Abstract
Autophagy is a central mechanism by which hepatocytes catabolize lipid droplets (LDs). Currently, the regulatory mechanisms that control this important process are poorly defined. The small GTPase Rab7 has been implicated in the late endocytic pathway and is known to associate with LDs, although its role in LD breakdown has not been tested. In this study, we demonstrate that Rab7 is indispensable for LD breakdown (“lipophagy”) in hepatocytes subjected to nutrient deprivation. Importantly, Rab7 is dramatically activated in cells placed under nutrient stress; this activation is required for the trafficking of both multivesicular bodies (MVBs) and lysosomes to the LD surface during lipophagy, resulting in the formation of a lipophagic “synapse.” Depletion of Rab7 leads to gross morphological changes of MVBs, lysosomes, and autophagosomes, consequently leading to attenuation of hepatocellular lipophagy.
Conclusion
These findings provide additional support for the role of autophagy in hepatocellular LD catabolism while implicating the small GTPase Rab7 as a key regulatory component of this essential process.
Keywords: lipid droplets, autophagy, multivesicular bodies, lysosomes, kiss-and-run
Introduction
The liver is known as a central organ of lipid storage and is highly susceptible to steatosis when exposed to a high fat diet, alcohol or infection. Most hepatic lipid is stored in the hepatocyte as cytosolic lipid droplets (LDs), neutral lipid depots composed of triacylglycerol (TG) and cholesteryl esters. These dynamic organelles are a principal source of fatty acids for β-oxidation, membrane lipid biosynthesis, protein modification, and numerous other physiological processes. There is now great interest in defining the basic mechanisms of LD formation versus catabolism, as an imbalance in these processes can lead to non-alcoholic steatohepatitis (NASH and non-alcoholic fatty liver disease (NAFL), as well as alcohol-induced hepatic steatosis and diabetes (1, 2).
The canonical pathway for LD catabolism involves cellular lipases that dock to the LD surface, catalyzing the hydrolysis of TG and downstream metabolites (3). In addition to the action of these cytoplasmic lipases, LDs are also degraded by autophagy, a degradative process in which the LDs are engulfed by an autophagosome (AP) that subsequently fuses with lysosomes to form autolysosomes. Within the autolysosome, acid lipases break down TG into its glycerol and fatty acid components. Conversely, inhibition of the autophagic pathway in cells and mice is known to lead to hepatocellular steatosis (4, 5).
Despite the importance of the lipophagic process, our understanding of the cellular mechanisms that support and regulate the interactions between LDs and APs is modest. It is known that many components of the endosomal pathway are utilized in AP formation and maturation. For example, phosphatidylinositol 3-kinases (PI3Ks) initiate AP formation and modulate endosome maturation (6), while Rab GTPases function as key organizers of membrane trafficking events (7) and promote the interaction between endosomal compartments and the APs (8). Further evidence also links downstream effectors of Rab proteins to AP maturation (9, 10). It is possible, although unclear, that these endocytic components are used to support AP-LD targeting, docking and engulfment; however, the exact mechanisms underlying these processes are almost totally undefined.
It has been demonstrated that Rab7, known to regulate the transport and maturation of the late endocytic pathway (11-13) is a conserved component of the LD membrane (10). Currently, how this late endocytic Rab protein functions in the context of LD dynamics remains unclear but is an attractive candidate to both regulate and mediate the interaction of this important organelle with the autophagic pathway. In this study, we observe that Rab7 is markedly recruited to LDs in live hepatocellular carcinoma cells when placed under nutrient deprivation. Most importantly, Rab7 is highly activated under these conditions and is required to promote direct physical interactions between MVBs/lysosomes and LDs. This Rab7 mediated interaction is essential for the subsequent utilization of LDs as an energy source. The role of this regulatory GTPase as a key player in promoting the formation of a LD-AP “synapse” is discussed.
Materials and Methods
A detailed description of the materials and methods used can be found in the Supporting Information section.
Results
Starvation activates LD-localized Rab7 to mediate lipolysis
To verify that the Rab7 GTPase resides on LDs in the hepatocyte cell models utilized in our study (HuH-7 and Hep3B), we first isolated LDs by subcellular fractionation, in parallel with microscopy of cells expressing fluorescently-tagged Rab7. As shown in Figure 1a, Rab7 was clearly associated with LDs isolated from HuH-7 cells cultured in regular medium containing 10% FBS (“resting”) or starved for 2h in HBSS (“starved”). Interestingly, no difference in the amount of Rab7 associated with the purified LDs was observed between resting and starvation conditions. In transfected cells, modest levels of GFP-Rab7wt could be resolved surrounding the LD surfaces. Notably, Rab7 also localized to a cytoplasmic vesicular compartment that, in many instances, associated intimately with LDs (Fig. 1b, wt). This Rab7wt-LD association appeared to increase in cells exposed to low serum (0.1% FBS) starvation for 24h, but was especially pronounced upon the expression of a constitutively active GFP-Rab7 form (Q67L). In these mutant-expressing cells, most of the Oil Red O (ORO)-stained LDs were surrounded by or completely engulfed within those compartments containing active Rab7. In strong contrast, cells expressing GFP-Rab7-T22N, a mutant defective in GTP binding, exhibited virtually no interactions with the LD under all nutrient conditions tested (Fig.1b).
Figure 1. Rab7 localizes to lipid droplets (LDs) and is activated upon starvation.
