Abstract
Background
Neurocognitive deficits in schizophrenia (SZ) are established and the Consortium on the Genetics of Schizophrenia (COGS) investigated such measures as endophenotypes in family-based (COGS-1) and case-control (COGS-2) studies. By requiring family participation, family-based sampling may result in samples that vary demographically and perform better on neurocognitive measures.
Methods
The Penn computerized neurocognitive battery (CNB) evaluates accuracy and speed of performance for several domains and was administered across sites in COGS-1 and COGS-2. Most tests were included in both studies. COGS-1 included 328 patients with SZ and 497 healthy comparison subjects (HCS) and COGS-2 included 1195 patients and 1009 HCS.
Results
Demographically, COGS-1 participants were younger, more educated, with more educated parents and higher estimated IQ compared to COGS-2 participants. After controlling for demographics, the two samples produced very similar performance profiles compared to their respective controls. As expected, performance was better and with smaller effect sizes compared to controls in COGS-1 relative to COGS-2. Better performance was most pronounced for spatial processing while emotion identification had large effect sizes for both accuracy and speed in both samples. Performance was positively correlated with functioning and negatively with negative and positive symptoms in both samples, but correlations were attenuated in COGS-2, especially with positive symptoms.
Conclusions
Patients ascertained through family-based design have more favorable demographics and better performance on some neurocognitive domains. Thus, studies that use case-control ascertainment may tap into populations with more severe forms of illness that are exposed to less favorable factors compared to those ascertained with family-based designs.
Keywords: Neurocognition, schizophrenia, family-based, case-control, ascertainment
1.0 Introduction
Methods of ascertainment are pivotal across biomedical research and are an important consideration in the research design. In genetic studies, the utility and statistical approach of family-based and unrelated case-controls studies has been discussed (e. g. Hiekkalinna et al. 2012). The incorporation of endophenotypes to genetic investigations of schizophrenia (SZ) has grown significantly with neurocognitive measures (Gur et al. 2007; Lee et al. this issue; Nuechterlein et al. this issue; Stone et al. this issue) and neurophysiological measures (Swerdlow et al. 2014; Light et al. this issue, Turetsky et al. this issue) playing key roles. Family-based designs enable testing the endophenotype criteria (Braff et al. 2007; Braff et al. this issue; Gottesman and Gould, 2003) and, when sufficiently powered, allow for the examination of heritability, association with the disease phenotype and co-segregation within families (Glahn et al. 2014; Greenwood et al. 2007, 2011, 2013).
Several meta-analyses have reported that adult relatives of probands with SZ show intermediate deficits in neurocognitive measures including executive functions, such as working memory and attention, verbal fluency and sensori-motor speed (Faraone et al. 2001; Kremen and Hoff, 2004; Sitskoorn et al. 2004; Snitz et al. 2006). Similar deficits have also been observed in younger relatives (Niemi et al. 2003; Seidman et al. 2006; Keshavan et al. 2010; Agnew-Blais and Seidman, 2013). The neurocognitive domains implicated in family-based studies are similar to deficits observed in case-control studies (Gur et al. 2001b). Yet, direct evaluation of these complementary ascertainment strategies applying the same measures has not been conducted. The Penn computerized neurocognitive battery (CNB) used in the Consortium on the Genetics of Schizophrenia (COGS) provides a unique opportunity to evaluate effects of ascertainment methods –family-based (COGS-1) vs. case control (COGS-2) - with the same neurocognitive battery across the participating sites.
The CNB, developed in concert with functional neuroimaging studies (Gur et al. 2010), has been validated in healthy participants and people with SZ (Gur et al. 2001a,b) and is sensitive to the effects of age and sex (Gur et al. 2012; Irani et al. 2012). The battery, which provides measures of performance accuracy and response time, was applied in three independent large-scale family-based genetic studies. The Multiplex Multigenerational Investigation of Schizophrenia (MGI; Gur et al. 2007) reported that probands demonstrated greatest impairment relative to healthy controls, with intermediate performance of family members. Liability for SZ affected the speed-accuracy tradeoff differently for specific neurocognitive domains. Significant heritability estimates were obtained for accuracy of verbal, facial, and spatial memory and spatial and emotion processing. For speed, estimates of heritability were significant for abstraction and mental flexibility, attention, face memory, and spatial and sensorimotor processing. The results of the Project among African- Americans to Explore Risks for Schizophrenia (PAARTNERS) revealed that patients with SZ exhibited less accuracy and speed in most neurocognitive domains than their relatives, who were impaired relative to HCS in most domains. Significant heritabilities were observed for most neurocognitive domains, with the highest for accuracy of abstraction and mental flexibility, verbal memory, face memory, spatial processing, and emotion processing and for speed of attention (Calkins et al. 2010).
In COGS-1 all of the measures applied from the Penn CNB (Abstraction and Mental Flexibility, Face Memory, Spatial Memory, Spatial Processing, Sensorimotor Dexterity, and Emotion Recognition) were significantly heritable with heritability estimates ranging from 24% for Spatial Memory to 55% for Spatial Processing (Greenwood et al. 2007). These heritabilities are in the same range as the heritability of SZ itself in the COGS-1 families (Light et al. 2014). Furthermore, we noted sex differences in familiality effects with male probands’ performance predictive of performance of their unaffected relatives (Calkins et al. 2013). The subsequent application of the CNB in the case-control design of COGS-2 enabled evaluation of the pattern of performance of individuals with SZ, compared to HCS, ascertained in family-based and case-control designs. We noted that in some endophenotypic measures in COGS-1 probands were less impaired than observed in other samples of patients with SZ (Greenwood et al. 2007). The major ascertainment difference between the samples is that patients recruited for COGS-1 required the availability of parents and siblings while COGS-2 permitted participation of patients regardless of family availability (Swerdlow et al. this issue). This difference likely affects multiple demographic characteristics related to age, education, socioeconomic status as well as severity of illness, favoring COGS-1. We hypothesized that while the profile of impairment would be similar, probands in the COGS-1 family-based ascertainment would perform better than those ascertained as cases in COGS-2.
