Abstract
Chronic liver disease mediated by activation of hepatic stellate cells (HSCs) leads to liver fibrosis. Here, we postulated that the immune regulatory properties of HSCs might promote the profibrogenic activity of B cells. Fibrosis is completely attenuated in carbon tetrachloride (CCl4)-treated B cell deficient μMT mice showing that B cells are required. The retinoic acid produced by HSCs augmented B cell survival, plasma cell marker CD138 expression, and IgG production. These activities were reversed following the addition of the retinoic acid inhibitor, LE540. Transcriptional profiling of fibrotic liver B cells revealed an increased expression of genes related to NF-κB activation, proinflammatory cytokine production and CD40 signaling suggesting that these B cells are activated and may be acting as inflammatory cells. Biological validation experiments also revealed increased activation (CD44 and CD86 expressions), constitutive IgG production and secretion of the proinflammatory cytokines TNF-α, MCP-1 and MIP1-α. Likewise targeted deletion of B-cell-intrinsic MyD88 signaling, an innate adaptor with involvement in RA signaling, resulted in reduced infiltration of migratory CD11c+ dendritic cells and Ly6C++ monocytes, and hence reduced liver pathology.
Conclusion
Our findings demonstrate that liver fibrosis occurs through a mechanism of HSC-mediated augmentation of innate B cell activity and highlight B cells as an important ‘first responders’ of the intrahepatic immune environment.
Keywords: Hepatic stellate cells, retinoic acid, B cells, liver fibrosis, MyD88 signaling
Introduction
Liver fibrosis is a clinical disease resulting from chronic inflammation of the liver, mediated by the activation of hepatic stellate cells (HSCs).1 Increasing evidence suggests, however, that HSCs are also key modulators of immunity within the liver through a number of cytokine-mediated and receptor-driven mechanisms.2,3 For example, TLR-and RIG-I-signaling in HSCs results in the innate secretion of type I and III IFNs, which can reduce viral replication in hepatocytes.4 Data from our own laboratory has shown that HSCs can skew T cell development towards a regulatory T cell fate, through the secretion of the vitamin A metabolite, retinoic acid (RA).5 In addition, upregulation of B7 family member B7-H4 by activated HSCs allows for the direct inhibition of T cell proliferation and cytokine secretion.6 While direct stimulation of CD4+, CD8+, and NK T cells by IFNγ-activated HSCs has been documented, HSCs are inefficient antigen presenting cells (APCs) compared to other populations such as dendritic cells (DCs). Rather, modulation of immunity through cytokine secretion or receptor ligation is thought to be the primary mechanism by which HSCs exert their influence.7 HSC-derived RA may play a pleiotropic role in this process, through binding to its specific nuclear receptors (RAR, RARα, RARβ and RXRα).8 At this time, the in vivo relevance of HSC-mediated involvement in intrahepatic tolerance and immunity are unknown.
It’s now been suggested that, within the liver, a novel pathogenic role for B cells also exists in the propagation of liver fibrosis.9 This report in mice has documented attenuated liver fibrosis in the absence of B cells through an unknown mechanism. Moreover, the involvement of B cells in αCD40-induced necroinflammatory liver disease revealed a proinflammatory role for B cells that depended on the presence of macrophages but not T cells.10 In humans, B cells are important in the pathogenesis of numerous inflammatory diseases, such as rheumatoid arthritis and systemic lupus erythematosus11,12 Importantly, there are also known associations between chronic liver disease and B cell proliferative disorders such as mixed cryoglobulinema (MC) and non-Hodgkin’s B cell lymphoma, suggesting that a pathogenic dysregulation of B cell homeostasis may be occurring.13,14 Whether this type of B cell activity affects earlier stages of fibrotic liver disease is unknown.
While a role for antibody production in the pathogenesis of liver fibrosis has been ruled out 9, the many other functions of B cells, such as opsonization, complement fixation, antigen presentation and cytokine production are currently unknown. Here we investigated whether the profibrogenic activity of B cells is initiated through their interactions with HSCs within the liver. We found that HSC-derived retinoic acid augmented B cell survival, plasmablast differentiation, and IgG production. Interestingly, HSC-mediated effect on B cells was reversible by treatment with the RA inhibitor LE540. Furthermore, the transcriptional profiling and computational modeling highlighted the importance of NFκB signaling in fibrotic liver B cells, and the activation of pathways related to TLR activity, cytokine production, and CD40 signaling. The biological importance of these pathways during fibrosis was also demonstrated, as fibrotic liver B cells exhibited increased state of activation as measured by CD44 and CD86 expressions, constitutive IgG production and proinflammatory behavior. MyD88 signaling was an important contributor to the observed pathology, as mice having a B cell-restricted deficiency in MyD88 signaling demonstrated reduced fibrosis, and reduced liver infiltration of other inflammatory cell types such as monocytes and dendritic cells. Our study demonstrates that HSC-derived RA is responsible for the dysregulated activity of intrahepatic B cells. MyD88 signaling and liver B cell production of proinflammatory cytokines and chemoattractants is a prerequisite for mononuclear cell recruitment and thus, B cells serve to amplify fibrotic processes through a novel innate activity.
Materials and Methods
Mice
Eight week old male C57BL/6 (WT), B cell deficient mice (μMT), and MyD88fl (B6.129P2(SJL)-Myd88tm1Defr/J) mice additionally crossed to CD19-Cre (B6.129P2-Lyz2tm1(cre)Ifo/J), obtained from Jackson Laboratory, Bar harbor, ME on a C57BL/6 background were used for the study and maintained in accordance with AAALAC and IACUC guidelines.
CCl4-induced Liver Fibrosis
CCl4 (Sigma-Aldrich, St. Louis, MO) was as mixed with a common olive oil at a 1:10 ratio and injected intraperitoneally (i.p.) into mice at 0.5 μl CCl4/g body weight twice per week for six weeks as described earlier.15 For the therapeutic administration of LE540, mice were treated with CCl4 plus LE540 (10 μg, Wako Pure Chemical Industries Ltd., Osaka, Japan) for six weeks. Fibrosis was quantified based on a detail observation of hepatocyte necrosis, degeneration, fibrosis, and inflammation described as lesion severity score (0 = no significant lesion, 1 = mild lesion, 2 = moderate lesion and 3 = severe lesion).
Liver Cells Isolation
Briefly, liver cells were isolated by perfusion with HBSS, then 0.4% protease (Sigma-Aldrich), and 0.01% collagenase (Sigma-Aldrich) solutions in DMEM/F12 medium. After gentle mechanical disruption, the resulting suspension was incubated at 37°C in 0.4% collagenase solution in DMEM/F12 with 0.5mg/mL DNAse for 20 min. The digested suspension was filtered through 70μm mesh and centrifuged at 50g for 3 min. HSCs were then isolated from the supernatant using the Optiprep method (Sigma-Aldrich). Intrahepatic and splenic B cells were isolated using CD19 microbeads (Miltenyi Biotec, Bergish Gladbach, Germany), and splenic dendritic cells were isolated using CD11c microbeads (Miltenyi Biotec).
