Abstract
Objectives
To identify and characterize an association between persistent asthma and cardiovascular disease (CVD) risk in the Multi-Ethnic Study of Atherosclerosis (MESA).
Approach and Results
MESA is a longitudinal prospective study of an ethnically diverse cohort of individuals free of known CVD at its inception. Presence and severity of asthma were assessed in the MESA at Exam 1. Persistent asthma was defined as asthmatics using controller medications (inhaled corticosteroids, leukotriene inhibitors, oral corticosteroids) and intermittent asthma as asthmatics not using controller medications. Participants were followed for a mean (standard deviation) 9.1 (2.8) years for development of incident CVD (coronary death, myocardial infarction, angina, stroke, and CVD death). Multivariable Cox regression models were used to assess associations of asthma and CVD.
The 6,792 participants were 62.2 (standard deviation 10.2) years old: 47% male (28% African-American, 22% Hispanic, 12% Chinese). Persistent asthmatics (N=156), compared to intermittent (N=511) and non-asthmatics (N=6125), respectively had higher C-reactive protein (1.2 [1.2] vs 0.9 [1.2] vs 0.6 [1.2] mg/L) and fibrinogen (379 [88] vs 356 [80] vs 345 [73] mg/dL) levels. Persistent asthmatics had the lowest unadjusted CVD-free survival rate of 84.1%, 95% confidence interval (78.9–90.3%) compared with intermittent asthmatics 91.1% (88.5–93.8%) and non-asthmatics 90.2% (89.4–91%). Persistent asthmatics had greater risk of CVD events than non-asthmatics (HR 1.6 [95% 1.01–2.5, p=0.040]), even after adjustment for age, sex, race, CVD risk factors, and anti-hypertensive and lipid medication use.
Conclusions
In this large multi-ethnic cohort, persistent asthmatics had a higher CVD event rate than non-asthmatics.
Keywords: Atherosclerosis, Asthma, Epidemiology, Risk Factors
Introduction
Asthma is an inflammatory disorder that afflicts over 25 million individuals in the United States.1 The increasing prevalence of asthma over the previous decade poses a significant public health burden.1 Current pharmacotherapeutic management of asthma targets the underlying inflammatory mechanism of the disease. Cardiovascular disease (CVD) is the leading cause of death among adults in the United States.2 Similar to asthma, inflammation mediates the initiation and progression of atherosclerosis and is intricately involved in plaque rupture and acute CVD events.3 Individuals with other chronic inflammatory diseases such as human immunodeficiency virus infection and rheumatoid arthritis are at increased CVD risk, as are individuals with higher levels of subclinical systemic inflammation.3–8
Animal models suggest that increased leukotriene production may cause an overlap between the inflammatory pathogenesis of asthma and CVD. Leukotrienes are potent pro-inflammatory substances found in excess in asthmatic bronchioles; emerging data indicate that leukotrienes may also be active in atherosclerotic plaques.9, 10 Despite the shared inflammatory pathophysiology of asthma and CVD, few studies have investigated a potential association between asthma and CVD.11–18 To our knowledge, our study represents the largest contemporary, multi-ethnic, long-term, prospective cohort to analyze the association of asthma and CVD. We hypothesized that persistent asthma is associated with higher CVD risk in the Multi-Ethnic Study of Atherosclerosis.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
Descriptive Characteristics
The 6,792 MESA participants were followed for a mean (standard deviation) 9.1 (2.8) years for development of CVD. At baseline participants were 62.2 (10.3) years old and 47.1% were male, 38.4% were Caucasian, 27.8% African-American, 22.0% Hispanic, and 11.8% Chinese (Table 1). The 156 participants with persistent asthma and the 511 participants with intermittent asthma were compared to the 6,125 participants without asthma. The distribution of risk factors between those with persistent asthma and those with intermittent asthma differed slightly compared to those without asthma (Table 1). Those with asthma were more likely to be female (64% vs. 52%) and on anti-hypertensive medications (41% vs 37%).
Table 1.
