Abstract
Non-small cell lung cancer (NSCLC) is highly correlated with smoking and has very low survival rates. Multiple studies have shown that stem-like cells contribute to the genesis and progression of NSCLC. Our results show that the transcriptional co-activator YAP1, which is the oncogenic component of the Hippo signaling pathway, is elevated in the stem-like cells from NSCLC and contributes to their self-renewal and ability to form angiogenic tubules. Inhibition of YAP1 by a small molecule or depletion of YAP1 by siRNAs suppressed self-renewal and vascular mimicry of stem-like cells. These effects of YAP1 were mediated through the embryonic stem cell transcription factor, Sox2. YAP1 could transcriptionally induce Sox2 through a physical interaction with Oct4; Sox2 induction occurred independent of TEAD2 transcription factor, which is the predominant mediator of YAP1 functions. The binding of Oct4 to YAP1 could be detected in cell lines as well as tumor tissues; the interaction was elevated in NSCLC samples compared to normal tissue as seen by proximity ligation assays. YAP1 bound to Oct4 through the WW domain, and a peptide corresponding to this region could disrupt the interaction. Delivery of the WW domain peptide to stem-like cells disrupted the interaction and abrogated Sox2 expression, self-renewal and vascular mimicry. Depleting YAP1 reduced the expression of multiple EMT genes and prevented the growth and metastasis of tumor xenografts in mice; overexpression of Sox2 in YAP1 null cells rescued these functions. These results demonstrate a novel regulation of stem-like functions by YAP1, through the modulation of Sox2 expression.
Keywords: self-renewal, vascular mimicry, side-population cells, non-small cell lung cancer, transcriptional regulation
INTRODUCTION
Lung cancer is the leading cause of cancer related mortality in the US 1, with majority of this (85%) resulting from non-small cell lung cancer (NSCLC). Patients with early stage disease are treated by surgery, but they develop recurrent tumors 2–4. NSCLC patients acquire resistance to multiple therapeutic modalities, lead to a 5 year survival rate of about 15% 5,6. Thus, understanding the mechanisms that lead to the initiation and progression of these cancers is highly warranted. In this context, it has been hypothesized that tumor initiating cells or cancer stem-like cells might contribute to the initiation, progression, metastasis and recurrence of multiple tumor types 7–10 and this idea is gaining significant traction in the lung cancer arena 11.
Cancer stem-like cells (CSCs) are marked by their ability to initiate tumors as well as their ability to remain dormant in a quiescent state 7,9,12. They can give rise to multiple components of the tumor including the vasculature upon receiving appropriate microenvironmental cues 13–16. CSCs can be isolated from various solid tumors and cancer cell lines based on the expression of surface markers 17–20. Our earlier work had shown that Hoechst 33342 dye excluding side-population (SP) cells are enriched in cells having stem-like properties 21. SP cells displayed high self-renewal capacity, were highly drug resistant, and could form metastatic tumors in immunocompromised mice from as low as 1,000 cells 21.
At the molecular level, SP cells showed the expression of mesenchymal markers, reminiscent of epithelial-mesenchymal transition (EMT) and altered expression of Bcl2 family members, contributing to the observed drug resistance 21,22. Interestingly, the expression of stem cell factors Oct4, Sox2 and Nanog were elevated in SP cells, and Sox2 was found to be indispensable for the self-renewal of SP cells 21. Studies described in this manuscript demonstrate that YAP1 (Yes Associated Protein 1) transcriptional co-activator, which is the major downstream effector of the Hippo signaling cascade, plays a major role in the upregulation of Sox2 in stem-like SP cells from NSCLC and facilitates multiple stem-like functions of these cells.
Hippo signaling pathway is known to regulate organ size and is known to modulate the pluripotency of embryonic stem cells in various organisms 23–25. In addition, Hippo pathway has been demonstrated to have significant tumor suppressive role in various cancers, including those of the lung 24,26–28. Activation of the Hippo pathway by upstream regulators Merlin/NF2, Mst1/2, Lats1/2, Mob and Sav1 inactivates the oncogenic YAP1 protein (or its paralogue TAZ) by cytoplasmic sequestration or degradation 29–32. While it has been reported that YAP1 promotes the growth of lung cancers, the underlying molecular mechanisms have not been elucidated. Here we report that YAP1 mRNA and protein is expressed at a higher level in stem-like SP cells from NSCLC. Depletion of YAP1, or inhibition using the small molecule inhibitor Visudyne abolished the self-renewal property as well as the ability of the SP cells to form angiogenic tubules. Interestingly, we find that YAP1 induces Sox2 expression, and this required a physical interaction of YAP1 with Oct4. YAP1 could act as a transcriptional co-activator for Oct4 and the disruption of this interaction using a specific peptide abrogated the induction of Sox2, self-renewal as well as angiogenic tubule formation of SP cells. Further, YAP1-Oct4 interaction was elevated in lung tumor tissue compared to adjacent normal tissues, suggesting that the interaction between these two proteins might have contributed to the onset of NSCLC. Thus, the studies presented here identify a novel mechanism of regulation of Sox2 by the Hippo pathway effector YAP1, through its interaction with the Oct4 transcription factor.
MATERIALS AND METHODS
Cell lines
The human NSCLC cell lines A549, H1650 and H1975 were purchased from ATCC, A549 cells were maintained in Ham’s F12K medium (Cell Gro) supplemented with 10 % fetal bovine serum (Atlas Biologicals) while H1650 and H1975 cells were grown in RPMI 1640 (Gibco, Life Technologies) containing 10 % FBS. hMSCs (human Mesenchymal Stem cells) were purchased from Lonza and were grown in MSCBM medium (Lonza) supplemented with MSCGM kit (Lonza). All the cultures were maintained at 5 % CO2 at 37°C. Detailed experimental procedures applied in the present study are provided in supplementary methods section.
RESULTS
YAP1 levels are elevated in lung cancers and stem-like SP cells of NSCLC
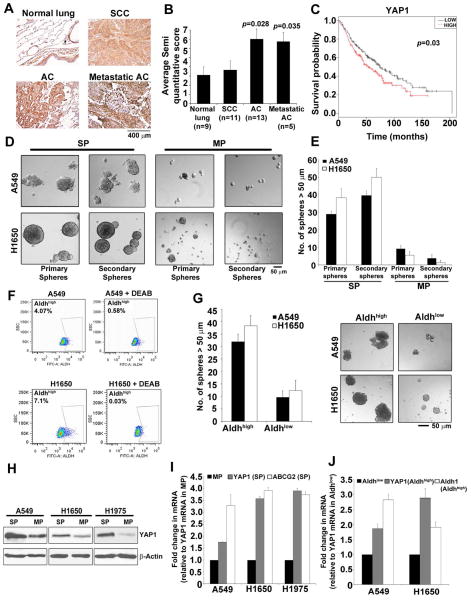
Immunohistochemistry conducted on a human lung cancer tissue microarray using a YAP1 antibody showed that YAP1 levels were significantly elevated in both primary and metastatic lung adenocarcinoma samples compared to normal tissue (Figure 1A); in contrast, YAP1 levels in squamous cell carcinomas were comparable to that in normal tissues (Figure 1B). Analysis of expression data from Director’s challenge set 33 showed a significant correlation between higher levels of YAP1 and poor prognosis (Figure 1C; p=0.03). These data suggested that YAP1 might be contributing to the genesis of lung adenocarcinomas, and its elevated levels correlate with poor overall survival.
Figure 1. Higher expression of YAP1 in NSCLC CSCs.
(A) Elevated YAP1 staining is seen in human lung adenocarcinoma and its metastatic sites as compared to the normal human lung tissue in TMA. SCC, Squamous cell carcinoma; AC, Adenocarcinoma. (B) Quantitation of IHC performed on the TMA shows a 3-fold increase in YAP1 expression in human lung adenocarcinoma tissue. (C) Significant correlation between higher levels of YAP1 and poor prognosis is observed by survival analysis of data from Director’s challenge set. (D) SP cells from A549 and H1650 cells show the ability to self-renew in serial sphere-formation assays in stem cell selective medium. (E) Quantitation of the average number of spheres formed from 1000 cells plated for serial sphere formation assay exhibit higher number of primary and secondary spheres in SP cells as compared to MP cells from A549 and H1650 cells. (F) Aldefluor staining of A549 and H1650 cells shows the presence of Aldhhigh population in both A549 and H1650 cells. Aldh activity inhibitor DEAB, is used to gate and separate the Aldhhigh cells. (G) Sphere formation assay and its quantitation show the ability of Aldhhigh population to form spheres in stem cell selective medium in both A549 and H1650 cells. The numbers of spheres are more in Aldhhigh population than the Aldhlow from both A549 and H1650 cells. (H) Western blot analysis on isolated SP and MP cells from NSCLC cell lines for YAP1 protein exhibit higher YAP1 protein levels in A549, H1650 and H1975 SP cells as compared to MP cells. β-Actin is used to show equal amounts of protein in the lanes. (I) qRT-PCR analysis of mRNA from SP and MP cells of A549, H1650 and H1975 cell lines reveal higher levels of YAP1 mRNA in SP cells. ABCG2 mRNA expression is used as positive control for SP cells (J) Elevated levels of YAP1 mRNA seen in sorted Aldhhigh cells compared to Aldhlow cells from A549 and H1650. The mRNA expression of Aldehyde dehydrogenase-1 (Aldh1) is used as a positive control.
