Abstract
Background
Although rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) is considered standard therapy for diffuse large B-cell lymphoma (DLBCL), patterns of use and the impact of R-CHOP on survival in patients >80 years are less clear.
Methods
We used the Surveillance, Epidemiology, and End Results (SEER)-Medicare database to characterize presentation, treatment, and survival patterns in DLBCL patients diagnosed from 2002–2009. Chi-squared tests compared characteristics and initial treatments of DLBCL patients >80 years and ≤80 years. Multivariable logistic regression models examined factors associated with treatment selection in patients >80 years; standard and propensity score-adjusted multivariable Cox proportional hazards models examined relationships between treatment regimen, treatment duration, and survival.
Results
Among 4,635 patients with DLBCL, 1,156 (25%) were >80 years. Patients >80 were less likely to receive R-CHOP and more likely to be observed or receive rituximab, cyclophosphamide, vincristine, and prednisone (R-CVP); both p<0.0001. Marital status, stage, disease site, performance status, radiation therapy, and growth factor support were associated with initial R-CHOP in patients >80. In propensity score-matched multivariable Cox proportional hazards models examining relationships between treatment regimen and survival, R-CHOP was the only regimen associated with improved OS (hazard ratio (HR) = 0.45, 95% confidence interval (CI) = 0.33–0.62) and LRS (HR=0.58, 95% CI 0.38–0.88).
Conclusions
Although DLBCL patients >80 years were less likely to receive R-CHOP, this regimen conferred the longest survival and should be considered for this population. Further studies are needed to characterize the impact of DLBCL treatment on quality of life in this age group.
Keywords: Lymphoma, Large B-Cell, Diffuse, Aged, 80 and over, Treatment Outcome, Hematologic Neoplasms
INTRODUCTION
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma (NHL) in the western world.1 It is also a disease of the elderly, with a median age at diagnosis of 70 years2 and an incidence that rises with increasing age.3 As the United States (U.S.) population ages, the proportion of the population ≥65 years is projected to increase from 14.8% in 2015 to 20.3% in 2030.4 Moreover, the number of people aged ≥80 in the U.S. is expected to increase from 11.5 million in 2010 to 12.8 million by 2020.5 An aging population coupled with an age-associated increase in DLBCL incidence will lead to a greater need for management of DLBCL in the very elderly, defined in this study as individuals older than 80 years.
This expected increase in DLBCL in the very elderly warrants a determination of current treatment patterns and the most effective management strategies for this population. Although DLBCL patients >80 are rarely included in studies, there is some evidence that standard-of-care rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) defined for younger patients should be used for this age group.6–13 Our study sought to determine presentation, treatment, and survival patterns in a large U.S. population-based cohort of very elderly DLBCL patients. Previous studies have identified DLBCL treatment disparities based on race and insurance status,14,15 but age-related disparities have not been studied as comprehensively. This study investigated whether there is a relationship between patient age and receipt of R-CHOP in a cohort of DLBCL patients who were all Medicare-insured. Specifically, we investigated factors associated with treatment selection and examined the impact of age and treatment regimen on overall survival (OS) and lymphoma-related survival (LRS). We hypothesized patients >80 were more likely to have comorbidities and poor performance status, and thus were more likely to undergo initial observation. Among patients receiving chemoimmunotherapy, we hypothesized patients >80 were less likely to receive R-CHOP. We also hypothesized very elderly patients who received R-CHOP would have superior OS and LRS, even after controlling for demographic and clinical factors.
METHODS
Data Source
We used the Surveillance, Epidemiology, and End Results (SEER) registry linked to Medicare claims data to examine elderly DLBCL patients diagnosed from 2002–2009. The National Cancer Institute (NCI) SEER program collects and reports cancer incidence and survival data from U.S. registries, covering approximately 28% of the population as of 2014.16 Data collected by SEER include patient demographics, tumor histopathology, disease stage, primary site of tumor, initial treatment, and date of death.
The linkage of SEER with Medicare claims data allows for the identification of specific treatments received by elderly cancer patients. Among persons aged ≥65, 97% are eligible for Medicare, and 93% who are listed in SEER are linked to the Medicare enrollment file.17 At the time of this study, the SEER-Medicare database included all Medicare-eligible individuals who appeared in SEER through 2009, and their Medicare claims through 2010. Since the SEER-Medicare database does not include patient identifiers, this study did not require Institutional Review Board approval; however, a data use agreement was signed prior to initiating this study.
