Abstract
Purpose
To determine if quantitative magnetic resonance (MR) imaging techniques (sodium MR imaging, glycosaminoglycan [GAG] chemical exchange saturation transfer [CEST], and T2* mapping) could be used as potential markers for biochemical changes in the Achilles tendon induced by ciprofloxacin intake.
Materials and Methods
The ethics committee of the Medical University of Vienna approved the protocol (number 1225/2012), and all patients gave written informed consent. Fourteen ankles from seven men (mean age, 32 years ± 12 [standard deviation]) were included in the study. All patients underwent 7-T MR imaging examinations of the Achilles tendon at baseline and 10 days and 5 months after ciprofloxacin intake. Sodium signal and T2* maps were acquired with the variable echo-time sequence and the GAG CEST values were acquired with a three-dimensional radiofrequency spoiled gradient-recalled-echo sequence.
Results
The mean sodium signal was significantly decreased by 25% in the whole tendon (from baseline to 10 days after ciprofloxacin intake, 130 arbitrary units [au] ± 8 to 98 au ± 5, respectively; P = .023) and returned to baseline after 5 months (116 au ± 10), as observed also at the tendon insertion (baseline, 10 days after ciprofloxacin intake, and 5 months after ciprofloxacin intake, 134 au ± 8, 105 au ± 5, and 119 au ± 9, respectively; P = .034). The mean GAG CEST value in the whole tendon was parallel to the sodium signal with a decrease from baseline to 10 days after ciprofloxacin intake, 4.74% ± 0.75 to 4.50% ± 0.23, respectively (P = .028) and an increase at 5 months after ciprofloxacin intake to 4.88% ± 1.02.
Conclusion
In conclusion, this study demonstrates a ciprofloxacin-induced reversible reduction of the normalized sodium MR imaging signal and the GAG CEST effect in the Achilles tendon of healthy volunteers. Changes in sodium MR imaging and GAG CEST in men may reflect a decrease of GAG content in the Achilles tendon after ciprofloxacin intake.
Fluoroquinolones (FQs) are frequently prescribed antibiotics and they are well established in both inpatient and outpatient settings for urinary and respiratory tract infections and skin, bone, joints, abdominal, and gastrointestinal infections (1). In addition to gastrointestinal, central nervous system, and skin adverse effects, and prologantion of the QT interval prolongation, cumulative evidence suggests that FQ might be associated with Achilles tendinopathy (2-6). The occurrence of FQ-associated tendinopathy seems to be dose independent, and some risk factors were described in patients who develop FQ-related tendinopathy: age older than 60 years, (additional) glucocorticoid or immunosuppressive therapy, and renal failure (7-11). However, cases of FQ-associated tendinopathy in the absence of these risk factors were described (12). Symptoms of tendinopathy include acute onset of tendon pain, tenderness, and swelling that affects the function of the tendon.
Because the biochemical composition of the Achilles tendon is closely related to its function, and biochemical alterations precede morphologic changes, the detection of biochemical changes can help elucidate the risk of developing tendinopathy (13,14). Pathologic alterations include an increase in the amount of glycosaminoglycans (GAGs) (15), which is also accompanied by an increased sodium concentration. For proteoglycans, the sulfate and carboxyl groups associated with GAGs predominate, and they provide proteoglycans with a net negative charge. These negatively charged molecules preferentially attract positive counter ions (16). Recently, several MR imaging methods were introduced that are capable of non-invasive evaluation of the ultrastructural composition of the Achilles tendon. The similar principle of the direct proportion of the sodium ions and GAG content as known in cartilage was used to investigate the increase of GAG content in Achilles tendinopathy (17). In addition, this method offers the opportunity to assess changes in the sodium concentration of the cartilage (17,18).
Furthermore, chemical exchange saturation transfer (CEST) also provides the ability to analyze the GAG content in cartilage. The most common method for acquisition of a CEST data set is to acquire multiple image data sets with presaturation at different offset frequencies around the water resonance and one reference data set without saturation or with saturation at a very large offset frequency (19). The normalized signal as a function of the presaturation offset (termed the z-spectrum) can then be used to determine and quantify CEST effects, which are asymmetric with respect to the water resonance (ie, a CEST effect appears either up- or down-field from water and therefore can be extracted from the z-spectrum via analysis of its asymmetry with respect to the water resonance) (20).
Collagen matrix was investigated by using mono- and biexponentially calculated T2* with a two-dimensional ultrashort echo time or a three-dimensional variable echo-time sequence (21-23). T2* reflects the interplay between water molecules and collagen fiber content and organization, and it has been shown that this parameter is sensitive to early degenerative changes in the Achilles tendon. These methods might also be capable of detecting the biochemical changes in the Achilles tendon that are caused by FQ antibiotics.
Therefore, the aim of this study was to determine whether quantitative MR imaging techniques (sodium MR imaging, GAG CEST, and T2* mapping) could be used as potential markers for biochemical changes in the Achilles tendon induced by ciprofloxacin intake.
Materials and Methods
Patients
The ethics committee of the Medical University of Vienna approved the protocol (ethics committee number 1225/2012), and all subjects gave written, informed consent. Seven healthy men (mean age, 32 years ± 12 [standard deviation]) were recruited by advertisement in the public areas of the Medical University of Vienna between September 2012 and September 2013 and were included in our prospective study, and both ankles were measured. Exclusion criteria were as follows: known allergy against antibiotic agents; history of tendinopathy, tendon rupture, or joint diseases; and heavy exercise, which was defined as engaging in physical activity or sports for more than 3 hours per week. Further exclusion criteria included acute or chronic inflammatory diseases, history of long-term treatment with corticosteroids, known chronic diseases (such as liver or kidney diseases, diseases of the central nervous system, history of seizures, history of cardiac arrhythmia or long-QT syndrome, glucose-6-phosphate dehydrogenase deficiency, and psychiatric disorders).