Sorting of HSCs and Quantification of α-SMA
After density gradient enrichment, NPCs were stained with Abs to CD146 (ME-9F1; Miltenyi Biotec), and CD45 (104; BD Bioscience, San Jose, CA) and sorted on a FACSAria Cell Sorter II (BD Bioscience). HSCs were identified as UV autofluorescence–positive (UVAF+), CD45-ve and CD146-ve population. For the detection of HSC-associated marker, purified liver cells were stained intracellularly with Abs to α-SMA (1A4; R&D Systems) and analyzed using FlowJo software (Treestar, Ashland, OR).
Histopathology & Immunofluorescence
Mouse liver biopsies were fixed in 10% phosphate-buffered formalin, embedded in paraffin and cut into sections. Sections for histopathological examination were stained with Sirius red, Masson’s trichrome and Hematoxylin and eosin (H&E) stain using standard procedures. For immunofluorescence, antibodies specific to α-SMA were used at 1:50 dilution according to manufacturer’s instructions and analyzed on an immunofluorescence microscope (Zeiss).
Co-culture Assay
B cells (2×105) enriched from spleen of WT mice were cocultured with HSCs (1×104) or CD11c+ DC (1×105) in presence of αIgM (affinipure F(ab′)2-fragment goat anti-mouse IgM, μ chain specific) and αCD40 (HM40-3, 2 μg/mL each), or with LPS (2 μg/mL, Sigma-Aldrich). In some conditions, RA (100nM) and/or LE540 (1 μM) were added to the B cell: HSC co-culture. On 5 d, supernatants were collected from the culture and analyzed for IgG levels by ELISA. Similarly, intrahepatic and splenic B cells from control or CCl4-treated mice were isolated, co-cultured with DC or HSCs and stimulated with αIgM and αCD40, or LPS. For trans-well experiments, splenic B cells and HSCs were separated using a trans-well insert and cocultured for 4 d as described above.
Intracellular Cytokine Staining and Luminex Assay
ICS
CD19+ B cells (2×105) isolated from the livers and spleen of mice were cultured unstimulated, or stimulated with αIgM and αCD40 (2 μg/mL), or LPS (2 μg/mL). After incubation, Brefeldin A (Sigma-Aldrich) was added and culture proceeded for 5 additional hours. Cultured cells were stained with Abs to B220 (RA3-6B2), and TNF-α (MP6-XT22) and IL-10 (JES5-16E3) using a standard intracellular staining procedure. Stained cells were acquired using an LSRII (BD Bioscience) and analyzed using FlowJo (Tree Star).
Luminex
Supernatants from the hepatic and splenic B cell cultures were collected at day 5 and analyzed using the Luminex 200 system according to the manufacturer’s instructions (Invitrogen, Burlingame, CA).
Microarray Analysis for B Cells
A detail protocol has been provided in Supplementary material section. Briefly, B cells (1×105) enriched from livers and spleens of oil or CCl4-treated mice were resuspended with 350 μl of RLT lysis buffer (Qiagen) mixed with 1% β-mercaptoethanol. Isolated RNA (5 ng) was reverse transcribed and cDNA strand was synthesized. Purified cDNA (5 μg) of SS-cDNAs were fragmented and chemically labeled with biotin to generate biotinylated ST-cDNA using Encore biotin module V2. Data were analyzed using Spotfire DecisionSite with OmicsOffice for Microarrays (Integrmics Biomarker Discovery). Primary microarray data has been submitted to Gene Expression Omnibus (GEO) in accordance with proposed Mimimum Information About a Microarray Experiment (MIAME) standards.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism Software (Version 6.0c, GraphPad Software, Inc., San Diego, CA). Two-tailed student’s t test was used to determine the significance, unless stated (*P<0.05, **P<0.01).
Results
B Cells Are Required for Liver Fibrogenesis
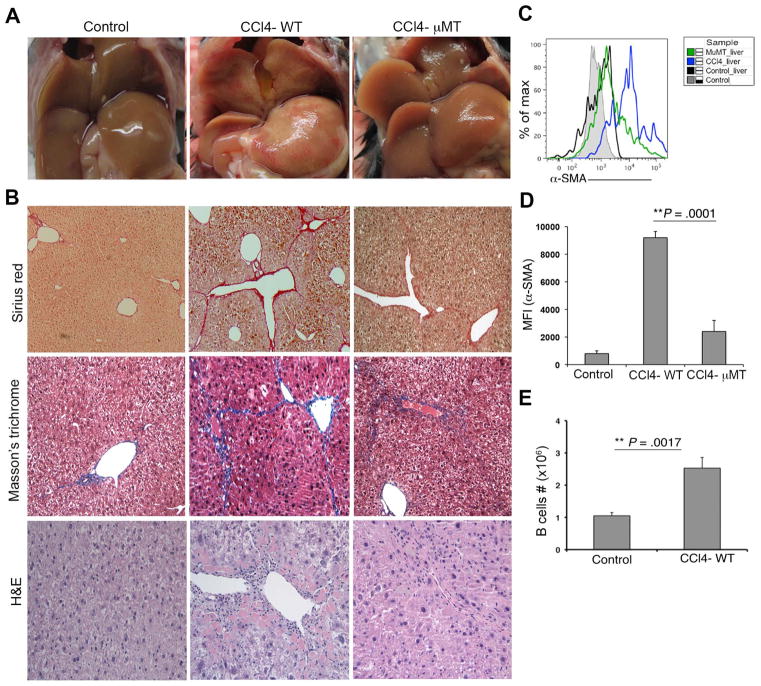
In this study, our aim was to identify the mechanistic contributions of B cells to fibrosis and disease progression using CCl4-model of induced liver fibrosis. We found that mice undergoing six weeks of treatment with CCl4 exhibited hepatic parenchyma with moderate to severe periportal to bridging fibrosis as measured by H&E, Masson’s trichrome and Sirius red stainings (Fig. 1A–B). Frequent hepatocyte karyomegaly and cytomegaly with an occasional periportal individual hepatocyte necrosis intermixed with a characteristic periportal collagen deposition, mononuclear cells infiltrations with an elevated serum ALT levels were also observed (Fig. 1A–B, Supplementary Fig. 1A). Phenotypic analysis of purified HSCs using FACS cell sorting based on vitamin A-mediated autofluorescence from healthy and fibrotic mice also revealed an increased expression of α-SMA by HSCs during fibrosis, signifying an increased state of activation (Supplementary Fig. 1, Fig. 1C–D). To assess the role of B cells in the observed liver pathology, CCl4 treatments were also given to mice having a targeted deletion of Igμ heavy chain (μMT) which lack mature B cells.16 Compared to WT mice fibrosis lesion (average score 3), μMT mice exhibited reduced fibrosis (average score 1) with reduced collagen deposition, immune cells infiltration, ALT levels and HSC expression of α-SMA (Fig. 1A–D, Supplementary Fig. 1 & Supplementary Table 1). Furthermore, hepatic B cells (CD19+) were markedly increased in the liver of CCl4-treated WT mice (Fig. 1E). Together our data reveal an important pathogenic role for B cells in the initiation, propagation, or maintenance of liver fibrotic processes.