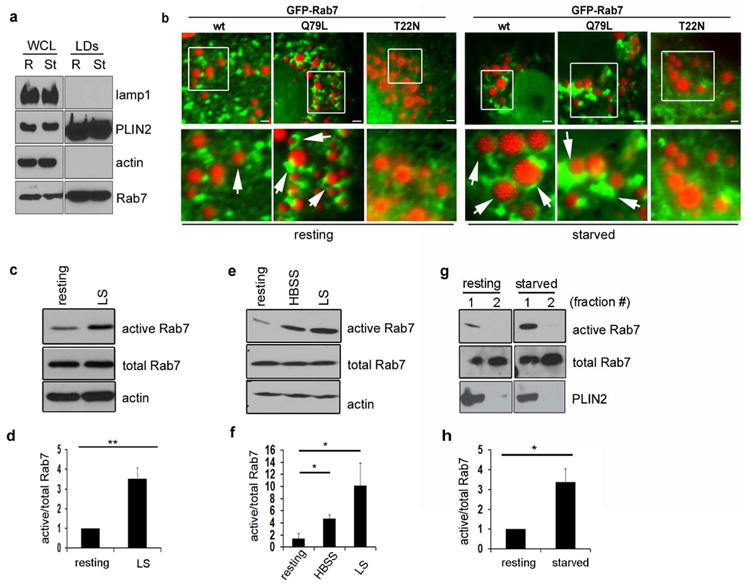
(a) LDs were purified from HuH7 cells under resting and starved conditions and the purity of the isolated fractions was tested using specific antibodies to various compartments. Note that Rab7 associates with the purified LDs under both conditions. (b) Hep3B cells expressing GFP-Rab7-wt, or the GTPase active (-Q67L) or inactive (-T22N) forms were loaded with 150 μM oleate overnight (“resting”, upper rows) and then starved for 24h in medium containing 0.1% FBS (“starved”, lower rows). LDs were visualized using Oil Red O (ORO). While no obvious changes in the Rab7 localization to LDs are observed upon starvation, cells expressing wt or active forms exhibit a marked association with LDs while the Rab7-T22N mutant does not. Scale bars represent 1 μm. (c) Rab7 activity increases 3 to 4 fold in Hep3B hepatoma cells, as assessed by a RILP pulldown assay. Cells were exposed to overnight loading with oleate followed by a 24h low serum starvation. (d) Quantitation of at least three independent experiments as described in (c). Data are presented as mean ± S.E., p value (**) = 0.001. (e) Activation of Rab7 in HuH7 cells exposed to 2h HBSS or 24h low serum starvation, as assessed by a RILP pulldown assay. (f) Quantitation of at least three independent experiments as described for (e). Data are presented as mean ± S.E., p values (*) ≤ 0.03. (g) A subpopulation of LD-associated Rab7 is activated upon 2h HBSS starvation in HuH7 cells. Cells were starved and the isolated LDs were subjected to a RILP pulldown assay. (h) Quantitation of three independent experiments as described in (g) reveals a 3-fold activation of Rab7 on LDs. Data are presented as mean ± S.E., p value (*) = 0.02. In all cases, total Rab7 was normalized to the actin loading control and active Rab7 was then normalized to the corrected total Rab7.
As Rab7-positive compartments displayed an increased association with LDs following nutrient deprivation or expression of constitutively active Rab7, we postulated that serum depletion could promote the activation of Rab7. To test this hypothesis, Rab7 activity was assessed by a RILP pulldown assay (19) in Hep3B and HuH-7 cells subjected to either short-term (2h) starvation in HBSS or long-term (24h) starvation in medium containing 0.1% serum. This assay works similarly to those used for other small GTPases (20) and is based on the fact that downstream effectors preferentially bind to the active form of the GTPase. Importantly, as measured by the RILP pulldown assay, all cell types tested exhibited a significant increase (≥ 3 fold) in Rab7 activity when subjected to starvation (Figs. 1c-f). Most interestingly, LDs purified from HuH7 cells also revealed a 2-3 fold increase in active Rab7 upon starvation compared to resting conditions (Figs. 1g and h), consistent with the premise of an activated late endosomal pathway following exposure to nutrient stress.
Because Rab7 has been implicated in the dynamics of late endocytic and autophagic compartments, we postulated that its activation following nutrient deprivation could represent the initiation of autophagy-centric LD catabolism, measurable by a reduction in both LD size and number (14). Indeed, Hep3B cells treated with either non-targeting (NT) control or Rab7-directed siRNA (siRab7), and subsequently transfected to re-express GFP alone or distinct Rab7-GFP-tagged mutants, exhibited substantial differences in the capacity to mobilize stored lipid when subjected to low-serum starvation for 48h post-oleate loading. While Rab7 depletion significantly reduced the breakdown of LDs during starvation (Figs. 2a, b), this impairment could be corrected by the re-expression of the wild-type and constitutively-active (Q67L) forms of Rab7, but not by the dominant-negative (T22N) mutant (Figs. 2a, b). Importantly, Rab7 depletion did not affect LD formation as demonstrated in Figs. 2c and d (Fig. 2f shows a representative blot of the knockdown efficiency). These observations are consistent with the concept that activation of Rab7 promotes LD breakdown. As these data were obtained in hepatoma cells, it was important to confirm that Rab7-dependent lipohagy also occurs in primary hepatocytes. To this end, Hep3B hepatoma cells and primary mouse hepatocytes were treated with the Rab7-specific inhibitor CID1067700 (21, 22) during lipophagy induction. Indeed, cells treated with the Rab7 inhibitor were greatly impaired in their ability to breakdown LDs when challenged by nutrient deprivation (Fig. S1).
Figure 2. Rab7 depletion impairs starvation-induced LD breakdown but not LD formation.
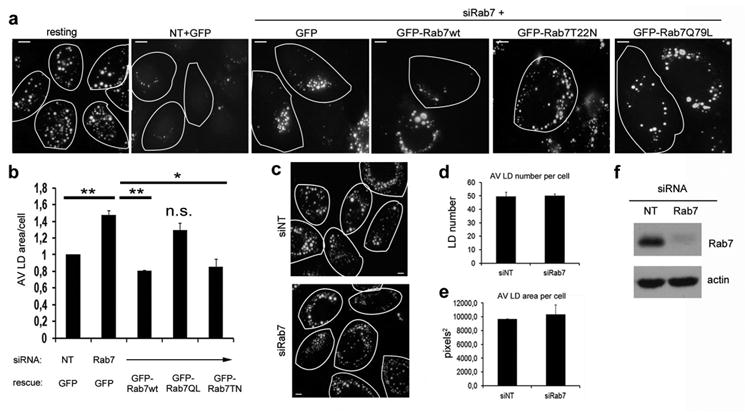
(a) IF of Hep3B cells treated with control (siNT) or Rab7 siRNA (siRab7), then transfected with either GFP control vector, GFP-Rab7-wt, -Q67L or T22N, loaded overnight with oleate and then starved for 48 h in medium containing 0.1% FBS. LDs were visualized by ORO and the area of LDs per cell was assessed. While control cells metabolize significant numbers of LDs, Rab7-depleted cells do not. This phenotype was rescued by Rab7-wt and the active –Q67L form, but not by the dominant negative –T22N form of Rab7. Encircled cells express GFP alone or GFP-Rab7 as indicated. (b) Quantitation of the average LD area per cell from at least three independent experiments as described in (a). Data are presented as mean ± S.E., p values (**) ≤ 0.004 and (*) = 0.048, respectively. (c) Representative images from a loading experiment in control (siNT) and Rab7 depleted cells (siRab7). 48h post-transfection, cells were loaded with oleate overnight and LDs were stained using ORO. Cell borders are outlined. (d and e) Average LD number (d) and average LD area (e) per cell in Hep3B control cells (siNT) or cells depleted of Rab7 (siRab7). Data are presented as mean ±S.E. and no significant difference in the ability to form LDs was observed between control and Rab7 knockdown cells. (f) Representative blot showing Rab7 knockdown efficiency in Hep3B cells. Scale bars = 10 μm.
Together, these data indicate that starvation-induced activation of Rab7 both on the LD surface as well as on endomembranes ultimately promotes LD breakdown.