All patients underwent MR imaging at three time points: at baseline, at 10 days, and at 5 months after ciprofloxacin intake (1000 mg/day in two doses—500 mg in the morning and 500 mg in the evening for 10 days). The first ciprofloxacin dose was taken after the baseline MR examination, and the last dose in the morning before the second MR examination.
MR Examination
All participants underwent MR examinations on a 7-T investigational MR unit (Siemens, Erlangen, Germany) with a 28-channel knee coil (Quality Electrodynamics, Mayfield Village, Ohio) for proton imaging and a 15-channel knee coil (Qed; Quality Electrodynamics) for sodium imaging. Morphologic imaging sequences and collagen- and GAG-specific MR imaging methods were used. Morphologic assessment was determined with a sagittal intermediate-weighted turbo spin-echo sequence with fat saturation by using the Vienna Morphologic Achilles Tendon Score suggested by Apprich et al (24). This score is based on four characteristics of the Achilles tendon (thickness, continuity, signal intensity, and associated pathologies), and the scores from 0 to 100, with 0 being the worst and 100 being the best.
To acquire the information on water and collagen content, the variable echotime sequence, exploiting the highly asymmetric readout to decrease the effective echo time below 1 msec (25), was used to generate the T2* maps. Ten echo times were used [1.12, 2.21, 5.11, 7.11, 10.2, 12.2, 17.34, 19.34, 24.48, and 26.48 msec].
For sodium imaging, the variable echo-time sequence adapted to x-nuclei capabilities was used. In the interest of time, the two-dimensional mode with three sections was used, and the echo time was 2.45 msec.
For GAG CEST imaging, CEST effects were prepared by a train of Gaussian radiofrequency pulses, followed by signal readout with a three-dimensional radiofrequency spoiled gradient-recalled-echo sequence similar to cartilage protocol used previously (26). The saturation parameters were as follows: the radiogrequency magnetic field (B1) continuous wave amplitude equivalent, 0.8 μT; pulse duration, 99 msec; interpulse delay, 100 msec; and five CEST pulses.
Other parameters of the GAG CEST sequence, as well as other sequences, are summarized in Table 1.
Table 1. Overview of Sequence Parameters.
| Parameter | Morphologic Evaluation | Variable Echo-time Sequence T2* | Sodium MR Imaging | GAG CEST |
|---|---|---|---|---|
| Sequence type | Sagittal PD TSE | 3D variable echo-time | 2D variable echo-time | GRE |
| Orientation | Sagittal | Sagittal | Sagittal | Sagittal |
| TE (msec) | 30 | 1.12 to 26.48 | 2.45 | 3.2 |
| TR (msec) | 4350 | 34 | 40 | 7.3 |
| FOV read (mm) | 170 | 127 | 200 | 154 |
| FOV phase (mm) | 170 | 180 | 200 | 190 |
| Signal average | 1 | 2 | 175 | 1 |
| No. of sections | 27 | 40 | 3 | 24 |
| Section thickness (mm) | 2.5 | 1.5 | 6 | 2.7 |
| Bandwidth (Hz/pixel) | 222 | 548 | 130 | 250 |
| Base resolution | 922 × 1024 | 608 × 432 | 256 × 256 | 208 × 256 |
| Total acquisition time (sec) | 249 | 657 | 899 | 585 |
Note.—FOV = field of view, PD = proton-density weighted, phase = phase direction, read = read-out direction, TE = echo time, TR = repetition time, TSE = turbo spin echo, 2D = two-dimensional, 3D = three-dimensional.
Data Processing
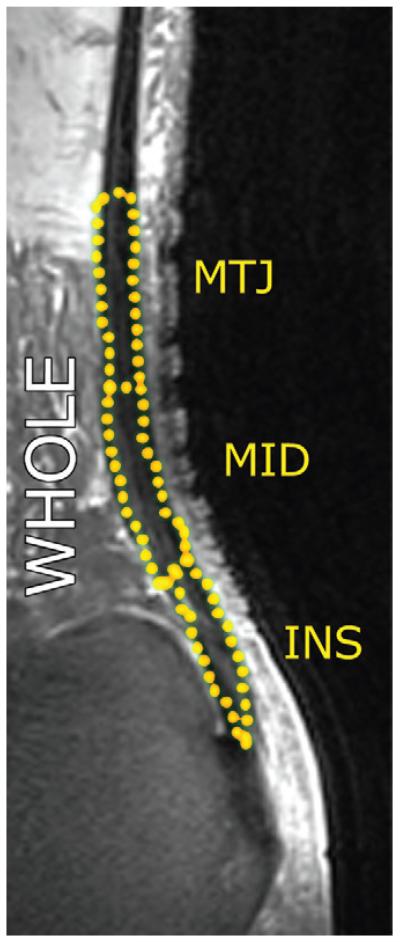
MR imaging parameters were calculated with a manually drawn region-of-interest (ROI) analysis in the three regions of the Achilles tendon (insertion, middle, and muscle-tendon junction; Fig 1). ROIs were drawn on two and three consecutive sections for sodium and other MR parameters (CEST, short component of T2* [T2*s], and long component of T2* [T2*l]), respectively. The length of each of the parts was defined as a third of the total Achilles tendon length, measured from the most proximal to the most distal. Values were also recorded for the sum of all three regions, hereafter referred to as the whole tendon.
Figure 1.
MR image shows Achilles tendon, and the yellow dots show the segmentation. INS = insertion of the tendon into the calcaneus, MID = middle portion of the tendon, MTJ = musculotendinous junction, and WHOLE = the whole tendon.
