Abstract
The endoplasmic reticulum (ER) membrane is closely apposed to the outer mitochondrial membrane (OMM), which facilitates communication between these organelles. These contacts, known as mitochondria-associated membranes (MAM), facilitate calcium signaling, lipid transfer, as well as antiviral and stress responses. How cellular proteins traffic to the MAM, are distributed therein, and interact with ER and mitochondrial proteins are subject of great interest. The human cytomegalovirus UL37 exon 1 protein or viral mitochondria-localized inhibitor of apoptosis (vMIA) is crucial for viral growth. Upon synthesis at the ER, vMIA traffics to the MAM and OMM, where it reprograms the organization and function of these compartments. vMIA significantly changes the abundance of cellular proteins at the MAM and OMM, including proteins that regulate calcium homeostasis and cell death. Through the use of superresolution imaging, we have shown that vMIA is distributed at the OMM in nanometer scale clusters. This is similar to the clusters reported for the mitochondrial calcium channel, VDAC, as well as electron transport chain, translocase of the OMM complex, and mitochondrial inner membrane organizing system components. Thus, aside from addressing how vMIA targets the MAM and regulates survival of infected cells, biochemical studies and superresolution imaging of vMIA offer insights into the formation, organization, and functioning of MAM. Here, we discuss these insights into trafficking, function, and organization of vMIA at the MAM and OMM and discuss how the use of superresolution imaging is contributing to the study of the formation and trafficking of viruses.
Keywords: Cytomegalovirus, Superresolution imaging, vMIA, MAM, Mitochondria, MSIM, PALM, STED
Introduction
Contacts between organelles allow communication needed for maintaining basal metabolism, coordinated cellular responses and cell survival. However, the small distances between apposed organellar membranes are below the diffraction limit imposed by visible light, making it impractical to use conventional microscopy to resolve protein location and functional organization in these compartments. Superresolution imaging can provide valuable insight into the nanoscale organization of viral and cellular proteins. Here, we focus on the use of superresolution microscopy to study the human cytomegalovirus (HCMV) viral mitochondria-localized inhibitor of apoptosis (vMIA) or UL37 exon 1 protein (pUL37x1). This protein traffics from the endoplasmic reticulum (ER) to the outer mitochondrial membrane (OMM) and prominently localizes to the ER subdomain where it contacts the mitochondria, known as the mitochondria-associated membrane (MAM) [1–7].
Apposed ER and OMM membranes are 10–30 nm apart but do not fuse [8, 9]. As these distances are below the diffraction limit of visible light, the best imaging of ER–mitochondrial contacts has been performed by electron tomography [8, 9]. Protein tethers stabilize these contacts even when the organelles move along the cytoskeleton [10–12]. In mammalian cells, mitofusin 2 (Mfn2) [11], mitostatin [13], PACS-2 [14], and a Ca2+ signaling complex [10] have been implicated in stabilizing contacts between the ER and the OMM. These contacts are the hub of communication between the ER and mitochondria and are called the MAM [15]. MAM facilitated ER–mitochondrial cross talk helps maintain basal mitochondrial metabolism and bioenergetics [16], transfer essential lipids to mitochondria [17], coordinate ER stress responses [8, 18], induce mitochondrial-mediated apoptosis [19], amplify innate immune signaling [20, 21], and mark mitochondrial fission sites [9].
The intracellular trafficking itinerary of vMIA
Upon infection, the HCMV genome is expressed sequentially through a temporally orchestrated set of gene expression. The earliest virally encoded proteins expressed are called the immediate early (IE) proteins. Of these proteins, vMIA increases HCMV growth in permissive human fibroblasts and imparts a potent antiapoptotic function to the infected cell [22, 23].