Rab7 mediates the docking of APs, MVBs, and lysosomes to LDs during lipophagy
Based on these results, we aimed to determine the mechanism by which Rab7 regulates the autophagic catabolism of LDs. APs fuse either directly with lysosomes or first with late endosomes/MVBs, forming an intermediate compartment, the amphisome, prior to lysosomal association (23, 24). As Rab7 coordinates the structural organization of and transport between all compartments involved in this process, we focused on potential morphological changes in APs, MVBs and lysosomes, monitoring their interactions with LDs following Rab7 perturbations.
As lipophagy is an autophagic process, we first analyzed whether Rab7 depletion affects AP function in hepatocytes, as this had not been studied. Indeed, siRNA-mediated Rab7 depletion significantly increased the number of LC3-positive spots in Hep3B hepatoma cells expressing GFP-LC3 and led to increased levels of endogenous LC3 (Fig. S2, a-d). Interestingly, while the increase in LC3 spots upon Rab7 depletion has been reported (18, 25), changes in total LC3 levels were not observed in other cell types (18) for unknown reasons. Use of the lysosomal inhibitor leupeptin in our studies revealed that the elevated LC3 levels observed in Rab7-depleted cells are due to an inhibition of AP degradation as leupeptin treatment did not result in a further increase in LC3 levels (Fig. S2c and d). This was confirmed through the use of a dually-fluorescent mRFP-EGFP-LC3 reporter, which can be used to monitor AP-to-autolysosome maturation by virtue of the pH sensitivity of EGFP(17). While numerous “red only” puncta were observed under starvation conditions in the control cells, indicative of AP maturation, their formation was impaired following Rab7 depletion (Fig. S2e). Further, the enlargement and clustering of the APs caused by Rab7 depletion clearly interfered with their ability to associate with LDs, rendering them unable to be primed for degradation (Fig. S2f).
Based on the observations described above together with the previously reported functions of Rab7 (26), we predicted that the Rab7-positive membranous structures that attach to and surround the “primed” LDs likely represent late endocytic/lysosomal compartments that drive the autophagic process. To test this prediction, we analyzed the interaction between LDs and MVBs, to determine whether amphisome formation is necessary prior to subsequent associations with the lysosomal compartment, all in the context of various Rab7 perturbations.
We first mimicked the starvation-induced activated status of Rab7 by over-expressing GFP-tagged Rab7-Q67L and observed an increase in CD63-positive MVB association with LDs, greater than that seen when GFP-Rab7-wt was expressed (Fig. 3a). These data support the concept that starvation-activated Rab7 recruits MVBs to the LD-AP interface to promote amphisome formation prior to lipophagic breakdown.
Figure 3. The interaction between MVBs and LDs is regulated by active Rab7.
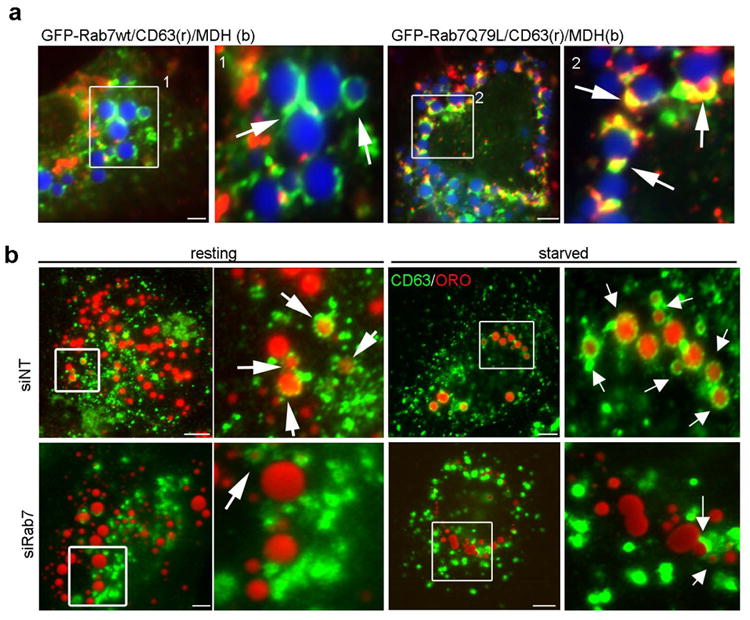
(a) IF images of Hep3B cells expressing GFP-Rab7-wt or the active – Q67L mutant loaded with oleate overnight. LDs were stained with MDH (blue) while MVBs were identified using an antibody specific to CD63 (red). In cells expressing wt Rab7, few LD-MVB interactions were observed whereas a substantial increase in the association between these compartments was observed in cells expressing the active Rab7-Q67L mutant. (b) MVB–LD association increases upon starvation and is inhibited by Rab7 depletion. Hep3B cells treated with siNT or siRab7 were loaded with oleate 24 h post transfection and then starved for 24 h in low serum medium. LDs were visualized using ORO. In control cells, punctate CD63-positive MVBs exhibit physical interactions with LDs that are markedly increased upon starvation (arrows). In contrast, CD63-labeled MVBs appear enlarged and aggregated with only limited LD interactions in Rab7 depleted cells under either resting or starved conditions. Scale bars = 5 μm.
To confirm this hypothesis, we tested if Rab7 depletion interferes with MVB recruitment to the LD. Indeed, while CD63-positive MVBs interacted with LDs in the control cells and showed an impressive envelopment of the LDs upon nutrient deprivation, Rab7 depletion almost completely abolished this process (Fig. 3b). Moreover, the CD63-positive compartment appeared enlarged and clustered upon Rab7 depletion (Fig. 3b, “siRab7”), consistent with EM data from previous reports (27). The importance of the MVB pathway in hepatic lipophagy was confirmed by inhibiting MVB maturation (28). Hep3B cells expressing GFP-SKD1-E235Q, a dominant-negative form of the mammalian ATPase required for ESCRT and MVB function (29), were challenged with nutrient deprivation for 48h. Consistent with the findings that utilized Rab7 disruption (Figs.2, 3), we observed that the mutant cells possessed an impaired MVB compartment and, subsequently, failed to catabolize LDs (Fig. S3).
With the establishment of a MVB-LD interaction mediated by active Rab7 to promote amphisome formation, we next assessed whether active Rab7 also regulates the subsequent step of lysosome-LD/amphisome docking by analyzing LD-lysosome interaction using LAMP1 as a marker. Again, while expression of constitutively-active GFP-Rab7-Q67L increased the recruitment of LAMP1-positive lysosomes to the LDs compared to wt-expressing cells (Fig. 4a), Rab7 knockdown caused an enlargement and clustering of the lysosomal structures (Fig. 4b, “resting”) and interfered with their ability to interact with LDs (Fig. 4b, “starved”). Interestingly, in contrast to the MVBs, the lysosomes did not fully wrap around the LDs under starvation conditions and rather appeared as a vesicular compartment that transiently interacts with the LDs, resembling a “kiss-and-run”-like mechanism. Indeed, when analyzed in living cells, the Rab7-mediated interaction between the LAMP1-positive lysosomes and the MDH-labeled LDs (arrow and arrowhead) seemed brief in nature, where the lysosome is recruited to the Rab7-positive subdomain of the LD, docks for a short period of time, and then eventually departs the LD (Figs. 4c and d, movies 1 and 2). In this way, one LD can be repeatedly bombarded by several lysosomes to facilitate LD breakdown in an energy-efficient way (see Fig. 4d, second arrow at the 28′ time frame).