Fig. 1.
B cells are required for liver fibrosis. WT and μMT mice were treated with either olive oil or CCl4 as described in Materials and Methods. (A) Representative livers from control, CCl4-treated WT and μMT mice are shown. (B) Histological analysis of liver specimens by Sirius red, Masson’s trichrome, and H&E stainings (magnification 100×). (C) Analysis of α-SMA expression on HSCs from mouse livers as described in Materials and Methods. (D) MFI value of α-SMA expression from control, CCl4-treated WT and μMT is shown. (E) Total B cells (CD19+) count from Control and CCl4-treated WT mice is shown. (* P<0.05, ** P<0.01).
HSC-derived RA Modulates B Cell Survival, Differentiation, and Function
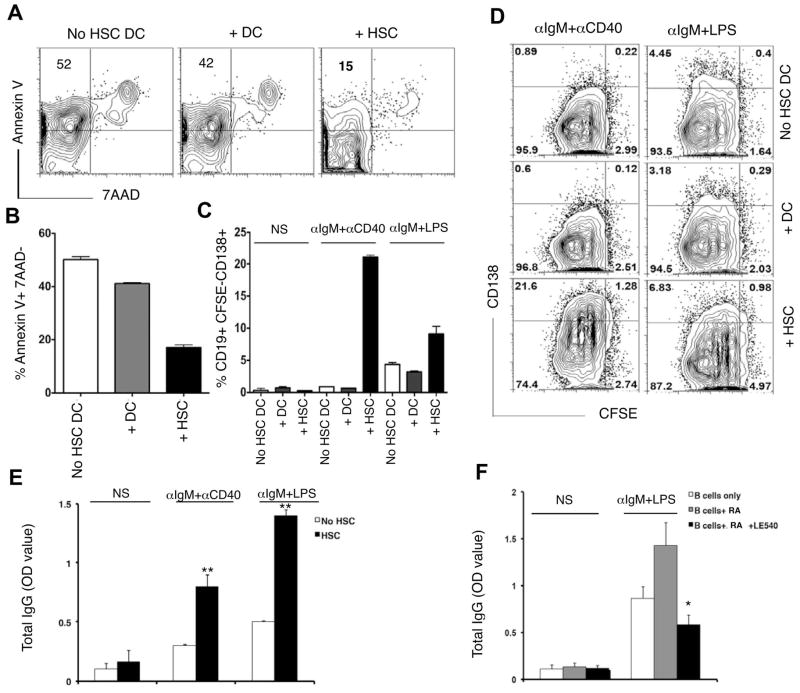
Following liver injury, HSCs transdifferentiate into myofibroblast like cells and become potent proinflammatory modulators of immunity and repair within the liver.1,17 In μMT mice, liver HSC activation and fibrosis were markedly diminished. One possibility is that liver fibrosis is initiated through a mechanism of crosstalk between liver HSCs and B cells in vivo. To address whether such interactions might exist, we analyzed the effects of HSC: B cell interactions on B cell activity using an in vitro co-culture system. HSCs were isolated from the liver of WT mice and activated in vitro according to a previously described method.5 Purified CD19+ splenic or hepatic B cells were then cultured for 4 days with the activated HSCs, alone, or with splenic CD11c+ DCs for comparison. When B cells were either cultured alone or in the presence of splenic DCs, 40–50% of them had entered an early stage of apoptosis, defined by an annexin V+ 7AAD-phenotype (Fig. 2A, Supplementary Fig. 2). In contrast, B cell co-cultured with activated HSCs appeared to offer a survival advantage, as the proportion of CD19+ cells staining annexin V+ or 7AAD+ was markedly reduced by comparison (Fig. 2A–B). Interestingly, HSC-mediated increase in B cell survival also correlated with upregulation of plasma B cell marker CD138 expression (Fig. 2C–D), and increased IgG production (Fig. 2E), signifying that activated HSCs might promote B cell survival through modulation of B cell differentiation or function.
Fig. 2.
HSCs promote survival and IgG production by B cells. CD19-enriched splenic B cells were co-cultured with splenic CD11c+ DC or plate-activated HSCs (isolated from livers of WT mice) for 4 d with stimulations as indicated. (A) B cells were stained for Annexin V and 7AAD and (B) percentage (%) of Annexin V+ 7AAD-cells shown. (C) CFSE-labeled B cells from co-culture were stained for CD138 and % CD19+ CFSE-CD138+ cells shown, and (D) FACS plot shown. (E) B cells were co-cultured with or without plate-activated HSCs for 5 d as indicated and analyzed for IgG production by ELISA. (F) B cells were cultured alone or in presence of RA (100nM) or RA+LE540 (1 μm) for 5 d and analyzed for IgG production by ELISA. Data are representative of 3 independent experiments (* p<0.05, ** p<0.01).
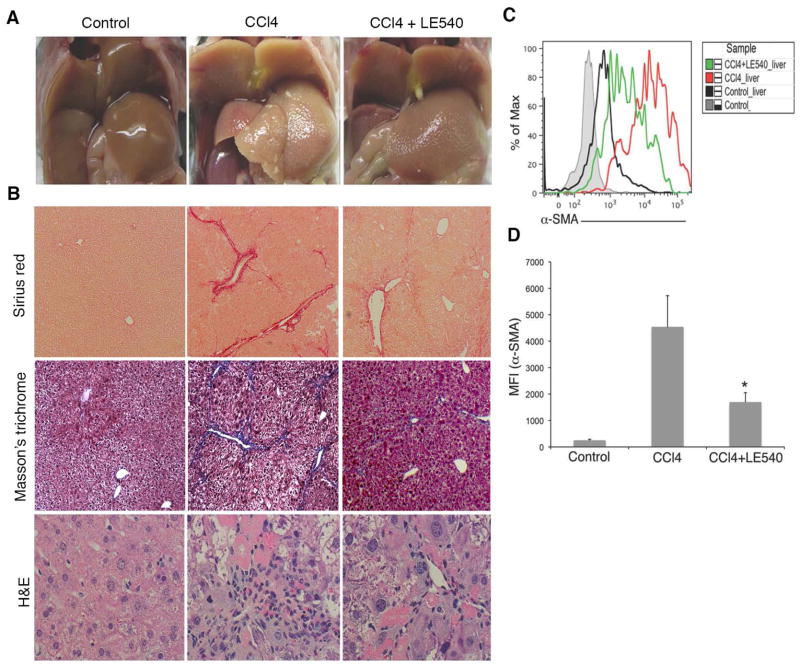
To test whether the HSC effect was due to a contact-dependent mechanism, we next used trans-well inserts to separate the two populations during the co-culture period. We observed that the HSC-mediated effects on B cell differentiation to a plasma cell phenotype were contact independent, allowing for the possibility that an HSC-derived soluble factor might be responsible (Supplementary Fig. 3A–B). We have previously shown that one such factor, HSC-derived retinoic acid (RA), has bystander effects in modulating T cell development towards a regulatory T cell phenotype. To test the role of RA signaling in our co-culture system, we next administered the RA inhibitor LE540 to our co-culture and monitored the effects of drug treatment on the HSC-induced modulation of B cell activity. Addition of LE540 resulted in a reduced production of IgG by B cells co-cultured with activated HSCs, signifying a reversal of the modulatory effect (Fig. 2F). Our data suggested that in vitro, in addition to any bystander effects on T cell development, HSC-derived RA might function to promote pathogenic B cell behavior through modulation of B cell development and/or effector functions. Importantly, the therapeutic administration of LE540 in vivo resulted in markedly reduced periportal to bridging liver fibrosis and immune cells infiltrates in CCl4-treated animals as indicated by histological analysis (Fig. 3A–B) and activation of HSCs (Fig. 3C–D).
