Abstract
Objectives
Enzymatically glycosylated proteins partake in multiple biological processes, including glucose transport and inflammation. We hypothesized that a novel biomarker (GlycA) of N-acetyl methyl groups originating mainly from N-acetylglucosamine moieties of acute-phase glycoproteins is related to incident type 2 diabetes and compared it with high-sensitivity C-reactive protein.
Approach and Results
In 26,508 initially healthy women free of diabetes, baseline GlycA and high-sensitivity C-reactive protein were quantified by nuclear magnetic resonance spectroscopy and immunoturbidimetry respectively. During median follow-up of 17.2 years, 2,087 type 2 diabetes cases occurred. In Cox models with adjustment for age, race, smoking, alcohol, activity, menopausal status, hormone use, family history, and body-mass index, quartile 4 vs 1 hazard ratios and 95% confidence intervals were 2.67 (2.26–3.14) for GlycA and 3.93 (3.24–4.77) for high-sensitivity C-reactive protein; both p-trend <0.0001. Associations for GlycA and high-sensitivity C-reactive protein were attenuated after additionally adjusting for lipids: 1.65 (1.39–1.95) and 2.83 (2.32–3.44), respectively, both p-trend <0.0001, and after mutual adjustment: 1.11 (0.93–1.33, p-trend=0.10) and 2.57 (2.09–3.16, p-trend<0.0001), respectively.
Conclusions
Our finding of an association between a consensus glycan sequence common to a host of acute phase reactants and incident type 2 diabetes provides further support for inflammation in the development of type 2 diabetes. Additional studies exploring the role of enzymatic glycosylation in the prevention of type 2 diabetes are warranted.
INTRODUCTION
Enzymatic protein glycosylation represents the most abundant post translational modification process, with nearly 70% of human plasma proteome bearing a glycan (sugar) structure.1 The significance of glycan attachments is exemplified by their role in modulating biological processes including cell trafficking, signal transduction, regulation of metabolism, and host pathogen recognition 2. Of clinical importance is that glycan biosynthesis is exquisitely responsive to the cellular milieu, 3 and an altered glycosylation pattern may therefore reflect the development of disease, hence the interest in studying glycans as early indicators of disease. However the diagnostic utility of glycans is hampered by technological limitations in quantitative glycan analysis due in part to their non-template derived nature 3. It is noteworthy to differentiate non-enzymatic glycation products, such as hemoglobin A1c (HbA1c) and advanced glycation end products, from glycans which are indeed different biomolecular species.
Amongst other techniques, proton nuclear magnetic resonance (NMR) spectroscopy is emerging as a promising high-throughput method for measuring protein glycans 3. Bell et al. 4 documented the first report of an NMR spectrum common to glycoproteins of acute-phase reactants. Furthermore, their work suggested that glycan modification of acute-phase reactants may be useful for the detection, prognosis, and therapeutic monitoring of tissue damage marked by inflammation 4. LipoScience Inc. (Raleigh, NC) recently developed an application for an NMR-derived biomarker referred to as “GlycA”, which originates from the N-acetyl methyl groups of N-acetylglucosamine residues located on specific glycan branches of plasma proteins mainly from the acute phase glycoproteins α1-acid glycoprotein, haptoglobin, α1-antitrypsin, α1-antichymotrypsin and transferrin.5 The proton NMR spectrum to which GlycA was assigned overlaps with the region identified by Bell et al.,4 and hence GlycA may be an integrative marker of systemic inflammation.
Low grade chronic systemic inflammation is known to trigger the development of insulin resistance and β-cell dysfunction.6 The association of circulating levels of inflammatory proteins, in particular acute-phase reactants, with the clinical expression of type 2 diabetes is also well described in prospective epidemiological studies.7, 8 Enzymatic glycosylation modulates the functions of these proteins, and analysis of the ensuing glycans may have important implications for the pathogenesis of type 2 diabetes. Whether a glycosylation pattern common to a host of acute phase reactants have potential utility as a marker of future risk of type 2 diabetes is unknown. We therefore examined the relation of baseline GlycA, quantified by NMR spectroscopy, with incident type 2 diabetes, and compared it with high sensitivity C-reactive protein (hsCRP), a commonly used clinical marker of inflammation. Because metabolic derangements can induce glycan changes relevant to glucose control,9 we also pre-specified to assess for effect modification by HbA1c, body-mass index (BMI), and smoking status.
MATERIALS AND METHODS
Materials and methods are available in the online-only Data Supplement.
RESULTS
Baseline characteristics and correlations
Mean (SD) age of the study population at baseline was 54.6 (7.1) years. Median (25th–75th percentile) baseline concentrations for GlycA and hsCRP were 368 (325–414) μmol/L and 1.97 (0.79–4.24) mg/L, respectively. Participants with higher levels of GlycA were more likely to be hormone users, postmenopausal, current smokers, and to report a first-degree relative with diabetes (Table 1). They also had higher BMI, hsCRP, and dyslipidemia; and were less likely to consume alcohol or exercise. HbA1c levels differed minimally across quartiles of GlycA, although achieved statistical significance given the large study sample.
