Abstract
Mammalian sex determination is the unique process whereby a single organ, the bipotential gonad, undergoes a developmental switch that promotes its differentiation into either a testis or an ovary. Disruptions of this complex genetic process during human development can manifest as disorders of sex development (DSDs). Sex development can be divided into two distinct processes: sex determination, in which the bipotential gonads form either testes or ovaries, and sex differentiation, in which the fully formed testes or ovaries secrete local and hormonal factors to drive differentiation of internal and external genitals, as well as extragonadal tissues such as the brain. DSDs can arise from a number of genetic lesions, which manifest as a spectrum of gonadal (gonadal dysgenesis to ovotestis) and genital (mild hypospadias or clitoromegaly to ambiguous genitalia) phenotypes. The physical attributes and medical implications associated with DSDs confront families of affected newborns with decisions, such as gender of rearing or genital surgery, and additional concerns, such as uncertainty over the child’s psychosexual development and personal wishes later in life. In this Review, we discuss the underlying genetics of human sex determination and focus on emerging data, genetic classification of DSDs and other considerations that surround gender development and identity in individuals with DSDs.
Introduction
Sex development is a critical component of mammalian development that provides a robust mechanism for continued generation of genetic diversity within a species. In mammals, sex development occurs in two distinct and sequential stages: sex determination and sex differentiation.1 Mammalian sex determination is dictated by the complement of sex chromosomes within an organism and refers to the mechanisms that lead to specification of either a male or female gonad from a single undifferentiated bipotential gonad.2 The presence of a Y chromosome drives the bipotential gonad toward testis-specific differentiation and formation of a male-specific gonad, whereas the absence of a Y chromosome results in development of an ovary, which is the female-specific gonad. In the process of male sex determination, expression of SRY, which is located on the Y chromosome, initiates a cascade of gene expression within Sertoli cells that ultimately drives the morphological differentiation of the testis.3,4 In sex differentiation, secretion of endocrine and local factors, such as testosterone, dihydrotestosterone and anti-Müllerian hormone, by the testis results in the development of male internal and external genitalia (prostate, vas deferens, penis and scrotum) and a reciprocal regression of the Müllerian ducts, which are the precursors of female internal genital structures (Fallopian tubes, uterus and vagina).5,6 Disorders of sex development (DSDs) arise when this tightly regulated process is disrupted, which occurs primarily as a result of genetic mutations that interfere with either the development of the testes or ovaries or the actions of endocrine and local factors in extragonadal tissues.7,8
This Review covers the current classifications and emerging knowledge of genetic mechanisms that contribute to sex and gender development among individuals with errors in sex determination that culminate in the development of DSDs. Current concepts of human sex determination and data from mouse models that have contributed to understanding of mammalian gonadal development are discussed. Aberrations in the process of sex differentiation (for example, defects in androgen biosynthesis) are also known to result in DSDs; however, these mechanisms have been reviewed elsewhere.9,10
Classification of DSDs
In 2006, the Consensus Statement on Management of Intersex Disorders was drawn up by international experts and patient advocates under the auspices of the Pediatric Endocrine Society and the European Society for Paediatric Endocrinology. The consensus conference was convened to review clinical management practices in DSDs given new advances in diagnosis, introduction of modified and new surgical techniques, studies of psychosocial factors pertinent to outcomes and challenges to clinical practices voiced by patient advocacy organizations. A key consensus recommendation involved the introduction of new terminology to replace confusing and potentially stigmatizing terms, including intersex, pseudohermaphroditism, hermaphroditism and sex reversal.7,8 Instead, the expert panel recommended that all variations of sex development should be incorporated under the superordinate term DSDs, which they defined as “congenital conditions in which development of chromosomal, gonadal, or anatomic sex is atypical”. Within this definition, DSDs can have a wide range of gonadal phenotypes, such as partial or complete gonadal dysgenesis and ovotestis, and external genital phenotypes, such as hypospadias, clitoromegaly and ambiguous genitalia or fully masculinized or feminized genitalia that are discordant with karyotype or gonadal phenotypes. Using this inclusive definition, DSDs are estimated to occur in approximately one in 100 live births.8,11 However, the incidence of 46,XY gonadal dysgenesis, in which genetic mutations result in disruption of testis development, is estimated at less than one in 10,000 live births. Given the rarity of this condition, neither the distribution of genetic mutations within these patients nor the long-term consequences of the diagnostic and management challenges associated with 46,XY gonadal dysgenesis are well understood. To address this issue and other gaps in our understanding, large multicentre studies in both the USA12,13 and Europe11,14 are ongoing.
Despite rapid and broad acceptance of the consensus definition of DSDs by clinical and research communities, controversy remains regarding whether particular conditions (such as Turner and Klinefelter syndromes) and genital phenotypes (such as hypospadias) constitute DSDs and, therefore, require a multidisciplinary approach to clinical management. The continuing uncertainty regarding definitions of DSDs carries with it a number of potential negative consequences. Most important among these drawbacks is the reduced likelihood that a patient with a DSD will receive comprehensive and integrated care if seen outside of a multidisciplinary DSD clinic.7,8 For example, endocrine abnormalities in a patient who has descended testes but has a history of mild or severe hypospadias might remain undetected until puberty.15,16 Additionally, despite positive and objectively-assessed cosmetic results following surgery to repair hypospadias, patients are frequently less satisfied with sexual function and less likely to experience intimate relationships than unaffected individuals, highlighting the importance of behavioural and/or sexual health involvement in the model of care.17 Incorporation of a multidisciplinary healthcare team that includes psychological counselling at the time of diagnosis could contribute to identification and prevention of adverse effects consequent to the DSD. One barrier to providing multidisciplinary care to a large population of patients with DSDs is the perception of a lack of resources. Contrary to this misperception, increased numbers of patients can make the logistics of multidisciplinary clinics practical. With regard to behavioural health services only a small proportion of patients with a DSD and their families would be expected to require services beyond anticipatory guidance and even fewer patients and families would be expected to need additional targeted interventions, such as addressing pre-existing psychosocial stressors that include financial problems or family conflict. Finally, clinical treatment would be reserved for patients and/or their families who exhibit multiple risk factors for maintaining distress, such as chronic and marked anxiety, depressive symptoms or other individual or family-based psychosocial problems.18 Triaging of resources can be effectively accomplished using psychometrically robust psychosocial screening tools, such as the Psychosocial Assessment Tool and Child Behavior Checklist.19
The distinction and clarification of the terms DSD and intersex is important and necessary. The term DSD was introduced to emphasize underlying genetic and hormonal factors responsible for atypical somatic sex development. However, there are individuals with DSDs who have assumed the term intersex as an identity and reject the notion that the human body must be dichotomous. These individuals view the term DSD as a negative label that implies that atypical sex anatomy must be corrected with surgical or hormonal interventions.20,21 Supporters of this position recognize that some interventions may be necessary in order to maintain physical health, such as removal of dysgenetic gonads in patients at high risk for malignancy, but call for a clear distinction between what is medically necessary versus what is elective or cosmetic.22–24
Genetic pathways of sex determination
Testis
The identification of genes involved in sex determination was spurred on by the discovery and characterization of the testis-determining gene SRY, which is a Y-chromosome-linked gene that encodes a transcription factor. In humans, levels of SRY mRNA are upregulated in the urogenital ridge at 7 weeks after conception and drive the bipotential gonad towards testis formation in 46,XY individuals.25,26 After translation, the SRY protein translocates to the nucleus and binds to the enhancer regions of SOX9, to drive the differentiation and proliferation of Sertoli cells and testis tubule organization.27–29 Definitive evidence that SRY is the initiating factor in human male sex determination was provided by the discovery of missense mutations that disrupt the DNA-binding region of SRY, nonsense mutations, and deletions in the 5′ or 3′ regulatory regions of SRY that alter the timing or levels of SRY expression and lead to 46,XY gonadal dysgenesis.3,30–33
The second major gene involved in male sex determination is the gene that encodes the SRY-related transcription factor SOX9. In humans, autosomal dominant mutations in SOX9 cause campomelic dysplasia with a 46,XY DSD and external genitalia ranging from ambiguous genitals to a female phenotype.34,35 SOX9 protein expression is required for testis determination and, in conjunction with SRY and the transcription factor NR5A1, the SOX9 protein binds to its own promoter to perpetuate a positive feedback loop that maintains high levels of SOX9 expression. In mouse models, Sox9 mRNA expression is also maintained by activation of the Fgf9–Fgfr236–38 and prostaglandin D2 (PGD2) signalling pathways.39 Clinical syndromes associated with skeletal dysplasias have been described for mutations in FGFR2.40 However, mutations that result in gonadal dysgenesis and DSDs in humans have not yet been identified in components of the FGF9–FGFR2 or PGD2 signalling pathways, which suggests that phenotypes associated with such mutations might result in embryonic lethality or have redundant functions in the genetic networks that drive sex determination.