Figure 4. Rab7 mediates the recruitment of lysosomes to the LD surface.
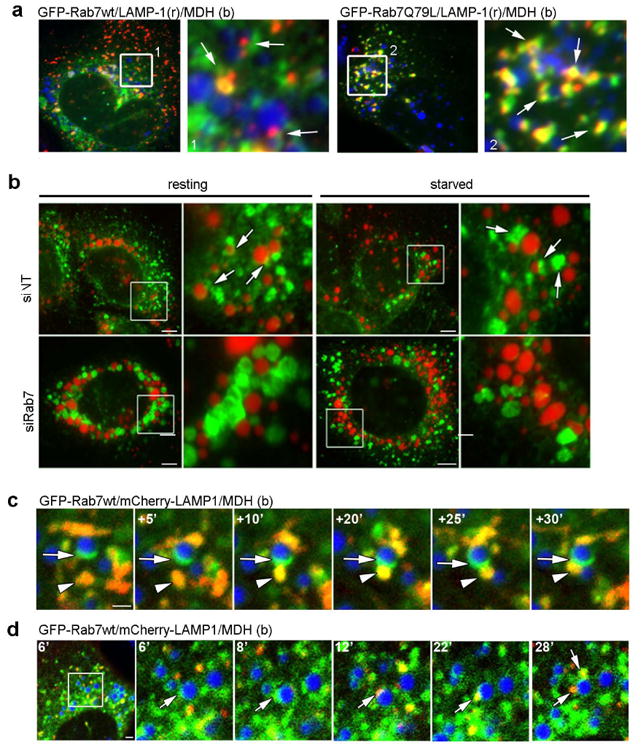
(a) IF images of Hep3B cells expressing GFP-Rab7-wt or the active – Q67L mutant loaded with oleate overnight. LDs are stained with MDH (blue) while lysosomes are visualized by LAMP1 staining (red). In cells expressing wt Rab7, few lysosome-associated LDs were observed while a substantial increase in LD-lysosome interactions (yellow) occurred in cells expressing the starvation-mimicking active Rab7-Q67L mutant. (b) As a reciprocal approach to expressing active Rab7, Hep3B cells were treated with either a non-targeting siRNA (siNT) or a Rab7 targeting siRNA (siRab7), stained for LAMP-1 (green) and labeled with MDH for visualizing lipid droplets (pseudolabeled red). Cells were fixed directly following oleate loading (resting) or following a 24h starvation period in low serum media. Few LAMP-1 positives vesicles associate with LDs compared to control cells, instead appearing to cluster throughout the cell. (c and d) Still images taken from a confocal time-lapse series of an oleate-loaded Hep3B cell co-expressing Rab7-GFP and LAMP1-mCherry proteins (MDH-labeled lipid droplets are shown in the blue channel). Putative Rab7-GFP positive MVBs associate with the surface of an LD to form a docking platform (arrows) for a LAMP-1 positive vesicle (red, arrowheads in c and arrows in d). Evidence of membrane exchange between the two compartments is observed by the increased yellow signal within the fusing vesicle.
To directly visualize and quantify the consequences of Rab7 perturbations on LD-lysosome docking/fusion both in either living or fixed cells, a fluorescent reporter incorporating the LD-specific structural protein perilipin-2 (PLIN2), mRFP1-EGFP-PLIN2, was engineered based on the mRFP1-EGFP-LC3 construct used above for analysis of AP maturation. As for the LC3 reporter (Fig. S2), an increase in “red only” PLIN2-positive structures can be used as a readout for LD-lysosome docking/fusion within the cell (Fig. S4a). We found that Hep3B cells grown under serum-starved conditions showed a clear labeling of ring-like structures around LDs in both the red and green channels (Fig. S4b). In addition, the formation of numerous “red-only”, punctate structures were also observed throughout the cell, many of which stained positive for LysoTracker Blue, denoting interactions between LD-resident PLIN2 and an acidic compartment (Fig. S4b). As an additional control, cells expressing the PLIN2 reporter were starved in the presence of chloroquine (CQ), an inhibitor of lysosomal acidification (30); this treatment resulted in a near complete abrogation of red puncta indicating that fusion with functional lysosomes was required for the docking of the two compartments (Figs. S4c and d).
When viewed in living cells (Fig 5a; movie 3), this reporter supported the concept of transient lysosome-LD interactions as displayed in the time-lapse movies (Fig. 4). Indeed, imaging cells expressing this probe reveals LysoTracker Blue-positive vesicles arriving at PLIN2-tagged LDs, where they interact briefly, and turn red. Most importantly, the number of cytoplasmic red puncta produced by starved, control siRNA-treated cells was significantly higher compared to the Rab7 depleted cells (Fig.5 b, c, d) consistent with the concept that Rab7 is required for structural interactions between the “primed” LDs and lysosomes.
Figure 5. Rab7 depletion inhibits LD-lysosome docking.
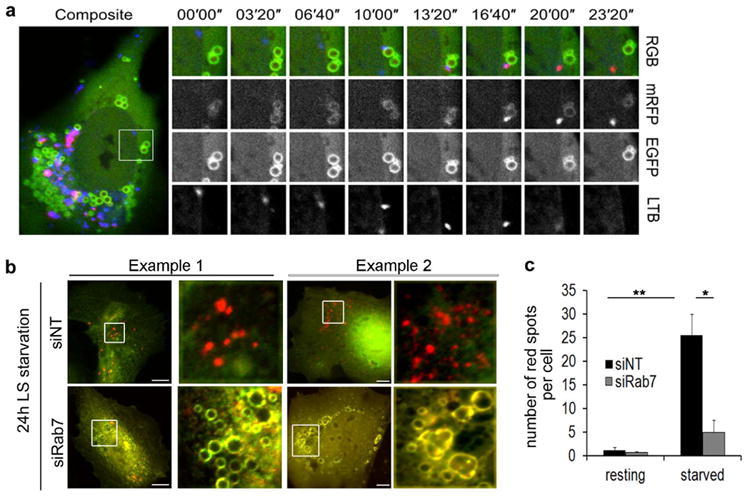
(a) Still images taken from a confocal time-lapse series of an oleate loaded Hep3B cell expressing mRFP1-EGFP-PLIN2. The yellow PLIN2 structure gives rise to a “red only” spot by the transient docking of and acidic lysosome (LysoTracker Blue, arrow) that quenches the GFP-portion of the tag. Note the blue to red spectral shift exhibited by the lysosome post docking (16”) that occurs in a “kiss-and-run”-like fashion. (b) Fluorescence images of fixed Hep3B cells, treated with siNT or siRab7 that were transfected to express mRFP1-EGFP-PLIN2, loaded overnight with oleate and then starved for 24h in medium containing 0.1% FBS (LS). In both examples, starvation induces an increase of red PLIN2 puncta, indicative of a lysosomal “sampling” of the PLIN2- reporter protein. This shift is abrogated in the Rab7 knockdown cells which displayed mainly yellow PLIN2 rings reminiscent of the PLIN2 localization in resting cells and indicative of little to no interaction between LDs and lysosomes. Scale bar = 10 μm. (c) Quantitation of three independent experiments as described in (b). Data are presented as mean ± S.E., p values = 0.005 (**) and 0.015 (*), respectively. At least 25 cells per condition were counted.