2.0 Materials and Methods
2.1 Participants
Details on the COGS-1 and COGS-2 samples’ ascertainment, inclusion and exclusion criteria and clinical assessment are provided elsewhere in this issue (Braff et al.; Swerdlow et al.). Briefly, COGS-1, a family-based design, and COG-2, a case-control design, included probands 18–65 years old who met DSM-IV criteria for schizophrenia based on established diagnostic procedures. COGS-1 required that both biological parents were available for genotyping, and that at least one full sibling, unaffected with schizophrenia, was available for endophenotyping and genotyping. Probands with one available parent but two or more available siblings, with at leas one unaffected by schizophrenia, were also included, as were probands with no available parents but three or more available siblings (≥1 unaffected by schizophrenia). COGS-2 had the same diagnostic requirements for probands and controls as COGS-1, but the availability of family members was not required. Here we focus on COG- 1 and COGS-2 patients and controls who completed the CNB testing. COGS-1 included 328 patients and 497 controls and COGS-2 included 1195 patients and 1009 controls. Demographic information is presented at the top portion of Table 1. As can be seen, COGS-1 patients did not differ from their controls in age, or parental education, but had lower education and lower reading level with moderate effect sizes. COGS-2 patients were significantly older than their controls as well as less educated with lower parental education and Wide Range Achievement Test (WRAT4, Wilkinson and Robertson, 2006) scores, with effect sizes ranging from moderate to large. COGS-1 controls were younger, attained higher educational level, had higher paternal education and higher WRAT scores compared to COGS-2 controls, but all these effect sizes were small (< 2 SD). COGS-1 patients were younger, had higher educational attainment, higher parental education and higher WRAT compared to COGS-2 patients and these effect sizes were moderate to large. Notably, the variances did not differ between the samples on most measures (Satterthwaite’s correction was used for these p values).
Table 1.
Demographics, raw performance data and effect sizes (Cohen’s d) in COGS-1 and COGS-2.
| COGS-1 SZ | COGS-1 HCS | SZ VS HCS | COGS-2 SZ | COGS-2 HCS | SZ VS HCS | SZ1 vs SZ2 | HCS1 vs HCS2 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | P | ES (d) | N | Mean | SD | N | Mean | SD | P | ES (d) | P | ES | P | ES | |||
| Age | 328 | 34.86 | 11.00 | 497 | 36.20 | 12.67 | 0.0878 | −0.11 | 1195 | 46.24 | 11.30 | 1009 | 38.62 | 13.18 | <0.0001 | 0.63 | <.0001 | −1.01 | 0.0008 | −0.19 | ||
| Education | 328 | 13.62 | 2.17 | 497 | 15.39 | 2.34 | <0.0001 | −0.78 | 1195 | 12.69 | 2.13 | 1009 | 14.97 | 2.22 | <0.0001 | −1.05 | <.0001 | 0.43 | 0.0056 | 0.18 | ||
| Mother education | 323 | 14.41 | 2.96 | 496 | 14.00 | 3.04 | 0.0579 | 0.13 | 1130 | 12.01 | 3.88 | 999 | 13.85 | 3.31 | <0.0001 | −0.51 | <.0001* | 0.65 | 0.1292 | 0.05 | ||
| Father education | 320 | 14.95 | 3.38 | 483 | 14.36 | 3.40 | 0.0161 | 0.16 | 1069 | 11.81 | 4.66 | 979 | 13.77 | 3.91 | <0.0001 | −0.45 | <.0001* | 0.71 | 0.0058* | 0.16 | ||
| DOMAIN | TEST | WRAT | 328 | 102.23 | 11.13 | 497 | 107.16 | 10.61 | <0.0001 | −0.46 | 1195 | 95.65 | 12.80 | 1009 | 105.65 | 10.74 | <0.0001 | −0.84 | <.0001 | 0.53 | 0.005 | 0.14 |
| (CONSTRUCT) | ||||||||||||||||||||||
| Abstraction/Flex | PCET | ACCURACY | 321 | 1.79 | 1.15 | 489 | 2.41 | 0.96 | <0.0001 | −0.66 | 1145 | 1.36 | 0.88 | 991 | 2.09 | 0.92 | <0.0001 | −0.80 | <.0001* | 0.46 | <.0001 | 0.35 |
| (ABF) | SPEED | 321 | 3058.64 | 1887.20 | 489 | 2316.19 | 1251.25 | <0.0001 | 0.62 | 1145 | 3517.44 | 1914.26 | 991 | 2480.85 | 1159.78 | <0.0001 | 0.64 | 0.001 | −0.24 | 0.0004 | −0.14 | |
| LNB | ACCURACY | 297 | 25.20 | 3.80 | 493 | 28.14 | 2.32 | <0.0001 | −0.95 | 1107 | 15.40 | 3.38 | 992 | 18.53 | 1.89 | <0.0001 | −0.77 | |||||
| (WM) | SPEED | 297 | 592.79 | 164.72 | 493 | 537.75 | 129.79 | <0.0001 | 0.43 | 1107 | 625.26 | 176.74 | 992 | 548.77 | 125.52 | <0.0001 | 0.49 | 0.0219 | −0.19 | 0.2624 | −0.09 | |