Images from the variable echo-time sequence were analyzed by using a custom-written script (Interactive Data Language 6.3; Research Systems, Boulder, Colo). A biexponential fitting procedure was performed on all variable echo-time sequence data sets on a pixel-by-pixel basis by using the following function.
where S is measured MR signal, exp is exponent, TE is echo time, A1 corresponds to T2*s, A3 corresponds to T2*l, and A0 and A2 are the component ratios expressed further as a percentage value of A0 + A2. The short component of Fs is 100 · A0/(A0 + A2) and the long component of Fl is 100 · A2/(A0 + A2), and F is the component ratio. A4 is the offset given primarily by noise. Two maps for each section were stored for further use (T2*s maps and T2*l maps).
The sodium signal was normalized by the signal from the reference tube measured along each ankle with a known sodium concentration. The CEST curves were calculated for each pixel and were shifted for the water resonance to appear at 0 parts per million of the z-spectrum. Asymmetric magnetization transfer ratio curves were determined. The CEST values were calculated by determining the GAG transfer ratio. The GAG transfer ratio is equivalent to asymmetrical musculotendinous ratio (1.3 parts per million)/[1 − average (asymmetrical musculotendinous ratio) (0 parts per million − 2.35 parts per million)]/average [asymmetric magnetization transfer ratio (0 parts per million − 2.35 parts per million)] and saturation transfer, which is equivalent to (CEST + 1.3 parts per million) − (CEST − 1.3 parts per million)/(CEST + 1.3 parts per million).
To define ROIs on relatively low-resolution sodium images, these images were rescaled to the pixel resolution of morphologic proton images and overlaid on images by using a picture editing software program (Adobe Photoshop CS2, version 9.0; Adobe Systems, Mountain View, Calif). ROIs were defined to cover the full thickness of the tendon and the ROI of each region had a length of 30 mm. Sketched ROIs were subsequently transferred to the Digital Imaging and Communications in Medicine evaluation tool (JiveX; Visus Technology Transfer GmbH, Bochum, Germany). Mean sodium signal intensity and standard deviation were recorded for each Achilles tendon region and for the whole tendon.
Test-Retest Reliability and Intraobserver and Interobserver Variation
To perform test-retest reliability validation, three more healthy men (aged 31 years ± 5) were imaged between May 2014 and July 2014 at baseline and 10 days thereafter without taking any drug. Evaluation was performed according to Bland and Altman (27).
To calculate the interobserver variability for all MR parameters (normalized sodium signal, CEST values, T2*s, and T2*l), three blinded independent readers evaluated 10 ankles. The variation between observers was expressed as a coefficient of variation in percentage. Intraobserver variability was calculated from three independent evaluations of seven patients by one blinded observer, with a delay of 1 week between the evaluations, and was expressed as an intraclass correlation coefficient.
Statistical Analysis
All statistical calculations were performed by using statistical software (SPSS version 21.0, SPSS, Chicago, Ill; pROC version 1.5.4 of R Statistical Package, R Foundation, Vienna, Austria). Descriptive statistics were performed to calculate the mean and standard deviation of age, T2*s, T2*l, normalized sodium signal, and CEST values in the Achilles tendon and cartilage separately for various time points. To compare average T2*s, T2*l, normalized sodium signal, and CEST values in different ankles (right and left), we used a repeat-measure analysis of variance. The longitudinal aspect was modeled by using time points as level 1 and laterality as level 2 nested within individuals (level 3) and by modeling covariance structures. We also applied a diagonal, unstructured first-order autoregressive covariance matrix but presented only the results from the unstructured covariance matrix as it provided the best model fit. The relationship among three variables (MR parameter, time points, and laterality) is referred to as interaction.
A P value equal to or below .05 was considered to indicate statistically significant results.
Results
The coefficient of variation for interobserver variation was found to be 6.96% and the intraclass correlation coefficient for intraobserver variation was 0.969%, on average. The results for different tendon segments and MR parameters are summarized in Table 2. On average for the whole tendon, the correlation coefficient in test-retest reliability validation was 0.997 for normalized sodium signal, 0.888 for GAG CEST values, and 0.955 for short component of T2*s.
Table 2. Summary of Coefficient of Variation and Intraclass Correlation Coefficient for Inter-and Intraobserver Variation.
| Parameter | Whole Tendon | Insertion | Midportion | Musculotendinous Junction |
|---|---|---|---|---|
| Normalized sodium signal | ||||
| CV | 6.86 | 5.76 | 6.61 | 5.15 |
| ICC | 0.932 | 0.907 | 0.934 | 0.979 |
| GAG CEST | ||||
| CV | 8.21 | 5.28 | 6.55 | 10.17 |
| ICC | 0.963 | 0.954 | 0.969 | 0.972 |
| T2*s | ||||
| CV | 6.53 | 5.93 | 5.85 | 6.62 |
| ICC | 0.983 | 0.987 | 0.992 | 0.986 |
| T2*l | ||||
| CV | 6.43 | 6.92 | 6.41 | 6.25 |
| ICC | 0.989 | 0.988 | 0.990 | 0.986 |
Note.—CV = coefficient of variation, ICC = intraclass correlation coefficient.