Eligibility Criteria
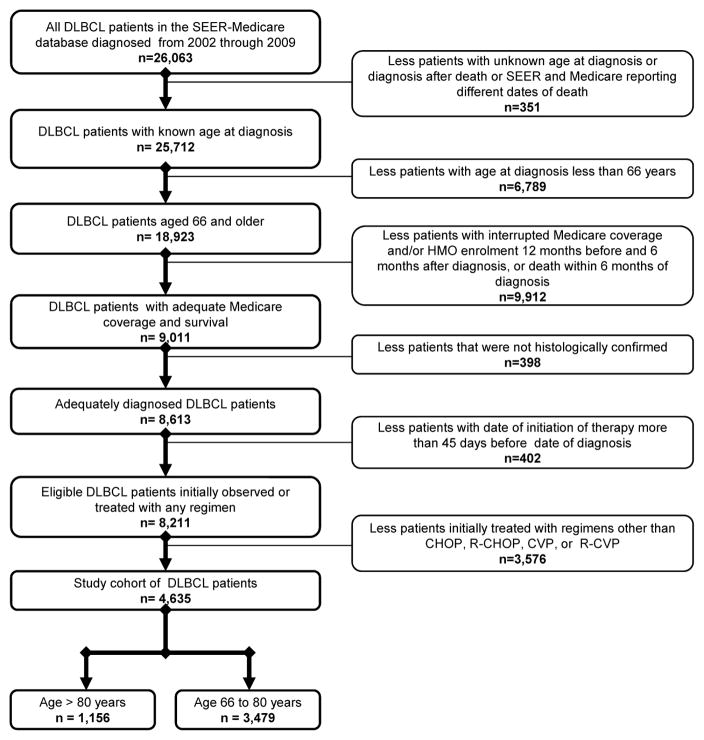
Patients were eligible for analysis if they were diagnosed with DLBCL between 1/1/2002 and 12/31/2009, and were aged ≥66 at diagnosis. The minimum required age was 66 in order to ensure that patients had been enrolled in Medicare for ≥12 months prior to diagnosis. DLBCL cases were identified using the World Health Organization International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3) histology codes 9680 and 9684.18 Exclusion criteria are displayed in Figure 1. Treatment regimens were limited for these analyses to examine factors associated with the use of anthracyclines (e.g. doxorubicin); all first-line regimens received by DLBCL patients in the SEER-Medicare database from 2002–2009 are listed in Supplemental Table 1.
Figure 1.
Selection of the study cohort. DLBCL=diffuse large B-cell lymphoma; SEER=Surveillance, Epidemiology, and End Results; HMO=health maintenance organization; CHOP=cyclophosphamide, doxorubicin, vincristine, prednisone; R-CHOP=rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone; CVP=cyclophosphamide, vincristine, prednisone; R-CVP=rituximab, cyclophosphamide, vincristine, prednisone.
Patient Characteristics
Patients were stratified into two groups by age at diagnosis (66–80 or >80). Self-reported race was classified as Caucasian, African-American, or “other”; in SEER data, “other” most commonly refers to those of Asian, Native American, Pacific Islander, or Alaskan Native ancestry.19 The SEER-Medicare dataset utilizes information from the 2000 U.S. Census regarding the characteristics of the census tract in which each patient lives (% of residents living in poverty and % with only a high school education) as surrogates for socioeconomic status (SES), as described in other SEER-Medicare studies.20–22 Other demographic variables included in this study were sex, marital status, and type of geographical area (less urban/rural, urban, or metropolitan).
Patients were also classified with regard to the following: Ann Arbor stage (I/II, III/IV, or unknown), primary site of disease (nodal or extranodal), presence of B-symptoms, receipt of radiation therapy beyond 6 months after diagnosis, receipt of granulocyte colony-stimulating factor (G-CSF) or granulocyte-macrophage colony-stimulating factor (GM-CSF) [Healthcare Common Procedure Coding System codes J1440, J1441, and J2820], performance status, NCI Comorbidity Index score (0, 1, or ≥2), and year of diagnosis. We classified performance status as poor if a patient had claims for any of the following: hospice, home health agency, skilled nursing facility, oxygen, or wheelchair/related supplies. Similar claims-based measures of performance status have been utilized in other cancer studies.23–26 We calculated NCI Comorbidity Index scores using the Deyo adaptation of the Charlson Comorbidity Index (CCI) to identify from Medicare claims the 15 non-cancer comorbidities included in the CCI.27,28
Treatment, Toxicity, and Mortality Classification
Initial management strategies were determined from Medicare claims made within 6 months of diagnosis; initial observation was defined as no treatment within this time frame. Information regarding the receipt of oral medications without an intravenous equivalent is not available in SEER-Medicare. Patients with claims for cyclophosphamide, doxorubicin, and vincristine were classified as receiving CHOP. Those with claims for cyclophosphamide and vincristine were categorized as receiving CVP. Patients with the same claims who also received rituximab were categorized as receiving R-CHOP and R-CVP, respectively. The treatment duration for each regimen was categorized as either ≤4 or >4 cycles. In order to examine treatment-related toxicities, we searched inpatient and emergency room claims from the date of treatment initiation to one year past diagnosis. We identified treatment-related toxicities using ICD-9 codes or Medicare Provider Analysis and Review claims for the following conditions: anemia, thrombocytopenia, febrile neutropenia, infections, cardiovascular disease, and any hospitalization.
