Abstract
Background and Aims
Growing evidence suggests abnormalities in brain morphology including hippocampal structure in patients with methamphetamine (MA) dependence. Yet little is known about the possible gender difference. This study was performed to examine hippocampal volume in abstinent male and female MA users, and to further explore its relationship with cognitive function.
Methods
27 abstinent MA users (19 males and 8 females) with average 5.75 months of duration and 29 healthy controls (19 males and 10 females) age 18 to 45 years old were recruited for clinical assessment and imaging scan. FreeSurfer was used to segment the hippocampus bilaterally, and hippocampal volumes were extracted for group and gender comparisons. Cognitive function was measured using the CogState Battery Chinese language version (CSB-C).
Results
Analysis of covariance (ANCOVA) controlling for education showed a significant group by gender interaction for right hippocampal relative volume adjusted for total brain size (p=0.002). Female patients showed significantly less volume compared with female healthy controls; there was no significant difference in volume between male patients and male healthy controls. Within female patients, there were significant negative relationships between right hippocampal volume and average dose of MA use (p=0.001), as well as the total error scores on the Continuous Paired Association Learning Task (CPAL) in CSB-C (p=0.013).
Conclusions
There seems to be a gender difference in how MA affects hippocampal volume and cognitive function in abstinent MA users. Hippocampus might be an important treatment target for cognitive improvement and functional recovery in this patient population, especially in females.
Keywords: methamphetamine dependence, gender difference, hippocampal volume, cognitive function
1. Introduction
Amphetamine-type stimulants (ATS) are a group of drugs including amphetamine and methamphetamine (MA). The use of ATS drugs has been spreading rapidly across many countries, and has become a worldwide problem in recent years (1). In the United States, over 12 million people have used ATS in their lifetimes, and 1.2 million people reported using ATS in the past year (2). China also faces a serious drug use problem. The number of registered drug users rose from 70,000 in 1990 to 2.14 million in 2013, according to figures from the Ministry of Public Security (3, 4). Heroin and other opiate drugs used to be the primary drugs used and abused in China. After the implementation of “Anti-drug campaign” in 2005, the frequency and prevalence of heroin and illicit opiate drug use has been reduced; however, the use of ATS has increased in China over the past decade. Overall, the prevalence of ATS users amongst known drug addicts has risen from 6.7% in 2005 to 34.4% in 2012 based on data from the China State Food and Drug Administration (SFDA) (4).
MA is the most frequently used of the ATS. Studies have suggested various negative health consequences related to MA use, such as brain abnormalities and cognitive impairment, cardiovascular disease, and HIV/AIDS (5). As a psychostimulant, MA has specific effects on the neurotransmitter system, causing the release of dopamine from vesicular storage pools into the cytoplasm and increasing cytosolic levels of monoamines by inhibiting the activity of monoamine oxidase (5, 6), therefore leading to functional and structural changes in the brain.
The hippocampus is vital in forming new memories, learning, and emotions. Growing evidence from both human (7–9) and animal (10–12) studies have found that MA users demonstrate dysfunction in these hippocampal functions and are linked to the clinical criteria of substance use disorders. Gardner et al. reported acute hippocampal toxicity from structural MRI, following chronic use of MA (13). Thompson et al. reported hippocampal volume reduction in recent abstinent MA users compared to control subjects (14). Similar findings have also been found in psychotic MA users (15).
Cognitive dysfunction in MA users, especially memory impairment, has been reported widely (5, 14, 16). It has been suggested that hippocampus might be involved in MA-related cognitive impairment. For example, one human study found that hippocampal metabolic and structural measures predict the performance on a vigilance test of sustained attention, which could be related to cognitive deficits observed in MA abusers (9). Other human studies suggested that hippocampus is a brain region related to episodic memory and empathy; the damage in this area in MA abusers may contribute to altered emotional experience and misunderstanding of others, leading to deteriorated interpersonal communication and social interactions in this patient population (7, 8). Animal studies found that extracellular signal-regulated kinase (ERK) and cAMP response element-binding protein (CREB) signaling pathway in the hippocampus might be involved in MA induced spatial memory changes (10). The interruption of hippocampal integrity by even modest doses of MA could cause significant clinical symptoms in MA abusers (12).