Since YAP1 plays a role in maintaining stemness in embryonic stem cells (ESCs) 34, we investigated whether it contributes to the functions of cancer stem-like cells from NSCLC. Earlier studies from our laboratory had shown that SP cells from NSCLC cell lines or patient tumor explants have stem-like properties, and have the ability to self-renew and to initiate tumors in mice 21,22. Supplementary Figure S1A shows a schematic of SP and MP cell isolation, based on Hoechst 33342 dye exclusion. SP cells could form spheres in serial sphere formation assays in stem cell selective medium, whereas MP cells did not (Figure 1D and 1E, Supplementary Figure S1B). Similar to the SP cells, aldehyde dehydrogenase high (Aldhhigh) cells from A549 and H1650 cells also formed spheres efficiently (Figure 1F and 1G), confirming the existence of a sub-population of cells within these cell lines that could self-renew and display stem-like properties. Western blotting showed that YAP1 expression was significantly higher in A549, H1650 and H1975 SP cells compared to MP cells (Figure 1H); these results were supported by qRT-PCR experiments (Figure 1I). YAP1 mRNA levels were higher in stem-like Aldhhigh cells compared to Aldhlow cells from both A549 and H1650 cell lines (Figure 1J), indicating that YAP might be contributing to their self-renewal.
Loss of YAP1 decreases the self-renewal potential of NSCLC cancer stem cells
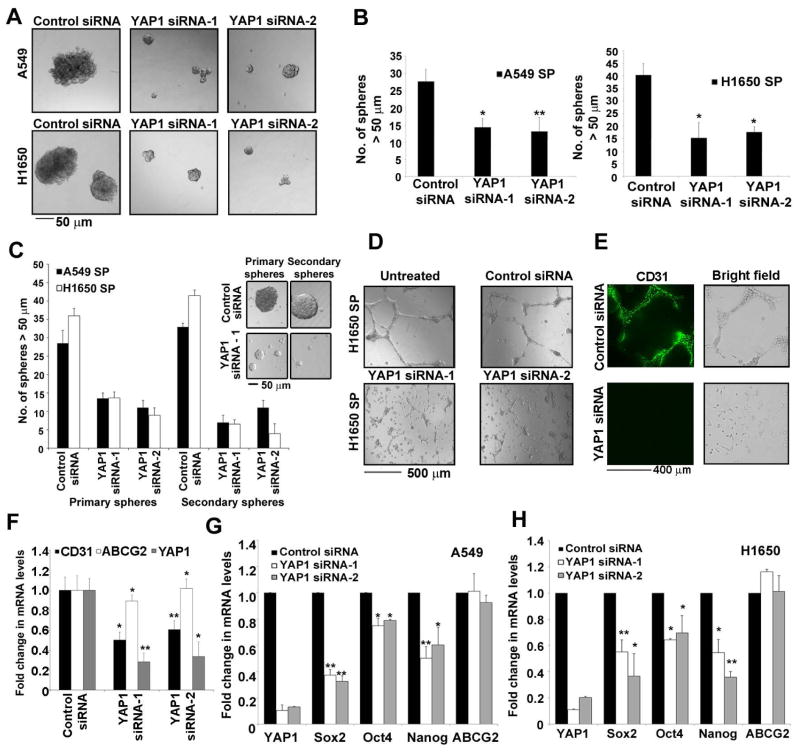
Attempts were made to determine whether YAP1 contributes to the stem-like functions of SP cells. It was found that depletion of YAP1 in A549 and H1650 cells using siRNAs resulted in lower frequency of SP cells (Supplementary Figure S1C and S1D). The effect of depleting YAP1 on the self-renewal of SP cells was examined by sphere formation assays. SP cells from both A549 and H1650 cells transfected with YAP1 siRNAs formed significantly less number of spheres compared to control siRNA transfected cells as seen in case of Sox2 siRNA treated SP cells (Figure 2A and 2B, Supplementary figure S1E); the spheres were markedly smaller as well, suggesting that YAP1 is necessary for the self-renewal of SP cells. Confirming these results, the YAP1 siRNA treated cells could not form spheres upon serial passage to a second generation (Figure 2C).
Figure 2. YAP1 silencing abrogates the self-renewal ability of CSCs.
(A) Sphere formation assay with SP cells from A549 and H1650 transfected with two different siRNA against YAP1 show smaller spheres as compared to a non-target control siRNA (B) Average number of spheres generated from 1000 SP cells reveal fewer number of spheres in YAP1 siRNAs treated cells as compared to the control siRNA in both cell lines. (C) Quantitation of serial sphere formation assay show fewer spheres in SP cells isolated from A549 and H1650 cells transfected with YAP1 siRNAs as compare to control siRNA treatment. Bright field images of the spheres are presented here. (D) SP cells isolated from YAP1 siRNAs treated H1650 cells show abrogation of angiogenic tubule-like structure formation when grown on Matrigel in endothelial growth medium. (E) Loss of CD31 expression on angiogenic tubule-like structures in SP cells from YAP1 siRNA treated H1650 as visualized by immunofluorescence. (F) Real time PCR analysis of YAP1 siRNA treated H1650 cells show a decrease in CD31 mRNA expression as compared to control siRNA treated cells. (G–H) Real time PCR analysis of YAP1 siRNAs transfected A549 (G) and H1650 (H) cells respectively for YAP1, Sox2, Oct4, Nanog and ABCG2 genes show decrease in Sox2, Oct4, Nanog expression. The above data is expressed as mean ± SD of three independent experiments. * represents p < 0.05, ** represents p < 0.01.
Many tumors have been shown to demonstrate the capacity for vascular mimicry, where certain cells acquire endothelial features and give rise to angiogenic tubules 35, 36–38; this property has been reported in glioma stem cells 13,39,40. SP cells from NSCLC cell lines, could form CD31+ angiogenic tubules like structures in matrigel, but MP cells could not 22; interestingly, while SP cells from untransfected or control siRNA transfected H1650 cells formed tubular structures in matrigel, depletion of YAP1 with siRNAs significantly impaired the formation of tubules (Figure 2D). The vascular nature of the tubules was confirmed by the expression of CD31, as seen by immunofluorescence (Figure 2E). Depletion of YAP1 also reduced the levels of CD31 mRNA, as seen by qRT-PCR; ABCG2 was used a control, since it governs the side population phenotype (Figure 2F).
To understand the mechanisms by which YAP1 regulates self-renewal, we examined the expression of ES cell transcription factors Sox2, Oct4 and Nanog by qRT-PCR in A549 and H1650 cells that were transfected with two different siRNAs to YAP1 or a non-targeting control siRNA. Sox2 mRNA was reduced in both the cell lines upon depletion of YAP1 (Figure 2G and 2H). Similarly, Oct4 and Nanog expression was also reduced upon YAP1 depletion, but to a lesser extent (Figure 2G and 2H). There was no reduction in the levels of ABCG2 mRNA; these results indicate that loss of YAP1 lowers the self-renewal ability of SP cells by reducing the expression of ES cell transcription factors. Since we had found that Sox2 is the major modulator of stemness of SP cells from NSCLC 21, we hypothesized that YAP1 regulates self-renewal by modulating Sox2.