Fig. 3.
Blocking of RA signaling reduces liver fibrosis. WT mice were treated with oil, or CCl4, or CCl4+ LE540 as described in Materials and Methods. (A) Representative livers from control, CCl4, and CCl4+ LE540-treated mice are shown. (B) Histological analysis of liver biopsies by Sirius red (magnification 100×), Masson’s trichrome (100×) and H&E staining (200×). Data are representative of 3 independent experiments. (C) The histogram showing α-SMA expression from control, CCl4, and CCl4+LE540 mice liver is representative of 2 independent experiments. (D) MFI value of α-SMA expression from control, CCl4 and CCl4+LE540 treated mice livers is shown. (* P<0.05).
Transcriptional Profiling Reveals Innate Molecular Signatures of Hepatic B Cells
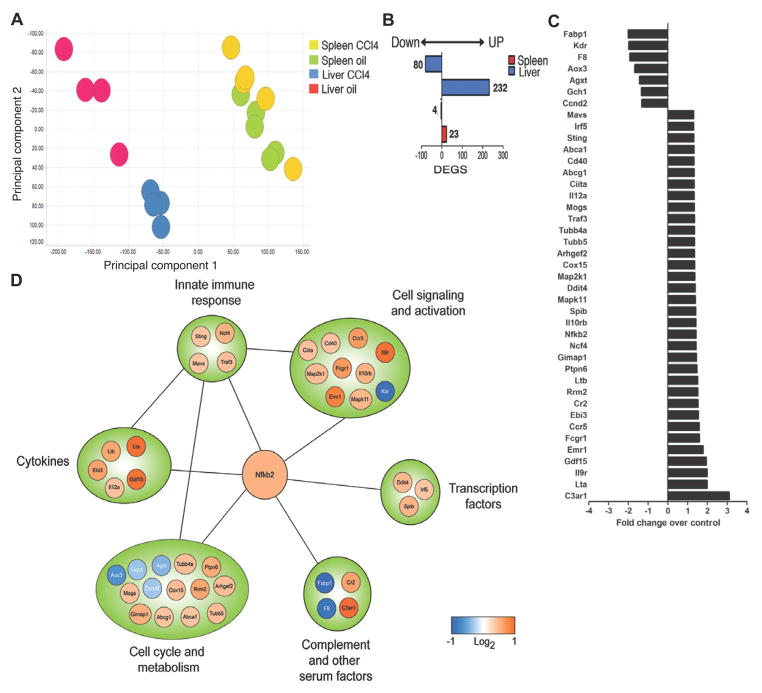
To better understand how RA signaling in vivo might influence a pathogenic role for B cells mechanistically, we used a systems biology approach to assess changes in the molecular signature of hepatic B cells that occur during fibrosis. B cells were isolated from the liver of healthy and fibrotic of mice and compared through the use of gene expression profiling and computational models. Principal component analysis suggested that hepatic B cells from fibrotic livers are distinct in their gene expression profiles as compared to B cells from healthy spleens or livers (Fig. 4A). Indeed, we identified 312 differentially expressed genes (DEGs; defined as 1.3 fold change with a P<0.001; 232 upregulated; 80 downregulated) in fibrotic liver B cells compared to healthy liver B cells (Fig. 4B). Surprisingly, computational modeling and enrichment analysis revealed 312 DEGs to be primarily associated with canonical pathways involved in innate immune signaling. These included: HSP60 and HSP70/TLR signaling pathway, Anti-apoptotic TNFs/NF-κB/Bcl-2 pathway, Lymphotoxin-beta receptor signaling, Role of HMGB1 in dendritic cell maturation and migration, and CD40 signaling (Table 1). Importantly, we also observed NF-κB to be a central regulatory node, linking the expression of transcription factors (IRF5, SPIB), innate immune signaling components (MAVS, STING, TRAF3), cytokines (IL12a, EBI3, Lta, Ltb, GDF15), and cytokine receptors (CD40, CCR5, IL12rb, IL9r, IL10rb) (Fig. 4C–D). When taken together, systems biology and computational modeling revealed both innate immune and activated molecular signatures for hepatic B cells during fibrosis. Surprisingly, our data suggest that liver B cells would function in a capacity similar to other inflammatory populations, such as monocytes, during liver fibrogenesis.
Fig. 4.
Transcriptional profile of hepatic B cells. (A) Principle component analysis of B cells isolated from the livers and spleens of oil and CCl4-treated mice. (B) Numbers of differentially expressed genes (DEGS; downregulated (left) or upregulated (right)) in each treatment condition shown. (C) Fold induction of candidate genes from network analysis comparing hepatic B cells isolated from CCl4-treated to oil-treated mice. (D) Network analysis of NF-κB regulated genes.
Table 1.
Liver B Cells Top Metacore Canonical Pathways
| Metacore Canonical Pathways | * B-H P value |
|---|---|
| HSP60 and HSP70/TLR signaling pathways | 1.43E-04 |
| Anti-apoptotic TNFs/NF-kB/Bcl-2 pathway | 3.63E-04 |
| Lymphotoxin-beta receptor signaling | 3.63E-04 |
| Role of HMGB1 in dendritic cell maturation and migration | 5.61E-04 |
| CD40 signaling | 1.35E-03 |
Benjamini-Hochberg (B-H) P value. Top five metacore canonical pathways are shown.