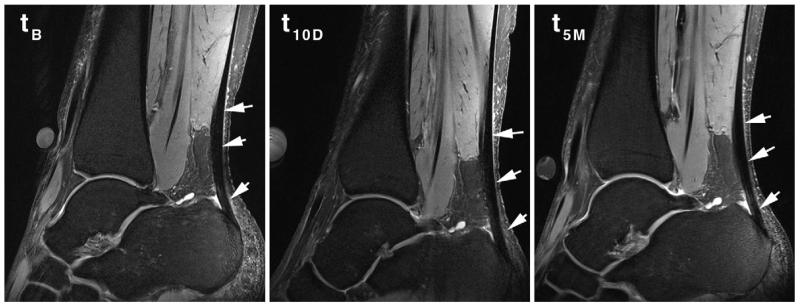
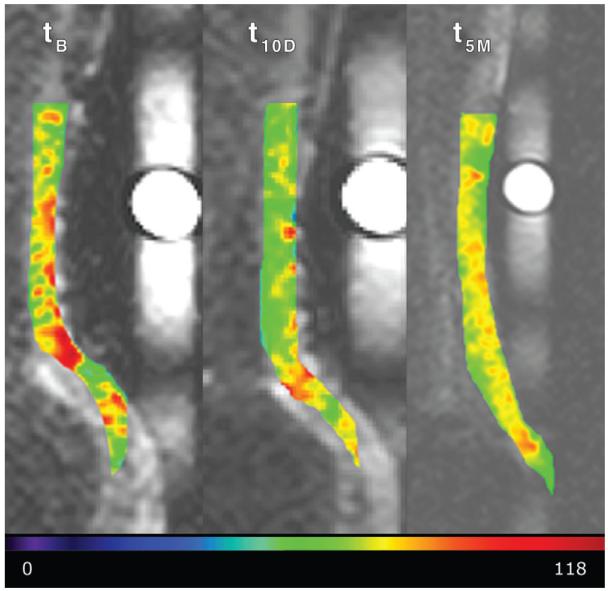
The mean Vienna Morphological Achilles Tendon Score was 89.6 ± 6.9 for the baseline, 93.75 ± 5.96 10 days after ciprofloxacin intake, and 93.75 ± 5.69 5 months thereafter. None of the volunteers experienced any clinical symptoms from the intake of ciprofloxacin. At the three time points for the whole tendon, the mean normalized sodium signal was 130 arbitrary units (au) ± 8, 98 au ± 5, and 116 au ± 10, respectively; for T2*s, it was 0.45 msec ± 0.13, 0.45 msec ± 0.23, and 0.57 msec ± 0.39, respectively; and for GAG CEST, it was 4.74 % ± 0.75, 4.50 % ± 0.41, and 4.88 % ± 1.02, respectively. All MR parameters at the three different time points are summarized in Table 3. A comparison of the morphologic appearance of a patient at three different time points is depicted in Figure 2. The example images of individual MR parameter maps are depicted in Figures 3-5.
Table 3. Summary of MR Imaging Parameters Measured at Different Time Points and at Different Tendon Areas (n = 14).
| Normalized Sodium Signal (au) |
GAG CEST (%) |
T2*s (msec) |
T2*l (msec) |
|||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| Insertion time point | ||||||||
|
| ||||||||
| 1 | 134.29 | 7.56 | 4.85 | 0.60 | 0.55 | 0.13 | 12.79 | 4.23 |
|
| ||||||||
| 2 | 104.51 | 5.47 | 4.64 | 0.83 | 0.58 | 0.20 | 13.13 | 2.28 |
|
| ||||||||
| 3 | 118.47 | 8.96 | 4.98 | 0.60 | 1.28 | 1.47 | 15.25 | 5.56 |
|
| ||||||||
| Midportion time point | ||||||||
|
| ||||||||
| 1 | 127.12 | 7.35 | 4.68 | 0.70 | 0.42 | 0.15 | 11.87 | 4.07 |
|
| ||||||||
| 2 | 103.87 | 7.02 | 4.52 | 0.65 | 0.46 | 0.25 | 12.48 | 4.40 |
|
| ||||||||
| 3 | 115.60 | 10.58 | 5.15 | 1.17 | 0.42 | 0.11 | 11.70 | 2.86 |
|
| ||||||||
| Musculotendinous junction time point | ||||||||
|
| ||||||||
| 1 | 125.85 | 8.86 | 4.77 | 0.84 | 0.37 | 0.26 | 13.78 | 6.07 |
|
| ||||||||
| 2 | 97.99 | 7.46 | 4.78 | 0.71 | 0.33 | 0.25 | 12.04 | 3.97 |
|
| ||||||||
| 3 | 113.75 | 10.42 | 5.07 | 0.69 | 0.29 | 0.11 | 12.99 | 4.56 |
|
| ||||||||
| Whole tendon time point | ||||||||
|
| ||||||||
| 1 | 129.82 | 7.77 | 4.74 | 0.75 | 0.45 | 0.13 | 12.75 | 3.72 |
|
| ||||||||
| 2 | 98.08 | 5.42 | 4.50 | 0.41 | 0.45 | 0.23 | 12.36 | 3.33 |
|
| ||||||||
| 3 | 115.76 | 9.92 | 4.88 | 1.02 | 0.57 | 0.39 | 12.90 | 1.70 |
Note.—SD = standard deviation.
Figure 2.
Morphologic image of a patient at baseline before ciprofloxacin intake (tB), 10 days after ciprofloxacin intake (t10D), and at the 5-month follow-up examination (t5M). There were no significant morphologic changes observed in any of the patients at 10 days and 3 months after ciprofloxacin intake (arrows show the top, middle, and bottom tendon parts, which are the insertion, midportion, and musculotendinous junction, respectively).
Figure 3.
The normalized sodium signal in the Achilles tendon at baseline before ciprofloxacin intake (tB), 10 days after ciprofloxacin intake (t10D), and at the 5-month follow-up examination (t5M). Images were scaled equally. The decrease in the sodium signal in the tendon after intake is shown. The scale at the bottom of the image indicates the normalized sodium signal in arbitrary units.