| Episodic Memory | WORD | ACCURACY | 201 | 32.38 | 4.19 | 309 | 34.80 | 3.49 | <0.0001 | −0.69 | 1173 | 31.98 | 4.23 | 999 | 34.20 | 3.51 | <0.0001 | −0.57 | 0.1712 | 0.09 | 0.0089 | 0.17 |
| (VME) | SPEED | 201 | 1602.15 | 1126.86 | 309 | 1233.33 | 342.65 | <0.0001 | 1.09 | 1173 | 2035.80 | 758.00 | 999 | 1532.50 | 336.00 | <0.0001 | 0.84 | <.0001 | −0.53 | <.0001 | −0.89 | |
| FACE | ACCURACY | 333 | 31.36 | 4.13 | 499 | 34.29 | 3.34 | <0.0001 | −0.87 | 1171 | 30.36 | 3.96 | 999 | 33.31 | 3.40 | <0.0001 | −0.80 | 0.0003 | 0.25 | <.0001 | 0.29 | |
| (FME) | SPEED | 333 | 1928.42 | 955.70 | 498 | 1639.07 | 535.08 | <0.0001 | 0.61 | 1171 | 2195.94 | 802.45 | 999 | 1791.03 | 435.39 | <0.0001 | 0.61 | <.0001 | −0.32 | <.0001* | −0.32 | |
| SHAPE | ACCURACY | 328 | 14.07 | 2.57 | 495 | 15.72 | 2.12 | <0.0001 | −0.73 | 1146 | 13.44 | 2.30 | 993 | 15.25 | 2.31 | <0.0001 | −0.78 | 0.0021 | 0.27 | 0.0013 | 0.21 | |
| (SME) | SPEED | 315 | 1532.40 | 738.90 | 487 | 1372.43 | 389.38 | 0.0004 | 0.41 | 1141 | 1746.36 | 664.24 | 993 | 1465.27 | 393.58 | <0.0001 | 0.51 | <.0001 | −0.31 | <.0001 | −0.24 | |
| Complex cognition | JOLO | ACCURACY | 308 | 21.81 | 5.96 | 489 | 22.46 | 5.31 | 0.1209 | −0.12 | 1160 | 18.98 | 6.44 | 1000 | 21.93 | 5.26 | <0.0001 | −0.50 | <.0001 | 0.45 | 0.094 | 0.10 |
| (SPA) | SPEED | 307 | 3364.79 | 1511.92 | 488 | 2940.26 | 1119.65 | <0.0001 | 0.36 | 1157 | 4516.18 | 2327.27 | 1000 | 3168.83 | 1198.42 | <0.0001 | 0.71 | <.0001* | −0.53 | 0.0006 | −0.19 | |
| Social cognition | ER40 | ACCURACY | 328 | 30.68 | 4.70 | 497 | 33.69 | 2.73 | <0.0001 | −1.02 | 1139 | 30.25 | 4.57 | 993 | 33.35 | 3.05 | <0.0001 | −0.79 | 0.4343 | 0.09 | 0.0351 | 0.11 |
| (EMO) | SPEED | 328 | 2803.23 | 1159.52 | 497 | 2037.94 | 584.93 | <0.0001 | 1.35 | 1139 | 3087.86 | 1059.63 | 993 | 2183.37 | 560.07 | <0.0001 | 1.05 | 0.0036 | −0.26 | <.0001 | −0.26 | |
| Sensorimotor | MPRACT | SPEED | 327 | 955.28 | 468.18 | 497 | 747.08 | 258.07 | <0.0001 | 0.97 | 1195 | 1190.46 | 478.06 | 1009 | 805.18 | 190.92 | <0.0001 | 1.03 | <.0001 | −0.49 | <.0001 | −0.27 |
| TAP (SM) | SPEED | 1176 | 94.09 | 16.73 | 1002 | 106.84 | 13.27 | <0.0001 | −0.84 | |||||||||||||
| Attention | CPT | ACCURACY | 1158 | 108.86 | 12.62 | 999 | 115.10 | 8.97 | <0.0001 | −0.56 | ||||||||||||
| (ATT) | SPEED | 1157 | 533.19 | 69.81 | 999 | 483.92 | 49.57 | <0.0001 | 0.80 | |||||||||||||
Variances are significantly different and p values are Satterthwaite corrected.
2.2 The Computerized Neurocognitive Battery (CNB)
The Penn CNB (Gur et al. 2001a,b) was administered in the COGS along with other candidate endophenotypes. It was abbreviated to reduce redundancy with other core endophenotypes. COGS-1 and COGS-2 CNB differed in three ways. First, for COGS-1 Degraded Stimulus CPT and CPT – Identical Pairs were use to cover the attention domain (Nuechterlein et al. this issue), while in COGS-2 the Penn CPT data was also added to allow the full CNB to be represented. Second, for measuring working memory, different forms of the letter n-back test were used in COGS-1 and COGS-2. Third, many participants from COGS-1 did not receive the delayed recognition tests because the CNB was administered last and time limitations and fatigue attenuated the test sessions.
The CNB was administered on Macintosh computers (Apple Inc., Cupertino, California) in a fixed order and included brief standardized rest periods, for a total administration time of about 60 minutes. The following neurocognitive domains were assessed (Gur et al. 2010, 2012): 1) Executive Functions: abstraction and mental flexibility (Penn Conditional Exclusion Test, PCET); working memory (WM, letter n-back, 1-back and 2-back conditions). 2) Episodic Memory: word memory (Penn Word Memory Test); face memory (Penn Face Memory Test); spatial memory (Visual Object Learning Test). 3) Complex Cognition: spatial processing (Computerized Judgment of Line Orientation, JOLO); 4) Social Cognition: emotion processing (Penn Emotion Recognition Test). 5. Sensorimotor Speed: motor praxis and finger tapping test. For all but the last domain, two summary functions were calculated: accuracy of responses and speed, the median response time for correct answers.
