of supplemental selenium intake on prostate cancer risk (Algotar 2013; Marshall 2011; SELECT 2009). An earlier ecological analysis of a nationwide programme to increase selenium intake with fortification in Finland also found no evidence of a protective effect against prostate cancer (Vinceti 2000a).
Overall, little evidence suggests an association between selenium exposure and cancer risk in women; if existent, it is likely to be small. Our meta‐analyses do not support a protective association between higher selenium exposure and breast or colorectal cancer in women.
It has been argued that gender‐related outcomes may reflect different exposure levels at baseline possibly related to gender‐specific nutritional behaviour, which might be true for comparisons of distinct women‐only and men‐only cohorts (Michaud 2005). However, comparisons by gender within studies also point to a differential effect at similar exposure levels. We cannot rule out that sex or gender differences may be observed by chance only, but laboratory and animal research has suggested sex differences in selenium metabolism and biology. Also sex‐specific tumour biology and a predominance of specific cancer types may contribute to differential health outcomes in women and men. However, we cannot estimate the magnitude that sex or gender differences possibly contribute to observed differential health outcomes in men and women. These considerations are of special interest, as selenium supplements are aggressively marketed, especially to women, with regard to breast cancer prevention and treatment, and this is not supported by data from observational or clinical investigations.
Heterogeneity between studies was not much reduced by gender stratification in our meta‐analyses. Furthermore, we expected that non–gender‐stratified data from observational studies would more or less reflect a combination of gender‐stratified results for a specific tumour type, but this was not always the case. In lung cancer meta‐analysis, for example, risk reduction by higher selenium levels seems to be greater in data for both genders combined than in data for women and men separately. This underlines the influence of other sources of heterogeneity on study outcomes. Reporting of gender‐stratified results in mixed‐gender cohort studies, which has become increasingly common over the years, might therefore reflect other factors related to study design, such as better evaluation of possible confounders in more recently published studies. Socioeconomic position could be one such possible confounder, leading to an overestimation of a protective effect of selenium. Several studies have found selenium levels to be positively associated with adult socioeconomic position in both men and women (Gundacker 2006; Niskar 2003).
Therefore, doubts about whether observed associations point to a real causal relation between selenium biomarker levels and cancer risk are fully justified.
RCTs and preventive efficacy—specific cancer types
Non‐melanoma skin cancer
The increase in risk of non‐melanoma skin cancer associated with selenium supplements found in the NPCT (NPCT 2002), and apparently confirmed in Dreno 2007 and in Algotar 2013 (although in the latter case without evidence of a dose‐response relation), raises strong concern about the safety of selenium yeast supplementation in both men and women with reference to this cancer type. Increased risk of non‐melanoma skin cancer could be more pronounced in or restricted to high‐risk populations, or could be observable only above certain selenium levels, which the NPCT suggested to be around 105 µg/L (Duffield 2003, see: SELECT 2009). Uncertainty over the size and precision of the risk associated with selenium supplementation from our analysis makes relevant data for this cancer type from the SELECT trial, in the light of its power and its low risk of bias, of fundamental importance for elucidating the hypothesis of an excess skin cancer risk associated with selenium exposure.
Liver and other gastrointestinal cancers
Bjelakovic 2008 conducted a systematic review of antioxidant supplements for prevention of gastrointestinal (GIT) cancers. Review authors meta‐analysed RCT data for liver cancer prevention with selenium‐containing supplements and reported a protective effect in both genders (RR 0.56, 95% CI 0.42 to 0.76). Three of the four trials in their meta‐analysis were also included in this systematic review (Li 2000; Yu 1991; Yu 1997). The remaining RCT (Li 2004b) used a combination of selenium with allitridum, a synthetic garlic extract, in the intervention and therefore did not meet our inclusion criteria. Li 2004b found a preventive efficacy of high‐dose allitridum/100 µg sodium selenite supplementation on total and gastric cancer incidence in men but not in women. No effect on liver cancer was seen in participants of either gender. Allitridum was considered the main intervention by Li and colleagues in their paper, and the contribution of selenium to the overall effect remained unclear. The more recent RCT by Qu 2007 found no effect of 50 µg selenium yeast in combination with beta‐carotene and alpha‐tocopherol on liver cancer mortality.
We calculated a summary risk estimate for the RCTs on liver cancer included in this review, but limitations of these trials, particularly with reference to their risk of bias, strongly hamper evaluation of their results and suggest extreme caution in interpreting the findings concerning liver cancer. An additional analysis from the SELECT trial with reference to liver cancer would help to assess the potential relation of this site‐specific cancer to antecedent selenium exposure.
We could not identify RCTs that investigated other GIT cancers as primary outcomes. The NPCT reported reduced risk of colorectal and oesophageal cancer as a secondary outcome in the selenium group. Other studies using multi‐component selenium‐containing supplements found divergent results, which also indicated potential sex or gender differences (Blot 1993; Hercberg 2004)
The SELECT trial included colorectal cancer but no other gastrointestinal cancers or overall gastrointestinal cancers among the secondary outcomes investigated (Lippman 2009, see: SELECT 2009). Trial results showed no change whatsoever in colorectal cancer risk in selenium‐supplemented participants compared with placebo‐receiving individuals. Because no reduction in overall cancer risk was seen among this selenium‐supplemented male population, a major effect on other frequent cancer types such as different gastrointestinal cancers seems unlikely to have occurred. Unfortunately, no low‐bias trials have been carried out in females.
We consider that the availability of supplemental results from the SELECT study regarding liver cancer and other gastrointestinal neoplasms, as well as other outcomes, would be of major importance for an adequate assessment of the relation between risk of these cancers and antecedent selenium exposure.
Other cancers and diseases
Data on a variety of other cancers were reported in NPCT and in SELECT. It is worthy of note that results for the primary outcome of the NPCT (i.e. the incidence of non‐melanoma skin cancer) received less attention in the public debate than those for secondary outcomes, especially those in favour of selenium supplementation. Underrepresentation of women in the NPCT decreased the power to detect sex‐/gender‐specific effects (Duffield‐Lillico 2002, see: NPCT 2002) and is a matter of concern, as a high but statistically imprecise risk of breast cancer was detected in the selenium group (HR 1.89, 95% CI 0.69 to 5.14). All possible beneficial effects on cancer incidence were confined to men in this study.
The SELECT trial investigated as secondary outcomes a variety of cancers in addition to prostate cancer (the primary outcome): lung cancer, colorectal cancer, other cancers, overall cancer and cardiovascular events (haemorrhagic stroke and other cardiovascular disease) (SELECT 2009). No evidence of a beneficial effect of selenium supplementation on any of these outcomes emerged, with the partial exception of a slight and statistically very unstable decrease in cardiovascular events (HR 0.91, 99% CI 0.66 to 1.24); for this issue, we refer to a recent Cochrane review (Rees 2013) and a trial sequential analysis (Brigo 2014). Estimates for lung cancer (HR 1.10, 99% CI 0.63 to 1.61) were also very imprecise and suggested higher risk, mirroring the results of a recent trial in participants with a history of lung cancer (Karp 2013), which investigated the efficacy of selenium supplementation (200 µg/selenium/d as selenised yeast vs placebo) for the prevention of second primary tumor and second primary lung cancer in participants with resected non–small‐cell lung cancer (Karp 2013; RCT_ECOG 2002). Results of this trial could not be included in the present meta‐analyses because of its late publication date, but they appear to be consistent with results of the most recent RCTs (Algotar 2013; Marshall 2011; SELECT 2009) and therefore seem to confirm the findings of this review.
SELECT also reported a slightly elevated risk for type 2 diabetes in the selenium group (RR 1.07, 99% CI 0.94 to 1.22), which decreased in the longer, unblinded follow‐up study of the same study population after cessation of selenium supplementation (RR 1.04, 99% CI 0.93 to 1.17; Klein 2011, see: SELECT 2009). This increase was a matter of concern, especially in the light of detection in 2007 of an excess risk of diabetes associated with selenium supplementation in a secondary analysis of NPCT results (Stranges 2007). Based on our computations using data provided in the reports, two subsequent smaller trials (Algotar 2013; Karp 2013) had an increased (although statistically very unstable) diabetes risk among selenium‐supplemented participants (incidence rate ratio 1.7, 95% CI 0.6 to 5.1 and 1.2, 0.6 to 2.6, respectively). Therefore, the possibility that selenium supplementation represents a risk factor for diabetes deserves to be considered carefullyand appears to be under active investigation (Pounis 2014; Rocourt 2013).
Authors' conclusions
Observational studies have provided some evidence that intake of the metalloid selenium may influence cancer risk in humans, both in men and in women, but a role of bias, and of confounding in particular, cannot not be ruled out in these investigations because of methodological shortcomings. Results from the most recent randomised controlled trials, which were carried out in men and had a low risk of bias, have failed to provide evidence of any beneficial effect of selenium supplementation on risk of all cancers, prostate cancer or other site‐specific cancers. Additionally, RCTs have raised concern about possible toxicities from long‐term intake of supplemental selenium, such as excess risk of non‐melanoma skin cancer and type 2 diabetes. The findings of our review do not provide evidence to support supplementation with selenium to prevent cancer.
Some questions regarding selenium, such as whether selenium might influence cancer risk in individuals with very low or very high baseline exposure to this element, or in individuals with different genotypes, have not been fully resolved, although currently available evidence from randomised trials offers little support for such hypotheses. For ethical reasons, in the light of potential toxicity of selenium supplementation and failure of the most recent and well‐conducted experimental cohort studies to find beneficial effects, new randomised trials on the selenium and cancer relation are unlikely to be undertaken in the future. Therefore expanding the results of the SELECT trial to examine additional outcomes (liver cancer and non‐melanoma skin cancer) and subgroups with specific characteristics (baseline selenium exposure levels and genetic factors) may be the best available option to clarify these issues. Unfortunately, SELECT results cannot address the possible occurrence of gender differences because this trial enrolled only males.
It is definitively known from a number of studies that the various chemical forms of selenium have very different nutritional and toxicological properties. However, for the most part, observational studies have assessed only total selenium exposure. Future observational studies would contribute greatly to a better understanding of the selenium and cancer relation by including selenium speciation in their exposure assessment methodology in evaluating cancer risk associated with intake or tissue levels of specific inorganic and organic species of this metalloid.
Feedback
2 Selenium for preventing cancer, 30 October 2014
Summary
Comment: Selenium for preventing cancer; The Cochrane Library 2014, Issue 3 Vinceti M, Dennert G, Crespi CM, Zwahlen M, Brinkman M, Zeegers MPA, Horneber M, D’Amico R, Del Giovane C
We are pleased to see that a revised version of the review has now been published though it has taken longer than we would have wished. In the updated review, the authors have remedied some of the shortcomings which we pointed out, but not all. I have attached detailed comments on what we think still needs to be changed and hope that these points can be remedied in the very near future.
Comments by section are given below.
Abstract 1. Selection criteria refer to including RCTs with “healthy adult participants”. However, it is clear that SELECT was the only trial that included “healthy adult participants”, all other trials included participants with a high risk of cancer (Li, Yu 1991, Yu 1997, Marshall, Algotar, Dreno) or a previous history of cancer (NPCT 2002, Reid 2008). The word “healthy” should be removed and the statement should be modified to reflect the high proportion of participants at high risk of cancer.
2. The main results of the pooled analysis of RCTs overwhelmingly reflect the results of by far the largest trial, SELECT. However, SELECT was carried out in a population of high selenium status. This needs to be mentioned either under “Main results” or under “Authors’ conclusions”. Not to mention it is to ignore a fact that is likely to be highly relevant to the outcome.
3. The “Authors’ conclusions” assert that there is “little evidence of any influence of baseline selenium status”, but that lack of evidence all relates to trials in populations of much higher baseline selenium status than the NPCT where such an effect was seen: baseline plasma Se was 114 µg/L in the NPCT compared to 126.1 µg/L in Algotar and 135.2‐138.1 µg/L in Marshall. [No such effect was seen in SELECT, but baseline selenium status was also high ‐ 136 µg/L (Kristal et al. 2014).]
Plain language summary The sentence that begins “Recent trials that were judged to be well conducted and reliable… “ should be modified to read “Recent trials that were judged to be well conducted and reliable, though conducted in high‐selenium populations, have found no effects of selenium supplementation on reducing the overall risk of cancer or on reducing the risk of particular cancers, including prostate cancer”.
Main text Page 5 column 2: We previously pointed out that having inclusion criteria that allowed RCTs of only four‐weeks’ length to be included is unjustifiable. While no studies as short as that were included, clearly a four‐week intervention with Se is insufficient to alter cancer risk so what is the justification retaining this inclusion criterion?
Page 21 column 1: We previously objected to the description of an increased risk of diabetes mellitus type 2 being found in SELECT yet such a description is there again: “An increase in diabetes mellitus type 2 was seen in the selenium‐alone group (RR 1.07, 99% CI 0.94 to 1.22)”, despite the confidence interval spanning 1. The only trial in which an increased risk of type‐2 diabetes was seen was the NPCT. The authors also refer to a short‐term effect of selenium supplementation on type‐2 diabetes risk. However, there is no mention, either here or elsewhere, of our RCT that found no increased risk of type‐2 diabetes in 500 people treated with 100, 200 or 300 µg selenium or placebo for a period of six months (Rayman et al. A randomized trial of selenium supplementation and risk of type‐2 diabetes, as assessed by plasma adiponectin. PLoS One. 2012;7:e45269).
Page 20‐21: There should have been some mention of baseline selenium status in this section. Clearly SELECT was showing evidence of toxicity, which is unsurprising given the high baseline status and substantial level of supplementation.
Page 23 column 2: In discussing the change from a protective to a possibly detrimental effect, the authors should be aware of the possibility of a threshold effect that may relate to a mechanism dependent on selenoprotein concentration/activity. Furthermore, discussing the relationship between selenium status and the risk of non‐melanoma skin cancer and type‐2 diabetes in the same breath ignores the likelihood of totally different mechanisms applying.
Page 23 column 2: The sentence “Little evidence of a beneficial effect of selenium supplementation was noted among participants with the lowest baseline selenium exposure (plasma selenium < 106 μg/L) in either the prostate cancer trial of Marshall et al. (Marshall 2011) or the prostate cancer trial of Algotar et al. (Algotar 2013), despite the fact that 45% of the participants in that study had baseline plasma selenium levels < 123 μg/L – the suggested threshold for beneficial effects of selenium supplementation according to the NPCT (NPCT 2002)”, should be qualified by pointing out that both the Marshall and Algotar trials were in men at high risk for prostate cancer and in whom prostate cancer was probably already initiated. Thus this is not an appropriate test for evidence of benefit of selenium supplementation for primary prevention in those with low selenium status.
Page 24 column 2: SNPs could be mentioned as a potential explanation of “the … unexplained heterogeneity in the reaction of participants’ plasma selenium levels to selenium supplementation”.
Page 25 column 1: As explained in our criticisms of the primary review, we and others profoundly disagree with the statement that “measurements of nutritional intake might provide better exposure estimates than do biomarkers, which may considerably mis‐classify the exposure to inorganic and organic selenium sources”. This is particularly true of exposure to selenium where food concentration data differ very considerably from one part of the world to another and many countries have no such data.
Page 28 column 1: The paragraph that contains the sentence “These ideas stimulated the largest ever cancer prevention trial, SELECT, which failed to provide support for this hypothesis, and two additional prostate cancer trials (Algotar 2013; Marshall 2011),whose results were in line with the SELECT findings in failing to find a beneficial effect of selenium”, needs to point out that SELECT, Algotar 2013 and Marshall 2011 were all carried out in high‐selenium populations and that Algotar 2013 and Marshall 2011 were both in men at high risk of prostate cancer.
Page 29 column 2: It is not especially accurate or informative to say that the Blot and Hercberg trials produced divergent results. Although they were both RCTs, they used very different designs in hugely different populations with different baseline selenium levels. It could equally fairly be said that they produced comparable results in that they both saw beneficial effects (of one sort or another).
Page 30 column 1: Karp was a secondary prevention trial in lung‐cancer patients. In relation to that trial, there should be some mention of the likely difference in mechanisms of primary prevention and those relevant to prevention of secondary tumours in already initiated patients.
Page 30 column 1: the previous RCT that found no increased risk of type‐2 diabetes in 500 people treated with 100, 200 or 300 µg selenium or placebo for a period of six months should be mentioned (Rayman et al. A randomized trial of selenium supplementation and risk of type‐2 diabetes, as assessed by plasma adiponectin. PLoS One. 2012;7:e45269).
Page 30 column 1: Under the heading, “Implications for practice”, it should be made clear that the “Results from the most recent randomised controlled trials, which were carried out in men and had a low risk of bias” were all in men of high selenium status.
Page 30 column 2: Under “Implications for research”, there is a statement that needs qualifying, “whether selenium might influence cancer risk in individuals with very low or very high baseline exposure to this element …….. have not been fully resolved, although currently available evidence from randomised trials offers little support for such hypotheses”. It needs to be acknowledged that there are no cancer trials of selenium as a single nutrient in people with low baseline selenium status.
Even if the results of SELECT are expanded to look at other endpoints, they will still not apply to low‐selenium populations and cannot compare truly low to higher levels; this also needs to be specified.
A question that remains ignored by this review, by design, is whether selenium in combination with other agents may be beneficial in cancer. This deserves some sort of comment under “Implications for research”.
Errors Page 4, column 2: Though we pointed out in our previous set of comments that SU.VI.M.AX was incorrect, it has not been corrected.
Page 6 column 2: 78.96 is described as the molecular weight of selenium; it should be atomic weight.
Page 28 column 2: we have previously pointed out that selenium supplement are not aggressively marketed to women with regard to breast cancer prevention and treatment.
Contributors (in alphabetical order): Professor Regina Brigelius‐Flohé, University of Potsdam, German Institute of Human Nutrition Professor GF Combs Jr, Grand Forks Human Nutrition Research Center, ARS/USDA, USA Dr Cindy D Davis, Office of Dietary Supplements, NIH, USA Dr Fiona R Green, Reader in Functional Genomics, University of Surrey, UK Professor John Hesketh, Institute for Cell & Molecular Biosciences, University of Newcastle, UK Professor Josef Köhrle, Charité Universitätsmedizin Berlin, Germany Dr Alan Kristal, Fred Hutchinson Cancer Research Center, Seattle, USA Professor Margaret Rayman, Faculty of Health and Medical Sciences, University of Surrey, UK Professor Lutz Schomburg, Charité Universitätsmedizin Berlin, Germany Dr Phil Taylor, Division of Cancer Epidemiology and Genetics, NCI, USA Piet van den Brandt, Professor of Epidemiology, Maastricht University, The Netherlands Professor David J. Waters, Purdue University, USA Professor Phil Whanger, Oregon State University, USA
I agree with the conflict of interest statement below:
I certify that I have no affiliations with or involvement in any organization or entity with a financial interest in the subject matter of my feedback.
Reply
21‐1‐2015
We wish to thank Dr. Brigelius‐Flohé and colleagues for their interest in our Cochrane review on selenium for preventing cancer.
Before addressing the specific points in their letter, we would like to clarify that our publication Vinceti et al. ‘Selenium for preventing cancer, Cochrane Database Syst Rev. 2014 Mar 30;3:CD005195’ was not a revised version of the previous Cochrane but rather an update, taking into account the additional three years of scientific literature on the topic, according to standard procedures of the Cochrane Collaboration.
With regard to the use of the term ‘healthy’ in RCTs, we used the term ‘healthy’ adult participants to mean that the (adult) individuals enrolled in the studies were free at the beginning of the trial from the disease representing the primary outcome, an incident cancer, as required when we deal with primary prevention trials. Being at high, low or intermediate risk of cancer, or affected by any other disease, or previously affected by another cancer, was not considered to be an exclusion criteria and did not preclude the term ‘healthy’ with respect to the trial outcome(s), which in all cases consisted of the incidence of a primary cancer. In our review, we specifically listed in detail the enrolment criteria for the trials, and before performing the meta‐analysis we excluded studies retrieved with our literature search that were not based on healthy adults (397 studies removed – see Figure 1 of our review). Being ‘totally’ healthy– i.e., apparently free from any disease and at a low risk for cancer or other chronic disease, was not a selection criteria for any of the selenium (Se) trials, including SELECT itself (for example, we used the term ‘apparently healthy men’ for the SELECT population in page 23).
As noted by Brigelius‐Flohé et al., the pooled analysis is obviously influenced by the largest trial, SELECT, and this is even more true when we limited the analysis, as recommended by the Cochrane review guidelines, to the trials at low risk of bias. SELECT has been of fundamental importance in selenium (Se) research for its large size, long follow‐up, and broad range of outcomes, all of which are important for defining the so far uncertain relation between Se and primary prevention of cancer and the adverse health effects of the metalloid. Results from SELECT, which are continuing to emerge in the literature (Kristal et al., JNCI; Martinez et al., Cancer Prev Res; Albanes et al., Cancer Prev Res 2014), in addition to other recent relevant trials (Karp et al., 2013), have been systematically confirmed by all the high‐quality, low‐bias trials so far carried out (some of which could unfortunately not be included in our review, having been published after our literature search deadline), with the exception of the excess high‐grade prostate cancer risk in the Se‐supplemented individuals with the highest baseline selenium status recently reported in SELECT (Kristal et al., JNCI 2014), an unexpected and concerning finding so far not investigated in the other trials with the partial exception of Marshall et al. (Cancer Prev Res 2011).
Assuming that the SELECT population was a group with ‘high Se status’ while NPC subjects had a low Se status, and suggesting that their different results were likely due to this, as claimed by Brigelius‐Flohé et al., is not well‐founded. Defining a low‐Se status and a high‐Se status is very subjective and debatable, but whatever approach is chosen, no such difference between these two trial populations emerges. We would argue that the more important distinctions between the two trials are that one had low risk of bias and high statistical power (SELECT), while the other one had high risk of bias and much lower power (NPC). The two trials also used different Se preparations. In fact, if we estimate Se intake though its relation with serum/plasma level computed with the rule of thumb proposed by Haldimann et al. (J Trace Elem Med Biol 1996) in the 30‐120 µg/l of plasma or serum Se, average baseline dietary exposure corresponding to their blood Se levels was around 90 µg/day for SELECT participants, and 76 for NPC subjects. If we compare these values to the Se recommended dietary intake (or comparable indexes defined as ‘recommended intake level’, ‘dietary reference value’, ‘average nutrient requirement’ etc.), both are well above these reference values for Se, whether using the 26‐34 µg/day recommended intake of the World Health Organization and Food Agriculture Organization (WHO‐FAO 2004), the 25‐35 µg/day range of the Japanese Ministry of Health Labour and Welfare (2005), the 55 µg/day of the US Institute of Medicine and Food Nutrition Board (2000), the 70 µg/day of European Food Safety Authority (EFSA 2014) or the 55 µg/day of the Italian Human Nutrition Society (SINU, Milan 2014). For a comprehensive review of this issue we refer to among other sources the Eurreca database at www.eurreca.org, Cavellaars et al., Eur J Clin Nutr 2010, Vinceti et al. 2013 Sci Total Environ, and to the EFSA journal, 2014. Thus, according to all of these standards, both the SELECT and NPC populations should be defined as having a ‘high Se status’. This would be further strengthened should we use the 110 µg/l serum Se cutpoint for Se toxicity (increased prevalence of depressive symptoms and higher levels of urinary 8‐oxo‐7,8‐dihydroguanine) recently suggested by two observational studies (Galan‐Chilet et al., Free Rad Biol Med 2014 and Conner et al., J Nutr 2014): according to such threshold values, all the RCTs included in our review including SELECT and NPC, with the exception of the Chinese ones, should be considered as carried out in populations with ‘very high’ Se status.
In our review, given the uncertainties and complexity of the issue, we consciously avoided labeling the populations in RCTs as low or high Se status, preferring instead to report baseline exposure levels and to use relative measures for their comparison (such as ‘lower status’, ‘the lowest exposure category’ instead of ‘low Se status’, and the converse for higher exposures). This was done particularly for the most influential studies in the review, the RCTs, to facilitate assessment of whether baseline Se exposure may influence the response to Se supplementation in terms of cancer risk and comparison of distributions of baseline Se exposure. We refer Brigelius‐Flohé et al. to our analysis in the review (pages 22/24), which found the following points, among others: the marginal difference in intake of around 15 µg/day between the SELECT and NPC populations, in contrast with usual differences of Se intake at the population and the individual level, which span hundreds of micrograms; the occurrence of adverse effects even in the trials with the lowest baseline exposure level, such as the increased incidence of skin cancer in NPC and of type 2 diabetes in all trials which so far investigated this outcome; and the considerable overlap of Se exposure levels between the various RCTs. Finally, in our review we had to state that ‘analyses stratified by baseline Se status are not available for SELECT: Such analyses would greatly help to elucidate this issue.’ Fortunately, such evaluation (though so far only for prostate cancer) has been subsequently published (Kristal et al., JNCI 2014, and specifically its table 4). As it happens, their finding based on quintiles of baseline Se exposure is consistent with our previous assessment. In fact, the abstract of that paper reported that ‘Se supplementation did not benefit men with low Se status but increased the risk of high‐grade PCa among men with high Se status.’
When programming the update of this Cochrane review, we decided not to further restrict the inclusion criteria for studies compared with the 2011 review, but rather to relax them somewhat. For example, meta‐analysis was carried out for site‐specific cancer types when only 2 randomized trials were available. We even discussed whether to include trials reported only as abstracts and not in extenso, but decided against this due to lack of consensus, even though this precluded consideration of at least two possible relevant RCTs, the Karp et al. trial for prevention of second primary tumors in patients with resected lung cancer (Karp et al., J Clin Oncol 2010) and a trial on the risk of cancer in BRCA1 carriers (Lubinski et al., Hered Cancer Clin Pract 2011). We agree with Dr. Brigelius‐Flohé et al. that a trial with only 4 weeks of supplementation would be very unsatisfactory, even in case of ‘mega‐dose’ Se administration, and such a dosage scheme would not have passed un‐remarked upon in our literature review, had we found such a study.
As far as Brigelius‐Flohé et al. comments about the excess diabetes incidence in SELECT among subjects allocated to Se administration, we are surprised to see this objection: reporting and commenting on the adverse effects of RCTs is mandatory according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins JPT and Green S, Chapter 4 ‘Adverse effects’) and more generally according to ethical and scientific issues. We also note that Brigelius‐Flohé et al. when commenting on the excess diabetes incidence rely entirely on statistical significance testing (‘..despite the confidence interval spanning 1’), an approach generally considered to be inappropriate for evaluating findings from epidemiologic studies (Sterne and Davey Smith, BMJ 2001; Rothman, Greenland and Lash, Modern Epidemiology 2008; Stang, Poole and Kuss, Eur J Epidemiol 2010), especially for adverse effects that the studies were not necessarily powered to detect. The excess diabetes risk was one of the concerning findings yielded by SELECT (Vinceti et al., Rev Environ Health 2009), mirroring the observation of an increased diabetes incidence detected in the previous NPC trial (Stranges et al., 2007). We also noted that such excess risk was found in all four RCTs that investigated this outcome, and this was also supported by some biological plausibility, though we did not carry out an in‐depth investigation of the diabetes & Se relation, for which we refer to recent literature (Steinbrenner 2013; Vinceti et al., J Trace Elem Med Biol 2015). Contrary to the claims of Brigelius‐Flohé et al., we did not mention the 2012 Rayman et al. trial published in PLoS One for the obvious reason that it did not include cancer nor diabetes among the outcomes under investigation.
The comment by Brigelius‐Flohé et al. stating that ‘Clearly SELECT was showing evidence of toxicity, which is unsurprising given the high baseline Se status and substantial level of supplementation’ is also unfounded. Being ourselves among the few investigators who have systematically reviewed the human health risks of chronic low‐dose Se overexposure, (Vinceti et al., Rev Environ Health 2001 and 2009; Vinceti et al., Sci Total Environ 2013; Vinceti et al., Toxicol Lett 2014), we must point out that the upper limit of ‘safe’ Se exposure was and is set at a higher level than that of the SELECT study groups allocated to Se administration, i.e. at 400 µg/day (US Institute of Medicine 2000; World Health Organization Food Agriculture Organization 2004, and the Office of Dietary Supplements of the National Institute of Health accessed at ods.od.nih.gov/factsheets/Selenium‐HealthProfessional/ on January 20, 2015).
Brigelius‐Flohè et al. challenge discussing the excess risk of diabetes and of non‐melanoma risk cancer ‘in the same breath’ since this would ‘ignore the likelihood of totally different mechanisms’. This misrepresents the review, which makes no claim that risk of non‐melanoma skin cancer and diabetes operate through the same mechanisms.
Brigelius‐Flohè et al. state that the participants in the Marshall et al. and Algotar et al. studies were at high risk for prostate cancer (as we mentioned in our review) and that prostate cancer was probably already initiated in them. The participants in these trials were biopsy‐negative for prostate cancer, and therefore the latter statement by Brigelius‐Flohè et al. is speculation not supported by the available evidence. Contrary to the claims of Brigelius‐Flohè et al., the Marshall et al. 2011 trial and the Algotar et al. 2013 trial were important, not only since they confirmed key results of SELECT trial, but also since they addressed the issue of influence of baseline Se status on the effect of Se supplementation on (prostate) cancer risk. We refer Brigelius‐Flohè et al. to pages 23 and 24 of our review where we analyzed this issue in‐depth, and specifically to the following text: “Little evidence of a beneficial effect of Se supplementation was noted among participants with the lowest baseline Se exposure (plasma Se < 106 μg/L) in either the prostate cancer trial of Marshall et al. (Marshall 2011) or the prostate cancer trial of Algotar et al. (Algotar 2013), despite the fact that 45% of the participants in that study had baseline plasma Se levels < 123 μg/L‐the suggested threshold for beneficial effects of Se supplementation according to the NPCT (NPCT 2002)”. In addition, as previously mentioned, a 2014 report published after final submission of our review showed that SELECT subjects in the lowest baseline status categories did not benefit from Se supplementation with regard to (prostate) cancer risk, though they did not experience the increased risk of high‐grade prostate cancer induced by the Se supplementation observed in the highest exposure groups (Kristal et al. JNCI 2014).
We did not mention SNPs as a potential explanation of “the .. unexplained heterogeneity in the reaction of participants” since we were specifically reporting the comments of Ashton et al. Am J Clin Nutr 2009, who did not primarily focus on this possibility. However, as Brigelius‐Flohè et al. may note from several statements within our review, we agree about the potential importance of SNPs, and this is why we frequently mention the potential role of genetic factors in our review.
Page 25, column 1 (assessment of Se exposure): though we could not review in‐depth all studies concerning methods for assessing Se exposure and related issues, we wanted to mention the human studies finding an association between dietary and biomarker Se, those unable to find it, and the advantages and limitations of all these approaches. We refer Brigelius‐Flohè et al. to specific reviews or research papers on this important issue, which show that inadequate Se exposure classification made on the basis of dietary intake or of hair, blood, urine and toenail levels may have had a major role in the inconsistencies among various observational studies and between the observational and the experimental investigations. We stand behind the brief statement in our review concerning Se exposure assessment methods in the human body.
Contrary to the claims of Brigelius‐Flohé et al., the Blot and Hercberg trials indeed produced divergent results, and the statement about these two trials that ‘both saw beneficial effects’ is untrue. Though the effects of these trials administering (different) mixtures of vitamins and minerals and carried out in very different populations cannot be adequately summarized in few words, it can be easily appreciated that the Chinese trial found beneficial effects on decreased mortality, mainly due to reduced cancer rates (especially for stomach cancer) (Blot et al., JNCI 1993 and Am J Clin Nutr 1995) while the second trial found beneficial, null and adverse effects of supplementation overall as well as specifically for cancer (Hercberg et al., Arch Intern Med 2004 and Br J Nutr 2006). Among the adverse effects following supplementation, Hercberg et al. found an alteration of the lipid profile (Hercberg et al., Lipids 2005) and an increase in melanoma incidence (Hercberg et al., J Nutr 2007), later shown to decrease during the post‐intervention follow‐up, further supporting a causative role of the treatment (Ezzedine et al., Eur J Cancer 2010). However, since these two trials did not include an intervention arm receiving Se alone, they were excluded from our meta‐analysis as were all trials that administered Se together with other substances. They were included in a different Cochrane review (Bjelakovic et al. Cochrane Database Syst Rev. 2012).
Page 30, column 1: contrary to the statements of Brigelius‐Flohé et al., the Karp et al. trial, published in extenso in J Clin Oncol 2014, was not a secondary prevention trial, but a primary prevention trial, as we indicated in our review. As literally abstracted from the Karp paper, study objectives were “to evaluate the efficacy of Se supplementation in reducing the incidence of lung second primary tumors in patients who had been treated for stage I non–small‐cell lung cancer; to evaluate the qualitative and quantitative toxicity of daily Se supplementation; and to compare the incidence of specific cancers, mortality from cancer, and overall survival of patients treated with Se supplementation versus placebo”. The study population was therefore comparable to that of the NPC trial in the sense that both included participants with a recent history of cancer: the first trial comprised 1561 individuals who had been treated for stage I non–small‐cell lung cancer with complete surgical resection, while the second RCT included 1312 individuals with a history of two or more basal cell carcinomas or one squamous cell carcinoma of the skin, with one of these occurring within the year prior to randomization. We note that the results of the low‐bias Karp et al. trial, which could not be meta‐analyzed in our review having been published in extenso beyond the literature search deadline, were fully consistent with the conclusions of our review.
Brigelius‐Flohé et al. state that ‘A question that remains ignored by this review, by design, is whether Se in combination with other agents may be beneficial in cancer’. As they correctly recognize, this was not included among the objectives of our review. However, we agree with Brigelius‐Flohé et al. concerning the use of selenium compounds in cancer therapy warranting strong attention and in‐depth investigation, as stated in our section ‘Se as a potential cancer therapeutic agent’ in Vinceti et al., J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 2013. However, caution must be used when addressing this issue, also due to the concerning results of a recent study in patients affected by nonmetastatic prostate cancer, where supplementation of ≥140 µg/day Se was found to be associated with excess mortality from prostate cancer (Kenfield et al., JNCI 2015).
We wish to thank Brigelius‐Flohé et al. for their search for typos and mistakes in our 193 page review. They claim that three errors were found; however, these were not errors. The acronym SU.VI.M.AX was sometimes used by the authors of that trial, and we used it in our review only when citing a reference titled with that form of the acronym (Arnaud et al., J Trace Elem Med Biol 2007), while we used the more common ‘SU.VI.MAX’ for the remaining papers. As far as the 78.96 ‘molecular weight’ of Se is concerned, we recognize that the adjective ‘atomic’ is more commonly used than ‘molecular’, but the latter may also be used in connection with ‘weight’ for Se, as it may be observed at the PubChem Open Chemistry database of the US National Institute of Health (http://pubchem.ncbi.nlm.nih.gov/compound/Se, accessed January 20, 2015) or the US Center for Disease Control and Prevention ‐ National Institute for Occupational Safety and Health website (http://www.cdc.gov/niosh/docs/81‐123/pdfs/0550.pdf, accessed January 20, 2015). Finally, aggressive marketing of Se supplements for breast cancer can be detected through a simple Google Internet search. Admittedly, this is also true for other cancers, including of course prostate cancer, and more generally for chronic disease or conditions claimed to be due to oxidative stress and alleged to be prevented by Se. However, such marketing approaches differed depending on the diseases, populations, sources of information, strategies, and periods involved, and were not analyzed because they were outside the scope of our current review.
Contributors
Marco Vinceti, Gabriele Dennert, Catherine M Crespi, Marcel Zwahlen, Maree Brinkman, Maurice PA Zeegers, Markus Horneber, Roberto D’Amico, Cinzia Del Giovane
Selenium for preventing cancer, 23 November 2011
Summary
Re: Dennert et al., Selenium for preventing cancer, The Cochrane Library, 2011, Issue 5. As selenium scientists with considerable knowledge of the selenium‐cancer field, we wish to draw to the attention of The Cochrane Collaboration the shortcomings of the recent review cited above. We contend that the quality of this review is not up to the expected standard of Cochrane systematic reviews. We are not criticising the way in which the analyses were performed, but rather the ways they were interpreted and summarised, which we believe to be overly negative and rather biased. For these reasons, we find the resulting report to be misleading to the reader. Some of the weaknesses are listed below. Abstract and Plain Language Summary: These sections do not fairly represent the findings of the review. Contrary to the impression given in these summaries, the review itself demonstrates that there is in fact a considerable body of evidence, much of it from prospective observational studies, for a beneficial effect of selenium on a number of cancers. The stated summary of RCT findings is more conclusive than it should be, given the very small number of published clinical trials with selenium alone and the limited trial data that the review authors arbitrarily chose to consider. Furthermore, the NPCT is treated very harshly, and its secondary findings (lung, colorectal and prostate cancers) are more or less discounted. Body of the Paper: 1. Lack of appreciation of the importance of baseline selenium status in influencing trial outcomes (i.e. the fact that only people with a low selenium status profited from supplementation). For example, no acknowledgement was made of the fact that lack of benefit of a 200 μg/d dose of selenium for cancer risk in SELECT occurred in participants with relatively high baseline serum selenium concentrations—well above those found to confer benefit from selenium supplementation in the NPC trial (NPCT). This point was raised by us previously (Rayman et al. JAMA 2010). 2. Lack of discrimination between trials in which supplementation with selenium had the capacity to maximise selenoprotein expression/concentration (e.g. NPCT) and those (e.g. SELECT) in which selenoprotein expression/concentration would already have been maximised at baseline. 3. Lack of appreciation that, despite the high selenium status of SELECT men, the effects of selenium supplementation on type 2 diabetes risk were not significant. 4. Failure to understand that biomarkers of selenium status are considerably more reliable than dietary data, which we know to be much more error‐prone. 5. Frequent failure to distinguish between significant and non‐significant findings. 6. Lack of familiarity with the relevant selenium literature. 7. No mention of oesophageal or gastric cardia cancer results (although RCT results for these are not based on selenium alone) and, in relation to colorectal cancer, no mention of adenoma data. 8. In 'Implications for research', no mention is made of the need to carry out randomised controlled trials in low‐selenium populations, nor to take into consideration selenoprotein genotype, which has been shown to affect selenium metabolism. The relevance of the species of selenium administered in various trials is not mentioned.
Reply
The authors wish to thank the colleagues Doctors Brigelius‐Flohé, Combs, Davis, Green, Hesketh, Köhrle, Kristal, Rayman, Schomburg, Taylor, van den Brandt, Waters and Whanger for their detailed commentary on the selenium review.
Their comments captured some of the same concerns that we had regarding the methodological challenges associated with conducting a systematic review in the field of selenium and cancer.
In response to the commentary, we will first address concerns related to the specific setting of this review as a Cochrane review and will then respond to concerns regarding the content of the review.
We strongly agree with the concerns that it is difficult to capture all differentiations elaborated on by the review in the abstract and summary, which are limited to a certain length. Similarly, length limitations were applied to the background section. We also share the opinion that some headings in the review do not adequately reflect the content of the text that follows. For readers who have not authored Cochrane reviews themselves, we wish to explain that Cochrane reviews are submitted in an electronic format that does not allow for all adaptations authors might wish to make. The headings, for example, cannot be changed. This electronic format is optimised for reviews on intervention studies. Our review included both RCTs and epidemiological studies, and so we encountered several structural challenges throughout the review process. We hope that both the commentary of our colleagues and our experiences will contribute to the continuing work of advancing the structural processes of The Cochrane Collaboration, including the electronic software Review Manager, and to developing a more inclusive format for reviews, which encompasses epidemiological studies.
Has the condensation of information in the abstract and the plain text summary led to a distortion in the presentation of the review results?
The abstract and the plain text summary present to readers the body of evidence that was reviewed as the main results for both study questions. Our aim was to report the answers to our research questions, and although space was a limitation for the abstract and summary results sections, we have endeavoured to provide across the entire review all the best available evidence for the role of selenium in preventing cancer.
We agree with our colleagues that no studies can be found on the association of selenium with cancer in children or on the preventive efficacy of selenium supplements in children. Hence, as stated in the abstract, there is currently no convincing evidence that selenium supplementation may prevent cancer in children. However, we are completely happy not to mention children in the abstract if this may be considered misleading.
We agree with our colleagues that long‐term supplementation is more likely than short‐term supplementation to influence cancer risk, if any effect exists. The minimum of four weeks has been chosen arbitrarily. However, no consistent current agreement has indicated where to draw the line between short‐term and long‐term selenium supplementation, so any cutoff would be arbitrary to some extent. In addition, we wished to avoid making assumptions about supplementation effects in our inclusion criteria and decided rather to address the question of the effect of shorter supplementation periods in the review discussion, if any trial would have been identified.
To our knowledge, there is currently no universal recommended daily allowance for selenium intake or upper tolerable level; therefore recommending a selenium dose or level of safe intake would not be appropriate in this instance. This is clearly an area for further research, taking into account some of the potential influencing factors cited in our review (e.g. baseline levels, gender, population, source). We would like to thank the commentators for the hint to the RNI (reference nutrient intake) values for selenium in the UK, which we are happy to include in a future update of the review. Nevertheless, regarding the RNI, we would like to draw attention to the latest draft of a position paper on selenium by the Scientific Advisory Committee on Nutrition (2011), which notes “that the selenium dietary reference value was set on very limited data and could be set too high” (p74).
Dr Brigelius‐Flohé and colleagues commented that “Quoted recommendations such as 30 and 40 μg/d for men and women (WHO 2004) are no longer credible to anyone with up‐to‐date knowledge of the endpoints and biomarkers (SePP, GPx activity) that we have in 2011. There is no justification for quoting the Vinceti 2009a opinion that 20 μg/day organic selenium should be the maximum safe level.”