Baseline and Follow-up Descriptive Statistics
| Variables | No asthma (n=6125) |
Intermittent Asthma (n=511) |
Persistent Asthma (n=156) |
|---|---|---|---|
| Age, year | 62.3(10.2) | 59.7(10.1)* | 63.6(10.1) |
| Body-mass index, kg/m2 | 28.1(5.3) | 30.0 (6.4) * | 30.4(7.0) * |
| Male sex, n (%) | 2962(48.4) | 194(38.0) * | 46 (29.5) * |
| Race/Ethnicity % (N) | |||
| Caucasian, n (%) | 2354(38.4) | 187(36.6) | 68(43.6) |
| Chinese, n (%) | 755(12.3) | 40(7.8) * | 8(5.1) * |
| African-American, n (%) | 1671(27.3) | 161(31.5) * | 55(35.3) * |
| Hispanics, n (%) | 1345(22.0) | 123(24.1) | 25(16.0) |
| Smoking | |||
| Never smoker, n (%) | 3085(50.5) | 251(49.4) | 73(47.4) |
| Former smoker, n (%) | 2226(36.4) | 189(37.2) | 63(40.9) |
| Current smoker, n (%) | 797(13.05) | 68(13.39) | 18(11.7) |
| Total cholesterol, mg/dL | 193.98(35.3) | 195.2(39.4) | 196.87(38.0) |
| High-density lipoprotein cholesterol, mg/dL | 50.8(14.9) | 51.28(14.4) | 56.12(14.6) * |
| Systolic blood pressure, mmHg | 126.62(21.5) | 125.68(21.9) | 129.1(20.4) |
| Family history of coronary heart disease, n (%) | 2437(42.4) | 217(44.7) | 70(49.0) |
| Diabetes Mellitus, n (%) | 756(12.4) | 76(14.9) * | 21(13.5) |
| Income >$35,000/year, n (%) | 3235(55.1) | 287(58.0) | 88(57.9) |
| Anti-hypertension medication use, n (%) | 2255(36.8) | 193(37.8) * | 78(50.0) * |
| Lipid-lowering medication use, n (%) | 978(16.0) | 81(15.9) | 36(23.1) * |
| Oral corticosteroid medication use, n (%) | 72(1.2) | 0(0) | 32(20.5) |
| Leukotriene receptor antagonist medication use, n (%) | 7(0.1) | 0(0) | 45(28.9) |
| Inhaled corticosteroid medication use, n (%) | 28(0.5) | 0(0) | 118(75.6) |
age adjusted p<0.05, no asthma group as reference
Asthma and Cardiovascular Events
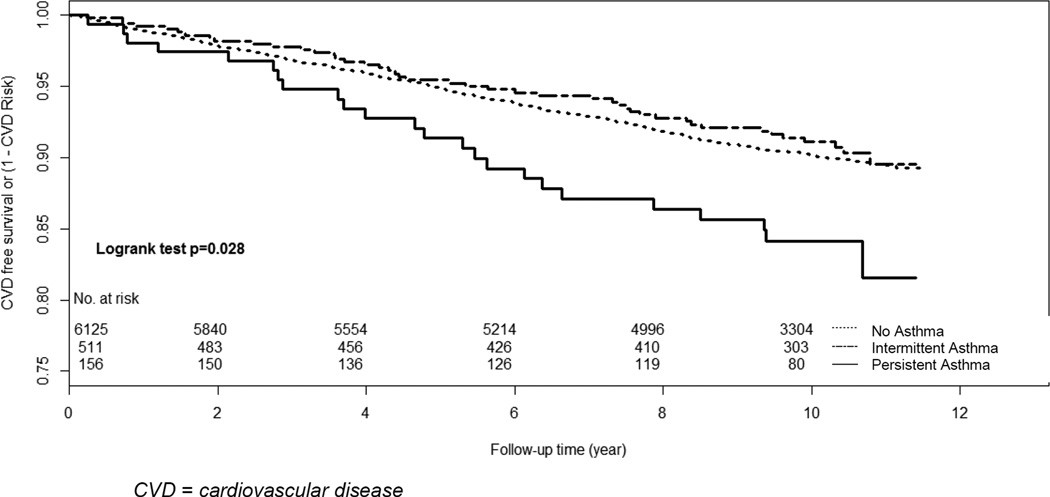
A total of 642 CVD events (249 hard endpoint coronary heart disease events, 188 angina, 167 stroke, 3 stroke death, 35 CVD deaths) occurred during the observation period. The incidence rate for CVD was higher in those with persistent asthma. The 10-year CVD-free survival rates are 89.5% (95% CI [87.0% –91.9%]) for those with asthma, and 90.2% (89.4%, –91.0%) for those that did not report a diagnosis of asthma. Among those with asthma, the 10-year CVD-free survival rates are 84.1% (78.4% –90.3%) for those with persistent asthma and 91.1% (88.5% –93.8%) for those with intermittent asthma. In multivariate models adjusted for potential confounders, having persistent asthma was associated with a significantly higher risk of CVD events. In models adjusted for age, race, and gender (Table 2, Model 1), participants with persistent asthma had a higher risk of CVD events (HR 1.72 [(1.14–2.59], p=0.01). This association persisted in models fully adjusted for potential confounders (HR 1.59 [1.01–2.5], p=0.04) (Table 2, Model 4). Participants with intermittent asthma, however, had no difference in CVD events compared to those without asthma in unadjusted (HR 1.13 [0.83–1.50], p=0.45) and fully adjusted models (HR 1.10 [9 0.78–1.48], p=0.66). We found no sex or race interactions with asthma and CVD outcomes (data not shown). Associations of asthma with stroke and all-cause mortality are in the Supplementary Table.