The effects of MA exposure on the brain and cognition have been well established. However, little is known about the gender difference in these effects. Although drug use occurs more often in males than in females, females appear more vulnerable to the effect of drugs than males (17). Several studies demonstrated that females are more sensitive than males in the test of spontaneous locomotion following MDMA treatment (18). Moreover, female rats showed a greater behavioral response to cocaine in estrus after the striatal dopaminergic system was stimulated by gonadal hormones (19, 20).
Based on previous imaging findings of reduced hippocampal volume and the lack of study of gender differences in hippocampal volume changes, we examined hippocampal volume in male and female abstinent MA users in the present cross-sectional study and explored the relationship between hippocampal volume and cognitive function.
2. Methods
2.1 Subjects
In China, according to the “Narcotic Control Law,” newly identified drug users will receive mandatory inpatient treatment; after discharge, they will continue to receive rehabilitation treatment in the community under the supervision of social workers. If they relapse, drug users will be sent to the Compulsory Isolation Center for Drug Rehabilitation, which is under the judicial system. Routine physical exam and HIV test will be performed before they are admitted to the center. Those with serious physical illness or tested HIV positive will be referred to other appropriate medical facilities to receive treatment.
Abstinent MA users who met the diagnostic criteria for MA dependence according to Diagnostic and Statistical Manual of Mental Disorders criteria (DSM-IV) were recruited from Shanghai Compulsory Rehabilitation Treatment Center. Eleven of them also had an additional diagnosis of MA- induced psychosis. Other inclusion criteria included: 1) age 18 to 45 years old; 2) more than 9 years of education; 3) normal or corrected-to-normal vision and hearing; 4) no current medication treatment except oral contraceptives or vitamins; 5) capacity to provide informed consent. Patient participants also fulfilled the following exclusion criteria: 1) major medical or neurological disorders, including HIV; 2) comorbid psychiatric disorders including schizophrenia, bipolar disorder, and use of other illicit drugs as preferred drug of abuse; 3) contraindication to MRI. In total, 32 individuals were approached, 5 of them were excluded because they did not fit the inclusion or exclusion criteria, and therefore 27 patients were enrolled in the study.
Twenty-nine healthy controls matched by age were recruited from the community through advertising. All of them received a comprehensive physical and psychiatric assessment by the research psychiatrists (J.D. and H.J.). These subjects fulfilled the same inclusion and exclusion criteria as the patient group, but had no current and history drug abuse or dependence (except nicotine).
All participants signed the informed consent forms, which were approved by Shanghai Mental Health Center Institutional Review Board, prior to the start of the study.
2.2 Clinical and cognitive measures
Clinical interview and cognitive assessment were conducted by the research psychiatrists (J.D. and H.J.). Demographic information and drug use/abuse history were collected from patients. Questions about drug use history included age of onset (age when using MA for the first time); types of drugs ever used in addition to MA; duration of abstinence (time interval between last time using MA and imaging date); duration of drug use (time interval between first and last time using MA); duration of continuous drug use (the longest time using MA daily continuously); average dose of MA use (average dose of MA per use in the past one year before abstinence).
The CogState Battery Chinese language version (CSB-C) was used to assess cognitive function. The detailed procedure has been described elsewhere (21), and more information can be found on the website http://www.Cogstate.com. The CSB-C contains eight computerized tasks; among them, we selected 5 that are closely related to hippocampus function. These 5 tasks includes the One Card Learning Task (OCL, visual learning and memory), Two Back Task (TWOB, working memory), International shopping List Task (ISLT, verbal learning and memory), the Groton Maze Learning Task (GML, problem solving/error monitoring), and Continuous Paired Association Learning Task (CPAL, spatial working memory).