A number of mutations in the genes encoding additional transcription factors that are involved in testis determination have been identified in human DSDs. NR5A1 (also known as steroidogenic factor 1, or SF-1), encodes an orphan nuclear receptor, which is a major contributor to the development of the hypothalamic–pituitary–gonadal–adrenal axis.41–43 High expression of Nr5a1 in the mouse bipotential gonad suggested a role for this gene in cell proliferation prior to the onset of testis determination and in the upregulation of both Sry and Sox9 gene expression.44 Initial reports highlighted the involvement of NR5A1 mutations in gonadal and adrenal dysgenesis in 46,XY individuals with a female phenotype.43 In pre-Sertoli cells, NR5A1 synergizes with the transcription factor GATA4 at the onset of testis determination and binds to the SRY promoter to upregulate SRY expression.45 Further analysis of NR5A1 in individuals with DSDs or with infertility issues has shown that mutations in this gene are associated with a variable range of phenotypes from mild hypospadias to ambiguous genitalia46,47 as well as infertility in adulthood.48,49 Emerging studies have also demonstrated that 46,XY individuals with mutations in NR5A1 who are phenotypically female may present with clitoromegaly that is secondary to elevated testosterone levels at the onset of puberty despite their dysgenetic gonads.50
NR0B1 (also known as DAX1) is located on the X chromosome (at p21.3) and like NR5A1 encodes an orphan nuclear receptor that has a function in mammalian sex determination.51 Duplications encompassing NR0B1 in humans52 or transgenic overexpression of Nr0b1 in mice53,54 lead to dose-dependent XY gonadal dysgenesis and a female phenotype. As XY individuals have only one copy of this gene, a duplication that results in NR0B1 overexpression is sufficient to block testis determination. One of the molecular mechanisms identified in XY mice transgenic for Nr0b1 is through direct inhibition of Nr5a1-mediated transcription of Sox9.54 In 46,XX female individuals, the two functioning copies of the NR0B1 gene are crucial to preventing testis formation. Loss-of-function mutations or deletions of NR0B1 lead to congenital adrenal hypoplasia and life-threatening adrenal failure that is associated with abnormalities in male genital development owing to decreased steroidogenesis.55
The GATA4 and ZFPM2 (also known as FOG2) genes encode transcription factors that are critical for testis development. The discovery of a familial heterozygous missense mutation in GATA4 that resulted in a Gly221Arg mutation and development of a 46,XY DSD, which was also associated with congenital heart disease, underscores the fundamental role of GATA4 in both gonad and cardiac development.56 Genetic associations between two unrelated patients with rare mutations in ZFPM2 who also have 46,XY gonadal dysgenesis have recently been described, which further underscores the important role of GATA4–ZFPM2 interactions in testis determination.57 In mouse models, mutations that disrupt associations between Gata4 and Zfpm2 give rise to abnormal testis development.58 In a porcine model, GATA4 directly activated the SRY promoter; however, in humans and mice direct activation of SRY expression has only been observed when the WT1 protein is coexpressed.59 The studies in mice support the findings in patients with mutations in these genes and offer additional mechanistic insights into sex determination. Mutations in Gata4 or Zfpm2 resulted in decreased interactions between Gata4 and Zfpm2 proteins, which led to decreased ability of either gene (independently or when coexpressed) to activate transcription of target genes such as Amh, Sry, and Sox9.56,57,59
Deletions that encompass the region surrounding the DMRT1 and DMRT2 genes, which are located on chromosome 9p, have been identified in multiple cases of 46,XY gonadal dysgenesis with ambiguous genitalia.60,61 Fine mapping of this region has narrowed the minimal region associated with 46,XY gonadal dysgenesis to a small 260 kb region upstream of the DMRT1 and DRMT2 genes.60 In addition to its role in somatic cell determination within the gonads, a highly significant locus on chromosome 9p near DMRT1 was identified in genome-wide association studies performed in patients with gonadal germ-cell tumours.62 The finding that DMRT1 is associated with a propensity towards germ-cell tumours is consistent with the phenotype of Dmrt1-knockout mice, which have a profound failure in postnatal maintenance of the germline63,64 and, on specific genetic backgrounds, also have increased susceptibility to gonadal germ-cell tumour formation.65
Within testis differentiation, DMRT1 is critical for the maintenance of Sertoli cell fate. Once the testis fate has been established, the phenotypes of the heterogeneous cells within the testis must be actively maintained. In mice, postnatal expression of Dmrt1 simultaneously promotes testis-specific gene expression in Sertoli cells, through maintenance of high levels of Sox9 expression, and represses ovary-specific granulosa cell differentiation. Loss of Dmrt1 from Sertoli cells in the postnatal mouse testis results in transdifferentiation of these cells into granulosa cells.66
An individual with multiexonic deletions in the WWOX gene on chromosome 16 presented with 46,XY gonadal dysgenesis (see Table 1),67 which suggested that this gene was also involved in testis development. Pathological analysis of the gonads of this patient revealed immature testis and the presence of premalignant gonadal germ cells.67 Given this phenotype it is possible that WWOX may function in the same developmental pathway as DMRT1 to promote somatic cell differentiation of Sertoli cells and maintain germ cells. Additional studies in mice support this hypothesis, as XY mice homozygous for a hypomorphic Wwox allele have testicular atrophy and decreased fertility.68
Table 1.
Copy number variations in 46,XX testicular or ovotesticular DSDs and 46,XY gonadal dysgenesis
| Chromosomal region | Duplication or deletion | Sex determination gene(s) | DSD phenotype(s) | Other phenotypes | Reference |
|---|---|---|---|---|---|
| 9p | Heterozygous deletion | DMRT1, DMRT2 | Complete gonadal dysgenesis to ambiguous genitalia | None | Tannour-Louet et al. (2010)60 |
| 10q25q26 | Heterozygous deletion | Possibly FGFR2 | 46,XY gonadal dysgenesis, ambiguous genitalia with bilateral cryptorchidism | IUGR, congenital heart disease, mental retardation, cranial dysmorphology | Tannour-Louet et al. (2010)60 Irving et al. (2003)118 Chung et al. (1998)158 Courtens et al. (2006)159 |
| 22q11.2 | Duplication | Possibly SOX10 | 46,XX gonadal dysgenesis with ambiguous genitalia | Developmental delay, mental retardation, Wilms tumour, tetralogy of Fallot | Tannour-Louet et al. (2010)60 |
| Xp21 | Duplication | NR0B1 | 46,XY gonadal dysgenesis | None | Barbaro et al. (2007)52 White et al. (2011)101 |
| 1p36.33 | Heterozygous deletion | Possibly WNT4 | 46,XX cloacal exstrophy, prominent labioscrotal folds, no apparent genital tubercle | Midline defect, imperforate anus, left foot anomaly | Tannour-Louet et al. (2010)60 Battaglia et al. (2008)160 |
| 8p23.1 | Heterozygous deletion | 3′ to GATA4 | 46,XY with complete gonadal dysgenesis, ambiguous genitalia | Adrenal hypoplasia congenita | White et al. (2011)101 |
| Xq27.1 | Deletion or duplication | 5′ or 3′ to SOX3 or including SOX3 | 46,XX testicular DSD with male phenotype | Developmental delay, microcephaly, gender dysphoria, short stature | Moalem et al. (2012)99 Sutton et al. (2011)100 |
| 16q23.1 | Multi-exon deletion | WWOX | 46,XY gonadal dysgenesis with ambiguous genitalia, immature testis | None | White et al. (2012)67 |
| 17q24.3 | Deletions or duplication | 5′ to SOX9, sometimes including SOX9 | 46,XY gonadal dysgenesis with female phenotype or ambiguous genitalia or 46,XX DSD with male phenotype | Skeletal anomalies in a subset of patients with a translocation involving upstream regions of the SOX9 gene | Huang et al. (2011)95 Cox et al. (2011)96 Xiao et al. (2013)97 Benko et al. (2011)98 |
| 1p35-p31 | Duplication | Possibly WNT4 and RSPO1 | 46,XY DSD with female phenotype | Microcephaly, developmental delay, IUGR, dysmorphic features | Jordan et al. (2001)161 |
| 19q12 | Heterozygous deletion | Unknown | 46,XY DSD with ambiguous genitalia and hypospadias | IUGR, ectrodactyly, developmental delay, feeding problems | Tannour-Louet et al. (2010)60 Chowdhury et al. (2014)121 |
| 13q33.2 | Heterozygous deletion | Possibly EFNB2 | 46,XY with ambiguous genitalia | Mental retardation, brain malformations eye malformations, gastrointestinal tract malformations, IUGR | Andresen et al. (2010)119 |
Abbreviations: DSD, disorder of sex development; IUGR, intrauterine growth retardation.
Ovary
Before the emergence of molecular and genomic tools in the past decade, ovarian sex determination was considered a passive default pathway that occurred in the absence of SRY expression and testis development. An equivalent gene to SRY has not yet been identified for ovarian differentiation. Furthermore, few morphological changes indicative of ovarian development have been identified that occur at the equivalent developmental time point as when an XY bipotential gonad begins to develop the organizational structure of the testis.
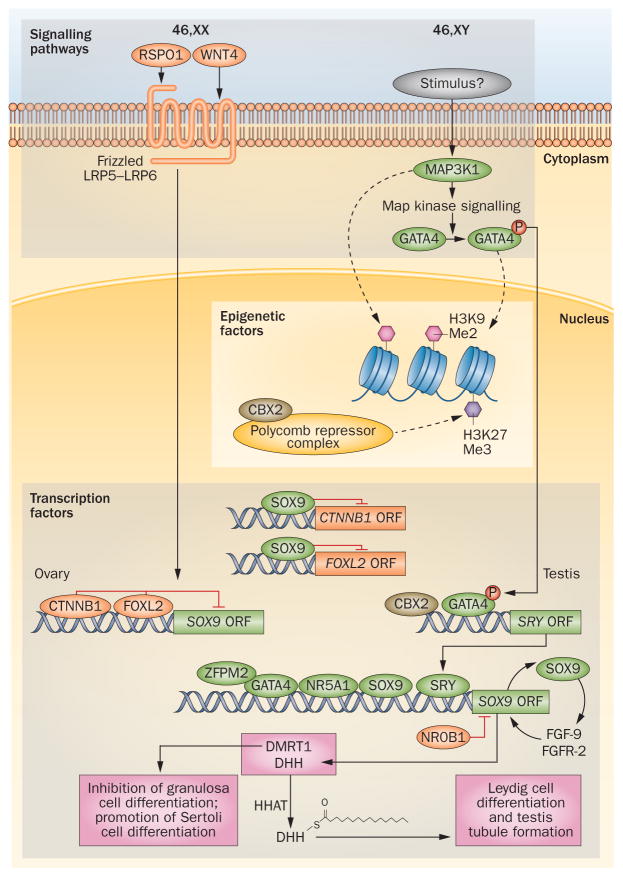
Despite the lack of visible morphological changes, gene expression patterns within XX somatic cells in the bipotential gonad have been shown to drive differentiation of granulosa cells and steroid-producing theca cells.69 The primary signals for initiation of granulosa cell differentiation are unclear. Nevertheless, high mRNA levels of the signalling factors WNT4 and RSPO1 upregulate expression of and stabilize the transcription factor CTNNB1 (also known as β-catenin), which suppresses male-specific SOX9 expression, maintains WNT4 gene expression and promotes germ-cell proliferation (Figure 1).70–73
Figure 1.
Genetic pathophysiology of human sex determination. Within the developing gonad, regulation of gene transcription occurs through cellular signalling pathways (WNT4–RSPO1 in ovary determination, Map-kinase in testis determination) that activate genes through alteration of chromatin structures and modulation of epigenetic factors or by direct activation of transcriptional networks. In 46,XX individuals, WNT4 and RSPO1 act through Frizzled or LRP5–LRP6 receptors to activate β-catenin (CTNNB1) transcription. β-catenin and FOXL2 promote expression of ovary-specific genes while inhibiting the expression of testis factors such as SOX9. In 46,XY individuals, Map-kinase signalling through MAP3K1 may alter chromatin conformation indirectly through histone modifications (dotted arrow). Map-kinase signalling also increases phosphorylation of transcription factors such as GATA4, which is thought to alter chromatin (dotted arrow) upstream of SRY, and was shown to directly bind to SRY promoter (solid arrow) to activate transcription. Within the nucleus, transcription factors GATA4 and ZFPM2 bind and transactivate SRY and SOX9. Other important factors are CBX2 that has been shown to directly bind the SRY promoter and that, in conjunction with the NR5A1 protein, binds to the SOX9 promoter. The SRY protein can then turn on downstream genes such as SOX9, which initiates the testis gene expression network and represses ovarian-specific genes such as RSPO1 and β-catenin. Ovary-promoting transcription factors are noted in orange and testis-promoting factors are noted in green. Abbreviations: ORF, open reading frame; P, phosphate.
Morphological differentiation of human ovaries occurs at week 7 of gestation, which is the developmental stage in which female germ cells enter the first steps of meiosis. Much of the knowledge we have of this developmental process has been gleaned from studies in animal models. In mice, meiosis in female germ cells is triggered by cell-extrinsic factors, such as retinoic acid and expression of Stra8.74,75 At this point, high levels of Wnt4 signalling through either Frizzled or Lrp5–Lrp6 receptors drive both germ cell meiosis and differentiation of theca cells.76 Similar to the developing testis, after the point of fetal ovarian determination, the ovarian phenotype and granulosa cell differentiation are actively maintained by expression of the FoxL2 protein and the estrogen receptors α and β.77–79 Loss of Foxl2 expression in mouse adult granulosa cells results in upregulation of Sox9 and transdifferentiation towards a Sertoli cell phenotype. Thus, like male sex determination, initial ovarian determination is dependent on Wnt4 and Rspo1 signalling, while maintenance of germ cells and the ovarian phenotype requires other proteins, such as FoxL2 and the estrogen receptors.