Table 2.
Association of Asthma with Cardiovascular Disease Events
| Persistent Asthma * | Intermittent Asthma * | |||
|---|---|---|---|---|
| Model | Hazard Ratio (95% CI) | P value | Hazard Ratio (95% CI) | P value |
| Model 1 | 1.72 (1.14–2.59) | 0.010 | 1.13 (0.83–1.53) | 0.452 |
| Model 2 | 1.83 (1.21–2.76) | 0.004 | 1.10 (0.79–1.47) | 0.626 |
| Model 3 | 1.75 (1.16–2.64) | 0.008 | 1.10 (0.80–1.47) | 0.613 |
| Model 4 | 1.59 (1.01–2.50) | 0.040 | 1.10 (0.78–1.48) | 0.655 |
No asthma group as reference
CI = confidence interval
Model 1: Adjusted for age, race, sex
Model 2: Model 1 + total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, smoking, diabetes mellitus
Model 3: Model 2 + anti-hypertensive and lipid-lowering medication use at baseline
Model 4: Model 3 + body-mass index, family history of CVD, income
Asthma and Inflammatory Biomarkers
Inflammatory markers were analyzed to assess the burden of systemic inflammation between the groups with persistent asthma, intermittent asthma, and those without asthma. Those with persistent asthma had the highest levels of systemic inflammatory markers (Table 3). Persistent asthmatics, compared to intermittent asthmatics and non-asthmatics respectively had higher age-adjusted levels of C-reactive protein (1.2 [1.2] vs 0.9 [1.2] vs 0.6 [1.2] mg/L) and fibrinogen (379 [88] vs 356 [80] vs 345 [73] mg/dL) mean differences were significant using Tukey's criteria. IL-6 levels were highest in persistent asthmatics 0.44 (0.61) compared to intermittent asthmatics 0.25 (0.67) and no asthma 0.20 (0.66) (Table 3). When C-reactive protein and D-Dimer were added to the a priori fully adjusted model, they were not statistically significant predictors of CVD outcomes and did not attenuate the asthma effect. When added to these models, IL-6 and fibrinogen were statistically significant predictors of CVD outcomes but did not notably attenuate the asthma effect (the HR was 1.55–1.59 in all models).
Table 3.