The suggestion of an upper safe limit of organic selenium of 20 µg/d was made by Vinceti et al. on the basis of preliminary results of the ORDET study (Vinceti 2009b), published in 2010 (Stranges 2010), and of other studies (please see for a review Vinceti 2009a). The recent availability of new data on endocrine (Lippman 2009; Stranges 2007) and dermatological (Lippman 2009) toxicity of low doses of organic selenium adds new findings supporting the recommendations by the WHO Group. We would like to draw attention to other recent studies on selenium toxicity (reviewed by Vinceti 2009a and Nogueira/Rocha 2011) and the issue of risk assessment of selenium (including the use of uncertainty factors (UF) or alternative approaches) (Aggett 2010; Douron 2010; Renwick 2006; Renwick/Walker 2008).
The diverse recommendations and the controversial discussions clearly underline the need for a systematic review in this field.
To address our research question—What evidence exists on the efficacy of selenium supplementation for cancer prevention?—we restricted our focus to RCTs with mono‐selenium supplementation. Multicomponent interventions, such as those chosen in the SU.VI.MAX, involve several nutritional/antioxidant supplements (e.g. 120 mg of ascorbic acid, 30 mg of vitamin E, 6 mg of beta carotene, 100 µg of selenium, and 20 mg of zinc in SU.VI.MAX), some of which are reportedly thought to have a potentially synergistic effect with selenium (Willett 1983); others may act as antagonists (Schrauzer/White/Schneider 1977) or may have an unknown biological interaction. Although all these factors are important considerations for the overall efficacy of selenium in the long term, we thought that inclusion of these studies in attempts to elucidate an actual anticarcinogenic role for selenium in its own right could potentially conceal the true effects (positive or negative) of selenium. By including the four studies that were mentioned in the commentary, which used multicomponent interventions, we may have gained numbers but lost out in trying to elucidate the actual effects of selenium. Therefore, these RCTs, which use selenium in combination with other nutritional factors, were outside the scope of the current review process but have been addressed in the background and discussions and could be the focus of future valuable investigations.
To avoid any potential preferential and non‐systematic selection of studies and hence results, we established a set of a priori inclusion criteria during the initial stages of the study design. These were outlined in the protocol of the review, which has been available on The Cochrane Library website and for comment since 2005.
The details of all selenium supplementation have been reported for each RCT, including the form of selenium when available, and we emphasised the importance of carefully evaluating the different biological activity and toxicity of each selenium compound. Please refer to the plain language summary: “In general there are two types of selenium supplements: one type uses the salt of selenium as the main ingredient, the other type uses organic selenium. These two types may act differently in the human body when ingested,” and in the RCTs and preventive efficacy section: “Interpretation of the results of clinical trials using selenium supplements should consider the different biological forms as well as their potential differential health effects when supplemented”; and please refer to the table Characteristics of included studies, for details on each RCT.
References are made throughout the review text to the baseline selenium status of study participants and potential interactions with study results. Please refer to Section 2.3. Adverse effects, “The RR for developing type II diabetes mellitus was higher in the participants in the upper two tertiles of plasma selenium levels, indicating a possible interaction with baseline exposure status”, for instance, or page 38 in our review: “SELECT participants had a higher selenium level at randomisation than men in the NPCT. While the mean plasma selenium concentration was 113 to 114 μg/l in the NPCT, median serum concentration was 135 to 138 μg/l in the different study arms in SELECT. Lower prostate cancer incidence in the NPCT trial was confined to men with baseline selenium levels in the lower two thirds (below 121 μg/l). Subgroup analyses of the SELECT trial are underway to investigate a possible modification by pre‐intervention selenium levels“.
Regarding the findings of NPCT and SELECT for type 2 diabetes, we would like to refer our readers to Section 2.3. Adverse effects, “A statistically non‐significant increase in diabetes mellitus type II in the selenium‐alone group (HR 1.07 (99% CI 0.94: 1.22)) was seen. An increased risk for diabetes mellitus type II was also observed in the NPCT (Stranges 2007, in: NPCT 1996). A secondary analysis of participants who did not have diabetes at start of the study revealed an excess risk in the selenium group (adjusted HR 1.55 (95% CI 1.03 to 2.33))”. We have previously outlined the section that referred to the fact that selenium baseline levels were higher in this group and would like to cite the original paper by Stranges et al. (2007), which stated: “Despite the lack of statistically significant interactions between treatment group and baseline co‐variates, the risk for type 2 diabetes was consistently higher in the selenium group within all subgroups of baseline age, sex, smoking
status, and BMI.” (p220). Regarding the issue of a potential diabetogenic effect of selenium supplements and gender, we would like to draw attention to a recent observational cohort study by Stranges (2010), which documented an excess risk of diabetes among a large cohort of women from Varese, Northern Italy. Such a diabetogenic effect of selenium is also supported by suggestive laboratory evidence, recently reviewed by Steinbrenner al. (2011).
Lippman et al. (2009) stated in their publication about the SELECT trial: “The data and safety monitoring committee had some concern over the statistically non‐significant increase in prostate cancer in the vitamin E‐alone group (P=.09 per interim data of August 1, 2008) and over a non‐significant increase in diabetes mellitus associated with selenium (P=.08 per interim data of August 1, 2008)” (p45).
The observation from SELECT (Klein 2011) that the effect diminished over time may suggest exactly the opposite to that hypothesised by Dr Brigelius‐Flohé and colleagues. A decrease in the diabetogenic effect of selenium administration over time after interruption of such administration may well indicate a decreasing adverse effect over time, as expected, of a causal association. This was what occurred in the SU.VI.MAX study, in which administration of selenium/vitamins C‐E/beta‐carotene/zinc led to an excess incidence of skin cancer, including melanoma (Hercberg 2004), which entirely disappeared after interruption of the intervention (Ezzedine 2010). The investigators interpreted such decreasing risk as an indication of the causal effect of the treatment of skin cancer and the origin of melanoma (Ezzedine 2010).
Regarding the interaction of baseline PSA levels with selenium effects in the NPCT, we would like to quote the original publication: “The protective effect of SS [selenium supplements; GD] appeared to be confined to those with a baseline PSA level of <= 4 ng/mL (0.35, 0.13–0.87), although the interaction of baseline PSA and treatment was not statistically significant“ (p608, Duffield‐Lillico 2003a). To summarise, no statistically significant interaction was noted between baseline PSA levels and prostate cancer incidence, as reported by the study authors.
Dr Brigelius‐Flohé highlighted a sentence on page 4 that might be misunderstood if taken out of its context (“risk ratios (RRs) with confidence intervals (CIs) were not calculated because of low numbers”). Our colleagues rightly stated that Hercberg et al. (2004) provided hazard ratios for cancer incidence by gender. However, the sentence our colleagues quoted from our review reads in the context as follows: “In the more recent French SU.VI.M.AX trial (Hercberg 2004), a supplementation with beta‐carotene, vitamin C, vitamin E and 100 μg selenium‐enriched yeast did not alter the incidence of cancer of the digestive tract after a median period of 7.5 years in women. In men, the incidence rate was lower in the intervention group than in the placebo group, but risk ratios (RRs) with confidence intervals (CIs) were not calculated because of low numbers”. The part of the sentence our colleagues cited about the men’s incidence rate refers to cancer of the digestive tract. Site‐specific cancer rates were not calculated or reported by gender: “We were not able to analyze differences in site‐specific cancers between men and women because of low statistical power” (p2340, Hercberg 2004).
Our colleagues highlighted another sentence on page 39: “Results from two randomised controlled trials (NPCT and SELECT) have failed to provide evidence that non‐melanoma skin cancer or prostate cancer can be prevented by selenium supplementation in men”. This statement refers to the primary study outcomes of both investigations, which were non‐melanoma skin cancer in NPCT and prostate cancer in SELECT, and is correct. Contrary to what was stated by Dr Brigelius‐Flohé and colleagues, the outcome measures in the NPCT were incident basal cell carcinomas and squamous cell carcinomas, and recurrent skin tumors were excluded from analysis, as summarised in the report of the primary NPCT endpoint by Duffield‐Lillico et al. (2003b). We clearly stated in our review that the NPCT was carried out among non‐melanoma skin cancer participants at baseline.
Our conclusions have been based on the available evidence, and we have highlighted the paucity of literature and data available from RCTs. Please refer to the 'Implications for research' section: “Potential differential effects of sex/gender and the use of selenium supplements in populations with a high burden of specific types of cancer diseases and differing selenium exposure levels, e.g. known low nutritional selenium intake, require further examination”.
Dr Brigelius‐Flohé and colleagues have also expressed concerns regarding our inclusion criteria for epidemiological studies and the ways results of epidemiological studies were included and presented in the systematic review.
In reply to their concern, we might have omitted three relevant studies for gastrointestinal cancers; we would like to refer them to the detailed references to both studies, Mark 2000 and Wei 2004, throughout the review. The Steevens (2010) study has not been included, as it was not available at the time of our review process and submission to The Cochrane Collaboration Group (please refer to Methods section, Search strategy). As reported in Section 1.1.6 of the review, the strength of association varied according to what was included in analyses (e.g. cardia vs non‐cardia cancers, gender), thus preventing any clear and concise conclusion to be drawn between selenium levels and upper gastrointestinal cancers in the observational summary results.
As we understood the publications Wei 2004 and Mark 2000, Wei 2004 reports on a population that was part of the population at risk in Mark 2000. Participants in Wei 2004 were the disease‐free controls for the cases of Mark 2000. Because of this overlap, we decided to report the papers jointly and put emphasis on the detailed description of both papers and their study populations (please refer to the Characteristics of included studies).
Dr Brigelius‐Flohé and colleagues criticised inclusion in the review of observational studies assessing selenium exposure as intake (e.g. with food frequency questionnaires).
Regarding the problems associated with dietary assessment, please refer to the section 'Bias and confounding': “Assessment of total selenium intake from food‐frequency questionnaires (FFQ) or interviews has proven difficult in other investigations because of the lack of food composition data which adequately reflects regional and seasonal variations in selenium concentration”. Additionally, “The FFQ overestimated the mean selenium intake in study participants when compared with laboratory analyses of duplicate meals” and ”Validity problems, possibly leading to misclassification, have also been reported when questionnaires are used to assess supplement use”.
However, studies using dietary assessment add a valuable perspective to the discussion of the relationship between selenium exposure and cancer risk. Furthermore, in addition to the literature cited by Dr Brigelius‐Flohé, other studies (van den Brandt PA et al, 1993; Longnecker et al., 1996; Haldimann et al., 1996) have reported a direct correlation between dietary and body selenium (please also see for a review of this topic Vinceti et al. 2000b and Vinceti et al. in press).
We consider the issue of selenium exposure assessment to be more complex than has been implicated by our colleagues´ comments. Assessment of selenium intake, despite the difficulties associated with its variability and possible individual variability in absorption, in some cases might even yield better estimates of actual exposure compared with biomarkers. This adds an important perspective to the discussion of why several observational studies have suggested a protective effect of higher selenium exposure towards cancer risk and others have not.
With regard to toxicity, animal studies have demonstrated that the intake of equivalent amounts of selenium, when administered in different species, might induce a stronger effect even when retained to a lesser extent (Panter et al., 1996), as shown for the inorganic compounds. The wealth of toxicological data from laboratory studies is clearly and, for obvious ethical reasons, much greater than those yielded by human studies. The same is true for studies investigating tissue distribution and biological activity of the different selenium compounds (see: Hatfield/Berry/Gladyshev 2012). We consider references to laboratory and animal studies as a necessary and valuable contribution to the understanding of selenium effects in humans.
Dr Brigelius‐Flohé and colleagues asked why our summary of the findings of the review of Ashton (2009) on the use of biomarkers for selenium measurement did not mention singular nucleotide polymorphisms (p34 in our review). We summarised the findings of Ashton 2009 that were relevant for the discussion of bias and confounding in our review. Genetic polymorphisms were not included in the analyses of heterogeneity between study results by Ashton (2009). Instead, Ashton et al. proposed singular nucleotide polymorphisms in their discussion as an area for future research and stated: “Also, for all potential biomarkers, more information is needed to understand the limitations of applicability for different population groups, the possible effects of genotype, supplementation doses, duration, baseline status, etc” (p2037S).
The criticism that we failed to distinguish between significant and non‐significant findings in epidemiological studies points to a fundamental difference in the interpretation of epidemiological study results. Indeed, we consider ‘statistical’ significance as an inappropriate approach to data analysis and interpretation with regard to observational studies, as has been long recognised (Rothman KJ 1978; Sterne/Davey Smith 2001; Greenland 2011), with no connection with ‘biological significance’. Pitfalls of statistical significance testing encompass dismissing so called `non‐significant values´ in small studies or putting undue emphasis on ‘statistically significant’ results without attempting to integrate potential biases for a study finding that would affect the estimates from that study (see: e.g. Rothman, Greenland & Lash 2008; Stang/Poole/Kuss 2010). This may lead to confusion between the validity of an investigation and its statistical stability.
Analysis and interpretation of results in biomedical research must be based on a number of considerations, comprising both study design and data analysis. We made a conscious effort in our selenium review to avoid use of an approach that dichotomised study results according to which were statistically significant and which were not. We consider this effort a major strength of our review.
We have attempted to be prudent with our conclusions by highlighting important considerations associated with the results of epidemiological studies that we reported. Both the current literature and our review indicate that although some associations have been noted between selenium levels and risk of cancer at certain body sites (e.g. prostate, bladder), more research and information are clearly required before it can be concluded that these results are “convincing” for a protective effect of selenium. The World Cancer Research Fund’s Second Expert Report (2007) also suggests the possibility of residual confounding between selenium levels and healthy lifestyles (p109).
We admit that the sentence about the marketing situation of selenium in our discussion section expresses a valuation, and we acknowledge that other colleagues might assess the marketing situation differently and as such might disagree with this sentence.
In the last part of our reply, we will address the concerns by Dr Brigelius‐Flohé and colleagues regarding the content of the background section of the review.
The reference Rodriguez 1995, which is listed in the MEDLINE database, in contrast to what our colleagues stated (please refer to PubMed ID 7605824), is an early study that investigated urinary selenium in healthy men and women and addressed the study question of the relationship between factors such as gender/sex, etc., and urinary selenium. It found gender/sex differences in urinary selenium excretion, as well as influences of health behaviours (physical activity), as stated in our background text.
We do not agree that studies investigating primarily the relationship between selenium status, thyroid volume and gland echostructure (Derumeaux 2003) or the relationship between baseline plasma selenium concentration and occurrence of dysglycaemia (Akbaraly 2010) would have been more suitable references for the statement that we made regarding gender differences.
We also would like to recapitulate the Vinceti et al. (2000a) paper because we feel that Dr Brigelius‐Flohé and colleagues misreported the methods and findings of this study. The Vinceti et al. studies in an unusual Northern Italy setting evaluated the health effects of selenium in its inorganic hexavalent form—the one usually found in underground and drinking water—together with the tetravalent species (Vinceti 2010). This study was a ‘natural experiment’, considered to be ‘the paradigm of non‐experimental epidemiologic research’, as in this type of study, ‘nature emulates the sort of experiment the investigator might have conducted, but for ethical and cost constraints’ (p94, Rothman/Greenland/Lash 2008). Study authors assessed the potential for confounding by lifestyle by assessing the socioeconomic status of exposed and unexposed cohorts, and labeling this study as a natural experiment was allowed only after the similarity of the two populations was confirmed. Dr Brigelius‐Flohé stated that Vinceti et al. admitted that their results are consistent with “no effect”, as standardised mortality ratios were generally inconsistent between men and women at most sites, and most site‐specific estimates had limited precision. The citation in the original publication reads: “The results of our study are consistent with either no effect or, particularly among the elderly, unfavourable effects of long‐term exposure to inorganic selenium on cancer mortality”. Then Vinceti et al. analyzed the strengths and limitations of their study, both for the melanoma association and more generally for the effects on cancer risk. Excess melanoma risk, despite different study designs and strengths of association, has been documented to be associated with selenium exposure in a number of studies (Garland 1995; Vinceti 1998; Duffield‐Lillico 2002; Vinceti et al., in press) and has been causally associated with administration of selenium in combination with zinc and vitamins in SU.VI.MAX (Hercberg 2007). In general, we would like to propose caution when dealing with the possible selenium‐melanoma association.
In conclusion, we express our appreciation to our commentators for scrutinising our review, offering their criticisms and supporting the scientific endeavour of enclosing epidemiological as well as intervention studies in a Cochrane review. We are hopeful that the review and the commentary of our colleagues will contribute to the important and continuing discussion about the health effects of selenium and selenium supplements globally and in diverse populations.
Aggett PJ. (2010). Toxicity due to excess and deficiency. J Toxicol Environ Health A: 73(2):175‐80.
Akbaraly TN, Arnaud J, Rayman MP, Hininger‐Favier I, Roussel AM, Berr C, Fontbonne A. (2010). Plasma selenium and risk of dysglycemia in an elderly French population: results from the prospective Epidemiology of Vascular Ageing Study. Nutr Metab 18:21. Electronic publication: doi:10.1186/1743‐7075‐7‐2.
Ashton K, Hooper L, Harvey LJ, Hurst R, Casgrain A, Fairweather‐Tait SJ. (2009). Methods of assessment of selenium status in humans: a systematic review. Am J Clin Nutr 89(6):2025S‐39S.
Derumeaux H, Valeix P, Castetbon K, Bensimon M, Boutron‐Ruault MC, Arnaud J, Hercberg S. (2003). Association of selenium with thyroid volume and echostructure in 35‐ to 60‐year‐old French adults. Eur J Endocrinol 148(3):309‐15.
Douron M. (2010). U‐shaped dose‐response curves: implications for risk characterization of essential elements and other chemicals. J Toxicol Environ Health A 73(2):181‐6.
Duffield‐Lillico AJ, Reid ME, Turnbull BW, Combs GF Jr, Slate EH, Fischbach LA, et al. (2002). Baseline characteristics and the effect of selenium supplementation on cancer incidence in a randomized clinical trial: a summary report of the Nutritional Prevention of Cancer Trial. Cancer Epidemiol Biomarkers Prev 11(7):630‐9.
Duffield‐Lillico AJ, Dalkin BL, Reid ME, Turnbull BW, Slate EH, Jacobs ET, et al. (2003a). Selenium supplementation, baseline plasma selenium status and incidence of prostate cancer: an analysis of the complete treatment period of the Nutritional Prevention of Cancer Trial. BJU Int 91(7):608‐12.
Duffield‐Lillico AJ, Slate EH, Reid ME, Turnbull BW, Wilkins PA, Combs GF Jr, et al. (2003b). Selenium supplementation and secondary prevention of nonmelanoma skin cancer in a randomized trial. J Natl Cancer Inst Cancer Spectrum 95(19):1477‐81.
Ezzedine K, Latreille J, Kesse‐Guyot E, Galan P, Hercberg S, Guinot C, Malvy D. (2010). Incidence of skin cancers during 5‐year follow‐up after stopping antioxidant vitamins and mineral supplementation. Eur J Cancer 46(18):3316‐22.
Garland M, Morris JS, Stampfer MJ, Colditz GA, Spate VL, Baskett CK, et al. (1995). Prospective study of toenail selenium levels and cancer among women. J Natl Cancer Inst 87(7):497‐505.
Greenland S. (2011). Null misinterpretation in statistical testing and its impact on health risk assessment. Preventive Med 53:225–228.
Haldimann M, Venner TY, Zimmerli B. (1996). Determination of selenium in the serum of healthy Swiss adults and correlation to dietary intake. J Trace Elem Med Biol 10(1):31‐45.
Hatfield DL, Berry MJ, Gladyshev VN (Editors). (2012). Selenium. Its Molecular Biology and Role in Human Health. 3rd edition. Springer, New York, 2012, and springer ebooks: 10.1007/978‐1‐4614‐1025‐6.
Hercberg S, Galan P, Preziosi P, Bertrais S, Mennen L, Malvy D, Roussel AM, Favier A, Briançon S. (2004). The SU.VI.MAX Study: a randomized, placebo‐controlled trial of the health effects of antioxidant vitamins and minerals. Arch Intern Med 164(21):2335‐42.
Hercberg S, Ezzedine K, Guinot C, Preziosi P, Galan P, Bertrais S, Estaquio C, Briancon S, Favier A, Latreille J, Malvy D. (2007). Antioxidant supplementation increases the risk of skin cancers in women but not in men. J Nutr 137:2098‐2105.
Klein EA, Thompson IM Jr, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, Minasian LM, Ford LG, Parnes HL, Gaziano JM, Karp DD, Lieber MM, Walther PJ, Klotz L, Parsons JK, Chin JL, Darke AK, Lippman SM, Goodman GE, Meyskens FL Jr, Baker LH. (2011). Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 306:1549‐56.
Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, et al. (2009). Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 301(1):39‐51.
Longnecker MP, Stram DO, Taylor PR, Levander OA, Howe M, Veillon C, et al. (1996). Use of selenium concentration in whole blood, serum, toenails, or urine as a surrogate measure of selenium intake. Epidemiology 7(4):384‐90.
Mark SD, Qiao YL, Dawsey SM, Wu YP, Katki H, Gunter EW, et al. (2000). Prospective study of serum selenium levels and incident esophageal and gastric cancers. J Natl Cancer Inst 92(21):1753‐63.
Nogueira CW, Rocha JB. (2011). Toxicology and pharmacology of selenium: emphasis on synthetic organo‐selenium compounds. Arch Toxicol 85(11):1313‐59.
Panter KE, Hartley WJ, James LF, Mayland HF, Stegelmeier BL, Kechele PO. (1996). Comparative toxicity of selenium from seleno‐DL‐methionine, sodium selenate, and Astragalus bisulcatus in pigs. Appl Toxicol 32(2):217‐23.
Renwick AG. (2006). Toxicology of micronutrients: adverse effects and uncertainty. J Nutr 136:493S‐501S.
Renwick AG, Walker R. (2008). Risk assessment of micronutrients.Toxicol Lett 180(2):123‐30.
Rothman KJ (1978). A show of confidence. N Engl J Med 299:1362‐3.
Rothman KJ, Greenland S, Lash TL. (2008). Modern Epidemiology. Philadelphia: Lippincott Williams & Wilkins.
Schrauzer GN, White DA, Schneider CJ. (1977). Cancer mortality correlation studies—IV: associations with dietary intakes and blood levels of certain trace elements, notably Se‐antagonists. Bioinorg Chem 7(1):35‐56.
Scientific Advisory Committee on Nutrition. (2011). Paper for discussion: Draft Selenium and Health position statement, 19/10/11. http://www.sacn.gov.uk/pdfs/sacn1113_sacn_selenium_and_health_position_paper.pdf (accessed on 20 December 2011).
Stang A, Poole C, Kuss O. (2010). The ongoing tyranny of statistical significance testing in biomedical research. Eur J Epidemiol 25:225‐30.
Steevens J, van den Brandt PA, Goldbohm RA, Schouten LJ. (2010). Selenium status and the risk of esophageal and gastric cancer subtypes: the Netherlands cohort study. Gastroenterology 138(5):1704‐13.
Steinbrenner H, Speckmann B, Pinto A, Sies H. (2011). High selenium intake and increased diabetes risk: experimental evidence for interplay between selenium and carbohydrate metabolism. J Clin Biochem Nutr 48(1):40‐5.
Sterne JA, Davey Smith G. (2001). Sifting the evidence—what's wrong with significance tests? BMJ 322:226‐31.
Stranges S, Marshall JR, Natarajan R, Donahue RP, Trevisan M, Combs GF, et al. (2007). Effects of long‐term selenium supplementation on the incidence of type 2 diabetes: a randomized trial. Ann Intern Med 147:217‐23.
Stranges S, Sieri S, Vinceti M, Grioni S, Guallar E, Laclaustra M, Muti P, Berrino F, Krogh V. (2010). A prospective study of dietary selenium intake and risk of type 2 diabetes. BMC Public Health 10:564. http://www.biomedcentral.com/1471‐2458/10/564.
van den Brandt PA, Goldbohm RA, vant´t Veer P, Bode P, Hermus RJ, Sturmans F. (1993). Predictors of toenail selenium levels in men and women. Cancer Epidemiol Biomarkers Prev 2(2):107‐112.
Vinceti M, Rothman KJ, Bergomi M, Borciani N, Serra L, Vivoli G. (1998). Excess melanoma incidence in a cohort exposed to high levels of environmental selenium. Cancer Epidemiol Biomarkers Prev 7(10):853‐6.
Vinceti M, Nacci G, Rocchi E, Cassinadri T, Vivoli R, Marchesi C, et al. (2000a). Mortality in a population with long‐term exposure to inorganic selenium via drinking water. J Clin Epidemiol 53(10):1062‐8.
Vinceti M, Rovesti S, Bergomi M, Vivoli G. (2000b). The epidemiology of selenium and human cancer. Tumori 86(2):105‐18.
Vinceti M, Maraldi T, Bergomi M, Malagoli C. (2009a). Risk of chronic low‐dose selenium overexposure in humans: insights from epidemiology and biochemistry. Rev Environ Health 24(3):231‐48.
Vinceti M, Stranges S, Sieri S, Grioni S, Malagoli C, Muti P, Berrino F, Krogh V. (2009b). Association between high selenium intake and subsequent increased risk of type 2 diabetes in an Italian population. ISEE 2009 Conference Abstracts Supplement; Epidemiology: 20: S47.
Vinceti M, Bonvicini F, Rothman KJ, Vescovi L, Wang F. (2010). The relation between amyotrophic lateral sclerosis and inorganic selenium in drinking water: a population‐based case‐control study. Environ Health 6:77. Electronic citation: doi 10.1186/1476‐069X‐9‐77.
Vinceti M, Crespi CM, Malagoli C, Bottecchi I, Ferrari A, Sieri S, Krogh V, Alber D, Bergomi M, Seidenari S, Pellacani G. (in press). A case‐control study of the risk of cutaneous melanoma associated with three selenium exposure indicators. Tumori.
Wei WQ, Abnet CC, Qiao YL, Dawsey SM, Dong ZW, Sun XD, et al. (2004). Prospective study of serum selenium concentrations and esophageal and gastric cardia cancer, heart disease, stroke, and total death. Am J Clin Nutri 79(1):80‐5.
Willett WC, Polk BF, Morris JS, Stampfer MJ, Pressel S, Rosner B, Taylor JO, Schneider K, Hames CG. (1983). Prediagnostic serum selenium and risk of cancer. Lancet 2(8342):130‐4.
World Cancer Research Fund International. (2007). Food, Nutrition, Physical Activity, and the Prevention of Cancer. A Global Perspective. Washington, DC: AICR.
World Health Organization (WHO). (2004). Joint FAO/WHO Expert Consultation on Human Vitamin, Mineral Requirements. (1998: Bangkok, Thailand). Vitamin and mineral requirements in human nutrition: report of a joint FAO/WHO expert consultation, Bangkok, Thailand, 21‐30 September 1998. http://whqlibdoc.who.int/publications/2004/9241546123.pdf (accessed on 23 January 2011).
Contributors
Professor Regina Brigelius‐Floh, Professor GF Combs Jr, Dr Cindy D Davis, Dr Fiona R Green, Professor John Hesketh, Professor Josef Köhrle, Dr Alan Kristal, Fred Hutchinson, Professor Margaret P Rayman, Professor Lutz Schomburg, Phil Taylor, Piet van den Brandt, Professor David J. Waters, Professor Phil Whanger.
Maree Brinkman, Gabriele Dennert and Marco Vinceti on behalf of the review authors.
Further discussion on 'Selenium for preventing cancer'
Summary
We are pleased with your positive response to our concerns and the expressed willingness of the review authors to make changes as appropriate. In particular, we welcome the following proposed modifications.
A more accurate (and longer) abstract and plain language summary to take account of the concerns we specified in our letter and in the first of our “General criticisms”.
Modification of the review by ensuring that differences in baseline selenium exposure between trials are clarified and placed in the proper context.
More careful use of language in relation to statistical significance, as, for instance, in the two examples you cite in your letter. The preferred form you quote is much better than the misleading use of “lower” or “higher” for “non‐significant” effects, as occurred frequently in the review.
Removal of constraints on the use of section headings so that more appropriate headings can be used.
There is little point in revisiting all of our criticisms as they were clearly set out in our original letter and document, and most still stand. We would like to see the review amended as soon as possible to take account of those criticisms and specifically to correct the inaccuracies that we have noted. The review authors have replied with a number of points that we would like to challenge.
p2: Re the suggestion of an upper safe limit of organic selenium of 20 μg/d by Vinceti et al., the authors now justify the original inclusion of that statement on the basis of a study (ORDET) based on a semiquantitative FFQ at baseline and follow‐up for development of type 2 diabetes 16 years later. Based on that same study (p4), the authors refer to “Such a diabetogenic effect of selenium….”. A prospective study, especially one with a very weak study design such as ORDET, can only show an association—hardly a good basis for making such a statement in a Cochrane review. Furthermore, an upper safe limit of organic selenium of 20 μg/d would be just above that at which Keshan disease is seen—11 μg/d in a Chinese man, which translates to 14 μg/d in a man of Western body weight.[1]
p2: The authors say, “The recent availability of new data about endocrine (Lippman 2009; Stranges 2007) and dermatologic (Lippman 2009) toxicity of low doses of organic selenium adds new findings which support the recommendations by the WHO group.” The authors seem still not to have taken on board the fact that Lippman et al. 2009 doesnot show any endocrine toxicity of selenium. Furthermore, the dose given—200 μg/d—was not low.
p4: Diminution of the effect on type 2 diabetes over time. Proper interpretation of SELECT is that there was a null result during the trial (RR 1.07, P value 0.16) and a similarly null result with postintervention follow‐up time included (RR 1.04, P value 0.34). If trial‐only data versus post‐trial‐only data were compared, it is probably unlikely that there would be any difference statistically. However, we do understand the point the review authors make: Interpretation depends on how one thinks selenium acts. If we were talking about an effect that occurred immediately after starting a drug (e.g. platelet effect of aspirin, blood pressure reduction from antihypertensive) and stopped more or less immediately after cessation of the drug, then the review authors’ interpretation would have better credibility.
In contrast to the week or so that the effect of aspirin on platelets lasts, selenomethionine has a long half‐life of 252 d [363 d (turnover time) × 0.693 (from kinetic modelling)] (Swanson et al. AJCN 1991, 54:917‐26). In medicine, when calculating dosing intervals for drugs, it is typical to give doses every five to six half‐lives. When first‐order kinetics is applied, five half‐lives for total body selenium is 1260 days (3.45 years), and six half‐lives is 1512 days (4.14 years). Although it is true that the amount of the original dose still remaining is small after five (6.25%) or six (3.13%) half‐lives, excess residual selenium remains from the supplementation. So, on the basis of both observed effects with cancer and pharmacokinetic data, the events that occurred in the post‐trial period for SELECT participants (34 additional months) should still be considered a period of selenium exposure and thereforeincompatible with the review authors’ hypothesis.
p6: We hotly dispute the assertion of the review authors (none of whom is a nutritionist) that “The assessment of selenium intake, despite the difficulties associated to its variability and the possible individual variability in absorption, in some cases might even yield better estimates of actual exposure compared with biomarkers”.
p7: Gender differences: The Schomburg references would have been preferable; Schomburg is the accepted authority in this area.
We very much hope that our original comments and those contained in this letter will help the review authors, guided by the editors, to revise the review, so that it sits more comfortably with the opinion of experienced researchers in the selenium‐cancer field.
Yours sincerely,
Professor Regina Brigelius‐Flohé, University of Potsdam, German Institute of Human Nutrition Professor GF Combs Jr, Grand Forks Human Nutrition Research Center, ARS/USDA, USA Dr Cindy D Davis, Office of Dietary Supplements, NIH, USA Dr Fiona R Green, Reader in Functional Genomics, University of Surrey, UK Professor John Hesketh, Institute for Cell & Molecular Biosciences, University of Newcastle, UK Professor Josef Köhrle, Charité Universitätsmedizin Berlin, Germany Dr Alan Kristal, Fred Hutchinson Cancer Research Center, Seattle, USA Professor Margaret P Rayman, Faculty of Health and Medical Sciences, University of Surrey, UK Professor Lutz Schomburg, Charité Universitätsmedizin Berlin, Germany Dr Phil Taylor, Division of Cancer Epidemiology and Genetics, NCI, USA Professor Piet van den Brandt, Department of Epidemiology, Maastricht University, The Netherlands Professor David J Waters, Purdue University, USA Professor Phil Whanger, Oregon State University, USA [1] National Academy of Sciences, Institute of Medicine’s Food and Nutrition Board, Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium and Carotenoids. http://fnic.nal.usda.gov/nal_display/index.php?info_center=4&tax_level=4&tax_subject=256&topic_id=1342&level3_id=5141&level4_id=10591.
Reply
We would like to thank Drs Brigelius‐Flohé and colleagues for their continuing interest in our research activity on selenium.
We decided to shortly respond to some of their discussion points (citations from Dr Brigelius‐Flohé et al are provided in italics):
“more careful use of language in relation to statistical significance as, for instance, in the two examples you cite in your letter. The preferred form you quote is much better than the misleading use of “lower” or “higher” for “non‐significant” effects as occurred frequently in the review”
Dr Brigelius‐Flohé and colleagues do not acknowledge the limitations of their approach based on ‘statistical significance’ (please refer to the references provided in our previous reply). Their approach appears to have had major consequences for a number of considerations and statements in their two letters. It is of interest to note that even the SELECT “Data and Safety Monitoring Committee” expressed its concern “over a non‐significant increase in diabetes mellitus associated with selenium (P = 0.08 per interim data of August 1, 2008)” (cited from Lippman et al., JAMA 2009), which we consider a very correct approach given the decision‐making responsibility of such a Committee.
“The authors have replied with a number of points that we would like to challenge"
p2: "Re the suggestion of an upper safe limit of organic selenium of 20 μg/d by Vinceti et al., the authors now justify the original inclusion of that statement on the basis of a study (ORDET) based on a semi‐quantitative FFQ at baseline and follow‐up for development of type‐2 diabetes 16 years later. Based on that same study (p4), the authors refer to “Such a diabetogenic effect of selenium….”. A prospective study, especially one with a very weak study design such as ORDET, can only show an association—hardly a good basis for making such a statement in a Cochrane review. Furthermore, an upper safe limit of organic selenium of 20 μg/d would be just above that at which Keshan Disease is seen—11 mg/d in a Chinese man, which translates to 14 μg/d in a man of Western body weight.
As written in our original response, the suggestion of a safe upper limit of 20 µg/L was based on the ORDET study results already availableand published as an abstract in Epidemiology in 2009. Stating that the ORDET study, one of the first and most methodologically sound European prospective studies, started in the 1980s by the Italian National Cancer Institute in Milan, was ‘weak’ is unacceptable. Its methodological value has been largely recognised in the scientific community and in the epidemiological literature.
Our review, however, never aimed at summarising the large epidemiological and laboratory literature addressing the issue of safe upper limit of Se exposure in humans, particularly the most recent studies.
p2: The authors say, “The recent availability of new data about endocrine (Stranges 2007; Lippman 2009) and dermatologic (Lippman 2009) toxicity of low doses of organic selenium adds new findings which support the recommendations by the WHO group.” The authors seem still not to have taken on board the fact that Lippman et al. 2009 shows no endocrine toxicity of selenium. Furthermore, the dose given—200 mg/d—was not low.
The relation between selenium and excess diabetes risk is an extremely important issue that clearly would require extensive review, but this was not the aim of our Cochrane review;therefore we would like to refer Dr Brigelius‐Flohé and colleagues to the most recent studies and reviews on the topic. It would also be useful to remind Dr Brigelius‐Flohé and colleagues that the SELECT trial found an excess risk of diabetes, which understandably caused concern for its “Data and safety monitoring Committee” (see above) and contributed to the anticipated ending of the trial. We took note that Dr Brigelius‐Flohé and colleagues do not consider the SELECT supplemental dose of 200 mg/Se/d to be a ‘low’ dose; actually, it was so high that it could be toxic.
p6: "We hotly dispute the assertion of the authors (none of whom is a nutritionist) that “The assessment of selenium intake, despite the difficulties associated to its variability and the possible individual variability in absorption, in some cases might even yield better estimates of actual exposure compared with biomarkers”.
Different exposure assessment methods have different advantages and disadvantages. What we stated in our review was, “A concern, which we cannot clarify to date, is that biomarkers do not adequately reflect intake of both organic and inorganic selenium species”. We still think there is currently no way of clarifying this.
We were very surprised in reading comments such as ‘None of the authors is a nutritionist’, not just because this is incorrect (one of the review authors, MB, is an accredited and practicing dietician and nutritionist), but also for the underlying and clearly ‘biased’ concept: that the right to conduct independent research should be determined by subjective value judgements by one’s peers.
Despite the detailed comments made by Dr Brigelius‐Flohé et al regarding key statements we have made and details of the studies we have identified in preparing the review, we remain convinced that the conclusions drawn from the original version of the review remain valid: We have not demonstrated a protective effect of selenium against cancer in men, women or children.
Contributors
Marco Vinceti, Maree Brinkman, Gabriele Dennert and Marcel Zwahlen on behalf of the review authors.
Acknowledgements
We thank the CGCG Editorial Team for their advice, in particular, Jane Hayes for designing the search strategy and Clare Jess for her contribution to the editorial process.
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Gynaecological Cancer Group. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the NIHR, the NHS or the Department of Health.
Appendices
Appendix 1. Electronic search strategies
| Database | Date of most recent literature search | Search strategy | Comment |
| www.cancer.gov | 4 Feb 2011 | medication: selenium indication: prevention | |
| Cancerlit | Oct 2004 | 1 selen* OR organoselen* OR natriumselen* 2 random* OR placebo* OR clinical trial* OR controlled trial* OR controlled clinical trial* OR double blind* OR single blind* 3 epidemiologic stud* OR cohort OR case‐control stud* OR nested case‐control* OR case‐control design* OR prospectiv* 4 2 OR 3 5 1 AND 4 | Now included in MEDLINE database |
| Clinical Contents in Medicine (CCMed) | 4 Feb 2011 | selen* OR organoselen* OR natriumselen* | |
| CENTRAL | 2013, Issue 1 | #1 MeSH descriptor: [Selenium] this term only #2 MeSH descriptor: [Selenium Compounds] explode all trees #3 MeSH descriptor: [Organoselenium Compounds] explode all trees #4 selen* #5 #1 or #2 or #3 or #4 #6 MeSH descriptor: [Neoplasms] explode all trees #7 (neoplasm* or cancer* or tumor* or tumour* or carcino* or malignan* or adenocarcinoma* or sarcoma* or adenoma* or chondrosarcoma* or fibrosarcoma* or dermatofibrosarcoma* or neurofibrosarcoma* or hemangiosarcoma* or leiomyosarcoma* or liposarcoma* or myosarcoma* or rhabdomyosarcoma* or myxosarcoma* or osteosarcoma* or lymphoma*) #8 #6 or #7 #9 #5 and #8 |
|
| metaRegister of Controlled Trials (mRCT, www.controlled‐trials.com) | 4 Feb 2011 | selen AND cancer | |
| EMBASE Ovid | 2013 week 6 | 1 selenium/ 2 selen*.mp. 3 selenium derivative/ 4 methylseleninic acid/ 5 methylselenium.mp. 6 exp organoselenium derivative/ 7 1 or 2 or 3 or 4 or 5 or 6 8 exp neoplasm/ 9 (neoplasm* or cancer* or tumor* or tumour* or carcino* or malignan* or adenocarcinoma* or sarcoma* or adenoma* or chondrosarcoma* or fibrosarcoma* or dermatofibrosarcoma* or neurofibrosarcoma* or hemangiosarcoma* or leiomyosarcoma* or liposarcoma* or myosarcoma* or rhabdomyosarcoma* or myxosarcoma* or osteosarcoma* or lymphoma*).mp. 10 8 or 9 11 7 and 10 12 exp clinical study/ 13 crossover procedure/ 14 double‐blind procedure/ 15 single‐blind procedure/ 16 cohort analysis/ 17 observational study/ 18 (random* or factorial* or crossover* or cross‐over* or cross over* or placebo* or (double adj blind*) or (singl* adj blind*) or assign* or allocat* or volunteer* or observ* or cohort* or prospectiv* or (case* and control*)).mp. 19 12 or 13 or 14 or 15 or 16 or 17 or 18 20 11 and 19 21 (exp animal/ or nonhuman/ or exp animal experiment/) not human/ 22 20 not 21 key: [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] |
|
| German Cancer Study Register: www.studien.de |
4 Feb 2011 | selen | |
| MEDLINE (via Ovid) | Feb 2013 week 1 | 1 Selenium/ 2 exp Selenium Compounds/ 3 exp Organoselenium Compounds/ 4 selen*.mp. 5 1 or 2 or 3 or 4 6 exp Neoplasms/ 7 (neoplasm* or cancer* or tumor* or tumour* or carcino* or malignan* or adenocarcinoma* or sarcoma* or adenoma* or chondrosarcoma* or fibrosarcoma* or dermatofibrosarcoma* or neurofibrosarcoma* or hemangiosarcoma* or leiomyosarcoma* or liposarcoma* or myosarcoma* or rhabdomyosarcoma* or myxosarcoma* or osteosarcoma* or lymphoma*).mp. 8 6 or 7 9 5 and 8 10 randomized controlled trial.pt. 11 controlled clinical trial.pt. 12 randomized.ab. 13 placebo.ab. 14 drug therapy.fs. 15 randomly.ab. 16 trial.ab. 17 groups.ab. 18 exp case‐control studies/ 19 exp Cohort Studies/ 20 (cohort* or observ* or prospectiv* or (case* and control*)).mp. 21 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 22 9 and 21 23 exp animals/ not humans.sh. 24 22 not 23 key: mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier pt=publication type ab=abstract fs=floating subheading |
|
| SIGLE | Oct 2004 | ?selen? | database discontinued in 2005 |
Appendix 2. Newcastle‐Ottawa Scale for Cohort Studies
((*) means that a 'star' was assigned to the study for the corresponding item)
1) Selection
1.1) representativeness of the exposed cohort a) truly representative of the average _____________ (target population) in the community (*) b) somewhat representative of the average _____________ (target population) in the community (*) c) selected group of users, e.g. volunteers / nurses d) no description of the derivation of the cohort 1.2) selection of the non‐exposed cohort a) drawn from the same community as the exposed cohort (*) b) drawn from a different source c) no description 1.3) ascertainment of selenium exposure a) secure record (biochemical records) (*) b) structured interview (*) c) written self report or medical record only d) no description 1.4) demonstration that outcome of interest was not present at start of study a) no history of disease or exclusion of cases that occurred in the first 12 months (*) b) not stated
2) Comparability
2.1.) comparability of cohorts on the basis of the design or analysis a) study controls for AGE (*) b) study controls for SMOKING (*)
3) Outcome
3.1) assessment of outcome a) independent blind validation (> 1 person/record/time/process to extract information or reference to primary source such as X‐rays/hospital records) (*) b) record linkage (e.g. ICD codes in databases) (*) c) self report d) no description 3.2) Was follow‐up long enough for outcomes to occur? a) yes (> 3 years) b) no 3.3) adequacy of follow up of cohorts a) complete follow‐up of all subjects (*) OR b) subjects lost to follow‐up unlikely to introduce bias (< 5% lost to follow‐up or description provided of lost people) (*) c) follow‐up‐rate < 95% and no description of those lost d) no statement
Appendix 3. Additional Newcastle‐Ottawa Scale for Nested Case‐Control Studies
((*) means that a 'star' was assigned to the study for the corresponding item)
1) Selection
1.1) case definition a) independent validation (> 1 person/record/time/process to extract information or reference to primary source such as X‐rays/hospital records) (*) b) record linkage (e.g. ICD codes in databases) or self‐report with no reference to primary record c) no description 1.2) representativeness of cases: a) all eligible cases with outcome of interest over a defined period, cases in a defined catchment area/hospital etc. or an appropriate/random sample of those cases (*) b) not satisfying requirements in part (a) or not stated 1.3) selection of controls: a) community controls (same community and would be cases if had outcome) (*) b) hospital controls (within the same population e.g. city as cases) c) no description 1.4) definition of controls a) cases had no history of outcome controls had no history of outcome OR case had new (not necessarily first) occurrence of outcome controls with previous occurrence of outcome should not be excluded (*) b) no mention of history of outcome
2) Comparability
(validated in cohort assessment in question 2 ‐ number of stars was copied)
3) Exposure
3.1) ascertainment of selenium exposure: (validated in cohort assessment in question 1.3 ‐ number of stars was copied) 3.2) Same method of ascertainment for cases and controls a) yes (*) b) no 3.3) non‐response rate a) same rate for both groups (*) b) non‐respondents described c) rate different and no designation
Data and analyses
Comparison 1.