Table 1.
Baseline Characteristics According to Quartiles of GlycA
| Quartiles of GlycA | ||||
|---|---|---|---|---|
|
| ||||
| ≤325 μmol/L n=6 718 |
326–368 μmol/L n=6 599 |
369–414 μmol/L n=6 587 |
>414 μmol/L n=6 604 |
|
|
| ||||
| Age, years | 51 (48, 57) | 53 (49, 59) | 54 (49, 60) | 54 (50, 60) |
|
| ||||
| Race/ethnicity | ||||
| Caucasian | 94.5 | 95.7 | 95.8 | 96.0 |
| Hispanic | 1.0 | 1.2 | 1.0 | 0.9 |
| African-American | 1.7 | 1.6 | 1.6 | 2.1 |
| Other | 2.8 | 1.41 | 1.6 | 1.0 |
|
| ||||
| Hormone use, % | 36.9 | 41.9 | 46.8 | 51.2 |
|
| ||||
| Postmenopausal, % | 45.5 | 53.6 | 57.2 | 60.6 |
|
| ||||
| Family history of Diabetes | 21.9 | 22.9 | 26.1 | 28.0 |
|
| ||||
| Current smoking, % | 7.5 | 10.8 | 12.2 | 16.1 |
|
| ||||
| Rare/never alcohol intake, % | 36.9 | 41.2 | 45.2 | 50.5 |
|
| ||||
| Exercise, MET-h/wk | 11.4 (4.1–24.0) | 10.0 (3.3–21.4) | 8.1 (2.7–19.5) | 6.5 (2.0–16.4) |
|
| ||||
| Body mass index, Kg/m2 | 22.8 (21.2, 25.1) | 24.1 (22.1, 26.6) | 25.6 (23.1, 28.8) | 27.4 (24.3,31.2) |
|
| ||||
| hsCRP, mg/L | 0.71 (0.35–1.50) | 1.47 (0.69, 2.80) | 2.53 (1.32, 4.44) | 4.90 (2.71, 8.04) |
|
| ||||
| Fibrinogen, mg/dl | 314 (279–349) | 340 (303–383) | 361 (320–407) | 399 (350–453) |
|
| ||||
| † Interleukin 6, μg/dl | 1.16 (0.77–1.79) | 1.32 (0.92–1.97) | 1.54 (1.00–2.13) | 1.93 (1.27–3.53) |
|
| ||||
| sICAM-1, ng/ml | 316 (281–359) | 335 (296–381) | 348 (307–398) | 370 (326–425) |
|
| ||||
| † TNF-αR2, mg/ml | 3043 (2654.17–3700) | 3154 (2623–3782) | 3036 (2561–3797) | 3201 (2636–3833) |
|
| ||||
| Hemoglobin A1c, % | 4.9 (4.8, 5.1) | 5.0 (4.8, 5.1) | 5.0 (4.9, 5.2) | 5.1 (4.9, 5.3) |
|
| ||||
| LDL cholesterol, mg/dl | 113 (94, 133) | 121 (100, 143) | 125 (104, 148) | 128 (106, 152) |
|
| ||||
| HDL cholesterol, mg/dl | 57 (48, 68) | 53 (45, 64) | 50.7 (42.2, 60.7) | 47.6 (39.9, 56.9) |
|
| ||||
| Triglycerides, mg/dl | 86 (65, 115) | 109 (81, 152) | 132 (96, 183) | 165 (118, 230) |
Values shown are medians (25th–75th percentile) or percentages. P values were obtained from Wilcoxon rank sum test for quantitative variables and χ2 tests for qualitative variables. All p values for trend across quartiles were <0.0001 except for TNF- αR2 (p=0.87).
n is 342 for Interleukin 6 and 345 for TNF-αR2. MET-h/wk is metabolic equivalent hours per week; hsCRP is high-sensitivity C-reactive protein; sICAM-1 is soluble intercellular adhesion molecule-1; TNF- αR2 is tumor necrosis factor α receptor type 2; LDL is low-density lipoprotein; HDL is high-density lipoprotein.
GlycA correlated positively with hsCRP (Spearman correlation coefficient 0.61, p<0.0001) Metabolic risk indicators also correlated with GlycA: range of coefficients, in increasing magnitude, with HbA1c, interleukin-6, soluble intracellular adhesion molecule-1, BMI, fibrinogen and triglycerides were 0.21 to 0.46, p for all <0.0001; and with HDL-cholesterol (−0.26, p<0.0001). GlycA was not correlated with tumor necrosis factor α receptor type 2, spearman correlation coefficient was 0.02 (p=0.77).