2.3 Image acquisition and processing
T1-weighted Spoiled Gradient Echo (SPGR) images were obtained from all subjects on a 3.0 Tesla scanner (Siemens Verio 1) at the Imaging center of Shanghai Mental Health Center. All scans were clinically reviewed by a neuroradiologist during a continuous scan period. Each subject was asked to stay awake, lie on his/her back with body remaining still and eyes closed. A foam mat was used to limit head motion. The acquisition protocol included the following pulse sequence and parameters: repetition time (TR)= 2530 ms, echo time (TE)= 2.34 ms, flip angle 7°, field of view (FOV)= 200×200×200 mm, matrix size 314×448, slice thickness 2 mm, echo spacing 11.7 ms, voxel dimension 1×1×1mm. All data acquisitions were performed in the coronal plane, which was perpendicular to the anterior commissure–posterior commissure (AC–PC) line. Scans were optimized by high contrast in the gray/white and gray/CSF boundaries to obtain the best structure and surface segmentation images.
MRI data were coded and catalogued, and transferred to the Child and Adolescent NeuroDevelopment Initiative (CANDI) lab, Department of Psychiatry at University of Massachusetts Medical School (UMMS) for blinded analysis. The FreeSurfer software (http://surfer.nmr.mgh.harvard.edu/) was used to segment T1-weighted SPGR images into cortical and subcortical gray and white matter regions, as well as total intracranial volume, for each subject (22). The hippocampus has been shown to have comparable accuracy as in manual labeling and sufficient sensitivity to robustly detect change in volume.
2.4 Statistical analysis
Demographic and clinical characteristics data were compared between the patient and control groups using t test for continuous variables and Fisher’s exact test for categorical variables.
Hippocampus volume comparison was performed based on relative volumes to correct for variations in head size (absolute volume divided by intracranial volume). Analysis of covariance (ANCOVA) was used with group and gender as fixed factors and potential confounding variables as covariates.
In addition, Spearman’s rho correlation or Pearson correlation analysis was performed as appropriate to examine the relationship between hippocampal volume and clinical or cognitive measures. Further, partial correlation was performed to control for potential confounding variables. For all statistical analyses, the significance level was set at p<0.05 (two tailed).
3. Results
Table 1 shows demographic and clinical characteristics for the study sample. There were no significant differences in age and gender between the two groups; however, the patient group had significantly fewer years of education than the control group (t(54) =3.002, p=0.004). Within the patient group, only duration of abstinence had significant gender difference (male: 6.53±1.06 months; female: 3.88±0.92 months, t(25) =6.150, p<0.001).
Table 1.
Demographic and clinical characteristics of the study sample
| Characteristics | Control (N=29) (Mean±SD) | Patient (N=27) (Mean±SD) | t | P | ||
|---|---|---|---|---|---|---|
| Gender (Male/Female) | 19/10 | 19/8 | Fisher’s exact test | 0.779 | ||
|
| ||||||
| Age (years) | 32.93±7.00 | 33.26±6.65 | −0.180 | 0.858 | ||
| Education (years) | 11.65±2.61 | 9.76±2.06 | 3.002 | 0.004 | ||
|
| ||||||
| Total | Male (N=19) | Female (N=8) | ||||
|
| ||||||
| Age (years) | 34.26±4.85 | 30.88±9.72 | 0.938 | 0.374 | ||
| Education (years) | 9.50±1.92 | 10.38±2.39 | −0.919 | 0.378 | ||
| Duration of abstinence (months) | N/A | 5.75±1.59 | 6.53±1.06 | 3.88±0.92 | 6.150 | <0.001 |
| Age of onseta | N/A | 26.74±7.25 | 27.79±6.54 | 24.25±8.68 | 1.166 | 0.255 |
| Duration of drug use (months) | N/A | 76.52±44.23 | 77.42±30.57 | 74.39±69.68 | 0.160 | 0.874 |
| Duration of continuous drug useb (months) | N/A | 5.85±12.48 | 7.09±14.57 | 2.90±4.28 | 0.791 | 0.436 |
| Average dose of MA usec (g) | N/A | 0.56±0.35 | 0.84±0.72 | −1.055 | 0.321 | |
Age of onset: age at first time using methamphetamine (MA)
Duration of continuous drug use: the duration of the longest time using drug daily
Average dose of MA use: average dose of MA per use in the last one year before abstinence
Figure 1 shows the representative bilateral hippocampus labels extracted from FreeSurfer. Analysis of covariance (ANCOVA) for hippocampus relative volume using education as a covariate found no group or gender main effect for either left or right hippocampal volume (p’s>0.05). However, there was a significant group by gender interaction for the right (p=0.002) but not left (p=0.138) relative hippocampal volume. Follow-up independent sample t test of right hippocampus relative volume found that: 1) there was a significant subgroup difference between female patients and female controls (t(16) =2.915, p=0.010) but such a difference did not exist between male patients and male controls (t(36) = −0.929, p=0.359) (Figure 2); 2)there was a significant subgroup difference between male controls and female controls (t(27) = −4.443, p<0.001) but there was no such a difference between male patients and female patients (t(25) = −0.014, p=0.989) (Figure 3).