Observational studies: highest versus lowest selenium exposure
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total cancer incidence and mortality | 14 | Odds Ratio (Random, 95% CI) | Subtotals only | |
| 1.1 Incidence | 8 | Odds Ratio (Random, 95% CI) | 0.69 [0.53, 0.91] | |
| 1.2 Mortality | 6 | Odds Ratio (Random, 95% CI) | 0.60 [0.39, 0.93] | |
| 2 Total cancer incidence and mortality (men) | 8 | Odds Ratio (Random, 95% CI) | Subtotals only | |
| 2.1 Incidence | 5 | Odds Ratio (Random, 95% CI) | 0.66 [0.42, 1.05] | |
| 2.2 Mortality | 3 | Odds Ratio (Random, 95% CI) | 0.56 [0.38, 0.81] | |
| 3 Total cancer incidence and mortality (women) | 5 | Odds Ratio (Random, 95% CI) | Subtotals only | |
| 3.1 Incidence | 2 | Odds Ratio (Random, 95% CI) | 0.90 [0.45, 1.77] | |
| 3.2 Mortality | 3 | Odds Ratio (Random, 95% CI) | 0.92 [0.79, 1.07] | |
| 4 Total cancer incidence and mortality (ascending order of selenium levels) | 12 | Odds Ratio (Random, 95% CI) | Subtotals only | |
| 4.1 Incidence | 6 | 1297 | Odds Ratio (Random, 95% CI) | 0.69 [0.52, 0.91] |
| 4.2 Mortality | 6 | 1041 | Odds Ratio (Random, 95% CI) | 0.60 [0.39, 0.93] |
| 5 Breast cancer risk (women) | 7 | Odds Ratio (Random, 95% CI) | 1.00 [0.77, 1.29] | |
| 5.1 Breast cancer (all) | 6 | Odds Ratio (Random, 95% CI) | 1.01 [0.74, 1.36] | |
| 5.2 Breast cancer (premenopausal) | 1 | Odds Ratio (Random, 95% CI) | 1.10 [0.46, 2.65] | |
| 6 Bladder cancer risk | 5 | Odds Ratio (Random, 95% CI) | 0.67 [0.46, 0.97] | |
| 6.1 All (male + female) | 2 | Odds Ratio (Random, 95% CI) | 0.65 [0.46, 0.92] | |
| 6.2 Male | 3 | Odds Ratio (Random, 95% CI) | 0.82 [0.41, 1.62] | |
| 6.3 Female | 1 | Odds Ratio (Random, 95% CI) | 0.36 [0.14, 0.92] | |
| 7 Lung cancer risk (gender‐aggregated data) | 12 | Odds Ratio (Random, 95% CI) | 0.83 [0.61, 1.13] | |
| 7.1 Incidence | 10 | Odds Ratio (Random, 95% CI) | 0.75 [0.54, 1.03] | |
| 7.2 Mortality | 2 | Odds Ratio (Random, 95% CI) | 1.34 [0.93, 1.93] | |
| 8 Lung cancer risk (gender‐disaggregated data) | 12 | Odds Ratio (Random, 95% CI) | 0.84 [0.65, 1.09] | |
| 8.1 All (female + male) | 4 | Odds Ratio (Random, 95% CI) | 0.58 [0.39, 0.86] | |
| 8.2 Female | 4 | Odds Ratio (Random, 95% CI) | 0.83 [0.43, 1.61] | |
| 8.3 Male | 7 | Odds Ratio (Random, 95% CI) | 0.98 [0.68, 1.39] | |
| 9 Lung cancer risk | 12 | Odds Ratio (Random, 95% CI) | 0.83 [0.61, 1.13] | |
| 9.1 Intake | 1 | Odds Ratio (Random, 95% CI) | 0.98 [0.41, 2.35] | |
| 9.2 Serum or plasma | 9 | Odds Ratio (Random, 95% CI) | 0.91 [0.70, 1.18] | |
| 9.3 Toenail | 2 | Odds Ratio (Random, 95% CI) | 1.05 [0.11, 10.36] | |
| 10 Lung cancer risk (ascending order of selenium levels) | 8 | 1867 | Odds Ratio (Random, 95% CI) | 0.97 [0.74, 1.27] |
| 11 Prostate cancer risk | 17 | Odds Ratio (Random, 95% CI) | 0.79 [0.69, 0.90] | |
| 12 Prostate cancer risk (by selenium measurement) | 17 | Odds Ratio (Random, 95% CI) | 0.79 [0.69, 0.90] | |
| 12.1 Biochemical selenium level | 15 | Odds Ratio (Random, 95% CI) | 0.76 [0.67, 0.88] | |
| 12.2 Estimated selenium intake | 2 | Odds Ratio (Random, 95% CI) | 1.00 [0.73, 1.36] | |
| 13 Prostate cancer risk (by exposure assessment) | 17 | Odds Ratio (Random, 95% CI) | 0.79 [0.69, 0.90] | |
| 13.1 Intake | 2 | Odds Ratio (Random, 95% CI) | 1.00 [0.73, 1.36] | |
| 13.2 Serum or plasma | 12 | Odds Ratio (Random, 95% CI) | 0.82 [0.72, 0.93] | |
| 13.3 Toenail | 3 | Odds Ratio (Random, 95% CI) | 0.53 [0.35, 0.81] | |
| 14 Prostate cancer risk (by continent) | 17 | Odds Ratio (Random, 95% CI) | 0.79 [0.69, 0.90] | |
| 14.1 Europe | 6 | Odds Ratio (Random, 95% CI) | 0.86 [0.73, 1.02] | |
| 14.2 North America | 11 | Odds Ratio (Random, 95% CI) | 0.73 [0.60, 0.88] | |
| 15 Prostate cancer risk (by country) | 17 | Odds Ratio (Random, 95% CI) | 0.79 [0.69, 0.90] | |
| 15.1 Several European countries | 3 | Odds Ratio (Random, 95% CI) | 0.87 [0.71, 1.07] | |
| 15.2 Finland | 2 | Odds Ratio (Random, 95% CI) | 1.24 [0.75, 2.05] | |
| 15.3 The Netherlands | 1 | Odds Ratio (Random, 95% CI) | 0.69 [0.48, 0.99] | |
| 15.4 US | 11 | Odds Ratio (Random, 95% CI) | 0.73 [0.60, 0.88] | |
| 16 Prostate cancer risk (ascending order of selenium levels) | 12 | 2982 | Odds Ratio (Random, 95% CI) | 0.82 [0.72, 0.93] |
| 17 Stomach cancer risk | 5 | Odds Ratio (Random, 95% CI) | 0.66 [0.43, 1.01] | |
| 17.1 Stomach | 4 | Odds Ratio (Random, 95% CI) | 0.65 [0.35, 1.19] | |
| 17.2 Stomach: cardia cancer | 1 | Odds Ratio (Random, 95% CI) | 0.47 [0.33, 0.66] | |
| 17.3 Stomach: non‐cardia cancer | 1 | Odds Ratio (Random, 95% CI) | 1.07 [0.55, 2.08] | |
| 18 Stomach cancer risk (by gender) | 5 | Odds Ratio (Random, 95% CI) | 0.66 [0.42, 1.04] | |
| 18.1 All (female + male) | 2 | Odds Ratio (Random, 95% CI) | 0.75 [0.41, 1.36] | |
| 18.2 Female | 2 | Odds Ratio (Random, 95% CI) | 0.73 [0.12, 4.35] | |
| 18.3 Male | 3 | Odds Ratio (Random, 95% CI) | 0.43 [0.14, 1.32] | |
| 19 Colorectal cancer risk | 5 | Odds Ratio (Random, 95% CI) | 0.89 [0.65, 1.23] | |
| 19.1 Colon and rectal cancer | 2 | Odds Ratio (Random, 95% CI) | 1.11 [0.50, 2.46] | |
| 19.2 Colon cancer | 3 | Odds Ratio (Random, 95% CI) | 0.80 [0.56, 1.15] | |
| 20 Colorectal cancer risk (by gender) | 5 | Odds Ratio (Random, 95% CI) | 0.89 [0.65, 1.23] | |
| 20.1 All (female + male) | 1 | Odds Ratio (Random, 95% CI) | 1.22 [0.52, 2.86] | |
| 20.2 Female | 3 | Odds Ratio (Random, 95% CI) | 1.06 [0.57, 2.00] | |
| 20.3 Male | 3 | Odds Ratio (Random, 95% CI) | 0.69 [0.42, 1.12] |
Comparison 2.
Randomised controlled trials: highest versus lowest selenium exposure
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any cancer risk | 2 | 18698 | Risk Ratio (IV, Random, 95% CI) | 0.90 [0.70, 1.17] |
| 2 Cancer mortality | 2 | 18698 | Risk Ratio (IV, Random, 95% CI) | 0.81 [0.49, 1.32] |
| 3 Liver cancer risk | 3 | 4765 | Risk Ratio (IV, Random, 95% CI) | 0.50 [0.35, 0.71] |
| 4 Non‐melanoma skin cancer risk | 3 | Risk Ratio (Random, 95% CI) | 1.44 [0.95, 2.17] | |
| 5 Prostate cancer risk | 4 | 19110 | Risk Ratio (IV, Random, 95% CI) | 0.90 [0.71, 1.14] |
| 6 Prostate cancer risk for studies with low RoB | 3 | 18183 | Risk Ratio (IV, Random, 95% CI) | 1.02 [0.90, 1.14] |
| 7 Lung cancer risk | 2 | 18698 | Risk Ratio (IV, Random, 95% CI) | 0.94 [0.62, 1.42] |
| 8 Bladder cancer risk | 2 | 18698 | Risk Ratio (IV, Random, 95% CI) | 1.14 [0.81, 1.61] |
| 9 Colorectal cancer risk | 2 | 18698 | Risk Ratio (IV, Random, 95% CI) | 0.77 [0.37, 1.62] |
What's new
Last assessed as up‐to‐date: 15 February 2013.
| Date | Event | Description |
|---|---|---|
| 21 September 2016 | Amended | Contact details updated. |
History
Protocol first published: Issue 2, 2005 Review first published: Issue 5, 2011
| Date | Event | Description |
|---|---|---|
| 10 February 2015 | Amended | Minor edit made to feedback response |
| 3 February 2015 | Feedback has been incorporated | Feedback and author's response added |
| 18 March 2014 | New citation required but conclusions have not changed | New trials added. Meta‐analysis of data from RCTs was applied when at least two studies were available for each outcome |
| 15 February 2013 | New search has been performed | Search strategy updated |
| 9 January 2013 | Amended | Authors' list changed |
| 14 August 2012 | Feedback has been incorporated | Additional feedback and author response incorporated. |
| 8 March 2012 | Feedback has been incorporated | Feedback submitted and author's reply added. |
| 6 December 2011 | Amended | Sources of support amended. |
| 6 July 2011 | Amended | Search dates added to abstract. |
Differences between protocol and review
In the previous Cochrane review, the risk of bias assessment for RCTs, which was introduced by The Cochrane Collaboration after publication of our protocol, was adapted; the Jadad score and the Delphi list were also used to assess the quality of RCTs, but because the results of these checklist assessments were of no relevance for this review, they have been omitted.
With respect to the protocol, in this updated review, we decided to perform meta‐analysis of RCTs when at least two studies were available, and to emphasise the analysis conducted for all RCTs and for RCTs at low risk of bias to highlight the most reliable and recent evidence on the selenium and cancer relation, which comes from well‐designed experimental studies. As in the previous version of the review, we included in our analysis both primary and secondary outcomes of the RCTs.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Agalliu 2011
| Methods | Nested case‐cohort study Country: Canada |
|
| Participants |
Name of parent cohort: Canadian Study of Diet, Lifestyle and Health (CSDLH) Participants: 22.975 participants (alumni associations of the University of Western Ontario, 67% of 34.291) Recruitment: between 1995 and 1998 Outcome assessment: December 2003 Number of cases: Prostate cancer: 661 Case definition: incidence Years of follow‐up: 4.3 to 7.7 years mean Type of selenium marker: supplementation |
|
| Interventions | d.n.a. | |
| Outcomes | Statistical methods: Cox proportional hazard model Variables controlled in analysis: age at baseline, race, BMI, exercise activity, and education | |
| Risk estimates [95% CI] |
Reference category: zero Results: Prostate cancer highest quartile: HR 0.76 (95% CI 0.43 to 1.33) |
|
| Selenium levels in exposure categories | lowest quartile (median value): 15.7 µg highest quartile (median value): 105.0 µg | |
| Notes | ||
Akbaraly 2005
| Methods | Cohort/sub‐cohort controlled cohort study Country: France |
|
| Participants |
Name of parent cohort: Etude du Vieillissement Antériel Study (EVA study) Participants: 1389 participants (41% male, 59% female) Inclusion criteria: 59 to 71 years of age; residents of Nantes; able to undergo examination at study centre Recruitment: 1991 to 1993 Outcome assessment: December 2001 Number of cases: Any cancer: 45 (male/female: n.r.) Case definition: mortality Years of follow‐up: 9.0 years Type of selenium marker: plasma |
|
| Interventions | d.n.a. | |
| Outcomes | Statistical methods: Cox proportional hazard model Variables controlled in analysis: gender, smoking, alcohol intake, medication use, obesity, diabetes mellitus, hypertension, CVD, age, education, dyslipidaemia, low cognitive function | |
| Risk estimates [95% CI] |
Reference category: highest quartile Results: Any cancer both genders: lowest quartile: RR 4.06 (95% CI 1.51 to 10.92) |
|
| Selenium levels in exposure categories | lowest quartile: 0.18 to 0.95 µmol/l highest quartile: 1.22 to 1.97 µmol/l | |
| Notes | ||
Algotar 2013
| Methods | Randomised controlled trial Allocation: random Sequence generation: unclear Concealment: The study agent (two doses) and matched placebo caplets were coated with titanium oxide to ensure identical appearance, weight, taste, and smell. Blinding: only described as double‐blinded Dropouts/withdrawals: study dropouts percentage was 34.1%, 41.9%, and 40.8% for placebo, 200 mg/ day selenium group and 400 mg/day selenium group respectively (P=0.173). Intention‐to‐treat‐analysis: yes Recruitment period: not specified Treatment duration: not specified Observation period/dermatologic follow‐up: Subjects were followed every 6 months for up to 5 years Detection of cases: Tissue samples from the subject’s qualifying biopsy were requested from the subject’s physician and compiled in a biospecimen repository Informed consent: An external Data and Safety Monitoring Committee (DSMC) was established before study initiation.This committee was responsible for reviewing protocol amendments, consent forms, accrual and retention rates, adverse events, and data analysis reports |
|
| Participants | 699 male participants with a negative prostate biopsy Country: US and NZ Number of patients: 699 (randomised to selenium 200 ug/day group: 234, to selenium 400 ug/day group: 233; to placebo group: 233) Condition: male patients at high risk for prostate cancer (prostate specific antigen (PSA) >4 ng/ml and/or suspicious digital rectal examination and/or PSA velocity >0.75 ng/ml/year), but with a negative prostate biopsy Demographics: mean age 65.2± SD 8 years (selenium 200ug/day), 65.5±7.7 years (selenium 400ug/day), 65.5±7.4 years (placebo); Recruitment and setting: from urology offices at 20 sites in the United States and New Zealand |
|
| Interventions |
Intervention: 200 µg/day selenium supplied as selenium yeast 400 µg/day selenium supplied as selenium yeast Control: placebo Recruitment: not reported End of the blinded treatment period: For subjects in the US, participation was complete at 5 years, whereas subjects in New Zealand received intervention for no more than 3 years. |
|
| Outcomes |
Primary outcome measure: the incidence of biopsyproven prostate cancer over the course of the study. Other reported outcomes: The secondary endpoint was the rate of change of PSA over time (i.e., PSA velocity) using biannual PSA measurements. |
|
| Risk estimates [95% CI] |
Primary outcomes: The hazard ratios [95% confidence intervals] for risk of developing prostate cancer in the selenium 200 mg/day or the selenium 400 mg/day group were 0.94 [0.52, 1.7] and 0.90 [0.48, 1.7], respectively. Other reported outcomes: PSA velocity in the selenium arms was not significantly different from that observed in the placebo group (P= 0.18 and P = 0.17, respectively) |
|
| Selenium levels in exposure categories | d.n.a. | |
| Notes | The DSMC recommended that the trial be stopped before all participants completed the full intervention duration Adverse effects: No significant differences were seen in the incidences of cataract/glaucoma or in hair/nail changes in the three treatment groups HR: adjusted for: age at baseline, baseline PSA, baseline selenium concentrations. |
|
Allen 2008
| Methods | Matched, nested case‐control study Countries: Denmark, Germany, Greece, Italy, the Netherlands, Spain, Sweden, the UK |
|
| Participants |
Participants: approximately 130,000 men Inclusion criteria: male participants of the EPIC study Name of parent cohort: European Prospective Investigation into Cancer and Nutrition (EPIC) Recruitment: 1992 to 2000 Outcome assessment: at each country's study closure date (between June 1999 and January 2003) Number of cases: Prostate cancer: 959 (male/female: 959/0) Case definition: incidence Years of follow‐up: median 2.6 years (Greece) to 9.2 years (Sweden) Type of selenium marker: plasma |
|
| Interventions | d.n.a. | |
| Outcomes |
Statistical methods: conditional logistic regression Variables controlled in analysis: BMI, smoking, alcohol consumption, physical activity, marital status, education Variables controlled by matching: age, study centre, time of day of blood collection, time between blood collection and last meal, sex |
|
| Risk estimates [95% CI] |
Reference category: lowest quintile Results: Prostate cancer highest quintile: OR 0.96 (95% CI 0.70 to 1.31) |
|
| Selenium levels in exposure categories | lowest quintile < 62.0 µg/l highest quintile ≥ 84.1 µg/l |
|
| Notes | ||
Bates 2011
| Methods | Cohort Study Country: UK |
|
| Participants |
Participants: 1,054 men and women Inclusion criteria: people aged 65 years and over Name of parent cohort: British National Diet and Nutrition Survey Recruitment: 1994 to 1995 Outcome assessment: September 2008 Number of cases: Cancer deaths: 140 Case definition: mortality Type of selenium marker: plasma concentration |
|
| Interventions | d.n.a. | |
| Outcomes | Statistical methods: Cox proportional hazard regression Variables controlled in analysis: age, sex, a1‐antichymotrypsin (an acute‐phase indicator), plasma creatinine (a renal status indicator), plasma total and HDL‐cholesterol concentrations and plasma albumin concentration. | |
| Risk estimates [95% CI] |
Cancer deaths HR 0.72; 95 % CI 0.58 to 0.89 |
|
| Selenium levels in exposure categories | plasma concentrations | |
| Notes | ||
Bleys 2008
| Methods | Cohort Study Country: US |
|
| Participants |
Participants: 13,887 men and women Inclusion criteria: male and female adults, aged 20 to 90 years, participating in the NHANES III: "stratified, multistage probability cluster to provide data representing the noninstitutionalized US population" (Bleys 2008, p. 404) Name of parent cohort: Third National Health and Nutrition Examination Survey (NHANES III) Recruitment: 1988 to 1994 Outcome assessment: 15 December 2000 Number of cases: Cancer deaths: 457 (male/female: n.r.) Case definition: mortality Years of follow‐up: 6 to 12 years Type of selenium marker: serum |
|
| Interventions | d.n.a. | |
| Outcomes | Statistical methods: Cox proportional hazard regression Variables controlled in analysis: age, sex, race, education, annual family income, post‐menopausal status (women), cigarette smoking, serum cotinine level, alcohol consumption | |
| Risk estimates [95% CI] |
Reference category: lowest tertile Results: Cancer deaths both genders: highest tertile: HR 0.69 (95% CI 0.53 to 0.90) both genders: highest tertile: HR 0.68 (95% CI 0.48 to 0.97); cases at baseline were excluded |
|
| Selenium levels in exposure categories | lowest tertile < 117.31 ng/ml highest tertile ≥ 130.39 ng/ml | |
| Notes | ||
Brooks 2001
| Methods | Matched, nested case‐control study Country: US |
|
| Participants |
Name of parent cohort: Baltimore Longitudinal Study of Aging Participants: 1555 men Inclusion criteria: n.r. Recruitment: n.r. Outcome assessment: n.r. Number of cases: prostate cancer: 52 (male/female: 52/0) Case definition: incidence Years of follow‐up: n.r. Type of selenium marker: plasma |
|
| Interventions | d.n.a. | |
| Outcomes | Analysed cases: analysis for 52 of 133 cases (reason for non‐inclusion: plasma and/or histological confirmation of diagnosis not available) Statistical methods: logistic regression Variables controlled in analysis: years between blood donation and diagnosis/follow‐up, age, age by years before diagnosis interaction, BMI, smoking history, alcohol use Variables controlled by matching: age | |
| Risk estimates [95% CI] |
Reference category: lowest quartile Results: Prostate cancer highest quartile: OR 0.24 (95% CI 0.07 to 0.77) |
|
| Selenium levels in exposure categories | lowest quartile: 8.20 to 10.70 µg/dl highest quartile: 13.30 to 18.20 µg/dl | |
| Notes | ||
Clark 1985
| Methods | Cohort/sub‐cohort‐controlled cohort study Country: US |
|
| Participants |
Participants: 177 participants; no information on gender Inclusion criteria: persons at high risk of non‐melanoma skin cancer Recruitment: n.r. Outcome assessment: n.r. Number of cases: skin (non‐melanoma): 19 (male/female: n.r.) Case definition: incidence Years of follow‐up: mean: 3.0 years Type of selenium marker: plasma |
|
| Interventions | d.n.a. | |
| Outcomes | Statistical methods: Cox proportional hazard model | |
| Risk estimates [95% CI] |
Reference category: lower half Results: Skin (non‐melanoma) gender n.r.: higher half: RR 0.77 (CI not reported) |
|
| Selenium levels in exposure categories | n.r. | |
| Notes | ||
Coates 1988
| Methods | Matched, nested case‐control study Country: US |
|
| Participants |
Participants: number of participants n.r.; both genders Inclusion criteria: employees of two Seattle companies Recruitment: 1972 to 1973 and 1976 Outcome assessment: not stated Number of cases: Any cancer: 154 (male/female: n.r.) Gastrointestinal cancer: 28 (male/female: n.r.) Breast cancer: 20 (male/female: 0/20) Prostate cancer: 13 (male/female: 13/0) Haematological cancers: 12 (male/female: n.r.) Cervical cancer: 12 (male/female: 0/12) Lung cancer: 11 (male/female: n.r.) Other: 58 (male/female: n.r.) Case definition: incidence Years of follow‐up: n.r. Type of selenium marker: serum and plasma |
|
| Interventions | d.n.a. | |
| Outcomes | Analysed cases: 154 (133 serum, 21 plasma) of 195 cases analysed (reason for non‐inclusion: no sample available for analysis or no control available) Statistical methods: conditional logistic regression Variables controlled by matching: age, gender, race/ethnicity, year/month of sample collection, employer, plasma or serum sample | |
| Risk estimates [95% CI] |
Reference category: lowest Results: Any cancer both genders: highest quintile: OR 1.0 (95% CI 0.5 to 1.8) Gastrointestinal cancer both genders: highest tertile: OR 1.0 (CI not reported) Breast cancer highest tertile: OR 3.4 (CI not reported) Prostate cancer highest tertile: OR 0.3 (CI not reported) Haematological cancers both genders: highest tertile: OR 0.6 (CI not reported) Cervical cancer highest tertile: OR 1.1 (CI not reported) Lung cancer both genders: highest tertile: OR 0.8 (CI not reported) Other cancers both genders: highest tertile: OR 0.9 (CI not reported) |
|
| Selenium levels in exposure categories | serum: lowest quintile: 98 to 142 µg/l highest quintile: 181 to 240 µg/l lowest tertile: 98 to 148 µg/l highest tertile: 171 to 240 µg/l plasma: lowest quintile: 115 to 129 µg/l highest quintile: 157 to 207 µg/l lowest tertile: 115 to 137 µg/l highest tertile: 151 to 207 µg/l |
|
| Notes | Primary publication: Coates 1988 Secondary publication: Coates 1987 | |
Combs 1993
| Methods | Cohort/sub‐cohort‐controlled cohort study Country: US |
|
| Participants |
Participants: 1239 men and women Inclusion criteria: participants of the NPCT with valid selenium measurement at baseline Name of parent cohort: Nutritional Prevention of Cancer Trial (NPCT) Recruitment: see: Nutritional Prevention of Cancer Trial Outcome assessment: not stated Number of cases: Squamous cell cancer: 204 (male/female: n.r.) Case definition: incidence Years of follow‐up: 2.0 years Type of selenium marker: plasma |
|
| Interventions | d.n.a. | |
| Outcomes | Statistical methods: Cox proportional hazard model Variables controlled in analysis: age, gender, current smoking, alcohol drinking | |
| Risk estimates [95% CI] |
Reference category (unadjusted RR): lower half Results: Squamous cell cancer both genders: higher half: unadjusted RR 0.69 (95% CI 0.51 to 0.92) both genders: "interquartile contrast" (high versus low), adjusted RR 0.79 (95% CI 0.67 to 0.94) |
|
| Selenium levels in exposure categories | lower half: ≤ 114.00 µg/l higher half: ≥ 114.10 µg/l | |
| Notes | ||
Comstock 1997
| Methods | Matched, nested case‐control study Country: US |
|
| Participants |
Participants: number of participants n.r.; both genders Inclusion criteria: residents of Washington County Name of parent cohort: CLUE I and II Cohort Recruitment: 1974/75 or 1989 Outcome assessment: n.r. Number of cases: Lung cancer: 258 (male/female: 157/101) Case definition: incidence Years of follow‐up: n.r. Type of selenium marker: serum/plasma |
|
| Interventions | d.n.a. | |
| Outcomes | Statistical methods: conditional logistic regression Variables controlled by matching: age, gender, race/ethnicity, year and month of sample collection, participant of Clue I or Clue II cohort | |
| Risk estimates [95% CI] |
Reference category: lowest quintile Results: Lung cancer both genders: highest quintile: OR 0.65 (CI not reported) |
|
| Selenium levels in exposure categories | n.r. | |
| Notes | ||
Dong 2008
| Methods | Cohort study Country: US |
|
| Participants |
Participants: 339 participants (275 men; 64 women) Inclusion criteria: participants of a surveillance programme for men and women with Barrett's oesophagus, no prior history of oesophageal cancer or diagnosis of cancer within first three months of baseline Name of parent cohort: Seattle Barrett's Esophagus Program Recruitment: 1983 to 2004, baseline assessment for this study: 1 February 1995 to 1 July 2004 Outcome assessment: n.r. Number of cases: oesophageal adenocarcinoma: 37 (32 men, 5 women) Case definition: incidence Years of follow‐up: mean: 5 years Type of selenium marker: intake of selenium supplements (self administered food frequency questionnaire) |
|
| Interventions | d.n.a. | |
| Outcomes | Statistical methods: Cox proportional hazards regression Variables controlled in analysis: age, sex, fruit and vegetable consumption, percent energy from fat, waist‐hip ratio, cigarette smoking, non‐steroidal anti‐inflammatory drug use | |
| Risk estimates [95% CI] |
Reference category: no supplemental selenium intake (lowest exposure category) Results: both genders: supplement intake ≥ 50 µg/day: HR 0.27 (95% CI 0.03 to 2.21) |
|
| Selenium levels in exposure categories | lowest category: no supplemental selenium intake middle category: supplemental selenium intake < 50 µg/day highest category: supplemental intake ≥ 50 µg/day |
|
| Notes | ||
Dorgan 1998
| Methods | Matched, nested case‐control study Country: US |
|
| Participants |
Participants: 6426 women Inclusion criteria: female volunteers with serum available at the Breast Cancer Serum Bank in Columbia (Missouri)/U.S.A; no history of cancer at baseline; missing serum sample for analysis excluded Recruitment: 1987 to 1997 Outcome assessment: 1982 to 1983, 1989 Number of cases: Breast cancer: 105 (male/female: 0/105) Case definition: incidence Years of follow‐up: median: 2.7 years Type of selenium marker: serum |
|
| Interventions | d.n.a. | |
| Outcomes | Statistical methods: conditional logistic regression Variables controlled in analysis: serum cholesterol, packs of cigarettes / day, BMI Variables controlled by matching: age, year and month of sample collection, diagnosis of benign breast disease within two years prior to study enrolment, "sequence number of blood draw" for women who donate blood more than one time | |
| Risk estimates [95% CI] |
Reference category: lowest quartile Results: Breast cancer highest quartile: OR 0.9 (95% CI 0.4 to 1.8) |
|
| Selenium levels in exposure categories | lowest quartile: ≤ 1.43 µmol/l highest quartile: 1.67 to 1.98 µmol/l | |
| Notes | ||
Dreno 2007
| Methods | Multicentre, randomised, placebo‐controlled, parallel‐group trial Allocation: random Sequence generation: unclear Concealment: unclear Blinding: only described as double‐blinded Dropouts/withdrawals: During the treatment phase, 38 in the selenium group and 37 in the placebo group withdrew from the study. This distribution was similar in both treatment groups. Intention‐to‐treat‐analysis: unclear Recruitment period: not specified Treatment duration: 3 years of treatment Observation period/dermatologic follow‐up: Subjects were followed for 2 years more after treatment Detection of cases: Patients were seen by a dermatologist before grafting; and any patients presenting with a non‐malignant or malignant skin keratosis or viral warts that had been present for less than 3 months were not selected. Within 10 weeks following the graft, a second visit was performed by a dermatologist to check that no new cutaneous lesion had appeared. Informed consent:The protocol and consent form had been approved by a National Ethics Committee prior to starting the study. Written informed consent was mandatory. |
|
| Participants | 184 participants Number of patients: 184 (randomised to selenium 200 ug/day group: 91, to placebo group: 93) Condition: organ transplant recipient population Demographics: mean age 44.3± SD 13 years (selenium 200ug/day), 44.4± 10.7 years (placebo); |
|
| Interventions |
Intervention: 200 µg/day selenium supplied as selenium yeast Control: placebo |
|
| Outcomes |
Primary outcome measure: Occurrence rates of warts and various keratoses Other reported outcomes: skin cancers |
|
| Risk estimates [95% CI] | Primary outcome: events in selenium group=33 (36.3%), events in placebo group=31 (33.3%); odds‐ratio 1.09, P = 0.72 Secondary outcome: events in selenium group=6 (6.6%), events in placebo group=2 (2.2%); odds‐ratio 3.08, P = 0.15 |
|
| Selenium levels in exposure categories | ||
| Notes | ||
Epplein 2009
| Methods | Matched, nested case‐control study (Epplein 2009, Gill 2009) Country: US |
|
| Participants |
Inclusion criteria: participants of the Multiethnic Cohort, aged 45 to 75 years (native Hawaiians: aged 42 years and older), blood sample provided before cancer diagnosis between 1997 and 2006 Name of parent cohort: Multiethnic Cohort Recruitment: 1993 to 1996 Case definition: incidence Type of selenium marker: serum Epplein 2009: Participants: 67,594 (male: 29,009 / female: 38,585) men and women Outcome assessment: 2006 Number of cases: Lung cancer: 207 (male/female: 136/71) Years of follow‐up: 0 to 10 years Gill 2009: Participants: 29,009 men Outcome assessment: n.r. Number of cases: Prostate cancer: 467 (male/female: 467/0) Years of follow‐up: n.r. |
|
| Interventions | d.n.a. | |
| Outcomes |
Statistical methods: conditional logistic regression Epplein 2009: Variables controlled in analysis: age, fasting hours, pack‐years, pack‐years squared, years of schooling, family history of lung cancer Variables controlled by matching: age, sex, race/ethnicity, date of sample collection, time of day of sample collection, fasting status, smoking Gill 2009: Analysed cases: 450 of 467 cases analysed Variables controlled in analysis: age, fasting hours, BMI, family history of prostate cancer, education Variables controlled by matching: age, race/ethnicity, date of sample collection, geographic site (California, Hawaii), time of day of sample collection, fasting status |
|
| Risk estimates [95% CI] |
Epplein 2009:
Reference category: lowest tertile Results: Lung cancer male: highest tertile: OR 0.70 (95% CI 0.37 to 1.33) female: highest tertile: OR 0.98 (95% CI 0.42 to 2.29) Gill 2009: Reference category: lowest quartile Results: Prostate cancer highest quartile: OR 0.82 (95% CI 0.59 to 1.14) |
|
| Selenium levels in exposure categories |
Epplein 2009: lowest tertile: median 0.12 µg/g of sodium highest tertile: median 0.15 µg/g of sodium Gill 2009: lowest quartile: median 0.12 µg/g highest quartile: median 0.16 µg/g |
|
| Notes | Primary publication: Epplein 2009 Other publications: Gill 2009 | |
Fex 1987
| Methods | Matched, nested case‐control study Country: Sweden |
|
| Participants |
Participants: 7935 men Inclusion criteria: 46 to 48 years of age; residents of Malmo/Sweden; no restriction regarding malignant disease at baseline (11 of 35 cases were diagnosed with cancer at baseline screening examination and/or died during first year of follow‐up) Name of parent cohort: Malmo Preventive Programme Recruitment: 1975 to 1979 Outcome assessment: June 1981 Number of cases: Any cancer: 35 (male/female: 35/0) Case definition: mortality Years of follow‐up: 3.5 to 8.0 years Type of selenium marker: plasma |
|
| Interventions | d.n.a. | |
| Outcomes | Analysed cases: 35 of 61 cases analysed (reason for non‐inclusion: no plasma sample available) Statistical methods: logistic regression, Mantel‐Haenszel Variables controlled by matching: age, month of sample collection | |
| Risk estimates [95% CI] |
Reference category: highest quintile Results: Any cancer male: lowest quintiles: OR 3.8 (CI not reported) |
|
| Selenium levels in exposure categories | n.r. | |
| Notes | ||
Fujishima 2011
| Methods | Prospective cohort study Country: northern part of Japan |
|
| Participants |
Participants: 1,041 men and women Inclusion criteria: adult haemodialysis patients Name of parent cohort: ‘Kaleidoscopic Approaches to patients with end‐stage RENal disease Study’ (the KAREN Study) Recruitment: June 2003 to March 2004 Number of cases: malignant disease‐related death: 17 Case definition: mortality Years of follow‐up: 5‐year Type of selenium marker: serum |
|
| Interventions | d.n.a. | |
| Outcomes | Statistical methods: Cox logistic regression Variables controlled by matching: age, male gender, BMI, hypertension, dyslipidaemia, diabetes mellitus, serum albumin levels, high‐sensitivity CRPlevels, history of myocardial infarction, history of stroke, history of malignant disease, smoking status and regular drinking habit | |
| Risk estimates [95% CI] |
Reference category: lowest quartile Results: malignant disease‐related death highest quartile: HR 2.98 (95% CI 0.62 to 14.35) |
|
| Selenium levels in exposure categories | lowest quartile: 18.4‐85.3 pg/L highest quartile: 114.2‐226.2 pg/L | |
| Notes | ||
Garland 1995
| Methods | Matched, nested case‐control study Country: US |
|
| Participants |
Participants: 62,641 women Inclusion criteria: female registered nurses in 11 U.S. states; aged 30 to 55 years at baseline; completed questionnaire in 1976 and provision of toenail sample in 1982; no history of cancer at baseline Name of parent cohort: Nurses' Health Study (NHS) Recruitment: 1976 (toenail sample collection in 1982) Outcome assessment: 1 June 1986 Garland 1995: Number of cases: Any cancer (without breast): 503 (male/female: 0/503) Colon and rectal cancer: 89 (male/female: 0/89) Melanoma: 63 (male/female: 0/63) Ovarian cancer: 58 (male/female: 0/58) Lung cancer: 47 (male/female: 0/47) Other: 155 (male/female: 0/155) Uterine cancer: 91 (male/female: 0/91) Hunter 1990: Number of cases: Breast cancer: 434 (0/434) Case definition: incidence Years of follow‐up: 2.0 to 4.4 years Type of selenium marker: toenail |
|
| Interventions | d.n.a. | |
| Outcomes | Statistical methods: logistic regression, conditional logistic regression Variables controlled in analysis: smoking status Variables controlled by matching: age, year and month of sample collection Hunter 1990 additionally controlled in analysis for: age at first birth, age at menarche, alcohol use, history of benign breast disease, menopausal status, maternal breast cancer, breast cancer in sister(s), oral contraceptive use, parity, relative weight | |
| Risk estimates [95% CI] |
Reference category: lowest quintile, lowest tertile Results: Garland 1995: Any cancer (without breast) female: highest quintile: OR 1.44 (95% CI 0.97 to 2.13) Colon and rectal cancer female: highest tertile: OR 2.04 (95% CI 0.88 to 4.75) Melanoma female: highest tertile: OR 1.66 (95% CI 0.71 to 3.85) Ovarian cancer highest tertile: OR 1.22 (95% CI 0.44 to 3.38) Lung cancer female: highest tertile: OR 4.33 (95% CI 0.54 to 34.60) Other cancer female: highest tertile: OR 0.97 (95% CI 0.55 to 1.71) Uterine cancer highest tertile: OR 1.38 (95% CI 0.62 to 3.08) Hunter 1990: Breast cancer highest quintile: OR 1.10 (95% CI 0.70 to 1.72) |
|
| Selenium levels in exposure categories |
Garland 1995: lowest quintile: ≤ 0.71 µg/g highest quintile: ≥ 0.95 µg/g Hunter 1990: lowest quintile: ≤ 0.705 µg/g highest quintile: ≥ 0.906 µg/g |
|
| Notes | Primary publication: Garland 1995 Other publications: Hunter 1990 | |
Glattre 1989
| Methods | Matched, nested case‐control study Country: Norway |
|
| Participants |
Participants: 100,000 men and women Inclusion criteria: serum available at Janus serum bank (Norwegian serum bank which is consolidated from several sources and maintained by the Norwegian Cancer Society for research purposes) Recruitment: 1972 to 1985 Outcome assessment: end of 1985 Number of cases: thyroid cancer: 43 (male/female: 12/31) Case definition: incidence Years of follow‐up: 0.0 to 14.0 years Type of selenium marker: serum |
|
| Interventions | d.n.a. | |
| Outcomes | Statistical methods: conditional logistic regression Variables controlled by matching: age, gender, year of sample collection, county of residence | |
| Risk estimates [95% CI] |
Reference category: highest tertile Results: Thyroid cancer both genders: lowest tertiles: OR 7.7 (95% CI 1.3 to 44.7) men: lowest tertiles: OR 6.5 (95% CI 0.2 to 201.9) women: lowest tertiles: OR 8.3 (95% CI 0.9 to 78.5) |
|
| Selenium levels in exposure categories | lowest tertile: ≤ 1.25 µmol/l highest tertile: ≥ 1.65 µmol/l | |
| Notes | ||
Goodman 2001
| Methods | Matched, nested case‐control study Country: US |
|
| Participants |
Participants: 18,314 men and women Inclusion criteria: asbestos workers: 45 to 74 years of age; smokers > 20 pack‐years: 50 to 69 years of age; cohort of a RCT for lung cancer prevention in high risk populations Name of parent cohort: Caret (Carotene and Retinol Efficacy Trial) Recruitment: 1988 to 1994 Outcome assessment: April 1999 Number of cases: Lung cancer: 235 (male/female: n.r.) Prostate cancer: 356 (male/female: 356/0) Case definition: incidence Years of follow‐up: 6.0 to 12.0 years Type of selenium marker: serum |
|
| Interventions | d.n.a. | |
| Outcomes |
Analysed cases: 235 of 236 prostate cancer cases analysed (reason for non‐inclusion: no sample available for analysis or no control available); 356 of 385 lung cancer cases analysed (reason for non‐inclusion: missing selenium values for case‐control pairs) Statistical methods: conditional logistic regression Variables controlled by matching: age, smoking status at randomisation, year of randomisation, year of sample collection, treatment arm, exposure population |
|
| Risk estimates [95% CI] |
Reference category: lowest quartile Results: Lung cancer both genders: highest quartile: OR 1.20 (95% CI 0.77 to 1.88) male: highest quartile: OR 1.53 (95% CI 0.83 to 2.82) female: highest quartile: OR 0.76 (95% CI 0.29 to 2.01) Prostate cancer highest quartile: OR 1.02 (95% CI 0.65 to 1.60) |
|
| Selenium levels in exposure categories | Lung cancer: lowest quartile: 6.39 to 10.55 µg/dl highest quartile: 12.94 to 17.23 µg/dl Prostate cancer: lowest quartile: 5.07 to 10.12 µg/dl highest quartile: 12.60 to 21.96 µg/dl |
|
| Notes | ||
Grundmark 2011
| Methods | Cohort study Country: Swedish |
|
| Participants |
Participants: 2322 males Inclusion criteria: male residents in Uppsala county in January 1970, born in 1920‐24 Name of parent cohort: Uppsala Longitudinal Study of Adult Men (ULSAM). Recruitment: 1991 to 1995 Outcome assessment: 31/12/2003 Number of cases: Prostate cancer: 208 Case definition: incidence Years of follow‐up: 26.5‐years (median) Type of selenium marker: serum |
|
| Interventions | d.n.a. | |
| Outcomes | Statistical methods: proportional hazard model | |
| Risk estimates [95% CI] |
Reference category: lowest level Results: Prostate cancer: highest level: RR 0.83 (95% CI 0.60 to 1.16) |
|
| Selenium levels in exposure categories | lowest level: <=70 µg/l highest level: >81 µg/l | |
| Notes | ||
Hartman 1998
| Methods | Cohort/sub‐cohort‐controlled cohort study Country: Finland |
|
| Participants |
Participants: 29,133 men Inclusion criteria: 50 to 69 years of age; smokers; no history of cancer (other than non‐melanoma skin cancer) at baseline; no severe physical or psychiatric illness; intake of vitamin E/A/beta‐carotene supplements in excess of defined amounts Name of parent cohort: Alpha‐Tocopherol, Beta‐Carotene Cancer Prevention (ATBC) Study Recruitment: 1985 to 1988 Outcome assessment: 30 April 1993 Number of cases: Prostate cancer: 302 (male/female: 302/0) Case definition: incidence Years of follow‐up: 5.0 to 8.0 years Type of selenium marker: intake(food use questionnaire) |
|
| Interventions | d.n.a. | |