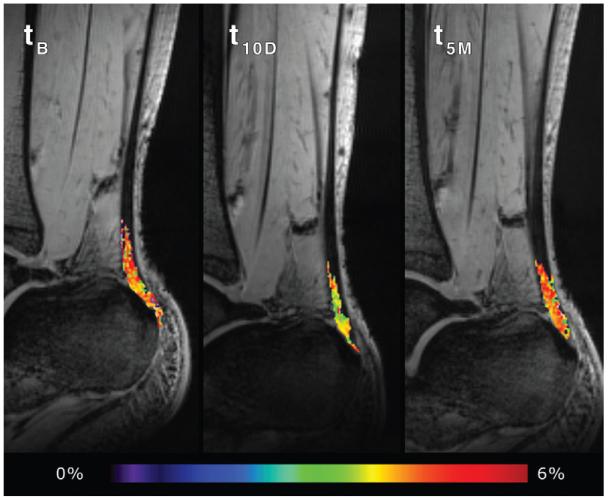
Figure 4.
The GAG CEST map of the Achilles tendon (the segmented pseudocolor-coded tendon is overlaid on CEST image acquired at zero resonant frequency offset) at baseline ciprofloxacin intake (tB), 10 days after ciprofloxacin intake (t10D), and at the 5-month follow-up (t5M). The black areas within the ROI represent the pixels equal to or lower than zero after GAG CEST processing which were excluded from statistics.
Figure 5.
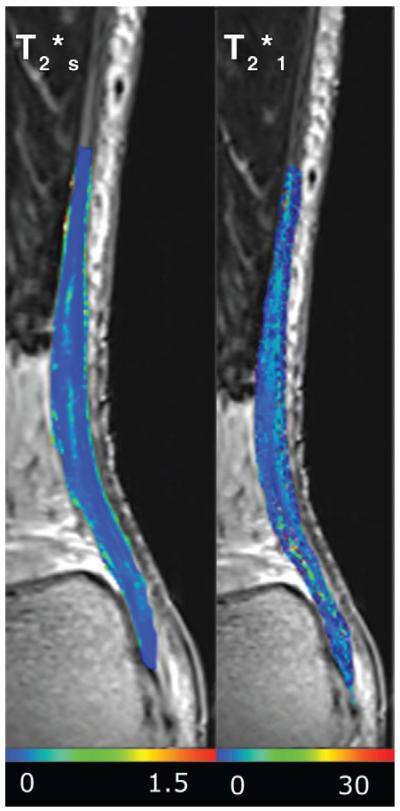
Map of T2*s and T2*l overlaid on the variable echo-time sequence image, acquired with an echo time of 1.12 msec. Neither T2*s nor T2*l was significantly different between baseline before ciprofloxacin intake, 10 days after ciprofloxacin intake, and at the 5-month follow-up examination. The scales indicate the T2* values in milliseconds.
Using the repeated measures analysis of variance, a statistically significant difference was found between imaging at baseline and 10 days after for both whole tendon and the insertion in normalized sodium signal. Five months after ciprofloxacin intake, there was no significant change compared with baseline. As for the other parameters, neither showed the ability to distinguish between the respective time points (Tables 4, 5), except for the GAG CEST value, considering the statistical interaction according to side (ie, left vs right ankle).
Table 4. Summary of P Values Calculated by Using a Hierarchical Linear Model in Different Tendon Areas and Different Interactions.
| Parameter | Normalized Sodium Signal | GAG CEST | T2*s | T2*l |
|---|---|---|---|---|
| Insertion | ||||
| Time point | 0.034* | 0.089 | 0.054 | 0.212 |
| Side | 0.062 | 0.574 | 0.048 | 0.894 |
| Time point and side | 0.734 | 0.399 | 0.067 | 0.244 |
| Midportion | ||||
| Time point | 0.153 | 0.321 | 0.765 | 0.681 |
| Side | 0.164 | 0.021* | 0.281 | 0.009 |
| Time point and side | 0.550 | 0.581 | 0.909 | 0.822 |
| Musculotendinous junction | ||||
| Time point | 0.109 | 0.742 | 0.923 | 0.845 |
| Side | 0.203 | 0.287 | 0.314 | 0.150 |
| Time point and side | 0.347 | 0.633 | 0.693 | 0.154 |
| Whole tendon | ||||
| Time point | 0.023* | 0.523 | 0.134 | 0.981 |
| Side | 0.101 | 0.266 | 0.453 | 0.076 |
| Time point and side | 0.894 | 0.028* | 0.165 | 0.909 |
Note.—Side = left or right Achilles tendon, time point = imaging at baseline, imaging 5 months after ciprofloxacin intake, and imaging at 10 days.
Statistically significant.
Table 5. Detailed Findings of the Difference between Time Points in Normalized Sodium Signal and GAG CEST Values.
| Parameter | Time Point | P Value* |
|---|---|---|
| Normalized sodium signal (region) | ||
| Insertion | tB to t10D | .002† |
| Insertion | tB to t5M | .553 |
| Insertion | t5M to t10D | .122 |
| Whole tendon | tB to t10D | .005† |
| Whole tendon | tB to t5M | .417 |
| Whole tendon | t5M to t10D | .231 |
| GAG CEST (region) | ||
| Insertion | tB to t10D | >.999 |
| Insertion | tB to t5M | >.999 |
| Insertion | t5M to t10D | .657 |
| Whole tendon | tB to t10D | >.999 |
| Whole tendon | tB to t5M | >.999 |
| Whole tendon | t5M to t10D | .568 |
Note.—tB = imaging at baseline, t5M = imaging S months after ciprofloxacin intake, t10D = imaging at 10 days.
Bonferroni corrected.
Statistically significant.
Discussion
Our study demonstrates that sodium MR imaging is likely to detect changes in GAG content in the Achilles tendon after ciprofloxacin intake in healthy men. The changes were observed 10 days after ciprofloxacin intake, while the sodium signal returned to normal after 5 months. There were no visible morphologic changes in the Achilles tendon between the respective time points.