Membrane-bound Rab7 promotes LD breakdown via an interaction with its downstream effector RILP
The findings of this study have thus far suggested that activated Rab7 promotes the cellular utilization of LDs as an energy source by regulating the interaction between the LDs and degradative compartments. To further define the mechanisms by which Rab7 might support this process we manipulated the capability of Rab7 to interact with membranes or, alternatively, its downstream effector RILP (31). Two Rab7 mutants were therefore designed: a “CAAX” mutant (Rab7-C205A, Fig. 6a), that is prenylation defective and therefore cannot insert into membranes, rendering it inactive (32, 33); and a RILP- binding mutant (Rab7-V180A, Fig. 6a; (34)), that perturbs the interaction of Rab7-associated vesicles with microtubules, thereby interfering with transport to the lysosomes (35, 36). Applying a similar knockdown/re-expression approach to that described above (Fig. 2), we saw that neither the Rab7-C205A nor the -V180A mutants reverted the lipophagy defect in the Rab7 knockdown cells (Fig. 6b and c), indicating that both membrane association and microtubule-based transport via RILP are necessary for the regulatory function of Rab7 in starvation-induced LD breakdown. Interestingly, the RILP binding mutant (V180A), but not the CAAX mutant (C205A), remained associated with the LDs, suggesting that the sub-cellular localization alone is not responsible for the observed defect in lipophagy (Fig. 6d). Both mutants exhibited decreased RILP binding (Fig. 6d and e), and a separate assay, measuring Rab7 protein binding to GTP-bound beads and thus activity per se (37), showed that only the CAAX mutant of Rab7 was impaired in activity (Fig. 6f). These data demonstrate that membrane association of Rab7 is required for its activation during lipophagy and that its ability to connect to the microtubule network via RILP is important for efficient regulation of starvation-induced LD breakdown.
Figure 6. Rab7 membrane association and RILP interaction are required for LD breakdown.
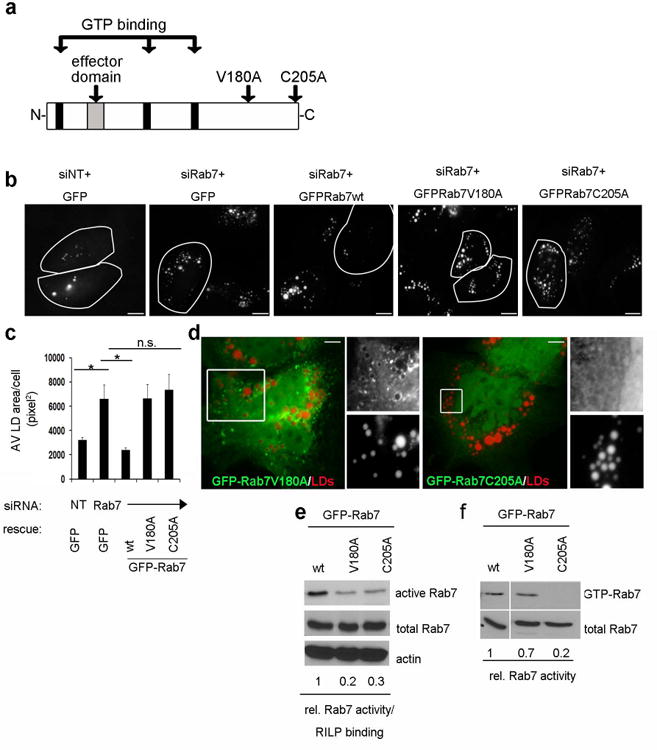
(a) Schematic representation of the Rab7 domain structure showing the GTPase domain (black insets), the effector region (gray inset) and the two point mutations that were generated (V180A= RILP binding defective, C205A= CAAX mutant). (b) Hep3B cells, treated with either control (siNT) or Rab7-targeting siRNA (siRab7) were transfected to re-express either Rab7wt or the two mutants as indicated above. Cells were loaded overnight with oleate and then starved for 48h in low serum media. LDs were visualized by ORO post-starvation. Cells re-expressing the indicated constructs are outlined. Note that only the Rab7-wt, but neither mutant, rescues the defect in lipophagy in Rab7-depleted cells. Scale bar = 2.5 μm. (c) Quantitation of three independent experiments as described in (b). Data are presented as mean ± S.E., p values ≤ 0.05 (*). (d) The Rab7-RILP binding mutant (GFP-Rab7V180A) localizes to ORO-stained LDs (red) while the Rab7-CAAX mutant (GFP-Rab7C205A) appears cytosolic. The boxed areas are enlarged on the right side of each image. Scale bars = 2.5 μm. (e) RILP pulldown assay from Hep3B cells expressing either Rab7-wt the Rab7-C205A, or -V180A mutant, respectively. The numbers underneath the blot represent the average (fold difference) of precipitated Rab7 protein compared to the wt from three independent experiments. The differences in Rab7 activity/RILP binding are significant compared to the wt (p values ≤ 0.0001, not shown). Note that the RILP pulldown assay does not distinguish between the binding mutant per se and the activity-defective mutant. (f) GTP pulldown assay to assess Rab7 activity only in Hep3B cells expressing Rab7wt, Rab7-V180A or- C205A, respectively. The numbers underneath the blot represent the average of precipitated Rab7 protein, compared to the wt from three independent experiments. Note that the RILP binding mutant (V180A) exhibits only a slight decrease in activity (p=0.3), while the activity of the membrane binding mutant (C205A) is reduced significantly (p=0.01).
Taken together, the data presented in this study support a working model for the role of Rab7 in the lipophagic breakdown of LDs as summarized in Figure 7, emphasizing the concept that starvation-activated Rab7 promotes LD breakdown by coordinating the recruitment and docking of degradative compartments to those LDs primed for autophagic breakdown.
Figure 7. Rab7 mediates LD breakdown by recruiting degradative compartments: a working model.