| Outcomes | Analysed cases: 302 of 317 cases included in analysis (reason for non‐inclusion: no dietary information available) analysis stratified by randomisation status according to active interventions or placebo interventions in the RCT results reported separately for total selenium intake and non‐supplemental selenium intake Statistical methods: Cox regression Variables controlled in analysis: age, living in urban area, beta‐carotene intervention, total energy, BPH | |
| Risk estimates [95% CI] |
Reference category: lowest quartile Results: Prostate cancer: Total (nutritional and supplemental) selenium intake in participants without active alpha‐tocopherol intervention: highest quartile: RR 1.27 (95% CI 0.70 to 2.20) Total (nutritional and supplemental) selenium intake in participants with alpha‐tocopherol intervention: highest quartile: RR 0.84 (95% CI 0.43 to 1.67) Nutritional selenium intake in participants without active alpha‐tocopherol intervention: highest quartile: RR 1.32 (95% CI 0.70 to 2.47) Nutritional selenium intake in participants with alpha‐tocopherol intervention: highest quartile: RR 0.72 (95% CI 0.33 to 1.55) |
|
| Selenium levels in exposure categories | Total nutritional and supplemental selenium intake: lowest quartile: ≤ 71.51 µg/day highest quartile: ≥ 111.06 µg/day Nutritional selenium intake: lowest quartile: ≤ 70.10 µg/day highest quartile: ≥ 105.65 µg/day |
|
| Notes | ||
Helzlsouer 2000
| Methods | Matched, nested case‐control study Country: US |
|
| Participants |
Participants: 10,456 men Inclusion criteria: residents of Washington county; cases with second malignancy or missing pathologic confirmation excluded Name of parent cohort: CLUE II Cohort Recruitment: 1989 Outcome assessment: September 1996 Number of cases: prostate cancer: 117 (male/female: 117/0) Case definition: incidence Years of follow‐up: 6.8 to 7.8 years Type of selenium marker: toenail |
|
| Interventions | d.n.a. | |
| Outcomes | Analysed cases: 117 of 145 cases analysed (reason for non‐inclusion: no toenail clipping available) Statistical methods: conditional logistic regression Variables controlled in analysis: BMI at age 21, education, hours since last meal Variables controlled by matching: age, race/ethnicity, year and month of sample collection, size of toenail clipping | |
| Risk estimates [95% CI] |
Reference category: lowest quintile Results: Prostate cancer highest quintile: OR 0.38 (95% CI 0.17 to 0.85) |
|
| Selenium levels in exposure categories | lowest quintile: ≤ 0.69 ppm highest quintile: ≥ 0.92 ppm | |
| Notes | ||
Hotaling 2011
| Methods | Cohort study Country: US |
|
| Participants |
Participants: 77,050 men and women, aged 50 to 76 years, participants recruited from subscribers of commercial mailing list, residents of western Washington state, non‐whites excluded, no malignant disease at baseline Name of parent cohort: Vitamins and lifestyle (VITAL) study Recruitment: 1 October 2000 to 31 December 2002 Outcome assessment: 31/12/2007 Number of cases: Urothelial carcinoma: 330 Case definition: incidence Years of follow‐up: 6 years (median) Type of selenium marker: supplemental intake (questionnaire: use of supplements over the last 10 years, mean supplemental intake / day calculated) |
|
| Interventions | d.n.a. | |
| Outcomes | Statistical methods: cox proportional hazards regression, Variables controlled in analysis: age, gender, race (white, black, other), education, family history of bladder cancer, smoking (never; former, quit more than 10 years before start of VITAL; former, quit less than 10 years before start of VITAL; current), pack‐years (never smoker and tertiles), and fruit and vegetable intake | |
| Risk estimates [95% CI] |
Reference category: nonuse Results: highest level: HR 0.97 (95% CI 0.72 to 1.31) |
|
| Selenium levels in exposure categories | lowest level: nonuse highest quartile: 20 mcg | |
| Notes | ||
Kabuto 1994
| Methods | Matched, nested case‐control study Country: Japan |
|
| Participants |
Participants: 20,000 men and women Inclusion criteria: survivors of the atomic bomb in Hiroshima or Nagasaki; serum available for analysis Name of parent cohort: Adult Health Study Hiroshima and Nagasaki Recruitment: 1960 (blood samples drawn in 1970 to 1972) Outcome assessment: 1983 Number of cases: Stomach cancer: 201 (male/female: 113/88) Lung cancer: 77 (male/female: 43/34) Case definition: incidence Years of follow‐up: 12.0 to 14.0 years Type of selenium marker: serum |
|
| Interventions | d.n.a. | |
| Outcomes | Statistical methods: conditional logistic regression Variables controlled in analysis: radiation dose, smoking, age, gender Variables controlled by matching: age, gender, year/month of sample collection, city | |
| Risk estimates [95% CI] |
Reference category: highest quartile Results: Stomach both genders: lowest quartile: OR 1.0 (95% CI 0.5 to 1.9) Lung cancer both genders: lowest quartile: OR 1.8 (95% CI 0.7 to 5.0) |
|
| Selenium levels in exposure categories | lowest quartile ≤ 98.90 ng/ml highest quartile ≥ 128.10 ng/ml | |
| Notes | ||
Karagas 1997
| Methods | Matched, nested case‐control study Country: US |
|
| Participants |
Participants: 1805 men and women Inclusion criteria: at least one basal cell or squamous cell cancer before study entry; participants of an RCT for non‐melanoma skin cancer prevention with oral beta‐carotene supplementation Name of parent cohort: Skin Cancer Prevention Study Recruitment: February 1983 to February 1986 Outcome assessment: 30 September 1989 Number of cases: Squamous cell cancer: 131 (89% male/11% female) Case definition: incidence Years of follow‐up: 3.0 to 5.0 years Type of selenium marker: plasma |
|
| Interventions | d.n.a. | |
| Outcomes | Statistical methods: conditional logistic regression Variables controlled in analysis: cigarette smoking Variables controlled by matching: age, gender, study centre of RCT, time in study (diagnosis date) | |
| Risk estimates [95% CI] |
Reference category: lowest quartile Results: Squamous cell cancer both genders: highest quartile: OR 0.86 (95% CI 0.47 to 1.58) |
|
| Selenium levels in exposure categories | lowest quartile: ≤ 0.12 ppm highest quartile: ≥ 0.14 ppm; | |
| Notes | ||
Knekt 1990
| Methods | Matched, nested case‐control study (Knekt 1990, Hakama 1990, Knekt 1988, Knekt 1996) Cohort study (Knekt 1991) Country: Finland |
|
| Participants |
Inclusion criteria: no history of cancer at baseline Name of parent cohort: Social Insurance Institution's Mobile Clinic Health Examination Survey Recruitment: 1968 to 1972 Knekt 1990: Participants: 39,268: 21,172 men and 18,096 women Outcome assessment: 31 December 1980 Number of cases: Any cancer: 1096 (male/female: 597/499) Stomach cancer: 95 (male/female: 58/37) Colon and rectal cancer: 91 (male/female: 32/59) Lung cancer: 198 (male/female: 189/9) Prostate cancer: 51 (male/female: 51/0) Urinary tract cancer: 47 (male/female: 34/13) Pancreatic cancer: 45 (male/female: 22/23) Breast cancer: 90 (male/female: 0/90) Gynaecological cancer (without breast): 86 (male/female: 0/86) Basal cell carcinoma (skin): 126 (male/female: 64/62) Other: 267 (male/female: 147/120) Hakama 1990: Participants: number of participants n.r.; both genders Inclusion criteria: aged 15 years and older Outcome assessment: 1977 Number of cases: Any cancer: 766 (male/female: n.r.) Lung cancer: 151 (male/female: 151/0) Breast cancer: 67 (male/female: 0/67) Stomach cancer: 76 (male/female: n.r.) Prostate cancer: 37 (male/female: 37/0) Knekt 1988: Participants: 36,265: 21,172 men and 15,093 women Outcome assessment: 31 December 1977 Number of cases: Oesophageal and stomach cancer: 86 (male/female: 51/35) Colon and rectal cancer: 57 (male/female: 21/36) Knekt 1991: Participants: 4538 men Inclusion criteria: aged 20 to 69 years, with dietary history taken Outcome assessment: 1986 Number of cases: Lung cancer: 117 (male/female: 117/0) Knekt 1996: Participants: 1896 women Outcome assessment: 1980 Number of cases: Ovarian cancer: 24 (male/female: 0/24) Case definition: incidence Years of follow‐up: 9 to 20 years Type of selenium marker: serum (Knekt 1990, Hakama 1990, Knekt 1988, Knekt 1996), intake (Knekt 1991: dietary history) |
|
| Interventions | d.n.a. | |
| Outcomes |
Knekt 1990:
Statistical methods: conditional logistic regression Variables controlled in analysis: smoking Variables additionally controlled in analysis of highest four quintiles versus lowest quintile: occupation, BMI, parity, cholesterol, haematocrit Variables controlled by matching: age, gender, municipality, time of baseline examination, duration of storage of sample Hakama 1990: Analysed cases: 766 of 864 cases analysed (reason for non‐inclusion: no serum sample) Statistical methods: conditional logistic regression Variables controlled in analysis: smoking Variables additionally controlled in analysis of highest four quintiles versus lowest quintile: retinol level, alpha‐tocopherol level Variables controlled by matching: age, gender, municipality, time of baseline examination, duration of storage of sample Knekt 1988: Statistical methods: n.r. Variables controlled in analysis: smoking, serum cholesterol Variables controlled by matching: age, gender, municipality, time of baseline examination, duration of storage of sample Knekt 1991: Statistical methods: Cox‐proportional hazards model Variables controlled in analysis: age, smoking (data stratified according to smoking status) Knekt 1996: Statistical methods: conditional logistic regression Variables controlled by matching: age, gender, municipality, time of baseline examination, duration of storage of sample |
|
| Risk estimates [95% CI] |
Knekt 1990:
Reference category: lowest quintile Results: Any cancer male: highest quintile: OR 0.41 (CI not reported) above 20th percentile: OR 0.67 (CI not reported); cases during first 2 years of follow‐up excluded: 476 cases: OR 0.65 (95% CI 0.48 to 0.89) female: highest quintile: OR 0.86 (CI not reported) above 20th percentile: OR 0.93 (CI not reported); cases during first 2 years of follow‐up excluded: 423 cases: OR 0.97 (95% CI 0.68 to 1.39) Stomach cancer male: highest quintile: OR 0.09 (CI not reported) above 20th percentile: OR 0.26 (CI not reported); cases during first 2 years of follow‐up excluded: 43 cases: OR 0.24 (95% CI 0.09 to 0.69) female: highest quintile: OR 0.27 (CI not reported) above 20th percentile: OR 0.59 (CI not reported); cases during first 2 years of follow‐up excluded: 30 cases: OR 0.48 (95% CI 0.14 to 1.66) Colon and rectal cancer male: highest quintile: OR 0.53 (CI not reported) above 20th percentile: OR 0.69 (CI not reported); cases during first 2 years of follow‐up excluded: 29 cases: OR 1.01 (95% CI 0.18 to 5.65) female: highest quintile: OR 0.80 (CI not reported) above 20th percentile: OR 1.26 (CI not reported); cases during first 2 years of follow‐up excluded: 48 cases: OR 1.10 (95% CI 0.42 to 2.92) Lung cancer male: highest quintile: OR 0.30 (CI not reported) above 20th percentile: OR 0.60 (CI not reported); cases during first 2 years of follow‐up excluded: 153 cases: OR 0.66 (95% CI 0.37 to 1.19) female: third highest quintile: OR 4.62 (CI not reported) (quintile 4 and 5 did not contain any cases) Prostate cancer highest quintile: OR 1.15 (CI not reported) above 20th percentile: OR 1.13 (CI not reported); cases during first 2 years of follow‐up excluded: 46 cases: OR 1.00 (95% CI 0.42 to 2.40) Urinary tract cancer male: highest quintile: OR 0.81 (CI not reported) above 20th percentile: OR 0.89 (CI not reported); cases during first 2 years of follow‐up excluded: 26 cases: OR 0.34 (95% CI 0.06 to 2.06) female: highest quintile: OR 4.12 (CI not reported) above 20th percentile: not reported; cases during first 2 years of follow‐up excluded: 9 cases: OR 2.51 (95% CI 0.13 to 47.9) Pancreatic cancer male: fourth quintile versus lowest: OR 0.58 (CI not reported) (highest quintile did not contain any cases) above 20th percentile: OR 0.11 (CI not reported); cases during first 2 years of follow‐up excluded: not reported female: highest quintile: OR 3.49 (CI not reported) above 20th percentile: not reported; cases during first 2 years of follow‐up excluded: 22 cases: OR 0.86 (95% CI 0.21 to 3.52) Breast cancer highest quintile: OR 0.64 (CI not reported) above 20th percentile: OR 0.52 (CI not reported); cases during first 2 years of follow‐up excluded: 74 cases: OR 0.57 (95% CI 0.18 to 1.81) Gynaecological cancer (without breast) highest quintile: OR 0.96 (CI not reported) above 20th percentile: OR 0.91 (CI not reported); cases during first 2 years of follow‐up excluded: 70 cases: OR 1.03 (95% CI 0.43 to 2.50) Basal cell carcinoma (skin) male: highest quintile: OR 0.54 (CI not reported) above 20th percentile: OR 0.65 (CI not reported); cases during first 2 years of follow‐up excluded: 54 cases: OR 0.86 (95% CI 0.35 to 2.12) female: highest quintile: OR 1.55 (CI not reported) above 20th percentile: OR 1.73 (CI not reported); cases during first 2 years of follow‐up excluded: 52 cases: OR 1.54 (95% CI 0.64 to 3.73) Other or unspecified cancer: male: highest quintile: OR 0.42 (CI not reported) above 20th percentile: OR 0.72 (CI not reported); cases during first 2 years of follow‐up excluded: 110 cases: OR 0.70 (95% CI 0.36 to 1.36) female: highest quintile: OR 0.71 (CI not reported) above 20th percentile: OR 0.87 (CI not reported); cases during first 2 years of follow‐up excluded: 111 cases: OR 0.92 (95% CI 0.44 to 1.92) Hakama 1990: Reference category: highest quintile Results: Any cancer male: lowest quintile: OR 2.40 (CI not reported) lowest quintile vs. four highest quintiles: OR 1.60 (CI not reported) female: lowest quintile: OR 1.20 (CI not reported) lowest quintile vs. four highest quintiles:0.90 (CI not reported) Lung cancer male: lowest quintile vs. four highest quintiles: OR 1.80 (CI not reported) Breast cancer lowest quintile vs. four highest quintiles: OR 3.10 (CI not reported) Stomach cancer male: lowest quintile vs. four highest quintiles: OR 6.70 (CI not reported) female: lowest quintile vs. four highest quintiles: OR 2.00 (CI not reported) Prostate cancer lowest quintile vs. four highest quintiles: OR 0.80 (CI not reported) Knekt 1988: Reference category: highest quintile Results: Oesophageal and stomach cancer male: lowest tertile: OR 2.20 (CI not reported) lowest quintile vs. four highest quintiles: OR 3.3 (95% CI 1.3 to 9.1) female: lowest tertile: OR 1.50 (CI not reported) lowest quintile vs. four highest quintiles: OR 2.4 (95% CI 0.7 to 8.3) Colon and rectal cancer male: lowest tertile: OR 0.90 (CI not reported) lowest quintile vs. four highest quintiles: OR 1.7 (95% CI 0.4 to 7.7) female: lowest tertile: OR 0.60 (CI not reported) lowest quintile vs. four highest quintiles: OR 0.8 (95% CI 0.2 to 2.4) Knekt 1991: Reference category: highest tertile Results: Lung cancer male non‐smokers: lowest tertile: OR 1.03 (CI not reported) male smokers: lowest tertile: OR 0.83 (CI not reported) Knekt 1996: Reference category: highest tertile Results: Ovarian cancer lowest tertile: OR 1.15 (95% CI 0.19 to 4.06) |
|
| Selenium levels in exposure categories |
Knekt 1990: lowest quintile: ≤ 48.90 µg/l; highest quintile ≥ 78.00 µg/l Hakama 1990: quintiles: not specified Knekt 1988: both genders: lowest tertile: ≤ 56.90 µg/l; highest tertile ≥ 70.10 µg/l lowest quintile: ≤ 50 µg/l; highest four quintiles > 50 µg/l Knekt 1991: tertiles: n.r. Knekt 1996: lowest tertile: ≤ 56.90 µg/l; highest tertile: ≥ 68.10 µg/l |
|
| Notes | Primary publication: Knekt 1990 Other publications: Hakama 1990, Knekt 1988, Knekt 1991, Knekt 1996 | |
Knekt 1998
| Methods | Matched, nested case‐control study Country: Finland |
|
| Participants |
Participants: 9101 men and women Inclusion criteria: 19 years or older; no history of cancer at baseline; serum sample available for analysis Name of parent cohort: Social Insurance Institution's Mobile Clinic Health Examination Survey Recruitment: 1973 to 1976 Outcome assessment: end of 1991 Number of cases: Lung cancer: 91 (male/female: approximately 95%/5%) Case definition: incidence Years of follow‐up: 16.0 to 19.0 years Type of selenium marker: serum |
|
| Interventions | d.n.a. | |
| Outcomes | Analysed cases: 91 of 95 (male/female: 90/5) cases analysed Statistical methods: conditional logistic regression Variables controlled in analysis: smoking, alpha‐tocopherol, serum cholesterol, copper, orosomucoid, BMI Variables controlled by matching: age, gender, municipality, season of sample collection, length of storage of sample | |
| Risk estimates [95% CI] |
Reference category: lowest tertile Results: Lung cancer analysis adjusted for smoking only: both genders: highest tertiles: OR 0.44 (95% CI 0.21 to 0.89) analysis adjusted for all variables (number of cases: 77): highest tertiles: OR 0.41 (95% CI 0.17 to 0.94) |
|
| Selenium levels in exposure categories | lowest tertile: ≤ 45.49 µg/l highest tertile: ≥ 60.60 µg/l | |
| Notes | ||
Kok 1987a
| Methods | Matched, nested case‐control study Country: the Netherlands |
|
| Participants |
Participants: 10,532 men and women Inclusion criteria: inhabitants of Zoetermeer; 5 years or older Name of parent cohort: EPOZ Cohort (Epidemiologisch onderzoek naar risico‐indicatoren voor hart‐ en vaatziekten) Recruitment: 1975 to 1978 Outcome assessment: 31 December 1983 Number of cases: Any cancer: 69 (male/female: 40/29) Case definition: mortality Years of follow‐up: 6.0 to 9.0 years Type of selenium marker: serum |
|
| Interventions | d.n.a. | |
| Outcomes | Analysed cases: 69 of 114 cases analysed (reason for non‐inclusion: serum or baseline data not available, deaths in first year of follow‐up excluded) Statistical methods: not specified Variables controlled in analysis: age, smoking, serum cholesterol, serum vitamin A and E, systolic and diastolic blood pressure, BMI, week of blood collection, years of education, gender (in group of both genders) Variables controlled by matching: age, gender, smoking status | |
| Risk estimates [95% CI] |
Reference category: highest four quintiles Results: Any cancer both genders: lowest quintile: OR 1.9 (90% CI 1.0 to 3.5) male: lowest quintile: OR 2.7 (90% CI 1.2 to 6.2) female: lowest quintile: OR 1.5 (90% CI 0.5 to 4.5) |
|
| Selenium levels in exposure categories | both genders: lowest quintile: ≤ 102.79 µg/l highest four quintiles: ≥ 102.80 µg/l men: lowest quintile: ≤ 100.79 µg/l highest four quintiles: ≥ 100.80 µg/l women: lowest quintile: ≤ 107.29 µg/l highest four quintiles: ≥ 107.30 µg/l |
|
| Notes | Primary publication: Kok 1987b Other publication: Kok 1987a | |
Kornitzer 2004
| Methods | Matched, nested case‐control study Country: Belgium |
|
| Participants |
Participants: cohort size not reported; men and women Inclusion criteria: 25 to 74 years of age Name of parent cohort: Belgian Interuniversity Study on Nutrition and Health Recruitment: 1980 to 1984 Outcome assessment: n.r. Number of cases: Any cancer: 193 (male/female: 143/50) Case definition: mortality Years of follow‐up: 10.0 years Type of selenium marker: serum |
|
| Interventions | d.n.a. | |
| Outcomes | Analysed cases: 143 male/50 female cases analysed from 252 male/91 female cases (reason for non‐inclusion: no selenium measurement available) Statistical methods: not specified Variables controlled in analysis: BMI, total energy, total fat, saturated fat, alcohol intake, fibre, retinol, vitamin C, smoking, beta‐carotene Variables controlled by matching: age, gender | |
| Risk estimates [95% CI] |
Reference category: highest tertile Results: Any cancer male: lowest tertile: OR 2.2 (95% CI 1.3 to 3.7) female: lowest tertile: OR 0.7 (95% CI 0.3 to 1.6) |
|
| Selenium levels in exposure categories | lowest tertile ≤ 72.00 µg/l highest tertile ≥ 85.00 µg/l | |
| Notes | ||
Kromhout 1987
| Methods | Cohort/sub‐cohort‐controlled cohort study Country: the Netherlands |
|
| Participants |
Participants: 878 men Inclusion criteria: 40 to 59 years of age; random sample of general male population at specific age in Zutphen Name of parent cohort: Zutphen Study Recruitment: 1960 Outcome assessment: 1985 Number of cases: lung cancer: 63 (male/female: 63/0) Case definition: mortality Years of follow‐up: 25.0 years Type of selenium marker: intake (interview) |
|
| Interventions | d.n.a. | |
| Outcomes | Statistical methods: Cox proportional hazard model Variables controlled in analysis: age, pack years of smoking | |
| Risk estimates [95% CI] |
Reference category: lowest quartile Results: Lung cancer male: highest quartile: RR 0.98 (95% CI 0.41 to 2.36) |
|
| Selenium levels in exposure categories | lowest quartile: ≤ 55.00 µg/day highest quartile: ≥ 72.10 µg/day | |
| Notes | ||
Li 2000
| Methods | Randomised controlled trial Allocation: randomised, "based on their residence area" Sequence generation: unclear, not described Concealment: unclear, not described Blinding: of participants: adequate (placebo), of investigators and doctors: unclear, not described Dropouts/withdrawals: no significant difference between percentage of drop‐outs in intervention and control group (absolute numbers not reported) Intention‐to‐treat‐analysis: unclear Recruitment period: unclear, not described Observation period: 3 years, started in 1996 Study period: unclear, not described Detection of cases: unclear, the study followed the diagnostic menu published by the National Cancer Control and Prevention Center, follow‐up procedures not described Informed consent: unclear, not described |
|
| Participants |
Country: China Number of participants: 2065 (selenium group: 1112; placebo group: 953) Condition: HBsAg carriers with negative AFP and normal ALT living in Qidong, Jiangsu province Demographics: men only; aged 20 to 65 years (screening group) Recruitment and setting: recruitment of 2065 HBsAg carriers from 17 villages out of a screening group of 18,000 men |
|
| Interventions |
Intervention: 0.5 mg sodium selenite p.o. daily for 3 years Control: placebo |
|
| Outcomes |
Primary outcome measure: incidence of primary liver cancer Other: blood selenium levels, activity of glutathione peroxidase Results: person‐year incidence rate (number of cases/total number of persons) in intervention and control group: 1st year of follow‐up: selenium group 899.25/100,000 (10/1112); placebo group: 1,888.77/100,000 (18/953) 2nd year of follow‐up: selenium group 1,708.60/100,000 (19/1112); placebo group: 4,302.20/100,000 (41/953) 3rd year of follow‐up: selenium group 3,057.55/100,000 (34/1112); placebo group: 5,981.11/100,000 (57/953) |
|
| Risk estimates [95% CI] | n.r. | |
| Selenium levels in exposure categories | d.n.a. | |
| Notes | adverse effects were not mentioned | |
Li 2004a
| Methods | Matched, nested case‐control study Country: US |
|
| Participants |
Participants: 14,916 men Inclusion criteria: participants of Physicians' Health Study who provided blood sample (healthy male physicians); no history of cancer at baseline; several physical conditions excluded at baseline: chronic renal failure, unstable angina pectoris, liver disease, peptic ulcer, history of TIA/stroke/myocardial infarction/gout; no use of vitamin A or beta‐carotene supplements Name of parent cohort: Physicians' Health Study Recruitment: 1982 Outcome assessment: 1995 Number of cases: Prostate cancer: 586 (male/female: 586/0) Case definition: incidence Years of follow‐up: 13.0 years Type of selenium marker: plasma |
|
| Interventions | d.n.a. | |
| Outcomes | Statistical methods: logistic regression Variables controlled in analysis: age at baseline, smoking status, duration of follow‐up Variables controlled by matching: age, smoking status | |
| Risk estimates [95% CI] |
Reference category: lowest quintile Results: Prostate cancer highest quintile: OR 0.78 (95% CI 0.54 to 1.13) |
|
| Selenium levels in exposure categories | lowest quintile: 0.060 to 0.090 ppm highest quintile: 0.121 to 0.190 ppm | |
| Notes | ||
Marshall 2011
| Methods | Randomised controlled trial Allocation: random Sequence generation: unclear Concealment: unclear Blinding: only described as double‐blinded. The central pathologist was also blinded to study assignment. Dropouts/withdrawals: 13/227 in the selenium arm and 12/225 in the placebo arm were lost to follow‐up. Intention‐to‐treat‐analysis: yes Recruitment period: not specified Treatment duration: not specified Observation period/dermatologic follow‐up: Subjects were followed for three years. They were seen in clinic at baseline and every six months thereafter. Detection of cases: Tissue blocks and corresponding pathology reports for all prostate procedures were to be submitted to the central study pathologist for review Informed consent: All patients gave oral and written informed consent in accordance with institutional and federal guidelines. The protocol was approved by the Institutional Review Boards at participating institutions, and was monitored by the Data and Safety Monitoring Committee of SWOG |
|
| Participants |
Country: US Number of patients: 452 (randomised to selenium 200 ug/day group: 227, to placebo group: 225) Condition: 40 years of age or older; digital rectal examination; biopsy‐ confirmed diagnosis of HGPIN with no evidence of cancer; upper limit of prostate‐specific antigen (PSA) of 10 ng/mL (as measured locally); American Urological Association (AUA) symptom score of less than 20 (41), signifying no debilitating urinary problems; ambulatory and able to carry out work of a light or sedentary nature. Demographics: Selenium and placebo patients were well balanced with respect to age, race, ethnicity, pre‐study PSA category, vitamin E supplements, and number of cores in the initial biopsy. They also were well balanced in body mass index, baseline blood selenium, performance status, and number of cores revealing HGPIN |
|
| Interventions | Subjects were randomised in fashion to placebo or 200 mcg/day of selenium, with daily treatment scheduled for three years or until a PC diagnosis. Recruitment: not reported End of the blinded treatment period: at 3 years |
|
| Outcomes |
Primary outcome measure: progression of HGPIN to PC over a three‐year period |
|
| Risk estimates [95% CI] |
Primary outcomes: Adjusted OR=0.913, P= 0.727, 95%CI= 0.55‐1.52 for risk of prostate cancer as a function of treatment group (with placebo as referent group) is: adj OR= |
|
| Selenium levels in exposure categories | d.n.a. | |
| Notes | The OR estimate was given from the author | |
McNaughton 2005
| Methods | Matched, nested case‐control study (McNaughton 2005b) Cohort study (Heinen 2007, van der Pols 2009) Country: Australia |
|
| Participants |
Name of parent cohort: Nambour Skin Cancer Study Recruitment: 1992 to 1996 Case definition: incidence McNaughton 2005b: Participants: approximately 1000 men and women Inclusion criteria: randomly selected adults, aged 20 to 69 years; recruited for participation in a randomised controlled trial for skin cancer prevention with beta‐carotene supplements and sunscreen application in 1992; living in the Nambour community; free of SCC at baseline; with blood sample and FFQ provided in 1996; participants with extreme energy intakes in FFQ excluded Outcome assessment: December 2001 Number of cases: Basal cell carcinoma of the skin: 90 (male/female: 39/51) Years of follow‐up: 5.5 years Type of selenium marker: serum and nutritional intake (FFQ) Heinen 2007: Participants: 1001 men and women Inclusion criteria: randomly selected adults, aged 20 to 69 years; recruited for participation in randomised controlled trial for skin cancer prevention with beta‐carotene supplements and sunscreen application in 1992; living in the Nambour community; with blood sample and FFQ provided in 1996; participants with extreme energy intakes in FFQ and missing consumption frequencies for more than 10% of food items excluded Outcome assessment: 31 December 2004 Number of cases: Basal cell carcinoma of the skin: 149 (male/female: 87/62) participants with 321 BCC tumours Squamous cell carcinoma of the skin: 116 (male/female: 70/46) participants with 221 SCC tumours, Case definition: incidence (tumour‐based incidence and person‐based incidence) Years of follow‐up: 8 years Type of selenium marker: nutritional intake (FFQ) van der Pols 2009: Participants: 485 (male/female: 223/262) men and women Inclusion criteria: randomly selected adults, aged 20 to 69 years; recruited for participation in randomised controlled trial for skin cancer prevention with beta‐carotene supplements and sunscreen application in 1992; randomised to placebo in the intervention trial; living in the Nambour community; free of SCC at baseline; with blood sample and FFQ provided in 1996; participants with extreme energy intakes in FFQ excluded Outcome assessment: 31 December 2004 Number of cases: Basal cell carcinoma of the skin: 77 (male/female: 46/31) participants with 173 BCC tumours Squamous cell carcinoma of the skin: 59 (male/female: 38/21) participants with 124 SCC tumours, Years of follow‐up: 8 years Type of selenium marker: serum |
|
| Interventions | d.n.a. | |
| Outcomes |
McNaughton 2005b:
Statistical methods: conditional logistic regression Variables controlled in analysis: age, gender Variables controlled by matching: age, gender Heinen 2007: Statistical methods: generalised linear models Variables controlled in analysis: age, sex, intervention arm in RCT, energy intake, skin colour, elastosis of the neck, smoking, use of dietary supplements, history of skin cancer van der Pols 2009: Statistical methods: generalised linear models Variables controlled in analysis: age, sex, pack‐years of smoking, alcohol intake, time spent outdoors on weekdays, history of skin cancer before 1996 |
|
| Risk estimates [95% CI] |
McNaughton 2005b: Reference category: lowest quartile Results: Basal cell carcinoma (skin) both genders: highest quartile: OR 0.86 (95% CI 0.38 to 1.96) biochemical selenium level both genders: highest quartile: OR 1.13 (95% CI 0.47 to 2.74) selenium intake Heinen 2007: Reference category: lowest tertile Results: Basal cell carcinoma (skin) both genders: highest tertile: RR 0.95 (95% CI 0.59 to 1.50) Squamous cell carcinoma (skin) both genders: highest tertile: RR 1.3 (95% CI 0.77 to 2.3) van der Pols 2009: Reference category: lowest exposure category Results: Basal cell carcinoma (skin) both genders: highest exposure category: RR 0.58 (95% CI 0.32 to 1.07) Squamous cell carcinoma (skin) both genders: highest exposure category: RR 0.49 (95% CI 0.24 to 0.99) |
|
| Selenium levels in exposure categories |
McNaughton 2005b: n.r. Heinen 2007: lowest tertile ≤ 76.20 µg/day highest tertile ≥ 89.31 µg/day van der Pols 2009: lowest exposure category ≤ 1.0 µmol/l highest exposure category ≥ 1.3 µmol/l |
|
| Notes |
Primary publication:McNaughton 2005b
Other publication: Heinen 2007, van der Pols 2009 tumour‐based incidence: number of newly developed histologically confirmed BCC or SCC divided by the person‐years of follow‐up accumulated over follow‐up period person‐based incidence: number of persons newly affected by BCC or SCC during the same person‐years of follow‐up time as calculated for the tumour‐based analysis |
|
Menkes 1986
| Methods | Matched, nested case‐control study Country: US |
|
| Participants |
Participants: 25,804 men and women Inclusion criteria: female and male inhabitants of Washington county/Maryland; history of cancer at baseline excluded Name of parent cohort: CLUE I Cohort Recruitment: September to November 1974 Menkes 1986b: Outcome assessment: 1983 Number of cases: Lung cancer: 99 (69% male/31% female) Helzlsour 1996: Inclusion criteria: women only; women who used hormones at baseline excluded Outcome assessment: 1989 Number of cases: Ovarian cancer: 35 (male/female: 0/35) Breslow 1995: Outcome assessment: 1994 Number of cases: Melanoma: 23 (male/female: n.r.) Basal cell carcinoma (skin): 17 (male/female: n.r.) Squamous cell cancer: 37 (male/female: n.r.) Zheng 1993: Outcome assessment: 1990 Number of cases: Oral and pharyngeal: 28 (male/female: n.r.) Batieha 1993: Inclusion criteria: 15,161 women Outcome assessment: 31 May 1990 Number of cases: Cervical cancer: 50 (male/female: 0/50) Helzlsour 1989: Inclusion criteria: 20,305 men and women Outcome assessment: 1986 Number of cases: Bladder cancer: 35 (male/female: n.r.) Burney 1989: Outcome assessment: 1986 Number of cases: Pancreatic cancer: 22 (male/female: 9/13) Ko 1994: Outcome assessment: 25 September 1991 Number of cases: Colon cancer: 121 (male/female: 50/71) Case definition: incidence Years of follow‐up: 8.0 to 16.8 years Type of selenium marker: serum |
|
| Interventions | d.n.a. | |
| Outcomes |
Menkes 1986b:
Statistical methods: conditional logistic regression Variables controlled by matching: age, gender, race/ethnicity, smoking status, year and month of sample collection Helzlsour 1986: Statistical methods: conditional logistic regression Variables controlled by matching: Age, race/ethnicity, day time of blood sample collection, hours since last meal, time since last menstrual period (post‐menopausal: years, pre‐menopausal: days) Breslow 1995: Statistical methods: conditional logistic regression Analysed cases: 17 of 98 basal cell carcinoma cases, and 23 of 30 melanoma cases (and all squamous cell carcinoma cases) included in analysis Variables controlled by matching: age, gender, race/ethnicity Zheng 1993: Statistical methods: n.r. Variables controlled in analysis: smoking Variables controlled by matching: age, gender, race/ethnicity, year and month of sample collection, hours between previous meal and blood collection Batieha 1993: Statistical methods: conditional logistic regression Analysed cases: 50 of 60 cases (CIS and invasive cervical cancer) analysed (reason for non‐inclusion: no matched control available) Variables controlled by matching: age, race/ethnicity, year and month of blood collection, hours since last meal, time since last menstrual period Helzlsour 1989: Statistical methods: n.r. Variables controlled in analysis: cigarette smoking, use of vitamin supplements Variables controlled by matching: age, gender, race/ethnicity, hours since last meal (all samples collected in same year) Burney 1989: Statistical methods: n.r. Variables controlled by matching: age, gender, race/ethnicity, hours since last meal Ko 1994: Analysed cases: 121 of 154 cases analysed (reason for non‐inclusion: no serum sample available, tumour pathology or localisation unclear) Statistical methods: conditional logistic regression Variables controlled by matching: age, gender, race/ethnicity, year and month of sample collection, hours since last meal, women: time since last menstrual period, women: use of hormones/hormonal contraceptives |
|
| Risk estimates [95% CI] |
Menkes 1986b:
Reference category: highest quintile Results: Lung cancer both genders: lowest quintile: OR 0.68 (CI not reported) Helzlsouer 1986: Reference category: lowest tertile Results: Ovarian cancer highest tertiles: OR 0.58 (95% CI 0.20 to 1.70) Breslow 1995: Reference category: lowest tertile Results: Melanoma both genders: highest tertile: OR 0.9 (95% CI 0.3 to 2.5) Basal cell carcinoma (skin) both genders: highest tertile: OR 0.8 (95% CI 0.1 to 4.5) Squamous cell cancer both genders: highest tertile: OR 0.6 (95% CI 0.2 to 1.5) Zheng 1993: Reference category: lowest tertile Results: Oral and pharyngeal cancer both genders: highest tertile: OR 5.43 (CI not reported) Batieha 1993: Reference category: highest tertile Results: Cervical cancer lowest tertile: OR 1.12 (95% CI 0.50 to 2.53) Helzlsour 1989: Reference category: highest tertile Results: Bladder cancer both genders: lowest tertile: OR 2.06 (95% CI 0.67 to 6.35) Burney 1989: Reference category: highest tertile Results: Pancreatic cancer both genders: lowest tertile: OR 4.5 (CI not reported) (unmatched analysis) both genders: lowest tertile vs. higher two tertiles: OR 3.90 (95% CI 1.13 to 13.2) (matched analysis) male: 12.5 (95% CI 1.8 to 84.0) (unmatched analysis) female: 1.2 (95% CI 0.6 to 2.5) (unmatched analysis) Ko 1994: Reference category: highest quartile Results: Colon cancer both genders: lowest quartile: OR 0.82 (95% CI 0.35 to 1.92) |
|
| Selenium levels in exposure categories |
Menkes 1986b: quintiles: n.r. Helzlsouer 1986: women: lowest tertile: ≤ 10.50 µg/dl highest tertile: ≥ 11.61 µg/dl Breslow 1995: tertiles: n.r. Zheng 1993: tertiles: n.r. Batieha 1993: women: lowest tertile: ≤ 0.109 ppm highest tertile: ≥ 0.124 ppm Helzlsour 1989: both genders: lowest tertile: ≤ 10.90 µg/dl highest tertile: ≥ 11.91 µg/dl Burney 1989: lowest: 0.99 to 1.26 µmol/l; highest: 1.44 to 1.81 µmol/l Ko 1994: lowest quartile: ≤ 9.90 µg/dl highest quartile: ≥ 11.81 µg/dl |
|
| Notes | Primary publication: Menkes 1986b Other publications: Helzlsour 1996, Breslow 1995, Zheng 1993, Batieha 1993, Helzlsour 1989, Burney 1989, Ko 1994, Schober 1987 (cases included in Ko 1994), Menkes 1986a (cases included in Menkes 1986b) | |
Michaud 2002
| Methods | Matched, nested case‐control study Country: Finland |
|
| Participants |
Participants: 29,133 men Inclusion criteria: 50 to 69 years of age; smokers; no history of cancer (other than non‐melanoma skin cancer) at baseline; no severe physical or psychiatric illness; intake of vitamin E/A/beta‐carotene supplements in excess of defined amounts Name of parent cohort: Alpha‐Tocopherol, Beta‐Carotene Cancer Prevention (ATBC) Study Recruitment: 1985 to 1988 Outcome assessment: 30 April 1993 Number of cases: Bladder cancer: 133 (male/female: 133/0) Case definition: incidence Years of follow‐up: 5.0 to 8.0 years Type of selenium marker: toenail |
|
| Interventions | d.n.a. | |
| Outcomes | Statistical methods: conditional logistic regression Variables controlled in analysis: smoking dose and duration Variables controlled by matching: age, year/month of sample collection, intervention group status in RCT (only male smokers included in cohort) | |
| Risk estimates [95% CI] |
Reference category: lowest tertile/quartile Results: Bladder cancer male: highest tertile: OR 0.90 (95% CI 0.45 to 1.78) male: highest quartile: OR 0.87 (95% CI 0.30 to 2.52) |
|
| Selenium levels in exposure categories | n.r. | |
| Notes | ||
Michaud 2005
| Methods | Matched, nested case‐control study Country: US |
|
| Participants |
Participants: 101,950: 33,737 men, 68,213 women Inclusion criteria: cohort of HPFS (men) and NHS (women); no history of cancer at baseline Name of parent cohort: Health Professional Follow‐Up Study (HPFS) and Nurses' Health Study (NHS) Recruitment: 1987 (HPFS), 1983 (NHS) Outcome assessment: 2000 Number of cases: Bladder cancer: 337 (male/female: 221/116) Case definition: incidence Years of follow‐up: 13.0 to 17.0 years Type of selenium marker: toenail |
|
| Interventions | d.n.a. | |
| Outcomes | Statistical methods: conditional logistic regression Variables controlled in analysis: pack‐years of smoking, heavy smoking at baseline Variables controlled by matching: age, gender, smoking status, month of sample collection | |
| Risk estimates [95% CI] |