Fibrosis Proceeds Through Innate B Cell Activity
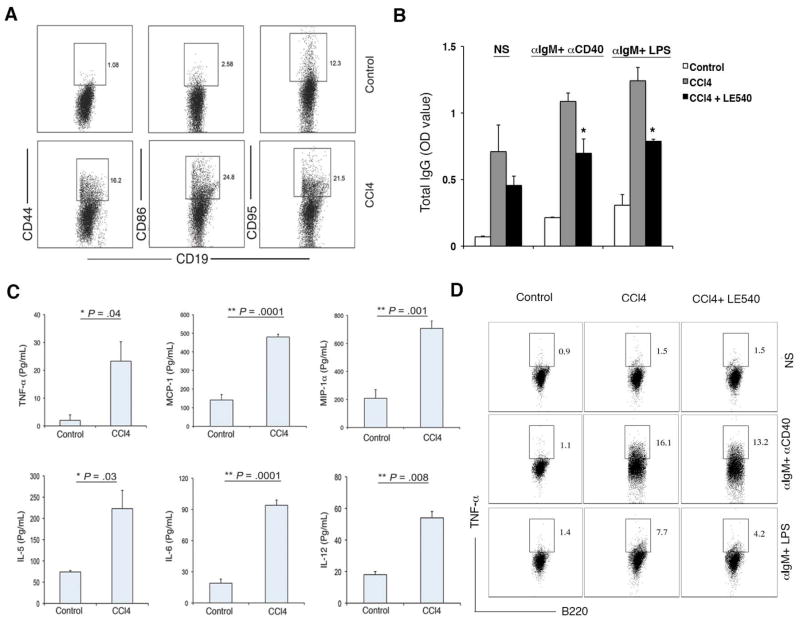
We next sought to validate the computational models using a biological system by measuring activation and function of liver B cells in healthy and fibrotic mice. Following 6 weeks of CCl4-treatment, intrahepatic B cells from fibrotic mice were found to be in an increased state of activation, characterized by the increased expression of activation markers CD44, CD86 and CD95 (Fas) (Fig. 5A). Consistent with our in vitro co-culture data, liver B cells from fibrotic but not healthy mice constitutively produced IgG directly ex vivo, even in the absence of stimulation (Fig. 5B). Therapeutic administration of LE540 to fibrotic animals reduced this activity, implicating HSC-derived RA in the modulation of B cell activation and function in vivo (Figure 5B).
Fig. 5.
Properties of Hepatic B cells from CCl4-treated mice. (A) B cells isolated from the livers of oil and CCl4-treated mice were analyzed for the expression of CD44, CD86 and CD95 by FACS analysis. B cells (2×105) isolated from control and CCl4-treated mice were cultured in presence of stimulations as indicated and supernatants were collected on day 5 and analyzed for (B) IgG production by ELISA and (C) Cytokine production by multiplex luminex assay (LPS stimulation shown). (D) B cells were cultured in presence of stimulations as indicated and stained for TNF-α production by intracellular staining. FACS plots were confirmed in 3 independent experiments. (*P< 0.05, **P <0.01).
To determine whether hepatic B cells were also functioning in an innate capacity in vivo, we also analyzed inflammatory cytokine/chemokine production by liver B cells following stimulation ex vivo. First, liver B cells from fibrotic and healthy mice were stimulated with either α-IgM/ α-CD40 antibodies or LPS as indicated, and the secretion profile of fibrotic liver B cells was assessed. Consistent with their function as an innate immune cell, liver B cells from fibrotic mice produced greater proinflammatory cytokines in response to stimulation when compared to liver B cells from healthy mice. These included the innate cytokines TNF-α, MCP-1, MIP-1α, KC, IL-5, IL-6 and IL-12, potent molecules involved in the activation and recruitment of inflammatory cells (Fig. 5C–D). Importantly, administration of LE540 to fibrotic animals reduced TNF-α production by B cells as detected by FACS analysis (Fig. 5D). Together, the data confirm that the innate activity of B cells is enhanced during fibrosis, and suggest that B cell production of proinflammatory cytokines/chemokines may be an important contribution to liver fibrogenesis.
B-cell-intrinsic MyD88 Signaling is Central to RA-induced B Cell Pathology
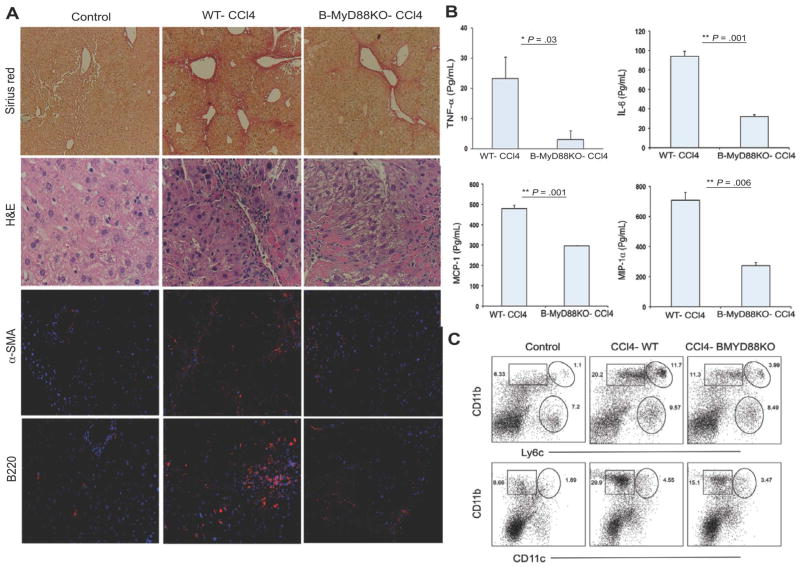
For B cells, innate signaling through MyD88 is indispensible for proper activation and proinflammatory cytokine production.18,19 In addition, MyD88 has known involvement in the developmental and modulatory effects of RA 20, which we show to be involved in the upregulation of TLR4 and TLR9 expression on B cells (Supplementary Fig 4). To understand the mechanistic contributions of MyD88 signaling to hepatic fibrosis in vivo, we used a MyD88fl-CD19-Cre system to generate B-cell-specific MyD88-deficient mice (B-MyD88KO).19 Consistent with a role for MyD88 in B cell survival and activity, liver B cells from fibrotic B-MyD88KO mice also demonstrated remarkably reduced proliferation capacity as determined by Ki-67 staining, and expressed decreased levels of activation marker CD44 in response to LPS (Supplementary Fig. 5A). Following six weeks of CCl4-treatment, absence of MyD88 signaling in B cells resulted in significantly reduced liver periportal bridging fibrosis with reduced serum ALT, collagen deposition, mononuclear cells infiltrates, and HSC activation with reduced fibrosis score ranging from 1 to 2 (Fig. 6A, Supplementary Fig. 5B). In addition, liver B cells from fibrotic B-MyD88KO mice also produced decreased levels of proinflammatory cytokines and IgG than MyD88 competent liver B cells (Fig. 6B, Supplementary Fig. 5C). Importantly, B cell intrinsic MyD88 signaling had mechanistic effects on other populations, as inspection of liver mononuclear cell infiltration in fibrotic B-MyD88KO animals also revealed a reduced frequency and reduced total number of Ly6C++ monocytes, CD11b+ DCs and neutrophils (Fig. 6C). Myeloid cells in particular, like monocytes and DCs, have known contributions to liver fibrosis following MCP-1-mediated recruitment.21,22 Our data now demonstrate a significant mechanistic role for B-cell-intrinsic MyD88 signaling in the recruitment of these populations. We therefore conclude that MyD88 signaling in B cells contributes to their heightened innate activity following RA exposure in vivo. The resultant ‘innate’ B cells function to amplify fibrotic processes through liver mononuclear cell recruitment.
Fig. 6.