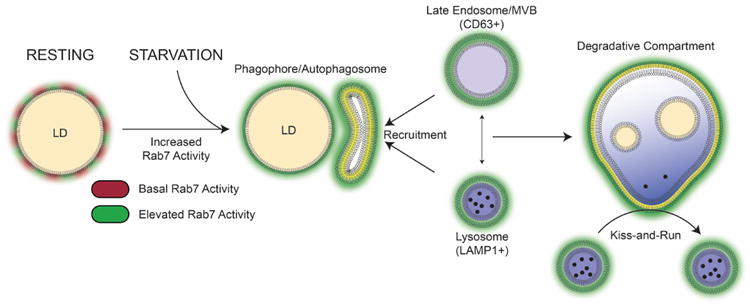
Under control conditions, LD-associated Rab7 (red) is activated upon starvation (green) to recruit LC3-positve APs that are also enriched in the active GTPase. This process is the first step in the lipophagic breakdown of the LDs. In a second step, the APs fuse with Rab7-positive degradative compartments to form an autolysosome for lipid degradation. This process may occur either via the formation of an amphisome (AP-MVB intermediate) that in turn fuses with the lysosome or via direct fusion of the AP with the lysosome (similar to classic macroautophagy). The direct interaction between lysosomes and APs can also occur in a “kiss-and-run”-like fashion allowing constant sampling of the autophagic LDs that will eventually result in LD degradation as observed by a reduction in LD number and area. Thus, functional Rab7 is indispensable for proper lipophagy as it controls both the integrity of the degradative compartments and their fusion with the LDs.
Discussion
In this study, we provide new insights into the molecular mechanisms regulating the interactions between LDs and endocytic degradative compartments during starvation induced lipophagy in hepatocytes. Rab7 appears as a fundamental component of both LD and endolysosomal membranes and its activation, and subsequent recruitment, under conditions of nutritional stress is necessary for initiation of LD catabolism by autophagy (Figs. 1-2). In addition to the LD itself, this activation occurs on several relevant compartments, including MVBs and late endosomes/lysosomes resulting in tight associations between these organelles (Figs. 3-5). Lipophagy can then proceed, provided that the activated Rab7 is able to associate with the membranes of all necessary compartments as mutations in Rab7 that prevent membrane interactions attenuate this process (Fig. 6). We therefore propose a model for Rab7 as a central regulator in the coordination of LD-autophagic interactions during nutrient-limited conditions (Fig. 7).
Rab7 as a Central Regulator of Hepatocellular Lipophagy
In hepatocytes, lipophagy is important for regulating energy homeostasis and lipid content. As a consequence, inhibition of lipophagy by pharmacological or genetic means leads to increases in cellular TG, as well as in LD number and size resulting in impaired β-oxidation (1). Little is known however about the regulatory elements in this important process. For example, under basal conditions LDs are constitutively degraded by the lysosome (5), though how the hepatocyte responds to nutritional changes to amplify the LD-targeted autophagic response is unclear, as is the process by which “primed” LDs are transported to the lysosome.
The data presented in this study is the first to position the small GTPase Rab7, an important regulator of late endocytic trafficking, as a central player in hepatic lipophagy, providing evidence for a regulated targeting and fusion of “primed” autophagic LDs with lysosomes. This is manifested as an intimate association of Rab7-positive degradative compartments with LDs upon starvation, conditions under which the levels of Rab7 protein on the LD itself do not appear to increase (Fig 1). Interestingly, LD-associated Rab7 is highly activated upon a nutritional challenge and appears to drive the formation of a LD-MVB platform that could facilitate the subsequent interaction with lysosomes. The formation of these Rab7 platforms can become robust in starved cells and even more so in cells expressing the active Rab7Q67L mutant.; expression of the inactive T22N form of the protein, however, precludes their formation. Likewise, siRNA-mediated depletion of Rab7 or expression of a mutant defective in RILP binding also interferes with the recruitment/docking of the degradative compartments, ultimately attenuating LD breakdown (Figs. 2 to 6).
Active Rab7 promotes the formation of a “lipophagy synapse”
APs deliver their cargo for degradation either by directly fusing with the lysosomes or via the amphisome, a hybrid compartment emerging from the fusion between APs and MVBs/late endosomes (38-40). The resulting autolysosome provides the enzymes required for the degradation of the inner membrane of the AP and its lumenal cargo. Subsequently, the macromolecules are released to and recycled in the cytosol (41, 42). In hepatocytes, autophagic LDs can directly fuse with the lysosomal compartment, or first associate with MVBs/late endosomes (forming amphisomes) prior to lysosomal fusion. Both pathways seem equally important in the lipophagic process as depletion of Rab7 results in enlarged, clustered MVBs (Fig.4, (27)) and lysosomes that are unable to interact with LDs to promote their breakdown (Fig 5.). The MVB may provide an intermediate compartment that possesses the appropriate docking and fusion machinery to link the LD surface with that of the autophagic machinery, and Rab7 appears to be essential in this process. Whether the formation of amphisomes is absolutely required for lipophagy is unclear.
Dynamics of LD-Lysosome interactions are mediated by active Rab7and its effectors
A detailed analysis of the association of the MVBs and lysosomes with LDs revealed two interesting interactions: a) LDs surrounded by MVBs and b) LDs interacting with vesicular, LAMP1-positive lysosomal structures. These interactions may reflect different stages of the lipophagic process that are regulated by Rab7 on either compartment. While the fully engulfed LDs likely represent amphisomes, the interaction between lysosomes and LDs also occur via a “kiss-and-run” process. Kiss-and-run type interactions between lysosomes and vesicle-bound substrates have been previously reported (43, 44) and are predicted to represent cargo “sampling” that may require less of an energy cost as compared to the fully committed engulfment of large structures such as LDs. Rab7 may regulate this process by transporting the primed autophagic LDs to the perinuclear region in close proximity to the lysosomes by its interaction with RILP. Such transport would link Rab7-positive structures to microtubules via the dynein motor, an interaction that is required for efficient LD turnover (Fig. 6). This observation is consistent with other studies in mammalian cells demonstrating that a) the recruitment and activation of Rab7 alone is insufficient to induce fusion of phagosomes with late endosomes and lysosomes (45), and b) that Rab7 binding to RILP is required for this process, as it drives the phagosomes into a centripetal direction, inducing the extension of phagosomal tubules that contact late endocytic compartments (46). Further recruitment and activation of Rab7 on the resulting AP as well as the degradative compartments could then regulate their interaction and ultimately promote the fusion of the LDs with lysosomes for breakdown (Fig. 7).
Interestingly, Rab7 has recently been shown to participate in the maturation and motility of APs via its interaction with yet another motor adaptor, FYCO1 (FYVE and coiled-coil domain-containing 1). This effector supports the formation of a complex between Atg8 (LC3), phosphoinositol-3-phosphate, and kinesin (47). Additionally, the HOPS (homotypic fusion and protein sorting) complex (48), another Rab7 effector, has been implicated in AP-lysosome fusion through its interaction with the SNARE Syntaxin 17 (49). Perhaps most relevant is a recent study by Lizaso and coworkers, suggesting that agonist-induced activation of lipolysis in adipocytes results in a release of perilipin 1 from the LD surface to allow the subsequent recruitment of Rab7 and autophagic components for lysosomal degradation (50). In summary, these data emphasize the role of Rab7 as a central player at the confluence between the autophagosomal and degradative pathways, directing the proper fusion and docking of multiple compartments to facilitate lipophagy.