Associations with Incident Type 2 Diabetes
During a median follow-up of 17.2 years, a total of 2,087 cases of incident type 2 diabetes occurred. In Cox models adjusting for age, race/ethnicity, smoking, alcohol consumption, physical activity, menopausal status, hormone use, family history, BMI, and randomized trial assignments, HRs and 95% CIs for type 2 diabetes across increasing quartiles of GlycA were: 1.00, 1.43 (1.20–1.71), 1.90 (1.60–2.24), and 2.67 (2.26–3.14), and for hsCRP: 1.00, 1.86 (1.52–2.28), 2.96 (2.44–3.59) and 3.93 (3.24–4.77); both p-trend <0.0001 (Table 2). The associations were attenuated but remained statistically significant after further adjusting for lipids and hemoglobin A1c: top vs bottom quartile was 1.43 (1.21–1.69, p-trend<0.0001) and 2.43 (2.00–2.96, p-trend <0.0001), respectively. In models additionally adjusting for the other biomarker, these HRs became 1.04 (0.87–1.24, p-trend=0.35) and 2.31 (1.88–2.85, p-trend <0.0001) for GlycA and hsCRP respectively. Results were similar when the biomarkers were examined per-1-SD. Sensitivity analysis performed after exclusion of type 2 diabetes cases reported during the first 5 years of follow-up (428 events) yielded similar results.
Table 2.
Hazard ratios (95% confidence intervals) of GlycA and high-sensitivity C-reactive protein with incident type 2 diabetes
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | p Trend | Per 1 SD* | p Value | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| GlycA | |||||||
| Range, μmol/L | ≤325 | 326–368 | 369–414 | ≥415 | |||
| Incidence rate/1 000 person-years | 1.9 | 3.3 | 5.5 | 10.1 | |||
| Basic model | 1 | 1.43 (1.20–1.71) | 1.90 (1.60–2.24) | 2.67 (2.26–3.14) | <0.0001 | 1.40 (1.34–1.46) | <0.0001 |
| Basic model + lipids + HbA1c | 1 | 1.16 (0.97–1.39) | 1.29 (1.09–1.53) | 1.43 (1.21–1.69) | <0.0001 | 1.14 (1.09–1.20) | <0.0001 |
| Basic model + lipids +hsCRP | 1 | 1.01 (0.84–1.21) | 1.03 (0.87–1.23) | 1.11 (0.93–1.33) | 0.10 | 1.05 (0.99–1.11) | 0.12 |
| HsCRP | |||||||
| Range, mg/L | <0.80 | 0.80–1.97 | 1.98–4.24 | >4.24 | |||
| Incidence rate/1 000 person-years | 1.3 | 3.0 | 5.9 | 10.5 | |||
| Basic model | 1 | 1.86 (1.52–2.28) | 2.96 (2.44–3.59) | 3.93 (3.24–4.77) | <0.0001 | 1.58 (1.49–1.66) | <0.0001 |
| Basic model + lipids + HbA1c | 1 | 1.51 (1.23–1.85) | 2.14 (1.76–2.61) | 2.43 (2.00–2.96) | <0.0001 | 1.35 (1.27–1.43) | <0.0001 |
| Basic model + lipids + GlycA | 1 | 1.47 (1.19–1.80) | 2.04 (1.68–2.49) | 2.57 (2.09–3.16) | <0.0001 | 1.43 (1.34–1.53) | <0.0001 |
Basic model is adjusted for age, race, treatment assignments, smoking status, alcohol intake, physical activity, menopausal status, hormone replacement use, family history of diabetes and body mass index
Lipids include LDL-cholesterol, HDL-cholesterol and log triglycerides.
SD represents standard deviation (68 μmol/L for GlycA and 5.7 mg/L for hsCRP)
hsCRP was log transformed.
In stratified models adjusting for risk factors and lipids, the relative risk for GlycA with type 2 diabetes was somewhat higher for individuals with baseline BMI < vs ≥25 kg/m2: HRs for top vs bottom quartile were 2.20 (1.58–3.13. p-trend <0.0001) and 1.37 (1.13–1.67, p-trend <0.0001) respectively, p for interaction=0.006. No significant interaction was noted for BMI and hsCRP: corresponding HRs 2.22 (1.57–3.16, p-trend <0.0001) and 2.66 (2.06–3.45, p-trend <0.0001) respectively, p for interaction =0.61. Both GlycA and hsCRP were significantly associated with the risk of incident type 2 diabetes within all stratified categories of exercise, although p for interaction were marginally significant (p for interaction = 0.03 and 0.04 respectively) Associations of GlycA with type 2 diabetes was consistent in stratified analyses based on baseline HbA1c and smoking categories, with p for interactions of 0.15 and 0.36, respectively; and similarly for hsCRP (Table 3). A statistically significant interaction was observed between hsCRP and the association of GlycA with incident type 2 diabetes (p for interaction was 0.039), with a borderline significant tend for GlycA to be associated with the risk of type 2 diabetes at levels only at levels of hsCRP <1mg/L (p-trend =0.054). (Table 3). Results were similar when hsCRP was analyses as tertiles. hsCRP remained associated with incident type 2 diabetes across all tertiles of GlycA (p for interaction was 0.13).
Table 3.