Systemic Inflammatory Markers Based on Asthma Status
| Variables | No asthma | Intermittent Asthma | Persistent Asthma |
|---|---|---|---|
| IL-6, pg/mL, log, mean (SD) | 0.20 (0.66) | 0.25 (0.67) | 0.44 (0.61)a,b |
| CRP, mg/L, log, mean (SD) | 0.62 (1.16) | 0.85 (1.2)a | 1.19 (1.16)a,b |
| D-Dimer, ug/mL, log, mean (SD) | −1.5 (0.93) | −1.54 (0.87) | −1.28 (0.87)a,b |
| Fibrinogen, mg/dL, mean (SD) | 345.2 (72.89) | 355.7 (79.51)a | 378.73 (87.55)a,b |
significantly different at the p<0.05 level compared to No Asthma group
significantly different from asthma not on controller medication at the p<0.05 level
Discussion
After a decade of prospective observation, persistent asthmatics had a 1.6 fold higher risk of CVD events than non-asthmatics in models fully adjusted for potential confounders. Despite being treated for their asthma, persistent asthmatics on controller medications had the highest burden of systemic inflammation. To our knowledge, our study represents the largest contemporary, multi-ethnic, long-term, prospective cohort to analyze the association of asthma and CVD. Prior studies investigating the association between asthma and CVD have been limited by case-control and cross-sectional designs, studied retrospective insurance claims, or contained a homogenous group of individuals with older asthma treatments.11–18 Furthermore, some studies noted no association between asthma and CVD or described limited associations of women only or associations only with stroke.12, 15, 17 Two previous reports from the population-based Atherosclerosis Risk in Communities (ARIC) study cohort investigated the association of asthma with incident CVD. In the first report, there was an association of stroke with asthma with a higher risk in women but no association with CHD.12 In the second, there was an association of CHD and stroke amongst women with adult-onset asthma, but not men.18 We observed an association of persistent asthmatics, but not intermittent asthmatics, with CVD events.
Our study results and design differ from the previous studies in several ways. First, we did not observe effect modification by female sex.11, 14, 18 The three investigations that observed effect modification by sex had more participants, but two used insurance claims databases and the other, a report from the ARIC study, was discussed above. Our study may not have had enough statistical power to identify effect modification by sex. 11, 14, 18 Second, a strength of our paper is that we accounted for the heterogeneous nature of asthma and classified it by severity (intermittent vs persistent). Thus, our clinical classification of asthma was different than in prior studies11, 14, 18 and was validated by its association with serum inflammatory markers. Third, other studies had limited data on systemic markers of inflammation; only one study demonstrated that women with adult-onset asthma had higher fibrinogen levels.18 Previous population-based studies have demonstrated a positive association between asthma severity and levels of systemic inflammatory markers such as hs-CRP and fibrinogen levels.17, 19 Our data demonstrated graded relationships between inflammatory markers, asthma classification. Finally, our study was contemporary and included large numbers of ethnic minorities. There is a higher prevalence of asthma among ethnic minorities and there have been significant changes in asthma treatment and CVD prevention over the antecedent two decades. Inhaled corticosteroids currently are the mainstay of therapy for asthma management and statins reduce CVD risk. MESA is a prospective cohort study of participants enrolled from 2000–2002 and followed through 2012, all of which were free of CVD at baseline with the purpose of investigating the development and progression of subclinical atherosclerosis. We observed a longitudinal association of persistent asthma and CVD events in a contemporary, multi-ethnic cohort with adjustment for traditional risk factors and additional adjustment for statin and anti-hypertensive medication use.
The most recent investigation that analyzed the association of asthma and CVD was from the Kaiser Permanente Northern California health care claims database. It demonstrated an increased risk of CVD events among asthmatics with a similar hazard ratio to our finding. with stronger associations for those on asthma medications and in women.14 In contrast to the Kaiser Permanente analysis which used International Classification of Diseases, Ninth Revision (ICD-9) codes to identify CVD events, all CVD events in MESA were adjudicated. This is especially important because asthma exacerbations need to be accurately distinguished from heart failure events. Because of their overlap in clinical presentations, we did not include heart failure as an endpoint in the current study to avoid misclassification bias. Also, claims databases do not have patient level laboratory, physical exam, and medical or family history information. The use proxy measures to adjust for potential biologic confounders such as using ICD-9 codes, which can increase residual confounding. Since these codes primarily are used for billing purposes, they are prone to "upcoding" to more severe conditions such as heart failure for higher reimbursement.20 Finally, given the breadth of data collected in MESA, we were able to adjust for additional potential risk factor and socio-demographic confounders of the association between asthma and CVD. Despite the differences in study design, the current investigation found a similar magnitude of association between asthma and CVD events in fully adjusted models compared to the Kaiser Permanente claims database study; however, in the current study, we did not see effect modification by female sex.