For mortality classification, we used SEER date and cause of death. Since SEER only reports month and year of diagnosis, date of diagnosis for survival analyses was assigned as the 15th day of the reported month of diagnosis. Patients were followed until death, enrollment in a health maintenance organization, or last date of available Medicare claims. Two different survival endpoints were examined: OS measured from the date of diagnosis until death censored at last follow-up and LRS measured from the date of diagnosis until death from lymphoma censored at last follow-up or death from other causes. Since SEER date of death was only complete through December 31, 2009, patients diagnosed in 2009 were excluded from the LRS analysis to allow a minimum follow-up of 1 year.
Statistical Analysis
Patients >80 were compared to patients aged 66–80 using chi-squared tests, which were also used to compare R-CHOP toxicity in these two age groups. Multivariable logistic regression models were employed to investigate the relationships between patient characteristics and initial observation or R-CHOP, and odds ratios (OR) with 95% confidence intervals were calculated. Multivariable regression models were adjusted for the following demographic and clinical variables: sex, race, marital status, percent in census tract living in poverty, percent in census tract with only a high school education, type of geographical area, receipt of radiation therapy, growth factor support, stage, primary site of disease, presence of B-symptoms, NCI comorbidity index score, performance status, and year of diagnosis. The goodness of fit of the logistic regression model was assessed using the Hosmer-Lemeshow test and was found to fit the data well.
Kaplan-Meier curves were constructed to examine the effectiveness of initial management strategies in patients >80 and the effect of age on OS and LRS. Cox proportional hazards models assessed the effect of treatments on OS and LRS. Cox models were adjusted for the same variables described above. The global proportional hazards assumption was tested with the Wald test. Proportional hazards assumptions for individual covariates were tested by assessing the Schoenfeld residuals. No violations were detected. Sensitivity analyses were performed using propensity score methods to adjust for imbalances in observable covariates between treatment groups. Two propensity score adjustment methodologies were applied: the first adjusted for propensity by matching the R-CHOP and R-CVP groups to the observation group, and the second estimated the Cox proportional hazards models with inverse probability weighting.29,30 Since only 1 patient in the observation group received growth factors, growth factor use was excluded in the calculation of propensity scores. We set α=0.05 to determine statistical significance, and all p-values were two-sided. Data were analyzed using SAS 9.4 (Cary, NC) and Stata 13 (StataCorp LP, TX).
RESULTS
Patient Characteristics
We identified a cohort of 4,635 DLBCL patients including 1,156 patients (25%) >80 at diagnosis. In the cohort >80, the median age at diagnosis was 84 and the mean age at diagnosis was 85 (standard deviation 3). Characteristics of each age category are displayed in Supplemental Table 2. Compared to patients ≤80, those >80 were more likely to be female, widowed, live in a metropolitan area, have extranodal disease, have poor performance status, and be diagnosed after 2004; patients >80 were less likely to live in a lower SES census tract, have stage III/IV disease, have B-symptoms, and be treated with radiation or growth factors (all p<0.05).
Treatment Selection
Compared to patients 66–80, those >80 were more likely to undergo observation and less likely to receive R-CHOP or CHOP; p<0.0001 [Table 1]. Patients >80 were more likely to receive CVP or R-CVP (p<0.0001). Among patients >80, the initial receipt of R-CHOP was more commonly associated with being married (OR 1.67, 1.04–2.69), stage III/IV disease (OR 1.36, 1.03–1.80), radiation therapy (OR 1.96, 1.37–2.80), and growth factor treatment (OR 2.87, 2.07–4.00) [Supplemental Table 3]. The initial receipt of R-CHOP was less commonly associated with extranodal disease (OR 0.68, 0.52–0.88) and poor performance status (OR 0.64, 0.48–0.85). Patients with poor performance status were more likely to undergo observation (OR 2.03, 1.46–2.84). Among patients >80 years who underwent observation, 80% had ≤1 comorbidity, 40% had a poor performance status, and only 6% ultimately received radiation [Supplemental Table 5].
Table 1.
First-line management strategies, by age category.
| Age 66–80 years N (%) |
Age >80 years N (%) |
χ2 p-value |
|
|---|---|---|---|
| Observation | 403 (11.6) | 300 (26.0) | <0.0001 |
| CVP | 30 (0.9) | 23 (2.0) | |
| R-CVP | 169 (4.9) | 165 (14.3) | |
| CHOP | 296 (8.5) | 73 (6.3) | |
| R-CHOP | 2,581 (74.2) | 595 (51.5) |
Note: CVP=cyclophosphamide, vincristine, prednisone; R-CVP=rituximab + CVP; CHOP=cyclophosphamide, doxorubicin, vincristine, prednisone; R-CHOP=rituximab + CHOP
Note: percentages may not add up to 100.0% due to rounding.