Stratified hazard ratios (95% confidence intervals) of GlycA and high-sensitivity C-reactive protein with incident type 2 diabetes
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | p Trend | Per 1 SD* | p Value for Interaction | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| GlycA, μmol/L | |||||||
| BMI | 0.006 | ||||||
| <25 Kg/m2 | 1 | 1.38 (1.00–1.91) | 1.27 (0.90–1.79) | 2.20 (1.58–3.13) | <0.0001 | 1.38 (1.23–1.55) | |
| ≥25 Kg/m2 | 1 | 0.99 (0.80–1.23) | 1.14 (0.94–1.39) | 1.37 (1.13–1.67) | <0.0001 | 1.17 (1.11–1.23) | |
| HbA1c | 0.15 | ||||||
| <5 % (<31mmol/mol) | 1 | 1.21 (0.84–1.75) | 1.48 (1.04–2.11) | 1.57 (1.09–2.26) | 0.02 | 1.15 (1.03–1.29) | |
| ≥5 % (≥31mmol/mol) | 1 | 1.10 (0.90–1.35) | 1.15 (0.95–1.40) | 1.42 (1.18–1.72) | <0.0001 | 1.17 (1.11–1.23) | |
| Smoking† | 0.36 | ||||||
| Current | 1 | 1.30 (0.69–2.46) | 1.53 (0.83–2.82) | 1.93 (1.07–3.49) | 0.02 | 1.28 (1.12–1.46) | |
| Past | 1 | 1.21 (0.89–1.63) | 1.32 (0.99–1.77) | 1.87 (1.40–2.49) | <0.0001 | 1.26 (1.16–1.37) | |
| Never | 1 | 1.11 (0.88–1.41) | 1.28 (1.02–1.60) | 1.46 (1.17–1.84) | 0.0003 | 1.16 (1.09–1.25) | |
| hsCRP, mg/L | 0.039 | ||||||
| <1 | 1 | 1.28 (0.89–1.84) | 1.33 (0.89–1.99) | 1.59 (0.92–2.76) | 0.054 | 1.25 (1.02–1.53) | |
| 1–3 | 1 | 0.85 (0.65–1.12) | 0.98 (0.75–1.27) | 1.12 (0.85–1.48) | 0.11 | 1.04 (0.93–1.16) | |
| >3 | 1 | 0.90 (0.65–1.26) | 0.85 (0.62–1.16) | 1.01 (0.75–1.36) | 0.37 | 1.11 (1.04–1.18) | |
| Exercise, MET-h/wk | 0.02 | ||||||
| Tertile 1 | 1 | 1.32 (0.98–1.76) | 1.45 (1.10–1.92) | 1.71 (1.30–2.26) | <0.0001 | 1.21 (1.13–1.30) | |
| Tertile 2 | 1 | 1.18 (0.87–1.60) | 1.31 (0.98–1.75) | 1.60 (1.19–2.14) | 0.002 | 1.23 (1.12–1.34) | |
| Tertile 3 | 1 | 0.91 (0.65–1.28) | 1.10 (0.80–1.52) | 1.64 (1.19–2.25) | 0.0001 | 1.18 (1.07–1.31) | |
|
| |||||||
| hsCRP, mg/L | |||||||
| BMI | 0.61 | ||||||
| <25 Kg/m2 | 1 | 1.22 (0.88–1.69) | 2.01 (1.46–2.78) | 2.22 (1.57–3.16) | <0.0001 | 1.46 (1.29–1.66) | |
| ≥25 Kg/m2 | 1 | 1.47 (1.11–1.93) | 1.94 (1.49–2.51) | 2.66 (2.06–3.45) | <0.0001 | 1.44 (1.34–1.54) | |
| HbA1c | 0.27 | ||||||
| <5 % (<31mmol/mol) | 1 | 1.55 (1.02–2.35) | 2.26 (1.52–3.38) | 2.72 (1.80–4.10) | <0.0001 | 1.48 (1.30–1.68) | |
| ≥5 % (≥31mmol/mol) | 1 | 1.43 (1.13–1.81) | 1.97 (1.58–2.47) | 2.52 (2.02–3.15) | <0.0001 | 1.40 (1.32–1.50) | |
| Smoking† | 0.27 | ||||||
| Current | 1 | 1.66 (0.86–3.18) | 2.46 (1.32–4.59) | 2.66 (1.41–5.00) | 0.008 | 1.28 (1.08–1.51) | |
| Past | 1 | 1.57 (1.11–2.22) | 2.34 (1.68–3.25) | 2.99 (2.15–4.16) | <0.0001 | 1.48 (1.34–1.63) | |
| Never | 1 | 1.46 (1.11–1.92) | 1.98 (1.52–2.58) | 2.81 (2.15–3.67) | <0.0001 | 1.51 (1.40–1.64) | |
| GlycA, μmol/L | 0.13 | ||||||
| Tertile 1 | 1 | 1.67 (1.21–2.30) | 2.10 (1.46–3.00) | 3.02 (1.94–4.71) | <0.0001 | 1.59 (1.35–1.88) | |
| Tertile 2 | 1 | 1.35 (0.96–1.89) | 1.95 (1.41–2.70) | 2.50 (1.79–3.51) | <0.0001 | 1.56 (1.38–1.76) | |
| Tertile 3 | 1 | 0.99 (0.63–1.58) | 1.46 (0.95–2.24) | 1.86 (1.22–2.84) | <0.0001 | 1.32 (1.22–1.44) | |
| Exercise, MET-h/wk | 0.04 | ||||||
| Tertile 1 | 1 | 1.43 (1.02–2.00) | 1.98 (1.44–2.73) | 2.61 (1.89–3.59) | <0.0001 | 1.43 (1.31–1.57) | |
| Tertile 2 | 1 | 1.64 (1.15–2.35) | 2.45 (1.74–3.46) | 3.17 (2.24–4.48) | <0.0001 | 1.51 (1.37–1.67) | |
| Tertile 3 | 1 | 1.37 (0.94–1.98) | 1.86 (1.30–2.67) | 2.59 (1.80–3.72) | <0.0001 | 1.45 (1.28–1.63) | |
Adjusted for age, race and treatment assignments, smoking status, alcohol intake, physical activity, menopausal status, hormone replacement use, family history of diabetes, body mass index, LDL-cholesterol, HDL-cholesterol and log triglycerides.