Reference category: lowest quartile Results: Bladder cancer male: highest quartile: OR 1.17 (95% CI 0.66 to 2.07) female: highest quartile: OR 0.36 (95% CI 0.14 to 0.91) |
|
| Selenium levels in exposure categories | men: lowest quartile: ≤ 0.722 µg/g highest quartile: ≥ 0.912 µg/g women: lowest quartile: ≤ 0.686 µg/g highest quartile: ≥ 0.840 µg/g |
|
| Notes | ||
Nomura 1987
| Methods | Unmatched, nested case‐control study Country: US |
|
| Participants |
Participants: 6860 men Inclusion criteria: born 1900 to 1919; Japanese ancestry; inhabitants of Oahu/Hawaii; participants in the Honolulu Heart Program (1965 to 68) Name of parent cohort: Honolulu Heart Program Recruitment: 1971 to 1975 Outcome assessment: n.r. Number of cases: Any cancer: 280 (male/female: 280/0) Stomach cancer: 66 (male/female: 66/0) Rectal cancer: 32 (male/female: 32/0) Lung cancer: 71 (male/female: 71/0) Colon cancer: 82 (male/female: 82/0) Bladder cancer: 29 (male/female: 29/0) Case definition: incidence Years of follow‐up: 11.0 years Type of selenium marker: serum |
|
| Interventions | d.n.a. | |
| Outcomes | Statistical methods: proportional hazards regression/Cox regression Variables controlled in analysis: age at examination, cigarettes/day (any cancer, lung cancer, bladder cancer) age at examination (stomach, rectum, colon) | |
| Risk estimates [95% CI] |
Reference category: highest quintile Results: Stomach cancer: male: lowest quintile: OR 0.9 (CI not reported) Rectal cancer male: lowest quintile: OR 1.6 (CI not reported) Lung cancer male: lowest quintile: OR 1.1 (CI not reported) Colon cancer male: lowest quintile: OR 1.8 (CI not reported) Bladder cancer male: lowest quintile: OR 3.1 (CI not reported) All five types of cancer male: lowest quintile: OR 1.3 (CI not reported) |
|
| Selenium levels in exposure categories | lowest quintile: ≤ 10.30 µg/dl highest quintile: ≥ 13.31 µg/dl | |
| Notes | N.B.: "Any cancer" in this study comprises all cancer cases for stomach, rectal, lung, colon and bladder cancer. | |
Nomura 2000
| Methods | Matched, nested case‐control study Country: US |
|
| Participants |
Participants: 9345 men Inclusion criteria: no cancer diagnosis at baseline, blood sample available for analysis, men from two cohorts: sub‐cohort one: participants of Nomura 1987; sub‐cohort 2: brothers of participants in Nomura 1987 Recruitment: 1971 to 1977 Outcome assessment: 1995 Number of cases: Prostate cancer: 249 (male/female: 249/0) Case definition: incidence Years of follow‐up: 19.0 to 25.0 years Type of selenium marker: serum |
|
| Interventions | d.n.a. | |
| Outcomes | Analysed cases: random sample of 249 (out of 360) cases analysed because of limited resources Statistical methods: generalised linear model Variables controlled in analysis: cigarette smoking history, age Variables controlled by matching: age, year/month of sample collection, recruitment in sub‐cohort 1 or 2 | |
| Risk estimates [95% CI] |
Reference category: lowest quartile Results: Prostate cancer highest quartile: OR 0.5 (95% CI 0.3 to 0.9) |
|
| Selenium levels in exposure categories | lowest quartile: ≤ 119.29 ng/ml highest quartile: ≥ 147.20 ng/ml | |
| Notes | ||
NPCT 2002
| Methods | Randomised controlled trial Nutritional Prevention of Cancer Trial (NPCT) Allocation: random, block/stratified by clinic Sequence generation: computer generated random numbers Concealment: central assignment (sealed pill bottles) Blinding: participant blinded, doctor blinded, outcome assessor/pathologist unclear, review/coding of medical records blinded Dropouts/withdrawals: “9 patients (5 in the selenium group and 4 in the placebo group) declined to provide additional illness information” (Clark 1996, p. 1959) ‐ 0 participants lost to vital follow‐up Intention‐to‐treat‐analysis: yes Recruitment period: 1983 to 1991 End of predefined study period: 31 December 1993 Blinded intervention continued until the end of the blinded period: 31 January 1996 Intervention duration: 31 December 1993 (end of study period): mean = 4.5 years 31 January 1996 (end of blinded period): mean = 7.9 years Observation period/dermatologic follow‐up: 31 December 1993 (end of study period): mean = 6.4 years 31 January 1996 (end of blinded period): mean = 7.4 years Detection of cases: dermatologic examination and interview every 6 months during follow‐up; incident BCC and SCC were diagnosed by biopsy and confirmed by another dermatopathologist Informed consent: written informed consent forms, approval by institutional review board of participating institutions |
|
| Participants |
Country: US Number of participants: 1312 (randomised to selenium group: 653, to placebo group: 659) Condition: male and female participants with history of 2 or more squamous cell or basal cell skin cancers Demographics: mean age 63.4 years (selenium)/63.0 years (placebo); 73.8% men (selenium). 75.6% men (placebo) Recruitment and setting: seven dermatological clinics (three academic units, four private practices) in the US |
|
| Interventions |
Intervention: 200 µg selenium supplied as 500 mg selenium yeast tablets p.o./daily. Control: placebo |
|
| Outcomes |
Primary outcome measure: incidence of basal and squamous cell carcinoma of the skin: all analyses were based on 1250 participants with initial blood collection within four days after randomisation (621 in the selenium group and 629 in the placebo group) Other reported outcomes and secondary outcome measures: Reported in Clark 1996: Incidence of lung cancer, prostate cancer, colorectal cancer, any cancer, head and neck cancer, bladder cancer, oesophageal cancer, breast cancer, melanoma, haematologic cancer, Reported in Duffield‐Lillico 2002: Overall cancer mortality |
|
| Risk estimates [95% CI] |
Primary outcomes: 1) at the end of study period (31 December 1993) (Clark 1996): BCC: RR 1.10 (95% CI 0.95 to 1.28); cases: selenium group: 377, placebo group: 350; incidence per person‐year under follow‐up: selenium group 0.16, placebo group 0.15 SCC: RR 1.14 (95% CI 0.93 to 1.39); cases: selenium group 218, placebo group: 190; incidence per person‐year under follow‐up: selenium group 0.07, placebo group 0.06 2) at the end of blinded period (31 January 1996) (Duffield‐Lillico 2003): BCC: RR 1.17 (95% CI 1.02 to 1.35), HR 1.09 (95% CI 0.94 to 1.26); number of cases not reported; incidence per person‐year under follow‐up: selenium group: 0.16, placebo group 0.13 SCC: RR 1.32 (95% CI 1.09 to 1.60), HR 1.25 (95% CI 1.03 to 1.51); number of cases not reported; incidence per person‐year under follow‐up: selenium group: 0.05, placebo group 0.07 NMSC: RR 1.27 (95% CI 1.11 to 1.45) HR 1.17 (95% CI 1.02 to 1.34); number of cases not reported; incidence per person‐year under follow‐up: selenium group: 0.20, placebo group 0.16 Other reported outcomes and secondary outcomes: 1) at the end of study period (31 December 1993) (Clark 1996): lung cancer RR 0.54 (95% CI 0.30 to 0.98), adjusted HR 0.56 (95% CI 0.31 to 1.01) cases selenium: 17, placebo: 31 prostate cancer RR 0.37 (95% CI 0.18 to 0.71), adjusted HR 0.35 (95% CI 0.18 to 0.65) cases selenium: 13, placebo: 35 colorectal cancer RR 0.42 (95% CI 0.18 to 0.95), adjusted HR 0.39 (95% CI 0.17 to 0.90) cases selenium: 8, placebo: 19 any cancer RR 0.63 (95% CI 0.47 to 0.85), adjusted HR 0.61 (95% CI 0.46 to 0.82) cases selenium: 77, placebo: 119 head and neck cancer RR 0.74 (95% CI 0.21 to 2.43), adjusted HR 0.77 (95% CI 0.27 to 2.24) cases selenium: 6, placebo: 8 bladder cancer RR 1.32 (95% CI 0.40 to 4.61), adjusted HR 1.27 (95% CI 0.44 to 3.67) cases selenium: 8, placebo: 6 oesophageal cancer RR 0.33 (95% CI 0.03 to 1.84), adjusted HR 0.30 (95% CI 0.06 to 1.49) cases selenium: 2, placebo: 6 breast cancer RR 2.88 (95% CI 0.72 to 16.5), adjusted HR 2.95 (95% CI 0.80 to 10.9) cases selenium: 9, placebo:3 melanoma RR 0.97 (95% CI 0.32 to 2.96), adjusted HR 0.92 (95% CI 0.34 to 2.45) cases selenium: 8, placebo: 8 haematological cancer RR 1.58 (95% CI 0.46 to 6.14), adjusted HR 1.50 (95% CI 0.49 to 4.60) cases selenium: 8, placebo: 5 other specific carcinomas RR 0.55 (95% CI 0.14 to 1.82), adjusted HR 0.54 (95% CI 0.18 to 1.62), cases selenium: 5, placebo: 9 total carcinoma RR 0.55 (95% CI 0.40 to 0.77), adjusted HR 0.54 (95% CI 0.39 to 0.75), cases selenium: 59; placebo: 104 leukaemia /lymphomas RR 1.58 (95% CI 0.46 to 6.14), adjusted HR 1.50 (95% CI 0.49 to 4.60), cases selenium: 8, placebo 5 other specific non‐carcinomas RR 0.99 (95% CI 0.13 to 7.37), HR 0.99 (95% CI 0.20 to 4.94), cases selenium: 3, placebo: 3 total non‐carcinomas RR 1.17 (95% CI 0.57 to 2.44), adjusted HR 1.16 (95% CI 0.60 to 2.27), cases selenium: 19; placebo: 16 2) at the end of the blinded period (31 January 1996) (Duffield‐Lillico 2002): lung cancer RR 0.70 (95% CI 0.40 to 1.21), adjusted HR 0.74 (95% CI 0.44 to 1.24), cases selenium: 25, placebo: 35 prostate cancer RR 0.51 (95% CI 0.29 to 0.87), adjusted HR 0.48 (95% CI 0.28 to 0.80), cases selenium: 22, placebo: 42 colorectal cancer RR 0.46 (95% CI 0.19 to 1.08), adjusted HR 0.46 (95% CI 0.21 to 1.02), cases selenium: 9, placebo: 19 any cancer RR 0.75 (95% CI 0.58 to 0.98), adjusted HR 0.75 (95% CI 0.58 to 0.97), cases selenium: 105, placebo: 137 head and neck cancer RR 1.27 (95% CI 0.42 to 4.01), adjusted HR 1.27 (95% CI 0.47 to 3.42), cases selenium: 9, placebo: 7 bladder cancer RR 1.24 (95% CI 0.44 to 3.61), adjusted HR 1.28 (95% CI 0.50 to 3.25), cases selenium: 10, placebo: 8 oesophageal cancer RR 0.39 (95% CI 0.04 to 2.41), adjusted HR 0.40 (95% CI 0.08 to 2.07), cases selenium: 2, placebo: 5 breast cancer RR 1.82 (95% CI 0.62 to 6.01), adjusted HR 1.89 (95% CI 0.69 to 5.14), cases selenium: 11, placebo: 6 melanoma RR 1.21 (95% CI 0.46 to 3.30), adjusted HR 1.18 (95% CI 0.49 to 2.85), cases selenium: 11, placebo: 9 haematological cancer (lymphoma and leukaemia) RR 1.32 (95% CI 0.40 to 4.61), adjusted HR 1.25 (95% CI 0.43 to 3.61), cases selenium: 8, placebo: 6 cancer mortality, all sites RR 0.59 (95% CI 0.39 to 0.89), adjusted HR 0.59 (95% CI 0.39 to 0.87), cases selenium: 40, placebo: 66 other carcinomas RR 0.66 (95% CI 0.19 to 2.07), adjusted HR 0.67 (95% CI 0.24 to 1.88), cases selenium: 6, placebo:9 other non‐carcinomas RR 0.59 (95% CI 0.09 to 3.04), adjusted HR 0.59 (95% CI 0.14 to 2.47), cases selenium: 3, placebo: 5 |
|
| Selenium levels in exposure categories | d.n.a. | |
| Notes | Adverse effects: Clark 1996: 35 participants (21 in selenium and 14 in control group) complained of adverse effects, mostly involving gastrointestinal upset, and withdrew treatment. Post‐hoc introduced secondary outcomes were: all‐cause mortality, total cancer mortality, total cancer incidence and incidence of lung / prostate / colorectal cancers HR: adjusted for sex, age, smoking status, clinic site, plasma selenium concentration, clinical sun damage, sunscreen use at baseline and number of BCCs/SCCs/NMSCs in the 12 months before randomisation |
|
Overvad 1991
| Methods | Cohort/sub‐cohort‐controlled cohort study Country: Channel Islands |
|
| Participants |
Participants: 5162 women Inclusion criteria: ≥ 35 years of age; ostensibly healthy inhabitants of Guernsey Name of parent cohort: Channel Island Cohort Recruitment: 1967 to 1976 Outcome assessment: end of 1985 Number of cases: Breast cancer: 46 (male/female: 0/46) Case definition: incidence Years of follow‐up: mean: 11 years for cases Type of selenium marker: plasma |
|
| Interventions | d.n.a. | |
| Outcomes | Analysed cases: 46 of 88 cases analysed (reason for non‐inclusion: no plasma available) Statistical methods: logistic regression Variables controlled in analysis: age, age at menarche, age at first baby, parity, BMI | |
| Risk estimates [95% CI] |
Reference category: highest quartile Results: Breast cancer lowest quartile: RR 0.80 (95% CI 0.29 to 2.19) |
|
| Selenium levels in exposure categories | lowest quartile: ≤ 84.90 µg/l highest quartile: ≥ 116.00 µg/l | |
| Notes | ||
Peleg 1985
| Methods | Matched, nested case‐control study Country: US |
|
| Participants |
Participants: 2530 men and women Inclusion criteria: 15 years of age and older; residents of Evans county; cases within first two years of follow‐up excluded Name of parent cohort: Evans County Study Recruitment: 1967 to 1969 Outcome assessment: January 1981 Number of cases: Any cancer: 130 (male/female: 78/52) Case definition: incidence Years of follow‐up: 11.0 to 14.0 years Type of selenium marker: serum |
|
| Interventions | d.n.a. | |
| Outcomes | Statistical methods: n.r. Variables controlled by matching: age, gender, race/ethnicity, year/month of sample collection | |
| Risk estimates [95% CI] |
Reference category: highest quartile Results: Any cancer both genders: lowest quartile: OR 1.0 (CI not reported) |
|
| Selenium levels in exposure categories | lowest quartile: ≤ 0.103 µg/ml highest quartile: ≥ 0.127 µg/ml | |
| Notes | ||
Persson 2000
| Methods | Matched, nested case‐control study Country: Sweden |
|
| Participants |
Participants: approximately 9500 men (exact figure not reported) Inclusion criteria: 46 to 48 years; residents of Malmo/Sweden Name of parent cohort: Malmö Preventive Programme Recruitment: 1974 to 1982 Outcome assessment: end of 1988 Number of cases: Any cancer: 302 (male/female: 302/0) Gastrointestinal cancer: 115 (male/female: 115/0) Respiratory tract cancer: 69 (male/female: 69/0) Other: 61 (male/female: 61/0) Urinary tract cancer: 57 (male/female: 57/0) Case definition: incidence Years of follow‐up: 6.0 to 15.0 years Type of selenium marker: plasma selenium P |
|
| Interventions | d.n.a. | |
| Outcomes | Analysed cases: 302 of 400 cases analysed (reason for non‐inclusion: no sample available) Statistical methods: logistic regression, Mantel‐Haenszel Variables controlled in analysis: smoking Variables controlled by matching: age, year/month/date of sample collection | |
| Risk estimates [95% CI] |
Reference category: highest tertile/quintile Results: Any cancer male: lowest quintile: OR 5.2 (95% CI 1.2 to 23.4) Gastrointestinal cancer male: lowest tertile: OR 3.4 (95% CI 1.1 to 10.2) Respiratory tract cancer male: lowest tertile: OR 6.0 (95% CI 1.5 to 24.2) Other cancers: male: lowest tertile: OR 0.6 (95% CI 0.2 to 2.1) Urinary tract cancer male: lowest tertile: OR 0.2 (95% CI 0.0 to 1.4) |
|
| Selenium levels in exposure categories | ||
| Notes | Arbitrary unit: Concentration of selenoprotein was expressed in arbitrary units (AU) relative to a standard of pooled plasma. 0.3 AU equal one standard deviation. | |
Peters 2007
| Methods | Matched, nested case‐control study Country: US |
|
| Participants |
Participants: 26,975 white non‐Hispanic men Inclusion criteria: 55 to 74 years of age; excluded: no baseline questionnaire/informed consent/blood sample, no further contact after screening Name of parent cohort: Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial Recruitment: September 1993 to June 2001 Outcome assessment: 1 October 2001 Number of cases: Prostate cancer: 724 (male/female: 724/0) Case definition: incidence Years of follow‐up: 0.3 to 8.0 years Type of selenium marker: serum |
|
| Interventions | d.n.a. | |
| Outcomes | Analysed cases: 724 of 803 cases included in analysis (reason for non‐inclusion: no selenium measurement available) Statistical methods: n.r. Variables controlled in analysis: age, time since initial screening, year of blood collection, study centre Variables controlled by matching: age, month of sample collection, time since initial screening | |
| Risk estimates [95% CI] |
Reference category: lowest quartile Results: Prostate cancer highest quartile: OR 0.84 (95% CI 0.62 to 1.14) |
|
| Selenium levels in exposure categories | lowest quartile: 50.5 to 126.7 ng/ml highest quartile: 158.0 to 253.0 ng/ml | |
| Notes | ||
Peters 2008
| Methods | Cohort study Country: US |
|
| Participants |
Inclusion criteria: aged 50 to 76 years, participants recruited from subscribers of commercial mailing list, residents of western Washington state, non‐whites excluded, no malignant disease at baseline Name of parent cohort: Vitamins and lifestyle (VITAL) study Recruitment: 1 October 2000 to 31 December 2002 Type of selenium marker: supplemental intake (questionnaire: use of supplements over the last 10 years, mean supplemental intake / day calculated) Case definition: incidence Peters 2008: Participants: 35,242 men Outcome assessment: 31 December 2004 Number of cases: Prostate cancer: 818 (male/female: 818/0) Years of follow‐up: 2 to 4 years Asgari 2009: Participants: 69,671 men and women Outcome assessment: 31 December 2006 Number of cases: Melanoma: 461 (male/female: n.r.) Years of follow‐up: 4 to 5 years |
|
| Interventions | d.n.a. | |
| Outcomes |
Peters 2008: Analysed cases: 818 of 830 cases analysed (reason for non‐inclusion: not reported) Statistical methods: Cox proportional hazard regression analysis Variables controlled in analysis: age, family history of prostate cancer, BPH, income, multivitamin use Asgari 2009: Analysed cases: one case not analysed (reason for non‐inclusion: not reported) Statistical methods: Cox proportional hazard regression Variables controlled in analysis: age, sex, education, family history of melanoma, personal history of non‐melanoma skin cancer, mole removal, freckles, sunburns, hair colour, reaction to sunlight exposure |
|
| Risk estimates [95% CI] |
Reference category: no supplemental selenium intake (lowest exposure category) Peters 2008: Results: Prostate cancer highest exposure category: RR 0.90 (95% CI 0.62 to 1.30) Asgari 2009: Results: Melanoma highest exposure category HR 0.98 (95% CI 0.69 to 1.41) |
|
| Selenium levels in exposure categories | stratification according to supplemental selenium intake Peters 2008: lowest category: no supplemental intake highest category ≥ 51 µg/day Asgari 2009: lowest exposure category: no supplemental intake highest exposure category ≥ 50 µg/day |
|
| Notes | ||
Ratnasinghe 2000
| Methods | Matched, nested case‐control study Country: China |
|
| Participants |
Participants: 9143 men Inclusion criteria: 35 years or older; tin miners employed by the Yunnan Tin Corporation; 10 or more years of underground mining / smelting; no history of cancer at baseline Recruitment: 1992 to 1997 Outcome assessment: 1997 Number of cases: Lung cancer: 108 (male/female: 108/0) Case definition: incidence Years of follow‐up: ≈ 3 years Type of selenium marker: serum |
|
| Interventions | d.n.a. | |
| Outcomes | Analysed cases: plasma was available for 108 of a total of 339 identified cases Statistical methods: logistic regression, conditional logistic regression, Wilcoxon rank sum test Variables controlled in analysis: radon exposure, smoking Variables controlled by matching: age, year and month of sample collection | |
| Risk estimates [95% CI] |
Reference category: lowest tertile Results: Lung cancer highest tertile: OR 1.2 (95% CI 0.6 to 2.4) |
|
| Selenium levels in exposure categories | lowest tertile: 20 to 39 ng/ml highest tertile: 55 to 121 ng/ml | |
| Notes | ||
Reid 2008
| Methods | Randomised controlled trial Sub‐study of the Nutritional Prevention of Cancer Trial (NPCT 2002) Allocation: random Sequence generation: computer generated random numbers Concealment: central assignment (sealed pill bottles) Blinding: participant blinded, doctor blinded, outcome assessor/pathologist unclear, review/coding of medical records blinded Dropouts/withdrawals: two participants declined to provide additional illness information, no participant lost to vital follow ‐up Intention‐to‐treat‐analysis: yes Recruitment period: 1989‐1992 Treatment duration: Blinded intervention continued until the end of the blinded period; 1 February 1996. Observation period/dermatologic follow‐up: 1 February 1996 Detection of cases: dermatological examination and interview every 6 months during follow‐up; incident BCC and SCC were diagnosed by biopsy and confirmed by another dermatopathologist Informed consent: written informed consent forms, approval by institutional review board of participating institutions |
|
| Participants | 423 male and female participants with prior non‐melanoma skin cancer Country: US Number of patients: 423 (randomised to selenium group: 210, to placebo group: 213) Condition: male and female patients with history of 2 or more squamous cell or basal cell skin cancers Demographics: mean age 63.8 years (selenium)/63.8 years (placebo); 66.2% men (selenium). 68.2% men (placebo) Recruitment and setting: dermatologic clinic in Macon, Georgia |
|
| Interventions |
Intervention: 400 µg selenium supplied as selenium yeast tablets p.o./daily. Control: placebo 400 µg/day of selenium yeast or identical‐appearing low selenium yeast placebo Recruitment: 12 September 1989 to 3 April 1992 End of the blinded treatment period: 2 February 1996 |
|
| Outcomes |
Primary outcome measure: incidence of basal and squamous cell carcinoma of the skin: all analyses were based on n = 423 participants with initial blood collection within 4 days after randomizations Other reported outcomes: total internal cancer incidence |
|
| Risk estimates [95% CI] |
Primary outcomes: BCC: RR 0.90 (95% CI 0.65 to 1.24); cases: selenium group: 76, placebo group: 83; adjusted HR: 0.95 (95% CI 0.69 to 1.29) SCC: RR 1.05 (95% CI 0.71 to 1.56); cases: selenium group: 56, placebo group: 53; adjusted HR: 1.05 (95% CI 0.72 to 1.53) NMSC: RR 0.88 (95% CI 0.66 to 1.16); cases: selenium group: 98, placebo group: 108; adjusted HR: 0.91 (95% CI 0.69 to 1.20) NMSC in women: RR 0.40 (95% CI 0.20 to 0.80) Other reported outcomes: total internal cancer incidence: RR 1.10 (95% CI 0.57 to 2.17); cases: selenium group: 21, placebo group: 19 |
|
| Selenium levels in exposure categories | d.n.a. | |
| Notes | Information on study design, which was not reported in Reid 2008, was taken from the information available on the Nutritional Prevention of Cancer Trial. Adverse effects: not reported HR: adjusted for: age (continuous), smoking status (never, former, current), gender |
|
Ringstad 1988
| Methods | Matched, nested case‐control study Country: Norway |
|
| Participants |
Participants: 9364 men and women Inclusion criteria: 20 to 54 years of age (men), 20 to 49 years of age (women); inhabitants of Tromso; blood sample provided in 1979; no history of cancer at baseline Name of parent cohort: Tromso Heart Study II Recruitment: 1979 to 1980 Outcome assessment: 1985 Number of cases: Any cancer: 60 (male/female: 26/34) Case definition: incidence Years of follow‐up: 5.0 to 7.0 years Type of selenium marker: serum |
|
| Interventions | d.n.a. | |
| Outcomes | Analysed cases: 60 of 72 cases analysed (reason for non‐inclusion: no sample available) Statistical methods: n.r. Variables controlled by matching: age, gender, smoking status, month of sample collection, place of residence (district of Tromso) | |
| Risk estimates [95% CI] |
Reference category: highest three quartiles Results: Any cancer both genders: lowest quartile: OR 1.4 (95% CI 0.6 to 3.5) |
|
| Selenium levels in exposure categories | lowest quartile: ≤ 114.49 µg/l highest three quartiles: 114.50 to 114.51 µg/l | |
| Notes | ||
Sakoda 2005
| Methods | Matched, nested case‐control study Country: China |
|
| Participants |
Participants: 41,563 men and women Inclusion criteria: inhabitants of Haiman city of Chinese origin; written consent; toenail clipping available Recruitment: January 1993 to December 1993 Outcome assessment: 30 September 2000 Number of cases: Primary liver cancer: 166 (male/female: 154/12) Case definition: mortality Years of follow‐up: 6.8 to 7.8 years Type of selenium marker: toenail |
|
| Interventions | d.n.a. | |
| Outcomes | Analysed cases: 166 of 455 observed cases included in analysis (only cases with questionnaire, blood sample and toenail specimen analysed after 2000 due to different methods of selenium analysis) Statistical methods: not specified Variables controlled in analysis: both genders: age, gender, HBsAg‐status, alcohol intake, history of acute hepatitis, occupation men: age, HBsAg‐status, alcohol intake, history of acute hepatitis, family history of HCC, occupation women: HBsAg‐status, age, history of acute hepatitis Variables controlled by matching: age, gender, township of residence | |
| Risk estimates [95% CI] |
Reference category: lowest quartile Results: Primary liver cancer both genders: highest quartile: OR 0.50 (95% CI 0.28 to 0.90) male: highest quartile: OR 0.57 (95% CI 0.31 to 1.05) female: highest three quartiles: OR 0.18 (95% CI 0.03 to 1.13) |
|
| Selenium levels in exposure categories | both genders and men: lowest quartile: 0 to 1.70 ppm highest quartile: ≥ 4.43 ppm women: lowest quartile: 0.00 to 1.70 ppm highest three quartiles ≥ 1.71 ppm |
|
| Notes | ||
Salonen 1984
| Methods | Matched, nested case‐control study Country: Finland |
|
| Participants |
Participants: 8113 men and women Inclusion criteria: 31 to 59 years of age; random sample of inhabitants of two Finnish provinces; initially free of cancer Name of parent cohort: North Karelia Project Recruitment: February to April 1972 Outcome assessment: 31 December 1978 Number of cases: Any cancer: 128 (male/female: n.r.) Case definition: incidence Years of follow‐up: 8.5 years Type of selenium marker: serum |
|
| Interventions | d.n.a. | |
| Outcomes | Statistical methods: logistic regression / paired‐sample OR Variables controlled in analysis: tobacco consumption, serum cholesterol, beer consumption, dietary saturated fats, years of education, study area Variables controlled by matching: age, gender, smoking (tobacco use/day), total serum cholesterol | |
| Risk estimates [95% CI] |
Reference category: above 30th percentile Results: Any cancer both genders: ≤ 30th percentile: OR 3.1 (95% CI 1.5 to 7.7) both genders: ≤ 0th percentile: OR 3.0 (95% CI 1.2 to 21.9) |
|
| Selenium levels in exposure categories | 1 to 10th percentile ≤ 34.00 µg/l above 30th percentile ≥ 45.00 µg/l | |
| Notes | ||
Salonen 1985
| Methods | Matched, nested case‐control study Country: Finland |
|
| Participants |
Participants: 12,155 men and women Inclusion criteria: 30 to 64 years of age; random sample of residents of two Finnish provinces; initially free of cancer Name of parent cohort: North Karelia Project Recruitment: January to March 1977 Outcome assessment: 31 December 1980 Number of cases: Any cancer: 51 (male/female: 30/21) Case definition: mortality Years of follow‐up: 3.7 years Type of selenium marker: serum |
|
| Interventions | d.n.a. | |
| Outcomes | Analysed cases: 51 out of 56 cases (reason for non‐inclusion: no serum sample available) Statistical methods: logistic regression Variables controlled by matching: age, gender, smoking (tobacco use/day) | |
| Risk estimates [95% CI] |
Reference category: highest two tertiles Results: Any cancer both genders: lowest tertile: OR 5.8 (95% CI 1.2 to 29.0) |
|
| Selenium levels in exposure categories | lowest tertile: ≤ 47.00 µg/l highest two tertiles ≥ 47.10 µg/l | |
| Notes | ||
SELECT 2009
| Methods | Randomised controlled trial SELECT (Selenium and Vitamin E Cancer Prevention Trial) Allocation: random, block/stratified by clinic Sequence generation: computer‐generated random numbers Concealment: central assignment (pill bottles) Blinding: participant blinded, doctor blinded, outcome assessor/pathologist blinded, review/coding of medical records blinded Dropouts/withdrawals: of 35,533 randomised participants, 645 were excluded from analysis because they had prior prostate cancer, did not give informed consent or participated at two study sites, which were excluded due to management and regulatory issues Intention‐to‐treat‐analysis: yes Recruitment period: 22 August 2001 to 24 June 2004 End of study period: 1 August 2009 Blinded intervention was discontinued on 23 October 2008 following the recommendation of the data safety and monitoring committee after the second formal interim analysis in September 2008 Detection of cases: Participants had clinic visits once every 6 months and reported prostate cancers to the study staff. Study staff obtained medical records to verify the diagnosis. Tissue and the corresponding pathology report were sent to the central pathology laboratory for confirmation. Informed consent: yes |
|
| Participants |
Countries: US, Canada, Puerto Rico Number of participants: 34,888 men, randomised to four groups: placebo (8696), vitamin E (8737), selenium (8752), selenium + vitamin E (8703) Condition: healthy men, aged 50 years or older (African American) or 55 years or older (all other), no prior diagnosis of prostate cancer, 4 ng/ml or less of PSA in serum, a digital rectal examination not suspicious for cancer, no current use of anticoagulant therapy other than 175 mg/day or less of acetylsalicylic acid or 81 mg/day or less of acetylsalicylic acid with clopidogrel bisulphate, no history of haemorrhagic stroke, normal blood pressure. Demographics: median age: 62.3‐62.6 years in all four intervention groups, 79% white in all four intervention groups Recruitment and setting: 427 participating sites |
|
| Interventions | Group 1: placebo + placebo Group 2: 400 IU/day all rac‐alpha‐tocopheryl acetate + placebo Group 3: 200 µg/day L‐selenomethionine + placebo Group 4: 400 IU/day all rac‐alpha‐tocopheryl acetate + 200 µg/day L‐selenomethionine |
|
| Outcomes |
Primary outcome: incidence of prostate cancer as determined by routine clinical management Secondary outcomes: incidence of any cancer / lung cancer / colorectal cancer, diabetes mellitus, cardiovascular events, death from any cause |
|
| Risk estimates [95% CI] | Results are presented for the comparison of selenium alone (group 3) versus placebo (group 1) Primary outcome: prostate cancer HR 1.04, (95% CI 0.90 to 1.18), (99% CI 0.87 to 1.24), cases: selenium 432 (5‐year rate: 4.56%), placebo 416 (5‐year rate 4.43%) Secondary outcomes: any cancer HR 1.01, (95% CI 0.89 to 1.15) lung cancer HR 1.12, (99% CI 0.73 to 1.72) colorectal cancer 1.05, (99% CI 0.66 to 1.67) other primary cancer (excluding prostate cancer, basal cell and squamous cell skin cancer) 0.95, (99% CI 0.77 to 1.17) diabetes mellitus 1.07, (99% CI 0.94 to 1.22) cardiovascular events 1.02, (99% CI 0.92 to 1.13) deaths 0.99, (99% CI 0.82 to 1.19) deaths from cancer 1.02, (99% CI 0.74 to 1.41) |
|
| Selenium levels in exposure categories | d.n.a. | |
| Notes | Adverse effects: alopecia RR 1.28, (99% CI 1.01 to 1.62) dermatitis grade 1‐2 RR 1.17, (99% CI 1.00 to 1.35) dermatitis grade 3‐4 RR 1.74, (99% CI 0.56 to 5.44) halitosis RR 1.17, (99% CI 0.99 to 1.38) nail changes RR 1.04, (99% CI 0.94 to 1.16) fatigue grade 1‐2 RR 1.09, (99% CI 0.95 to 1.26) fatigue grade 3‐4 RR 0.87, (99% CI 0.40 to 1.88) nausea grade 1‐2 RR 1.19, (99% CI 0.94 to 1.52) nausea grade 3 RR 0.99, (99% CI 0.30 to 3.34) |
|
Steevens 2010
| Methods | Cohort/sub‐cohort‐controlled cohort study Country: the Netherlands |
|
| Participants |
Name of parent cohort: Netherlands Cohort Study (NLCS) Recruitment: 1986 van den Brandt 1993b: Participants: 120,852: 58,279 men and 62,573 women; aged 55 to 69 years; returned baseline questionnaire; no history of cancer at baseline Outcome assessment: 31/12/2002 Number of cases: esophageal squamous cell carcinoma (ESCC): 64 (male/female: 40/24) esophageal adenocarcinoma (EAC): 112 (male/female: 93/19) gastric cardia adenocarcinoma (GCA): 114 (male/female: 97/17) Case definition: incidence Years of follow‐up: 16.3 years, Type of selenium marker: toenail |
|
| Interventions | d.n.a. | |
| Outcomes |
Analysed cases: esophageal squamous cell carcinoma (ESCC): 64 of 71 esophageal adenocarcinoma (EAC): 112 of 129 gastric cardia adenocarcinoma (GCA): 114 of 127 Statistical methods: Cox proportional hazards models Variables controlled in analysis: age, sex, cigarette smoking (current yes/no, number of cigarettes smoked daily, and number of smoking years), alcohol consumption (g/day), andBMI (kg/m2) |
|
| Risk estimates [95% CI] |
Reference category: lowest quartile Results: esophageal squamous cell carcinoma (ESCC): both genders: highest quartile: RR 0.37 (95% CI 0.16 to 0.86) men: highest quartile: RR 0.81 (95% CI 0.64 to 1.4) women: highest quartile: RR 0.79 (95% CI 0.63 to 0.99) esophageal adenocarcinoma (EAC): both genders: highest quartile: RR 0.76 (95% CI 0.41 to 1.40) men: highest quartile: RR 1.07 (95% CI 0.99 to 1.15) women: highest quartile: RR 0.72 (95% CI 0.61 to 0.84) gastric cardia adenocarcinoma (GCA): both genders: highest quartile: RR 0.52 (95% CI 0.27 to 1.02) men: highest quartile: RR 0.94 (95% CI 0.84 to 1.06) women: highest quartile: RR 0.73 (95% CI 0.56 to 0.95) |
|
| Selenium levels in exposure categories | lowest quartile: ≤ 0.498 µg/g highest quartile: ≥ 0.613 µg/g | |
| Notes | ||
Steinbrecher 2010
| Methods | Nested case‐control study Country: Germany |
|
| Participants |
Participants: 11928 men (from the total cohort of 25540 men and women) Name of parent cohort: EPIC‐Heidelberg cohort Recruitment: 1994‐1998. Outcome assessment: 2/2007 Number of cases: prostate cancer: 248 Case definition: incidence Years of follow‐up: mean: 3 years Type of selenium marker: serum selenium concentration |
|
| Interventions | d.n.a. | |
| Outcomes |
Statistical methods: conditional logistic regression Variables controlled in analysis: family history of prostate cancer, participation in PSA testing, smoking status, and vigorous physical activity. variables controlled in matching: age group and time of recruitment |
|
| Risk estimates [95% CI] |
Prostate cancer Reference category: lowest quartile highest quartile OR 1.10 (95% CI, 0.58‐2.09) |
|
| Selenium levels in exposure categories | lowest quartile: ≤ 78.9 µg/L highest quartile: ≥ 95 µg/L | |
| Notes | ||
Suadicani 2012
| Methods | Cohort Study Country: Denmark |
|
| Participants |
Participants: 3,333 males, male participants were derived from 14 workplaces in Copenhagen: the air force, army, navy, emergency management agency, postal service, customs service, a railroad company, national bank, a telephone company, three municipal service centres (for electricity and engineering and a fire brigade), a pharmaceutical company, and a building contractor company. Name of parent cohort: Copenhagen male study Recruitment: from 1970‐1971/1985‐1986 Outcome assessment: 1985‐1986/2001 Number of cases: deaths for lung cancer: 167 Case definition: death for lung cancer Years of follow‐up: 16 years Type of selenium marker: serum selenium concentration |
|
| Interventions | d.n.a. | |
| Outcomes | Statistical methods: Cox logistic regression Variables controlled in analysis: age, pack‐years of smoking, spirits intake and dietary markers | |
| Risk estimates [95% CI] |
Reference category: lowest exposure category: 0.4‐1.0 µmol.L^‐1 Results: Deaths for lung cancer highest exposure category: HR 1.43 (95% CI 0.96 to 2.14) |
|
| Selenium levels in exposure categories | lowest category: 0.4‐1.0 µmol.L^‐1 highest category: 1.3‐3.0 µmol.L^‐1 |
|
| Notes | ||
Thomson 2008
| Methods | Cohort Study Country: US |
|
| Participants |
Participants: 133,614 women Inclusion criteria: post‐menopausal participants (aged 50 to 79 years) of the WHI clinical trial and observational study Name of parent cohort: Women's Health Initiative (WHI) Recruitment: n.r. Outcome assessment: December 2004 Number of cases: ovarian cancer: 451 (0/451) Case definition: incidence Years of follow‐up: mean: 7 years Type of selenium marker: supplemental selenium intake (food frequency questionnaire) |
|
| Interventions | d.n.a. | |
| Outcomes | Statistical methods: Cox logistic regression Variables controlled in analysis: participation in observational or intervention study, age, log calories, number of relatives with breast/ovarian cancer, dietary modification randomisation arm, hysterectomy, minority race, pack‐years of smoking, physical activity, NSAID use, parity, infertility, duration of oral contraceptive use, number of lifetime ovulatory cycles, partial oophorectomy, age at menopause, hormone therapy at study entry | |
| Risk estimates [95% CI] |
Reference category: no intake of supplemental selenium (lowest exposure category) Results: Ovarian Cancer highest exposure category: HR 1.00 (95% CI 0.73 to 1.37) |
|
| Selenium levels in exposure categories | lowest exposure category: no supplemental selenium intake highest exposure category: > 20 µg/day supplemental selenium intake |
|
| Notes | ||
van den Brandt 1993a
| Methods | Cohort/sub‐cohort‐controlled cohort study Country: the Netherlands |
|
| Participants |
Name of parent cohort: Netherlands Cohort Study (NLCS) Recruitment: 1986 van den Brandt 1993b: Participants: 120,852: 58,279 men and 62,573 women; aged 55 to 69 years; returned baseline questionnaire; no history of cancer at baseline Outcome assessment: n.r. Number of cases: Stomach cancer: 104 (male/female: 84/20) Colon cancer: 234 (male/female: 121/113) Rectal cancer: 113 (male/female: 77/36) van den Brandt 1993a: Participants: 120,852: 58,279 men and 62,573 women; age 55 to 69 years; returned baseline questionnaire; no history of cancer at baseline Outcome assessment: n.r. Number of cases: Lung cancer: 370 (male/female: 335/35) van den Brandt 1994: Participants: 62,573 post‐menopausal women Outcome assessment: 1989 Number of cases: Breast cancer (post‐menopausal): 355 (male/female: 0/355) Breast cancer (post‐menopausal), multivariate analysis: 270 (male/female: 0/270) Zeegers 2002: Participants: 120,852: 58,279 men and 62,573 women Outcome assessment: December 1992 Number of cases: Bladder cancer: 431 (male/female: 372/59) van den Brandt 2003: Participants: 58,279 men Outcome assessment: n.r. (probably December 1992) Number of cases: Prostate cancer: 540 (male/female: 540/0) Case definition: incidence Years of follow‐up: 3.3 years (Brandt 1993a; Brandt 1993b; Brandt 1994), 6.3 years (Zeegers 2002; Brandt 2003) Type of selenium marker: toenail |
|
| Interventions | d.n.a. | |
| Outcomes |
van den Brandt 1993b:
Analysed cases: 234 of 351 colon cancer cases / 104 of 176 stomach cancer cases / 113 of 185 rectal cancer cases analysed (reasons for non‐inclusion: history of cancer at baseline not available, no pathological confirmation or CIS, no toenail clipping available) Statistical methods: Mantel‐Haenszel Variables controlled in analysis: age, gender van den Brandt 1993a: Analysed cases: 370 of 617 cases analysed (reasons for non‐inclusion: history of cancer at baseline not available, no toenail clipping, no pathological confirmation, problems with selenium measurement) Statistical methods: Statistical methods: Mantel‐Haenszel Variables controlled in analysis: age, gender van den Brandt 1994: Analysed cases: 355 of 553 cases analysed (reasons for non‐inclusion: history of cancer at baseline not available, CIS, no toenail sample or problems with selenium detection) Statistical methods: multivariate case‐cohort analysis Variables controlled in analysis: age, history of benign breast disease, maternal breast cancer, breast cancer in sister(s), age at menarche, age at menopause, oral contraceptive use, parity, age at first birth, body mass index, education, current cigarette smoking, alcohol intake, energy intake Zeegers 2002: Analysed cases: 431 of 619 cases analysed (reason for non‐inclusion: no toenails available) Statistical methods: exponentially distributed failure time regression models Variables controlled in analysis: age, gender, number of cigarettes/day, years of cigarette smoking van den Brandt 2003: Analysed cases: 540 of 704 cases analysed (reason for non‐inclusion: no toenail samples or selenium detection not possible) Statistical methods: exponentially distributed failure time regression models Variables controlled in analysis: age, family history of prostate cancer, number of cigarettes/day, years of cigarette smoking, level of education |