Survival and Treatment Toxicity
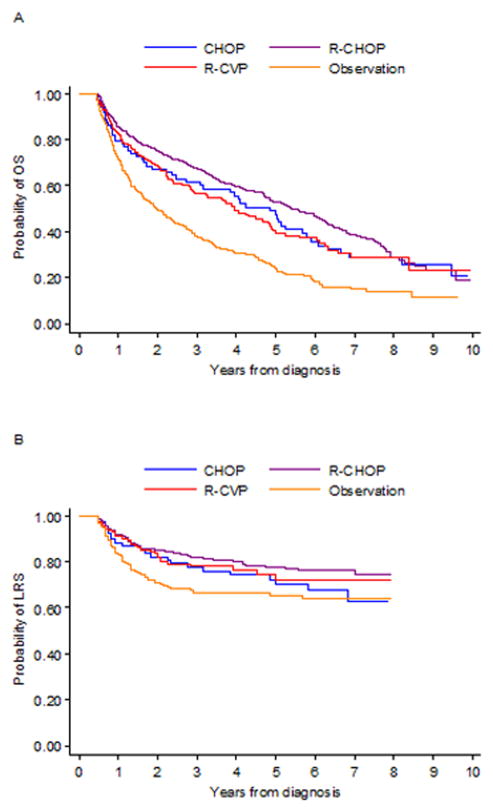
Kaplan-Meier survival curves in Figure 2 illustrate that in patients initially treated with R-CHOP, increasing age category was associated with decreased OS and LRS. In patients >80, R-CHOP was associated with the longest OS and LRS [Figure 3].
Figure 2.
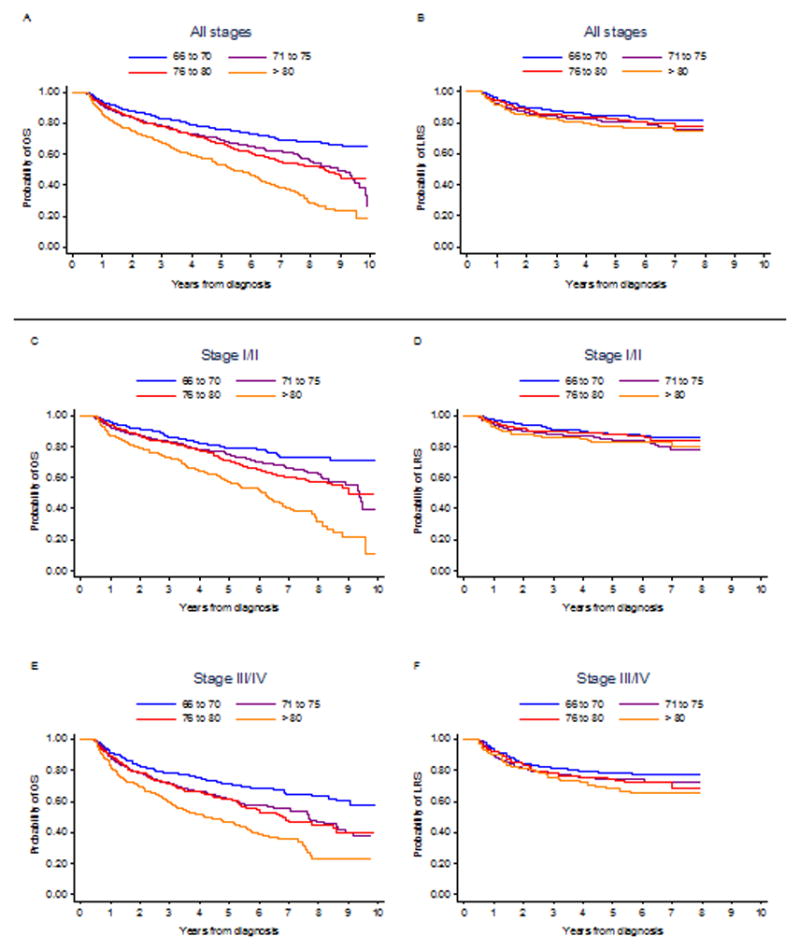
Overall survival curves (A: all stages, n=4,635; C: stage I/II, n=2,475; E: stage III/IV, n=1,832) and lymphoma-related survival curves (B: all stages, n=4,066; D: stage I/II, n=2,188; F: stage III/IV, n=1,584) by age category among patients initially receiving R-CHOP. OS=overall survival; LRS=lymphoma-related survival; R-CHOP=rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone.
Figure 3.
Overall survival curves (A: n=1,133) and lymphoma-related survival curves (B: n=984) for patients greater than 80 years old, by treatment regimen. CHOP=cyclophosphamide, doxorubicin, vincristine, prednisone; R-CHOP=rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone; R-CVP=rituximab, cyclophosphamide, vincristine, prednisone; OS=overall survival; LRS=lymphoma-related survival. Patients treated with CVP (cyclophosphamide, vincristine, prednisone) were removed in order to prevent patient identification.