Failure of correct ovarian development in individuals with the 46,XX karyotype can result in one of two distinct states: gonadal dysgenesis or the presence of some testicular tissue within a 46,XX gonad. Gonadal dysgenesis has been suggested to have a distinct aetiology from that of a DSD; however, we believe that failure of ovarian development, particularly during the early stages, is similar to 46,XY gonadal dysgenesis. The primary difference between 46,XX and 46,XY gonadal dysgenesis is that, in the former case, the phenotype of the internal and external genitalia is congruent with the complement of sex chromosomes. By contrast, in individuals with the 46,XY karyotype, there is some degree of phenotypic variability of the external genitals that can range from hypospadias to a female phenotype and can include ambiguous genitalia.7
Gonadal dysgenesis associated with the 46,XX karyotype manifests clinically as primary ovarian insufficiency, which is defined as premature depletion of ovarian follicles and onset of menopause before 40 years of age.80 This condition is represented by a range of phenotypes, from individuals who have not entered puberty by 15 years of age81 to those who experience early cessation of ovulation known as secondary amenorrhoea.80 Although primary ovarian insufficiency can occur as a result of a variety of nongenetic aetiologies (for example, chemotherapy, auto-immunity and environmental factors), a genetic basis is thought to be the primary cause in 10–15% of all cases.82,83
Female sex determination is intricately linked with the initiation of meiosis in germ cells.84 The most frequent genetic cause of gonadal dysgenesis in a phenotypic female is 45,X Turner syndrome, which is estimated to occur in one in 2,000 live female births.85 The follicle depletion observed among patients with 45,X Turner syndrome can result both from haploinsufficiency of critical genes on the X chromosome that escape X-inactivation and from incorrect pairing of the X chromosomes during meiosis.
Mutations or deletions that affect the expression of the autosomal gene FOXL2 can lead to 46,XX gonadal dysgenesis with blepharophimosis, ptosis and epicanthus inversus syndrome (BPES) in humans and goats.86,87 BPES in humans is divided into two types. BPES type I is a sex-limited, autosomal dominant form with the full spectrum of disease. This severe phenotype results from truncating mutations that lead to haploinsufficiency of FOXL2 protein.86 BPES type II is limited to the blepharophimosis phenotype, is present in both male and female individuals and has no gonadal phenotype. The subset of patients who have this limited form of the disease have small duplications within the FOXL2 gene.88 BPES types I and II can rarely occur together within a single family.88 Autosomal dominant mutations in the nuclear orphan receptor NR5A1, described in detail above, can also result in premature ovarian failure in 46,XX individuals and account for <3% of all genetic cases of this condition.48,89,90
In addition to FOXL2, ovarian determination is dependent on the presence of either functional WNT4 or functional RSPO1.91 Mutations in WNT4 and RSPO1 (Tables 1 and 2, Figure 1) are discussed in detail in the section of this Review focused on signal transduction pathways. Genetic mutations in the WNT4 gene can have a wide range of functional effects, including impaired lipid modification, defects in receptor signalling, and aggregate formation.92,93
Table 2.
Genetic mutations associated with disorders of sex development
| Gene involved in sex determination | Genomic region of mutation | Inheritance | |
|---|---|---|---|
| Noncoding | ORF | ||
| MAP3K1 | No | Yes | Autosomal dominant, gain-of-function |
| WNT4 | No | Yes | Autosomal recessive |
| RSPO1 | No | Yes | Homozygous recessive |
| CBX2 | No | Yes | Autosomal recessive |
| SRY | Yes | Yes | Sex-linked recessive |
| SOX9 | Yes | Yes | Autosomal dominant |
| NR5A1 (SF-1) | Yes | Yes | Autosomal dominant |
| GATA4 | Yes | Yes | Autosomal dominant |
| ZFPM2 (FOG2) | No | Yes | Autosomal dominant |
| NR0B1 | Yes | Yes | Sex-linked recessive |
| FOXL2 | Yes | Yes | Autosomal dominant |
| DMRT1 | Yes | Yes | Autosomal dominant |
Abbreviation: ORF, open reading frame.
Testicular or ovotesticular DSDs that are associated with the 46,XX genotype are rare and arise primarily as a result of ectopic expression of SOX-family genes that are related to the major testis determining gene, SRY, within the fetal bipotential gonad. Up to 90% of isolated 46,XX testicular DSDs involve a translocation of SRY to the X chromosome or to an autosome.94 A portion of the remaining cases can be explained by rare duplications or deletions in the promoter and enhancer regions of SOX genes, including SOX9,95–98 SOX399,100 and SOX10101,102 (Table 1). In such cases, testicular tissue develops and causes some degree of masculinization of both the internal and external genital structures. Individuals with rare syndromic 46,XX testicular or ovotesticular DSDs have been identified who harbour mutations in RSPO1.71
Pathways of sex determination
As discussed above, transcription factors have a key role in male sex determination; however, other factors and pathways have recently been recognized to play an important part in this process.
Signal transduction pathways
A number of studies have addressed the role of cellular proliferation in the bipotential gonad and in the period before the onset of sexually dimorphic changes in sex determination. At the initiation of male sex determination, the WNT4 and RSPO1 signalling pathways become sexually dimorphic, with downregulation of WNT4 and RSPO1 expression and upregulation of SOX9 expression in the developing testis.70,71,73
Mitogen-activated protein kinase (MAPK) signalling has is the dominant pathway in testis determination (Figure 1). Heterozygous missense mutations in MAP3K1 have been described in six published cases of 46,XY DSDs.103,104 Additionally, functional studies in human cell lines suggest that gain-of-function mutations in MAP3K1 shift the balance of sex determination from testis development (driven by SOX9–FGF9 signalling) towards ovarian development (mediated by WNT4 and β-catenin signalling).105 Furthermore, studies in mice have demonstrated that phosphorylation of the transcription factor Gata4 is regulated through MAP3K4 signalling. This pathway might also regulate Sry expression during fetal gonadal development via modulation of chromatin structures.106
Mutations in another signalling pathway, the desert hedgehog (DHH) pathway, have also been described in a small subset of patients with 46,XY DSDs with complete gonadal dysgenesis, as well as in a subset of patients with minifascicular neuropathy.107,108 Additionally, autosomal recessive mutations in the hedgehog acyltransferase gene, HHAT, have been identified in a patient with a 46,XY DSD with gonadal dysgenesis and chondroplasia.109 Gonad-specific deletion of Hhat in mice resulted in abnormal testis tubule formation, decreased gonadal size, and testicular dysgenesis. High expression of Sox9 and Cyp11a1 (which encodes a steroidogenic enzyme marker of Leydig cells) in these mice showed that differentiation of both Sertoli and Leydig cells was affected; however, Sry expression remained normal, which indicated that these disruptions occur downstream of Sry expression.109
In female sex determination, WNT4 and RSPO1 signalling via β-catenin is crucial for normal ovarian development. In humans, dominant missense mutations in WNT4 have been associated with Müllerian aplasia and hyperandrogenism,92,93 a finding that is supported by the phenotypes of female Wnt4-deficient mice.70 Rare recessive mutations in WNT4 cause SERKAL syndrome, which is associated with multiple developmental anomalies in the kidneys, adrenal gland and lungs, as well as testicular DSDs with ambiguous genitalia in individuals with the 46,XX karyotype.110 Findings in Wnt4-knockout mice predated the human discoveries and demonstrated early loss of germ cells and increased expression male steroidogenesis enzymes, which leads to elevated androgen levels driving Wolffian duct formation.70 Further studies have shown that Wnt4 also functions to upregulate retinoic acid signals that both initiate the onset of meiosis in germs cells and maintain germ cells throughout adult life.74,75,111
In conjunction with WNT4, RSPO1 is upregulated in the developing human ovary, and missense mutations in this gene result in syndromic forms of 46,XX testicular and ovotesticular DSDs that are associated with palmoplantar hyperkeratosis and a predisposition for development of squamous cell carcinoma in the skin.71,73 Findings in mouse models suggest that Rspo1 is required for ovarian determination in both normal XX individuals and in individuals with XY sex reversal.37,71 Thus, the interplay between signalling pathways and transcription factors in ovary determination is without clear hierarchy, unlike that which exists with SRY signalling in testis determination. What is clear in both ovary and testis determination is that multiple pathways are required to program the heterogeneous group of cells within the differentiating gonads.
Epigenetic pathways
The emergence of sequencing technologies has enabled dissection of epigenomes in vivo on a large scale and has brought heightened recognition of the importance of crosstalk between epigenetic and transcriptional factors in developmental processes. Epigenetic factors that influence the expression of SRY have a substantial role in sex determination.
Chromobox2 (CBX2) has been shown to modulate DNA histone marks, which alter the expression of downstream genes in other developmental processes. To date, a single case of an individual with compound heterozygote mutations in CBX2 that result in Pro98Leu and Arg443Pro amino acid substitutions has been reported.112 A follow-up study of 47 patients with 46,XY or 46,XX DSDs did not identify any pathogenic CBX2 mutations, which indicates that CBX2 is probably not a frequent cause of these disorders.113 The two CBX2 mutations were placed independently into the gene that encodes the long-CBX2 isoform and transfected into a variety of human cell lines. Introduction of each mutation independently disrupted DNA transactivation of downstream sex determination genes, such as NR5A1, and transfection of the same cell with a gene that carried both mutations had a synergistic effect and further decreased activation of the sex determination genes.112
Mechanistic studies using a human epithelial cell line grown in culture suggest that CBX2 functions as a component of the Polycomb repressive complex, which silences transcription through binding to H3K27me3 histone tags (Figure 1)114 In addition to CBX2’s known role as part of a polycomb repressive complex, emerging evidence in both Drosophila melanogaster and mouse models suggests that the long isoform of the protein may influence the transactivation of sex determination genes through its DNA-binding domain.115,116 Findings from mouse models of sex determination suggest that Cbx2 acts upstream of Sry to either directly or indirectly upregulate gene expression and suppress the ovarian determination pathway in XY mice.117 However, whether one or both of the known roles of CBX2 are important in human gonadal development has yet to be determined.
Copy number variations
The emergence of high-density single nucleotide polymorphism analyses to identify small and large copy number variations (CNVs) on a genome-wide scale has highlighted the importance of noncoding regions of the genomic DNA in the development of DSDs. A summary of duplications and deletions that have been implicated in DSDs is shown in Table 1.
Large and rare chromosomal duplications and deletions are associated with DSDs and these genomic regions are likely to harbour genes involved in sex determination. The most prominent chromosomal anomalies include 9p deletion60 (the region that contains the known sex determination genes DMRT1 and DMRT2), Xp duplication52,101 (the location of NR0B1), 10q deletion118,60 (encompassing FGFR2) and 22q duplication60,102 (the location of SOX10).