Figure 1.
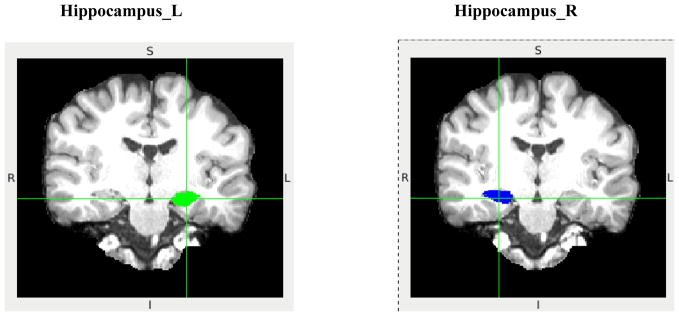
Representative coronal view of left (green) and right (blue) hippocampus labels extracted from FreeSurfer. L: left; R: right; S: superior; I: inferior.
Figure 2.
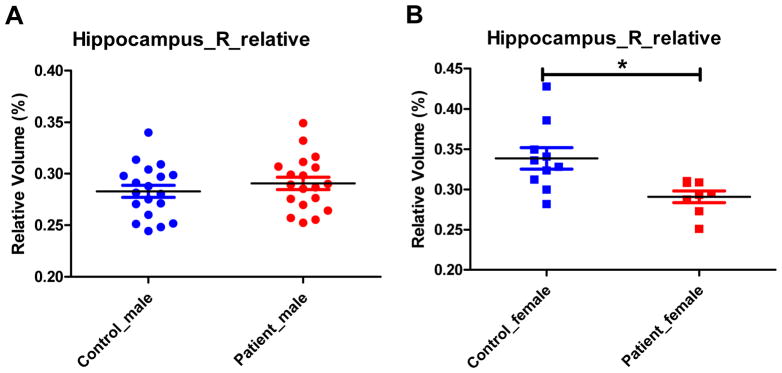
Comparison of right hippocampal relative volume between male controls and male patients (A), and between female controls and female patients (B). * 0.01<p<0.05.
Figure 3.
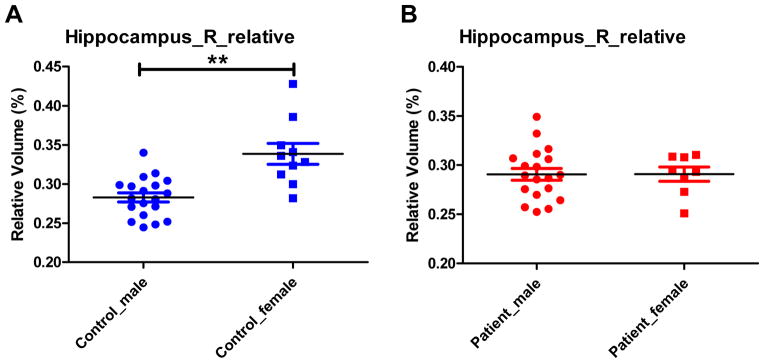
Comparison of right hippocampal relative volume between male controls and female controls (A), and between male patients and female patients (B). * 0.01<p<0.05; **p<0.01.