Our study further links ciprofloxacin intake to Achilles tendinopathy. Of interest, while previous studies offered suspicions that FQ-associated Achilles tendinopathy is closely related to the age of the patient, cortisone treatment, and renal failure, our study demonstrates that changes in the Achilles tendon can also be observed in healthy young men. Although verified by MR imaging, the changes did not lead to clinical symptoms of tendinopathy or tendon injury; however, all subjects were asked to refrain from intense physical activity at least 14 days after ciprofloxacin initiation.
Ciprofloxacin-associated tendinopathy and accompanying tendon rupture most commonly occur in the Achilles tendon, which is likely related to the weight-bearing role of the Achilles tendon (3,28,29). Other tendons, including the supraspinatus, patellar, and quadriceps tendons, may also be occasionally affected by FQ drugs (30). It is possible that the tendinitis observed in certain patients treated with FQs is secondary to an alteration in fibroblast cell homeostasis that results in the structural compromise of the tendon.
In their in vitro study, Williams et al (10) observed an inhibited incorporation of sulphate in fibroblast cells. Ciprofloxacin caused a 14%, 19%, and 53% decrease in Achilles tendon cell sulphate incorporation at 5, 10, and 50 μg/mL of administration, respectively. This resulted in increased matrix-degrading activity, decreased matrix synthesis, and decreased cell proliferation. In normal and pathologic conditions, tendon function is directly dependent on fibroblast metabolism. The increase in matrix-degrading activity, combined with the concomitant decrease in matrix synthesis, suggests a mechanism by which ciprofloxacin might predispose tendons to injury. Other mechanisms of the effect of FQ on tendons were described by Pouzaud et al (31) in their in vitro study. Their results suggest an involvement of oxidative stress in FQ toxicity. Moreover, they observed varying toxicity for different FQs (ciprofloxacin, levofloxacin, pefloxacin, and ofloxacin).
It was previously shown (17) that sodium MR imaging could serve as a marker for GAG changes in the Achilles tendon during degenerative processes. Unlike the degeneration of the Achilles tendon when stimulated synthesis of GAGs is observed (13,32), ciprofloxacin intake is accompanied by GAG inhibition (10,31). Sodium, as a counter-ion to negatively charged GAG molecules, is directly proportional to GAG content. The sensitivity of sodium MR imaging signal to GAG content has been described in cartilage: Maroudas et al (33) showed that the fixed-charge density of cartilage is correlated with GAG content. Similar mechanisms also apply to other connective tissues containing GAG, such as tendons.
Another GAG-sensitive MR imaging method, widely used in cartilage, is GAG CEST. The basic principle of CEST imaging is that the bulk water MR signal is reduced after off-resonant spins are selectively presaturated by radiofrequency irradiation and then these spins undergo chemical exchange with bulk water protons (34). The hydroxyl and amide protons of GAG provide exchange properties that render them exquisitely suited for CEST experiments. A recent study demonstrated that GAG CEST can be used to reliably detect GAG in the knee cartilage of patients who underwent cartilage repair surgery (26). In principle, GAG CEST might provide information about the GAG content in tissues other than cartilage, such as tendon, ligaments, or menisci. To date, there is no study that has used GAG CEST in the Achilles tendon. Compared with sodium MR imaging, the processing after GAG CEST imaging is much more complicated. It is essential to account for inhomogeneities of the static magnetic field B0 in a sample, and it is necessary to compensate for sample movement during the course of a measurement (35,36). In our study, we found a 5% decrease in GAG CEST asymmetry values between baseline and 10 days after ciprofloxacin intake (in whole tendon); however, this change was not statistically significant (P = .318). On the other hand, if the left and right ankles were considered as an interaction for hierarchical linear model analysis, the GAG CEST change was statistically significant (P = .037).
Recently, the variable echo-time sequence was shown to be a suitable sequence for T2* biexponential mapping in the human Achilles tendon in vivo, with the minimum echo time 0.8 msec. The short component of T2* provides a suitable marker of Achilles tendinopathy, because it reflects the changes in water content and collagen orientation (22). In our study, we did not find any statistically significant change between either of the time points for either of the T2* components. This suggests that the decomposition of the collagen matrix induced by ciprofloxacin intake is a slower process compared with GAG turnover. Also, because no morphologic changes were found, it is likely that the water content is only minimally altered.
The correlation coefficients of test-retest reliability showed high values for all three MR parameters, which suggests that there is no spontaneous change of the parameters over time. However, this is a confirmation rather than a proof of that expectation.
Our study has several limitations. There is a relatively low number of patients involved in the study and because only men were studied, the results cannot be generalized to women. Furthermore, the segmentation of the Achilles tendon is rather complicated on sodium images because of low resolution and blurring artifacts. We overcame this problem by segmenting the tendon on the morphologic images and by subsequently transferring the segmentations onto the sodium images. This might be, however, a drawback in clinical practice. The postprocessing method and evaluation of GAG CEST maps is also complex. During the GAG CEST values calculation, if the value was equal to or lower than zero, this pixel was excluded from the evaluation. This reduced the number of pixels in individual ROIs, which could have influenced the statistics. The largest reduction in pixels was observed in the musculotendinous junction (~40%), and it increased in the midportion (~12%) and the insertion (~8%). In the whole tendon, the reduction in pixels was approximately 19%. Another drawback of GAG CEST in tendons is the lower water content, which is unfavorable for the GAG CEST method.
In conclusion, our study demonstrates the changes in sodium MR imaging and GAG CEST in men after ciprofloxacin intake were very likely caused by a decrease of GAG content in the Achilles tendon. The observed changes in GAG content may contribute to the characterization of the pathomechanism of FQ-associated tendinopathy in the future.