There remain a number of rare DSD-associated CNVs in which a causative gene in sex determination has not yet been identified or in which the putative gene has not been well studied in the context of sex determination. Heterozygous deletions in the 13q33.2 chromosomal region result in a 46,XY male phenotype with ambiguous genitalia and growth restriction and, depending on the size of the deletion, multiple organ malformations. The critical region encompasses a 9.5 Mb region that contains over 20 genes, of which EFNB2 is a potential candidate given that the phenotype of mice with ephrin-b2 knocked out show defects in urorectal development.119,120
Deletions on 19q12q13 have been described in several patients with 46,XY DSDs characterized by cryptorchidism, ambiguous genitalia, intrauterine growth restriction and other developmental anomalies;60,121 however, no genes have been associated with DSDs located in this region. The smallest region of overlap in these patients that is associated with a DSD phenotype has been difficult to pinpoint, as deletions in this region have a wide range of variable phenotypes with approximately 75% of affected individuals demonstrating a DSD phenotype.121
Small chromosomal deletions or duplications that result in either haploinsufficiency or overexpression of a single sex determination gene can also lead to development of a DSD. For example, deletions in WWOX67 and duplications in NR0B152 can each lead to development of 46,XY DSDs. In addition, CNVs within noncoding regulatory regions of sex determining genes are associated with development of DSDs in individuals with 46,XY and 46,XX karyotypes.31,100,122 The exact mechanisms by which these noncoding CNVs function is incompletely understood; however, it has been postulated that they disrupt long-range repressor or activator elements or alter the balance between these elements, which are critical for correct spatiotemporal expression of nearby sex determination genes.100 Several noncoding CNVs have been described for SOX9.96,98,101 Nevertheless, it is important to note that while 98% of the human genome is non-protein-coding, emerging evidence suggests that a large proportion of the non-protein-coding DNA is transcribed into functional RNA molecules, such as long non-coding RNA and microRNA species. These regions may harbour causative mutations in non-protein-coding genome that might function to regulate developmental gene expression.123
Multigenic inheritance
The availability of next-generation sequencing technologies has provided the potential to identify concurrent mutations in genes that are associated with DSDs, which might contribute to pathogenesis in a cumulative fashion. One such example is the discovery of mutations in AKR1C4 and AKR1C2, which are involved in regulating fetal testosterone synthesis. In two families with multiple affected members who presented with 46,XY DSDs and varying degrees of undervirilization and cryptorchidism, mutations in AKR1C4 and AKR1C2 were identified using Sanger sequencing of coding regions of genes within an overlapping linkage peak. Variable inheritance of splicing and missense mutations in AKR1C4 and AKR1C2, respectively, were found to result in a dose-dependent 46,XY DSD with ambiguous genitalia.124 The variable expression that is often observed in large families with multiple members affected by DSDs might be influenced by co-inheritance of other mutations in sex determination genes or modifier genes.
The shift from traditional viewpoints of Mendelian genetic inheritance as relates to diseases has been cultivated as the complexities of the human genome continue to be unravelled. Mutations within the same developmental pathways may be additive or multiplicative and can result in an increased mutational load and increased severity of the DSD phenotype. An example of a DSD with complex inheritance is Bardet–Biedl syndrome. This syndrome is a ciliopathy characterized by dysfunction in multiple systems and is associated with obesity, developmental delays, renal malformations and genital anomalies. The pattern of inheritance of Bardet–Biedl syndrome displays substantial locus heterogeneity and has been shown to map to at least 15 loci with rare instances of triallelic inheritance.125,126 Advances in next-generation sequencing will enable rapid genetic diagnosis in patients with DSDs that arise from multigenic inheritance, such as Bardet–Biedl syndrome or in cases with multiple defects within the same steroidogenic pathway.
Environmental factors
In addition to the genetic factors addressed in this Review, the contribution of environmental factors to gonadal and genital development, which range from intra-uterine environment and placental insufficiency127,128 to low doses of endocrine disruptors, such as industrial and agricultural chemicals,129 cannot be discounted. Emerging evidence suggests that an association might exist between environmental factors and development of DSD-associated conditions such as cryptorchidism and hypospadias.129–131 However, precise understanding of the contributions of genetic, hormonal and environmental factors to genital development and their interactions with genes remains limited.
Genetic diagnosis
The genetic and clinical diagnosis of DSDs has become increasingly complex as a consequence of the rapid expansion of knowledge about the genetic mechanisms that cause DSDs over the past 20 years. Mutations in the same gene can result in a wide range of genital phenotypes among individuals with DSDs; conversely, similar genital and gonadal phenotypes in different individuals can be caused by mutations in any one of 20 or more genes.12 When an individual presents with a suspected DSD, a minimal initial endocrinological and genetic evaluation is required to rule out life-threatening adrenal disorders, such as congenital adrenal hyperplasia.132 However, technological advances have provided the tantalizing possibility that next-generation sequencing might be used for the primary identification of genetic mutations associated with DSDs, either using a targeted sequencing panel or whole-exome sequencing with a focus on genes known to be associated with DSDs.132,133
Given the limitations of current understanding of the human genome, it is imperative to evaluate the parental DNA alongside that of the affected child in order to narrow down the pathogenic genetic variant, particularly in the case of previously unidentified mutations. Many types of genetic mutations (missense, nonsense, small deletions, small insertions, deletions and duplications) and inheritance patterns can be ascertained using whole-exome sequencing, with the exception of deletions, duplications, or chromosomal inversions that are limited to the non-coding portion of the human genome.
Next-generation sequencing provides a rapid and unbiased approach to detection of genetic mutations compared with the approach of sequentially sequencing individual genes, which is both expensive and can delay diagnosis and initiation of appropriate treatments for individuals with DSDs. Next-generation sequencing can identify a genetic diagnosis in almost 40% of cases of DSDs, a number which is likely to be an underestimate, as only cases that are refractory to other diagnostic approaches, such as a thorough endocrine work-up and single-gene sequencing are currently sent for this type of analysis. Given the limitations of whole-exome sequencing mentioned above, the cases in which no causative mutation is identified should be further analysed by microarray or complete genomic hybridization for CNVs.
Using a next-generation-sequencing diagnostic approach, after one or more genetic mutations are identified an astute clinician may pursue functional validation of the predicted effects of the identified variant(s) in vivo using endocrinological and/or imaging approaches. For example, in cases in which a mutation in NR5A1 is identified as causative, one should perform adrenal function tests such as measurement of serum cortisol and adrenocorticotropic hormone levels. A mutation in GATA4 would prompt imaging of the heart to rule out the possibility of an associated congenital heart defect.
From the perspective of both clinicians and researchers, correct genetic classification is essential to best assess the pathophysiology and treatment outcomes in patients with DSDs. Use of terminology and classifications should be consistent and careful and a genetic diagnosis should be obtained for all patients with DSDs. Currently, only a small fraction of individuals with a DSD receive a genetic diagnosis,132 but patients and their families embrace such a diagnosis for the purposes of fully understanding and planning for the potential health-related and psychological issues that might be associated with an individual’s mutation(s). The particular genetic mutation(s) that underlie a specific DSD diagnosis can influence health-related considerations include management of complications such as cancer within the gonad and in other organ systems (for example Wilms tumours, skin tumours and adrenal tumours), whose risk might be increased; the potential need for hormone replacement therapy; and family planning considerations, both for the affected individual and for their parents.
Psychosexual differentiation
The terms sex and gender are often used interchangeably in studies with human patients; however, there are important distinctions between them. According to recommendations of an Institute of Medicine report, the term sex should be used to refer to classifications of male or female individuals according to physical attributes that are determined by an individual’s karyotype, specifically the reproductive organs and their functions. By contrast, the term gender is defined by the individual’s self-representation and identity as either a male or a female person, as well as by society-specific expectations regarding the appropriateness of attributes, activities, or behaviours for boys and men or girls and women.134
Psychosexual differentiation involves the developmental unfolding of gender identity, gender role and sexual orientation. Gender identity refers to a person’s identification of self as a girl (or woman), a boy (or man) or a mixture of both. Gender role refers to behaviours that differ in frequency or level between male individuals and female individuals in culture and time (such as toy play or maternal interest). Sexual orientation refers to sexual arousal to persons of the same sex (homosexual), opposite sex (heterosexual) or both sexes (bisexual), and is expressed in behaviour, fantasies and attractions.135 The consensus statement advises that gender identity, gender-typical behaviour, and sexual orientation should all be viewed as separate components. As such, if an individual with a DSD exhibits atypical gender role behaviour this is not an indication of having been reared in the wrong gender.7
The issue of gender identity in DSDs has been reviewed elsewhere.136–138 Gender identity has been suggested to generally follow gender of rearing,136,137 with the exception of syndromes that result from errors in biosynthesis of androgens, such as deficiencies of 5-α-reductase-2 and 17β-hydroxysteroid dehydrogenase-3.138–140 In these conditions, the masculinizing puberty that results from endogenous testosterone production is frequently associated with a shift in gender identity among individuals reared as girls.138 Unfortunately, the details provided in published case series are inadequate to test competing explanations for the observed change in gender identity in these individuals, such as the role of atypical genital appearance, masculinized gender behaviour, a contra-sexual puberty and cultural pressure that favours living as a man.141
Other challenging considerations from the perspective of gender assignment are those associated with non-hormonal DSDs, such as cloacal exstrophy and penile agenesis. A report of self-initiated gender change in a cohort of patients with 46,XY cloacal exstrophy who were reared as girls142 has been associated with a shift in gender assignment recommendations by paediatric urologists, from recommendations of rearing as a girl to recommendations of rearing as a boy for these patients.143,144 In a six-year follow-up survey of US paediatric urologists, 79% recommended gender assignment as a boy for patients with cloacal exstrophy, with 97% identifying ‘brain imprinting’ by prenatal androgens as an important factor in their decision. Nonetheless, a review of literature on gender identity stability in female-reared individuals with a 46,XY karyotype who have nonhormonal genital defects concluded that evidence is lacking to infer that gender identity is fully determined by prenatal androgen exposure.145
DSDs have been studied to test hormonal hypotheses of gender development that extend beyond gender identity. In contrast to the broadly accepted notions that gender is determined by both biological and social environmental factors,146 associations between DSDs and gender-atypical behavioural development have often been interpreted as directly resulting from prenatal androgen exposure.147,148
Prenatal androgens have been shown to exert a masculinizing effect on the development of behaviours that exhibit gender-related variability (for example, childrens’ toy and play preferences) and sex differences in neurocognitive function.149,150 As noted, less certainty exists regarding the role of early androgens in shaping gender identity and this is also the case for sexual orientation. The possibility that early androgen exposure shapes sexual orientation has been investigated, in particular among women with classic congenital adrenal hyperplasia, a population in which increased rates of non-heterosexual orientation have been reported.149,151,152 The reason for this observed correlation remains to be determined but could be attributed to a number of factors, such as a direct influence of androgens on sexual differentiation of the brain, or influences of early androgens on gender role expression. Additionally, the effects of having a chronic medical condition and its management on physical appearance and body image could also be involved, and the possibility that the correlation results from a combination of these and possibly other factors cannot be ruled out.152
Finally, animal experiments have demonstrated that early exposure to sex hormones during steroid-sensitive periods of brain development has long-lasting effects on neurocognitive function and sex-dimorphic behaviours.153 However, few studies have addressed the possibility of sex-hormone-independent effects on brain structure and function.154,155 Genetically modified mice have been used to separate gonadal phenotypes from chromosome complement. This separation is accomplished by moving Sry, which determines testes development, from the Y chromosome to an autosome. This paradigm has enabled investigators to consider whether observed sex differences in brain and behaviour are consequent to hormone exposure, genetic factors or both.154,155 Little evidence exists to support a role for genetic factors acting independently of sex hormones in shaping aspects of human psychosexual differentiation, such as gender identity, gender role or sexual orientation. Nonetheless, studies in mice have demonstrated sex differences in physiology and behaviour with similarities in humans that are unaccounted for by differential hormone exposure.155 Examples include autoimmune diseases such as multiple sclerosis, which are more common in female individuals,156 and more rapid escalation of substance abuse to the point of addiction in women than in men.157
Conclusions
The classification of patients with DSDs has been revolutionized by use of multidisciplinary approaches and large research networks that seek to better understand the pathophysiology of DSDs. By providing a consistent framework for diagnosis, clinicians can begin to identify patients with similar phenotypes and better understand the medical, social, and psychological factors that contribute to the overall well-being of patients with DSDs. Given the rarity of individuals with DSDs, multicenter studies are essential to identifying the methods that will lead to consistent diagnosis and optimal medical care for these people.