YAP1 inhibition by Visudyne abrogates self-renewal of CSCs from NSCLC
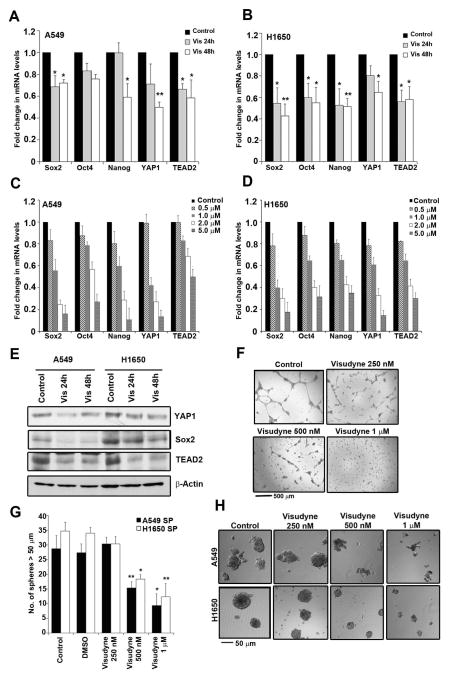
Visudyne (Verteporfin) is used as a photosensitizer in photodynamic therapy for neovascular macular degeneration, where its activation by light generates reactive oxygen species (ROS) 41,42. However, Visudyne was found to inhibit YAP1 by altering its conformation and disrupting its interaction with other proteins; this did not require light activation43. Attempts were made to assess whether treatment with Visudyne affected the expression of Sox2 and YAP1 in the cells. A qRT-PCR analysis showed that treatment with Visudyne for 24 and 48 h led to a decrease in the mRNAs of Sox2, Oct4, Nanog, YAP1 and TEAD2 in both A549 and H1650 cells (Figure 3A and 3B) in a dose dependent fashion (Figure 3C and 3D). Similarly, Sox2 and YAP1 protein levels were reduced in Visudyne treated cells, as seen by western blotting (Figure 3E). In addition, Visudyne treatment abrogated the ability of the H1650 SP cells to form angiogenic tubules in matrigel (Figure 3F). Additional experiments showed that Visudyne could reduce the number and size of spheres formed, suggesting that Visudyne can inhibit the self-renewal of SP cells (Figure 3G and 3H), probably by reducing the levels of Sox2. These results raise the possibility that Visudyne might be effective in reducing the self-renewal of CSCs and could be a potential therapeutic agent for lung cancer.
Figure 3. Visudyne inhibits self-renewal of CSCs.
(A–B) Real time PCR (qRT-PCR) analysis of 1 μM Visudyne treated A549 (A) and H1650 (B) for 24 and 48 h respectively show decrease in stem cell factors like Sox2, Oct4, Nanog as well as YAP1 and TEAD2. (C–D) qRT-PCR analysis of expression of Sox2, Oct4, Nanog, YAP1 and TEAD2 upon treatment with various concentrations of Visudyne (0.5, 1, 2 and 5 μM) in A549 (C) and H1650 (D) cells for 48 hr. (E) Western blot analysis with 1 μM Visudyne treated A549 and H1650 cells exhibit a decrease in YAP1, Sox2 and TEAD2. β-Actin levels show equal amount of protein in all samples. (F) Visudyne treatment on SP cells sorted from H1650 cell line show decrease in angiogenic tubule-like structures when grown on Matrigel in endothelial cell growth medium. (G) Quantitation of spheres formed in a sphere formation assay with 1000 cells per treatment show fewer number of spheres with increasing concentration of Visudyne in SP cells from both A549 and H1650 cell lines. (H) Bright field images of spheres formed by A549 and H1650 SP cells exhibit decrease in self renewal ability of SP cells with Visudyne treatment. The above data is expressed as mean ± SD of three independent experiments. * represents p < 0.05, ** represents p < 0.01.
Sox2 gene expression is regulated by YAP1
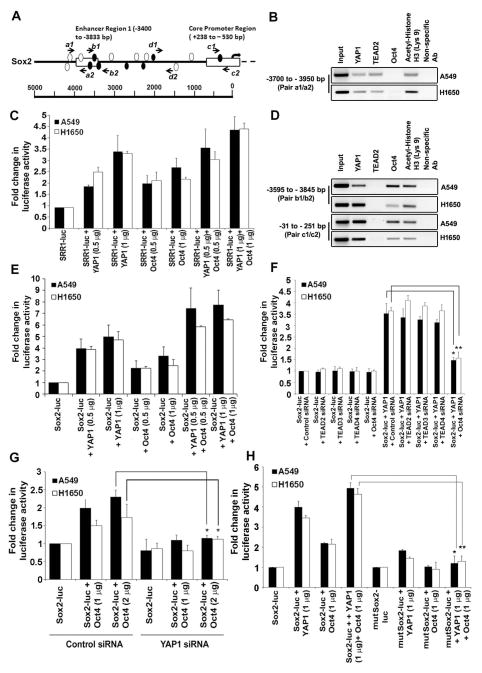
Since Sox2 was critical for the self-renewal of SP cells and because depletion of YAP1 reduced Sox2 expression, we examined whether YAP1 could regulate the expression of Sox2. An analysis of the upstream regulatory regions of Sox2 using Matinspector program showed the presence of multiple TEAD2 binding sites, especially in the Sox2 Regulatory Region 1 (SRR1) (clear ellipses in Figure 4A). Since YAP1 is a transcriptional co-activator of TEAD2, chromatin immunoprecipitation (ChIP) assays followed by qRT-PCR were conducted on A549 and H1650 cells to assess whether YAP1 and TEAD2 are associated with SRR1 enhancer upstream of the TSS 44; the location of the primers is indicated by arrows in the Figure 4A. YAP1 and TEAD2 could be detected on the SRR1 enhancer region (pair a1/a2) (Figure 4B and Supplementary Figure S2A); acetylated histone H3 was used as a positive control and a non-specific IgG was used a negative control for the assay. Transient transfection experiments were conducted to assess whether the association of YAP1 with the SRR1 region affected Sox2 expression. It was found that overexpression of YAP1 could induce a SRR1-luc reporter construct in a dose-dependent fashion in both A549 and H1650 cells (Figure 4C).
Figure 4. YAP1 regulates Sox2 gene expression through Oct4 transcription factor.
(A) A schematic representation of Sox2 proximal core promoter and enhancer region 1 showing potential TEAD2 binding sites represented as clear ellipses and Oct4 binding sites as filled ellipses. The arrows represent the position of primers spanning the TEAD2 and Oct4 binding sites tested for ChIP assays. (B) ChIP assays conducted on asynchronously growing A549 and H1650 cells with the indicated antibodies show presence of YAP1 on SRR1 region. Acetylated Histone H3 (Lys9) was used as positive control and non-specific IgG was used as the negative control for immunoprecipitation. (C) Transient transfection experiments in A549 and H1650 cells with co-transfection with YAP1 and Oct4 expression vectors showed an additive effect on SRR1 enhancer-luc activity. Control lanes had the reporter with empty vector. (D) Presence of YAP1 on Sox2 core promoter and SRR1 enhancer region through Oct4 binding site is confirmed by ChIP assays conducted on asynchronously growing A549 and H1650 cells using the indicated antibodies. (E) Transient transfection assays in A549 and H1650 cells transfected with YAP1 or Oct4 expression vectors showed an increase in Sox2 core promoter-luc activity. An additive effect on Sox2 core promoter-luc activity is observed when YAP1 and Oct4 are co-expressed. (F) Sox2 core promoter-luc activity is reduced in both A549 and H1650 cells upon depletion of Oct4 using siRNA in the presence of exogenous YAP1; transfection of TEAD2, TEAD3 and TEAD4 siRNA had no significant effect. (G) Transient transfections in YAP1 depleted cells with siRNA treatment show reduction in Sox2 core promoter-luc activity with Oct4 overexpression as compared to non-target control siRNA treated A549 and H1650 cells. (H) Transient transfections of Sox2 core promoter-luc with mutated Oct4 binding (mutSox2-luc) site exhibit significantly reduced promoter activity even with increased exogenous YAP1 and Oct4 expression. The above data is expressed as mean ± SD of three independent experiments. * represents p < 0.05, ** represents p < 0.01.
In addition to TEAD2, multiple binding sites for Oct4 transcription factor were also predicted on the SRR1 enhancer region (filled ellipses in Figure 4A). Further experiments were conducted to assess whether these sites in SRR1 were functional. Transient transfection experiments showed that Oct4 could induce the SRR1 enhancer region (Figure 4C); interestingly, co-transfection of YAP1 further increased the Oct4-mediated induction of SRR1 enhancer (Figure 4C). ChIP-qRT-PCR assays were conducted using a primer set that spans Oct4 binding sites, but not TEAD2 binding sites (primer pair b1/b2); YAP1 and Oct4 could be detected on the SRR1 region at the Oct4 binding site (Figure 4D and Supplementary Figure S2B). Since Oct4 is known to transcriptionally upregulate Sox2 expression in a reciprocal regulatory loop 45, these observations indicate that YAP1 can possibly act as a co-activator for Oct4 as well.