SD represents standard deviation (68 μmol/L for GlycA and 5.7 mg/L for hsCRP).
hsCRP was log transformed.
Not adjusted for smoking status
Adding GlycA to a model that included age, race, smoking status, alcohol intake, physical activity, menopausal status, hormone replacement use, family history of diabetes, body mass index, LDL-cholesterol, HDL-cholesterol, and log triglycerides, resulted in improved metrics of goodness of fit (p for likelihood ratio χ2 ≤0.0001). GlycA did not improve risk discrimination as indicated by the Harrell’s C-index and the integrated discrimination improvement (Supplementary Table). However, GlycA yielded a small improvement in category-free net reclassification index of 0.1848 (95% CI: 0.1269–0.2423).
DISCUSSION
This prospective study of 26,508 apparently healthy women followed for a median of 17.2 years provides the first evidence for the potential role of protein glycan side-chains early in the development of type 2 diabetes and specifically for GlycA levels as a biomarker for predicting the future risk of type 2 diabetes. High baseline levels of GlycA, a novel NMR-derived protein glycan biomarker, were associated with a graded increase in the risk of type 2 diabetes in a pattern similar to that observed for hsCRP. The magnitude of HRs were somewhat greater for hsCRP, and accounting for hsCRP attenuated the GlycA-type 2 diabetes association, consistent with a potential inflammatory role for GlycA and risk of type 2 diabetes. The association of GlycA with type 2 diabetes was significant even among individuals with baseline HbA1c< 5% (31mmol/mol). A quantitative interaction was noted between GlycA and BMI, with greater risk observed in participants with BMI<25 kg/m2.
Evidence for glycan differences in relation to cardiometabolic risk factors as observed in this study is consistent with a previous study which found significant associations between specific changes in plasma N-glycans and risk factors including body fat, lipids, and smoking. For instance hyperlipidemia and smoking were found to correlate with higher levels of galactosylation.10 These results suggest a role for lifestyle changes in modifying the expression of pathogenic glycans. Our finding of a higher GlycA attributable type 2 diabetes risk in individuals with BMI <25 kg/m2 is intriguing. Recently, Perry and Voight et al. identified a novel genetic variant whose magnitude of association with type 2 diabetes was higher in lean (BMI <25 kg/m2) compared to obese (≥ 30 kg/m2) cases of type 2 diabetes,11 also demonstrating that lean compared with obese type 2 diabetes cases were more enriched with known candidate type 2 diabetes loci.11 It will therefore be of interest to determine whether these genetic variants relate to the expression of the glycosylation machinery (i.e. specific glycosidases and glycosyltransferases) responsible for the GlycA signal.
Several proteins, mostly acute phase reactants, appear to bear the oligosaccharide chains unique to GlycA. Some of the major known contributors to the GlycA signal, including α1-acid glycoprotein, α1-antitrypsin, and transferrin, have been linked to type 2 diabetes.12–16 A previous analysis of the Atherosclerosis Risk and Communities Study showed that individuals with higher levels of α1-acid glycoprotein had increased risk for developing type 2 diabetes.12 Related studies have also linked deficiency and impairment of α1-antitrypsin with type 2 diabetes,14, 15 findings that are in support of α1-antitrypsin exerting a regulatory effect on inflammation.17 Transferrin levels were also found to be associated with new onset hyperglycemia despite being a negative acute phase reactant.16 One study found that α1-antichymotrypsin, another glycoprotein with major contributions to GlycA, was strongly associated with insulin resistance as determined by post-load insulin levels,18 although a cross-sectional study, this report is relevant in the context of insulin exerting a null effect on levels of α1-antichymotrypsin.19 In other words, mechanisms linked to ongoing insulin resistance and not insulin likely account for the observed findings. Furthermore, a set of other acute phase reactants, including fibrinogen, hsCRP and interleukin-6 make negligible contributions to the GlycA signal. As with hsCRP, several prospective studies have consistently shown a positive association between interleukin-6 and the risk of incident type 2 diabetes.7, 8 Non acute phase reactants such as apolipoprotein B also make negligible contribution to the GlycA signal. Thus the metabolic cluster concomitant with a preponderance of apolipoprotein B may partly account for the observed association between GlycA and type 2 diabetes as indicated by the attenuation in the magnitude of effect after adjusting for lipids. The present study adds to the growing body of literature on the link between inflammation and the pathogenesis of type 2 diabetes by showing that a unique glycan sequence presumably common to specific glycoforms of acute phase reactants may serve as a biomarker for the risk of type 2 diabetes. Nonetheless, despite the temporality of our findings, additional work needs to be done to evaluate if the glycans captured by GlycA are causal with respect to the pathogenesis of diabetes.