2.3 Statistical analysis
The performance scores were transformed to their standard equivalents (z-scores) within each sample (COGS-1, COGS-2), based on the controls in that sample. These z-scores were the dependent measures in a Mixed Model analysis (SAS PROC MIXED), with Sample and Diagnosis as grouping factors and neurocognitive Domain as a within-group factor and age and parental education (average of mother’s and father’s) as covariates. We did not use patient’s education as a covariate to avoid committing the “matching fallacy” because schizophrenia itself interferes with educational attainment and covarying or matching for it will falsely remove relevant variance (Meehl, 1970). Instead, we covaried parental education as recommended (Resnick, 1992) and widely practiced. Similarly, we did not covary for the WRAT score, as it is a measure of cognitive abilities that is highly correlated with performance on the neurocognitive battery. Again, covarying for it will remove relevant variance. The model was applied separately to the accuracy (7 domains) and speed (8 domains) scores. Mixed Model analysis was preferred to a MANOVA mainly because it can accommodate missing values while MANOVA would eliminate all subjects with missing data on any test. This would have affected mainly the first sample, where the word memory test was introduced in the middle of the COGS-1 study. Initially, the model included sex as a grouping factor, but since the main effects have been well established (e.g. better performance of males on spatial tests and of females on memory and emotion processing) and there were no interactions by cohort or diagnostic group, it was dropped from subsequent analyses. To examine the association between neurocognitive measures and clinical status, we correlated performance on the neurocognitive domains with clinical ratings on the Scale for the Assessment of Negative Symptoms (SANS, Andreasen, 1984a), the Scale of Assessment of Positive Symptoms (SAPS, Andreasen, 1984b) and the Global Assessment of Functioning (GAF, Hall and Parks, 1995).
3.0 Results
3.1 Performance Comparisons on the Computerized Neurocognitive Battery
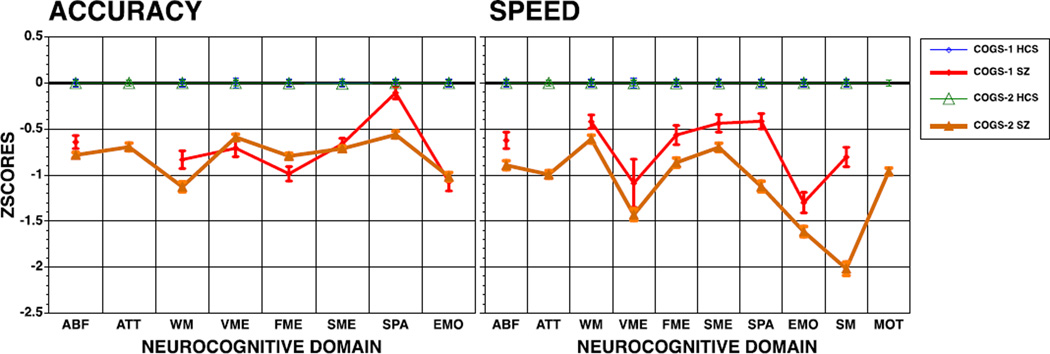
The means and standard deviations, as well as p values for student t-tests and effect sizes comparing patients and controls within each sample and between samples are presented in the bottom portion of Table 1. The z- scores of patients compared to their respective control groups are illustrated in Figure 1. As can be seen in Table 1, both COGS-1 patients and COGS-2 patients are impaired relative to their respective controls, although the effect sizes range from small to moderate in COGS-1, with six large effect sizes, while in COGS-2 all effects sizes are at least moderate and seven of them large. Notably, COGS-1 controls outperformed COGS-2 controls on all domains, but these effect sizes are generally small with only one large effect (speed of word memory). COGS-1 patients outperformed COGS-2 patients on most domains with effect sizes ranging from small to moderate.
Figure 1.
Neurocognitive performance of COG-1 and COGS-2 participants: Mean (±95% Confidence Interval) accuracy (left panel) and speed (right panel) of patients are presented in z-scores compared to the respective control groups (z-scores for response times are inverted for speed so that higher scores always reflects better performance).
SZ = Schizophrenia, HCS = Healthy Comparison Subjects; ABF = Abstraction and Mental Flexibility, ATT = Attention, WM = Working Memory, VME = Word Memory, FME = Face Memory, SME = Spatial Memory, SPA = Spatial Processing, EMO = Emotion Identification, SM = Sensori-Motor, MOT = Motor Speed.
The Mixed Model analysis on the accuracy scores showed main effects for sample, F(1, 17000) = 30.32, p <.0001, Diagnostic group, F(1, 17000) = 414.85, p <.0001, and Domain, F(6, 17000) = 18.46, p <.0001. Also significant were the 2-way interactions of Sample×Diagnosis, F(1, 17000) = 10.17, p = 0.0014, Sample×Domain, F(6, 17000) = 3.61, p = 0.0014, and Diagnosis×Domain, F(6, 17000) = 18.52, p <.0001. The 3-way interaction of Sample×Diagnosis×Domain was also significant, F(6, 17000) = 3.18, p = 0.004. The Mixed Model analysis on the speed scores showed main effects of sample, F(1, 20000) = 10.29, p = 0.0013, Diagnostic group, F(1, 20000) = 197.24, p <.0001 and Domain, F(7, 20000) = 65.89, p <.0001. Also significant were the 2-way interactions of Sample×Domain, F(7,20000) = 16.34, p <.0001, and Diagnosis×Domain, F(7,20000) = 66.02, p <.0001, but not Sample×Diagnosis, F(1, 20000) = 0.02, p = 0.8897. The 3-way interaction of Sample×Diagnosis×Domain was also significant, F(7,20000) = 16.34, p <.0001.
Decomposing the three-way interactions for accuracy indicated that COGS-1 patients were less impaired than COGS-2 counterparts, compared to their respective controls, in abstraction and mental flexibility (p < 0. 0001) and in spatial processing (p < 0. 0001). For speed, the three-way interaction reflected differentially greater impairment in COGS-2 patients for verbal memory (p < 0. 0001), spatial processing (p < 0. 0001) and sensorimotor speed (p < 0. 0001).
3. 2 Correlations of performance with clinical status
COGS-1 patients had less severe negative and positive symptoms and better functioning (Table 2). Significant correlations in the expected directions, namely negative for symptom severity and positive for GAF, were obtained in both cohorts (Table 3). They were comparable in the two cohorts for GAF, ranging generally from .3 to .47. The clinical severity ratings for COGS-1 correlated significantly with neurocognitive performance, with greater severity associated with poorer performance. These correlations were of similar magnitude for SANS and SAPS, hovering around −.3 and as high as −.5 for emotion processing speed. For COGS-2, the correlations between symptom severity and neurocognitive performance were considerably attenuated, hovering around −.1 and significant for SANS but only few for SAPS.