An examination of the Sox2 core promoter showed the presence of a single Oct4 binding site; given that YAP1 could induce SRR1 through the Oct4 binding sites, experiments were conducted to assess whether YAP1 could also induce the Sox2 core promoter 46,44. Interestingly, ChIP-qRT-PCR assays on A549 and H1650 cells detected the presence of endogenous YAP1 and Oct4 on the Sox2 core promoter (primer pair c1/c2) (Figure 4D and Supplementary Figure S2C). To rule out non-specific binding and amplification, we also performed ChIP-qRT-PCR analysis on another region upstream of Sox2 transcription start site (TSS) that is devoid of any TEAD or Oct4 predicted binding site (primer pair d1/d2 in Figure 4A). The assay showed no binding of YAP1, Oct4 and TEAD2 to this region (Supplementary Figure S2D). Functionality of the Oct4 binding site in the core promoter region was tested by transient transfection experiments. It was found that an Oct4 expression vector could induce a Sox2 core promoter-luc reporter by 2–3 fold (Figure 4E). Interestingly, co-transfection of a YAP1 expression vector with the Sox2 core promoter-luc reporter led to a 5-fold induction of the promoter, while co-transfection of YAP1 and Oct4 led to an additive induction of the promoter (Figure 4E). This suggests that the Oct4 binding site on the Sox2 core promoter is functional and YAP1 can induce Sox2 through this region, possibly through Oct4. This possibility was verified by additional transient transfection experiments. A549 and H1650 cells were transfected with siRNAs against TEAD2, TEAD3 or TEAD4, which are the TEAD proteins known to bind to YAP1, or a siRNA against Oct4; cells were then transfected with the Sox2 core promoter-luc reporter, along with a YAP1 expression vector. It was found that depletion of TEAD2, TEAD3 or TEAD4 had no effect on the YAP1-mediated induction of the Sox2 promoter in either cell line, while depletion of Oct4 significantly reduced the YAP1-mediated expression of the reporter (Figure 4F). This experiment suggests that YAP1 utilizes Oct4 to induce the Sox2 core promoter. In a complementary experiment, it was found that depletion of YAP1 significantly reduced the ability of Oct4 to induce the core promoter, suggesting that YAP1 is necessary for Oct4 to induce this promoter (Figure 4G).
To further confirm the role of Oct4 in YAP1-mediated induction of the Sox2 core promoter, the predicted binding site for Oct4 was mutated by site-specific mutagenesis. Asynchronous A549 and H1650 cells were transiently transfected with mutated Sox2 core promoter-luciferase construct (mutSox2-luc) along with YAP1 expression vector; it was found that Oct4 could not induce the promoter when the site was mutated, as expected (Figure 4H); interestingly, YAP1 could not induce this promoter either, clearly indicating that YAP1 induces the Sox2 promoter through the mediation of Oct4. These observations raise the possibility that YAP1 could be facilitating stemness and self-renewal through upregulating Oct4-mediated induction of Sox2.
YAP1 protein directly interacts with Oct4 transcription factor
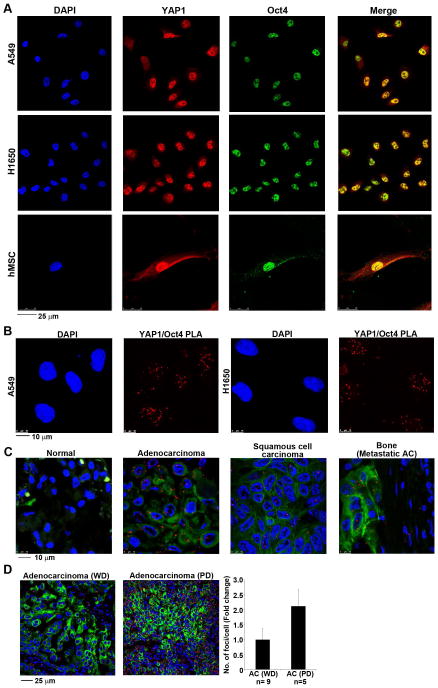
Since it was found that YAP1 could enhance Oct4-mediated induction of the Sox2 promoter, attempts were made to assess whether these proteins physically interact with each other. Towards this purpose, a double immunofluorescence experiment was conducted on A549 and H1650 cells and the localization of the proteins examined by confocal microscopy. It was found that a significant amount of Oct4 and YAP1 proteins were localized in the nucleus of these cells (Figure 5A); merging the images showed a significant co-localization of the two proteins in both the cell lines (Figure 5A, top two panels). A similar experiment was conducted to assess whether this interaction was present only in lung cancer cells, or can be detected also in primary stem cells. A double immunofluorescence experiment on human mesenchymal stem cells showed a similar co-localization of Oct4 with YAP1 in the nucleus (Figure 5A, bottom panels). We tested the physical interaction between these proteins using a proximity ligation assay (PLA) using the Duolink system 47–49. The physical interaction could be detected in A549 and H1650 cells as red foci (Figure 5B); there was no interaction when only one antibody was used (Supplementary Figure S3A). Taken together, these results clearly show that YAP1 can physically interact with Oct4 in the cells.
Figure 5. YAP1 directly associates with Oct4 transcription factor.
(A) Immunofluorescence staining reveal YAP1 co-localizing with Oct4 in asynchronous A549 and H1650 NSCLC cell lines (top) and in human mesenchymal stem cells (hMSCs) (bottom). Representative confocal images of are presented here. DAPI is used to stain the nucleus. (B) Detection of YAP1-Oct4 protein-protein interaction using proximity ligation assay (PLA) in asynchronous A549 and H1650 cells. Multiple PLA foci are observed in the nucleus of the cells showing the association of YAP1 and Oct4 protein in both cell lines. (C) Proximity ligation assay performed on a human lung tissue microarray show strong association of YAP1 and Oct4 in lung tumor tissue as compared to the normal lung tissue. The red foci represent the PLA detection for YAP1 and Oct4 association. The TMA is also treated with anti-pan cytokeratin to detect the tumor areas in the tissue samples. DAPI is used to stain the nucleus. Representative confocal images captured for normal lung, adenocarcinoma, squamous cell carcinoma and metastatic adenocarcinoma to bone are shown here. (D) Proximity ligation assay on human lung tissue display a higher interaction of YAP1 and Oct4 proteins in poorly differentiated as compared to well differentiated lung adenocarcinoma. The quantitation of fold change in number of foci per cell shows a 2-fold increase in the signal from poorly differentiated lung adenocarcinomas. Adenocarcinoma well differentiated (WD), Adenocarcinoma poorly differentiated (PD).
We investigated the association of YAP1 with Oct4 in lung tumor tissues by performing a PLA on a lung TMA; there was a higher interaction of these two proteins in lung adenocarcinomas compared to normal tissues (Figure 5C). The association between the two proteins, represented by the number of foci, was very low in the normal lung tissue samples (Supplementary Figure S3B). The interaction could also be detected in squamous cell carcinomas as well as metastatic adenocarcinomas, but the association was lesser than in those tissues as compared primary adenocarcinomas. (Figure 5C, Supplementary Figure S3B). Interestingly, the numbers of foci were higher in poorly differentiated adenocarcinoma versus well differentiated ones (Figure 5D). Such an observation was not made for squamous cell carcinoma. The interaction was also detected in tumor stroma and in the inflammatory cells suggesting that this association might play a role in tumor microenvironment as well (Figure 5C, Supplementary Figure S3B).
YAP1 interacts with Oct4 through its WW domain
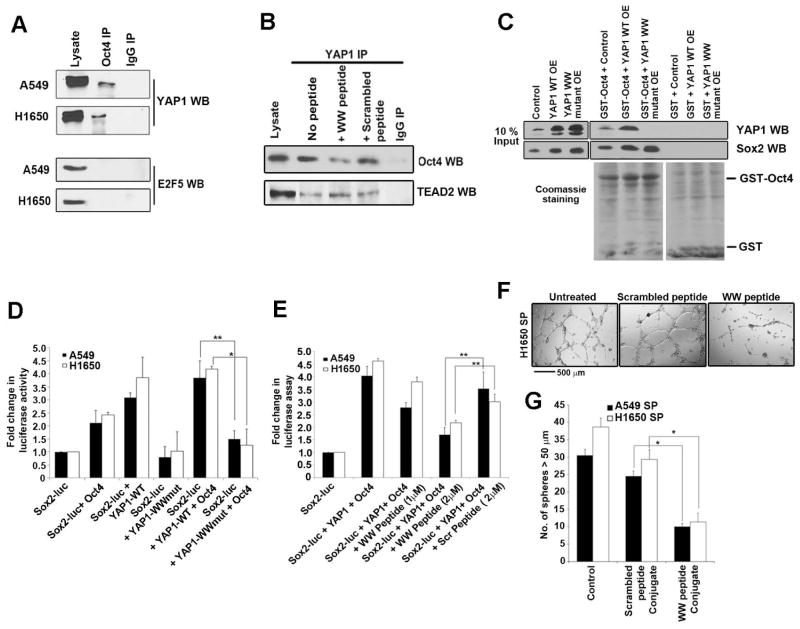
A co-immunoprecipitation (co-IP) experiment was performed to assess whether the binding of the YAP1 and Oct4 could be detected in cell lysates. A significant amount of YAP1 could be detected in the Oct4 immunoprecipitate, but not in the control IgG immunoprecipitate, confirming the specificity of the assay (Figure 6A). The same result was observed in a reverse immunoprecipitation experiment (data not shown). A western blot was conducted for E2F5, which does not bind to Oct4, as a negative control (Figure 6A).
Figure 6. YAP1 WW domain interacts with Oct4 transcription factor.