|
| Risk estimates [95% CI] |
Reference category: lowest quartile/quintile Results: van den Brandt 1993b: Stomach cancer both genders: highest quintile: RR 0.61 (95% CI 0.33 to 1.11); highest quintile: RR 0.64 (95% CI 0.33 to 1.27) (max. adj.) men: highest quintile: RR 0.40 (95% CI 0.17 to 0.96) (max. adj.) women: highest quartile: RR 1.68 (95% CI 0.43 to 6.54) (max. adj.) Colon cancer both genders: highest quintile: RR 0.77 (95% CI 0.49 to 1.19); highest quintile: RR 0.80 (95% CI 0.50 to 1.29) (max. adj.) men: highest quintile: RR 0.82 (95% CI 0.43 to 1.58) (max. adj.) women: highest quintile: RR 0.77 (95% CI 0.41 to 1.45) (max. adj.) Rectal cancer both genders: highest quintile: RR 1.01 (95% CI 0.55 to 1.84); highest quintile: RR 1.05 (95% CI 0.54 to 2.03) (max. adj.) men: highest quintile: RR 0.91 (95% CI 0.41 to 2.00) (max. adj.) women: highest quartile: RR 1.58 (95% CI 0.59 to 4.22) (max. adj.) van den Brandt 1993a: Lung cancer both genders: highest quintile: RR 0.40 (95% CI 0.27 to 0.59) men: highest quintile: RR 0.50 (95% CI 0.30 to 0.82) women: highest quartile: RR 0.40 (95% CI 0.13 to 1.24) van den Brandt 1994: Breast cancer multivariate analysis: highest quintile: RR 0.84 (95% CI 0.55 to 1.27) age‐stratified analysis: highest quintile: RR 0.93 (95% CI 0.65 to 1.33) Zeegers 2002: Bladder cancer both genders: highest quintile: RR 0.67 (95% CI 0.46 to 0.97) van den Brandt 2003: Prostate cancer highest quintile: RR 0.69 (95% CI 0.48 to 0.99) |
|
| Selenium levels in exposure categories |
van den Brandt 1993b: lowest quintile: ≤ 0.483 µg/g highest quintile: ≥ 0.631 µg/g lowest quartile: ≤ 0.497 µg/g highest quartile: ≥ 0.613 µg/g van den Brandt 1993a: both genders and men: lowest quintile: ≤ 0.483 µg/g highest quintile: ≥ 0.631 µg/g women: lowest quartile ≤ 0.497 µg/g highest quartile ≥ 0.613 µg/g van den Brandt 1994: women: lowest quintile: ≤ 0.499 µg/g highest quintile: ≥ 0.646 µg/g Zeegers 2002 : lowest quintile: ≤ 0.483 µg/g highest quintile: ≥ 0.631 µg/g van den Brandt 2003: men: lowest quintile: ≤ 0.467 µg/g highest quintile: ≥ 0.617 µg/g |
|
| Notes | Primary publication: van den Brandt 1993b Other publications: Zeegers 2002, van den Brandt 1993a, van den Brandt 1994, van den Brandt 2003 | |
van Noord 1987
| Methods | Matched, nested case‐control study Country: the Netherlands |
|
| Participants |
Participants: 8760 women Inclusion criteria: 42 to 52 years of age; pre‐menopausal; inhabitants of Utrecht Name of parent cohort: DOM (Diagnostic onderzoek mammacarcinoom) Study Recruitment: n.r. Outcome assessment: 1 February 1986 Number of cases: Breast cancer (pre‐menopausal): 27 (male/female: 0/27) Case definition: incidence Years of follow‐up: 0.6 to 3.5 years, mean: 2.1 years Type of selenium marker: toenail |
|
| Interventions | d.n.a. | |
| Outcomes |
Analysed cases: 7 cases were detected in the initial mammography screening in this study and not included in the analysis of incident cases Statistical methods: n.r. Variables controlled by matching: age, date of birth, pre‐menopausal status |
|
| Risk estimates [95% CI] |
Reference category: lowest quartile Results: Breast cancer (pre‐menopausal) highest quartile: OR 1.1 (95% CI 0.5 to 2.9) |
|
| Selenium levels in exposure categories | n.r. | |
| Notes | ||
Virtamo 1987
| Methods | Cohort/sub‐cohort‐controlled cohort study Country: Finland |
|
| Participants |
Participants: 1110 men Inclusion criteria: 55 to 74 years of age; inhabitants of Finnish rural areas; participants of prior study on CHD; serum sample available: cases within first year of follow‐up excluded Name of parent cohort: Men in rural East and West Finland Recruitment: 1974 Outcome assessment: 31 December 1983 Number of cases: Any cancer: 109 (male/female: 109/0) Case definition: incidence Years of follow‐up: 10.0 years Type of selenium marker: serum |
|
| Interventions | d.n.a. | |
| Outcomes | Statistical methods: conditional logistic regression Variables controlled in analysis: age, area of residence, smoking, serum cholesterol, alcohol intake | |
| Risk estimates [95% CI] |
Reference category: highest tertile Results: Any cancer lowest tertile OR 1.14 (95% CI 0.66 to 1.98) |
|
| Selenium levels in exposure categories | lowest tertile: 15 to 46µg/l highest tertile: 60 to 136µg/l | |
| Notes | ||
Walter 2011
| Methods | Cohort study Country: US |
|
| Participants |
Inclusion criteria: aged 50 to 76 years, participants recruited from subscribers of commercial mailing list, residents of western Washington state, non‐whites excluded, no malignant disease at baseline Name of parent cohort: Vitamins and lifestyle (VITAL) study Recruitment: 1 October 2000 to 31 December 2002 Outcome assessment: 31/12/2008 Type of selenium marker: supplemental intake (questionnaire: use of supplements over the last 10 years, mean supplemental intake / day calculated) Case definition: incidence Number of cases: hematologic malignancies: 588 |
|
| Interventions | d.n.a. | |
| Outcomes | Statistical methods: cox proportional hazards regression, Variables controlled in analysis: sex, race/ethnicity (white, Hispanic, other), education (high school graduate or less, some college, college or advanced degree), smoking (pack‐years), self‐rated health (excellent, very good, good, fair, poor), vegetable servings per day (excluding potato servings); fruit servings per day; history of coronary artery disease (defined as history of heart attack, coronary bypass surgery, angioplasty, and/or angina; yes, no), history of rheumatoid arthritis (yes, no), history of fatigue or lack of energy over the year prior to baseline (yes, no), and number of first‐degree relatives with a history of leukemia or lymphoma (none, 1, 2) | |
| Risk estimates [95% CI] |
Reference category: none Results: highest level: RR 0.95 (95% CI 0.75 to 1.20) |
|
| Selenium levels in exposure categories | lowest level: none highest level: 20.1–400.0 mg/d |
|
| Notes | ||
Wei 2004
| Methods | Frequency‐matched cohort controlled study Country: China |
|
| Participants |
Participants:Mark 2000: 29,584 men and women; Wei 2004: 1103 people who were originally selected as disease‐free controls in Mark 2000 Inclusion criteria: 40 to 69 years of age; healthy inhabitants of 4 Linxian communities; participants of a randomised controlled trial Name of parent cohort: General Population Trial Linxian Recruitment: 1985 Outcome assessment: May 1991 (Mark 2000); n.r. (Wei 2004) Number of cases: Wei 2004: oesophageal cancer: 75 (male/female: 49/26) mortality stomach, cardia cancer: 36 (male/female: 22/14) mortality stomach, non‐cardia cancer: 24 (male/female: 20/4) mortality other: 32 (male/female: 22/10) mortality Mark 2000: oesophageal cancer: 590 (male/female: 286/304) incidence oesophageal cancer: 332 (male/female: n.r.) mortality stomach, cardia cancer: 402 (male/female: 239/163) incidence stomach, cardia cancer: 232 (male/female: n.r.) mortality stomach, non‐cardia cancer: 87 (male/female: 66/21) incidence stomach, non‐cardia cancer: 68 (male/female: n.r.) mortality Case definition: mortality, incidence Years of follow‐up: unclear/approximately 9 years (Wei 2004), 6 years (Mark 2000) Type of selenium marker: serum |
|
| Interventions | d.n.a. | |
| Outcomes |
Statistical methods: cox‐proportional hazard model Variables controlled in analysis:Wei 2004: age, cholesterol, smoking, alcohol intake, BMI; Mark 2000: age Variables controlled by matching: age category, gender |
|
| Risk estimates [95% CI] |
Wei 2004:
Reference category: lowest quartile Results: Oesophageal cancer both genders: highest quartile: RR 0.35 (95% CI 0.16 to 0.81) Stomach, cardia cancer both genders: highest quartile: RR 0.31 (95% CI 0.11 to 0.87) Stomach, non‐cardia cancer both genders: highest quartile: RR 1.64 (95% CI 0.49 to 5.48) Other cancers both genders: highest quartile: RR 1.95 (95% CI 0.66 to 5.81) Mark 2000: Reference category: lowest quartile Results: Oesophageal cancer both genders / incidence: highest quartile: RR 0.56 (95% CI 0.44 to 0.71) both genders / mortality: highest quartile: RR 0.62 (95% CI 0.44 to 0.89) Stomach, cardia cancer both genders / incidence: highest quartile: RR 0.47 (95% CI 0.33 to 0.65) both genders / mortality: highest quartile: RR 0.59 (95% CI 0.39 to 0.90) Stomach, non‐cardia cancer both genders / incidence: highest quartile: OR 1.07 (95% CI 0.55 to 2.08) both genders / mortality: highest quartile: OR 1.03 (95% CI 0.85 to 2.02) |
|
| Selenium levels in exposure categories |
Wei 2004: lowest quartile: 0.00 to 0.76 µmol/l highest quartile ≥ 1.07 µmol/l Mark 2000: lowest quartile: 0.00 to 59.70 µg/l highest quartile ≥ 82.20 µg/l |
|
| Notes |
Primary publication: Wei 2004 Other publication: Mark 2000 Remark: Wei 2004 measured serum selenium in a sub‐cohort derived from 29,584 male and female participants of the Linxian Population Trial. The earlier publication of this study, Mark 2000 reported 332 fatal cases and 590 incident cases. The later publication, Wei 2004 reported deaths from oesophageal cancer in the disease‐free controls of Mark 2000 and analysed 75 fatal cases. |
|
Willett 1983
| Methods | Matched, nested case‐control study Country: US |
|
| Participants |
Participants: 10,940 men and women Inclusion criteria: 30 to 69 years of age; serum sample available (only 4480 samples of cohort were available because of freezer breakdown); participants of an RCT on hypertension; institutionalised and bedfast people were excluded Name of parent cohort: Hypertension Detection Follow‐Up Programme (HDFP) Recruitment: 1973 to 1974 Outcome assessment: n.r. Number of cases: Any cancer: 111 (male/female: 60/51) Case definition: incidence Years of follow‐up: 5.0 years Type of selenium marker: serum |
|
| Interventions | d.n.a. | |
| Outcomes | Statistical methods: logistic regression of unmatched data Variables controlled by matching: age, gender, race/ethnicity, smoking status, year/month of sample collection, initial blood pressure, use of antihypertensive medication, randomisation group in women: parity, menopausal status | |
| Risk estimates [95% CI] |
Reference category: highest quintile, highest three quintiles Results: Any cancer both genders: lowest quintile versus highest quintile: OR 2.0 (CI not reported) both genders: lowest quintile versus highest three quintiles: OR 1.9 (95% CI 1.1 to 3.3) |
|
| Selenium levels in exposure categories | lowest quintile: ≤ 0.114 µg/ml highest quintile: ≥ 0.154 µg/ml | |
| Notes | ||
Yoshizawa 1998
| Methods | Matched, nested case‐control study Country: US |
|
| Participants |
Participants: 33,737 men Inclusion criteria: 40 to 75 years of age; physicians from all 50 U.S. states; provision of toenails in 1987 and completed baseline questionnaire in 1986; exclusion of histologically confirmed prostate cancer at baseline and cases within first 2 years of follow‐up Name of parent cohort: Health Professionals Follow‐Up Study (HPFS) Recruitment: 1986 to 1987 Outcome assessment: 1994 Number of cases: Prostate cancer: 181 (male/female: 181/0) Case definition: incidence Years of follow‐up: 8.0 to 9.0 years Type of selenium marker: toenail |
|
| Interventions | d.n.a. | |
| Outcomes | Statistical methods: logistic regression, conditional logistic regression Variables controlled in analysis: quintiles of lycopene, saturated fat, calcium, family history of prostate cancer, BMI, vasectomy Variables controlled by matching: age, smoking status, year/month of sample collection | |
| Risk estimates [95% CI] |
Reference category: lowest quintile Results: Prostate cancer (advanced) highest quintile: OR 0.39 (95% CI 0.18 to 0.84) |
|
| Selenium levels in exposure categories | lowest quintile: 0.530 to 0.730 µg/g highest quintile: 0.941 to 7.090 µg/g | |
| Notes | ||
Yu 1991
| Methods | Randomised controlled trial Allocation: random Sequence generation: unclear, not described Concealment: unclear, not described Blinding: described as double‐blind; blinding of participants: adequate, placebo tablets; blinding of investigators and doctors: unclear Dropouts/withdrawals: unclear, not described Intention‐to‐treat‐analysis: unclear, not described Recruitment period: unclear, not described Observation period: 2 years Study period: 2 years Detection of cases: unclear, use of "national standards" for the diagnosis of liver cancer Informed consent: unclear, not described |
|
| Participants |
Country: China Number of participants: 2,474 Condition: first‐degree relatives within three generations of families with 2 or more cases of liver cancer during the period 1972 to 1985 Demographics: gender distribution not reported; age: 15 to 75 years Recruitment and setting: participants were residents in Qidong province |
|
| Interventions |
Intervention: 200 µg selenium as selenised yeast p.o. daily, intervention period unclear Control: placebo |
|
| Outcomes |
Primary outcome measure: incidence of primary liver cancer within 2 years after start of intervention Results: 13 cases in 1030 placebo subjects 10 cases in 1444 selenium subjects |
|
| Risk estimates [95% CI] | n.r. | |
| Selenium levels in exposure categories | d.n.a. | |
| Notes | Data were extracted from Yu 1991. We identified two later publications (Li 2002, Yu 1993), which we assumed to report on the same trial as Yu 1991. However, total number of participants differed from the initial report (N = 3849 in the later publications with 1485 receiving placebo and 2364 receiving selenium). The total number of cases was not reported in either Li 1992 or Yu 1993. The reported results were: Li 1992: person‐year incidence rate in intervention and control group: within one year of follow‐up: selenium group 175.36/100,000; placebo group: 414.65/100,000 within two years of follow‐up: selenium group 219.37/100,000; placebo group: 553.15/100,000 Yu 1993: cumulated incidence: after one year: selenium group 1.75/1000; placebo group: 4.15/1000 after two years: selenium group 2.19/1000; placebo group: 5.53/1000 We could not make contact with the study investigators to clarify these discrepancies. As we could not clarify the actual number of liver cancer cases in the later publications, we decided to use the data of Yu 1991 for this review. Adverse effects were not mentioned in Yu 1991 or Li 1992. Yu 1993 stated that no cases of selenosis were observed in the trial. |
|
Yu 1997
| Methods | Randomised controlled trial Allocation: random Sequence generation: unclear, not described Concealment: unclear, not described Blinding: of participants: adequate (placebo), of investigators and doctors: unclear, not described Dropouts/withdrawals: unclear, not described Recruitment period: unclear, not described Intention‐to‐treat‐analysis: unclear, not described Observation period: 1987 to 1994 Intervention period: 1987 to 1990 Detection of cases: unclear, monthly blood sample during follow‐up for liver enzymes (SGPT, ZnTT), use of "national standards" for the diagnosis of liver cancer Informed consent: unclear, not described |
|
| Participants |
Country: China Number of participants: 226 (selenium group: 113; placebo group 113) Condition: HBs‐antigen carriers with normal liver function Demographics: 95 men, 131 women; age: 21 to 63 years Recruitment and setting: recruitment “through screening in a village in the city Qidong” (Li 1992) |
|
| Interventions |
Intervention: 200 µg selenium as selenised yeast p.o. daily for 4 years Control: placebo |
|
| Outcomes |
Primary outcome measure: incidence of primary liver cancer (defined as increase of SGPT and ZnTT) Results: at the end of the intervention period: 0 cases in the selenium group; 7 cases in the placebo group in a total of 445 person years of observation (person‐time incidence rate: 1,573.03/100,000) |
|
| Risk estimates [95% CI] | n.r. | |
| Selenium levels in exposure categories | d.n.a. | |
| Notes |
Adverse effects: "No side effects have been found in these trials." (Yu 1997, p124) further data reported in: Li 1992 (Chinese, translated); Yu 1991 In Yu 1991 a different incidence in the selenium group was reported (5 cases). We could not clarify this discrepancy to the later papers Li 1992 and Yu 1997. |
|
Yu 1999
| Methods | Matched, nested case‐control study Country: China (Taiwan) |
|
| Participants |
Participants: 4841 men Inclusion criteria: 30 to 65 years of age; HBsAg‐positive or/and HCV‐positive; recruited at two centres: Government Employee Central Clinics or Liver Unit of Chang‐Gung Memorial Hospital Recruitment: August 1988 to June 1992 Outcome assessment: 31 December 1996 Number of cases: Primary liver cancer: 69 (male/female: 69/0) Case definition: incidence Years of follow‐up: 4.5 to 8.3 years Type of selenium marker: plasma |
|
| Interventions | d.n.a. | |
| Outcomes | Analysed cases: 69 of 73 cases analysed (reason for non‐inclusion: no sample available) Statistical methods: conditional logistic regression Variables controlled in analysis: age, cigarette smoking, alcohol intake, plasma levels of retinol/alpha‐tocopherol/alpha‐carotene/beta‐carotene/lycopene Variables controlled by matching: age, year and season of sample collection, recruitment clinic | |
| Risk estimates [95% CI] |
Reference category: lowest quintile Results: Primary liver cancer highest quintile: OR 0.62 (95% CI 0.21 to 1.86) |
|
| Selenium levels in exposure categories | lowest quintile ≤ 124.90 µg/l highest quintile ≥ 162.40 µg/l | |
| Notes | ||
(lower border; upper border) lower and upper border of the 95% CI (if not otherwise specified)
µ micro AFP alpha‐fetoprotein ALT alanine aminotransferase ATBC Alpha‐tocopherol, beta‐carotene cancer prevention study AU arbitrary unit BCC basal cell carcinoma BMI body‐mass‐index BPH benign prostate hyperplasia CARET Carotene and Retinol Efficacy Trial CHD coronary heart disease CI confidence interval CIS carcinoma in situ CVD cardiovascular disease dl deciliter d.n.a. does not apply DOM Diagnostic onderzoek mammacarcinoom EVA Etude du Vieillissement Antériel EPOZ Epidemiologisch onderzoek naar risico‐indicatoren voor hart‐ en vaatziekten FFQ food‐frequency questionnaire g gram HBsAg Hepatitis B surface antigen HCC hepatocellular carcinoma HCV hepatitis C virus HPFP Hypertension Detection Follow‐up Programme HPFS Health Professionals Follow‐up Study HR hazard ratio IU international unit l litre m milli max. adj. maximally adjusted MHC Mobile Health Clinic n nano NHS Nurses‘ Health Study NLCS Netherlands Cohort Study NMSC non‐melanoma skin cancer NPCT Nutritional Prevention of Cancer Trial n.r. not reported NSAID non‐steroidal antiinflammatory drugs OR Odds ratio p. page p.o. per os ppm parts per million PSA prostate‐specific antigen RCT randomised controlled trial RR relative risk SCC squamous cell carcinoma SGPT alanine aminotransferase TIA transient ischemic attack UK United Kingdom US United States of America VITAL Vitamins and Lifestyle Study ZnTT zinc turbidity test
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bostick 1993 | Cohort study: Iowa Women's Health Study cohort Selenium exposure not assessed according to eligibility: only intake of selenium supplements yes/no in questionnaire assessed |
| Brock 1991 | Case‐control study with precancerous condition (carcinoma in situ of the cervix) |
| Chen 1988 | Case‐control study |
| Chen 2003 | Case‐control study |
| Connelly‐Frost 2009 | Case‐control study |
| Costello 2001 | APPOSE (Australian Prostate Cancer Prevention Trial Using Selenium): publication describes study design, trial was not started |
| Criqui 1991 | Population‐based prospective case‐control study: Lipid Research Clinic Prevalence and Follow‐Up study Results not reported according to inclusion criteria: differences in mean selenium levels reported |
| Cui 2007 | Nested case‐control study Selenium exposure not assessed according to eligibility: selenium measurement conducted in tissue of benign breast disease |
| Davies 2002 | Nested case‐control study: EPIC Norfolk study cohort Results not reported according to inclusion criteria: RR estimate per unit increase in selenium level reported |
| Fleshner 2003 | Randomised Study of Vitamin E, Selenium, and Soy Protein Isolate in Patients with High‐Grade Prostatic Intraepithelial Neoplasia: Multicomponent Intervention |
| Hagmar 1992 | Historical cohort study |
| Harris 2012 | Cancer was not a study endpoint |
| Hartman 2002 | Nested case‐control study: ATBC cohort Results not reported according to inclusion criteria: differences in mean selenium levels reported; OR reported as graph and could not be calculated from reported data |
| Huzarski 2006 | Interventional study without control group with 1489 female participants with BRCA1 mutation who received a selenium‐containing nutritional supplement |
| Joniau 2007 | Intervention study without control group with male participants with high‐grade intraepithelial neoplasia of the prostate who received a selenium‐containing nutritional supplement |
| Kellen 2008 | Case‐control study |
| Kilander 2001 | Cohort study in Uppsala/Sweden Results not reported according to inclusion criteria: RR estimate per unit increase in selenium level reported |
| Knekt 1988a | Nested case‐control study: Mobile Health Clinic cohort Results not reported according to inclusion criteria: differences in mean selenium levels reported |
| Knekt 1988b | Nested case‐control study: Mobile Health Clinic cohort Results not reported according to inclusion criteria: differences in mean selenium levels reported |
| Knekt 1991 | Nested case‐control study: Mobile Health Clinic cohort Results not reported according to inclusion criteria: differences in mean selenium levels reported |
| Kok 1987b | Nested case‐control study: Zoetermeer cohort Results not reported according to inclusion criteria: differences in mean selenium levels reported |
| Kune 2006 | Case‐control study |
| Kuroda 1988 | Case‐control study |
| Lawson 2007 | Cohort study on multivitamin use and risk of prostate cancer |
| Le Marchand 2006 | Case‐control study |
| Li 2004b | RCT for gastric cancer prevention with multicomponent intervention (200 mg synthetic allitridum and 100 μg selenium per day) |
| Limburg 2005 | Randomised controlled trial: primary endpoint in this two‐by‐two factorial design trial with selenomethionine 200 µg daily and/or celecoxib 200 mg twice daily was the per‐participant change (regression, stable, progression) of preexisting oesophageal dysplasia—cancer incidence and mortality were not endpoints in this study |
| Linxian Pilot 2000 | Randomised controlled trial with selenium supplements and celecoxib in participants with oesophageal squamous dysplasia in Linxian, China Endpoint was "regression of disease", cancer was not an endpoint in this investigation |
| Neuhouser 2009 | Cohort study (Women's Health Initiative) on multivitamin use and risk of cancer and cardiovascular disease No data for selenium and cancer risk reported |
| Ray 2006 | Cohort study (Women's Health and Aging Studies I and II) on selenium and carotenoid serum levels and mortality No data for selenium and cancer mortality reported |
| Rayman 2001 | PRECISE trial (Prevention of Cancer by Intervention with Selenium): trial has been stopped |
| Rendon | Randomised controlled trial: Vitamin E, Selenium, and Soy Protein in Preventing Cancer in Patients with High‐Grade Prostate Neoplasia: Multicomponent Intervention |
| Steevens 2010b | Cancer was not a study endpoint |
| Thompson 2009 | Cohort study: Iowa Women's Health Study cohort Selenium exposure not assessed according to eligibility: only intake of selenium supplements yes/no in questionnaire assessed |
| Tsugane 1996 | Case‐control and cross‐sectional studies |
| Ujiie 2002 | A part of this study is a prospective cohort study in Miyagi/Japan Results not reported according to inclusion criteria: differences in mean selenium levels reported |
| van Noord 1992 | Nested case‐control study: DOM cohort Results not reported according to inclusion criteria: differences in mean selenium levels reported |
| van Noord 1993 | Nested case‐control study: DOM II cohort Results not reported according to inclusion criteria: RR estimate per unit increase of selenium level reported |
| van't Veer 1996 | Case‐control study |
| Wallace 2009 | Case‐control study |
| Watters 2009 | Cohort study on smoking and prostate cancer risk. Selenium not reported as independent variable |
| Wright 2004 | Cohort study: ATBC cohort Exposure to antioxidants was assessed using a self‐developed index |
| You 2005 | Randomised controlled trial to test retardation of the progression of precancerous gastric lesions among 3400 adults in Shandong, China. Intervention: vitamin C, vitamin E, selenium, garlic preparation Multicomponent intervention |
| Yuan 2006 | Nested case‐control study: Shanghai cohort study No data on selenium and cancer risk reported |
| Zeegers 2009 | Cohort study on factors influencing recurrence or progression of bladder cancer: West Midlands Bladder Cancer Prognosis Programme |
µ = micro
APPOSE = Australian Prostate Cancer Prevention Trial Using Selenium ATBC = alpha‐tocopherol, beta‐carotene cancer prevention study BRCA = breast cancer DOM = Diagnostic Onderzoek Mammacarcinoom EPIC = European Prospective Investigation of Cancer m = milli g = gram
OR = odds ratio PRECISE = Prevention of Cancer by Intervention with Selenium RCT = randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
Epi_Nomura 2002
| Trial name or title | Cancer Sero Epidemiology Among the Japanese in Hawaii |
| Methods | This is a sero‐epidemiological prospective study to identify biochemical markers related to common cancers Among the aims are (a) to see whether low serum selenium levels increase prostate cancer risk, and (b) to determine whether low serum selenium levels increase urinary bladder cancer risk in men |
| Participants | 9345 male American Japanese subjects, examined in Hawaii |
| Interventions | d.n.a. |
| Outcomes | Risk of prostate and urinary bladder cancer |
| Starting date | Project start: 15 September 1983, Project end planned for 30 June 2004 |
| Contact information | Abraham M. Nomura Kuakini Medical Center 347 N Kuakini St Honolulu, HI 96817 |
| Notes |
RCT_Cheng 2003
| Trial name or title | Selenium Supplementation for the Prevention of Hepatocellular Carcinomas in HBsAg Positive Patients (pilot study) |
| Methods | Randomised controlled trial |
| Participants | Men 45 to 64 years of age with positive HBsAg test, negative AFP test and normal ALT values |
| Interventions | Placebo or 200 mg (sic!)/d selenium as selenised yeast |
| Outcomes | Primary liver cancer |
| Starting date | 2003 |
| Contact information | Prof Kar Keung Cheng, University of Birmingham, UK |
| Notes | Study author contacted for further information, but no reply received Should probably say 200 µg/d selenium yeast as intervention in the publication |
RCT_Cheng 2006
| Trial name or title | Bladder Cancer Prognosis Programme (incorporating SELENIB trial) |
| Methods | Double‐blinded, placebo‐controlled, two‐by‐two factorial, randomised controlled trial (SELENIB), nested within a prospective observational cohort study (Bladder Cancer Prognosis Programme BCPP) |
| Participants | 1200 participants in the Bladder Cancer Prognosis Programme in the United Kingdom Inclusion criteria: Histopathologically confirmed non‐muscle invasive transitional cell carcinoma. Solitary grade 1 pTa larger than 3 cm and all other stage pTa, pT1 or pTcis Exclusion criteria: 1. Disease characteristics—solitary grade 1 pTa < 3 cm or stage pT2 and above 2. Patients who are pregnant or breastfeeding 3. Patients diagnosed with human immunodeficiency virus (HIV) infection 4. Patients who are on immunosuppressive therapy following organ transplantation 5. Patients taking cyclosporin 6. Any condition that, in the opinion of the local investigator, might interfere with the safety of the participant or with evaluation of trial objectives |
| Interventions | Four study arms: 1. Selenium 2. Alpha‐tocopherol 3. Selenium and alpha‐tocopherol 4. Placebo |
| Outcomes | Primary outcomes: recurrence‐free survival, progression‐free survival SELENIB trial—secondary outcomes 1. All‐cause mortality 2. Incidence of transitional cell carcinoma (TCC) outside the bladder 3. Incidence of all other malignancies clinically diagnosed 4. Incidence of cardiovascular events: myocardial infarction, stroke, death from cardiovascular causes 5. Quality of life—as assessed by quality of life instruments: European Organisation for Research and Treatment of Cancer (EORTC) QLQ‐C30, QLQ‐BLS24 and QLQ‐BLM30 |
| Starting date | |
| Contact information | |
| Notes | Ongoing trial Contact details: Prof K. K. Cheng The Public Health Building University of Birmingham Edgbaston Birmingham United Kingdom B15 2TT http://www.bcpp.bham.ac.uk |
RCT_ECOG 2002
| Trial name or title | Randomised Chemoprevention Study of Selenium in Participants With Previously Resected Stage I Non‐Small Cell Lung Cancer |
| Methods | Randomised controlled trial |
| Participants | Disease characteristics: Histologically confirmed, completely resected stage IA (pT1, N0) or IB (pT2, N0) non–small‐cell lung cancer (except carcinoid) Completion of treatment for stage I lung cancer within the past six to 36 months and currently disease free At least one mediastinal lymph node sampled at resection Age: 18 years and older Performance status: ECOG zero to one A total of 1960 participants (980 per arm) will be accrued for this study within four years |
| Interventions | Arm I: Participants receive oral selenium yeast daily for six months. Treatment repeats every six months for eight courses for a total of four years in the absence of unacceptable toxicity Arm II: Participants receive an oral yeast placebo as in arm I Participants are followed annually |
| Outcomes | Second incidence/recurrence of primary lung tumours Toxicity Incidence of specific cancers, mortality from cancer and overall survival |
| Starting date | October 2000 |
| Contact information | Eastern Cooperative Oncology Group Daniel Karp, MD, Protocol chair Phone: 713‐745‐7398; 800‐392‐1611 Southwest Oncology Group Omer Kucuk, MD, Protocol chair Phone: 313‐576‐8739; 800‐527‐6266 Email: kucuko@karmanos.org |
| Notes | Recruiting |
RCT_NBT_Stratton 2003
| Trial name or title | Negative Biopsy Trial (NBT) |
| Methods | This study is a phase III cancer chemoprevention study among men at high risk of prostate cancer because of a persistent elevation in PSA above 4 ng/mL and a negative initial biopsy |
| Participants | The trial will randomly assign at least 700 participants with persistently elevated PSA levels (> 4 ng/mL) and at least one negative biopsy for prostate cancer. The principal purpose of this trial is to assess the potential for treatment with the essential trace element of selenium to prevent prostate cancer (PCa) |
| Interventions | The trial will randomly assign participants to placebo or to one of two selenium dosages—200 µg/d or 400 µg/d |
| Outcomes | The primary endpoints for the trial are the incidence of PCa and the velocity of the primary serum marker of prostate cancer progression, prostate‐specific antigen (PSA). Safety endpoints for the trial include onset of mild symptoms of selenium toxicity and significant changes in liver and kidney enzyme levels |
| Starting date | 30 September 1999 |
| Contact information | M. Suzanne Stratton, Ph.D. Research Assistant Professor Arizona Cancer Center Prostate Cancer Prevention Program 2504 E Elm Street. Tucson, AZ 85716 http://www.selenium.arizona.edu |
| Notes |
µ = micro AARP = American Association of Retired Persons AFP = alpha‐fetoprotein ALT = alanine aminotransferase BCC = basal cell carcinoma BCPP = Bladder Cancer Prognosis Programme BRCA = breast cancer cm = centimeter d.n.a. = does not apply ECG = electrocardiogram ECOG = Eastern Cooperative Oncology Group EORTC = European Organisation for Research and Treatment of Cancer EPIC = European Prospective Investigation of Cancer g = gram HBs‐Ag = hepatitis B surface antigen HGPIN = high‐grade prostatic intraepithelial neoplasia HIV = human immunodeficiency virus iU = international unit l = liter m = milli n = nano n.r. = not reported NHANES = National Health and Nutrition Examination Survey NIH = National Institutes of Health p = page PSA = prostate‐specific antigen pT = tumour after pathological assessment, according to the tumour/nodules/metastases TNM staging system QLQ = Quality of Life Questionnaire RCT = randomised controlled trial SCC = squamous cell carcinoma SELECT = Selenium and Vitamin E Cancer Prevention Trial SELENIB = randomised controlled trial of selenium and vitamin E in the recurrence and progression of non‐muscle invasive bladder cancer TCC = transitional cell carcinoma UK = United Kingdom US = United States of America WHAS = Women’s Health and Aging Study WHI = Women’s Health Initiative WHO = World Health Organization
Contributions of authors
MV coordinated the current update, commented on the protocol and the review, screened the search results and updated the draft in collaboration with the other review authors.
GD is the primary author of the first version of the review and was involved in all steps of the present update, including commenting on the protocol and the manuscript, extracting data from papers and providing a methodological perspective.
CMC commented on the protocol and on the review, wrote part of the draft and provided a methodological perspective.
MZw commented on the protocol and the review and provided a methodological perspective.
MB commented on the protocol and provided feedback at various stages of the review.
MZe commented on the protocol and the review and provided feedback on different portions of these documents.
MH commented on the protocol, extracted data from papers and commented on the review text at various stages of the review.
RDA commented on the protocol and provided feedback at various stages of the review.
CDG commented on the protocol, extracted data from the added papers, conducted the data analyses, commented on the review, wrote part of the draft and provided a methodological perspective.
All review authors have reviewed and approved the final draft of this update.
Sources of support
Internal sources
-
Department of Diagnostic, Clinical and Public Health Medicine, University of Modena and Reggio Emilia, Modena, Italy.
This work was funded in part by the Department of Diagnostic, Clinical and Public Health Medicine, University of Modena and Reggio Emilia, Modena. The funding source had no role in designing, conducting or writing this systematic review. The contents of this systematic review are solely the responsibility of the review authors and do not necessarily represent the official views of this Department.
External sources
-
Dr. Ernst und Anita Bauer Foundation, Germany.
This work was partially funded by the Dr. Ernst and Anita Bauer Foundation. The funding source had no role in designing, conducting or writing this systematic review. The contents of this systematic review are solely the responsibility of the review authors and do not necessarily represent the official views of the Dr. Ernst and Anita Bauer Foundation.
-
EU (European Union) Project: Concerted action for complementary and alternative medicine in the cancer field (EU CAM‐Cancer) (Contract no.: QLG4‐CT‐2002‐00786), Other.
This work was funded in part by the EU CAM‐Cancer Project. The funding source had no role in designing, conducting or writing this systematic review. The contents of this systematic review are solely the responsibility of the review authors and do not necessarily represent the official views of the EU CAM‐Cancer Project.
-
Deutsche Krebshilfe (German Cancer Aid), Germany.
This work was funded in part by the German Cancer Aid. The funding source had no role in designing, conducting or writing this systematic review. The contents of this systematic review are solely the responsibility of the review authors and do not necessarily represent the official views of the German Cancer Aid.
-
NCCAM, USA.
This work was funded in part by Grant Number R24 AT001293 from the National Center for Complementary and Alternative Medicine (NCCAM). The funding source had no role in designing, conducting or writing this systematic review. The contents of this systematic review are solely the responsibility of the review authors and do not necessarily represent the official views of the NCCAM or the National Institutes of Health.
-
NIH, USA.
This work was partially funded by Grant Number CA16042 from the National Institutes of Health, National Cancer Institute (NCI). The funding source had no role in designing, conducting or writing this systematic review. The contents of this systematic review are solely the responsibility of the review authors and do not necessarily represent the official views of the NCI or the National Institutes of Health.
-
Italian League against Cancer‐LILT (Reggio Emilia), Italy.
- This work was funded in part by the Italian League against Cancer (LILT), Reggio Emilia section. The funding source had no role in designing, conducting or writing this systematic review. The contents of this systematic review are solely the responsibility of the review authors and do not necessarily represent the official views of LILT‐Reggio Emilia.
-
Fondazione Pietro Manodori (Reggio Emilia), Italy, Other.
- This work was funded in part by the Fondazione Pietro Manodori of Reggio Emilia. The funding source had no role in designing, conducting or writing this systematic review. The contents of this systematic review are solely the responsibility of the review authors and do not necessarily represent the official views of the Fondazione Pietro Manodori.
Declarations of interest
MV: None known.
GD: None known.
CMC: None known.
MZw: None known.
MB: None known.
MZe: Maurice Zeegers is the first investigator of one included observational study and one ongoing randomised controlled trial. He is second author of another included observational study.
MH: None known.
RDA: None known.
CDG: None known.