Table 2.
Clinical measures of patients in COGS-1 and COGS-2
| COGS% | COGS% | COGS%1(VS(2 | ||||||
|---|---|---|---|---|---|---|---|---|
| N | MEAN | SD | N | MEAN | SD | P | ES | |
| SANS | 461 | 4.09 | 5.68 | 1407 | 11.03 | 5.66 | <.0001 | ,1.22 |
| SAPS | 461 | 2.40 | 3.87 | 1405 | 6.92 | 4.02 | <.0001 | ,1.14 |
| GAF | 477 | 69.73 | 22.09 | 2460 | 62.08 | 22.72 | <.0001 | 0.34 |
Table 3.
Correlations between clinical measures and neurocognitive performance in COGS-1 and COGS-2.
| ACCURACY | SPEED | |||||||||||||||||
| COGS1 | ABF | ATT | WM | VME | FME | SME | SPA | EMO | ABF | ATT | WM | VME | FME | SME | SPA | EMO | SM | MOT |
| SANS | !0.303 | NA | !0.366 | !0.287 | !0.403 | !0.405 | !0.126 | !0.361 | !0.294 | NA | !0.240 | !0.365 | !0.189 | !0.219 | !0.268 | !0.550 | !0.470 | NA |
| SAPS | !0.265 | NA | !0.351 | !0.251 | !0.321 | !0.376 | !0.117 | !0.335 | !0.328 | NA | !0.185 | !0.257 | !0.173 | !0.227 | !0.220 | !0.514 | !0.493 | NA |
| GAF | 0.349 | NA | 0.356 | 0.313 | 0.403 | 0.375 | 0.083 | 0.371 | 0.291 | NA | 0.247 | 0.348 | 0.185 | 0.189 | 0.207 | 0.515 | 0.402 | NA |
| COGS2 | ||||||||||||||||||
| SANS | !0.107 | !0.148 | !0.113 | !0.111 | !0.083 | !0.105 | !0.068 | !0.128 | !0.073 | !0.118 | !0.120 | !0.129 | !0.108 | !0.133 | !0.090 | !0.140 | !0.153 | !0.145 |
| SAPS | !0.117 | !0.077 | !0.096 | !0.086 | !0.070 | !0.098 | !0.049 | !0.041 | !0.034 | 0.003 | !0.040 | !0.047 | !0.013 | !0.056 | !0.050 | !0.056 | !0.059 | !0.019 |
| GAF | 0.384 | 0.275 | 0.368 | 0.286 | 0.367 | 0.373 | 0.231 | 0.370 | 0.317 | 0.364 | 0.256 | 0.391 | 0.308 | 0.283 | 0.343 | 0.473 | 0.464 | 0.380 |
ABF = Abstraction and Mental Flexibility, ATT = Attention, WM = Working Memory, VME = Word Memory, FME = Face Memory, SME = Spatial Memory, SPA = Spatial Processing, EMO = Emotion Identification, SM = Sensori-Motor, MOT = Motor Speed.
4.0 Discussion
The Consortium on the Genetics of Schizophrenia (COGS) allowed for the comparison of neurocognitive performance deficits between individuals with schizophrenia ascertained through a family-based sampling (COGS-1) and those ascertained through case-control sampling (COGS-2) with their differing ascertainment strategies as discussed above and in this issue (cf. Swerdlow et al. this issue). The results indicated very similar neurocognitive deficit profiles, for COGS-1 and COGS-2 schizophrenia patients, strongly supporting the sensitivity of the neurocognitive battery to deficits characteristic of schizophrenia. Against this similarity of profiles, the results generally supported the hypothesis that patients ascertained through family sampling are less impaired than those ascertained as cases in a case-control design. The difference between the two groups of patients was robust even when controlled for age and parental education. The effect sizes comparing patients to controls were in the small to moderate range in the COGS-1 sample and in the moderate to large range in the COGS-2 sample reflecting the greater deficits in COGS-2 patients. Thus, studies using a case-control design that does not require availability of family and therefore is likely to include older and more chronic patients should expect patients with greater neurocognitive impairment than studies where family is engaged.
The significant diagnosis by domain by sample interactions indicate that the case-control ascertained sample did not perform more poorly to the same extent in all domains. The biggest difference between the samples was in spatial processing, both for accuracy and for speed, and for sensorimotor speed. Spatial processing was the only measure of complex cognition included in this iteration of the Penn CNB (Gur et al. 2010; Moore et al. 2014). It represents temporo-parietal functioning and it loads heavily on general intellectual abilities. Lower performance on this test is consistent with lower IQ estimates based on the WRAT scores. However, the COGS-2 sample was also differentially impaired in working memory suggesting that executive functioning may likewise play a role in their neurocognitive dysfunction.
Two tests were added to COGS-2 that were not included in COGS-1. The CPT in COGS-2 produced moderate effect size (−.56 SDs) for accuracy and a large effect size (.80 SD) for speed (see also Nuechterlein et al. this Issue). The other test that was not used in COGS-1 was finger tapping, which assessed motor-speed. This test produced a large effect size of −.84 SD. The sensitivity of finger tapping to deficits in schizophrenia has been reported (e.g., Da Silva et al. 2012). The present study suggests that both attention and motor speed are sensitive measures that should be included in neurocognitive batteries assessing deficits in schizophrenia.
As with neurocognitive performance, symptom severity was greater and functioning was poorer in patients from COGS-2 compared to COGS-1. Neurocognitive measures were significantly correlated with these clinical parameters in both samples. It is notable that the correlations were nearly identical in both samples for GAF, but differed for severity of symptoms. The lower correlations for SAPS than for SANS are consistent with previous studies, most using the case-control design (Nuechterlein et al. 2011). Our finding that they are of similar magnitude for SAPS and SANS in COGS-1 suggests that positive symptoms may have more adverse effect on functioning of younger patients who are still engaged with family. The higher correlations with symptoms in COGS-1 are not explained by greater variance of symptom severity or performance since these did not generally differ between the samples. Thus, studies that ascertain patients in a case-control design are more likely to find lower correlations between symptom severity and neurocognitive performance, with a magnitude especially low for positive symptoms. When such designs are used in treatment trials, they are therefore likely to underestimate the potential impact of improved neurocognitive performance on symptoms and functioning.