(A) YAP1-Oct4 binding is detected by immunoprecipitation with an Oct4 antibody followed by western blot analysis with YAP1 in asynchronous A549 and H1650 cells. Normal IgG was used as a control for the IP reaction, and western blot for E2F was a negative control for Oct4 binding. (B) YAP1-Oct4 interaction is disrupted by a WW domain peptide. The presence of Oct4 in YAP1 immunoprecipitation was abolished by the WW domain peptide, but not a scrambled peptide, as seen by western blotting; binding of TEAD2 to YAP1 was not affected. Normal IgG is used as a control for the IP reaction. (C) An in vitro binding assay using 293T cell lysate overexpressing wild type YAP1 (YAP1 WT OE) or WW mutant YAP1 (YAP1 WW mutant OE) and GST-Oct4 showing that mutation in the WW domain of YAP1 abrogates its binding to Oct4. (D) Expression of YAP1 WW domain mutant lacks the ability to induce the Sox2 core promoter-luc activity alone or in the presence of Oct4 exogenous expression as shown in a luciferase assay. The increased Sox2 core promoter-luc activity is seen when YAP1 WT construct was used alone or in combination with Oct4. 1 μg of DNA is used for all the plasmid constructs in this assay (E) WW domain peptide conjugated to penetratin (WW peptide conjugate) inhibited the YAP1 mediated induction of Sox2 core promoter-luc activity, but a scrambled peptide conjugated to penetratin (Scrambled peptide conjugate) did not. (F) WW peptide conjugate, but not a scrambled peptide conjugate, abrogates angiogenic tubule formation by H1650 SP cells (G) SP cells from A549 and H1650 cell lines form less number of spheres when with WW peptide conjugate, but not scrambled peptide conjugate. The above data is represented as mean ± SD of three independent experiments. * represents p < 0.05, ** represents p < 0.01.
Next we attempted to disrupt the YAP1-Oct4 interaction. Towards this, we synthesized a peptide corresponding to the WW domain of YAP1 while a peptide with the sequence scrambled was used as control; a cysteine residue was added to the C-terminus of both the peptides to facilitate coupling to carrier molecules. It was found that the WW peptide, but not the scrambled peptide, could significantly reduce the binding of YAP1 to Oct4, but not TEAD2, in IP-WB experiments (Figure 6B). YAP1 interacts with multiple transcription factors through its WW domain 50–52 and Oct4 has been shown to interact with WW domain proteins 53. Experiments were conducted to confirm that the WW domain mediates the Oct4-YAP1 interaction. An in vitro GST binding assay showed that lysates from cells overexpressing wild type YAP1 (YAP1 WT OE) bound to GST-Oct4 whereas lysates from cells overexpressing a WW domain mutant YAP1 (YAP1 WW mutant OE) did not (Figure 6C); Sox2 was used as a positive control. We next carried out a luciferase assay to assess whether WW domain of YAP1 is necessary for enhancing Oct4-mediated transcription. A549 and H1650 cells were transiently transfected with Sox2 core promoter luc construct with an Oct4 expression vector and either YAP1-WT or YAP1-WW-mutant expression vectors (Figure 6D). It was found that the YAP1-WW-mutant vector could not increase the promoter activity alone or in conjunction with Oct4 (Figure 6D).
Experiments were then conducted to assess how the disruption of the YAP1-Oct4 interaction using the WW peptide affects Sox2 transcription. To examine this, the WW peptide or the scrambled peptide was conjugated to the carrier molecule penetratin for delivery into cells. Disruption of the Oct4-YAP1 interaction significantly reduced the YAP1-mediated induction of Sox2 core promoter luc activity (Figure 6E). Further, delivery of the WW peptide, but not the scrambled peptide, significantly reduced angiogenic tubule formation (Figure 6F) as well as the self-renewal of SP cells (Figure 6G). These results suggest that YAP1 physically interacts with Oct4, and disrupting this interaction prevents self-renewal as well as differentiation into vascular structures.
YAP1 knockdown reduces the mesenchymal features of cells
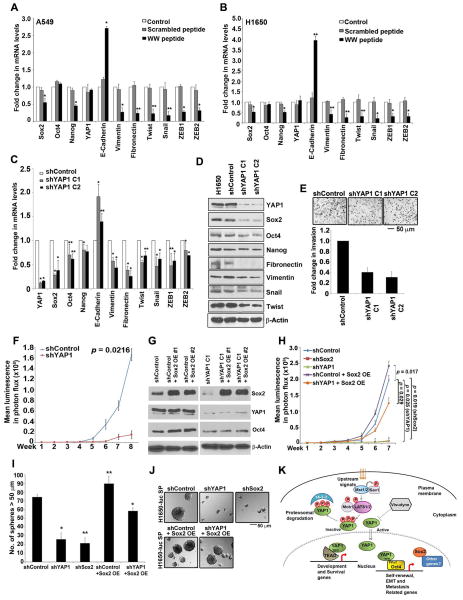
One of the features of CSCs is that they have EMT like properties 54, and our previous work had shown that SP cells from NSCLC express mesenchymal markers indicative of EMT 21,54. To assess whether YAP1-Oct4 interaction contributes to the mesenchymal phenotype, we examined the expression of EMT associated genes by qRT-PCR in A549 and H1650 cells treated with YAP1 WW peptide conjugate (Figure 7A–7B). A significant decrease in Fibronectin and Vimentin mRNA was observed upon treatment with WW peptide, but not scrambled peptide. Similarly, levels of Twist, Snail, ZEB1 and ZEB2 were also reduced upon WW peptide treatment (Figure 7A–7B) with a corresponding increase in E-cadherin mRNA. This suggests that the interaction of YAP1 with Oct4 might regulate EMT associated gene expression.
Figure 7. Sox2 expression rescues loss of stem-like and tumor growing features of YAP1 null cells.
(A–B) qRT-PCR analysis of A549 (A) and H1650 (B) cells treated with YAP1 WW peptide conjugated to penetratin for 48 h show a change in EMT related genes as compared to cells treated with Scrambled peptide penetratin conjugate. (C) Real time PCR analysis of two clones of YAP1 null H1650-luc cells (clones C1 and C2) show a reduction in YAP1, Sox2 and Oct4 mRNA expression as compared to shControl H1650-luc cells. Also, a significant change in mRNA of genes associated with epithelial and mesenchymal features is observed in both YAP1 null clones (C1 and C2). (D) Western blot analysis reveal decrease in YAP1 and Sox2 protein expression in YAP1 null H1650-luc cells as compared to shControl H1650-luc cells. A marked reduction in the levels of mesenchymal proteins that change during EMT is also seen with YAP1 knock down in H1650-luc cells. (E) YAP1 null H1650-luc cells (clone C1 and C2) show reduced invasive properties as seen in Boyden chamber assay in comparison with shControl H1650 cells. The representative bright field images of the cells on the membrane are shown here. (F) Tumor growth monitored in mice injected with shYAP1 H1650-luc cells showed significantly lower tumor growth as compared to mice injected with shControl cells. (G) Western blot analysis shows the overexpression of Sox2 protein in shControl and shYAP1 H1650-luc cells stably transfected with a Sox2 expression vector. (H) Depletion of Sox2 or YAP1 reduced the growth of orthotopically implanted tumors as compared to shControl H1650-luc cells. Stable over expression of Sox2 rescued the tumor growing abilities of YAP1 knock down H1650-luc cells. (I–J) SP cells from H1650-luc cells showed significantly reduced self-renewal when Sox2 or YAP1 was depleted, as seen in a sphere formation assay; sphere forming capacity of YAP1 knock depleted was rescued by stable overexpression of Sox2. The above data represents the mean ± SD, *, p < 0.05, **, p < 0.01 (K) Schematic representation of proposed mechanism of YAP1 mediated regulation of stemness and in NSCLC SP cells. TBD, Tead binding domain; WW, WW domain; PPxY, PPxY motif.
To further understand the role of YAP1 protein in EMT, a YAP1 knock down H1650-luc cell line was established by stably transfecting shRNA for YAP1; two different clones were validated and used for these studies. There was a significant reduction in the levels of Sox2 and Oct4 mRNA, as seen by qRT-PCR; Nanog levels were affected to a lesser extent (Figure 7C). In addition, there was a marked reduction in the expression of Fibronectin and Vimentin genes (Figure 7C). Further, the levels of Snail and Twist, which suppress E-cadherin, were reduced as well. The above changes were validated at the level of protein by Western blotting (Figure 7D). Similar results were observed by qRT-PCR (Supplementary Figure S4A) and western blot analysis (Supplementary Figure S4B) when YAP1 was depleted in H1650 cells using two different siRNAs.
We next investigated if YAP1 plays a role migration and invasion of cells. Migration was measured by a wound-healing assay on plastic; YAP1 knockdown cells migrated at a much lower rate compared to shControl cells (Supplementary Figure S4C). In addition, the YAP1 silenced cells showed significantly reduced capacity to invade in a Boyden chamber assay (Figure 7E).