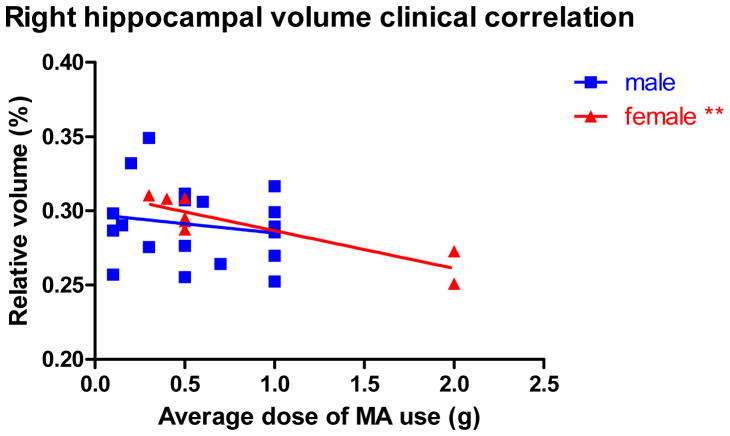
Within the patient group, there were no significant correlations between right hippocampus relative volume and any drug use history and demographicvariables, including average dose used, duration of abstinence, age, and education. Further analysis found a significant negative relationship between right hippocampus relative volume and average dose of MA use in female patients (n=8, rho= −0.932, p=0.001) but not in male patients (n=19, r= −0.068, p=0.781) (Figure 4). In addition, partial correlation analysis controlling for age, education and duration of abstinence did not show any significant correlations between right hippocampal volume and the scores of the 5 selected CSB-C tasks in entire patient group. Subgroup analysis found a significant negative relationship between right hippocampal volume and the total number of errors on the CPAL task in female patients (df=3, r= −0.950, p=0.013); but such a relationship did not exist in male patients (df=14, r= −0.067, p=0.805).
Figure 4.
Correlation between right hippocampal relative volume and average dose of MA use in male and female patients. Although Spearman’s rho correlation was used to test statistical significance, regression lines were plotted for the convenience of the readers. *0.01<p<0.05; **p<0.01.
4. Discussion
Using an automated imaging method to segment the brain and extract hippocampal volume, our study found reduced right hippocampus relative volume in female but not in male abstinent MA users. Only a few previous imaging studies have examined hippocampal volume in either abstinent or active MA users. One study evaluated primarily individuals who were recently abstinent from MA use using an automated segmentation program, and found that bilateral hippocampal volumes were smaller than in healthy controls; furthermore, those MA users with smaller hippocampal volumes had poorer memory performance on a word-recall test (14). Another study found significant gray matter volume reductions in bilateral amygdala and hippocampus in subjects with MA induced psychosis (15). Our study also found hippocampal volume reduction, but only in the right hippocampus and only in female subjects.
One possible explanation for the differences in this study compared to the others studies in the literature is the difference in duration of abstinence across different studies. Our subjects had a relatively long duration of abstinence (average 5.75 months), while the subjects in other two studies either had a much shorter duration of abstinence (14 days on average) (14), or active MA use (15). Previous studies have suggested that the brain morphological abnormalities associated with MA use in hippocampus, white matter, parietal cortical and basal ganglia are time sensitive; the volume reductions in these brain regions were observed during early abstinence (< 4 months), and disappeared or attenuated with longer abstinence (> 20 months) (23–26).
Our study, for the first time, examined the gender difference in hippocampal volume in abstinent MA users. We found no within the patient group. The observed gender difference within the control group is consistent with previous reports showing that the hippocampus is larger in women than in men after adjusted for total brain size (27). On the other hand, female patients showed right hippocampal volume reduction in our study, suggesting that female MA users might be more vulnerable to MA related brain damage, especially in hippocampus, than male MA users. Evidence also exists suggesting gender differences in various neurotransmitter systems within hippocampus (28), including the adrenergic, serotonergic, cholinergic, corticosterone, benzodiazepine and cholecystokinin systems. The absence of gender difference in hippocampal volume in our abstinent MA users might be contributed by the changes of these neurotransmitter systems. Our study also found, for the first time, that a higher average dose of MA use was associated with a smaller right hippocampal volume in females even after months of abstinence; but this relationship did not exist in males. (29–31). Our findings are consistent with previous reports regarding the dose effect of MA use on the brain. For example, an animal study found that altered hippocampal integrity by even modest doses of MA could account for pronounced brain pathology linked to MA abuse (12). Another human imaging study reported that MA compromised frontal lobe high-energy phosphate metabolism in a dose-responsive manner, and also suggested that this abnormality might be more prominent in female than in male MA users (32).