Although the structure of the glycans captured as GlycA is known to contain N-acetyl methyl group protons of the N-acetylglucosamine moieties located on the bi- tri- and tetraantennary branches of specific serum proteins, we do not know the exact carbohydrate structure of the side-chains of the contributing proteins during inflammation and how this may relate to the risk of incident type 2 diabetes. In this regard, a shift in the glycoform pattern of α1-acid glycoprotein characterized by increased fucosylation has been reported in relation to type 2 diabetes with similar changes observed on α1-acid glycoprotein isolated from sera of patients with marked inflammation.13 Whether the other glycoproteins contributing to the GlycA signal exhibit similar shifts in their glycan composition in relation to type 2 diabetes is unknown. Nonetheless, our findings indicate that a consensus glycosylation sequence on these proteins as captured by the GlycA signal may be useful in defining their specific role in promoting the development of type 2 diabetes through inflammatory pathways.
Enzymatic addition of glycans to proteins occurs through the coordinated expression of glycosidases and glycosyltransferases which regulate posttranslational modifications that alter protein function during metabolism and thus have implication for detecting disease onset. In this regard, the functional integrity of proteins essential to glucose trafficking have been correlated with specific glycosylation patterns, as exemplified in experiments demonstrating that altered glycosylation patterns of insulin signal transduction proteins impair glucose homeostasis and β-islet cell function.9, 20 But as yet it is unknown if the glycosylation machinery responsible for the glycan chains unique to GlycA distort the glycosylation pattern needed to preserve normal glucose metabolism. The proposed inflammatory origin of GlycA provides a more plausible mechanistic explanation for the observed association. Increased synthesis of pro-inflammatory cytokines including acute phase reactants is well known to characterize pre-clinical stages of type 2 diabetes with further graded increase facilitating disease progression.7 Proof-of-concept studies have also demonstrated the effect of biological agents targeting specific pro-inflammatory cytokines in improving parameters of glucose control.21, 22 From a therapeutic standpoint, GlycA may provide utility for evaluating novel strategies targeting the inflammatory network in the prevention of type 2 diabetes.
Persistent hyperglycemia contribute to impaired insulin responsiveness in target tissues, partly through increased conversion of glucose to donor sugars used in enzymatic glycosylation reactions that render the effector proteins of the insulin signaling cascade defective.23 Sustained hyperglycemia may therefore lead to the formation of protein glycans that in turn perpetuate insulin resistance. Robustness of our findings amongst participants with HbA1c in euglycemic range however implicate mechanisms other than chronic hyperglycemia. Moreover, results from the sensitivity analysis excluding cases occurring within the first 5 years i.e. potential cases of clinically silent type 2 diabetes, mitigate against chronic hyperglycemia as an explanation for our observation. These along with the prospective design of the present study thus minimizes the influence of reverse causality in the interpretation of our findings. It should also be pointed out that the monotonic pattern of HbA1c across levels of GlycA highlights an important distinction between glycation, which is a consequence of non-enzymatic glycosylation, and enzymatic glycosylation, a different process that is responsible for the glycan conjugation of proteins and other biopolymers.
Limitations of the present study warrants discussion. First, our study population was composed of healthy middle-aged and older women, thus our results may not be generalizable to other groups particularly men and women with a different metabolic risk profile. Second, measurements of GlycA were only available at baseline precluding our ability to assess the effect of time dependent changes in GlycA levels in relation to type 2 diabetes. Third, we cannot rule out the possibility of undetected type 2 diabetes at baseline, although our results remained robust in women with HbA1c levels<5% (31mmol/mol) as well as after excluding events occurring during the first 5 years of follow-up. Finally, we cannot exclude the potential of residual and unmeasured confounding in the interpretation of our findings. Notable strengths of our study include a large sample size, a long prospective follow-up, a systematic ascertainment of incident type 2 diabetes, and a detailed assessment of cardiometabolic risk factors.
In summary, we found elevated baseline levels of GlycA, a novel protein glycan, to be significantly associated with incident type 2 diabetes in a cohort of initially healthy middle aged women. This association was attenuated after adjusting for hsCRP, a clinical biomarker of systemic inflammation, and provides new evidence on the potential role of glycans early in the development of type 2 diabetes. More research is needed to verify our findings in other populations as well as to investigate the potential of GlycA in the prevention and treatment of type 2 diabetes.