In multivariable Cox regression models, treatment with R-CHOP for >4 cycles was associated with the most favorable OS (HR 0.44, 0.33–0.58). Patients with stage III/IV disease experienced similar outcomes when treated with R-CVP for >4 cycles (OS HR 0.33, 0.16–0.65; LRS HR 0.16, 0.05–0.57) or R-CHOP for >4 cycles (OS HR 0.39, 0.25–0.62; LRS HR 0.35, 0.18–0.72) [Table 2]. Propensity score-adjusted HRs were similar in magnitude and statistical significance demonstrating that R-CHOP was the only regimen associated with improved OS (HR 0.45, 0.33–0.62) and LRS (HR 0.58, 0.38–0.88) [see Supplemental Table 4; inverse probability weighting results are not shown but comparable]. In comparison to patients aged 66–80 years, patients >80 years who received R-CHOP had an increased risk of hospitalization post-treatment (74.9% vs. 66.2%, p=0.0001) and a decreased risk of febrile neutropenia (5.6% vs. 8.6%, p=0.0250).
Table 2.
Multivariable Cox proportional hazards survival analysis in patients >80 years, by stage.
| Stage I/II | Stage III/IV | ||||||
|---|---|---|---|---|---|---|---|
| OS Analysis (n=523)1 | LRS Analysis (n=454)1 | OS Analysis (n=348)1 | LRS Analysis (n=298)2 | ||||
| Median OS (years)3 | HR (95% CI) | HR (95% CI) | Median OS (years)3 | HR (95% CI) | HR (95% CI) | ||
| Observation | 2.2 | Reference | Reference | Observation | 2.1 | Reference | Reference |
| CVP (≤ 4 cycles) | 1.3 | 1.00 (0.23–4.34) | 3.31 (0.68–16.03) | CVP (All cycles)4 | 1.3 | 0.92 (0.42–2.02) | 0.99 (0.35–2.86) |
| CVP (>4 cycles) | 1.9 | 1.38 (0.42–4.56) | 0.75 (0.10–5.80) | ||||
| CHOP (≤4 cycles) | 5.7 | 0.51 (0.21–0.89) | 0.79 (0.33–1.92) | CHOP (≤4 cycles) | 2.7 | 0.73 (0.36–1.49) | 0.47 (0.15–1.49) |
| CHOP (>4 cycles) | 6.9 | 0.37 (0.11–1.22) | 2.47 (0.70–8.66) | CHOP (>4 cycles) | 1.1 | 1.60 (0.44–5.78) | 2.63 (0.59–11.68)s |
| R-CVP (≤4 cycles) | 4.3 | 0.74 (0.48–1.15) | 1.02 (0.52–2.01) | R-CVP (≤4 cycles) | 3.9 | 0.67 (0.36–1.25) | 0.61 (0.23–1.64) |
| R-CVP (>4 cycles) | 4.5 | 0.60 (0.32–1.15) | 0.18 (0.02–1.34) | R-CVP (>4 cycles) | 6.9 | 0.33 (0.16–0.65) | 0.16 (0.05–0.57) |
| R-CHOP (≤4 cycles) | 6.4 | 0.50 (0.35–0.71) | 0.61 (0.34–1.09) | R-CHOP (≤4 cycles) | 3.3 | 0.67 (0.44–1.04) | 0.39 (0.20–0.77) |
| R-CHOP (>4 cycles) | 7.2 | 0.51 (0.34–0.77) | 0.59 (0.30–1.17) | R-CHOP (>4 cycles) | 6.1 | 0.39 (0.25–0.62) | 0.35 (0.18–0.72) |
B-symptoms were dropped due to complete separation across years of diagnosis.
B-symptoms and percentage in census tract completing high school dropped due to complete separation.
Median OS was unadjusted for covariates.
Only 1 patient with stage III/IV DLBCL was treated with >4 cycles of CVP.
Note: OS=overall survival, LRS=lymphoma-related survival; HR=hazard ratio; CI=confidence interval; CVP=cyclophosphamide, vincristine, prednisone; R-CVP=rituximab + CVP; CHOP=cyclophosphamide, doxorubicin, vincristine, prednisone; R-CHOP=rituximab + CHOP
DISCUSSION
Our study of treatment patterns and outcomes in DLBCL patients >80 is one of the most comprehensive to date examining a large national cohort treated with modern chemoimmunotherapy. An unexpected finding was that standard-of-care R-CHOP was the most common initial management strategy in this age group. These data indicate the broad application of the best available evidence for DLBCL to the very elderly population following the 2002 publication of a randomized controlled trial demonstrating the superiority of R-CHOP over CHOP in patients >60.31 However, approximately 25% of patients >80 had no initial therapy recorded in the 6 months following diagnosis. This is somewhat surprising since <50% had a poor performance status and only 20% had a comorbidity score >1. Additional studies are needed to determine the reasons for observation in DLBCL patients > 80 years, since factors not captured in claims data appear to influence selection of this strategy.