Despite the focus on the identification of genetic mutations that result in disruptions to the processes of sex determination and sex differentiation, genetics alone cannot completely explain the full range of health or psychological issues that might be experienced by an individual with a DSD. However, mutations identified in critical developmental genes have provided much needed insight into pathophysiological mechanisms of DSDs as well as a means of classification, which can be used to follow the long-term effects of genetic mutations and treatment outcomes in these individuals.
Emerging technological advances have transformed the ability to identify mutations and CNVs in individuals with DSDs; however, the interpretation and validation of identified mutations that are the direct cause of the observed phenotypes remains challenging. As sequencing technologies advance and our appreciation of the degree of variation within the human genome continues to expand, the distinction between causative mutations versus normal genetic variation will increase. The complexity of the human genome is evident in multiple layers of regulation, including at the level of DNA, messenger and long non-coding RNA transcripts, post- transcriptional and post-translational regulation, as well as within non-protein-coding genomic regions and the epigenome. Thus, while a large proportion of patients with DSDs have been diagnosed with a genetic lesion within known genes, much of the remaining variation that contributes DSDs have yet to be fully explored. Studying embryonic gonads in humans is ethically and technically very difficult, as these investigations require using tissues appropriate to the stage in development being examined. For example, fetal gonads of around 7 weeks of gestation would be needed to assess the epigenetic status, levels of various RNA species and mechanisms of post-translational regulation if sex determination were to be investigated.
Our understanding of the mechanisms within sex determination processes is heavily dependent on knowledge of the human patients with DSDs and studies in mouse models, which can be manipulated to determine the effects of individual genetic mutations. Understanding the multiple layers of genetic regulation that govern gonadal development and how these layers interact using next-generation sequencing tools coupled with careful studies in both animal models and in affected individuals might unlock the key to fully appreciating and treating the health needs of people with DSDs.
Key points.
Disorders of sex development (DSDs) are defined as congenital conditions in which development of chromosomal, gonadal, or anatomic sex is atypical
Mutations in genes that encode transcription factors, signalling components and epigenetic modifiers that are involved in sex determination can result in 46,XX and 46,XY DSDs
At 6–8 weeks post-conception in human fetal development, upregulated expression of SRY in the bipotential gonad promotes testis determination, whereas activation of WNT4 and RSPO1 signalling promotes ovary determination
Gonadal phenotypes in patients with DSDs range from gonadal dysgenesis (in which the gonads are fibrous streak gonads) to varying degrees of ovotesis (in which both ovary and testicular tissue are present)
The complexity and interrelatedness of factors that contribute to the aetiology and the medical and psychological outcomes of DSDs demand a multidisciplinary team approach to health care
In contrast to gender differences in activities and interests, associations between prenatal exposure to androgens and development of gender identity or sexual orientation are unclear
Review criteria.
A search for articles published between 1991 and 2014 and focusing on disorders of sex development was performed in PubMed and MEDLINE. The search terms used were “sex determination”, “disorders of sex development”, “intersex”, “quality of life”, “gender”, “gender identity”, “gender role”, “sexual differentiation” and “psychosexual differentiation”. Specific search terms for genes were also used (“SRY”, “SOX9”, “WT1”, “NR5A1”, “NR0B1”, “WNT4”, “RSPO1”, “FOXL2”, “DMRT1”, “MAP3K1”, and “CBX2”). All articles identified were English-language full-text papers and abstracts.
Acknowledgments
Funding for this project was from the Doris Duke Foundation and the National Institute of Child Health and Human Development RO1HD06138 DSD-TRN (Platform for Basic and Translational Research) grant to E.V. and D.E.S, University of California Los Angeles institutional funds to V.A.A. and Patient-Centered Outcomes Research Institute contract funds to D.E.S.
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions
The authors contributed equally to all aspects of the article.
Contributor Information
Valerie A. Arboleda, Department of Human Genetics, David Geffen School of Medicine, University of California Los Angeles, 695 Charles E. Young Drive South, Los Angeles, CA 90095-7088, USA
David E. Sandberg, Department of Pediatrics, Division of Child Behavioral Health and Child Health Evaluation & Research (CHEAR) Unit, University of Michigan, 300 North Ingalls Street, Ann Arbor, MI 48109-5456, USA
Eric Vilain, Department of Human Genetics, David Geffen School of Medicine, University of California Los Angeles, 695 Charles E. Young Drive South, Los Angeles, CA 90095-7088, USA.
References
- 1.Arboleda VA, Vilain E. In: Yen and Jaffe’s Reproductive Endocrinology. 6. Strauss JF, Barbieri RL, editors. Ch 16. Saunders Elsevier; 2009. pp. 367–393. [Google Scholar]
- 2.Danon M, Sachs L. Sex chromosomes and human sexual development. Lancet. 1957;273:20–25. doi: 10.1016/s0140-6736(57)90575-5. [DOI] [PubMed] [Google Scholar]
- 3.Sinclair AH, et al. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature. 1990;346:240–244. doi: 10.1038/346240a0. [DOI] [PubMed] [Google Scholar]
- 4.Koopman P, Munsterberg A, Capel B, Vivian N, Lovell-Badge R. Expression of a candidate sex-determining gene during mouse testis differentiation. Nature. 1990;348:450–452. doi: 10.1038/348450a0. [DOI] [PubMed] [Google Scholar]
- 5.Jost A. Problems of fetal endocrinology: the gonadal and hypophyseal hormones. Recent Prog Horm Res. 1953;8:379–418. [Google Scholar]
- 6.Jost A. Studies on sex differentiation in mammals. Recent Prog Horm Res. 1973;29:1–41. doi: 10.1016/b978-0-12-571129-6.50004-x. [DOI] [PubMed] [Google Scholar]
- 7.Lee PA, Houk CP, Ahmed SF, Hughes IA. Consensus statement on management of intersex disorders. International Consensus Conference on Intersex. Pediatrics. 2006;118:e488–e500. doi: 10.1542/peds.2006-0738. [DOI] [PubMed] [Google Scholar]
- 8.Hughes IA, Houk C, Ahmed SF, Lee PA. Consensus statement on management of intersex disorders. J Pediatr Urol. 2006;2:148–162. doi: 10.1016/j.jpurol.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Auchus RJ, Miller WL. Defects in androgen biosynthesis causing 46, XY disorders of sexual development. Semin Reprod Med. 2012;30:417–426. doi: 10.1055/s-0032-1324726. [DOI] [PubMed] [Google Scholar]
- 10.Biason-Lauber A, Boscaro M, Mantero F, Balercia G. Defects of steroidogenesis. J Endocrinol Invest. 2010;33 doi: 10.1007/BF03346683. [DOI] [PubMed] [Google Scholar]
- 11.Lux A, Kropf S, Kleinemeier E, Jurgensen M, Thyen U. Clinical evaluation study of the German network of disorders of sex development (DSD)/intersexuality: study design, description of the study population, and data quality. BMC Public Health. 2009;9:110. doi: 10.1186/1471-2458-9-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baxter RM, Vilain E. Translational genetics for diagnosis of human disorders of sex development. Annu Rev Genomics Hum Genet. 2013;14:371–392. doi: 10.1146/annurev-genom-091212-153417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Disorders of Sex Development Translational Research Network [online] 2014 https://dsdtrn.genetics.ucla.edu/
- 14.I-DSD Registry [online] 2014 https://www.i-dsd.org/
- 15.Moriya K, Mitsui T, Tanaka H, Nakamura M, Nonomura K. Long-term outcome of pituitary-gonadal axis and gonadal growth in patients with hypospadias at puberty. J Urol. 2010;184:1610–1614. doi: 10.1016/j.juro.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 16.Ogata T, Sano S, Nagata E, Kato F, Fukami M. MAMLD1 and 46,XY disorders of sex development. Semin Reprod Med. 2012;30:410–416. doi: 10.1055/s-0032-1324725. [DOI] [PubMed] [Google Scholar]
- 17.Rynja SP, de Jong TP, Bosch JL, de Kort LM. Functional, cosmetic and psychosexual results in adult men who underwent hypospadias correction in childhood. J Pediatr Urol. 2011;7:504–515. doi: 10.1016/j.jpurol.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Kazak AE, et al. An integrative model of pediatric medical traumatic stress. J Pediatr Psychol. 2006;31:343–355. doi: 10.1093/jpepsy/jsj054. [DOI] [PubMed] [Google Scholar]
- 19.Sandberg DE, Mazur T. In: Gender Dysphoria and Disorders of Sex Development: Progress in Care and Knowledge Focus on Sexuality Research. Kreukels BPC, Steensma TD, de Vries ALC, editors. Ch 5. Springer Science+Business Media; 2013. pp. 93–114. [Google Scholar]
- 20.Karkazis K, Feder EK. Naming the problem: disorders and their meanings. Lancet. 2008;372:2016–2017. doi: 10.1016/s0140-6736(08)61858-9. [DOI] [PubMed] [Google Scholar]
- 21.Reis E. Divergence or disorder?: The politics of naming intersex. Perspect Biol Med. 2007;50:535–543. doi: 10.1353/pbm.2007.0054. [DOI] [PubMed] [Google Scholar]
- 22.Tamar-Mattis A, Baratz A, Baratz Dalke K, Karkazis K. Emotionally and cognitively informed consent for clinical care for differences of sex development. Psychology & Sexuality. 2014;5:44–55. [Google Scholar]
- 23.Diamond M, Garland J. Evidence regarding cosmetic and medically unnecessary surgery on infants. J Pediatr Urol. 2014;10:2–6. doi: 10.1016/j.jpurol.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 24.Mouriquand P, Caldamone A, Malone P, Frank JD, Hoebeke P. The ESPU/SPU standpoint on the surgical management of Disorders of Sex Development (DSD) J Pediatr Urol. 2014;10:8–10. doi: 10.1016/j.jpurol.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 25.Clepet C, et al. The human SRY transcript. Hum Mol Genet. 1993;2:2007–2012. doi: 10.1093/hmg/2.12.2007. [DOI] [PubMed] [Google Scholar]