Supplementary Material
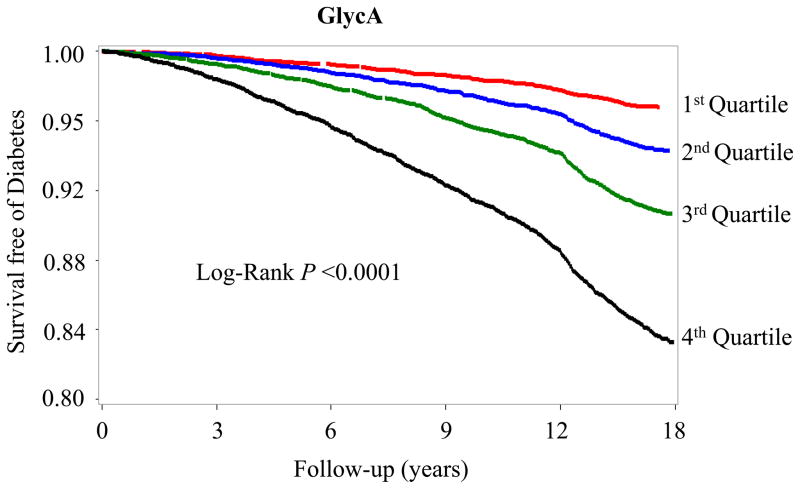
Figure 1.
Kaplan-Meier curves for survival free of incident type 2 diabetes according to quartiles of GlycA.
Quartile concentrations were ≤325, 326–368, 369–414 and ≥415μmol/L.
SIGNIFICANCE.
Several inflammatory proteins have been shown to be associated with the risk of type 2 diabetes. The biological role of glycans in modulating protein function provides a viable avenue for disease prevention. This study illustrates the potential role of glycans in prognosticating against the risk of type 2 diabetes by showing that baseline measurements of a unique glycan sequence conjugated to several acute phase proteins was associated with the risk of developing type 2 diabetes. Future work defining the biochemical properties of GlycA (the unique glycan sequence investigated in the current study) will provide additional biological information that if targeted, may enhance the diagnostic yield of GlycA. This effort will contribute to the understanding of the link between inflammation and type 2 diabetes while also serving as a template to initiate the discovery of new therapeutics.
Acknowledgments
Sources of Funding.
The research for this article was supported by the American Heart Association and by grants HL43851, HL 080467, and CA 47988 from the National Heart, Lung, and Blood Institute and the National Cancer Institute, National Institutes of Health, a charitable gift from the Molino Family Trust and with additional support from U01 HL108630 (Mechanism-Associated Phenotypes for Genetic analyses of Heart, Lung, Blood, and Sleep Diseases [MAPGen for HLBS]). LipoScience Inc. supplied the GlycA information at no additional cost. The funding agencies played no role in the design, conduct, data management, analysis, or manuscript preparation related to this study. Dr. Akinkuolie was supported by the National Heart, Lung, and Blood Institute (T32 HL007575).
Footnotes
Women’s Health Study URL http://clinicaltrials.gov/ct/show/NCT00000479 unique identifier NCT00000479
Disclosures.
Dr. Ridker is listed as a co-inventor on patents held by the Brigham and Women’s Hospital that relate to the use of inflammatory biomarkers in cardiovascular disease that have been licensed to AstraZeneca and Seimens. Dr. Mora has received institutional research support from AstraZeneca, Atherotech Diagnostics, and NHLBI; served as a consultant to Genzyme, Quest Diagnostics, Lilly, Pfizer, and Cerenis Therapeutics. The other authors report no conflicts.
References
- 1.Rabinovich GA, van Kooyk Y, Cobb BA. Glycobiology of immune responses. Ann N Y Acad Sci. 2012;1253:1–15. doi: 10.1111/j.1749-6632.2012.06492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126:855–867. doi: 10.1016/j.cell.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 3.Marino K, Bones J, Kattla JJ, Rudd PM. A systematic approach to protein glycosylation analysis: A path through the maze. Nat Chem Biol. 2010;6:713–723. doi: 10.1038/nchembio.437. [DOI] [PubMed] [Google Scholar]
- 4.Bell JD, Brown JC, Nicholson JK, Sadler PJ. Assignment of resonances for ‘acute-phase’ glycoproteins in high resolution proton nmr spectra of human blood plasma. FEBS Lett. 1987;215:311–315. doi: 10.1016/0014-5793(87)80168-0. [DOI] [PubMed] [Google Scholar]
- 5.Otvos JD, Shalaurova I, Wolak-Dinsmore J, Connelly MA, Mackey RH, Stein JH, Tracy RP. Glyca: A composite nuclear magnetic resonance biomarker of systemic inflammation. Clin Chem. 2015 doi: 10.1373/clinchem.2014.232918. [DOI] [PubMed] [Google Scholar]
- 6.Lontchi-Yimagou E, Sobngwi E, Matsha TE, Kengne AP. Diabetes mellitus and inflammation. Curr Diab Rep. 2013;13:435–444. doi: 10.1007/s11892-013-0375-y. [DOI] [PubMed] [Google Scholar]