B-cell-intrinsic MyD88 signaling is central to B cell pathology. WT and B-MyD88KO mice were treated with either oil or CCl4 for six wk. (A) Histological analysis by Sirius red (magnification 100×) and H&E (magnification 200×) stainings and analysis of α-SMA and B220 expressions by immunoflorescence analysis (Magnification 200×). (B) B cells enriched from the livers of WT and B-MyD88KO-CCl4-treated mice were cultured with LPS stimulation for 5 d and culture supernatants were collected and processed for cytokine/chemokine production by luminex assay. (C) NPCs from livers were processed for staining for CD11b, CD11c, GR1, and Ly6c by FACS analysis. Data are representative of two independent experiments. Two-tailed Student’s t test was applied for detection of significance (*P<0.05, **P<0.01).
Discussion
As lymphocytes, B cells are characteristically versatile and demonstrate a greater range of function through their expression of antigen-specific B cell receptors, immune modulatory receptors such as CD40, and pathogen targeting receptors such as FcRs, complement receptors, and TLRs.23–25 On one hand, the added dexterity of B cells may prove beneficial for organs having numerous biological functions such as the liver. At the same time, there may be dangers associated with an increased requirement and susceptibility for regulation, particularly during times of imbalance such as liver disease. Here, we report that B cells play an important role in hepatic fibrosis through their induction of a pathologic inflammatory milieu. In agreement with the reports 9,26, we observed a markedly diminished liver periportal bridging fibrosis in B-cell deficient (μMT) mice. Interestingly, hepatic B cells in fibrotic mice adapted a proinflammatory gene signature and pattern of behavior, producing TNF-α, IL-6, MCP-1 and MIP-1α in response to stimulation. The exacerbated innate functions of liver B cells were the result of HSC-derived RA, and mediated through B-cell-intrinsic MyD88 signaling. In the absence of MyD88 and RA signaling, HSC activation, intrahepatic mononuclear cell infiltration and liver collagen deposition were markedly reduced. Our data suggest that the mechanistic contributions of B cells in liver fibrosis occur through amplification of fibrotic processes.
While a mechanistic role for constitutive B cell antibody production during fibrosis was ruled out 9, such activity may have other unfortunate consequences for patients with liver disease. For example in humans, mixed cryoglobulinemia and other B cell disorders are the leading extrahepatic manifestations of advanced liver disease. Perturbations in B cell regulation are evident during chronic hepatitis C virus (HCV) infection, and non-viral diseases like non-alcoholic steatohepatitis (NASH) and alcoholic liver diseases (ALD).13,14,27,28 In HCV-infected individuals, mixed cryoglobulinemia (MC) present in up to 60% of patients, and associated with an increased long-term risk of developing B cell non-Hodgkin lymphoma.29 The mechanisms accounting for enhanced B cell activation and proliferation in these subjects is not known, however it’s been proposed that HCV itself may activate B cells, either through receptor-mediated stimulation or direct infection. During NASH and ALD there is an increased presence of circulating immunoglobulins and associated autoimmunity, but studies have focused on aberrant T cell responses, bacterial translocation, and neoantigen formation as potential incendiary mechanisms. Our findings suggest that interactions between B cell and HSCs within the liver likely contribute to the clinical manifestations of end-stage liver disease subjects. Further studies are needed to address the differentiation, activity and function of intrahepatic B cells in these subjects.
The recruitment, activation and inflammatory cytokine production of liver DCs, monocytes, and Kupffer cells during fibrosis is a well-characterized phenomenon, and thought to exacerbate fibrosis progression through effects on HSCs, tissue parenchyma and other innate immune populations.21,22,30 While the requirement of B cells in hepatic fibrosis has been addressed by a previous study 9, to our knowledge this is the first demonstration that liver B cells also function as innate immune cells, and induce liver fibrosis through their production of inflammatory cytokines and chemoattractants. The major discrepancies between previous and current studies include mouse strains, inoculation route of CCl4, and the time points of analysis, which perhaps explains the differences in our observations of monocytes/macrophages infiltration into the liver. In previous study, F4/80 macrophages were increased in the liver of B cell deficient (JH−/−) mice specifically at day 3 and 5 after a single gavage of CCl4. Consistent with recent studies that have established the role of monocytes/macrophages in liver fibrosis through activation of HSCs21,22, our study shows the increased infiltration of Ly6chigh monocytes, CD11b+ DCs and Gr1+ neutrophils after six weeks of CCl4 i.p. treatment. In our model, the early activation of B cells resulted in the production of soluble factors, which included both chemokines and cytokines like IL-12 and IL-6, which would have additional effects on intrahepatic lymphocyte populations. Therefore, it is likely that B cell pathology during liver fibrogenesis has general and broad effects on numerous immune cell populations. In agreement with our observations, a recent study in humans demonstrated an important role for B cells in the induction of atherosclerosis lesions, through CCl7-induced recruitment of inflammatory monocytes.31
Importantly, however, our data also reveal MyD88 as a dominant mechanistic requirement for the adaptation of innate function by liver B cells following RA exposure. The effects of RA signaling on DC activation and function within the gut were shown to be dependent on MyD88 signaling 20, consistent with our findings which we show to be involved in the upregulation of TLR4 and TLR9 expressions on B cells. MyD88 signaling is required for responsiveness to TLR agonists like LPS, which would be a significant stimulus in the liver due to the liver’s direct proximity to the gut and continuous exposure to commensal and pathogenic microbial components. In humans, end-stage liver disease is associated with instability of the gut epithelial barrier (leaky gut syndrome), characterized by circulating LPS, soluble CD14 and inflammatory cytokines within peripheral blood.32,33 In mice, enhancement of LPS/TLR4 signaling exacerbates liver disease following CCl4-treatment or bile duct ligation, and these effects were observed in animals deficient in CD14 and LPS binding protein.34,35 Direct comparison of TLR4 adaptors MyD88 and Trif using genetically engineered mice also revealed a greater mechanistic role for MyD88 in mediating fibrosis through the induction of TGF-β signaling.34 Furthermore, oral administration of broad spectrum of antibiotics has also proven successful in reducing fibrosis suggesting that microbial translocation is an important component of liver disease in general.34 One possibility is that therapeutic treatment of liver disease through disruption of RA signaling, as was performed in this study, may alleviate the LPS sensitivity of liver B cells, and consequential inflammation. Our study highlights an important pathogenic role for liver B cells that was previously unknown. We highlight the liver B cell as an important innate component of the liver immune microenvironment with significant potential as a therapeutic target.
Supplementary Material
Fig. 7.