Advances in Knowledge.
-
■
Changes in sodium MR imaging and glycosaminoglycan (GAG) chemical exchange saturation transfer (CEST) in men may reflect a decrease of GAG content in the Achilles tendon after ciprofloxacin intake.
-
■
Ciprofloxacin intake in healthy patients does not cause any persistent morphologic changes in the Achilles tendon nor any alteration in the collagen matrix that is detectable by T2* mapping.
Implication for Patient Care.
-
■
Sodium and GAG CEST measurement with MR imaging can detect biochemical alterations of the Achilles tendon after ciprofloxacin intake.
Abbreviations
- au
arbitrary units
- CEST
chemical exchange saturation transfer
- FQ
fluoroquinolone
- GAG
glycosaminoglycan
- ROI
region of interest
- T2*l
long component of T2*
- T2*s
short component of T2*
Footnotes
Supported by the Austrian Science Fund (FWF) P 25246 B24, Vienna Advanced Imaging Center (VIACLIC) FA102A0017, and Slovak Scientific Grant Agency VEGA (grant 2/0013/14).
Disclosures of Conflicts of Interest: V.J. disclosed no relevant relationships. Y.W. disclosed no relevant relationships. P.S. disclosed no relevant relationships. J.V. disclosed no relevant relationships. B.H. disclosed no relevant relationships. P.W. disclosed no relevant relationships. M.W. disclosed no relevant relationships. A.L. disclosed no relevant relationships. S.T. disclosed no relevant relationships.
References
- 1.Archer GL, Polk RE. Approach to therapy for bacterial diseases. In: Longo D, Fauci A, Kasper D, Hauser S, Jameson J, Loscalzo J, editors. Harrison’s principles of internal medicine. 18th ed. McGraw-Hill; New York, NY: 2012. [Google Scholar]
- 2.Szarfman A, Chen M, Blum MD. More on fluoroquinolone antibiotics and tendon rupture. N Engl J Med. 1995;332(3):193. [PubMed] [Google Scholar]
- 3.Zabraniecki L, Negrier I, Vergne P, et al. Fluoroquinolone induced tendinopathy: report of 6 cases. J Rheumatol. 1996;23(3):516–520. [PubMed] [Google Scholar]
- 4.Palin SL, Gough SC. Rupture of the Achilles tendon associated with ciprofloxacin. Diabet Med. 2006;23(12):1386–1387. doi: 10.1111/j.1464-5491.2006.02003.x. [DOI] [PubMed] [Google Scholar]
- 5.Khanzada Z, Rethnam U, Widdowson D, Mirza A. Bilateral spontaneous non-traumatic rupture of the Achilles tendon: a case report. J Med Case Reports. 2011;5:263. doi: 10.1186/1752-1947-5-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stephenson AL, Wu W, Cortes D, Rochon PA. Tendon injury and fluoroquinolone use: a systematic review. Drug Saf. 2013;36(9):709–721. doi: 10.1007/s40264-013-0089-8. [DOI] [PubMed] [Google Scholar]
- 7.van der Linden PD, Sturkenboom MC, Herings RM, Leufkens HG, Stricker BH. Fluoroquinolones and risk of Achilles tendon disorders: case-control study. BMJ. 2002;324(7349):1306–1307. doi: 10.1136/bmj.324.7349.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Linden PD, van Puijenbroek EP, Feenstra J, et al. Tendon disorders attributed to fluoroquinolones: a study on 42 spontaneous reports in the period 1988 to 1998. Arthritis Rheum. 2001;45(3):235–239. doi: 10.1002/1529-0131(200106)45:3<235::AID-ART254>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 9.Chhajed PN, Plit ML, Hopkins PM, Malouf MA, Glanville AR. Achilles tendon disease in lung transplant recipients: association with ciprofloxacin. Eur Respir J. 2002;19(3):469–471. doi: 10.1183/09031936.02.00257202. [DOI] [PubMed] [Google Scholar]
- 10.Williams RJ, 3rd, Attia E, Wickiewicz TL, Hannafin JA. The effect of ciprofloxacin on tendon, paratenon, and capsular fibroblast metabolism. Am J Sports Med. 2000;28(3):364–369. doi: 10.1177/03635465000280031401. [DOI] [PubMed] [Google Scholar]
- 11.Khaliq Y, Zhanel GG. Fluoroquinolone-associated tendinopathy: a critical review of the literature. Clin Infect Dis. 2003;36(11):1404–1410. doi: 10.1086/375078. [DOI] [PubMed] [Google Scholar]
- 12.Ozaras R, Mert A, Tahan V, et al. Ciprofloxacin and Achilles’ tendon rupture: a causal relationship. Clin Rheumatol. 2003;22(6):500–501. doi: 10.1007/s10067-003-0758-6. [DOI] [PubMed] [Google Scholar]
- 13.Samiric T, Parkinson J, Ilic MZ, Cook J, Feller JA, Handley CJ. Changes in the composition of the extracellular matrix in patellar tendinopathy. Matrix Biol. 2009;28(4):230–236. doi: 10.1016/j.matbio.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Schweitzer ME, Karasick D. MR imaging of disorders of the Achilles tendon. AJR Am J Roentgenol. 2000;175(3):613–625. doi: 10.2214/ajr.175.3.1750613. [DOI] [PubMed] [Google Scholar]
- 15.Parkinson J, Samiric T, Ilic MZ, Cook J, Feller JA, Handley CJ. Change in proteoglycan metabolism is a characteristic of human patellar tendinopathy. Arthritis Rheum. 2010;62(10):3028–3035. doi: 10.1002/art.27587. [DOI] [PubMed] [Google Scholar]