Future studies focusing on the spatiotemporal activation of Rab7 as well as a more detailed assessment of other Rab7 effector proteins involved in hepatic lipophagy will be required. In this context, it will be important to identify the signaling pathways that activate Rab7 under conditions of nutrient stress and determine whether fatty acid oxidation or ethanol metabolism might compromise the regulated process of lipophagy.
Supplementary Material
Acknowledgments
This study was supported by grants 5R37DK044650 (MAM), 5RO1AA020735 (MAM and CAC), NIH Challenge Grant AA19032 (MAM and CAC), the Robert and Arlene Kogod Center on Aging, and the Optical Morphology Core of the Mayo Clinic Center for Cell Signalling in Gastroenterology (MIDDK P30DK084567). The authors declare that they have no conflict of interest.
Abbreviations
- LD
lipid droplet
- TG
triacylglycerol
- MVB
multivesicular body
- Lys
lysosome
- AP
autophagosome
- PLIN2
perilipin 2
- RILP
Rab7 interacting lysosomal protein
References
- 1.Liu K, Czaja MJ. Regulation of lipid stores and metabolism by lipophagy. Cell Death Differ. 2013;20:3–11. doi: 10.1038/cdd.2012.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sahini N, Borlak J. Recent insights into the molecular pathophysiology of lipid droplet formation in hepatocytes. Prog Lipid Res. 2014;54:86–112. doi: 10.1016/j.plipres.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Walther TC, Farese RV., Jr Lipid droplets and cellular lipid metabolism. Annu Rev Biochem. 2012;81:687–714. doi: 10.1146/annurev-biochem-061009-102430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christian P, Sacco J, Adeli K. Autophagy: Emerging roles in lipid homeostasis and metabolic control. Biochimica et biophysica acta. 2013;1831:819–824. doi: 10.1016/j.bbalip.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 5.Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, et al. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Devereaux K, Dall'Armi C, Alcazar-Roman A, Ogasawara Y, Zhou X, Wang F, Yamamoto A, et al. Regulation of mammalian autophagy by class II and III PI 3-kinases through PI3P synthesis. PloS one. 2013;8:e76405. doi: 10.1371/journal.pone.0076405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 8.Gutierrez MG. Functional role(s) of phagosomal Rab GTPases. Small GTPases. 2013;4 doi: 10.4161/sgtp.25604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ao X, Zou L, Wu Y. Regulation of autophagy by the Rab GTPase network. Cell Death Differ. 2014;21:348–358. doi: 10.1038/cdd.2013.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hyttinen JM, Niittykoski M, Salminen A, Kaarniranta K. Maturation of autophagosomes and endosomes: a key role for Rab7. Biochimica et biophysica acta. 2013;1833:503–510. doi: 10.1016/j.bbamcr.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 11.Bucci C, Thomsen P, Nicoziani P, McCarthy J, van Deurs B. Rab7: a key to lysosome biogenesis. Molecular biology of the cell. 2000;11:467–480. doi: 10.1091/mbc.11.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng Y, Press B, Chen W, Zimmerman J, Wandinger-Ness A. Expression and properties of Rab7 in endosome function. Methods in enzymology. 2001;329:175–187. doi: 10.1016/s0076-6879(01)29078-8. [DOI] [PubMed] [Google Scholar]
- 13.Vonderheit A, Helenius A. Rab7 associates with early endosomes to mediate sorting and transport of Semliki forest virus to late endosomes. PLoS biology. 2005;3:e233. doi: 10.1371/journal.pbio.0030233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schulze RJ, Weller SG, Schroeder B, Krueger EW, Chi S, Casey CA, McNiven MA. Lipid droplet breakdown requires Dynamin 2 for vesiculation of autolysosomal tubules in hepatocytes. Journal of Cell Biology. 2013;203:315–326. doi: 10.1083/jcb.201306140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schroeder B, Srivatsan S, Shaw A, Billadeau D, McNiven MA. CIN85 phosphorylation is essential for EGFR ubiquitination and sorting into multivesicular bodies. Molecular biology of the cell. 2012;23:3602–3611. doi: 10.1091/mbc.E11-08-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE, Tsokos M, et al. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:3374–3379. doi: 10.1073/pnas.0712145105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimura S, Noda T, Yoshimori T. Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy. 2007;3:452–460. doi: 10.4161/auto.4451. [DOI] [PubMed] [Google Scholar]
- 18.Jager S, Bucci C, Tanida I, Ueno T, Kominami E, Saftig P, Eskelinen EL. Role for Rab7 in maturation of late autophagic vacuoles. Journal of cell science. 2004;117:4837–4848. doi: 10.1242/jcs.01370. [DOI] [PubMed] [Google Scholar]
- 19.Peralta ER, Martin BC, Edinger AL. Differential effects of TBC1D15 and mammalian Vps39 on Rab7 activation state, lysosomal morphology, and growth factor dependence. The Journal of biological chemistry. 2010;285:16814–16821. doi: 10.1074/jbc.M110.111633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benard V, Bokoch GM. Assay of Cdc42, Rac, and Rho GTPase activation by affinity methods. Methods in enzymology. 2002;345:349–359. doi: 10.1016/s0076-6879(02)45028-8. [DOI] [PubMed] [Google Scholar]
- 21.Agola JO, Hong L, Surviladze Z, Ursu O, Waller A, Strouse JJ, Simpson DS, et al. A competitive nucleotide binding inhibitor: in vitro characterization of Rab7 GTPase inhibition. ACS chemical biology. 2012;7:1095–1108. doi: 10.1021/cb3001099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong L, Simons P, Waller A, Strouse J, Surviladze Z, Ursu O, Bologa C, et al. A small molecule pan-inhibitor of Ras-superfamily GTPases with high efficacy towards Rab7. 2010 [PubMed] [Google Scholar]
- 23.Metcalf D, Isaacs AM. The role of ESCRT proteins in fusion events involving lysosomes, endosomes and autophagosomes. Biochemical Society transactions. 2010;38:1469–1473. doi: 10.1042/BST0381469. [DOI] [PubMed] [Google Scholar]
- 24.Rusten TE, Stenmark H. How do ESCRT proteins control autophagy? Journal of cell science. 2009;122:2179–2183. doi: 10.1242/jcs.050021. [DOI] [PubMed] [Google Scholar]