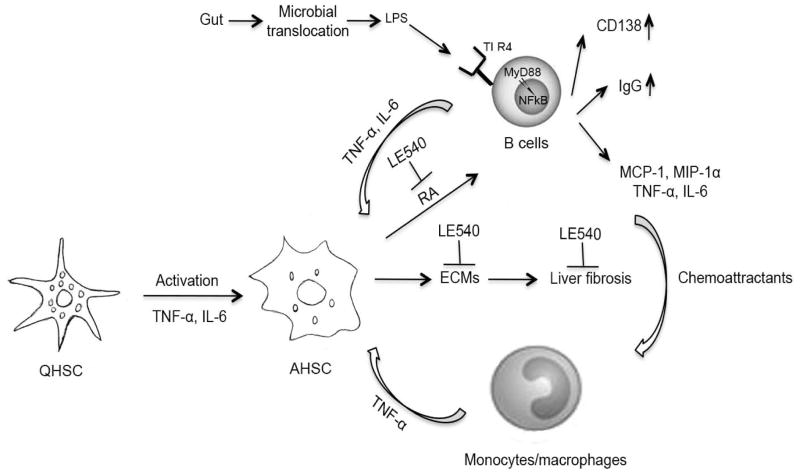
Schematic summary of B-cell-mediated liver fibrosis. HSC-derived retinoic acid (RA) augments B cell survival, plasmablast differentiation (CD138), and IgG production. HSC-mediated effect on B cells is reversible by treatment with the RA inhibitor LE540. Hepatic B cells from fibrotic mice adapt a proinflammatory gene signature and pattern of behavior, producing TNF-α, IL-6, MCP-1 and MIP-1α. Furthermore, the transcriptional profiling highlighted the importance of NFκB signaling in fibrotic liver B cells, and the activation of pathways related to TLR activity, cytokine production, and CD40 signaling. MyD88 is an important contributor to the observed pathology, as mice having a B cell-restricted deficiency in MyD88 signaling demonstrated reduced fibrosis, and reduced liver infiltration of other inflammatory cell types such as monocytes and dendritic cells. Increased gut microbial translocation and microbial products such as LPS to liver is most likely to mediate a greater activation of hepatic B cells through TLR4-MyD88 signaling pathways. In conclusion, MyD88 signaling and liver B cell production of proinflammatory cytokines and chemoattractants is a prerequisite for mononuclear cell recruitment and thus B cells serve to amplify fibrotic processes through a novel innate activity.
Acknowledgments
Financial support
We would like to acknowledge support from EVC/CFAR Flow Cytometry Core P30 AI050409, the Yerkes Research Center Base Grant RR-00165 (AG), PHS grants 1R01 DK062092-11, I01 BX001746-01 (FA), 5R37DK057665-11; 5R37AI048638-10, U19AI090023, HHSN266200700006C, U54AI057157, U19AI057266, and NO1 AI50025 (BP), a grant from the Bill & Melinda Gates Foundation (BP), and AI070101, DK083356 (AG). MSS is supported by the Children’s Healthcare of Atlanta, Emory Vaccine Center, the Georgia Research Alliance, and 5U19AI057266 and R03AI109194 from the National Institute of Health. MT was supported by the NRSA training grant T32 (2T32AI70081-06AI) and is currently supported by National Institute of Diabetes, Digestive and Kidney Diseases, National Institute of Health NRSA Fellowship (F32DK101163).
We thank John Altman, Kiran Gill and Barbara Cervasi (Flow Cytometry Core); Deepa Kodandera, Evan Dessasau and Claudia Patricia Cuellar (Pathology core) of Emory Vaccine Center (EVC) for their assistance respectively. We are grateful to Steven Bosinger, Gregory Tharp, and Nirav Patel (Genetics Core), Kalpana Patel and Benton Lawson (Virology Core), EVC for evaluation of microarray, luminex and real-time PCR data respectively. We thank Aryn Price, Surinder Kaur, Rajesh Nair, Paul Hakimata, and Toidi Adekambi for their cooperation and assistance throughout the study.
List of Abbreviations
- α-SMA
alpha-smooth muscle actin
- ALD
alcoholic liver disease
- ALT
alanine aminotransferase
- B7-H4
B7 family molecule H4
- CCl4
Carbon tetrachloride
- DCs
dendritic cells
- HCV
hepatitis C virus
- HSCs
hepatic stellate cells
- IFNs
interferons
- LE540
retinoic acid receptor antagonist LE540
- MC
mixed cryoglobulinemia
- MCP-1
monocyte chemoattractant protein-1
- MIP-1a
macrophage inflammatory protein-1a
- MyD88
Myeloid differentiation primary response gene (88)
- NASH
Nonalcoholic steatohepatitis
- NF-kB
nuclear factor κ light chain enhancer of activated B cells
- NPCs
non-parenchymal cells
- RA
Retinoic acid
- RIG-I
retinoic acid-inducible gene 1
- TLR
toll like receptor
- TNF-α
tumor necrosis factor-α
- WT
Wild type
Footnotes
Author Contributions:
MT conceived the study concept and design, performed the experiments, analyzed the data and wrote the manuscript.
RC performed the experiments, and analyzed the data.
VV analyzed the data, and wrote the manuscript.
DT and EE performed the experiments, and analyzed the data.
JHH, CI, AG (Andrew Gewirtz), FA, and BP contributed reagents and critical intellectual input to the manuscript.
PS analyzed histopathological slides, evaluated fibrosis data and contributed intellectual input.
MSS analyzed microarray data, contributed intellectual input and wrote the manuscript.
AG (Arash Grakoui) conceived the design of the experiments, analyzed the data and wrote the manuscript.