In addition, our study found that a smaller right hippocampal volume was associated with poorer performance in spatial learning memory, as measured by the total number of errors on the CPAL task of CSB-C, in female abstinent MA users. The role of hippocampus in learning and memory (9, 10, 33–35) including spatial learning and memory (34, 35) has been well established. Patients with right hippocampus damage have shown a selective spatial memory deficit (36). An fMRI study found that good between-participant performance in spatial processing tasks was predicted by right hippocampal activation (37). A volumetric analyses of structural MRI data indicated that the size of right posterior hippocampus predicted the ability to use spatial knowledge to make inferences about the relative positions of different buildings on campus (38). These studies indicate the dominance of right hippocampus on spatial memory, and our result is consistent with this indication.
Our study has a few limitations: First, our findings should be considered preliminary given the small sample size and no correction for correct for multiple comparisons. Second, MA related brain abnormalities might have been attenuated given the relatively long time duration of abstinence from MA use (on average 5 months). Third, the history of MA use should have been better characterized and quantified. Last but not the least, the automated imaging method we used to extract hippocampal volume is limited in accuracy, and need to be replicated by or compared with other methods such as manual tracing.
Future longitudinal studies with a larger sample size are needed to characterize brain morphological changes over the course of MA abstinence, to examine whether or not specific brain regions such as hippocampus can serve a biomarker to predict relapse, clinical symptoms and cognitive impairment. In addition, specific interventions, such as cognitive remediation, to improve neuroplasticity in hippocampus and possibly other brain regions seem to be promising with the hope to improve cognition and functional recovery, and also prevent relapse in the MA user population.
Acknowledgments
This work was supported by National Nature Science Foundation (81271468, 81130020), The Ministry of Science and technology project (2012BAI01B07), and. National Key Clinical Disciplines project from Ministry of Health (2011-873). The authors thank Shanghai Compulsory Rehabilitation Center with their collaboration and support. The effort of DNK was supported by the grants NIH-NIMH R01 MH083320 and NIH-NICHD P30 HD004147.
Footnotes
Conflict of interest declaration: none
Declarations of interest
None
References
- 1.United Nations Office on, D. & Crime, P. O. B. V. A. UNODC Annual Report 2009. Austria: 2009. [Google Scholar]
- 2.Rti International P.O. Box 12194 Research Triangle Park Nc, S. C. f. B. H. S. & Quality 1 Choke Cherry Road Rockville, M. D. Results From the 2012 National Survey on Drug Use and Health: Summary of National Findings. United States of America: 2013. [Google Scholar]
- 3.Chu TX, Levy JA. Injection drug use and HIV/AIDS transmission in China. Cell Res. 2005;15:865–9. doi: 10.1038/sj.cr.7290360. [DOI] [PubMed] [Google Scholar]
- 4.China, T. M. o. P. S. o. Annual Drug Abuse Report. 2012. [Google Scholar]
- 5.Panenka WJ, Procyshyn RM, Lecomte T, et al. Methamphetamine use: a comprehensive review of molecular, preclinical and clinical findings. Drug Alcohol Depend. 2013;129:167–79. doi: 10.1016/j.drugalcdep.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 6.Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol. 2005;75:406–33. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Kim YT, Lee JJ, Song HJ, et al. Alterations in cortical activity of male methamphetamine abusers performing an empathy task: fMRI study. Hum Psychopharmacol. 2010;25:63–70. doi: 10.1002/hup.1083. [DOI] [PubMed] [Google Scholar]