Edited (no change to conclusions)
References
References to studies included in this review
- Agalliu I, Kirsh VA, Kreiger N, Soskolne CL, Rohan TE. Oxidative balance score and risk of prostate cancer: results from a case‐cohort study. Cancer Epidemiology 2011;35(4):353‐61. [DOI] [PubMed] [Google Scholar]
- Akbaraly NT, Arnaud J, Hininger‐Favier I, Gourlet V, Roussel AM, Berr C. Selenium and mortality in the elderly: results from the EVA study. Clinical Chemistry 2005;51(11):2117‐23. [DOI] [PubMed] [Google Scholar]
- Algotar AM, Stratton MS, Ahmann FR, et al. Phase III clinical trial investigating the effect of selenium supplementation in men at high‐risk for prostate cancer. The Prostate 2013;73:328‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen NE. Reply to B Bekaert and MP Rayman. American Journal of Clinical Nutrition 2009;89(4):1277. [DOI] [PubMed] [Google Scholar]; Allen NE, Appleby PN, Roddam AW, Tjonneland A, Johnsen NF, Overvad K, et al. European Prospective Investigation into Cancer and Nutrition. Plasma selenium concentration and prostate cancer risk: results from the European Prospective Investigation into Cancer and Nutrition (EPIC). American Journal of Clinical Nutrition 2008;88(6):1567‐75. [DOI] [PubMed] [Google Scholar]
- Bates CJ, Hamer M, Mishra GD. Redox‐modulatory vitamins and minerals that prospectively predict mortality in older British people: the National Diet and Nutrition Survey of people aged 65 years and over. British Journal of Nutrition 2011;105(1):123‐32. [EPI_EU_Bates 2011] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleys J, Navas‐Acien A, Guallar E. Serum selenium levels and all‐cause, cancer, and cardiovascular mortality among US adults. Archive of Internal Medicine 2008;168(4):404‐10. [DOI] [PubMed] [Google Scholar]
- Brooks JD, Metter EJ, Chan DW, Sokoll LJ, Landis P, Nelson WG, et al. Plasma selenium level before diagnosis and the risk of prostate cancer development. The Journal of Urology 2001;166(6):2034‐8. [PubMed] [Google Scholar]
- Clark L, Graham G, Bray J. Nonmelanoma skin cancer and plasma selenium: a prospective cohort study. American Journal of Epidemiology 1985;122(3):528. [Google Scholar]
- Coates RJ. Cancer risk in relation to serum levels of selenium, retinol, and copper. Dissertation Abstract International (Sci)1987; Vol. 47, issue 12:4836. ; Coates RJ, Weiss NS, Daling JR, Morris JS, Labbe RF. Serum levels of selenium and retinol and the subsequent risk of cancer. American Journal of Epidemiology 1988;128(3):515‐23. [DOI] [PubMed] [Google Scholar]
- Combs GF Jr, Clark LC, Turnbull BW, Graham GF, Smith CL, Sanders B Jr, et al. Low plasma selenium (Se) predicts the 24 month incidence of squamous cell carcinoma of the skin in a cancer prevention trial. FASEB1993; Vol. 7, issue 3:A278.
- Comstock GW, Alberg AJ, Huang HY, Wu K, Burke AE, Hoffman SC, et al. The risk of developing lung cancer associated with antioxidants in the blood: ascorbic acid, carotenoids, alpha‐tocopherol, selenium, and total peroxyl radical absorbing capacity. Cancer Epidemiology, Biomarkers & Prevention 1997;6(11):907‐16. [PubMed] [Google Scholar]
- Dong LM, Kristal AR, Peters U, Schenk JM, Sanchez CA, Rabinovitch PS, et al. Dietary supplement use and risk of neoplastic progression in esophageal adenocarcinoma: a prospective study. Nutrition and Cancer 2008;60(1):39‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorgan JF, Sowell A, Swanson CA, Potischman N, Miller R, Schussler N, Stephenson HE Jr. Relationships of serum carotenoids, retinol, alpha‐tocopherol, and selenium with breast cancer risk: results from a prospective study in Columbia, Missouri (United States). Cancer Causes and Control 1998;9(1):89‐97. [DOI] [PubMed] [Google Scholar]
- Dreno B, Euvrard S, Frances C, Moyse D, Nandeuil A. Effect of selenium intake on the prevention of cutaneous epithelial lesions in organ transplant recipients. European Journal of Dermatology 2007;17(2):140‐5. [DOI] [PubMed] [Google Scholar]
- Epplein M, Franke AA, Cooney RV, Morris JS, Wilkens LR, Goodman MT, et al. Association of plasma micronutrient levels and urinary isoprostane with risk of lung cancer: the multiethnic cohort study. Cancer Epidemiology, Biomarkers & Prevention 2009;18(7):1962‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]; Gill JK, Franke AA, Steven MJ, Cooney RV, Wilkens LR, Marchand L, et al. Association of selenium, tocopherols, carotenoids, retinol, and 15‐isoprostane F(2t) in serum or urine with prostate cancer risk: the multiethnic cohort. Cancer Causes Control 2009;20(7):1161‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kolonel LN, 5P01CA033619‐20. Epidemiologic studies of diet and cancer in Hawaii. http://cancercontrol.cancer.gov/grants/abstract.asp?ApplID=6805844 (accessed 6 April 2011).
- Fex G, Pettersson B, Akesson B. Low plasma selenium as a risk factor for cancer death in middle‐aged men. Nutrition and Cancer 1987;10(4):221‐9. [DOI] [PubMed] [Google Scholar]
- Fujishima Y, Ohsawa M, Itai K, Kato K, Tanno K, Turin TC, et al. Serum selenium levels are inversely associated with death risk among hemodialysis patients. Nephrology, Dialysis, Transplantation 2011;26(10):3331‐8. [DOI] [PubMed] [Google Scholar]
- Garland M, Morris JS, Stampfer MJ, Colditz GA, Spate VL, Baskett CK, et al. Prospective study of toenail selenium levels and cancer among women. Journal of the National Cancer Institute 1995;87(7):497‐505. [DOI] [PubMed] [Google Scholar]; Hunter DJ, Morris JS, Stampfer MJ, Colditz GA, Speizer FE, Willett WC. A prospective study of selenium status and breast cancer risk. JAMA 1990;264(9):1128‐31. [PubMed] [Google Scholar]
- Glattre E, Thomassen Y, Thoresen SO, Haldorsen T, Lund‐Larsen PG, Theodorsen L, et al. Prediagnostic serum selenium in a case‐control study of thyroid cancer. International Journal of Epidemiology 1989;18(1):45‐9. [DOI] [PubMed] [Google Scholar]
- Goodman GE, Schaffer S, Bankson DD, Hughes MP, Omenn GS, The Carotene and Retinol Efficacy Trial (CARET) Co‐Investigators. Predictors of serum selenium in cigarette smokers and the lack of association with lung and prostate cancer risk. Cancer Epidemiology, Biomarkers & Prevention 2001;10(10):1069‐76. [PubMed] [Google Scholar]
- Grundmark B, Zethelius B, Garmo H, Holmberg L. Serum levels of selenium and smoking habits at age 50 influence long term prostate cancer risk; a 34 year ULSAM follow‐up. BMC Cancer 2011;11:431. [EPI_EU_Grundmark 2011] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman TJ, Albanes D, Pietinen P, Hartman AM, Rautalahti M, Tangrea JA, et al. The association between baseline vitamin E, selenium, and prostate cancer in the alpha‐tocopherol, beta‐carotene cancer prevention study. Cancer Epidemiology, Biomarkers & Prevention 1998;7(4):335‐40. [PubMed] [Google Scholar]
- Helzlsouer KJ, Huang HY, Alberg AJ, Hoffman S, Burke A, Norkus EP, et al. Association between alpha‐tocopherol, gamma‐tocopherol, selenium, and subsequent prostate cancer. Journal of the National Cancer Institute 2000;92(24):2018‐23. [DOI] [PubMed] [Google Scholar]
- Hotaling JM, Wright JL, Pocobelli G, Bhatti P, Porter MP, White E. Long‐term use of supplemental vitamins and minerals does not reduce the risk of urothelial cell carcinoma of the bladder in the VITamins And Lifestyle study. The Journal of Urology 2011;185(4):1210‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabuto M, Imai H, Yonezawa C, Neriishi K, Akiba S, Kato H, et al. Prediagnostic serum selenium and zinc levels and subsequent risk of lung and stomach cancer in Japan. Cancer Epidemiology, Biomarkers & Prevention 1994;3(6):465‐9. [PubMed] [Google Scholar]
- Karagas MR, Greenberg ER, Nierenberg D, Stukel TA, Morris JS, Stevens MM, et al. Risk of squamous cell carcinoma of the skin in relation to plasma selenium, alpha‐tocopherol, beta‐carotene, and retinol: a nested case‐control study. Cancer Epidemiology, Biomarkers & Prevention 1997;6(1):25‐9. [PubMed] [Google Scholar]
- Hakama M, Aaran RK, Alfthan G, Aromaa A, Hakulinen T, Knekt P, et al. Linkage of serum sample bank and cancer registry in epidemiological studies. Progress in Clinical and Biological Research, v. 346. New York: Wiley‐Liss, 1990:169‐78. [PubMed] [Google Scholar]; Knekt P, Aromaa A, Alfthan G, Maatela J, Hakama M, Hakulinen T, et al. Re: Prospective study of serum micronutrients and ovarian cancer. Journal of the National Cancer Institute 1996;88(19):1408. [DOI] [PubMed] [Google Scholar]; Knekt P, Aromaa A, Maatela J, Alfthan G, Aaran RK, Hakama M, et al. Serum selenium and subsequent risk of cancer among Finnish men and women. Journal of the National Cancer Institute 1990;82(10):864‐8. [DOI] [PubMed] [Google Scholar]; Knekt P, Aromaa A, Maatela J, Alfthan G, Aaran RK, Teppo L, et al. Serum vitamin E, serum selenium and the risk of gastrointestinal cancer. International Journal of Cancer 1988;42(6):846‐50. [DOI] [PubMed] [Google Scholar]; Knekt P, Jarvinen R, Seppanen R, Rissanen A, Aromaa A, Heinonen OP, et al. Dietary antioxidants and the risk of lung cancer. American Journal of Epidemiology 1991;134(5):471‐9. [DOI] [PubMed] [Google Scholar]
- Knekt P, Marniemi J, Teppo L, Heliovaara M, Aromaa A. Is low selenium status a risk factor for lung cancer?. American Journal of Epidemiology 1998;148(10):975‐82. [DOI] [PubMed] [Google Scholar]
- Kok FJ, Bruijn AM, Hofman A, Valkenburg HA. Selenium status and chronic disease mortality: Dutch epidemiological findings. International Journal of Epidemiology 1987a;16(2):329‐32. [DOI] [PubMed] [Google Scholar]; Kok FJ, Bruijn AM, Hofman A, Vermeeren R, Valkenburg HA. Is serum selenium a risk factor for cancer in men only?. American Journal of Epidemiology 1987b;125(1):12‐6. [DOI] [PubMed] [Google Scholar]
- Kornitzer M, Valente F, Bacquer D, Neve J, Backer G. Serum selenium and cancer mortality: a nested case‐control study within an age‐ and sex‐stratified sample of the Belgian adult population. European Journal of Clinical Nutrition 2004;58(1):98‐104. [DOI] [PubMed] [Google Scholar]
- Kromhout D. Essential micronutrients in relation to carcinogenesis. American Journal of Clinical Nutrition 1987;45(5 Suppl):1361‐7. [DOI] [PubMed] [Google Scholar]
- Li W, Zhu Y, Yan X, Zhang Q, Li X, Ni Z, et al. The prevention of primary liver cancer by selenium in high risk populations. Zhonghua Yu Fang Yi Xue Za Zhi [Chinese Journal of Preventive Medicine] 2000;34(6):336‐8. [Li 2000] [PubMed] [Google Scholar]
- Li H, Kantoff PW, Giovannucci E, Leitzmann MF, Gaziano JM, Stampfer MJ, et al. Manganese superoxide dismutase polymorphism, prediagnostic antioxidant status, and risk of clinical significant prostate cancer. Cancer Research 2005a;65(6):2498‐504. [DOI] [PubMed] [Google Scholar]; Li H, Stampfer MJ, Giovannucci EL, Morris JS, Willett WC, Gaziano JM, et al. A prospective study of plasma selenium levels and prostate cancer risk. Journal of the National Cancer Institute 2004;96(9):696‐703. [DOI] [PubMed] [Google Scholar]; Li H, Stampfer MJ, Giovannucci EL, Morris JS, Willett WC, Gaziano JM, et al. Plasma selenium levels associated with subsequent risk of prostate cancer. American Journal of Urology Review 2005b;3(1):28‐34. [Google Scholar]
- Marshall JR, Tangen CM, Sakr WA, Wood DP Jr, Berry DL, Klein EA, et al. Phase III trial of selenium to prevent prostate cancer in men with high‐grade prostatic intraepithelial neoplasia: SWOG S9917. Cancer Prevention Research 2011;4:1761‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinen MM, Hughes MC, Ibiebele TI, Marks GC, Green AC, Pols JC. Intake of antioxidant nutrients and the risk of skin cancer. European Journal of Cancer 2007;43(18):2707‐16. [DOI] [PubMed] [Google Scholar]; McNaughton SA, Marks GC, Gaffney P, Williams G, Green AC. Antioxidants and basal cell carcinoma of the skin: a nested case‐control study. Cancer Causes and Control 2005;16(5):609‐18. [DOI] [PubMed] [Google Scholar]; Pols JC, Heinen MM, Hughes MC, Ibiebele TI, Marks GC, Green AC. Serum antioxidants and skin cancer risk: an 8‐year community‐based follow‐up study. Cancer Epidemiology, Biomarkers & Prevention 2009;18(4):1167‐73. [DOI] [PubMed] [Google Scholar]
- Batieha AM, Armenian HK, Norkus EP, Morris JS, Spate VE, Comstock GW. Serum micronutrients and the subsequent risk of cervical cancer in a population‐based nested case‐control study. Cancer Epidemiology, Biomarkers & Prevention 1993;2(4):335‐9. [PubMed] [Google Scholar]; Breslow RA, Alberg AJ, Helzlsouer KJ, Bush TL, Norkus EP, Morris JS, et al. Serological precursors of cancer: malignant melanoma, basal and squamous cell skin cancer, and prediagnostic levels of retinol, beta‐carotene, lycopene, alpha‐tocopherol, and selenium. Cancer Epidemiology, Biomarkers & Prevention 1995;4(8):837‐42. [PubMed] [Google Scholar]; Burney PG, Comstock GW, Morris JS. Serologic precursors of cancer: serum micronutrients and the subsequent risk of pancreatic cancer. American Journal of Clinical Nutrition 1989;49(5):895‐900. [DOI] [PubMed] [Google Scholar]; Helzlsouer KJ, Alberg AJ, Norkus EP, Morris JS, Hoffman SC, Comstock GW. Prospective study of serum micronutrients and ovarian cancer. Journal of the National Cancer Institute 1996;88(1):32‐7. [DOI] [PubMed] [Google Scholar]; Helzlsouer KJ, Comstock GW, Morris JS. Selenium, lycopene, alpha‐tocopherol, beta‐carotene, retinol, and subsequent bladder cancer. Cancer Research 1989;49(21):6144‐8. [PubMed] [Google Scholar]; Ko W. The Associations of Serologic Precursors and the Anatomic‐Site Specific Incidence of Colon Cancer. Baltimore, MD: John Hopkins University, 1994. [Google Scholar]; Menkes MJ. Vitamins A, E, selenium and risk of lung cancer. Dissertation Abstract International (Sci) 1986a; Vol. 46, issue 11:3807. ; Menkes MS, Comstock GW, Vuilleumier JP, Helsing KJ, Rider AA, Brookmeyer R. Serum beta‐carotene, vitamins A and E, selenium, and the risk of lung cancer. New England Journal of Medicine 1986b;315(20):1250‐4. [DOI] [PubMed] [Google Scholar]; Schober SE. Vitamin A, vitamin E, selenium, and colon cancer risk. Dissertation Abstract International (Sci)1986; Vol. 46, issue 11:3808. ; Schober SE, Comstock GW, Helsing KJ, Salkeld RM, Morris JS, Rider AA, Brookmeyer R. Serologic precursors of cancer. I. Prediagnostic serum nutrients and colon cancer risk. American Journal of Epidemiology 1987;126(6):1033‐41. [DOI] [PubMed] [Google Scholar]; Zheng W, Blot WJ, Diamond EL, Norkus EP, Spate V, Morris JS, et al. Serum micronutrients and the subsequent risk of oral and pharyngeal cancer. Cancer Research 1993;53(4):795‐8. [PubMed] [Google Scholar]
- Michaud DS, Hartman TJ, Taylor PR, Pietinen P, Alfthan G, Virtamo J, et al. No association between toenail selenium levels and bladder cancer risk. Cancer Epidemiology, Biomarkers & Prevention 2002;11(11):1505‐6. [PubMed] [Google Scholar]
- Michaud DS, Vivo I, Morris JS, Giovannucci E. Toenail selenium concentrations and bladder cancer risk in women and men. British Journal of Cancer 2005;93(7):804‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura A, Heilbrun LK, Morris JS, Stemmermann GN. Serum selenium and the risk of cancer, by specific sites: case‐control analysis of prospective data. Journal of the National Cancer Institute 1987;79(1):103‐8. [PubMed] [Google Scholar]
- Nomura AMY, Lee J, Stemmermann GN, Combs GF Jr. Serum selenium and subsequent risk of prostate cancer. Cancer Epidemiology, Biomarkers & Prevention 2000;9(9):883‐7. [PubMed] [Google Scholar]
- Clark L, Krongrad A, Dalkin B, Witherington R, Herlong H, Carpenter D. Decreased incidence of prostate cancer with selenium supplementation: 1983‐96 results of a double‐blind cancer prevention trial. European Journal of Cancer Prevention1997; Vol. 6, issue 5:497‐8. [DOI] [PubMed]; Clark LC, Combs GF Jr, Turnbull BW, Slate EH, Chalker DK, Chow J, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin: a randomized controlled trial. JAMA 1996;276(24):1957‐63. [PubMed] [Google Scholar]; Clark LC, Dalkin B, Krongrad A, Combs GF, Turnbull BW, Slate EH, et al. Decreased incidence of prostate cancer with selenium supplementation: results of a double‐blind cancer prevention trial. British Journal of Urology 1998;81(5):730‐4. [DOI] [PubMed] [Google Scholar]; Clark LC, UARIZ‐CA49764 NCI‐P89‐0003. Double‐blind, randomized trial of selenium‐enriched brewer's yeast vs brewer's yeast placebo for the prevention of skin cancer in patients with a history of squamous or basal cell skin cancer (Summary Last Modified 05/91). http://www.cancer.gov. National Cancer Institute, (accessed 1 March 2004). ; Combs GF, Clark LC, Turnbull BW. Reduction of cancer mortality and incidence by selenium supplementation. Medizinische Klinik 1997;92(Suppl 3):42‐5. [DOI] [PubMed] [Google Scholar]; Combs GFJr, Clark LC, Turnbull BW. Reduction of cancer risk with an oral supplement of selenium. Biomedical and Environmental Sciences 1997;10(2‐3):227‐34. [PubMed] [Google Scholar]; Duffield‐Lillico AJ, Dalkin BL, Reid ME, Turnbull BW, Slate EH, Jacobs ET, et al. Nutritional Prevention of Cancer Study Group. Selenium supplementation, baseline plasma selenium status and incidence of prostate cancer: an analysis of the complete treatment period of the Nutritional Prevention of Cancer Trial. BJU International 2003b;91(7):608‐12. [DOI] [PubMed] [Google Scholar]; Duffield‐Lillico AJ, Reid ME, Turnbull BW, Combs GF Jr, Slate EH, Fischbach LA, et al. Baseline characteristics and the effect of selenium supplementation on cancer incidence in a randomized clinical trial: a summary report of the Nutritional Prevention of Cancer Trial. Cancer Epidemiology, Biomarkers & Prevention 2002;11(7):630‐9. [PubMed] [Google Scholar]; Duffield‐Lillico AJ, Slate EH, Reid ME, Turnbull BW, Wilkins PA, Combs GF Jr, et al. Nutritional Prevention of Cancer Study Group. Selenium supplementation and secondary prevention of nonmelanoma skin cancer in a randomized trial. Journal of the National Cancer Institute 2003a;95(19):1477‐81. [DOI] [PubMed] [Google Scholar]; Marshall JR, 5R01CA049764‐15. Nutritional Prevention of Cancer. http://crisp.cit.nih.gov (accessed 1 March 2004). ; Reid ME, Duffield‐Lillico AJ, Garland L, Turnbull BW, Clark LC, Marshall JR. Selenium supplementation and lung cancer incidence: an update of the Nutritional Prevention of Cancer Trial. Cancer Epidemiology, Biomarkers & Prevention 2002;11(11):1285‐91. [PubMed] [Google Scholar]; Reid ME, Duffield‐Lillico AJ, Slate E, Natarajan N, Turnbull B, Jacobs E, et al. The nutritional prevention of cancer: 400 mcg per day selenium treatment. Nutrition and Cancer 2008;60(2):155‐63. [DOI] [PubMed] [Google Scholar]; Stranges S, Marshall JR, Natarajan R, Donahue RP, Trevisan M, Combs GF, et al. Effects of long‐term selenium supplementation on the incidence of type 2 diabetes: a randomized trial. Annals of Internal Medicine 2007;147(4):217‐23. [DOI] [PubMed] [Google Scholar]
- Overvad K, Wang DY, Olsen J, Allen DS, Thorling EB, Bulbrook RD, Hayward JL. Selenium in human mammary carcinogenesis: a case‐cohort study. European Journal of Cancer 1991;27(7):900‐2. [DOI] [PubMed] [Google Scholar]
- Peleg I, Morris S, Hames CG. Is serum selenium a risk factor for cancer?. Medical Oncology and Tumor Pharmacotherapy 1985;2(3):157‐63. [DOI] [PubMed] [Google Scholar]
- Persson‐Moschos ME, Stavenow L, Akesson B, Lindgarde F. Selenoprotein P in plasma in relation to cancer morbidity in middle‐aged Swedish men. Nutrition and Cancer 2000;36(1):19‐26. [DOI] [PubMed] [Google Scholar]
- Peters U, Foster CB, Chatterjee N, Schatzkin A, Reding D, Andriole GL, et al. Serum selenium and risk of prostate cancer ‐ a nested case‐control study. American Journal of Clinical Nutrition 2007;85(1):209‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asgari MM, Maruti SS, Kushi LH, White E. Antioxidant supplementation and risk of incident melanomas: results of a large prospective cohort study. Archives of Dermatology 2009;145(8):879‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]; Peters U, Littman AJ, Kristal AR, Patterson RE, Potter JD, White E. Vitamin E and selenium supplementation and risk of prostate cancer in the Vitamins and lifestyle (VITAL) study cohort. Cancer Causes Control 2008;19(1):75‐87. [DOI] [PubMed] [Google Scholar]
- Ratnasinghe D, Tangrea JA, Forman MR, Hartman T, Gunter EW, Qiao YL, et al. Serum tocopherols, selenium and lung cancer risk among tin miners in China. Cancer Causes and Control 2000;11(2):129‐35. [DOI] [PubMed] [Google Scholar]
- Reid ME, Duffield‐Lillico AJ, Slate E, Natarajan N, Turnbull B, Jacobs E, et al. The nutritional prevention of cancer: 400 mcg per day selenium treatment. Nutrition and Cancer 2008;60(2):155‐63. [DOI] [PubMed] [Google Scholar]
- Ringstad J, Jacobsen BK, Tretli S, Thomassen Y. Serum selenium concentration associated with risk of cancer. Journal of Clinical Pathology 1988;41(4):454‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakoda LC, Graubard BI, Evans AA, London WT, Lin WY, Shen FM, et al. Toenail selenium and risk of hepatocellular carcinoma mortality in Haimen City, China. International Journal of Cancer 2005;115(4):618‐24. [DOI] [PubMed] [Google Scholar]
- Salonen JT, Alfthan G, Huttunen JK, Puska P. Association between serum selenium and the risk of cancer. American Journal of Epidemiology 1984;120(3):342‐9. [DOI] [PubMed] [Google Scholar]
- Salonen JT, Salonen R, Lappetelainen R, Maenpaa PH, Alfthan G, Puska P. Risk of cancer in relation to serum concentrations of selenium and vitamins A and E: matched case‐control analysis of prospective data. British Medical Journal (Clinical Research Edition) 1985;290(6466):417‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook ED. Selenium and Vitamin E Cancer Prevention Trial ‐ this one's for us. Journal of the National Medical Association 2002;94(9):856‐8. [PMC free article] [PubMed] [Google Scholar]; Cook ED, Moody‐Thomas S, Anderson KB, Campbell R, Hamilton SJ, Harrington JM, et al. Minority recruitment to the Selenium and Vitamin E Cancer Prevention Trial (SELECT). Clinical Trials 2005;2(5):436‐42. [DOI] [PubMed] [Google Scholar]; DeFrancesco L. Prostate cancer prevention trial launched. Nature Medicine 2001;7(10):1076. [DOI] [PubMed] [Google Scholar]; Dunn BK, Ryan A, Ford LG. Selenium and Vitamin E Cancer Prevention Trial: a nutrient approach to prostate cancer prevention. Recent Results in Cancer Research 2009;181:183‐93. [DOI] [PubMed] [Google Scholar]; FDA. Largest‐ever prostate cancer prevention trial. FDA Consumer 2001;35(5):8. [PubMed] [Google Scholar]; Ford LG, Minasian LM, McCaskill‐Stevens W, Pisano ED, Sullivan D, Smith RA. Prevention and early detection clinical trials: opportunities for primary care providers and their patients. CA: A Cancer Journal for Clinicians 2003;53(2):82‐101. [DOI] [PubMed] [Google Scholar]; Hoque A, Albanes D, Lippman SM, Spitz MR, Taylor PR, Klein EA, et al. Molecular epidemiologic studies within the Selenium and Vitamin E Cancer Prevention Trial (SELECT). Cancer Causes and Control 2001;12(7):627‐33. [DOI] [PubMed] [Google Scholar]; Kardinal C, Brooks J. Ochsner Cancer Institute studies vitamin E and selenium as prostate cancer prevention agents. Ochsner Journal 2003;5(2):51. [PMC free article] [PubMed] [Google Scholar]; Klein EA. Clinical models for testing chemopreventative agents in prostate cancer and overview of SELECT: the Selenium and vitamin E cancer prevention trial. Recent Results in Cancer Research 2003;163:212‐25. [DOI] [PubMed] [Google Scholar]; Klein EA. Selenium and vitamin E cancer prevention trial. Annals of the New York Academy of Sciences 2004;1031:234‐41. [DOI] [PubMed] [Google Scholar]; Klein EA, Atkins MB, Walther P, Klotz L, SWOG‐S000. Phase III randomized study of selenium and vitamin E for the prevention of prostate cancer (SELECT trial). http://clinicaltrials.gov/ (accessed 12 January 2004). ; Klein EA, Lippman SM, Thompson IM, Goodman PJ, Albanes D, Taylor PR, et al. The selenium and vitamin E cancer prevention trial. World Journal of Urology 2003;21(1):21‐7. [DOI] [PubMed] [Google Scholar]; Klein EA, Thompson IM Jr, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011;306(14):1549‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]; Klein EA, Thompson IM, Lippman SM, Goodman PJ, Albanes D, Taylor PR, et al. SELECT: The Selenium and Vitamin E Cancer Prevention Trial: rationale and design. Prostate Cancer and Prostatic Diseases 2000;3(3):145‐51. [DOI] [PubMed] [Google Scholar]; Klein EA, Thompson IM, Lippman SM, Goodman PJ, Albanes D, Taylor PR, et al. SELECT: the next prostate cancer prevention trial. Selenium and vitamin E cancer prevention trial. Journal of Urology 2001;166(4):1311‐5. [DOI] [PubMed] [Google Scholar]; Klein EA, Thompson IM, Lippman SM, Goodman PJ, Albanes D, Taylor PR, et al. SELECT: the selenium and vitamin E cancer prevention trial. Urologic Oncology 2003;21(1):59‐65. [DOI] [PubMed] [Google Scholar]; Lippman SM, Goodman PJ, Klein EA, Parnes HL, Thompson IM Jr, Kristal AR, et al. Designing the Selenium and Vitamin E Cancer Prevention Trial (SELECT). Journal of the National Cancer Institute 2005;97(2):94‐102. [DOI] [PubMed] [Google Scholar]; Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2009;301(1):39‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lotan Y, Goodman PJ, Youssef RF, Svatek RS, Shariat SF, Tangen CM, et al. Evaluation of vitamin E and selenium supplementation for the prevention of bladder cancer in SWOG coordinated SELECT. Journal of Urology 2012;187(6):205‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]; Miller M. Enrollment begins for largest‐ever prostate cancer prevention trial. Journal of the National Cancer Institute 2001;93(15):1132. [DOI] [PubMed] [Google Scholar]; Pak RW, Lanteri VJ, Scheuch JR, Sawczuk IS. Review of vitamin E and selenium in the prevention of prostate cancer: implications of the selenium and vitamin E chemoprevention trial. Integrative Cancer Therapies 2002;1(4):338‐44. [DOI] [PubMed] [Google Scholar]; South West Oncology Group. Selenium and Vitamin E Cancer Prevention Trial (SELECT). http://www.controlled‐trials.com/mrct/trial/SELENIUM/1059/42795.html (accessed 16 April 2004). ; South West Oncology Group. Selenium and Vitamin E in preventing prostate cancer. http://www.controlled‐trials.com/mrct/trial/SELENIUM/1059/32859.html. Southwest Oncology Group, (accessed 16 April 2004). ; Tangen CM, Goodman PJ, Crowley JJ, Thompson IM. Statistical design issues and other practical considerations for conducting phase III prostate cancer prevention trials. Journal of Urology 2004;171(2 Pt 2):S64‐7. [DOI] [PubMed] [Google Scholar]; Tom J. SELECT (opportunity for prostate cancer prevention). Hawaii Medical Journal 2002;61(6):126‐9. [PubMed] [Google Scholar]
- Steevens J, Brandt PA, Goldbohm RA, Schouten LJ. Selenium status and the risk of esophageal and gastric cancer subtypes: the Netherlands cohort study. Gastroenterology. 138. W.B. Saunders (Independence Square West, Philadelphia PA 19106‐3399, United States), 2010; Vol. 138, issue 5:1704‐13. [DOI] [PubMed]
- Steinbrecher A, Meplan C, Hesketh J, Schomburg L, Endermann T, Jansen E, et al. Effects of selenium status and polymorphisms in selenoprotein genes on prostate cancer risk in a prospective study of European men. Cancer Epidemiology, Biomarkers & Prevention. 19. American Association for Cancer Research Inc. (615 Chestnut Street, 17th Floor, Philadelphia PA 19106‐3483, United States), 2010; Vol. 19, issue 11:2958‐68. [DOI] [PubMed]
- Suadicani P, Hein HO, Gyntelberg F. Serum selenium level and risk of lung cancer mortality: a 16‐year follow‐up of the Copenhagen Male Study. The European Respiratory Journal 2012;39(6):1443‐8. [DOI] [PubMed] [Google Scholar]
- Thomson CA, Neuhouser ML, Shikany JM, Caan BJ, Monk BJ, Mossavar‐Rahmani Y, et al. The role of antioxidants and vitamin A in ovarian cancer: results from the Women's Health Initiative. Nutrition and Cancer 2008;60(6):710‐9. [DOI] [PubMed] [Google Scholar]
- Zeegers MPA, Goldbohm RA, Bode P, Brandt PA. Prediagnostic toenail selenium and risk of bladder cancer. Cancer Epidemiology, Biomarkers & Prevention 2002;11(11):1292‐7. [PubMed] [Google Scholar]; Brandt PA, Goldbohm RA, van't Veer P, Bode P, Dorant E, Hermus RJ, et al. A prospective cohort study on selenium status and the risk of lung cancer. Cancer Research 1993a;53(20):4860‐5. [PubMed] [Google Scholar]; Brandt PA, Goldbohm RA, van't Veer P, Bode P, Dorant E, Hermus RJ, et al. A prospective cohort study on toenail selenium levels and risk of gastrointestinal cancer. Journal of the National Cancer Institute 1993b;85(3):224‐9. [DOI] [PubMed] [Google Scholar]; Brandt PA, Goldbohm RA, van't Veer P, Bode P, Dorant E, Hermus RJ, et al. Toenail selenium levels and the risk of breast cancer. American Journal of Epidemiology 1994;140(1):20‐6. [DOI] [PubMed] [Google Scholar]; Brandt PA, Zeegers MPA, Bode P, Goldbohm RA. Toenail selenium levels and the subsequent risk of prostate cancer: a prospective cohort study. Cancer Epidemiology, Biomarkers & Prevention 2003;12(9):866‐71. [PubMed] [Google Scholar]
- Noord PAH, Collette HJA, Maas MJ, Waard F. Selenium levels in nails of premenopausal breast cancer patients assessed prediagnostically in a cohort‐nested case‐referent study among women screened in the DOM project. International Journal of Epidemiology 1987;16(2):318‐22. [DOI] [PubMed] [Google Scholar]
- Virtamo J, Valkeila E, Alfthan G, Punsar S, Huttunen JK, Karvonen MJ. Serum selenium and risk of cancer. A prospective follow‐up of nine years. Cancer 1987;60(2):145‐8. [DOI] [PubMed] [Google Scholar]
- Walter RB, Brasky TM, Milano F, White E. Vitamin, mineral, and specialty supplements and risk of hematologic malignancies in the prospective VITamins And Lifestyle (VITAL) study. Cancer Epidemiology, Biomarkers & Prevention 2011;20(10):2298‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark SD, Qiao YL, Dawsey SM, Wu YP, Katki H, Gunter EW, et al. Prospective study of serum selenium levels and incident esophageal and gastric cancers. Journal of the National Cancer Institute 2000;92(21):1753‐63. [DOI] [PubMed] [Google Scholar]; Wei WQ, Abnet CC, Qiao YL, Dawsey SM, Dong ZW, Sun XD, et al. Prospective study of serum selenium concentrations and esophageal and gastric cardia cancer, heart disease, stroke, and total death. American Journal of Clinical Nutrition 2004;79(1):80‐5. [DOI] [PubMed] [Google Scholar]
- Willett WC, Polk BF, Morris JS, Stampfer MJ, Pressel S, Rosner B, et al. Prediagnostic serum selenium and risk of cancer. Lancet 1983;2(8342):130‐4. [DOI] [PubMed] [Google Scholar]
- Yoshizawa K, Willett WC, Morris SJ, Stampfer MJ, Spiegelman D, Rimm EB, et al. Study of prediagnostic selenium level in toenails and the risk of advanced prostate cancer. Journal of the National Cancer Institute 1998;90(16):1219‐24. [DOI] [PubMed] [Google Scholar]
- Li WG. [Preliminary observations on effect of selenium yeast on high risk populations with primary liver cancer]. Zhonghua Yu Fang Yi Xue Za Zhi [Chinese Journal of Preventive Medicine] 1992;26(5):268‐71. [PubMed] [Google Scholar]; Yu S, Li W, Zhu Y. Chemoprevention of Liver Cancer. CCPC‐93: Second International Cancer Chemo Prevention Conference, April 28‐30, 1993, Berlin (Meeting Abstract)1993. ; Yu SY, Zhu YJ, Li WG, Huang QS, Huang CZ, Zhang QN, et al. A preliminary report on the intervention trials of primary liver cancer in high‐risk populations with nutritional supplementation of selenium in China. Biological Trace Element Research 1991;29(3):289‐94. [DOI] [PubMed] [Google Scholar]
- Li WG. [Preliminary observations on effect of selenium yeast on high risk populations with primary liver cancer]. Zhonghua Yu Fang Yi Xue Za Zhi [Chinese Journal of Preventive Medicine] 1992;26(5):268‐71. [PubMed] [Google Scholar]; Yu SY, Zhu YJ, Li WG. Protective role of selenium against hepatitis B virus and primary liver cancer in Qidong. Biological Trace Element Research 1997;56(1):117‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Yu SY, Zhu YJ, Li WG, Huang QS, Huang CZ, Zhang QN, et al. A preliminary report on the intervention trials of primary liver cancer in high‐risk populations with nutritional supplementation of selenium in China. Biological Trace Element Research 1991;29(3):289‐94. [DOI] [PubMed] [Google Scholar]
- Yu MW, Horng IS, Hsu KH, Chiang YC, Liaw YF, Chen CJ. Plasma selenium levels and risk of hepatocellular carcinoma among men with chronic hepatitis virus infection. American Journal of Epidemiology 1999;150(4):367‐74. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Bostick RM, Potter JD, McKenzie DR, Sellers TA, Kushi LH, Steinmetz KA, et al. Reduced risk of colon cancer with high intake of vitamin E: the Iowa Women's Health Study. Cancer Research 1993;53(18):4230‐7. [PubMed] [Google Scholar]
- Brock KE, Gridley G, Morris JS, Willett WC. Serum selenium level in relation to in situ cervical cancer in Australia. Journal of the National Cancer Institute 1991;83(4):292‐3. [DOI] [PubMed] [Google Scholar]
- Chen Q. [Protective effects of selenium, zinc and copper on lung cancer]. Zhonghua Yu Fang Yi Xue Za Zhi [Chinese Journal of Preventive Medicine] 1988;22(4):221‐4. [PubMed] [Google Scholar]
- Chen K, Qiu JL, Sui LM, Yu WP, Wang JY, Zhang LJ. Nutrient intake and gastric cancer in residents of Zhoushan Islands, China. Digestive and Liver Disease 2003;35(12):912‐3. [DOI] [PubMed] [Google Scholar]
- Connelly‐Frost A, Poole C, Satia JA, Kupper LL, Millikan RC, Sandler RS. Selenium, folate, and colon cancer. Nutrition and Cancer 2009;61(2):165‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello AJ. A randomized, controlled chemoprevention trial of selenium in familial prostate cancer: rationale, recruitment, and design issues. Urology 2001;57(4 Suppl 1):182‐4. [DOI] [PubMed] [Google Scholar]
- Criqui MH, Bangdiwala S, Goodman DS, Blaner WS, Morris JS, Kritchevsky S, et al. Selenium, retinol, retinol‐binding protein, and uric acid. Associations with cancer mortality in a population‐based prospective case‐control study. Annals of Epidemiology 1991;1(5):385‐93. [DOI] [PubMed] [Google Scholar]
- Cui Y, Vogt S, Olson N, Glass AG, Rohan TE. Levels of zinc, selenium, calcium, and iron in benign breast tissue and risk of subsequent breast cancer. Cancer Epidemiology, Biomarkers & Prevention 2007;16(8):1682‐5. [DOI] [PubMed] [Google Scholar]
- Davies TW, Treasure FP, Welch AA, Day NE. Diet and basal cell skin cancer: results from the EPIC‐Norfolk cohort. British Journal of Dermatology 2002;146(6):1017‐22. [DOI] [PubMed] [Google Scholar]
- Fleshner N, CAN‐CNIC‐PRP1. Phase II randomized study of vitamin E, selenium, and soy protein isolate in patients with high‐grade prostatic intraepithelial neoplasia. http://www.cancer.gov (accessed 1 April 2004).
- Hagmar L, Linden K, Nilsson A, Norrving B, Akesson B, Schutz A, et al. Cancer incidence and mortality among Swedish Baltic Sea fishermen. Scandinavian Journal of Work, Environment and Health 1992;18(4):217‐24. [DOI] [PubMed] [Google Scholar]
- Harris Holly R, Brgkvist L, Wolk A. Selenium intake and breast cancer mortality in a cohort of Swedish women. Breast Cancer Research and Treatment 2012;134(3):1269‐77. [DOI] [PubMed] [Google Scholar]
- Hartman TJ, Taylor PR, Alfthan G, Fagerstrom R, Virtamo J, Mark SD, et al. Toenail selenium concentration and lung cancer in male smokers (Finland). Cancer Causes & Control 2002;13(10):923‐8. [DOI] [PubMed] [Google Scholar]
- Huzarski T, Byrski T, Gronwald J, Kowalska E, Zajaczek S, Gorski B, et al. A lowering of breast and ovarian cancer risk in women with a BRCA1 mutation by selenium supplementation of diet. Hereditary Cancer in Clinical Practice 2006;4(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joniau S, Goeman L, Roskams T, Lerut E, Oyen R, Van PH. Effect of nutritional supplement challenge in patients with isolated high‐grade prostatic intraepithelial neoplasia. Urology 2007;69(6):1102‐6. [DOI] [PubMed] [Google Scholar]
- Kellen E, Zeegers MP, Bruckers L, Buntinx F. The investigation of a geographical cluster of bladder cancer. Acta Clinica Belgica 2008;63(5):313‐20. [DOI] [PubMed] [Google Scholar]
- Kilander L, Berglund L, Boberg M, Vessby B, Lithell H. Education, lifestyle factors and mortality from cardiovascular disease and cancer. A 25‐year follow‐up of Swedish 50‐year‐old men. International Journal of Epidemiology 2001;30(5):1119‐26. [DOI] [PubMed] [Google Scholar]
- Knekt P, Reunanen A, Aromaa A, Heliovaara M, Hakulinen T, Hakama M. Serum cholesterol and risk of cancer in a cohort of 39,000 men and women. Journal of Clinical Epidemiology 1988;41(6):519‐30. [DOI] [PubMed] [Google Scholar]
- Knekt P, Aromaa A, Maatela J, Aaran RK, Nikkari T, Hakama M, et al. Serum vitamin E and risk of cancer among Finnish men during a 10‐year follow‐up. American Journal of Epidemiology 1988;127(1):28‐41. [DOI] [PubMed] [Google Scholar]
- Knekt P, Aromaa A, Maatela J, Alfthan G, Aaran RK, Nikkari T, et al. Serum micronutrients and risk of cancers of low incidence in Finland. American Journal of Epidemiology 1991;134(4):356‐61. [DOI] [PubMed] [Google Scholar]
- Kok FJ, Duijn CM, Hofman A, Vermeeren R, Bruijn AM, Valkenburg HA. Micronutrients and the risk of lung cancer (letter). New England Journal of Medicine 1987c;316:1416. [DOI] [PubMed] [Google Scholar]
- Kune G, Watson L. Colorectal cancer protective effects and the dietary micronutrients folate, methionine, vitamins B6, B12, C, E, selenium, and lycopene. Nutrition and Cancer 2006;56(1):11‐21. [DOI] [PubMed] [Google Scholar]
- Kuroda M, Imura T, Morikawa K, Hasegawa T. Decreased serum levels of selenium and glutathione peroxidase activity associated with aging, malignancy and chronic hemodialysis. Trace Elements in Medicine 1988;5(3):97‐103. [Google Scholar]
- Lawson KA, Wright ME, Subar A, Mouw T, Hollenbeck A, Schatzkin A, et al. Multivitamin use and risk of prostate cancer in the National Institutes of Health‐AARP Diet and Health Study. Journal of the National Cancer Institute 2007;99(10):754‐64. [DOI] [PubMed] [Google Scholar]
- Marchand L, Saltzman BS, Hankin JH, Wilkens LR, Franke AA, Morris SJ, et al. Sun exposure, diet, and melanoma in Hawaii Caucasians. American Journal of Epidemiology 2006;164(3):232‐45. [DOI] [PubMed] [Google Scholar]
- Li H, Li HQ, Wang Y, Xu HX, Fan WT, Wang ML, et al. An intervention study to prevent gastric cancer by micro‐selenium and large dose of allitridum. Chinese Medical Journal 2004;117(8):1155‐60. [PubMed] [Google Scholar]
- Limburg PJ, Wei W, Ahnen DJ, Qiao Y, Hawk ET, Wang G, et al. Randomized, placebo‐controlled, esophageal squamous cell cancer chemoprevention trial of selenomethionine and celecoxib. Gastroenterology 2005;129(3):863‐73. [DOI] [PubMed] [Google Scholar]
- NCI‐OH95‐C‐N026NCI‐P00‐0157. Pilot randomized chemoprevention study of selenium and celecoxib, alone or in combination, in patients with esophageal squamous dysplasia who are residing in Linxian, People's Republic of China. http://www.cancer.gov/search/ViewClinicalTrials.aspx?cdrid=67930&version=HealthProfessional&protocolsearchid=5845119 (accessed 25 February 2009).