To further investigate the role of YAP1 in tumor growth and metastasis, we orthotopically implanted the H1650-luc shControl and shYAP1 cells into the right lung of the SCID mice. The growth of tumors in the mice was monitored weekly by imaging using an IVIS200 system. The mice implanted with shYAP1 cells showed significantly impaired tumor growth as compared to the mice implanted with shcontrol cells (Figure 7F; Supplementary Figure S4D Lung). Also, metastatic foci were detected in the liver, brain and adrenals of mice in shControl group but none of the mice in shYAP1 group showed distant metastases (Supplementary Figure S4D). These experiments strongly suggest that YAP1 plays a significant role in the growth and metastasis of NSCLC, through modulating genes involved in stemness as well as EMT.
We next designed experiments to confirm that these effects of YAP1 are mediated through Sox2. Towards this purpose, Sox2 was depleted in the above H1650-Luc cell lines by stable transfection of Sox2 shRNA. The downregulation of Sox2 in two clones (shSox2 A1 and A2) was confirmed by western blotting (Supplementary Figure S4E). To verify that the reduced tumor growth observed in YAP1 null cells was due to reduced levels of Sox2, we also stably overexpressed Sox2 in shYAP1 cells (shYAP1 Sox2 OE #1 and #2; Figure 7G).
We next examined the ability of shSox2 A2 clone, shControl Sox2 OE #1 and shYAP1 Sox2 OE #1 H1650-luc cells to form tumors, by orthotopically implanting them into the right lung of SCID mice. IVIS 200 imaging showed that Sox2 depleted H1650-luc (shSox2) cells had significantly reduced ability to form tumors, similar to shYAP1 H1650-luc cells (Figure 7H). In contrast, Sox2 overexpressing shControl H1650-luc cells formed tumors efficiently. Interestingly, Sox2 overexpression in YAP1 depleted cells (shYAP1 Sox2 OE) resulted in the formation of tumors that grew robustly, showing that Sox2 overexpression could rescue the loss of tumor formation resulting from the depletion of YAP1 (Figure 7H).
Supporting the tumor growth data, self-renewal was greatly reduced in Sox2 null cells, comparable to YAP1 depleted cells; Sox2 overexpressing cells formed spheres robustly, and overexpressing Sox2 could rescue the sphere formation in YAP1 null cells (Figure 7I and 7J). These results indicate that the effects of depleting YAP1 was possibly due to the loss of Sox2 expression and that Sox2 mediates the stem-like properties of SP cells downstream of YAP1.
DISCUSSION
Recent efforts to understand the biology behind the genesis and progression of NSCLC have led to the identification of stem-like cells with tumor initiating properties. Such cells have been identified based on the expression of a variety of cell surface markers 14,18,55. While these cells have tumor initiating properties and self-renew like embryonic stem cells, they display a limited range of differentiation. More obvious is their ability to differentiate into cells of tumor vasculature 13,39. The studies presented here provide novel insight into the role of the YAP1 transcriptional co-activator in the process of self-renewal and differentiation of stem-like side population cells.
The Hippo signaling cascade that controls organ size, has been shown to play a role in lung cancer progression 27,56,51. While it has been proposed that YAP1 and components of the Hippo pathway might contribute to stemness 34, our results provide mechanistic insights into the process. It is known that upstream signaling leads to activation of the core kinases of the Hippo signaling cascade, Mst1/2 and LATS1/2, resulting in a series of sequential phosphorylation events 23, leading to the proteosomal degradation of YAP1 (Figure 7K) 24. When Hippo pathway is turned off, YAP1 translocates into the nucleus where it functions as transcriptional co-activator, mainly by interacting with TEAD family of transcription factors 26,57. Here we show that YAP1 interacts with Oct4 transcription factor through the WW domain to play a significant role in NSCLC (Figure 7K), by promoting Sox2 expression, self-renewal and angiogenic tubule formation by stem-like cells.
It is established that the core embryonic stem cell transcriptional circuitry of Sox2, Oct4 and Nanog proteins contribute significantly to the self-renewal functions of CSCs 58. The studies presented here clearly show that YAP1 can transcriptionally induce Sox2, through a physical interaction with Oct4. Supporting this contention, our results show that depletion of YAP1 or its inhibition in NSCLC cell lines lowered the expression of Sox2, Oct4 and Nanog transcription factors in CSCs. Further, inhibition of YAP1 using Visudyne had similar effects on the SP cells suggesting the possibility of using Visudyne or its chemical/pharmacological analogs for therapeutic intervention, either alone or in combination with other agents. This idea is supported by the fact that GPCR signaling events as well as components of the mevalonate pathway are known to affect YAP1 localization and function, in addition to the classic Hippo pathway 59,60.
For tumors to survive there is a constant requirement of blood supply to sustain the uncontrolled growth and metastasis. One of the novel mechanisms with respect to tumor vasculature is vasculogenic mimicry. Vasculogenic mimicry is de novo formation of vasculogenic networks in highly aggressive tumors by the acquisition of endothelial phenotype by cancer cells 61. Multiple reports have now shown the ability of cancer stem cells to contribute directly in formation of de novo tumor vasculature by endothelial differentiation 13,14,39. In our earlier report 22 as well as in the present study, we have shown that the SP cells have the ability to transdifferentiate into CD31+ angiogenic tubules. Our results also indicated that depletion of YAP1 by siRNA or inhibition of YAP1 using Visudyne treatment abrogated the ability of the CSCs to form the angiogenic networks. These results therefore indicate the possible involvement of YAP1 in neovascularization through vascular mimicry by CSCs. Targeting YAP1 with Visudyne like agents therefore might also help address the issue of vascular mimicry in multiple cancer types.
The upregulation of Sox2 by YAP1 is particularly interesting in the context of lung cancer. In contrast to our findings, YAP1 has also been shown as a direct transcriptional target of Sox2 in osteoprogenitors and MSCs 62, suggesting that there could be a reciprocal regulation of these factors depending on the cell type. Sox genes encode a family of high mobility group transcription factors that have crucial roles in stemness, differentiation and development 63. In addition, Sox genes are now associated with cancer progression 63,64. Studies have shown that Sox2 expression correlates with poor prognosis in NSCLC patients 21,65. While Sox2 clearly contributes to the stemness of adenocarcinoma cells and is expressed only in a subset of cells, it is overexpressed significantly in most cells of squamous cell carcinomas 66,67. This discrepancy could probably be explained by the fact that the functional specificity of Sox transcription factors are largely determined by transcription regulators that associate with them 68,69, raising the possibility that YAP1 contributes to this 52. An earlier study indicated that YAP1 may associate with promoters of genes important for the pluripotency of embryonic stem cells, through the mediation of the TEAD transcription factor 34. However, the results presented here clearly demonstrate that YAP1 can physically associate with Oct4 to regulate the Sox2 promoter. A previous study by Beyer et al has demonstrated that YAP1-TEAD together was part of a multi-protein complex along with Oct4 transcription factor and this complex is associated with pluripotency gene promoters in human embryonic stem cells (hESCs) like Nanog and Oct4 70; this complex was absent on Sox2 gene promoter 70. This raises the possibility that YAP1 can form mono- or multimeric- protein complexes with Oct4 and regulate other targets which are necessary for the functioning of normal stem cells.
Our results also suggest that the WW domain of the YAP1 protein mediates its interaction with the Oct4 transcription factor. WW domains bind to short linear proline rich motifs (PPxY) in proteins 50–52. It has been suggested that the function of YAP1 WW domain can vary depending in the context of the cells and tissue type 71. Our findings show that the interaction of YAP1 WW domain with Oct4 occurs in the nucleus, raising the prospect of additional transcription factors carrying the PPxY motif as possible partners of YAP1 and agents that can target the interaction of these two domains might have anti-cancer activity. This is supported by the finding that the inhibition of the YAP1-Oct4 interaction with YAP1 WW domain peptide significantly reduced self-renewal of SP cells and the observation that the interaction between YAP1 and Oct4 was higher in lung tumor tissues as compared to the normal lung tissues. These results show that YAP1 affect transcription factor(s) other than TEAD family 72; its direct interaction with Oct4 is novel and has far reaching implications in stemness and tumorigenesis.