References
- 1.Kocabayoglu P, Friedman SL. Cellular basis of hepatic fibrosis and its role in inflammation and cancer. Front Biosci (Schol Ed) 2013;5:217–230. doi: 10.2741/s368. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Li J, Wang X, Sang M, Ho W. Hepatic stellate cells, liver innate immunity, and hepatitis C virus. J Gastroenterol Hepatol. 2013;28 (Suppl 1):112–115. doi: 10.1111/jgh.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88(1):125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y, Ye L, Wang X, Li J, Song L, Ho W. Retinoic acid inducible gene-I (RIG-I) signaling of hepatic stellate cells inhibits hepatitis C virus replication in hepatocytes. Innate Immun. 2013;19(2):193–202. doi: 10.1177/1753425912460414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunham RM*, Thapa M*, Velazquez VM, Elrod EJ, Denning TL, Pulendran B, et al. Hepatic stellate cells preferentially induce Foxp3+ regulatory T cells by production of retinoic acid. J Immunol. 2013;190(5):2009–2016. doi: 10.4049/jimmunol.1201937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chinnadurai R, Grakoui A. B7-H4 mediates inhibition of T cell responses by activated murine hepatic stellate cells. Hepatology. 2010;52(6):2177–2185. doi: 10.1002/hep.23953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147–163. doi: 10.1146/annurev.immunol.021908.132629. [DOI] [PubMed] [Google Scholar]
- 8.Paik J, Blaner WS, Sommer KM, Moe R, Swisshlem K. Retinoids, retinoic acid receptors, and breast cancer. Cancer Invest. 2003;21(2):304–312. doi: 10.1081/cnv-120016425. [DOI] [PubMed] [Google Scholar]
- 9.Novobrantseva TI, Majeau GR, Amatucci A, Kogan S, Brenner I, Casola S, et al. Attenuated liver fibrosis in the absence of B cells. J Clin Invest. 2005;115(11):3072–3082. doi: 10.1172/JCI24798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kimura K, Moriwaki H, Nagaki M, Saio M, Nakamoto Y, Naito M, et al. Pathogenic role of B cells in anti-CD40-induced necroinflammatory liver disease. Am J Pathol. 2006;168(3):786–795. doi: 10.2353/ajpath.2006.050314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ji H, Ohmura K, Mahmood U, Lee DM, Hofhuis FM, Boackle SA, et al. Arthritis critically dependent on innate immune system players. Immunity. 2002;16(2):157–168. doi: 10.1016/s1074-7613(02)00275-3. [DOI] [PubMed] [Google Scholar]
- 12.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3(10):944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 13.Gragnani L, Fognani E, Piluso A, Zignego AL. Hepatitis C virus-related mixed cryoglobulinemia: is genetics to blame? World J Gastroenterol. 2013;19(47):8910–8915. doi: 10.3748/wjg.v19.i47.8910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santer DM, Ma MM, Hockman D, Landi A, Tyrrell DL, Houghton M. Enhanced activation of memory, but not naive, B cells in chronic hepatitis C virus-infected patients with cryoglobulinemia and advanced liver fibrosis. PLoS One. 2013;8(6):e68308. doi: 10.1371/journal.pone.0068308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujii T, Fuchs BC, Yamada S, Lauwers GY, Kulu Y, Goodwin JM, et al. Mouse model of carbon tetrachloride induced liver fibrosis: Histopathological changes and expression of CD133 and epidermal growth factor. BMC Gastroenterol. 2010;10:79. doi: 10.1186/1471-230X-10-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350(6317):423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 17.Iwaisako K, Brenner DA, Kisseleva T. What’s new in liver fibrosis? The origin of myofibroblasts in liver fibrosis. J Gastroenterol Hepatol. 2012;27 (Suppl 2):65–68. doi: 10.1111/j.1440-1746.2011.07002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hou B, Saudan P, Ott G, Wheeler ML, Ji M, Kuzmich L, et al. Selective utilization of Toll-like receptor and MyD88 signaling in B cells for enhancement of the antiviral germinal center response. Immunity. 2011;34(3):375–384. doi: 10.1016/j.immuni.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirkland D, Benson A, Mirpuri J, Pifer R, Hou B, DeFranco AL, et al. B cell-intrinsic MyD88 signaling prevents the lethal dissemination of commensal bacteria during colonic damage. Immunity. 2012;36(2):228–238. doi: 10.1016/j.immuni.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Villablanca EJ, Wang S, de Calisto J, Gomes DC, Kane MA, Napoli JL, et al. MyD88 and retinoic acid signaling pathways interact to modulate gastrointestinal activities of dendritic cells. Gastroenterology. 2011;141(1):176–185. doi: 10.1053/j.gastro.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karlmark KR, Weiskirchen R, Zimmermann HW, Gassler N, Ginhoux F, Weber C, et al. Hepatic recruitment of the inflammatory Gr1+ monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology. 2009;50(1):261–274. doi: 10.1002/hep.22950. [DOI] [PubMed] [Google Scholar]
- 22.Ehling J, Bartneck M, Wei X, Gremse F, Fech V, Mockel D, et al. CCL2-dependent infiltrating macrophages promote angiogenesis in progressive liver fibrosis. Gut. 2014 doi: 10.1136/gutjnl-2013-306294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jagannathan M, Hasturk H, Liang Y, Shin H, Hetzel JT, Kantarci A, et al. TLR cross-talk specifically regulates cytokine production by B cells from chronic inflammatory disease patients. J Immunol. 2009;183(11):7461–7470. doi: 10.4049/jimmunol.0901517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamze M, Desmetz C, Guglielmi P. B cell-derived cytokines in disease. Eur Cytokine Netw. 2013;24(1):20–26. doi: 10.1684/ecn.2013.0327. [DOI] [PubMed] [Google Scholar]
- 25.Martin F, Chan AC. B cell immunobiology in disease: evolving concepts from the clinic. Annu Rev Immunol. 2006;24:467–496. doi: 10.1146/annurev.immunol.24.021605.090517. [DOI] [PubMed] [Google Scholar]
- 26.Shi Z, Wakil AE, Rockey DC. Strain-specific differences in mouse hepatic wound healing are mediated by divergent T helper cytokine responses. Proc Natl Acad Sci U S A. 1997;94(20):10663–10668. doi: 10.1073/pnas.94.20.10663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nouri-Aria KT, Alexander GJ, Portmann BC, Hegarty JE, Eddleston AL, Williams R. T and B cell function in alcoholic liver disease. J Hepatol. 1986;2(2):195–207. doi: 10.1016/s0168-8278(86)80078-2. [DOI] [PubMed] [Google Scholar]
- 28.Cotler SJ, Kanji K, Keshavarzian A, Jensen DM, Jakate S. Prevalence and significance of autoantibodies in patients with non-alcoholic steatohepatitis. J Clin Gastroenterol. 2004;38(9):801–804. doi: 10.1097/01.mcg.0000139072.38580.a0. [DOI] [PubMed] [Google Scholar]
- 29.Arcaini L, Merli M, Volpetti S, Rattotti S, Gotti M, Zaja F. Indolent B-cell lymphomas associated with HCV infection: clinical and virological features and role of antiviral therapy. Clin Dev Immunol. 2012;2012:638185. doi: 10.1155/2012/638185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Connolly MK, Bedrosian AS, Mallen-St Clair J, Mitchell AP, Ibrahim J, Stroud A, et al. In liver fibrosis, dendritic cells govern hepatic inflammation in mice via TNF-alpha. J Clin Invest. 2009;119(11):3213–3225. doi: 10.1172/JCI37581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zouggari Y, Ait-Oufella H, Bonnin P, Simon T, Sage AP, Guerin C, et al. B lymphocytes trigger monocyte mobilization and impair heart function after acute myocardial infarction. Nat Med. 2013;19(10):1273–1280. doi: 10.1038/nm.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin RS, Lee FY, Lee SD, Tsai YT, Lin HC, Lu RH, et al. Endotoxemia in patients with chronic liver diseases: relationship to severity of liver diseases, presence of esophageal varices, and hyperdynamic circulation. J Hepatol. 1995;22(2):165–172. doi: 10.1016/0168-8278(95)80424-2. [DOI] [PubMed] [Google Scholar]
- 33.Nolan JP. The role of intestinal endotoxin in liver injury: a long and evolving history. Hepatology. 2010;52(5):1829–1835. doi: 10.1002/hep.23917. [DOI] [PubMed] [Google Scholar]
- 34.Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13(11):1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 35.Isayama F, Hines IN, Kremer M, Milton RJ, Byrd CL, Perry AW, et al. LPS signaling enhances hepatic fibrogenesis caused by experimental cholestasis in mice. Am J Physiol Gastrointest Liver Physiol. 2006;290(6):G1318–1328. doi: 10.1152/ajpgi.00405.2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.