We also found that R-CHOP use in the very elderly varied with marital status, stage, disease site, radiation therapy, growth factor treatment, and performance status. These results suggest that the failure of very elderly DLBCL patients to receive standard treatment is associated with clinical factors, but also may vary across demographic factors such as marital status. This may reflect bias in the utilization of R-CHOP in very elderly patients that is not based on clinical parameters. Our observation that married patients were more likely to receive R-CHOP might be explained by an assumption that married patients enjoy a stronger support system,32 potentially leading to increased willingness to undergo and adhere to treatment. Indeed, several studies have found an association between being married and decreased mortality from cancer, including DLBCL.32–35
Our observation of survival benefit in patients initially treated with R-CVP is supported by previous studies describing improved survival in elderly DLBCL patients treated with non-anthracycline-based chemoimmunotherapy. Prior SEER-Medicare studies found that in DLBCL patients >65, those who received non-anthracycline-based chemoimmunotherapy had similar 3-year OS as those who received anthracycline-based chemotherapy without rituximab.36,37 Our study adds to these findings by using a more recent SEER-Medicare dataset, controlling for performance status, examining the effect of treatment duration on survival, and delineating a specific chemotherapy regimen with similar outcomes to R-CHOP in very elderly patients. We also compared toxicity of R-CHOP across age categories, and found that patients >80 years were less likely to develop febrile neutropenia but more likely to be hospitalized post-treatment. These findings may be explained by decreased use of intensive regimens and worse performance status in very elderly patients, respectively.
Although DLBCL treatment patterns and outcomes in very elderly patients previously have not been well-characterized, there is some evidence from prior studies that this group may benefit from standard treatment. A 1999 study found no difference in 5-year OS between DLBCL patients ≥80 and younger patients.6 A phase II study found that in DLBCL patients >80 with an Eastern Cooperative Oncology Group performance status of 0–2, low-dose R-CHOP conferred a survival benefit and was well-tolerated.7 A 2012 study showing markedly improved survival in DLBCL patients aged 80 in the rituximab era furthered the notion that this population may benefit from chemoimmunotherapy.8 Recently, investigators compared DLBCL patients aged ≥80 (n=40) with those aged 20–79 and found that the former group was significantly less likely to be treated with standard therapy and had significantly lower 1-year OS and event-free survival.9 This study also found that patients aged 80 who received standard therapy had significantly improved OS as compared to those who received no therapy, with the majority of untreated patients dying from lymphoma. Another recent study found that in 103 DLBCL patients aged ≥75, those who completed chemotherapy had a 70% 2-year OS, compared to 28% for those receiving incomplete chemotherapy and 21% for those receiving supportive care.10 Other studies examining outcomes in very elderly patients with NHL emphasized that standard treatments should be considered in this population to improve survival.11–13 In our study, R-CHOP for >4 cycles produced a median OS of >6 years among patients >80 and yielded optimal survival, even after controlling for comorbid diseases, performance status, and stage.
Strengths of our study include a large national cohort of DLBCL patients with validated SEER demographic and clinical information. Importantly, using claims data only may overestimate the proportion of patients who undergo observation, since some of these patients may have instead received oral chemotherapy or other regimens not captured by their Medicare claims. Thus, our use of patients receiving observation as a reference group for the OS and LRS Cox proportional hazards models provides a conservative estimate of the relative benefits of the treatment regimens. In contrast to our prior National Cancer Data Base study on the diffusion of chemoimmunotherapy over time,14 the present study contains more detailed information on the type of chemoimmunotherapy given and provides more complete capture of rituximab use. Our study also has limitations. First, we used claims data to indirectly assess comorbidity, performance status, and treatment, and we lacked direct clinical data on treatment dose and referral to hospice. However, we did examine the effectiveness of standard treatment versus abbreviated treatment cycles in our survival analysis, indicating that generally more cycles of therapy were associated with better outcomes. Additionally, we assumed that patients received prednisone when their claims data contained the other components of CHOP or CVP. This assumption may be inaccurate, especially given the toxicities associated with prednisone in elderly individuals. Despite these assumptions, our findings regarding the outcomes associated with anthracycline vs. non-anthracycline based chemoimmunotherapy regimens still hold. Of the patients excluded from our study population due to death within 6 months of diagnosis, 48% were over the age of 80. This represents 44% of the original population >80. Thus, our results may not be generalizable to patients >80 with the most advanced disease or the most severe comorbidity profile.
In conclusion, we found that in comparison to younger patients, those >80 years were less likely to receive R-CHOP and more likely to undergo observation or receive R-CVP (p<0.0001). R-CHOP was associated with the best survival outcomes in patients >80 of all stages, as has been demonstrated in younger patients in randomized controlled trials. These findings indicate that very elderly DLBCL patients who can tolerate R-CHOP should be treated with this regimen. R-CVP also appeared to be an effective treatment in DLBCL patients >80, particularly in those with stage III/IV disease, and can be considered a viable alternative with similar outcomes to R-CHOP in very elderly patients with advanced stage DLBCL. These data also establish a rationale for comparing a non-anthracycline-based regimen to R-CHOP in a randomized controlled trial involving previously untreated elderly DLBCL patients. Data from ongoing studies examining the role of bendamustine and rituximab in very elderly DLBCL patients (NCT01990144, NCT01234467) may provide a foundation for such a trial in the future. Clinicians should consider functional status and comorbidities when making treatment decisions for individual patients. Further studies are needed to characterize the impact of DLBCL treatment on quality of life and to determine optimal R-CHOP dosing for very elderly patients.