- 7.Badawi A, Klip A, Haddad P, Cole DE, Bailo BG, El-Sohemy A, Karmali M. Type 2 diabetes mellitus and inflammation: Prospects for biomarkers of risk and nutritional intervention. Diabetes Metab Syndr Obes. 2010;3:173–186. doi: 10.2147/dmsott.s9089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 9.Ohtsubo K, Chen MZ, Olefsky JM, Marth JD. Pathway to diabetes through attenuation of pancreatic beta cell glycosylation and glucose transport. Nat Med. 2011;17:1067–1075. doi: 10.1038/nm.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knezevic A, Gornik O, Polasek O, Pucic M, Redzic I, Novokmet M, Rudd PM, Wright AF, Campbell H, Rudan I, Lauc G. Effects of aging, body mass index, plasma lipid profiles, and smoking on human plasma n-glycans. Glycobiology. 2010;20:959–969. doi: 10.1093/glycob/cwq051. [DOI] [PubMed] [Google Scholar]
- 11.Perry JR, Voight BF, Yengo L, et al. Stratifying type 2 diabetes cases by BMI identifies genetic risk variants in lama1 and enrichment for risk variants in lean compared to obese cases. PLoS Genet. 2012;8:e1002741. doi: 10.1371/journal.pgen.1002741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt MI, Duncan BB, Sharrett AR, Lindberg G, Savage PJ, Offenbacher S, Azambuja MI, Tracy RP, Heiss G. Markers of inflammation and prediction of diabetes mellitus in adults (atherosclerosis risk in communities study): A cohort study. Lancet. 1999;353:1649–1652. doi: 10.1016/s0140-6736(99)01046-6. [DOI] [PubMed] [Google Scholar]
- 13.Higai K, Azuma Y, Aoki Y, Matsumoto K. Altered glycosylation of alpha1-acid glycoprotein in patients with inflammation and diabetes mellitus. Clin Chim Acta. 2003;329:117–125. doi: 10.1016/s0009-8981(02)00427-8. [DOI] [PubMed] [Google Scholar]
- 14.Sandstrom CS, Ohlsson B, Melander O, Westin U, Mahadeva R, Janciauskiene S. An association between type 2 diabetes and alpha-antitrypsin deficiency. Diabet Med. 2008;25:1370–1373. doi: 10.1111/j.1464-5491.2008.02584.x. [DOI] [PubMed] [Google Scholar]
- 15.Hashemi M, Naderi M, Rashidi H, Ghavami S. Impaired activity of serum alpha-1-antitrypsin in diabetes mellitus. Diabetes Res Clin Pract. 2007;75:246–248. doi: 10.1016/j.diabres.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 16.Fumeron F, Pean F, Driss F, Balkau B, Tichet J, Marre M, Grandchamp B Insulin Resistance Syndrome Study G. Ferritin and transferrin are both predictive of the onset of hyperglycemia in men and women over 3 years: The data from an epidemiological study on the insulin resistance syndrome (desir) study. Diabetes care. 2006;29:2090–2094. doi: 10.2337/dc06-0093. [DOI] [PubMed] [Google Scholar]
- 17.Churg A, Dai J, Zay K, Karsan A, Hendricks R, Yee C, Martin R, MacKenzie R, Xie C, Zhang L, Shapiro S, Wright JL. Alpha-1-antitrypsin and a broad spectrum metalloprotease inhibitor, rs113456, have similar acute anti-inflammatory effects. Lab Invest. 2001;81:1119–1131. doi: 10.1038/labinvest.3780324. [DOI] [PubMed] [Google Scholar]
- 18.Hak AE, Pols HA, Stehouwer CD, Meijer J, Kiliaan AJ, Hofman A, Breteler MM, Witteman JC. Markers of inflammation and cellular adhesion molecules in relation to insulin resistance in nondiabetic elderly: The rotterdam study. J Clin Endocrinol Metab. 2001;86:4398–4405. doi: 10.1210/jcem.86.9.7873. [DOI] [PubMed] [Google Scholar]
- 19.O’Riordain MG, Ross JA, Fearon KC, Maingay J, Farouk M, Garden OJ, Carter DC. Insulin and counterregulatory hormones influence acute-phase protein production in human hepatocytes. Am J Physiol. 1995;269:E323–330. doi: 10.1152/ajpendo.1995.269.2.E323. [DOI] [PubMed] [Google Scholar]
- 20.Konrad RJ, Kudlow JE. The role of O-linked protein glycosylation in beta-cell dysfunction. Int J Mol Med. 2002;10:535–539. [PubMed] [Google Scholar]
- 21.Larsen CM, Faulenbach M, Vaag A, Volund A, Ehses JA, Seifert B, Mandrup-Poulsen T, Donath MY. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356:1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 22.Goldfine AB, Fonseca V, Jablonski KA, Chen YD, Tipton L, Staten MA, Shoelson SE Targeting Inflammation Using Salsalate in Type 2 Diabetes Study T. Salicylate (salsalate) in patients with type 2 diabetes: A randomized trial. Ann Intern Med. 2013;159:1–12. doi: 10.7326/0003-4819-159-1-201307020-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.