Asthma and CVD share an inflammatory pathogenesis. Although there are differences in the inflammatory pathophysiologies between these two conditions, data from animal models suggests that there may be significant overlap. Inflammation in asthma is partly mediated through the 5-lipo-oxygenase enzymatic pathway. In this pathway, 5-lipo-oxygenase catalyzes conversion of arachidonic acid leading to the formation of leukotriene A4 which can then be converted into one of four different leukotrienes. Leukotrienes are paracrine inflammatory substances produced in immune cells that induce both acute and chronic inflammation.21 In bronchioles, leukotrienes act as potent smooth muscle constrictors, induce tissue edema, and are signals for migration of eosinophils. Atherosclerotic plaques also express 5-lipo-oxygenase and contain elevated levels of leukotrienes.10 In animal models, blockade of leukotriene B4 results in less monocyte recruitment and a reduction in atheroma progression.9 The 5-lipo-oxygenase pathway also has been implicated in CVD events as increased levels of 5-lipo-oxygenase and leukotrienes have been associated with plaque instability.21, 22 Systemic inflammation that increases risk of a CVD event also may affect asthma control. Additional studies have found associations between hs-CRP, airway hyper-responsiveness, and forced expiratory volume in one second among individuals free of CVD, suggesting that systemic inflammation also effects lung function.23 High-sensitivity CRP levels also have been used as surrogate marker of disease control in asthmatics, with lower levels indicating better disease control.24
In MESA, participants with persistent asthma had the highest level of systemic inflammatory markers. Furthermore, there was a graded relationship between those without asthma having the lowest levels of inflammation, those with intermittent asthma having intermediate levels, and those with persistent asthma having the highest levels of inflammatory markers. Previous studies have demonstrated that treatment of asthma with anti-inflammatory medications such as inhaled corticosteroids results in lower levels of markers of systemic inflammation.25, 26 The identification of elevated inflammatory markers among participants with persistent asthma in our study is hypothesis-generating; however, we note that their addition to the models did not attenuate the relationship between persistent asthma and CVD events, so we cannot imply a mediated effect. This may be due to partial treatment of inflammation by controller medications. It also is possible that asthma medications have adverse CVD effects via their effects on glucocorticoid metabolism, lipid metabolism, or in the case of long-acting beta antagonists, sympathetic activation. The elevated levels of systemic inflammatory markers observed among persistent asthmatics may have contributed to the increased CVD risk in this group; however, further studies are needed to elucidate this mechanism.
Limitations
Despite the numerous strengths of this study there are some limitations. As an observational study, the described associations do not confirm causation. Asthma was defined by self-report and may be prone to misclassification bias, though we classified participants by use or non-use of controller medications to improve the specificity of the diagnosis. We did not have lung function parameters at the baseline exam and given the heterogeneous nature of asthma severity, according to current guidelines, we defined use of controller medications as an indicator of more severe disease.27 The graded levels of inflammatory markers among the asthma classifications in this report support our approach, as those using controller medications had more systemic inflammation, and if anything, misclassification should lead to a null bias. The primary hypothesis of this study was to investigate the association between persistent asthma and CVD events. Given the design of MESA, this study cannot delineate the mechanism which led to higher CVD rates. Although we treated use of controller medications as a marker of more severe asthma and that supposition was supported by our biomarker data, we cannot exclude the possibility that controller or other asthma medications increased CVD risk. Certain classes of controller medications have been associated with CVD risk, especially among individuals with chronic obstructive pulmonary disease; however, these associations have been inconsistent and a few studies have noted a reduction in CVD events with the use of inhaled corticosteroids.28–30 Regression models were adjusted for measured risk factor and socio-demographic variables; however, unmeasured lifestyle exposures and risk factors that may be specific to each ethnicity cannot be accounted for. MESA is a United States cohort so the generalizability of these findings to populations outside the United States may be limited. The small number of CVD events limited our power to detect potential effect modification by sex or race. Finally, asthma is a heterogeneous disease that has complex genetic and environmental factors, thus generalizability to all subtypes in uncertain. To disentangle the limitations of observational studies like MESA, it would be useful if the large, randomized clinical trials of asthma medications had longer durations, measured inflammatory biomarkers, and formally adjudicated CVD events.
Conclusions
Persistent asthmatics taking daily controller medications were at increased risk of CVD events. Persistent asthmatics demonstrated higher levels of systemic inflammation than non-asthmatics or intermittent asthmatics. Given the increasing public health burden of asthma and the pathophysiological overlap of asthma and CVD, further investigations into this association are needed.
Supplementary Material
Figure 1.
Kaplan-Meier Cardiovascular Disease-Free Survival Estimates Based on Asthma Status
Significance.