- 26.Sadler TW. Langman’s Medical Embryology. 9. Lippincott Williams & Wilkins; 2004. [Google Scholar]
- 27.Poulat F, et al. Nuclear localization of the testis determining gene product SRY. J Cell Biol. 1995;128:737–748. doi: 10.1083/jcb.128.5.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hiramatsu R, et al. A critical time window of Sry action in gonadal sex determination in mice. Development. 2009;136:129–138. doi: 10.1242/dev.029587. [DOI] [PubMed] [Google Scholar]
- 29.Sekido R, Lovell-Badge R. Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature. 2008;453:930–934. doi: 10.1038/nature06944. [DOI] [PubMed] [Google Scholar]
- 30.McElreavy K, et al. XY sex reversal associated with a deletion 5′ to the SRY “HMG box” in the testis-determining region. Proc Natl Acad Sci USA. 1992;89:11016–11020. doi: 10.1073/pnas.89.22.11016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McElreavey K, et al. Loss of sequences 3′ to the testis-determining gene, SRY, including the Y pseudoautosomal boundary associated with partial testicular determination. Proc Natl Acad Sci USA. 1996;93:8590–8594. doi: 10.1073/pnas.93.16.8590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berta P, et al. Genetic evidence equating SRY and the testis-determining factor. Nature. 1990;348:448–450. doi: 10.1038/348448A0. [DOI] [PubMed] [Google Scholar]
- 33.Jager RJ, Anvret M, Hall K, Scherer G. A human XY female with a frame shift mutation in the candidate testis-determining gene SRY. Nature. 1990;348:452–454. doi: 10.1038/348452a0. [DOI] [PubMed] [Google Scholar]
- 34.Foster JW, et al. Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature. 1994;372:525–530. doi: 10.1038/372525a0. [DOI] [PubMed] [Google Scholar]
- 35.Wagner T, et al. Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell. 1994;79:1111–1120. doi: 10.1016/0092-8674(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 36.Bagheri-Fam S, et al. Loss of Fgfr2 leads to partial XY sex reversal. Dev Biol. 2008;314:71–83. doi: 10.1016/j.ydbio.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 37.Kim Y, et al. Fibroblast growth factor receptor 2 regulates proliferation and Sertoli differentiation during male sex determination. Proc Natl Acad Sci USA. 2007;104:16558–16563. doi: 10.1073/pnas.0702581104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmahl J, Kim Y, Colvin JS, Ornitz DM, Capel B. Fgf9 induces proliferation and nuclear localization of FGFR2 in Sertoli precursors during male sex determination. Development. 2004;131:3627–3636. doi: 10.1242/dev.01239. [DOI] [PubMed] [Google Scholar]
- 39.Moniot B, et al. The PGD2 pathway, independently of FGF9, amplifies SOX9 activity in Sertoli cells during male sexual differentiation. Development. 2009;136:1813–1821. doi: 10.1242/dev.032631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bellus GA, et al. Identical mutations in three different fibroblast growth factor receptor genes in autosomal dominant craniosynostosis syndromes. Nat Genet. 1996;14:174–176. doi: 10.1038/ng1096-174. [DOI] [PubMed] [Google Scholar]
- 41.Luo X, Ikeda Y, Parker KL. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell. 1994;77:481–490. doi: 10.1016/0092-8674(94)90211-9. [DOI] [PubMed] [Google Scholar]
- 42.Wong M, Ramayya MS, Chrousos GP, Driggers PH, Parker KL. Cloning and sequence analysis of the human gene encoding steroidogenic factor 1. J Mol Endocrinol. 1996;17:139–147. doi: 10.1677/jme.0.0170139. [DOI] [PubMed] [Google Scholar]
- 43.Achermann JC, Ito M, Hindmarsh PC, Jameson JL. A mutation in the gene encoding steroidogenic factor-1 causes XY sex reversal and adrenal failure in humans. Nat Genet. 1999;22:125–126. doi: 10.1038/9629. [DOI] [PubMed] [Google Scholar]
- 44.Tremblay JJ, Viger RS. A mutated form of steroidogenic factor 1 (SF-1 G35E) that causes sex reversal in humans fails to synergize with transcription factor GATA-4. J Biol Chem. 2003;278:42637–42642. doi: 10.1074/jbc.M305485200. [DOI] [PubMed] [Google Scholar]
- 45.Ikeda Y, Shen WH, Ingraham HA, Parker KL. Developmental expression of mouse steroidogenic factor-1, an essential regulator of the steroid hydroxylases. Mol Endocrinol. 1994;8:654–662. doi: 10.1210/mend.8.5.8058073. [DOI] [PubMed] [Google Scholar]
- 46.Kohler B, et al. Five novel mutations in steroidogenic factor 1 (SF1, NR5A1) in 46,XY patients with severe underandrogenization but without adrenal insufficiency. Hum Mutat. 2008;29:59–64. doi: 10.1002/humu.20588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kohler B, et al. The spectrum of phenotypes associated with mutations in steroidogenic factor 1 (SF-1, NR5A1, Ad4BP) includes severe penoscrotal hypospadias in 46,XY males without adrenal insufficiency. Eur J Endocrinol. 2009;161:237–242. doi: 10.1530/EJE-09-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lourenco D, et al. Mutations in NR5A1 associated with ovarian insufficiency. N Engl J Med. 2009;360:1200–1210. doi: 10.1056/NEJMoa0806228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bashamboo A, et al. Human male infertility associated with mutations in NR5A1 encoding steroidogenic factor 1. Am J Hum Genet. 2010;87 doi: 10.1016/j.ajhg.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tantawy S, et al. Testosterone production during puberty in two 46, XY patients with disorders of sex development and novel NR5A1 (SF-1) mutations. Eur J Endocrinol. 2012;167:125–130. doi: 10.1530/EJE-11-0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Swain A, Zanaria E, Hacker A, Lovell-Badge R, Camerino G. Mouse Dax1 expression is consistent with a role in sex determination as well as in adrenal and hypothalamus function. Nat Genet. 1996;12:404–409. doi: 10.1038/ng0496-404. [DOI] [PubMed] [Google Scholar]
- 52.Barbaro M, et al. Isolated 46, XY gonadal dysgenesis in two sisters caused by a Xp21.2 interstitial duplication containing the DAX1 gene. J Clin Endocrinol Metab. 2007;92:3305–3313. doi: 10.1210/jc.2007-0505. [DOI] [PubMed] [Google Scholar]
- 53.Swain A, Narvaez V, Burgoyne P, Camerino G, Lovell-Badge R. Dax1 antagonizes Sry action in mammalian sex determination. Nature. 1998;391:761–767. doi: 10.1038/35799. [DOI] [PubMed] [Google Scholar]
- 54.Ludbrook LM, et al. Excess DAX1 leads to XY ovotesticular disorder of sex development (DSD) in mice by inhibiting steroidogenic factor-1 (SF1) activation of the testis enhancer of SRY-box-9 (Sox9) Endocrinology. 2012;153:1948–1958. doi: 10.1210/en.2011-1428. [DOI] [PubMed] [Google Scholar]
- 55.Guo W, et al. Diagnosis of X-linked adrenal hypoplasia congenita by mutation analysis of the DAX1 gene. JAMA. 1995;274:324–330. [PubMed] [Google Scholar]
- 56.Lourenco D, et al. Loss-of-function mutation in GATA4 causes anomalies of human testicular development. Proc Natl Acad Sci USA. 2011;108:1597–1602. doi: 10.1073/pnas.1010257108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bashamboo A, et al. Mutations in the FOG2/ ZFPM2 gene are associated with anomalies of human testis determination. Hum Mol Genet. 2014;23:3657–3665. doi: 10.1093/hmg/ddu074. [DOI] [PubMed] [Google Scholar]
- 58.Tevosian SG, et al. Gonadal differentiation, sex determination and normal Sry expression in mice require direct interaction between transcription partners GATA4 and FOG2. Development. 2002;129:4627–4634. doi: 10.1242/dev.129.19.4627. [DOI] [PubMed] [Google Scholar]
- 59.Miyamoto Y, Taniguchi H, Hamel F, Silversides DW, Viger RS. A GATA4/WT1 cooperation regulates transcription of genes required for mammalian sex determination and differentiation. BMC Mol Biol. 2008;9:44. doi: 10.1186/1471-2199-9-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tannour-Louet M, et al. Identification of de novo copy number variants associated with human disorders of sexual development. PLoS ONE. 2010;5:e15392. doi: 10.1371/journal.pone.0015392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Calvari V, et al. A new submicroscopic deletion that refines the 9p region for sex reversal. Genomics. 2000;65:203–212. doi: 10.1006/geno.2000.6160. [DOI] [PubMed] [Google Scholar]
- 62.Turnbull C, et al. Variants near DMRT1, TERT and ATF7IP are associated with testicular germ cell cancer. Nat Genet. 2010;42:604–607. doi: 10.1038/ng.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim S, Bardwell VJ, Zarkower D. Cell type-autonomous and non-autonomous requirements for Dmrt1 in postnatal testis differentiation. Dev Biol. 2007;307:314–327. doi: 10.1016/j.ydbio.2007.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Krentz AD, et al. The DM domain protein DMRT1 is a dose-sensitive regulator of fetal germ cell proliferation and pluripotency. Proc Natl Acad Sci USA. 2009;106:22323–22328. doi: 10.1073/pnas.0905431106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Takashima S, et al. Regulation of pluripotency in male germline stem cells by Dmrt1. Genes Dev. 2013;27:1949–1958. doi: 10.1101/gad.220194.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Matson CK, et al. DMRT1 prevents female reprogramming in the postnatal mammalian testis. Nature. 2011;476:101–104. doi: 10.1038/nature10239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.White S, et al. A multi-exon deletion within WWOX is associated with a 46,XY disorder of sex development. Eur J Hum Genet. 2012;20:348–351. doi: 10.1038/ejhg.2011.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ludes-Meyers JH, et al. WWOX hypomorphic mice display a higher incidence of B-cell lymphomas and develop testicular atrophy. Genes Chromosomes Cancer. 2007;46:1129–1136. doi: 10.1002/gcc.20497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nef S, et al. Gene expression during sex determination reveals a robust female genetic program at the onset of ovarian development. Dev Biol. 2005;287:361–377. doi: 10.1016/j.ydbio.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 70.Vainio S, Heikkila M, Kispert A, Chin N, McMahon AP. Female development in mammals is regulated by Wnt-4 signalling. Nature. 1999;397:405–409. doi: 10.1038/17068. [DOI] [PubMed] [Google Scholar]
- 71.Parma P, et al. R-spondin1 is essential in sex determination, skin differentiation and malignancy. Nat Genet. 2006;38:1304–1309. doi: 10.1038/ng1907. [DOI] [PubMed] [Google Scholar]
