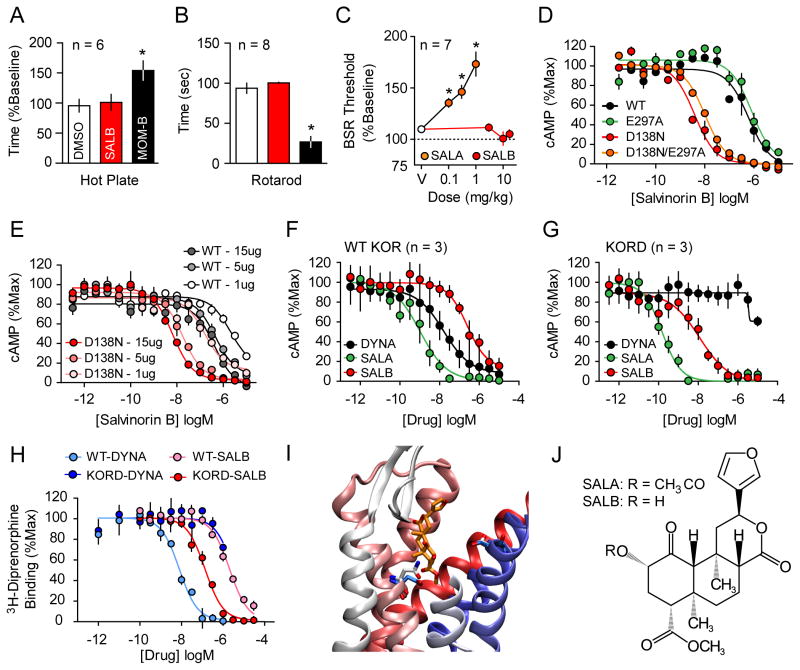
Figure 1. Rational design and in-vitro characterization of KORD.
SALB was initially validated as a DREADD ligand by demonstrating its apparent pharmacologic inertness in vivo using behavioral tests by comparing with MOM-SALB via (A) hot plate test and (B) impairment of motor performance using the rotarod test. In both tests SALB effects (red) were compared to vehicle (white) and a stabilized variant of SALA (MOM-SALB - black). (C) The lack of production of a KOR-like anhedonic states was tested using ICSS and compared to the effects of SALA. (D) We characterized the KORD comparing the Gi-mediated response of different KOR mutants and demonstrate an increased potency of SALB at D138N containing mutants. (E) The effect of receptor expression levels on SALB potency of WT KOR (gray) and KORD (red). As can be seen, DNA concentration is directly related in both WT-KOR and KORD to agonist potency yielding a right shift in potency of 1–2 orders of magnitude. Average Gi response of WT-KOR (F) and KORD (G) to classic KOR ligands Dynorphin A (DYNA, black), SALA (green), and the inert compound SALB (red) is shown. (H) Competition binding isotherms of WT and KORD for DYNA (1-13) (pKi values: 8.50 ± 0.12 and 5.79 ± 0.06 respectively) and SALB (pKi values: 5.53 ± 0.08 and 6.98 ± 0.13 respectively). An examination of a model of KORD docked with SALB (I) suggests that the DREADD mutation (D138N) eliminates unfavorable interactions between D138 and SALB. In WT KOR, D138 is turned away from the ligand binding site (cyan) while N138 in KORD (white) is interacting directly with the ligand. E297 in both models models assumes the same conformation reflecting the fact that it has no effect on DREADD activity. Deacetylation at position 2 of SALA results in SALB (J). Asterisk indicates p < 0.05.