Asthma is a significant public health burden afflicting more than 25 million individuals in the United States. Asthma and cardiovascular disease (CVD) share common inflammatory pathophysiologies; however, an association of asthma and CVD has not been identified in a contemporary, multi-ethnic cohort. In the current study we found that persistent asthmatics compared with non-asthmatics had a higher risk of a CVD event over almost a decade of observation. Despite treatment with controller medications, persistent asthmatics also had higher levels of systemic inflammatory markers compared with mild asthmatics and non asthmatics. These findings support the need for additional work to define whether the increased risk among persistent asthmatics is due to the systemic inflammation from asthma or if there is a contribution of the controller medications. These findings also support the need for CVD prevention and awareness of traditional risk factors and risk factor reduction in asthmatics.
Acknowledgements
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Sources of Funding
Drs. Tattersall and Gepner were supported by grant T32 HL07936. The MESA was supported by contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the NHLBI and by grants UL1-TR-000040 and UL1-TR-001079 from NCRR.
Abbreviations
- ARIC
Atherosclerosis Risk in Communities
- CRP
C-reactive protein
- CVD
cardiovascular disease
- MESA
Multi-Ethnic Study of Atherosclerosis
Footnotes
Disclosures
None
References
- 1.Akinbami LJ, Moorman JE, Bailey C, Zahran HS, King M, Johnson CA, Liu X. Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. NCHS Data Brief. 2012:1–8. [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 4.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 5.Solomon DH, Karlson EW, Rimm EB, Cannuscio CC, Mandl LA, Manson JE, Stampfer MJ, Curhan GC. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation. 2003;107:1303–1307. doi: 10.1161/01.cir.0000054612.26458.b2. [DOI] [PubMed] [Google Scholar]
- 6.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 7.Shrestha S, Irvin MR, Grunfeld C, Arnett DK. HIV, inflammation, and calcium in atherosclerosis. Arterioscler Thromb Vasc Biol. 2014;34:244–250. doi: 10.1161/ATVBAHA.113.302191. [DOI] [PubMed] [Google Scholar]
- 8.Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, Ledergerber B, Lundgren J, Neuhaus J, Nixon D, Paton NI, Neaton JD. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aiello RJ, Bourassa PA, Lindsey S, Weng W, Freeman A, Showell HJ. Leukotriene B4 receptor antagonism reduces monocytic foam cells in mice. Arterioscler Thromb Vasc Biol. 2002;22:443–449. doi: 10.1161/hq0302.105593. [DOI] [PubMed] [Google Scholar]
- 10.Spanbroek R, Grabner R, Lotzer K, et al. Expanding expression of the 5-lipoxygenase pathway within the arterial wall during human atherogenesis. Proc Natl Acad Sci U S A. 2003;100:1238–1243. doi: 10.1073/pnas.242716099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iribarren C, Tolstykh IV, Eisner MD. Are patients with asthma at increased risk of coronary heart disease? Int J Epidemiol. 2004;33:743–748. doi: 10.1093/ije/dyh081. [DOI] [PubMed] [Google Scholar]
- 12.Schanen JG, Iribarren C, Shahar E, Punjabi NM, Rich SS, Sorlie PD, Folsom AR. Asthma and incident cardiovascular disease: the Atherosclerosis Risk in Communities Study. Thorax. 2005;60:633–638. doi: 10.1136/thx.2004.026484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toren K, Lindholm NB. Do patients with severe asthma run an increased risk from ischaemic heart disease? Int J Epidemiol. 1996;25:617–620. doi: 10.1093/ije/25.3.617. [DOI] [PubMed] [Google Scholar]
- 14.Iribarren C, Tolstykh IV, Miller MK, Sobel E, Eisner MD. Adult asthma and risk of coronary heart disease, cerebrovascular disease, and heart failure: a prospective study of 2 matched cohorts. Am J Epidemiol. 2012;176:1014–1024. doi: 10.1093/aje/kws181. [DOI] [PubMed] [Google Scholar]