Supplementary Material
Acknowledgments
Acknowledgements of research support: This work was supported by Dr. Flowers’ National Cancer Institute R21 CA158686 and Dr. Nastoupil’s American Society of Hematology Clinical Scholars Award.
Footnotes
SEER-Medicare Disclaimer: This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
Financial Disclosures: Dr. Nastoupil has been a consultant for Celgene and has received research funding from Janssen and TG Therapeutics and honoraria from Genentech/Roche.
References
- 1.Flowers CR, Sinha R, Vose JM. Improving outcomes for patients with diffuse large B-cell lymphoma. CA Cancer J Clin. 2010;60:393–408. doi: 10.3322/caac.20087. [DOI] [PubMed] [Google Scholar]
- 2.Smith A, Howell D, Patmore R, Jack A, Roman E. Incidence of haematological malignancy by sub-type: a report from the Haematological Malignancy Research Network. Br J Cancer. 2011;105:1684–1692. doi: 10.1038/bjc.2011.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howlader N, Noone AM, Krapcho M, et al. [accessed June 3, 2014];SEER cancer statistics review, 1975–2011. 2013 Available from URL: http://seer.cancer.gov/csr/1975_2011/results_merged/sect_19_nhl.pdf.
- 4.United States Census Bureau. [accessed January 19, 2014];Percent distribution of the projected population by selected age groups and sex for the United States: 2015 to 2060. 2012 Table 3. Available from URL: http://www.census.gov/population/projections/data/national/2012/summarytables.html.
- 5.Vincent GK, Velkoff VA. [accessed May 14, 2014];The next four decades: the older population in the United States: 2010 to 2050. 2010 :10. Available from URL: http://www.census.gov/prod/2010pubs/p25-1138.pdf.
- 6.Zinzani PL, Storti S, Zaccaria A, et al. Elderly aggressive-histology non-Hodgkin’s lymphoma: first-line VNCOP-B regimen experience on 350 patients. Blood. 1999;94:33–38. [PubMed] [Google Scholar]
- 7.Peyrade F, Jardin F, Thieblemont C, et al. Attenuated immunochemotherapy regimen (R-miniCHOP) in elderly patients older than 80 years with diffuse large B-cell lymphoma: a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2011;12:460–468. doi: 10.1016/S1470-2045(11)70069-9. [DOI] [PubMed] [Google Scholar]
- 8.Hasselblom S, Stenson M, Werlenius O, et al. Improved outcome for very elderly patients with diffuse large B-cell lymphoma in the immunochemotherapy era. Leuk Lymphoma. 2012;53:394–399. doi: 10.3109/10428194.2011.616612. [DOI] [PubMed] [Google Scholar]
- 9.Varga C, Holcroft C, Kezouh A, et al. Comparison of outcomes among patients aged 80 and over and younger patients with diffuse large B-cell lymphoma: a population based study. Leuk Lymphoma. 2014;55:533–537. doi: 10.3109/10428194.2013.790968. [DOI] [PubMed] [Google Scholar]
- 10.Boslooper K, Kibbelaar R, Storm H, et al. Treatment with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone is beneficial but toxic in very elderly patients with diffuse large B-cell lymphoma: a population-based cohort study on treatment, toxicity and outcome. Leuk Lymphoma. 2014;55:526–532. doi: 10.3109/10428194.2013.810737. [DOI] [PubMed] [Google Scholar]
- 11.Bairey O, Benjamini O, Blickstein D, Elis A, Ruchlemer R. Non-Hodgkin’s lymphoma in patients 80 years of age or older. Ann Oncol. 2006;17:928–934. doi: 10.1093/annonc/mdl034. [DOI] [PubMed] [Google Scholar]
- 12.Nabhan C, Smith SM, Helenowski I, et al. Analysis of very elderly (>/=80 years) non-Hodgkin lymphoma: impact of functional status and co-morbidities on outcome. Br J Haematol. 2011;156:196–204. doi: 10.1111/j.1365-2141.2011.08934.x. [DOI] [PubMed] [Google Scholar]
- 13.Thieblemont C, Grossoeuvre A, Houot R, et al. Non-Hodgkin’s lymphoma in very elderly patients over 80 years. A descriptive analysis of clinical presentation and outcome. Ann Oncol. 2008;19:774–779. doi: 10.1093/annonc/mdm563. [DOI] [PubMed] [Google Scholar]
- 14.Flowers CR, Fedewa SA, Chen AY, et al. Disparities in the early adoption of chemoimmunotherapy for diffuse large B-cell lymphoma in the United States. Cancer Epidemiol Biomarkers Prev. 2012;21:1520–1530. doi: 10.1158/1055-9965.EPI-12-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han X, Jemal A, Flowers CR, Sineshaw H, Nastoupil LJ, Ward E. Insurance status is related to diffuse large B-cell lymphoma survival. Cancer. 2014;120:1220–1227. doi: 10.1002/cncr.28549. [DOI] [PubMed] [Google Scholar]
- 16.National Cancer Institute. [accessed June 3, 2014];Overview of the SEER program. Available from URL: http://seer.cancer.gov/about/overview.html.