It is well established that cancers when progresses towards malignancy, there is acquisition of mesenchymal properties with increased cell migration, invasion and resistance to apoptosis 73. YAP1 has been implicated to have a role in lung cancer metastasis recently 74. In agreement with another study, we also find that knock down of YAP1 in H1650-luc cells decreased migration and invasion, with a concomitant reduction in the levels of mesenchymal proteins, as well as reduced tumor growth and metastasis 72. Additionally our study also confirmed that loss of self-renewal and tumor growth in the absence of YAP1 could be rescued by stable expression of Sox2 stem cell factor in YAP1 depleted H1650-luc cells. The finding that inhibiting YAP1-Oct4 interaction with YAP1 WW peptide suppresses multiple genes important for EMT raises the prospect that YAP1 modulates such genes by affecting the transcriptional activity of Oct4, or its downstream targets like Sox2. Supporting this contention, we find that the YAP1-Oct4 interaction is more pronounced in poorly differentiated tumors than the well differentiated ones. The fact that this interaction was elevated in lung cancer samples and the observation that disrupting this interaction prevents self-renewal and vascular mimicry suggests that targeting this interaction might be a viable strategy to combat NSCLC.
CONCLUSION
Our studies show a novel mechanism in which YAP1 protein acts as a transcriptional co-activator of Oct4 and that the physical interaction between these two proteins is necessary for self-renewal and vascular mimicry of stem-like cells from NSCLC. Interestingly, in association with Oct4, YAP1 can transcriptionally induce Sox2 mRNA levels. The study also presents that YAP1 - Oct4 interaction is elevated in human lung cancer tissues and depletion of YAP1 leads to loss of mesenchymal features of NSCLC cancer cells as well as decrease in growth and metastases of tumor xenografts in immunodeficient mice. Our results raise the possibility that YAP1-Oct4 mediated gene regulation may lead to the genesis and progression of NSCLC.
Supplementary Material
Acknowledgments
We thank Dr. Angel Garcia Martin for generous gifts of the Sox2 luciferase constructs. Support of the Lung SPORE as well as Shared Resources at Moffitt Cancer Center is greatly appreciated. We thank Smitha Pillai, Matthew Smith and Eric Haura for helpful discussions. These studies were supported by grants CA127725 and CA139612 from the NCI.
Footnotes
AUTHOR CONTRIBUTIONS
N.B. and S.C. designed the study. N.B., J.N. and S.S. performed the experiments and data analysis. C.S. performed the hMSC immunofluorescence experiments. D.P. carried out the survival analysis of microarray dataset. D.C. analyzed and scored the IHC/PLA for lung TMA. N.B. and S.C. prepared the manuscript.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Demicheli R, Biganzoli E, Boracchi P, Greco M, Retsky MW. Recurrence dynamics does not depend on the recurrence site. Breast Cancer Res. 2008;10:R83. doi: 10.1186/bcr2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Demicheli R, et al. Recurrence dynamics for non-small-cell lung cancer: effect of surgery on the development of metastases. J Thorac Oncol. 2012;7:723–730. doi: 10.1097/JTO.0b013e31824a9022. [DOI] [PubMed] [Google Scholar]
- 4.Senthi S, Lagerwaard FJ, Haasbeek CJ, Slotman BJ, Senan S. Patterns of disease recurrence after stereotactic ablative radiotherapy for early stage non-small-cell lung cancer: a retrospective analysis. Lancet Oncol. 2012;13:802–809. doi: 10.1016/S1470-2045(12)70242-5. [DOI] [PubMed] [Google Scholar]
- 5.Seve P, Dumontet C. Chemoresistance in non-small cell lung cancer. Curr Med Chem Anticancer Agents. 2005;5:73–88. doi: 10.2174/1568011053352604. [DOI] [PubMed] [Google Scholar]
- 6.Lara PN, Jr, Lau DH, Gandara DR. Non-small-cell lung cancer progression after first-line chemotherapy. Curr Treat Options Oncol. 2002;3:53–58. doi: 10.1007/s11864-002-0041-0. [DOI] [PubMed] [Google Scholar]
- 7.Patel P, Chen EI. Cancer stem cells, tumor dormancy, and metastasis. Front Endocrinol (Lausanne) 2012;3:125. doi: 10.3389/fendo.2012.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allan AL, Vantyghem SA, Tuck AB, Chambers AF. Tumor dormancy and cancer stem cells: implications for the biology and treatment of breast cancer metastasis. Breast Dis. 2006;26:87–98. doi: 10.3233/bd-2007-26108. [DOI] [PubMed] [Google Scholar]
- 9.Giancotti FG. Mechanisms governing metastatic dormancy and reactivation. Cell. 2013;155:750–764. doi: 10.1016/j.cell.2013.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee N, Barthel SR, Schatton T. Melanoma stem cells and metastasis: mimicking hematopoietic cell trafficking? Lab Invest. 2014;94:13–30. doi: 10.1038/labinvest.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh S, Chellappan S. Lung cancer stem cells: Molecular features and therapeutic targets. Mol Aspects Med. 2013 doi: 10.1016/j.mam.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nature reviews. Cancer. 2008;8:755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 13.Ricci-Vitiani L, et al. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature. 2010;468:824–828. doi: 10.1038/nature09557. [DOI] [PubMed] [Google Scholar]
- 14.Chiao MT, Yang YC, Cheng WY, Shen CC, Ko JL. CD133+ glioblastoma stem-like cells induce vascular mimicry in vivo. Current neurovascular research. 2011;8:210–219. doi: 10.2174/156720211796558023. [DOI] [PubMed] [Google Scholar]
- 15.Kaur S, Bajwa P. A ‘tete-a tete’ between cancer stem cells and endothelial progenitor cells in tumor angiogenesis. Clin Transl Oncol. 2014;16:115–121. doi: 10.1007/s12094-013-1103-4. [DOI] [PubMed] [Google Scholar]
- 16.Fan YL, Zheng M, Tang YL, Liang XH. A new perspective of vasculogenic mimicry: EMT and cancer stem cells (Review) Oncol Lett. 2013;6:1174–1180. doi: 10.3892/ol.2013.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alamgeer M, Peacock CD, Matsui W, Ganju V, Watkins DN. Cancer stem cells in lung cancer: Evidence and controversies. Respirology. 2013;18:757–764. doi: 10.1111/resp.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang F, et al. Aldehyde dehydrogenase 1 is a tumor stem cell-associated marker in lung cancer. Molecular cancer research: MCR. 2009;7:330–338. doi: 10.1158/1541-7786.MCR-08-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sullivan JP, et al. Aldehyde dehydrogenase activity selects for lung adenocarcinoma stem cells dependent on notch signaling. Cancer research. 2010;70:9937–9948. doi: 10.1158/0008-5472.CAN-10-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang WC, et al. Glycine decarboxylase activity drives non-small cell lung cancer tumor-initiating cells and tumorigenesis. Cell. 2012;148:259–272. doi: 10.1016/j.cell.2011.11.050. [DOI] [PubMed] [Google Scholar]
- 21.Singh S, et al. EGFR/Src/Akt signaling modulates Sox2 expression and self-renewal of stem-like side-population cells in non-small cell lung cancer. Molecular cancer. 2012;11:73. doi: 10.1186/1476-4598-11-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh S, Bora-Singhal N, Kroeger J, Laklai H, Chellappan SP. betaArrestin-1 and Mcl-1 modulate self-renewal growth of cancer stem-like side-population cells in non-small cell lung cancer. PloS one. 2013;8:e55982. doi: 10.1371/journal.pone.0055982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piccolo S, Cordenonsi M, Dupont S. Molecular pathways: YAP and TAZ take center stage in organ growth and tumorigenesis. Clinical cancer research: an official journal of the American Association for Cancer Research. 2013;19:4925–4930. doi: 10.1158/1078-0432.CCR-12-3172. [DOI] [PubMed] [Google Scholar]
- 24.Halder G, Camargo FD. The hippo tumor suppressor network: from organ size control to stem cells and cancer. Cancer research. 2013;73:6389–6392. doi: 10.1158/0008-5472.CAN-13-2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hao J, et al. Role of Hippo signaling in cancer stem cells. Journal of cellular physiology. 2014;229:266–270. doi: 10.1002/jcp.24455. [DOI] [PubMed] [Google Scholar]
- 26.Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nature reviews. Cancer. 2013;13:246–257. doi: 10.1038/nrc3458. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, et al. Overexpression of yes-associated protein contributes to progression and poor prognosis of non-small-cell lung cancer. Cancer science. 2010;101:1279–1285. doi: 10.1111/j.1349-7006.2010.01511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou Z, et al. TAZ is a novel oncogene in non-small cell lung cancer. Oncogene. 2011;30:2181–2186. doi: 10.1038/onc.2010.606. [DOI] [PubMed] [Google Scholar]
- 29.Yu T, Bachman J, Lai ZC. Evidence for a tumor suppressor role for the large tumor suppressor genes LATS1 and LATS2 in human cancer. Genetics. 2013;195:1193–1196. doi: 10.1534/genetics.113.156372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang N, et al. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Developmental cell. 2010;19:27–38. doi: 10.1016/j.devcel.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramos A, Camargo FD. The Hippo signaling pathway and stem cell biology. Trends in cell biology. 2012;22:339–346. doi: 10.1016/j.tcb.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin XY, Zhang XP, Wu JH, Qiu XS, Wang EH. Expression of LATS1 contributes to good prognosis and can negatively regulate YAP oncoprotein in non-small-cell lung cancer. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2014 doi: 10.1007/s13277-014-1826-z. [DOI] [PubMed] [Google Scholar]