The study has several limitations and caveats. Most importantly, two tests were missing from one of the samples and therefore no comparable data were available. Secondly, the samples differed on several demographic variables including age and education. While these factors were controlled for a posteriori in the analyses, it is possible they relate to other factors associated with illness that may have affected the results. Medications can be related to cognitive performance, especially motor speed and these effects have not been evaluated in the present study.
These caveats notwithstanding, the present study offers robust support, with definitively large samples of carefully diagnosed and multi-site quality assured testing, to the presence in schizophrenia of a specific pattern of cognitive deficits that is related to dysfunctional brain systems (Roalf et al. 2014). The results also support the importance of ascertainment strategy (e.g. family-based vs. case-control ascertainment) and the hypothesis that family-based ascertainment will produce samples that are less neurocognitively impaired. This finding of large CNB case-control detected deficits offers a solid neurobiologically informed platform for genomic studies to follow in the large, well-characterized COGS-2 schizophrenia sample (Braff et al. 2014).
Acknowledgments
We thank the research staff who administered the CNB, Allison Mott CNB Implementation Manager, University of Pennsylvania and the patients, families and healthy volunteers who participated in the study.
Role of funding source
This work was supported by collaborative R01 grants from the National Institute of Mental Health. COGS-1 and COGS-2: MH065571 UCSD, MH065707 UCLA, MH065554 MSSM, MH065578 Penn, MH065558 Washington; COGS1 Only: MH065588 Colorado, MH065562 Harvard; Seidman and Stone are on a subcontract for COGS-2; COGS-2 Only: MH86135 Stanford.
Dr. Green has been a consultant to AbbVie, Biogen, DSP, EnVivo/Forum and Roche, and he is on the scientific advisory board of Mnemosyne. He has received research funds from Amgen. Dr. Lazzeroni is an inventor on a patent application filed by Stanford University on genetic polymorphisms associated with depression. Dr. Light has served as a consultant for Astellas, Forum, and Neuroverse. Dr. Nuechterlein has received unrelated research support from Janssen Scientific Affairs, Genentech, and Brain Plasticity, Inc., and has consulted to Genentech, Otsuka, Janssen, and Brain Plasticity, Inc. Dr. Swerdlow has been a consultant for Genco Sciences, Ltd.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
All authors significantly contributed to study design, data collection and reviewed and approved this manuscript.
Conflict of interest
All other authors declare that they have no conflict of interest.
References
- Agnew-Blais J, Siedman LJ. Neurocognition in youth adults under age 30 at familial risk for schizophrenia: A quantitative and qualitative review. Cogn. Neuropsychiatry. 2013;18(1–2):44–82. doi: 10.1080/13546805.2012.676309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS) Iowa City, Iowa: University of Iowa; 1984a. [Google Scholar]
- Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS) Iowa City, Iowa: University of Iowa; 1984b. [Google Scholar]
- Braff DL, Freedman R, Schork NJ, Gottesman II. Deconstructing schizophrenia: an overview of the use of endophenotypes in order to understand a complex disorder. Schizophr. Bull. 2007;33:21–32. doi: 10.1093/schbul/sbl049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braff DL. The importance of endophenotypes in schizophrenia research: Past, present and future. Schizophr. Res. doi: 10.1016/j.schres.2015.02.007. this issue. [DOI] [PubMed] [Google Scholar]
- Calkins ME, Tepper P, Gur RC, Ragland JD, Klei L, Wiener HW, Richard J, Savage RM, Allen TB, O'Jile J, Devlin B, Kwentus J, Aliyu MH, Bradford LD, Edwards N, Lyons PD, Nimgaonkar VL, Santos AB, Go RC, Gur RE. Project among African-Americans to explore risks for schizophrenia (PAARTNERS): evidence for impairment and heritability of neurocognitive functioning in families of schizophrenia patients. Am. J. Psychiatry. 2010;167(4):459–472. doi: 10.1176/appi.ajp.2009.08091351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins ME, Ray A, Gur RC, Freedman R, Green MF, Greenwood TA, Light GA, Nuechterlein KH, Olincy A, Radant AD, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Sugar C, Swerdlow NR, Tsuang DW, Tsuang MT, Turetsky BI, Braff DL, Lazzeroni LC, Gur RE. Sex differences in familiality effects on neurocognitive performance in schizophrenia. Biol Psychiatry. 2013;73(10):976–984. doi: 10.1016/j.biopsych.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva FN, Irani F, Richard J, Brensinger CM, Bilker WB, Gur RE, Gur RC. More than just tapping: index finger-tapping measures procedural learning in schizophrenia. Schizophr Res. 2012;137(1–3):234–240. doi: 10.1016/j.schres.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Green A, Seidman LJ, Tsuang MT. “Schizotaxia”: clinical implications and new directions for research. Schizophr Bull. 2001;27(1):1–18. doi: 10.1093/oxfordjournals.schbul.a006849. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Knowles EE, McKay DR, Sprooten E, Raventós H, Blangero J, Gottesman II, Almasy L. Arguments for the sake of endophenotypes: examining common misconceptions about the use of endophenotypes in psychiatric genetics. Am J Med Genet B Neuropsychiatr Genet. 