- 72.Maatouk DM, et al. Stabilization of β-catenin in XY gonads causes male-to-female sex-reversal. Hum Mol Genet. 2008;17:2949–2955. doi: 10.1093/hmg/ddn193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tomaselli S, et al. Human RSPO1/R-spondin1 is expressed during early ovary development and augments β-catenin signaling. PLoS ONE. 2011;6:e16366. doi: 10.1371/journal.pone.0016366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bowles J, et al. Retinoid signaling determines germ cell fate in mice. Science. 2006;312:596–600. doi: 10.1126/science.1125691. [DOI] [PubMed] [Google Scholar]
- 75.Koubova J, et al. Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc Natl Acad Sci USA. 2006;103:2474–2479. doi: 10.1073/pnas.0510813103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.He X, Semenov M, Tamai K, Zeng X. LDL receptor-related proteins 5 and 6 in Wnt/β-catenin signaling: arrows point the way. Development. 2004;131:1663–1677. doi: 10.1242/dev.01117. [DOI] [PubMed] [Google Scholar]
- 77.Uhlenhaut NH, et al. Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell. 2009;139:1130–1142. doi: 10.1016/j.cell.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 78.Couse JF, et al. Postnatal sex reversal of the ovaries in mice lacking estrogen receptors α and β. Science. 1999;286:2328–2331. doi: 10.1126/science.286.5448.2328. [DOI] [PubMed] [Google Scholar]
- 79.Dupont S, et al. Effect of single and compound knockouts of estrogen receptors α (ERα) and β (ERβ) on mouse reproductive phenotypes. Development. 2000;127:4277–4291. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- 80.Coulam CB. Premature gonadal failure. Fertil Steril. 1982;38:645–655. doi: 10.1016/s0015-0282(16)46688-4. [DOI] [PubMed] [Google Scholar]
- 81.Seminara SB, Oliveira LM, Beranova M, Hayes FJ, Crowley WF., Jr Genetics of hypogonadotropic hypogonadism. J Endocrinol Invest. 2000;23:560–565. doi: 10.1007/BF03343776. [DOI] [PubMed] [Google Scholar]
- 82.Persani L, Rossetti R, Cacciatore C. Genes involved in human premature ovarian failure. J Mol Endocrinol. 2010;45:257–279. doi: 10.1677/JME-10-0070. [DOI] [PubMed] [Google Scholar]
- 83.Bachelot A, et al. Phenotyping and genetic studies of 357 consecutive patients presenting with premature ovarian failure. Eur J Endocrinol. 2009;161:179–187. doi: 10.1530/EJE-09-0231. [DOI] [PubMed] [Google Scholar]
- 84.Spiller CM, Bowles J, Koopman P. Regulation of germ cell meiosis in the fetal ovary. Int J Dev Biol. 2012;56:779–787. doi: 10.1387/ijdb.120142pk. [DOI] [PubMed] [Google Scholar]
- 85.Stochholm K, Juul S, Juel K, Naeraa RW, Gravholt CH. Prevalence, incidence, diagnostic delay, and mortality in Turner syndrome. J Clin Endocrinol Metab. 2006;91:3897–3902. doi: 10.1210/jc.2006-0558. [DOI] [PubMed] [Google Scholar]
- 86.Crisponi L, et al. The putative forkhead transcription factor FOXL2 is mutated in blepharophimosis/ptosis/epicanthus inversus syndrome. Nat Genet. 2001;27:159–166. doi: 10.1038/84781. [DOI] [PubMed] [Google Scholar]
- 87.Pailhoux E, et al. A 11.7-kb deletion triggers intersexuality and polledness in goats. Nat Genet. 2001;29:453–458. doi: 10.1038/ng769. [DOI] [PubMed] [Google Scholar]
- 88.De Baere E, et al. Spectrum of FOXL2 gene mutations in blepharophimosis-ptosis- epicanthus inversus (BPES) families demonstrates a genotype–phenotype correlation. Hum Mol Genet. 2001;10:1591–1600. doi: 10.1093/hmg/10.15.1591. [DOI] [PubMed] [Google Scholar]
- 89.Voican A, et al. NR5A1 (SF-1) mutations are not a major cause of primary ovarian insufficiency. J Clin Endocrinol Metab. 2013;98:E1017–E1021. doi: 10.1210/jc.2012-4111. [DOI] [PubMed] [Google Scholar]
- 90.Philibert P, et al. NR5A1 (SF-1) gene variants in a group of 26 young women with XX primary ovarian insufficiency. Fertil Steril. 2013;99:484–489. doi: 10.1016/j.fertnstert.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 91.Ottolenghi C, et al. Loss of Wnt4 and Foxl2 leads to female-to-male sex reversal extending to germ cells. Hum Mol Genet. 2007;16:2795–2804. doi: 10.1093/hmg/ddm235. [DOI] [PubMed] [Google Scholar]
- 92.Biason-Lauber A, Konrad D, Navratil F, Schoenle EJ. A WNT4 mutation associated with Müllerian-duct regression and virilization in a 46,XX woman. N Engl J Med. 2004;351:792–798. doi: 10.1056/NEJMoa040533. [DOI] [PubMed] [Google Scholar]
- 93.Biason-Lauber A, et al. WNT4 deficiency--a clinical phenotype distinct from the classic Mayer–Rokitansky–Kuster–Hauser syndrome: a case report. Hum Reprod. 2007;22 doi: 10.1093/humrep/del360. [DOI] [PubMed] [Google Scholar]
- 94.Gao X, et al. Clinical, cytogenetic, and molecular analysis with 46,XX male sex reversal syndrome: case reports. J Assist Reprod Genet. 2013;30:431–435. doi: 10.1007/s10815-013-9939-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Huang B, Wang S, Ning Y, Lamb AN, Bartley J. Autosomal XX sex reversal caused by duplication of SOX9. Am J Med Genet. 1999;87:349–353. doi: 10.1002/(sici)1096-8628(19991203)87:4<349::aid-ajmg13>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 96.Cox JJ, Willatt L, Homfray T, Woods CG. A SOX9 duplication and familial 46,XX developmental testicular disorder. N Engl J Med. 2011;364:91–93. doi: 10.1056/NEJMc1010311. [DOI] [PubMed] [Google Scholar]
- 97.Xiao B, Ji X, Xing Y, Chen YW, Tao J. A rare case of 46,XX SRY-negative male with approximately 74-kb duplication in a region upstream of SOX9. Eur J Med Genet. 2013;56:695–698. doi: 10.1016/j.ejmg.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 98.Benko S, et al. Disruption of a long distance regulatory region upstream of SOX9 in isolated disorders of sex development. J Med Genet. 2011;48:825–830. doi: 10.1136/jmedgenet-2011-100255. [DOI] [PubMed] [Google Scholar]
- 99.Moalem S, et al. XX male sex reversal with genital abnormalities associated with a de novo SOX3 gene duplication. Am J Med Genet A. 2012;158A:1759–1764. doi: 10.1002/ajmg.a.35390. [DOI] [PubMed] [Google Scholar]
- 100.Sutton E, et al. Identification of SOX3 as an XX male sex reversal gene in mice and humans. J Clin Invest. 2011;121:328–341. doi: 10.1172/JCI42580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.White S, et al. Copy number variation in patients with disorders of sex development due to 46,XY gonadal dysgenesis. PLoS ONE. 2011;6:e17793. doi: 10.1371/journal.pone.0017793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Polanco JC, Wilhelm D, Davidson TL, Knight D, Koopman P. Sox10 gain-of-function causes XX sex reversal in mice: implications for human 22q-linked disorders of sex development. Hum Mol Genet. 2010;19:506–516. doi: 10.1093/hmg/ddp520. [DOI] [PubMed] [Google Scholar]
- 103.Pearlman A, et al. Mutations in MAP3K1 cause 46,XY disorders of sex development and implicate a common signal transduction pathway in human testis determination. Am J Hum Genet. 2010;87:898–904. doi: 10.1016/j.ajhg.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Das DK, Rahate SG, Mehta BP, Gawde HM, Tamhankar PM. Mutation analysis of mitogen activated protein kinase 1 gene in Indian cases of 46, XY disorder of sex development. Indian J Hum Genet. 2013;19:437–442. doi: 10.4103/0971-6866.124372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Loke J, et al. Mutations in MAP3K1 tilt the balance from SOX9/FGF9 to WNT/β-catenin signaling. Hum Mol Genet. 2014;23 doi: 10.1093/hmg/ddt502. [DOI] [PubMed] [Google Scholar]
- 106.Warr N, et al. Gadd45γ and Map3k4 interactions regulate mouse testis determination via p38 MAPK-mediated control of Sry expression. Dev Cell. 2012;23:1020–1031. doi: 10.1016/j.devcel.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Canto P, Soderlund D, Reyes E, Mendez JP. Mutations in the desert hedgehog (DHH) gene in patients with 46, XY complete pure gonadal dysgenesis. J Clin Endocrinol Metab. 2004;89:4480–4483. doi: 10.1210/jc.2004-0863. [DOI] [PubMed] [Google Scholar]
- 108.Umehara F, et al. A novel mutation of desert hedgehog in a patient with 46,XY partial gonadal dysgenesis accompanied by minifascicular neuropathy. Am J Hum Genet. 2000;67:1302–1305. doi: 10.1016/s0002-9297(07)62958-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Callier P, et al. Loss of function mutation in the palmitoyl-transferase HHAT leads to syndromic 46,XY disorder of sex development by impeding Hedgehog protein palmitoylation and signaling. PLoS Genet. 2014;10:e1004340. doi: 10.1371/journal.pgen.1004340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mandel H, et al. SERKAL syndrome: an autosomal-recessive disorder caused by a loss-of-function mutation in WNT4. Am J Hum Genet. 2008;82:39–47. doi: 10.1016/j.ajhg.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chassot AA, et al. WNT4 and RSPO1 together are required for cell proliferation in the early mouse gonad. Development. 2012;139:4461–4472. doi: 10.1242/dev.078972. [DOI] [PubMed] [Google Scholar]
- 112.Biason-Lauber A, Konrad D, Meyer M, DeBeaufort C, Schoenle EJ. Ovaries and female phenotype in a girl with 46,XY karyotype and mutations in the CBX2 gene. Am J Hum Genet. 2009;84:658–663. doi: 10.1016/j.ajhg.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Norling A, Hirschberg AL, Iwarsson E, Wedell A, Barbaro M. CBX2 gene analysis in patients with 46,XY and 46,XX gonadal disorders of sex development. Fertil Steril. 2013;99:819–826. e3. doi: 10.1016/j.fertnstert.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 114.Volkel P, Le Faou P, Vandamme J, Pira D, Angrand PO. A human Polycomb isoform lacking the Pc box does not participate to PRC1 complexes but forms protein assemblies and represses transcription. Epigenetics. 2012;7:482–491. doi: 10.4161/epi.19741. [DOI] [PubMed] [Google Scholar]
- 115.Milne TA, Sinclair DA, Brock HW. The Additional sex combs gene of Drosophila is required for activation and repression of homeotic loci, and interacts specifically with Polycomb and super sex combs. Mol Gen Genet. 1999;261:753–761. doi: 10.1007/s004380050018. [DOI] [PubMed] [Google Scholar]
- 116.Shirai M, et al. The Polycomb-group gene Rae28 sustains Nkx2.5/Csx expression and is essential for cardiac morphogenesis. J Clin Invest. 2002;110:177–184. doi: 10.1172/JCI14839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Katoh-Fukui Y, et al. Cbx2, a polycomb group gene, is required for Sry gene expression in mice. Endocrinology. 2012;153:913–924. doi: 10.1210/en.2011-1055. [DOI] [PubMed] [Google Scholar]