- 16.Maroudas A. Physiochemical properties of articular cartilage. Pinmal Medical; Kent, England: 1979. pp. 215–290. [Google Scholar]
- 17.Juras V, Zbýn S, Pressl C, et al. Sodium MR imaging of Achilles tendinopathy at 7 T: preliminary results. Radiology. 2012;262(1):199–205. doi: 10.1148/radiol.11110897. [DOI] [PubMed] [Google Scholar]
- 18.Trattnig S, Welsch GH, Juras V, et al. 23Na MR imaging at 7 T after knee matrix-associated autologous chondrocyte transplantation preliminary results. Radiology. 2010;257(1):175–184. doi: 10.1148/radiol.10100279. [DOI] [PubMed] [Google Scholar]
- 19.van Zijl PC, Yadav NN. Chemical exchange saturation transfer (CEST): what is in a name and what isn’t? Magn Reson Med. 2011;65(4):927–948. doi: 10.1002/mrm.22761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmitt B, Brix M, Domayer S. CEST imaging. Curr Radiol Rep. 2014;2(38) [Google Scholar]
- 21.Juras V, Zbyn S, Pressl C, et al. Regional variations of T2* in healthy and pathologic achilles tendon in vivo at 7 Tesla: preliminary results. Magn Reson Med. 2012;68(5):1607–1613. doi: 10.1002/mrm.24136. [DOI] [PubMed] [Google Scholar]
- 22.Juras V, Apprich S, Szomolanyi P, Bieri O, Deligianni X, Trattnig S. Bi-exponential T2 analysis of healthy and diseased Achilles tendons: an in vivo preliminary magnetic resonance study and correlation with clinical score. Eur Radiol. 2013;23(10):2814–2822. doi: 10.1007/s00330-013-2897-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Filho GH, Du J, Pak BC, et al. Quantitative characterization of the Achilles tendon in cadaveric specimens: T1 and T2* measurements using ultrashort-TE MRI at 3 T. AJR Am J Roentgenol. 2009;192(3):W117–W124. doi: 10.2214/AJR.07.3990. [DOI] [PubMed] [Google Scholar]
- 24.Apprich S, Friedrich K, Schöpf V, Trattnig S. VIMATS–Vienna Morphological Achilles Tendon Score [abstr]; Proceedings of the Twenty-First Meeting of the International Society for Magnetic Resonance in Medicine; Berkeley, Calif: International Society for Magnetic Resonance in Medicine. 2013.p. 3472. [Google Scholar]
- 25.Deligianni X, Bär P, Scheffler K, Trattnig S, Bieri O. High-resolution Fourier-encoded sub-millisecond echo time musculoskeletal imaging at 3 Tesla and 7 Tesla. Magn Reson Med. 2013;70(5):1434–1439. doi: 10.1002/mrm.24578. [DOI] [PubMed] [Google Scholar]
- 26.Schmitt B, Zbýn S, Stelzeneder D, et al. Cartilage quality assessment by using glycosaminoglycan chemical exchange saturation transfer and (23)Na MR imaging at 7 T. Radiology. 2011;260(1):257–264. doi: 10.1148/radiol.11101841. [DOI] [PubMed] [Google Scholar]
- 27.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–310. [PubMed] [Google Scholar]
- 28.Huston KA. Achilles tendinitis and tendon rupture due to fluoroquinolone antibiotics. N Engl J Med. 1994;331(11):748. doi: 10.1056/NEJM199409153311116. [DOI] [PubMed] [Google Scholar]
- 29.Ribard P, Audisio F, Kahn MF, et al. Seven Achilles tendinitis including 3 complicated by rupture during fluoroquinolone therapy. J Rheumatol. 1992;19(9):1479–1481. [PubMed] [Google Scholar]
- 30.McGarvey WC, Singh D, Trevino SG. Partial Achilles tendon ruptures associated with fluoroquinolone antibiotics: a case report and literature review. Foot Ankle Int. 1996;17(8):496–498. doi: 10.1177/107110079601700811. [DOI] [PubMed] [Google Scholar]
- 31.Pouzaud F, Bernard-Beaubois K, Thevenin M, Warnet JM, Hayem G, Rat P. In vitro discrimination of fluoroquinolones toxicity on tendon cells: involvement of oxidative stress. J Pharmacol Exp Ther. 2004;308(1):394–402. doi: 10.1124/jpet.103.057984. [DOI] [PubMed] [Google Scholar]
- 32.Rees SG, Dent CM, Caterson B. Metabolism of proteoglycans in tendon. Scand J Med Sci Sports. 2009;19(4):470–478. doi: 10.1111/j.1600-0838.2009.00938.x. [DOI] [PubMed] [Google Scholar]
- 33.Maroudas A, Muir H, Wingham J. The correlation of fixed negative charge with glycosaminoglycan content of human articular cartilage. Biochim Biophys Acta. 1969;177(3):492–500. doi: 10.1016/0304-4165(69)90311-0. [DOI] [PubMed] [Google Scholar]
- 34.Zhou JY, van Zijl PC. Chemical exchange saturation transfer imaging and spectroscopy. Prog Nucl Magn Reson Spectrosc. 2006;48(2-3):109–136. [Google Scholar]
- 35.Andris P, Frollo I. Optimized measurement of magnetic field maps using nuclear magnetic resonance (NMR) Meas Sci Technol. 2011;22(4):045501. [Google Scholar]
- 36.Schmitt B, Zaiss M, Zhou J, Bachert P. Optimization of pulse train presaturation for CEST imaging in clinical scanners. Magn Reson Med. 2011;65(6):1620–1629. doi: 10.1002/mrm.22750. [DOI] [PubMed] [Google Scholar]