- 17.National Cancer Institute. [accessed January 15, 2014];SEER-Medicare linked database. Available from URL: http://appliedresearch.cancer.gov/seermedicare.
- 18.ICD-O-3: International Classification of Diseases for Oncology. 3. Geneva: World Health Organization; 2000. [Google Scholar]
- 19.Fritz A, Ries L. NIH publication No. 98–1999. Bethesda, MD: National Cancer Institute; 1998. SEER Program Code Manual. [Google Scholar]
- 20.Satram-Hoang S, Reyes C, Hoang KQ, Momin F, Skettino S. Treatment practice in the elderly patient with chronic lymphocytic leukemia–analysis of the combined SEER and Medicare database. Ann Hematol. 2014;93:1335–1344. doi: 10.1007/s00277-014-2048-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Griffiths R, Gleeson M, Knopf K, Danese M. Racial differences in treatment and survival in older patients with diffuse large B-cell lymphoma (DLBCL) [published online November 12, 2010] BMC Cancer. doi: 10.1186/1471-2407-10-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang M, Burau KD, Fang S, Wang H, Du XL. Ethnic variations in diagnosis, treatment, socioeconomic status, and survival in a large population-based cohort of elderly patients with non-Hodgkin lymphoma. Cancer. 2008;113:3231–3241. doi: 10.1002/cncr.23914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griffiths RI, Gleeson ML, Mikhael J, Dreyling MH, Danese MD. Comparative effectiveness and cost of adding rituximab to first-line chemotherapy for elderly patients diagnosed with diffuse large B-cell lymphoma. Cancer. 2012;118:6079–6088. doi: 10.1002/cncr.27638. [DOI] [PubMed] [Google Scholar]
- 24.Griffiths R, Mikhael J, Gleeson M, Danese M, Dreyling M. Addition of rituximab to chemotherapy alone as first-line therapy improves overall survival in elderly patients with mantle cell lymphoma. Blood. 2011;118:4808–4816. doi: 10.1182/blood-2011-04-348367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davidoff AJ, Zuckerman IH, Pandya N, et al. A novel approach to improve health status measurement in observational claims-based studies of cancer treatment and outcomes. J Geriatr Oncol. 2013;4:157–165. doi: 10.1016/j.jgo.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davidoff AJ, Tang M, Seal B, Edelman MJ. Chemotherapy and survival benefit in elderly patients with advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:2191–2197. doi: 10.1200/JCO.2009.25.4052. [DOI] [PubMed] [Google Scholar]
- 27.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 28.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 29.Parsons LS. Using SAS® Software to Perform a Case-Control Match on Propensity Score in an Observational Study. Cary, NC. Proceedings of the Twenty-Fifth Annual SAS® Users Group International Conference; 2000. pp. 1166–1171. [Google Scholar]
- 30.Liu Y, Nickleach D, Lipscomb J. Propensity Score Matching for Multiple Treatment Comparisons in Observational Studies. Hong Kong. Proceedings of the Fifty-Ninth International Statistical Institute World Statistics Congress; 2013. pp. 4759–4764. [Google Scholar]
- 31.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 32.Aizer AA, Chen MH, McCarthy EP, et al. Marital status and survival in patients with cancer. J Clin Oncol. 2013;31:3869–3876. doi: 10.1200/JCO.2013.49.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tao L, Foran JM, Clarke CA, Gomez SL, Keegan TH. Socioeconomic disparities in mortality after diffuse large B-cell lymphoma in the modern treatment era. Blood. 2014;123:3553–3562. doi: 10.1182/blood-2013-07-517110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson NJ, Backlund E, Sorlie PD, Loveless CA. Marital status and mortality: the national longitudinal mortality study. Ann Epidemiol. 2000;10:224–238. doi: 10.1016/s1047-2797(99)00052-6. [DOI] [PubMed] [Google Scholar]
- 35.Cronin-Fenton DP, Sharp L, Deady S, Comber H. Treatment and survival for non-Hodgkin’s lymphoma: influence of histological subtype, age, and other factors in a population-based study (1999–2001) Eur J Cancer. 2006;42:2786–2793. doi: 10.1016/j.ejca.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 36.Link BK, Brooks J, Wright K, Pan X, Voelker M, Chrischilles E. Diffuse large B-cell lymphoma in the elderly: diffusion of treatment with rituximab and survival advances with and without anthracyclines. Leuk Lymphoma. 2011;52:994–1002. doi: 10.3109/10428194.2011.557167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tien YY, Link BK, Brooks JM, Wright K, Chrischilles E. Treatment of diffuse large B-cell lymphoma in the elderly: regimens without anthracyclines are common and not futile. Leuk Lymphoma. doi: 10.3109/10428194.2014.903589. published online April 22, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.