2014;165B(2):122–130. doi: 10.1002/ajmg.b.32221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am. J. Psychiatry. 2003;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Greenwood TA, Braff DL, Light GA, Cadenhead KS, Calkins ME, Dobie DJ, Freedman R, Green MF, Gur RE, Gur RC, Mintz J, Nuechterlein KH, Olincy A, Radant AD, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Swerdlow NR, Tsuang DW, Tsuang MT, Turetsky BI, Schork NJ. Initial heritability analyses of endophenotypic measures for schizophrenia: the consortium on the genetics of schizophrenia. Arch. Gen. Psychiatry. 2007;64(11):1242–1250. doi: 10.1001/archpsyc.64.11.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood TA, Lazzeroni LC, Murray SS, Cadenhead KS, Calkins ME, Dobie DJ, Green MF, Gur RE, Gur RC, Hardiman G, Kelsoe JR, Leonard S, Light GA, Neuchterlein KH, Olincy A, Radant AD, Schork NJ, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Swerdlow NR, Tsuang DW, Turetsky BI, Freedman R, Braff DL. Analysis of 94 candidate genes and 12 endophenotypes for schizophrenia from the Consortium on the Genetics of Schizophrenia. Am. J. Psychiatry. 2011;168(9):930–946. doi: 10.1176/appi.ajp.2011.10050723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood TA, Swerdlow NR, Gur RE, Cadenhead KS, Calkins ME, Dobie DJ, Freedman R, Green MF, Gur RC, Lazzeroni LC, Nuechterlein KH, Olincy A, Radant AD, Ray A, Schork NJ, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Sugar CA, Tsuang DW, Tsuang MT, Turetsky BI, Light GA, Braff DL. Genome-wide Linkage Analyses of 12 Endophenotypes for Schizophrenia from the Consortium on the Genetics of Schizophrenia. Am. J. Psychiatry. 2013;(170):521–532. doi: 10.1176/appi.ajp.2012.12020186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Richard J, Hughett P, Calkins ME, Macy L, Bilker WB, Brensinger C, Gur RE. A cognitive neuroscience-based computerized battery for efficient measurement of individual differences: standardization and initial construct validation. J Neurosci Methods. 2010;187(2):254–262. doi: 10.1016/j.jneumeth.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Ragland J, Moberg P, Turner T, Bilker WB, Kohler C, Siegel SJ, Gur RE. Computerized neurocognitive scanning: I. Methodology and validation in healthy people. Neuropsychopharmacol. 2001a;25(5):766–776. doi: 10.1016/S0893-133X(01)00278-0. [DOI] [PubMed] [Google Scholar]
- Gur RC, Ragland JD, Moberg PJ, Bilker WB, Kohler C, Siegel SJ, Gur RE. Computerized neurocognitive scanning: II. The profile of schizophrenia. Neuropsychopharmacol. 2001b;25(5):777–788. doi: 10.1016/S0893-133X(01)00279-2. [DOI] [PubMed] [Google Scholar]
- Gur RC, Richard J, Calkins ME, Chiavacci R, Hansen JA, Bilker WB, Loughead J, Connolly JJ, Qiu H, Mentch FD, Abou-Sleiman PM, Hakonarson H, Gur RE. Age group and sex differences in performance on a computerized neurocognitive battery in children age 8–21. Neuropsychol. 2012;26(2):251–265. doi: 10.1037/a0026712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RE, Nimgaonkar VL, Almasy L, Calkins ME, Ragland JD, Pogue-Geile MF, Kanes S, Blangero J, Gur RC. Neurocognitive endophenotypes in a multiplex multigenerational family study of schizophrenia. Am. J. Psychiatry. 2007;164(5):813–819. doi: 10.1176/ajp.2007.164.5.813. [DOI] [PubMed] [Google Scholar]
- Gur RE, Calkins ME, Gur RC, Horan WP, Nuechterlein KH, Seidman LJ, Stone WS. The Consortium on the Genetics of Schizophrenia: neurocognitive endophenotypes. Schizophr. Bull. 2007;33(1):49–68. doi: 10.1093/schbul/sbl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall RC, Parks J. The modified global assessment of functioning scale: addendum. Psychosomatics. 1995;36(4):416–417. doi: 10.1016/S0033-3182(95)71656-5. [DOI] [PubMed] [Google Scholar]
- Hiekkalinna T, Göring HH, Lambert B, Weiss KM, Norrgrann P, Schäffer AA, Terwilligeret JD. On the statistical properties of family-based association tests in datasets containing both pedigrees and unrelated case–control samples. Eur. J. Hum. Genet. 2012;20:217–223. doi: 10.1038/ejhg.2011.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irani F, Brensinger CM, Richard J, Calkins ME, Moberg PJ, Bilker W, Gur RE, Gur RC. Computerized neurocognitive test performance in schizophrenia: a lifespan analysis. Am J Geriatr Psychiatry. 2012;20(1):41–52. doi: 10.1097/JGP.0b013e3182051a7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavan M, Kulkarni S, Bhojraj T, Francis A, Diwadkar V, Montrose D, Seidman LJ, Sweeney J. Premorbid cognitive deficits in young relatives of schizophrenia patients. Front. Hum. Neurosci. 2010;62(3):1–14. doi: 10.3389/neuro.09.062.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen W, Hoff A. Neurocognitive deficits in biological relatives of individuals with schizophrenia. In: Stone W, Faraone S, Tsuang MM, editors. Early Clinical Intervention and Prevention in Schizophrenia. Totowa, NJ: Humana Press; 2004. pp. 133–154. [Google Scholar]