- 8.Kim YT, Song HJ, Seo JH, et al. The differences in neural network activity between methamphetamine abusers and healthy subjects performing an emotion-matching task: functional MRI study. NMR Biomed. 2011;24:1392–400. doi: 10.1002/nbm.1702. [DOI] [PubMed] [Google Scholar]
- 9.London ED, Berman SM, Voytek B, et al. Cerebral metabolic dysfunction and impaired vigilance in recently abstinent methamphetamine abusers. Biol Psychiatry. 2005;58:770–8. doi: 10.1016/j.biopsych.2005.04.039. [DOI] [PubMed] [Google Scholar]
- 10.Cao G, Zhu J, Zhong Q, et al. Distinct roles of methamphetamine in modulating spatial memory consolidation, retrieval, reconsolidation and the accompanying changes of ERK and CREB activation in hippocampus and prefrontal cortex. Neuropharmacology. 2013;67:144–54. doi: 10.1016/j.neuropharm.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ricoy UM, Martinez JL., Jr Local hippocampal methamphetamine-induced reinforcement. Front Behav Neurosci. 2009;3:47. doi: 10.3389/neuro.08.047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mandyam CD, Wee S, Crawford EF, et al. Varied access to intravenous methamphetamine self-administration differentially alters adult hippocampal neurogenesis. Biol Psychiatry. 2008;64:958–65. doi: 10.1016/j.biopsych.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gardner H, Lawn N, Fatovich DM, Archer JS. Acute hippocampal sclerosis following ecstasy ingestion. Neurology. 2009;73:567–9. doi: 10.1212/WNL.0b013e3181b2a684. [DOI] [PubMed] [Google Scholar]
- 14.Thompson PM, Hayashi KM, Simon SL, et al. Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci. 2004;24:6028–36. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orikabe L, Yamasue H, Inoue H, et al. Reduced amygdala and hippocampal volumes in patients with methamphetamine psychosis. Schizophr Res. 2011;132:183–9. doi: 10.1016/j.schres.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Diaz SD, Smith LM, LaGasse LL, et al. Effects of prenatal methamphetamine exposure on behavioral and cognitive findings at 7.5 years of age. J Pediatr. 2014;164:1333–8. doi: 10.1016/j.jpeds.2014.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carroll ME, Lynch WJ, Roth ME, Morgan AD, Cosgrove KP. Sex and estrogen influence drug abuse. Trends Pharmacol Sci. 2004;25:273–9. doi: 10.1016/j.tips.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Palenicek T, Votava M, Bubenikova V, Horacek J. Increased sensitivity to the acute effects of MDMA (“ecstasy”) in female rats. Physiol Behav. 2005;86:546–53. doi: 10.1016/j.physbeh.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 19.Sell SL, Scalzitti JM, Thomas ML, Cunningham KA. Influence of ovarian hormones and estrous cycle on the behavioral response to cocaine in female rats. J Pharmacol Exp Ther. 2000;293:879–86. [PubMed] [Google Scholar]
- 20.Peris J, Decambre N, Coleman-Hardee ML, Simpkins JW. Estradiol enhances behavioral sensitization to cocaine and amphetamine-stimulated striatal [3H]dopamine release. Brain Res. 1991;566:255–64. doi: 10.1016/0006-8993(91)91706-7. [DOI] [PubMed] [Google Scholar]
- 21.Pietrzak RH, Olver J, Norman T, et al. A comparison of the CogState Schizophrenia Battery and the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Battery in assessing cognitive impairment in chronic schizophrenia. J Clin Exp Neuropsychol. 2009;31:848–59. doi: 10.1080/13803390802592458. [DOI] [PubMed] [Google Scholar]