- 118.Irving M, et al. Deletion of the distal long arm of chromosome 10; is there a characteristic phenotype? A report of 15 de novo and familial cases. Am J Med Genet A. 2003;123A:153–163. doi: 10.1002/ajmg.a.20220. [DOI] [PubMed] [Google Scholar]
- 119.Andresen JH, Aftimos S, Doherty E, Love DR, Battin M. 13q33.2 deletion: a rare cause of ambiguous genitalia in a male newborn with growth restriction. Acta Paediatr. 2010;99:784–786. doi: 10.1111/j.1651-2227.2010.01683.x. [DOI] [PubMed] [Google Scholar]
- 120.Dravis C, et al. Bidirectional signaling mediated by ephrin-B2 and EphB2 controls urorectal development. Dev Biol. 2004;271:272–290. doi: 10.1016/j.ydbio.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 121.Chowdhury S, et al. Phenotypic and molecular characterization of 19q12q13.1 deletions: a report of five patients. Am J Med Genet A. 2014;164A:62–69. doi: 10.1002/ajmg.a.36201. [DOI] [PubMed] [Google Scholar]
- 122.Beysen D, et al. Deletions involving long-range conserved nongenic sequences upstream and downstream of FOXL2 as a novel disease-causing mechanism in blepharophimosis syndrome. Am J Hum Genet. 2005;77:205–218. doi: 10.1086/432083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fatica A, Bozzoni I. Long non-coding RNAs: new players in cell differentiation and development. Nat Rev Genet. 2014;15:7–21. doi: 10.1038/nrg3606. [DOI] [PubMed] [Google Scholar]
- 124.Fluck CE, et al. Why boys will be boys: two pathways of fetal testicular androgen biosynthesis are needed for male sexual differentiation. Am J Hum Genet. 2011;89:201–218. doi: 10.1016/j.ajhg.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Katsanis N, et al. Triallelic inheritance in Bardet–Biedl syndrome, a Mendelian recessive disorder. Science. 2001;293:2256–2259. doi: 10.1126/science.1063525. [DOI] [PubMed] [Google Scholar]
- 126.Mykytyn K, et al. Identification of the gene (BBS1) most commonly involved in Bardet–-Biedl syndrome, a complex human obesity syndrome. Nat Genet. 2002;31:435–438. doi: 10.1038/ng935. [DOI] [PubMed] [Google Scholar]
- 127.Jensen MS, et al. Cryptorchidism and hypospadias in a cohort of 934,538 Danish boys: the role of birth weight, gestational age, body dimensions, and fetal growth. Am J Epidemiol. 2012;175:917–925. doi: 10.1093/aje/kwr421. [DOI] [PubMed] [Google Scholar]
- 128.Yinon Y, et al. Hypospadias in males with intrauterine growth restriction due to placental insufficiency: the placental role in the embryogenesis of male external genitalia. Am J Med Genet A. 2010;152A:75–83. doi: 10.1002/ajmg.a.33140. [DOI] [PubMed] [Google Scholar]
- 129.Kalfa N, Philibert P, Baskin LS, Sultan C. Hypospadias: interactions between environment and genetics. Mol Cell Endocrinol. 2011;335:89–95. doi: 10.1016/j.mce.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 130.Meijer L, et al. Influence of prenatal organohalogen levels on infant male sexual development: sex hormone levels, testes volume and penile length. Hum Reprod. 2012;27:867–872. doi: 10.1093/humrep/der426. [DOI] [PubMed] [Google Scholar]
- 131.Fisch H, Hyun G, Hensle TW. Rising hypospadias rates: disproving a myth. J Pediatr Urol. 2010;6:37–39. doi: 10.1016/j.jpurol.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 132.Arboleda VA, et al. Targeted massively parallel sequencing provides comprehensive genetic diagnosis for patients with disorders of sex development. Clin Genet. 2013;83:35–43. doi: 10.1111/j.1399-0004.2012.01879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yang Y, et al. Clinical whole-exome sequencing for the diagnosis of Mendelian disorders. N Engl J Med. 2013;369:1502–1511. doi: 10.1056/NEJMoa1306555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wizemann TM, Pardue M-L, editors. Exploring the Biological Contributions to Human Health: Does Sex Matter? National Academy Press; 2001. [PubMed] [Google Scholar]
- 135.Stein MT, Sandberg DE, Mazur T, Eugster E, Daaboul J. A newborn infant with a disorder of sexual differentiation. J Dev Behav Pediatr. 2003;24:115–119. doi: 10.1097/00004703-200304000-00008. [DOI] [PubMed] [Google Scholar]
- 136.Dessens AB, Slijper FM, Drop SL. Gender dysphoria and gender change in chromosomal females with congenital adrenal hyperplasia. Arch Sex Behav. 2005;34:389–397. doi: 10.1007/s10508-005-4338-5. [DOI] [PubMed] [Google Scholar]
- 137.Mazur T. Gender dysphoria and gender change in androgen insensitivity or micropenis. Arch Sex Behav. 2005;34:411–421. doi: 10.1007/s10508-005-4341-x. [DOI] [PubMed] [Google Scholar]
- 138.Cohen-Kettenis PT. Gender change in 46,XY persons with 5α-reductase-2 deficiency and 17β-hydroxysteroid dehydrogenase-3 deficiency. Arch Sex Behav. 2005;34:399–410. doi: 10.1007/s10508-005-4339-4. [DOI] [PubMed] [Google Scholar]
- 139.Maimoun L, et al. Phenotypical, biological, and molecular heterogeneity of 5α-reductase deficiency: an extensive international experience of 55 patients. J Clin Endocrinol Metab. 2011;96:296–307. doi: 10.1210/jc.2010-1024. [DOI] [PubMed] [Google Scholar]
- 140.Chuang J, et al. Complexities of gender assignment in 17β-hydroxysteroid dehydrogenase type 3 deficiency: is there a role for early orchiectomy? Int J Pediatr Endocrinol. 2013;2013:15. doi: 10.1186/1687-9856-2013-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zucker KJ. Intersexuality and gender identity differentiation. Annu Rev Sex Res. 1999;10:1–69. [PubMed] [Google Scholar]
- 142.Reiner WG, Gearhart JP. Discordant sexual identity in some genetic males with cloacal exstrophy assigned to female sex at birth. N Engl J Med. 2004;350:333–341. doi: 10.1056/NEJMoa022236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Diamond DA, et al. Sex assignment for newborns with ambiguous genitalia and exposure to fetal testosterone: attitudes and practices of pediatric urologists. J Pediatr. 2006;148:445–449. doi: 10.1016/j.jpeds.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 144.Diamond DA, Burns JP, Huang L, Rosoklija I, Retik AB. Gender assignment for newborns with 46XY cloacal exstrophy: a 6-year followup survey of pediatric urologists. J Urol. 2011;186:1642–1648. doi: 10.1016/j.juro.2011.03.101. [DOI] [PubMed] [Google Scholar]
- 145.Meyer-Bahlburg HF. Gender identity outcome in female-raised 46,XY persons with penile agenesis, cloacal exstrophy of the bladder, or penile ablation. Arch Sex Behav. 2005;34:423–438. doi: 10.1007/s10508-005-4342-9. [DOI] [PubMed] [Google Scholar]
- 146.Ruble D, Martin C, Berenbaum S. In: Handbook of child psychology: Social, emotional, and personality. 6. Damon W, Lerner R, Eisenberg N, editors. Vol. 3. New York: Wiley; 2006. pp. 858–932. Ch. 14. [Google Scholar]
- 147.Stout SA, Litvak M, Robbins NM, Sandberg DE. Congenital adrenal hyperplasia: classification of studies employing psychological endpoints. Int J Pediatr Endocrinol. 2010;2010:191520. doi: 10.1155/2010/191520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jordan-Young RM. Hormones, context, and “brain gender”: a review of evidence from congenital adrenal hyperplasia. Soc Sci Med. 2012;74:1738–1744. doi: 10.1016/j.socscimed.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 149.Hines M. In: Multiple origins of sex differences in brain. Pfaff DW, Christen Y, editors. Springer; 2013. pp. 59–69. Series Ed. Christen, Y. Research and Perspectives in Endocrine Interactions. [Google Scholar]
- 150.Mueller SC, et al. Early androgen exposure modulates spatial cognition in congenital adrenal hyperplasia (CAH) Psychoneuroendocrinology. 2008;33:973–980. doi: 10.1016/j.psyneuen.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Frisen L, et al. Gender role behavior, sexuality, and psychosocial adaptation in women with congenital adrenal hyperplasia due to CYP21A2 deficiency. J Clin Endocrinol Metab. 2009;94:3432–3439. doi: 10.1210/jc.2009-0636. [DOI] [PubMed] [Google Scholar]
- 152.Meyer-Bahlburg HF, Dolezal C, Baker SW, New MI. Sexual orientation in women with classical or non-classical congenital adrenal hyperplasia as a function of degree of prenatal androgen excess. Arch Sex Behav. 2008;37:85–99. doi: 10.1007/s10508-007-9265-1. [DOI] [PubMed] [Google Scholar]
- 153.Arnold AP. The organizational-activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Horm Behav. 2009;55:570–578. doi: 10.1016/j.yhbeh.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Arnold AP. Mouse models for evaluating sex chromosome effects that cause sex differences in non-gonadal tissues. J Neuroendocrinol. 2009;21:377–386. doi: 10.1111/j.1365-2826.2009.01831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Arnold AP, Chen X. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front Neuroendocrinol. 2009;30:1–9. doi: 10.1016/j.yfrne.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Smith-Bouvier DL, et al. A role for sex chromosome complement in the female bias in autoimmune disease. J Exp Med. 2008;205:1099–1108. doi: 10.1084/jem.20070850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Quinn JJ, Hitchcott PK, Umeda EA, Arnold AP, Taylor JR. Sex chromosome complement regulates habit formation. Nat Neurosci. 2007;10:1398–1400. doi: 10.1038/nn1994. [DOI] [PubMed] [Google Scholar]
- 158.Chung YP, et al. Prenatal diagnosis of monosomy 10q25 associated with single umbilical artery and sex reversal: report of a case. Prenat Diagn. 1998;18:73–77. doi: 10.1002/(sici)1097-0223(199801)18:1<73::aid-pd215>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 159.Courtens W, Wuyts W, Rooms L, Pera SB, Wauters J. A subterminal deletion of the long arm of chromosome 10: a clinical report and review. Am J Med Genet A. 2006;140:402–409. doi: 10.1002/ajmg.a.31053. [DOI] [PubMed] [Google Scholar]
- 160.Battaglia A, et al. Further delineation of deletion 1p36 syndrome in 60 patients: a recognizable phenotype and common cause of developmental delay and mental retardation. Pediatrics. 2008;121:404–410. doi: 10.1542/peds.2007-0929. [DOI] [PubMed] [Google Scholar]
- 161.Jordan BK, et al. Up-regulation of WNT-4 signaling and dosage-sensitive sex reversal in humans. Am J Hum Genet. 2001;68:1102–1109. doi: 10.1086/320125. [DOI] [PMC free article] [PubMed] [Google Scholar]