- 25.Gutierrez MG, Munafo DB, Beron W, Colombo MI. Rab7 is required for the normal progression of the autophagic pathway in mammalian cells. Journal of cell science. 2004;117:2687–2697. doi: 10.1242/jcs.01114. [DOI] [PubMed] [Google Scholar]
- 26.Zhang M, Chen L, Wang S, Wang T. Rab7: roles in membrane trafficking and disease. Bioscience reports. 2009;29:193–209. doi: 10.1042/BSR20090032. [DOI] [PubMed] [Google Scholar]
- 27.Vanlandingham PA, Ceresa BP. Rab7 regulates late endocytic trafficking downstream of multivesicular body biogenesis and cargo sequestration. The Journal of biological chemistry. 2009;284:12110–12124. doi: 10.1074/jbc.M809277200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taelman VF, Dobrowolski R, Plouhinec JL, Fuentealba LC, Vorwald PP, Gumper I, Sabatini DD, et al. Wnt signaling requires sequestration of glycogen synthase kinase 3 inside multivesicular endosomes. Cell. 2010;143:1136–1148. doi: 10.1016/j.cell.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nara A, Mizushima N, Yamamoto A, Kabeya Y, Ohsumi Y, Yoshimori T. SKD1 AAA ATPase-dependent endosomal transport is involved in autolysosome formation. Cell Struct Funct. 2002;27:29–37. doi: 10.1247/csf.27.29. [DOI] [PubMed] [Google Scholar]
- 30.Hamano T, Gendron TF, Causevic E, Yen SH, Lin WL, Isidoro C, Deture M, et al. Autophagic-lysosomal perturbation enhances tau aggregation in transfectants with induced wild-type tau expression. Eur J Neurosci. 2008;27:1119–1130. doi: 10.1111/j.1460-9568.2008.06084.x. [DOI] [PubMed] [Google Scholar]
- 31.Barr FA. Review series: Rab GTPases and membrane identity: causal or inconsequential? The Journal of cell biology. 2013;202:191–199. doi: 10.1083/jcb.201306010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.ten Klooster JP, Hordijk PL. Targeting and localized signalling by small GTPases. Biol Cell. 2007;99:1–12. doi: 10.1042/BC20060071. [DOI] [PubMed] [Google Scholar]
- 33.Wright LP, Philips MR. Thematic review series: lipid posttranslational modifications. CAAX modification and membrane targeting of Ras Journal of Lipid Research. 2006;47:883–891. doi: 10.1194/jlr.R600004-JLR200. [DOI] [PubMed] [Google Scholar]
- 34.Wu M, Wang T, Loh E, Hong W, Song H. Structural basis for recruitment of RILP by small GTPase Rab7. The EMBO journal. 2005;24:1491–1501. doi: 10.1038/sj.emboj.7600643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Progida C, Malerod L, Stuffers S, Brech A, Bucci C, Stenmark H. RILP is required for the proper morphology and function of late endosomes. Journal of cell science. 2007;120:3729–3737. doi: 10.1242/jcs.017301. [DOI] [PubMed] [Google Scholar]
- 36.Wang T, Ming Z, Xiaochun W, Hong W. Rab7: role of its protein interaction cascades in endo-lysosomal traffic. Cellular signalling. 2011;23:516–521. doi: 10.1016/j.cellsig.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 37.Marino SF. High-level production and characterization of a G-protein coupled receptor signaling complex. FEBS J. 2009;276:4515–4528. doi: 10.1111/j.1742-4658.2009.07158.x. [DOI] [PubMed] [Google Scholar]
- 38.Fader CM, Colombo MI. Autophagy and multivesicular bodies: two closely related partners. Cell Death Differ. 2009;16:70–78. doi: 10.1038/cdd.2008.168. [DOI] [PubMed] [Google Scholar]
- 39.Fader CM, Sanchez DG, Mestre MB, Colombo MI. TI-VAMP/VAMP7 and VAMP3/cellubrevin: two v-SNARE proteins involved in specific steps of the autophagy/multivesicular body pathways. Biochimica et biophysica acta. 2009;1793:1901–1916. doi: 10.1016/j.bbamcr.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 40.Morvan J, Kochl R, Watson R, Collinson LM, Jefferies HB, Tooze SA. In vitro reconstitution of fusion between immature autophagosomes and endosomes. Autophagy. 2009;5:676–689. doi: 10.4161/auto.5.5.8378. [DOI] [PubMed] [Google Scholar]
- 41.Hansen TE, Johansen T. Following autophagy step by step. BMC Biol. 2011;9:39. doi: 10.1186/1741-7007-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klionsky DJ. Autophagy: from phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol. 2007;8:931–937. doi: 10.1038/nrm2245. [DOI] [PubMed] [Google Scholar]
- 43.Bright NA, Gratian MJ, Luzio JP. Endocytic delivery to lysosomes mediated by concurrent fusion and kissing events in living cells. Current biology : CB. 2005;15:360–365. doi: 10.1016/j.cub.2005.01.049. [DOI] [PubMed] [Google Scholar]
- 44.Desjardins M. Biogenesis of phagolysosomes: the ‘kiss and run’ hypothesis. Trends Cell Biol. 1995;5:183–186. doi: 10.1016/s0962-8924(00)88989-8. [DOI] [PubMed] [Google Scholar]
- 45.Vieira OV, Bucci C, Harrison RE, Trimble WS, Lanzetti L, Gruenberg J, Schreiber AD, et al. Modulation of Rab5 and Rab7 recruitment to phagosomes by phosphatidylinositol 3-kinase. Molecular and cellular biology. 2003;23:2501–2514. doi: 10.1128/MCB.23.7.2501-2514.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harrison RE, Bucci C, Vieira OV, Schroer TA, Grinstein S. Phagosomes fuse with late endosomes and/or lysosomes by extension of membrane protrusions along microtubules: role of Rab7 and RILP. Molecular and cellular biology. 2003;23:6494–6506. doi: 10.1128/MCB.23.18.6494-6506.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pankiv S, Alemu EA, Brech A, Bruun JA, Lamark T, Overvatn A, Bjorkoy G, et al. FYCO1 is a Rab7 effector that binds to LC3 and PI3P to mediate microtubule plus end-directed vesicle transport. The Journal of cell biology. 2010;188:253–269. doi: 10.1083/jcb.200907015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pawelec A, Arsic J, Kolling R. Mapping of Vps21 and HOPS binding sites in Vps8 and effect of binding site mutants on endocytic trafficking. Eukaryot Cell. 2010;9:602–610. doi: 10.1128/EC.00286-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang P, Nishimura T, Sakamaki Y, Itakura E, Hatta T, Natsume T, Mizushima N. The HOPS complex mediates autophagosome-lysosome fusion through interaction with syntaxin 17. Molecular biology of the cell. 2014;25:1327–1337. doi: 10.1091/mbc.E13-08-0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lizaso A, Tan KT, Lee YH. beta-adrenergic receptor-stimulated lipolysis requires the RAB7-mediated autolysosomal lipid degradation. Autophagy. 2013;9:1228–1243. doi: 10.4161/auto.24893. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