- 22.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–55. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 23.Chang L, Alicata D, Ernst T, Volkow N. Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction. 2007;102(Suppl 1):16–32. doi: 10.1111/j.1360-0443.2006.01782.x. [DOI] [PubMed] [Google Scholar]
- 24.Chang L, Cloak C, Patterson K, et al. Enlarged striatum in abstinent methamphetamine abusers: a possible compensatory response. Biol Psychiatry. 2005;57:967–74. doi: 10.1016/j.biopsych.2005.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jernigan TL, Gamst AC, Archibald SL, et al. Effects of methamphetamine dependence and HIV infection on cerebral morphology. Am J Psychiatry. 2005;162:1461–72. doi: 10.1176/appi.ajp.162.8.1461. [DOI] [PubMed] [Google Scholar]
- 26.Oh JS, Lyoo IK, Sung YH, et al. Shape changes of the corpus callosum in abstinent methamphetamine users. Neurosci Lett. 2005;384:76–81. doi: 10.1016/j.neulet.2005.04.082. [DOI] [PubMed] [Google Scholar]
- 27.Goldstein JM, Seidman LJ, Horton NJ, et al. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb Cortex. 2001;11:490–7. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- 28.Madeira MD, Lieberman AR. Sexual dimorphism in the mammalian limbic system. Prog Neurobiol. 1995;45:275–333. doi: 10.1016/0301-0082(94)00052-j. [DOI] [PubMed] [Google Scholar]
- 29.Dluzen DE, Liu B. Gender differences in methamphetamine use and responses: a review. Gend Med. 2008;5:24–35. doi: 10.1016/s1550-8579(08)80005-8. [DOI] [PubMed] [Google Scholar]
- 30.Cohen JB, Greenberg R, Uri J, Halpin M, Zweben JE. Women with methamphetamine dependence: research on etiology and treatment. J Psychoactive Drugs. 2007;(Suppl 4):347–51. doi: 10.1080/02791072.2007.10399896. [DOI] [PubMed] [Google Scholar]
- 31.Kim JY, Fendrich M. Gender differences in juvenile arrestees’ drug use, self-reported dependence, and perceived need for treatment. Psychiatr Serv. 2002;53:70–5. doi: 10.1176/appi.ps.53.1.70. [DOI] [PubMed] [Google Scholar]
- 32.Sung YH, Yurgelun-Todd DA, Shi XF, et al. Decreased frontal lobe phosphocreatine levels in methamphetamine users. Drug Alcohol Depend. 2013;129:102–9. doi: 10.1016/j.drugalcdep.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maren S, De Oca B, Fanselow MS. Sex differences in hippocampal long-term potentiation (LTP) and Pavlovian fear conditioning in rats: positive correlation between LTP and contextual learning. Brain Res. 1994;661:25–34. doi: 10.1016/0006-8993(94)91176-2. [DOI] [PubMed] [Google Scholar]
- 34.Quan MN, Tian YT, Xu KH, Zhang T, Yang Z. Post weaning social isolation influences spatial cognition, prefrontal cortical synaptic plasticity and hippocampal potassium ion channels in Wistar rats. Neuroscience. 2010;169:214–22. doi: 10.1016/j.neuroscience.2010.04.048. [DOI] [PubMed] [Google Scholar]
- 35.Wicking M, Nees F, Steiger F. Neuropsychological measures of hippocampal function. Front Neurol Neurosci. 2014;34:60–70. doi: 10.1159/000356425. [DOI] [PubMed] [Google Scholar]
- 36.Abrahams S, Pickering A, Polkey CE, Morris RG. Spatial memory deficits in patients with unilateral damage to the right hippocampal formation. Neuropsychologia. 1997;35:11–24. doi: 10.1016/s0028-3932(96)00051-6. [DOI] [PubMed] [Google Scholar]
- 37.Wegman J, Tyborowska A, Janzen G. Encoding and retrieval of landmark-related spatial cues during navigation: an fMRI study. Hippocampus. 2014;24:853–68. doi: 10.1002/hipo.22275. [DOI] [PubMed] [Google Scholar]
- 38.Schinazi VR, Nardi D, Newcombe NS, Shipley TF, Epstein RA. Hippocampal size predicts rapid learning of a cognitive map in humans. Hippocampus. 2013;23:515–28. doi: 10.1002/hipo.22111. [DOI] [PMC free article] [PubMed] [Google Scholar]