- Neuhouser ML, Wassertheil‐Smoller S, Tomson C, Aragaki A, Anderson GL, Manson JE, et al. Multivitamin use and risk of cancer and cardiovascular disease in the women's health initiative cohorts. Archives of Internal Medicine 2009;169(3):294‐304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray AL, Semba RD, Walston J, Ferrucci L, Cappola AR, Ricks MO, et al. Low serum selenium and total carotenoids predict mortality among older women living in the community: the women's health and aging studies. Journal of Nutrition 2006;136(1):172‐6. [DOI] [PubMed] [Google Scholar]
- Larsen EH. Prevention of cancer by intervention with Selenium (pilot) ‐ PRECISE‐PILOT. http://www.food.dtu.dk/Default.aspx?ID=20782 (accessed 6 April 2011). ; MRC Clinical Trials Unit, ISRCTN64336220. A randomised double‐blind placebo‐controlled cancer prevention trial with an estimated duration of 5 years with 52,000 subjects recruited from the general populations of the UK, Denmark, Sweden, Finland and the United States. http://www.controlled‐trials.com/ISRCTN64336220 (accessed 6 April 2011). ; Rayman M, ISRCTN25193534. UK prevention of cancer by intervention with selenium. http://www.controlled‐trials.com/ISRCTN25193534/ (accessed 6 April 2011). ; Rayman M, NCT00022165. Selenium in the prevention of cancer. http://clinicaltrials.gov/ct2/show/NCT00022165. England, W12 ONN, United Kingdom: Hammersmith Hospital London, (accessed 6 April 2011).
- Rendon RA, Fleshner N. Vitamin E, selenium and soy protein in preventing cancer in patients with high‐grade prostate neoplasia. http://clinicaltrials.gov/ct2/show/NCT00064194. www.clinicaltrials.gov, (accessed 6 April 2011).
- Steevens J, Schouten LJ, Driessen AL, Huysentruyt CJ, Keulemans YC, Goldbohm RA, et al. Toenail selenium status and the risk of Barrett's esophagus: the Netherlands Cohort Study. Cancer Causes Control 2010;21(12):2259‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CA, Habermann TM, Wang AH, Vierkant RA, Folsom AR, Ross JA, et al. Antioxidant intake from fruits, vegetables and other sources and risk of non‐Hodgkin lymphoma: the Iowa Women's Health Study. International Journal of Cancer 2009;(epub ahead of print):doi 10.1002/ijc.24830. [DOI: 10.1002/ijc.24830] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsugane S, Hamada GS, Karita K, Tsubono Y, Laurenti R. Cancer patterns and lifestyle among Japanese immigrants and their descendants in the city of Sao Paulo, Brazil. Gann Monographs on Cancer Research 1996;44:43‐50. [Google Scholar]
- Ujiie S, Kikuchi H. The relation between serum selenium value and cancer in Miyagi, Japan: 5‐year follow up study. Tohoku Journal of Experimental Medicine 2002;196(3):99‐109. [DOI] [PubMed] [Google Scholar]
- van't Veer P, Strain JJ, Fernandez‐Crehuet J, Martin BC, Thamm M, Kardinaal AF, et al. Tissue antioxidants and postmenopausal breast cancer: the European Community Multicentre Study on Antioxidants, Myocardial Infarction, and Cancer of the Breast (EURAMIC). Cancer Epidemiology, Biomarkers & Prevention 1996;5(6):441‐7. [PubMed] [Google Scholar]
- Noord PAH, Tweel I, Kaaks R, Waard F. Selenium levels and subsequent colorectal cancers: the efficiency gain of a sequential test to a cohort‐nested study with a 1:4 matching ratio. In: Noord PAH editor(s). Selenium and Human Cancer Risk: Nail Keratin as a Tool in Metabolic Epidemiology. Amsterdam: Thesis Publishers, 1992:139‐53. [Google Scholar]
- Noord PAH, Maas MJ, Tweel I, Collette C. Selenium and the risk of postmenopausal breast cancer in the DOM cohort. Breast Cancer Research and Treatment 1993;25(1):11‐9. [DOI] [PubMed] [Google Scholar]
- Wallace K, Kelsey KT, Schned A, Morris JS, Andrew AS, Karagas MR. Selenium and risk of bladder cancer: a population‐based case‐control study. Cancer Prevention Research (Phila Pa) 2009;2(1):70‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watters JL, Park Y, Hollenbeck A, Schatzkin A, Albanes D. Cigarette smoking and prostate cancer in a prospective US cohort study. Cancer Epidemiology, Biomarkers & Prevention 2009;18(9):2427‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright ME, Mayne ST, Stolzenberg‐Solomon RZ, Li Z, Pietinen P, Taylor PR, et al. Development of a comprehensive dietary antioxidant index and application to lung cancer risk in a cohort of male smokers. American Journal of Epidemiology 2004;160(1):68‐76. [DOI] [PubMed] [Google Scholar]
- You WC, Li JY, Zhang L, Jin ML, Chang YS, Ma JL, et al. Etiology and prevention of gastric cancer: a population study in a high risk area of China. Chinese Journal of Digestive Diseases 2005;6(4):149‐54. [DOI] [PubMed] [Google Scholar]
- Yuan JM, Gao YT, Ong CN, Ross RK, Yu MC. Prediagnostic level of serum retinol in relation to reduced risk of hepatocellular carcinoma. JNCI Cancer Spectrum 2006;98(7):482‐90. [DOI] [PubMed] [Google Scholar]
- Zeegers MP, Bryan RT, Langford C, Billingham L, Murray P, Deshmukh NS, et al. The West Midlands Bladder Cancer Prognosis Programme: rationale and design. BJU International 2010;705(6):784‐8. [DOI: 10.1111/j.1464-410X.2009.08849.x] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
- Nomura AM, 5R01CA033644‐19. Cancer sero epidemiology among the Japanese in Hawaii. http://crisp.cit.nih.gov/ (accessed 12 January 2004).
- Cheng KK, ISRCTN38534743. Selenium supplementation for the prevention of hepatocellular carcinomas in HBsAg positive patients (pilot study). http://www.controlled‐trials.com/isrctn/trial_print‐friendly.asp?ISRCTN=38534743 (accessed 16 April 2004).
- Cheng KK, ISRCTN13889738. Bladder Cancer Prognosis Programme (incorporating SELENIB trial). http://www.controlled‐trials.com/mrct/trial/232573/selenib (accessed 18 February 2009).
- Eastern Cooperative Oncology Group. Selenium in preventing tumor growth in patients with previously resected stage I non‐small cell lung cancer. http://clinicaltrials.gov/ct2/show/NCT00008385 (accessed 6 April 2011). ; Eastern Cooperative Oncology Group, ECOG‐5597. Phase III randomized chemoprevention study of selenium in participants with previously resected stage I non‐small cell lung cancer. http://www.cancer.gov/clinicaltrials/featured/trials/ecog‐5598. Eastern Cooperative Oncology Group, (accessed 6 April 2011). ; Karp DD. Phase III chemoprevention trial of selenium supplementation in persons with resected stage I non‐small‐cell lung cancer. Clinical Advances in Hematology and Oncology 2005;3(4):313‐5. [Google Scholar]
- Ahmann FR, 5R01CA077789‐05. Phase III Trial of Selenium for Prostate Cancer. http://crisp.cit.nih.gov/ (accessed 12 January 2004). ; Ahmann, F. Phase III Randomized Study of Selenium for Prostate Cancer Prevention. http://www.cancer.gov./search/ViewClinicalTrials.aspx?cdrid=654651 (accessed 20 October 2009). ; Clark LC, Marshall JR. Randomized, controlled chemoprevention trials in populations at very high risk for prostate cancer: elevated prostate‐specific antigen and high‐grade prostatic intraepithelial neoplasia. Urology 2001;57(4 Suppl 1):185‐7. [DOI] [PubMed] [Google Scholar]; Marshall JR. Larry Clark's legacy: randomized controlled, selenium‐based prostate cancer chemoprevention trials. Nutrition and Cancer 2001;40(1):74‐7. [DOI] [PubMed] [Google Scholar]; Nelson MA, Reid M, Duffield‐Lillico AJ, Marshall JR. Prostate cancer and selenium. The Urologic Clinics of North America 2002;29(1):67‐70. [DOI] [PubMed] [Google Scholar]; Stratton MS, Reid ME, Schwartzberg G, Minter FE, Monroe BK, Alberts DS, et al. Selenium and prevention of prostate cancer in high‐risk men: the negative biopsy study. Anticancer Drugs 2003;14(8):589‐94. [DOI] [PubMed] [Google Scholar]
Additional references
- Arnaud J, Arnault N, Roussel AM, Bertrais S, Ruffieux D, Galan P, et al. Relationships between selenium, lipids, iron status and hormonal therapy in women of the SU.VI.M.AX cohort. Journal of Trace Elements in Medicine and Biology 2007;21(Suppl 1):66‐9. [DOI] [PubMed] [Google Scholar]
- Arnaud J, Lorgeril M, Akbaraly T, Salen P, Arnout J, Cappuccio FP, et al. Gender differences in copper, zinc and selenium status in diabetic‐free metabolic syndrome European population ‐ the IMMIDIET study. Nutrition, Metabolism and Cardiovascular Disease 2012;22(6):517‐24. [DOI] [PubMed] [Google Scholar]
- Ashar BH. The dietary supplement health and education act: time for a reassessment. Archives of Internal Medicine 2010;170(3):261‐3. [DOI] [PubMed] [Google Scholar]
- Ashton K, Hooper L, Harvey LJ, Hurst R, Casgrain A, Fairweather‐Tait SJ. Methods of assessment of selenium status in humans: a systematic review. American Journal of Clinical Nutrition 2009;89(6):2025S‐39S. [DOI] [PubMed] [Google Scholar]
- Barany E, Bergdahl IA, Bratteby LE, Lundh T, Samuelson G, Schutz A, et al. Trace elements in blood and serum of Swedish adolescents: relation to gender, age, residential area, and socioeconomic status. Environmental Research 2002;89(1):72‐84. [DOI] [PubMed] [Google Scholar]
- Behne D, Gessner H, Kyriakopoulos A. Information on the selenium status of several body compartments of rats from the selenium concentrations in blood fractions, hair and nails. Journal of Trace Elements in Medicine and Biology 1996;103:174‐9. [DOI] [PubMed] [Google Scholar]
- Behne D, Alber D, Kyriakopoulos S. Long‐term selenium supplementation of humans: selenium status and relationships between selenium concentrations in skeletal muscle and indicator materials. Journal of Trace Elements in Medicine and Biology 2010;24(2):99‐105. [DOI] [PubMed] [Google Scholar]
- Bjelakovic G, Nikolova D, Simonetti RG, Gluud C. Antioxidant supplements for preventing gastrointestinal cancers. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD004183.pub3] [DOI] [PubMed] [Google Scholar]
- Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD007176] [DOI] [PubMed] [Google Scholar]
- Block E, Glass RS, Jacobsen NE, Johnson S, Kahakachchi C, Kaminski R, et al. Identification and synthesis of a novel selenium‐sulfur amino acid found in selenized yeast: rapid indirect detection NMR methods for characterizing low‐level organoselenium compounds in complex matrices. Journal of Agricultural and Food Chemistry 2004;52(12):3761‐71. [DOI] [PubMed] [Google Scholar]
- Blot WJ, Li JY, Taylor PR, Guo W, Dawsey S, Wang GQ, et al. Nutrition intervention trials in Linxian, China: supplementation with specific vitamin/mineral combinations, cancer incidence, and disease‐specific mortality in the general population. Journal of the National Cancer Institute 1993;85(18):1483‐92. [DOI] [PubMed] [Google Scholar]
- Bodnar M, Konieczka P, Namiesnik J. The properties, functions, and use of selenium compounds in living organisms. Journal of Environmental Science and Health, Part C, Environmental Carcinogenesis & Ecotoxicology Reviews 2012;30(3):225‐52. [DOI] [PubMed] [Google Scholar]
- Brigo F, Storti M, Lochner P, Tezzon F, Nardone F. Selenium supplementation for primary prevention of cardiovascular disease: proof of no effectiveness. Nutrition, Metabolism & Cardiovascular Disease 2014;24(1):e2‐3. [DOI] [PubMed] [Google Scholar]
- Brinkman M, Reulen RC, Kellen E, Buntinx F, Zeegers MP. Are men with low selenium levels at increased risk of prostate cancer?. European Journal of Cancer 2006;42(15):2463‐71. [DOI] [PubMed] [Google Scholar]
- Bruhn RL, Stamer WD, Herrygers LA, Levine JM, Noecker RJ. Relationship between glaucoma and selenium levels in plasma and aqueous humour. British Journal of Ophthalmology 2009;93(9):1155‐8. [DOI] [PubMed] [Google Scholar]
- Burri J, Haldimann M, Dudler V. Selenium status of the Swiss population: assessment and change over a decade. Journal of Trace Elements in Medicine and Biology 2008;22(2):112‐9. [DOI] [PubMed] [Google Scholar]
- Chen X, Mikhail SS, Ding YW, Yang G, Bondoc F, Yang CS. Effects of vitamin E and selenium supplementation on esophageal adenocarcinogenesis in a surgical model with rats. Carcinogenesis 2000;21(8):1531‐6. [PubMed] [Google Scholar]
- Chintala S, Najrana T, Toth K, Cao S, Durrani FA, Pili R, et al. Prolyl hydroxylase 2 dependent and Von‐Hippel‐Lindau independent degradation of hypoxia‐inducible factor 1 and 2 alpha by selenium in clear cell renal cell carcinoma leads to tumor growth inhibition. BMC Cancer 2012;12:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs GF Jr. Current evidence and research needs to support a health claim for selenium and cancer prevention. Journal of Nutrition 2005;135(2):343‐7. [DOI] [PubMed] [Google Scholar]
- Combs GF Jr, Jackson MI, Watts JC, Johnson LK, Zeng H, Idso J, et al. Differential responses to selenomethionine supplementation by sex and genotype in healthy adults. British Journal of Nutrition 2012;107(10):1514‐25. [DOI] [PubMed] [Google Scholar]
- Dalton SO, Schuz J, Engholm G, Johansen C, Kjaer SK, Steding‐Jessen M, et al. Social inequality in incidence of and survival from cancer in a population‐based study in Denmark, 1994‐2003: Summary of findings. European Journal of Cancer 2008;44(14):2074‐85. [DOI] [PubMed] [Google Scholar]
- Davis CD, Tsuji PA, Milner JA. Selenoproteins and cancer prevention. Annual Review of Nutrition 2012;32:73‐95. [DOI] [PubMed] [Google Scholar]
- Dennert G, Brinkman M, Vinceti M, Zeegers M, Zwahlen M, Horneber M. [P18‐179] How global is our knowledge? Population diversity in observational studies on selenium and cancer risk. http://www.cochrane.org/colloquium/2008/virtual_posters/?poster=168 16th Cochrane Colloquium, Freiburg, 3‐7 October 2008 (Poster).
- Driscoll MS, Kwon EK, Skupsky H, Kwon SY, Grant‐Kels JM. Nutrition and the deleterious side effects of nutritional supplements. Clinics in Dermatology 2010;28(4):371‐9. [DOI] [PubMed] [Google Scholar]
- Duffield AJ, Thomson CD. A comparison of methods of assessment of dietary selenium intakes in Otago, New Zealand. British Journal of Nutrition 1999;82(2):131‐8. [DOI] [PubMed] [Google Scholar]
- Egger M, Schneider M, Davey Smith G. Spurious precision? Meta‐analysis of observational studies. British Medical Journal 1998;316(7125):140‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairweather‐Tait SJ, Bao Y, Broadley MR, Collings R, Ford D, Hesketh JE, et al. Selenium in human health and disease. Antioxidants & Redox Signaling 2011;14(7):1337‐83. [DOI] [PubMed] [Google Scholar]
- Fan C, Chen J, Wang Y, Wong YS, Zhang Y, Zheng W, et al. Selenocystine potentiates cancer cell apoptosis induced by 5‐fluorouracil by triggering reactive oxygen species‐mediated DNA damage and inactivation of the ERK pathway. Free Radical Biology & Medicine 2013;65C:305‐16. [DOI] [PubMed] [Google Scholar]
- Fortmann SP, Burda BU, Senger CA, Lin JS, Whitlock EP. Vitamin and mineral supplements in the primary prevention of cardiovascular disease and cancer: an updated systematic evidence review for the U.S. Preventive Services Task Force. Annals of Internal Medicine 2013;159(12):824‐34. [DOI] [PubMed] [Google Scholar]
- Gammelgaard B, Jackson MI, Gabel‐Jensen C. Surveying selenium speciation from soil to cell‐‐forms and transformations. Analytical and Bioanalytical Chemistry 2011;399(5):1743‐63. [DOI] [PubMed] [Google Scholar]
- Gorlova OY, Zhang Y, Schabath MB, Lei L, Zhang Q, Amos CI, et al. Never smokers and lung cancer risk: a case‐control study of epidemiological factors. International Journal of Cancer 2006;118(7):1798‐804. [DOI] [PubMed] [Google Scholar]
- Guallar E, Stranges S, Mulrow C, Appel LJ, Miller ER III. Enough is enough: stop wasting money on vitamin and mineral supplements. Annals of Internal Medicine 2013;159(2):850‐1. [DOI] [PubMed] [Google Scholar]
- Gundacker C, Komarnicki G, Zodl B, Forster C, Schuster E, Wittmann K. Whole blood mercury and selenium concentrations in a selected Austrian population: does gender matter?. The Science of the Total Environment 2006;372(1):76‐86. [DOI] [PubMed] [Google Scholar]
- Haldimann M, Venner TY, Zimmerli B. Determination of selenium in the serum of healthy Swiss adults and correlation to dietary intake. Journal of Trace Elements in Medicine and Biology 1996;10(1):31‐45. [DOI] [PubMed] [Google Scholar]
- Hall C. [Investigations on the direct and indirect genotoxicity of sodium seleniute and selenomethionine] [Untersuchungen zur direkten und indirekten Genotoxizität von Natriumselenit und Selenmethionin]. Univ Diss. Berlin, Germany: Technische Universität Berlin, 2008. [Google Scholar]
- Hatfield DL, Yoo MH, Carlson BA, Gladyshev VN. Selenoproteins that function in cancer prevention and promotion. Biochimica et Biophysica Acta 2009;1790(11):1541‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield DL, Tsuji PA, Carlson BA, Gladyshev VN. Selenium and selenocysteine: roles in cancer, health, and development. Trends in Biochemical Sciences 2014;39(3):112‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazane‐Puch F, Champelovier P, Arnaud J, Garrel C, Ballester B, Faure P, et al. Long‐term selenium supplementation in HaCaT cells: importance of chemical form for antagonist (protective versus toxic) activities. Biological Trace Elements Research 2013;154(2):288‐98. [DOI] [PubMed] [Google Scholar]
- Heck JA, Parka AS, Qiub J, Cockburnc M, Ritz B. Risk of leukemia in relation to exposure to ambient air toxics inpregnancy and early childhood. International Journal of Hygiene and Environmental Health 2014:(in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hercberg S, Galan P, Preziosi P, Bertrais S, Mennen L, Malvy D, et al. The SU.VI.MAX Study: a randomized, placebo‐controlled trial of the health effects of antioxidant vitamins and minerals. Archives of Internal Medicine 2004;164(21):2335‐42. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. British Medical Journal 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG, Spiegelhalter DJ. A re‐evaluation of random‐effects meta‐analysis. Journal of the Royal Statistical Society Series A (Statistics in Society) 2009;172(1):137‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org2011.
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. Cochrane Bias Methods Group, Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. British Medical Journal 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter DJ, Morris JS, Chute CG, Kushner E, Colditz GA, Stampfer MJ, et al. Predictors of selenium concentration in human toenails. American Journal of Epidemiology 1990;132(1):114‐22. [DOI] [PubMed] [Google Scholar]
- Hurst R, Collings R, Harvey LJ, King M, Hooper L, Bouwman J, et al. EURRECA‐estimating selenium requirements for deriving dietary reference values. Critical Reviews in Food Science and Nutrition 2013;53(10):1077‐96. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet], 2013. http://globocan.iarc.fr/.
- Institute of Medicine. Dietary reference intakes: elements. http://www.iom.edu/Object.File/Master/54/395/DRIs.Elements.pdf (accessed 18 May 2009).
- Jablonska E, Gromadzinska J, Klos A, Bertrandt J, Skibniewska K, Darago A, et al. Selenium, zinc and copper in the Polish diet. Journal of Food Composition and Analysis 2013;31(2):259–65. [Google Scholar]
- Jackson MI, Combs GF Jr. Selenium and anticarcinogenesis: underlying mechanisms. Current Opinion in Clinical Nutrition and Metabolic Care 2008;11(6):718‐26. [DOI] [PubMed] [Google Scholar]
- Jaffé W. Selenio, un elemento esencial y toxico. Datos de Latinoamerica. Archivos Latinoamericanos de Nutrición 1992;42(2):90‐3. [PubMed] [Google Scholar]
- Jerome‐Morais A, Diamond AM, Wright ME. Dietary supplements and human health: for better or for worse?. Molecular Nutrition & Food Research 2011;55(1):122‐35. [DOI] [PubMed] [Google Scholar]
- Jossa F, Trevisan M, Krogh V, Farinaro E, Giumetti D, Fusco G, et al. Serum selenium and coronary heart disease risk factors in southern Italian men. Atherosclerosis 1991;87(2‐3):129‐34. [DOI] [PubMed] [Google Scholar]
- Kafai MR, Ganji V. Sex, age, geographical location, smoking, and alcohol consumption influence serum selenium concentrations in the USA: third National Health and Nutrition Examination Survey, 1988‐1994. Journal of Trace Elements in Medicine and Biology 2003;17(1):13‐8. [DOI] [PubMed] [Google Scholar]
- Kandas NO, Randolph C, Bosland MC. Differential effects of selenium on benign and malignant prostate epithelial cells: stimulation of LNCaP cell growth by noncytotoxic, low selenite concentrations. Nutrition and Cancer 2009;61(2):251‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kant AK, Graubard BI. Ethnicity is an independent correlate of biomarkers of micronutrient intake and status in American adults. Journal of Nutrition 2007;137(11):2456‐63. [DOI] [PubMed] [Google Scholar]
- Karita K, Sasaki S, Ishihara J, Tsugane S. Validity of a self‐administered food frequency questionnaire used in the 5‐year follow‐up survey of the JPHC Study to assess selenium intake: comparison with dietary records and blood levels. Journal of Epidemiology 2003;13(1 Suppl):S92‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp DD, Lee SJ, Keller SM, Wright GS, Aisner S, Belinsky SA, et al. Randomized, double‐blind, placebo‐controlled, phase III chemoprevention trial of selenium supplementation in patients with resected stage I non‐smallcell lung cancer: ECOG 5597. Journal of Clinical Oncology 2013;31(33):4179‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YY, Mahan DC. Comparative effects of high dietary levels of organic and inorganic selenium on selenium toxicity of growing‐finishing pigs. Journal of Animal Science 2001;79(4):942‐8. [DOI] [PubMed] [Google Scholar]
- Kim YW, Bae SM, Liu HB, Kim IW, Chun HJ, Ahn WS. Selenium enhances the efficacy of Radachlorin mediated‐photodynamic therapy in TC‐1 tumor development. Oncology Reports 2012;28(2):576‐84. [DOI] [PubMed] [Google Scholar]
- Lawlor DA, Davey SG, Kundu D, Bruckdorfer KR, Ebrahim S. Those confounded vitamins: what can we learn from the differences between observational versus randomised trial evidence?. Lancet 2004;363(9422):1724‐7. [DOI] [PubMed] [Google Scholar]
- Longnecker MP, Stram DO, Taylor PR, Levander OA, Howe M, Veillon C, et al. Use of selenium concentration in whole blood, serum, toenails, or urine as a surrogate measure of selenium intake. Epidemiology 1996;7(4):384‐90. [DOI] [PubMed] [Google Scholar]
- Marik PE, Flemmer M. Do dietary supplements have beneficial health effects in industrialized nations: what is the evidence?. JPEN. Journal of Parenteral and Enteral Nutrition 2012;36(2):159‐68. [DOI] [PubMed] [Google Scholar]
- Martinez ME, Jacobs ET, Baron JA, Marshall JR, Byers T. Dietary supplements and cancer prevention: balancing potential benefits against proven harms. Journal of the National Cancer Institute 2012;104(10):732‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayne ST, Ferrucci LM, Cartmel B. Lessons learned from randomized clinical trials of micronutrient supplementation for cancer prevention. Annual Review of Nutrition 2012;32:369‐90. [DOI] [PubMed] [Google Scholar]
- McNaughton SA, Marks GC, Green AC. Role of dietary factors in the development of basal cell cancer and squamous cell cancer of the skin. Cancer Epidemiology, Biomarkers & Prevention 2005;14(7):1596‐607. [DOI] [PubMed] [Google Scholar]
- Morris JS, Crane SB. Selenium toxicity from a misformulated dietary supplement, adverse health effects, and the temporal response in the nail biologic monitor. Nutrients 2013;5(4):1024‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyad MA. Heart healthy=prostate healthy: SELECT, the symbolic end of preventing prostate cancer via heart unhealthy and over anti‐oxidation mechanisms?. Asian Journal of Andrology 2012;14(2):243‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SP, Wilkens LR, Hankin JH, Foote JA, Monroe KR, Henderson BE, et al. Comparison of two instruments for quantifying intake of vitamin and mineral supplements: a brief questionnaire versus three 24‐hour recalls. American Journal of Epidemiology 2002;156(7):669‐75. [DOI] [PubMed] [Google Scholar]
- National Toxicology Program. Selenium sulfide. Report on carcinogens: carcinogen profiles/U.S. Dept. of Health and Human Services, Public Health Service, National Toxicology Program 2011;12:376‐7. [PubMed] [Google Scholar]
- Nishino Y, Tsubono Y, Tsuji I, Komatsu S, Kanemura S, Nakatsuka H, et al. Passive smoking at home and cancer risk: a population‐based prospective study in Japanese nonsmoking women. Cancer Causes and Control 2001;12(9):797‐802. [DOI] [PubMed] [Google Scholar]
- Niskar AS, Paschal DC, Kieszak SM, Flegal KM, Bowman B, Gunter EW, et al. Serum selenium levels in the US population: Third National Health and Nutrition Examination Survey, 1988‐1994. Biological Trace Element Research 2003;91(1):1‐10. [DOI] [PubMed] [Google Scholar]
- Novoselov SV, Calvisi DF, Labunskyy VM, Factor VM, Carlson BA, Fomenko DE, et al. Selenoprotein deficiency and high levels of selenium compounds can effectively inhibit hepatocarcinogenesis in transgenic mice. Oncogene 2005;24(54):8003‐11. [DOI] [PubMed] [Google Scholar]
- Office of Dietary Supplements, NIH Clinical Centers, National Institutes of Health. Dietary Supplement Fact Sheet: Selenium. http://ods.od.nih.gov/factsheets/selenium.asp (accessed 18 May 2009).
- Ovaskainen ML, Virtamo J, Alfthan G, Haukka J, Pietinen P, Taylor PR, et al. Toenail selenium as an indicator of selenium intake among middle‐aged men in an area with low soil selenium. The American Journal of Clinical Nutrition 1993;57(5):662‐5. [DOI] [PubMed] [Google Scholar]
- Panter KE, Hartley WJ, James LF, Mayland HF, Stegelmeier BL, Kechele PO. Comparative toxicity of selenium from seleno‐DL‐methionine, sodium selenate, and Astragalus bisulcatus in pigs. Fundamental and Applied Toxicology 1996;32(2):217‐23. [PubMed] [Google Scholar]
- Pearce N. The globalization of epidemiology: introductory remarks. International Journal of Epidemiology 2004;33(5):1127‐31. [DOI] [PubMed] [Google Scholar]
- Pestitschek M, Sonneck‐Koenne C, Zakavi SR, Li S, Knoll P, Mirzaei S. Selenium intake and selenium blood levels: a novel food frequency questionnaire. Wiener Klinische Wochenschrift 2013;125(5‐6):160‐4. [DOI] [PubMed] [Google Scholar]
- Pildal J, Hrobjartsson A, Jorgensen KJ, Hilden J, Altman DG, Gotzsche PC. Impact of allocation concealment on conclusions drawn from meta‐analyses of randomized trials. International Journal of Epidemiology 2007;36(4):847‐57. [DOI] [PubMed] [Google Scholar]
- Pounis G, Costanzo S, Persichillo M, Curtis A, Sieri S, Vinceti M, et al. Moli‐sani Project Investigators. Mushroom and dietary selenium intakes in relation to fasting glucose levels in a free‐living Italian adult population: the Moli‐sani Project. Diabetes & Metabolism 2014;40:34‐42; Vol. 40, issue 1:34‐42. [DOI] [PubMed]
- Qu CX, Kamangar F, Fan JH, Yu B, Sun XD, Taylor PR, et al. Chemoprevention of primary liver cancer: a randomized, double‐blind trial in Linxian, China. Journal of the National Cancer Institute 2007;99(16):1240‐7. [DOI] [PubMed] [Google Scholar]
- Rapiti E, Fioretta G, Schaffar R, Neyroud‐Caspar I, Verkooijen HM, Schmidlin F, et al. Impact of socioeconomic status on prostate cancer diagnosis, treatment, and prognosis. Cancer 2009;115(23):5556‐65. [DOI] [PubMed] [Google Scholar]
- Rayman MP. The importance of selenium to human health. Lancet 2000;356(9225):233‐41. [DOI] [PubMed] [Google Scholar]
- Rayman MP. The use of high‐selenium yeast to raise selenium status: how does it measure up?. British Journal of Nutrition 2004;92(4):557‐73. [DOI] [PubMed] [Google Scholar]
- Rayman MP, Infante HG, Sargent M. Food‐chain selenium and human health: spotlight on speciation. British Journal of Nutrition 2008;100(2):238‐53. [DOI] [PubMed] [Google Scholar]
- Rayman MP. Food‐chain selenium and human health: emphasis on intake. British Journal of Nutrition 2008;100(2):254‐68. [DOI] [PubMed] [Google Scholar]
- Rayman MP. Selenium and human health. Lancet 2012;379(9822):1256‐68. [DOI] [PubMed] [Google Scholar]
- Rees K, Hartley L, Day C, Flowers N, Clarke A, Stranges S. Selenium supplementation for the primary prevention of cardiovascular disease. The Cochrane Database of Systematic Reviews 2013;1:CD009671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocourt CR, Cheng WH. Selenium supranutrition: are the potential benefits of chemoprevention outweighed by the promotion of diabetes and insulin resistance?. Nutrients 2013;5(4):1349‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez Rodriguez EM, Sanz Alaejos MT, Diaz Romero C. Urinary selenium status of healthy people. European Journal of Clinical Chemistry and Clinical Biochemistry 1995;33(3):127‐33. [DOI] [PubMed] [Google Scholar]
- Rose AH, Bertino P, Hoffmann FW, Gaudino G, Carbone M, Hoffmann PR. Increasing dietary selenium elevates reducing capacity and ERK activation associated with accelerated progression of select mesothelioma tumors. The American Journal of Pathology 2014:(in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco JE, Dodd KW, Kirkpatrick SI, Tarasuk V. Voluntary food fortification in the United States: potential for excessive intakes. European Journal of Clinical Nutrition 2013;67(6):592‐7. [DOI] [PubMed] [Google Scholar]
- Satia JA, King IB, Morris JS, Stratton K, White E. Toenail and plasma levels as biomarkers of selenium exposure. Annals of Epidemiology 2006;16(1):53‐8. [DOI] [PubMed] [Google Scholar]
- Schrauzer GN, White DA, Schneider CJ. Cancer mortality correlation studies ‐ III: statistical associations with dietary selenium intakes. Biological Chemistry 1977;7(1):23‐31. [DOI] [PubMed] [Google Scholar]
- Shamberger RJ, Frost DV. Possible protective effect of selenium against human cancer. Canadian Medical Association Journal 1969;100(14):682. [PMC free article] [PubMed] [Google Scholar]
- Slavik P, Illek J, Brix M, Hlavicova J, Rajmon R, Jilek F. Influence of organic versus inorganic dietary selenium supplementation on the concentration of selenium in colostrum, milk and blood of beef cows. Acta Veterinaria Scandinavica 2008;50:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AM, Chang MP, Medeiros LC. Generational differences in selenium status of women. Biological Trace Element Research 2000;75(1‐3):157‐65. [DOI] [PubMed] [Google Scholar]
- Solovyev N, Berthele A, Michalke B. Selenium speciation in paired serum and cerebrospinal fluid samples. Analytical and Bioanalytical Chemistry 2013;405(6):1875‐84. [DOI] [PubMed] [Google Scholar]
- Sonaa E, Usha S, Ja In J. An ex vivo study of selenium, genistein on the morphological and nuclear changes in anticancer drug‐induced apoptosis in human peripheral blood lymphocytes. Biofactors 2013;39(3):279‐93. [DOI] [PubMed] [Google Scholar]
- Steen A, Strom T, Bernhoft A. Organic selenium supplementation increased selenium concentrations in ewe and newborn lamb blood and in slaughter lamb meat compared to inorganic selenium supplementation. Acta Veterinaria Scandinavica 2008;50:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbrenner H, Speckmann B, Sies H. Toward understanding success and failures in the use of selenium for cancer prevention. Antioxidants & Redox Signaling 2013;19(2):181‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranges S, Marshall JR, Natarajan R, Donahue RP, Trevisan M, Combs GF, et al. Effects of long‐term selenium supplementation on the incidence of type 2 diabetes: a randomized trial. Annals of Internal Medicine 2007;147(4):217‐23. [DOI] [PubMed] [Google Scholar]
- Stranges S, Sieri S, Vinceti M, Grioni S, Guallar E, Laclaustra M, et al. A prospective study of dietary selenium intake and risk of type 2 diabetes. BMC Public Health 2010;10:564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su YP, Tang JM, Tang Y, Gao HY. Histological and ultrastructural changes induced by selenium in early experimental gastric carcinogenesis. World Journal of Gastroenterology 2005;11(29):4457‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson CA, Longnecker MP, Veillon C, Howe M, Levander OA, Taylor PR, et al. Selenium intake, age, gender, and smoking in relation to indices of selenium status of adults residing in a seleniferous area. The American Journal of Clinical Nutrition 1990;52(5):858‐62. [DOI] [PubMed] [Google Scholar]
- Tiwary AK, Stegelmeier BL, Panter KE, James LF, Hall JO. Comparative toxicosis of sodium selenite and selenomethionine in lambs. Journal of Veterinary Diagnostic Investigation 2006;18(1):61‐70. [DOI] [PubMed] [Google Scholar]
- Brandt PA, Goldbohm RA, van't Veer P, Bode P, Hermus RJ, Sturmans F. Predictors of toenail selenium levels in men and women. Cancer Epidemiology, Biomarkers & Prevention 1993;2(2):107‐12. [PubMed] [Google Scholar]
- Vinceti M, Rothman KJ, Bergomi M, Borciani N, Serra L, Vivoli G. Excess melanoma incidence in a cohort exposed to high levels of environmental selenium. Cancer Epidemiology, Biomarkers & Prevention 1998;7(10):853‐6. [PubMed] [Google Scholar]
- Vinceti M, Rovesti S, Bergomi M, Vivoli G. The epidemiology of selenium and human cancer. Tumori 2000;86(2):105‐18. [DOI] [PubMed] [Google Scholar]
- Vinceti M, Nacci G, Rocchi E, Cassinadri T, Vivoli R, Marchesi C, et al. Mortality in a population with long‐term exposure to inorganic selenium via drinking water. Journal of Clinical Epidemiology 2000;53(10):1062‐8. [DOI] [PubMed] [Google Scholar]
- Vinceti M, Wei ET, Malagoli C, Bergomi M, Vivoli G. Adverse health effects of selenium in humans. Reviews on Environmental Health 2001;16(4):233‐51. [DOI] [PubMed] [Google Scholar]
- Vinceti M, Maraldi T, Bergomi M, Malagoli C. Risk of chronic low‐dose selenium overexposure in humans: insights from epidemiology and biochemistry. Reviews on Environmental Health 2009;24(3):231‐48. [DOI] [PubMed] [Google Scholar]
- Vinceti M, Crespi CM, Malagoli C, Bottecchi I, Ferrari A, Sieri S, et al. A case‐control study of the risk of cutaneous melanoma associated with three selenium exposure indicators. Tumori 2012;98(3):287‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinceti M, Crespi CM, Bonvicini F, Malagoli C, Ferrante M, Marmiroli S, et al. The need for a reassessment of the safe upper limit of selenium in drinking water. The Science of the Total Environment 2013;443:633‐42. [DOI] [PubMed] [Google Scholar]
- Vinceti M, Crespi CM, Malagoli C, Giovane C, Krogh V. Friend or foe? The current epidemiologic evidence on selenium and human cancer risk. Journal of Environmental Science and Health, Part C, Environmental Carcinogenesis & Ecotoxicology Reviews 2013;31(4):305‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinceti M, Solovyev N, Mandrioli J, Crespi CM, Bonvicini F, Arcolin E, et al. Cerebrospinal fluid of newly diagnosed amyotrophic lateral sclerosis patients exhibits abnormal levels of selenium species including elevated selenite. Neurotoxicology 2013;38:25‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinceti M, Mandrioli J, Borella P, Michalke B, Tsatsakis A, Finkelstein Y. Selenium neurotoxicity in humans: bridging laboratory and epidemiologic studies. Toxicology Letters 2013 (in press). [DOI] [PubMed]
- Waters DJ, Chiang EC, Cooley DM, Morris JS. Making sense of sex and supplements: differences in the anticarcinogenic effects of selenium in men and women. Mutation Research 2004;551(1‐2):91‐107. [DOI] [PubMed] [Google Scholar]
- Waters DJ, Shen S, Kengeri SS, Chiang EC, Combs GF, Morris JS, Bostwick DG. Proceedings of the 10th International Symposium on Selenium in Biology and Medicine. Berlin: Deutsche Forschungsgemeinschaft, 2013:62. [Google Scholar]
- Weekley CM, Aitken JB, Finney L, Vogt S, Witting PK, Harris HH. Selenium metabolism in cancer cells: the combined application of XAS and XFM techniques to the problem of selenium speciation in biological systems. Nutrients 2013;5(5):1734‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells GA, Shea B, O´Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Ottawa, (accessed 1 April 2004).
- Whanger PD. Selenium and its relationship to cancer: an update. British Journal of Nutrition 2004;91(1):11‐28. [DOI] [PubMed] [Google Scholar]
- Joint FAO/WHO Expert Consultation on Human Vitamin, Mineral Requirements. Vitamin and mineral requirements in human nutrition: report of a joint FAO/WHO expert consultation, Bangkok, Thailand, 21‐30 September 1998. http://whqlibdoc.who.int/publications/2004/9241546123.pdf. World Health Organization (WHO), 2004.
- WHO. Are the number of cancer cases increasing or decreasing in the world?. http://www.who.int/features/qa/15/en/index.html (accessed 9 September 2008).
- Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. British Medical Journal 2008;336(7644):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H, Uthus EO, Combs GF Jr. Mechanistic aspects of the interaction between selenium and arsenic. Journal of Inorganic Biochemistry 2005;99(6):1269‐74. [DOI] [PubMed] [Google Scholar]
- Zwolak I, Zaporowska H. Selenium interactions and toxicity: a review. Cell Biology and Toxicology 2012;28(1):31‐46. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
- Dennert G, Zwahlen M, Brinkman M, Vinceti M, Zeegers MP, Horneber M. Selenium for preventing cancer. Cochrane Database of Systematic Reviews 2011, Issue 5. [DOI: 10.1002/14651858.CD005195] [DOI] [PMC free article] [PubMed] [Google Scholar]