- Lee J, Green MF, Calkins ME, Greenwood TA, Gur RE, Gur RC, Lazzeroni LC, Light GA, Nuechterlein KH, Radant AD, Seidman LJ, Siever LJ, Silverman JM, Sprock J, Stone WS, Sugar CA, Swerdlow NR, Tsuang DW, Tsuang MT, Turetsky BI, Braff DL. Verbal working memory in schizophrenia: The moderating role of smoking status and antipsychotic medications. Schizophr. Res. doi: 10.1016/j.schres.2014.08.014. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light GA, Greenwood TA, Swerdlow NR, Calkins ME, Freedman R, Green MF, Gur RE, Gur RC, Lazzeroni LC, Nuechterlein KH, Olincy A, Radant AD, Seidman LJ, Siever LJ, Silverman JM, Sprock J, Stone WS, Sugar CA, Tsuang DW, Tsuang MT, Turetsky BI, Braff DL. Comparison of the heritability of schizophrenia and endophenotypes in the COGS-1 family study. Schizophr Bull. 2014 doi: 10.1093/schbul/sbu064. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light GA, Swerdlow NR, Thomas ML, Calkins ME, Green MF, Greenwood TA, Gur RE, Gur RC, Lazzeroni LC, Nuechterlein KH, Pela M, Radant AD, Seidman LJ, Sharp RF, Siever LJ, Silverman JM, Sprock J, Stone WS, Sugar CA, Tsuang DW, Tsuang MT, Braff DL, Turetsky BI. Validation of mismatch negativity and P3a for use in multi-site studies of schizophrenia: Characterization of demographic, clinical, cognitive, and functional correlates in the COGS-2. Schizophr. Res. doi: 10.1016/j.schres.2014.09.042. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehl PE. Nuisance variables and the ex post facto design. In: Radner M, Winokur S, editors. Minnesota Studies in the Philosophy of Science. Minneapolis: University of Minnesota Press; 1970. pp. 373–402. [Google Scholar]
- Moore TM, Reise ST, Gur RE, Hakonarson H, Gur RC. Psychometric properties of the Penn Computerized Neurocognitive Battery. Neuropsychol. 2014 doi: 10.1037/neu0000093. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemi L, Suvisaari J, Tuulio-Henriksson A, Lonnqvist J. Childhood developmental abnormalities in schizophrenia: evidence from high-risk studies. Schizophr. Res. 2003;60(2–3):239–258. doi: 10.1016/s0920-9964(02)00234-7. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, Calkins ME, Greenwood TA, Gur RE, Gur RC, Lazzeroni LC, Light GA, Radant AD, Seidman LJ, Siever LJ, Silverman JM, Sprock J, Stone WS, Sugar CA, Swerdlow NR, Tsuang DW, Tsuang MT, Turetsky BI, Braff DL. Attention/vigilance in schizophrenia: Performance results from a large multi-site study of the Consortium on the Genetics of Schizophrenia (COGS) Schizophr. Res. doi: 10.1016/j.schres.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuechterlein KH, Subotnik KL, Green MF, Ventura J, Asarnow RF, Gitlin MJ, Yee CM, Gretchen-Doorly D, Mintz J. Neurocognitive predictors of work outcome in recent-onset schizophrenia. Schizophr. Bull. 2011;37(Suppl 2):S33–S40. doi: 10.1093/schbul/sbr084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM. Matching for education in studies of schizophrenia. Arch Gen Psychiatry. 1992;49(3):246. doi: 10.1001/archpsyc.1992.01820030078011. [DOI] [PubMed] [Google Scholar]
- Roalf DR, Ruparel K, Gur RE, Bilker W, Gerraty R, Elliott MA, Gallagher RS, Almasy L, Pogue-Geile MF, Prasad K, Wood J, Nimgaonkar VL, Gur RC. Neuroimaging predictors of cognitive performance across a standardized neurocognitive battery. Neuropsycholo. 2014;28(2):161–176. doi: 10.1037/neu0000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman LJ, Giuliano AJ, Smith CW, Stone WS, Glatt SJ, Meyer E, Faraone SV, Tsuang MT, Cornblatt B. Neuropsychological functioning in adolescents and young adults at genetic risk for schizophrenia and affective psychoses: Results from the Harvard and Hillside Adolescent High Risk Studies. Schizophr. Bull. 2006;32(3):507–524. doi: 10.1093/schbul/sbj078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitskoorn MM, Aleman A, Ebisch SJ, Appels MC, Kahn RS. Cognitive deficits in relatives of patients with schizophrenia: a meta-analysis. Schizophr. Res. 2004;71(2–3):285–295. doi: 10.1016/j.schres.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Snitz BE, Macdonald AW, 3rd, Carter CS. Cognitive deficits in unaffected firstdegree relatives of schizophrenia patients: a meta-analytic review of putative endophenotypes. Schizophr. Bull. 2006;32(1):179–194. doi: 10.1093/schbul/sbi048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone WS, Braff DL, Calkins ME, Green MF, Greenwood TA, Gur RE, Gur RC, Lazzeroni LC, Light GA, Nuechterlein KH, Radant AD, Siever LJ, Silverman JM, Sprock J, Sugar CA, Swerdlow NR, Tsuang DW, Tsuang MT, Turetsky BI, Seidman LJ. CVLT-II performance in schizophrenia as a function of ascertainment strategy: Comparing the first and second phases of the Consortium on the Genetics of Schizophrenia (COGS) Schizophr. Res. doi: 10.1016/j.schres.2014.10.029. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Light GA, Sprock J, Calkins ME, Green MF, Greenwood TA, Gur RE, Gur RC, Lazzeroni LC, Nuechterlein KH, Radant AD, Ray A, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Sugar CA, Tsuang DW, Tsuang MT, Turestsky BI, Braff DL. Deficient prepulse inhibition in schizophrenia detected by the multi-site Consortium on the Genetics in Schizophrenia. Schizophr. Res. 2014;152:503–512. doi: 10.1016/j.schres.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Gur RE, Braff DL. Consortium on the Genetics of Schizophrenia (COGS) assessment of endophenotypes for schizophrenia: An introduction to this special issue of Schizophrenia Research. Schiz. Res. doi: 10.1016/j.schres.2014.09.047. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turetsky BI, Dress EM, Braff DL, Calkins ME, Green MF, Greenwood TA, Gur RE, Gur RC, Lazzeroni LC, Nuechterlein KH, Radant AD, Seidman LJ, Sieve LJ, Silverman JM, Sprock J, Stone WS, Sugar CA, Swerdlow NR, Tsuang DW, Ming T, Tsuang MT, Light GA. Schiz. Res. doi: 10.1016/j.schres.2014.09.024. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson GS, Robertson GJ. Psychological Assessment Resources. In: Lutz FL, editor. Wide Range Achievement. WRAT4 Introductory Kit; 2006. Test--Fourth Edition. [Google Scholar]