- 15.Bellia V, Pedone C, Catalano F, Zito A, Davi E, Palange S, Forastiere F, Incalzi RA. Asthma in the elderly: mortality rate and associated risk factors for mortality. Chest. 2007;132:1175–1182. doi: 10.1378/chest.06-2824. [DOI] [PubMed] [Google Scholar]
- 16.Lee HM, Truong ST, Wong ND. Association of adult-onset asthma with specific cardiovascular conditions. Respir Med. 2012;106:948–953. doi: 10.1016/j.rmed.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 17.Enright PL, Ward BJ, Tracy RP, Lasser EC The Cardiovascular Health Study Research Group. Asthma and its association with cardiovascular disease in the elderly. J Asthma. 1996;33:45–53. doi: 10.3109/02770909609077762. [DOI] [PubMed] [Google Scholar]
- 18.Onufrak SJ, Abramson JL, Austin HD, Holguin F, McClellan WM, Vaccarino LV. Relation of adult-onset asthma to coronary heart disease and stroke. Am J Cardiol. 2008;101:1247–1252. doi: 10.1016/j.amjcard.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jousilahti P, Salomaa V, Hakala K, Rasi V, Vahtera E, Palosuo T. The association of sensitive systemic inflammation markers with bronchial asthma. Ann Allergy Asthma Immunol. 2002;89:381–385. doi: 10.1016/S1081-1206(10)62039-X. [DOI] [PubMed] [Google Scholar]
- 20.Silverman E, Skinner J. Medicare upcoding and hospital ownership. J Health Econ. 2004;23:369–389. doi: 10.1016/j.jhealeco.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 21.Di Gennaro A, Haeggstrom JZ. The leukotrienes: immune-modulating lipid mediators of disease. Adv Immunol. 2012;116:51–92. doi: 10.1016/B978-0-12-394300-2.00002-8. [DOI] [PubMed] [Google Scholar]
- 22.Qiu H, Gabrielsen A, Agardh HE, Wan M, Wetterholm A, Wong CH, Hedin U, Swedenborg J, Hansson GK, Samuelsson B, Paulsson-Berne G, Haeggstrom JZ. Expression of 5-lipoxygenase and leukotriene A4 hydrolase in human atherosclerotic lesions correlates with symptoms of plaque instability. Proc Natl Acad Sci U S A. 2006;103:8161–8166. doi: 10.1073/pnas.0602414103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kony S, Zureik M, Driss F, Neukirch C, Leynaert B, Neukirch F. Association of bronchial hyperresponsiveness and lung function with C-reactive protein (CRP): a population based study. Thorax. 2004;59:892–896. doi: 10.1136/thx.2003.015768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujita M, Ueki S, Ito W, Chiba T, Takeda M, Saito N, Kayaba H, Chihara J. C-reactive protein levels in the serum of asthmatic patients. Ann Allergy Asthma Immunol. 2007;99:48–53. doi: 10.1016/S1081-1206(10)60620-5. [DOI] [PubMed] [Google Scholar]
- 25.Takemura M, Matsumoto H, Niimi A, Ueda T, Matsuoka H, Yamaguchi M, Jinnai M, Muro S, Hirai T, Ito Y, Nakamura T, Mio T, Chin K, Mishima M. High sensitivity C-reactive protein in asthma. Eur Respir J. 2006;27:908–912. doi: 10.1183/09031936.06.00114405. [DOI] [PubMed] [Google Scholar]
- 26.Karthikeyan R, Krishnamoorthy S, Maamidi S, Kaza AM, Balasubramanian N. Effect of inhaled corticosteroids on systemic inflammation in asthma. Perspect Clin Res. 2014;5:75–79. doi: 10.4103/2229-3485.128026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120:S94–S138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 28.Ferguson GT, Funck-Brentano C, Fischer T, Darken P, Reisner C. Cardiovascular safety of salmeterol in COPD. Chest. 2003;123:1817–1824. doi: 10.1378/chest.123.6.1817. [DOI] [PubMed] [Google Scholar]
- 29.Au DH, Lemaitre RN, Curtis JR, Smith NL, Psaty BM. The risk of myocardial infarction associated with inhaled beta-adrenoceptor agonists. Am J Respir Crit Care Med. 2000;161:827–830. doi: 10.1164/ajrccm.161.3.9904006. [DOI] [PubMed] [Google Scholar]
- 30.Camargo CA, Jr, Barr RG, Chen R, Speizer FE. Prospective study of inhaled corticosteroid use, cardiovascular mortality, and all-cause mortality in asthmatic women. Chest. 2008;134:546–551. doi: 10.1378/chest.07-3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.