- 33.Director’s Challenge Consortium for the Molecular Classification of Lung, A. et al. Gene expression-based survival prediction in lung adenocarcinoma: a multi-site, blinded validation study. Nature medicine. 2008;14:822–827. doi: 10.1038/nm.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lian I, et al. The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes & development. 2010;24:1106–1118. doi: 10.1101/gad.1903310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun B, et al. Vasculogenic mimicry is associated with high tumor grade, invasion and metastasis, and short survival in patients with hepatocellular carcinoma. Oncology reports. 2006;16:693–698. [PubMed] [Google Scholar]
- 36.Millimaggi D, et al. Vasculogenic mimicry of human ovarian cancer cells: role of CD147. International journal of oncology. 2009;35:1423–1428. doi: 10.3892/ijo_00000460. [DOI] [PubMed] [Google Scholar]
- 37.Qayum N, et al. Tumor vascular changes mediated by inhibition of oncogenic signaling. Cancer research. 2009;69:6347–6354. doi: 10.1158/0008-5472.CAN-09-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Upile T, et al. Vascular mimicry in cultured head and neck tumour cell lines. Head & neck oncology. 2011;3:55. doi: 10.1186/1758-3284-3-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang R, et al. Glioblastoma stem-like cells give rise to tumour endothelium. Nature. 2010;468:829–833. doi: 10.1038/nature09624. [DOI] [PubMed] [Google Scholar]
- 40.Soda Y, et al. Transdifferentiation of glioblastoma cells into vascular endothelial cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:4274–4280. doi: 10.1073/pnas.1016030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brown DM, et al. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: Two-year results of the ANCHOR study. Ophthalmology. 2009;116:57–65 e55. doi: 10.1016/j.ophtha.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 42.Belzacq AS, et al. Apoptosis induction by the photosensitizer verteporfin: identification of mitochondrial adenine nucleotide translocator as a critical target. Cancer research. 2001;61:1260–1264. [PubMed] [Google Scholar]
- 43.Liu-Chittenden Y, et al. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes & development. 2012;26:1300–1305. doi: 10.1101/gad.192856.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tomioka M, et al. Identification of Sox-2 regulatory region which is under the control of Oct-3/4-Sox-2 complex. Nucleic acids research. 2002;30:3202–3213. doi: 10.1093/nar/gkf435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chew JL, et al. Reciprocal transcriptional regulation of Pou5f1 and Sox2 via the Oct4/Sox2 complex in embryonic stem cells. Molecular and cellular biology. 2005;25:6031–6046. doi: 10.1128/MCB.25.14.6031-6046.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miyagi S, et al. The Sox2 regulatory region 2 functions as a neural stem cell-specific enhancer in the telencephalon. The Journal of biological chemistry. 2006;281:13374–13381. doi: 10.1074/jbc.M512669200. [DOI] [PubMed] [Google Scholar]
- 47.Soderberg O, et al. Characterizing proteins and their interactions in cells and tissues using the in situ proximity ligation assay. Methods. 2008;45:227–232. doi: 10.1016/j.ymeth.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 48.Soderberg O, et al. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nature methods. 2006;3:995–1000. doi: 10.1038/nmeth947. [DOI] [PubMed] [Google Scholar]
- 49.Smith MA, et al. Annotation of human cancers with EGFR signaling-associated protein complexes using proximity ligation assays. Science signaling. 2015;8:ra4. doi: 10.1126/scisignal.2005906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Komuro A, Nagai M, Navin NE, Sudol M. WW domain-containing protein YAP associates with ErbB-4 and acts as a co-transcriptional activator for the carboxyl-terminal fragment of ErbB-4 that translocates to the nucleus. The Journal of biological chemistry. 2003;278:33334–33341. doi: 10.1074/jbc.M305597200. [DOI] [PubMed] [Google Scholar]
- 51.Chen HI, Sudol M. The WW domain of Yes-associated protein binds a proline-rich ligand that differs from the consensus established for Src homology 3-binding modules. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:7819–7823. doi: 10.1073/pnas.92.17.7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yagi R, Chen LF, Shigesada K, Murakami Y, Ito Y. A WW domain-containing yes-associated protein (YAP) is a novel transcriptional co-activator. The EMBO journal. 1999;18:2551–2562. doi: 10.1093/emboj/18.9.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu H, et al. WWP2 promotes degradation of transcription factor OCT4 in human embryonic stem cells. Cell research. 2009;19:561–573. doi: 10.1038/cr.2009.31. [DOI] [PubMed] [Google Scholar]
- 54.Mani SA, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xin L, Lawson DA, Witte ON. The Sca-1 cell surface marker enriches for a prostate-regenerating cell subpopulation that can initiate prostate tumorigenesis. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:6942–6947. doi: 10.1073/pnas.0502320102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Y, et al. Overexpression of yes-associated protein contributes to progression and poor prognosis of non-small-cell lung cancer. Cancer Sci. 2010;101:1279–1285. doi: 10.1111/j.1349-7006.2010.01511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lamar JM, et al. The Hippo pathway target, YAP, promotes metastasis through its TEAD-interaction domain. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E2441–2450. doi: 10.1073/pnas.1212021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Das S, Levasseur D. Transcriptional regulatory mechanisms that govern embryonic stem cell fate. Methods in molecular biology. 2013;1029:191–203. doi: 10.1007/978-1-62703-478-4_13. [DOI] [PubMed] [Google Scholar]
- 59.Sorrentino G, et al. Metabolic control of YAP and TAZ by the mevalonate pathway. Nature cell biology. 2014;16:357–366. doi: 10.1038/ncb2936. [DOI] [PubMed] [Google Scholar]
- 60.Yu FX, et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kirschmann DA, Seftor EA, Hardy KM, Seftor RE, Hendrix MJ. Molecular pathways: vasculogenic mimicry in tumor cells: diagnostic and therapeutic implications. Clinical cancer research: an official journal of the American Association for Cancer Research. 2012;18:2726–2732. doi: 10.1158/1078-0432.CCR-11-3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Seo E, et al. SOX2 regulates YAP1 to maintain stemness and determine cell fate in the osteo-adipo lineage. Cell reports. 2013;3:2075–2087. doi: 10.1016/j.celrep.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu K, et al. The multiple roles for Sox2 in stem cell maintenance and tumorigenesis. Cellular signalling. 2013;25:1264–1271. doi: 10.1016/j.cellsig.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sarkar A, Hochedlinger K. The sox family of transcription factors: versatile regulators of stem and progenitor cell fate. Cell stem cell. 2013;12:15–30. doi: 10.1016/j.stem.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chou YT, et al. The emerging role of SOX2 in cell proliferation and survival and its crosstalk with oncogenic signaling in lung cancer. Stem cells. 2013;31:2607–2619. doi: 10.1002/stem.1518. [DOI] [PubMed] [Google Scholar]
- 66.Justilien V, et al. The PRKCI and SOX2 oncogenes are coamplified and cooperate to activate Hedgehog signaling in lung squamous cell carcinoma. Cancer cell. 2014;25:139–151. doi: 10.1016/j.ccr.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu K, et al. Sox2 cooperates with inflammation-mediated Stat3 activation in the malignant transformation of foregut basal progenitor cells. Cell stem cell. 2013;12:304–315. doi: 10.1016/j.stem.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen Y, et al. The molecular mechanism governing the oncogenic potential of SOX2 in breast cancer. The Journal of biological chemistry. 2008;283:17969–17978. doi: 10.1074/jbc.M802917200. [DOI] [PubMed] [Google Scholar]
- 69.Wilson M, Koopman P. Matching SOX: partner proteins and co-factors of the SOX family of transcriptional regulators. Current opinion in genetics & development. 2002;12:441–446. doi: 10.1016/s0959-437x(02)00323-4. [DOI] [PubMed] [Google Scholar]
- 70.Beyer TA, et al. Switch enhancers interpret TGF-beta and Hippo signaling to control cell fate in human embryonic stem cells. Cell reports. 2013;5:1611–1624. doi: 10.1016/j.celrep.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 71.Sudol M, Shields DC, Farooq A. Structures of YAP protein domains reveal promising targets for development of new cancer drugs. Seminars in cell & developmental biology. 2012;23:827–833. doi: 10.1016/j.semcdb.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shao DD, et al. KRAS and YAP1 Converge to Regulate EMT and Tumor Survival. Cell. 2014 doi: 10.1016/j.cell.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. The Journal of clinical investigation. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lau AN, et al. Tumor-propagating cells and Yap/Taz activity contribute to lung tumor progression and metastasis. The EMBO journal. 2014;33:468–481. doi: 10.1002/embj.201386082. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.