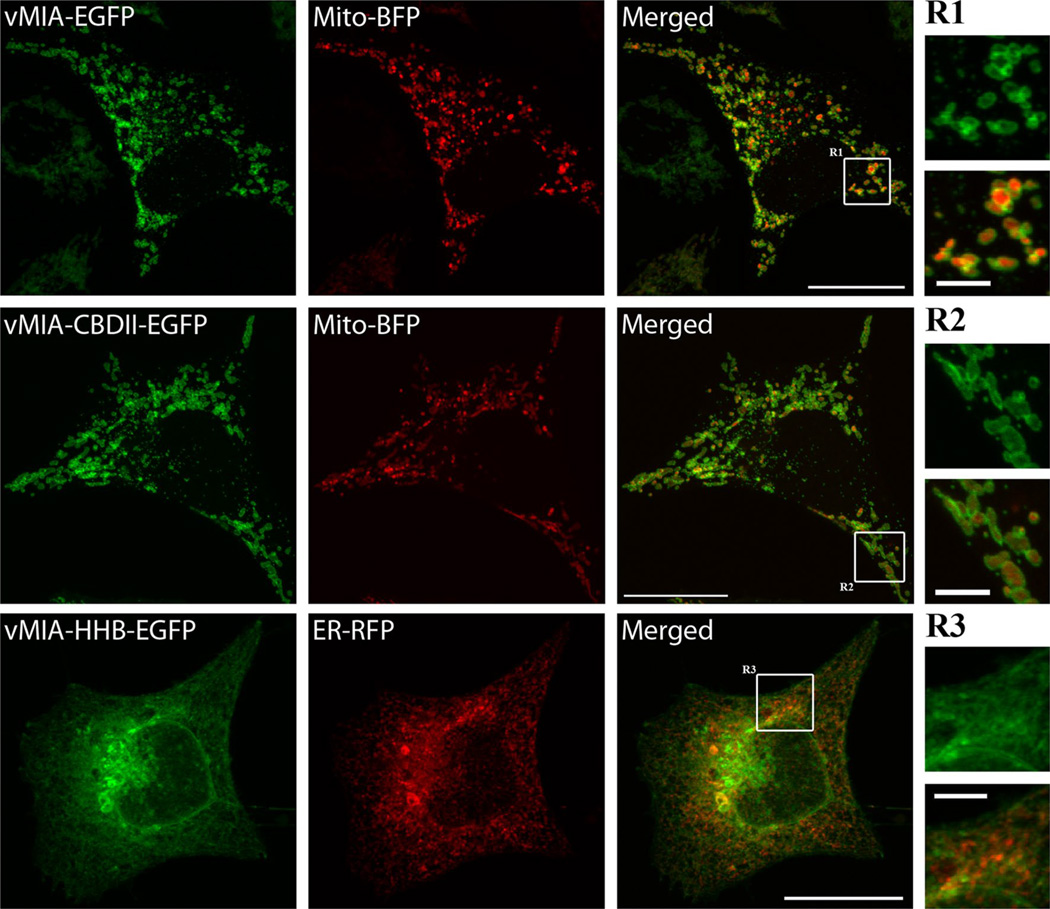
vMIA is synthesized in the ER and translocated in the ER membrane by a moderately hydrophobic leader [1, 2]. It is then directed by a bipartite signal sequence at its NH2-terminus from the ER to the MAM and to the OMM (Figs. 1, 2) [1, 2]. vMIA dual trafficking is determined by (1) the moderate hydrophobicity of its leader, (2) modification of its consensus protein kinase C site (21SY), and (3) its downstream proline-rich domain (33PLPP) [2]. Increasing the hydrophobicity of the vMIA leader retargets the high hydrophobicity B (HHB) mutant from the MAM to the ER secretory apparatus (Fig. 1). A consensus cholesterol-binding domain (CBD) in its leader allows vMIA to associate with detergent-resistant membranes (DRM) at the MAM [5]. However, mutation of the vMIA CBD domain (CBD II mutant) does not alter ER to OMM trafficking (Fig. 1) [5]. Throughout its sequential trafficking, vMIA remains signal-anchored in the corresponding ER, MAM, and OMM membranes, and MAM targeting is a common feature of all known UL37 protein isoforms [1]. This suggests that UL37 proteins, particularly vMIA, play important functions at the MAM throughout the process of HCMV infection. The vMIA C-terminal residues remain in the cytosol [1, 4], enabling it to interact with cytosolic proteins such as the cell death regulator Bax and the antiviral protein, viperin [24–27]. Due to interactions with cytosolic proteins, trafficking of vMIA alters the localization of these cellular proteins. For example, vMIA causes viperin to localize from ER to the mitochondria, where viperin affects the cellular ATP synthesis and facilitates disruption of the actin cytoskeleton [27].
Fig. 1.
Sub-cellular localization of vMIA wild type, and vMIA-CBDII, and vMIA-HHB mutants. HeLa cells were transiently transfected with plasmid vectors expressing vMIA-enhanced green fluorescent protein (EGFP), vMIA-CBDII-EGFP and vMIA-HHB-EGFP using Lipofectamine 2000 as previously described [93]. Plasmids expressing ER (ER-red fluorescent protein, RFP fused with KDEL) and mitochondrial (Mito-blue fluorescent protein, BFP) markers were used for co-transfection. Cells were fixed with 4 % paraformaldehyde for 15 min 20–24 h after transfection. Fixed cells were imaged using an Olympus FV1000 confocal microscope. Images shown are a single plane of vMIA-EGFP (green) and Mito-BFP (pseudocolored red) transfected HeLa cells (top row), vMIA-CBDII-EGFP (green) and Mito-BFP (pseudocolored red) transfected HeLa cells (middle row) and vMIA-HHB-EGFP (green) and ER-RFP (red) transfected cells (bottom row). Zooms of the regions R 1, 2, and 3 show the distribution of vMIA-EGFP (R1), vMIA-CBDII (R2), and vMIA-HHB (R3) in green (top) and merged (bottom) panels. The bars on the merged full-size images represent 20 µm and on the zoomed regions represent 4 µm
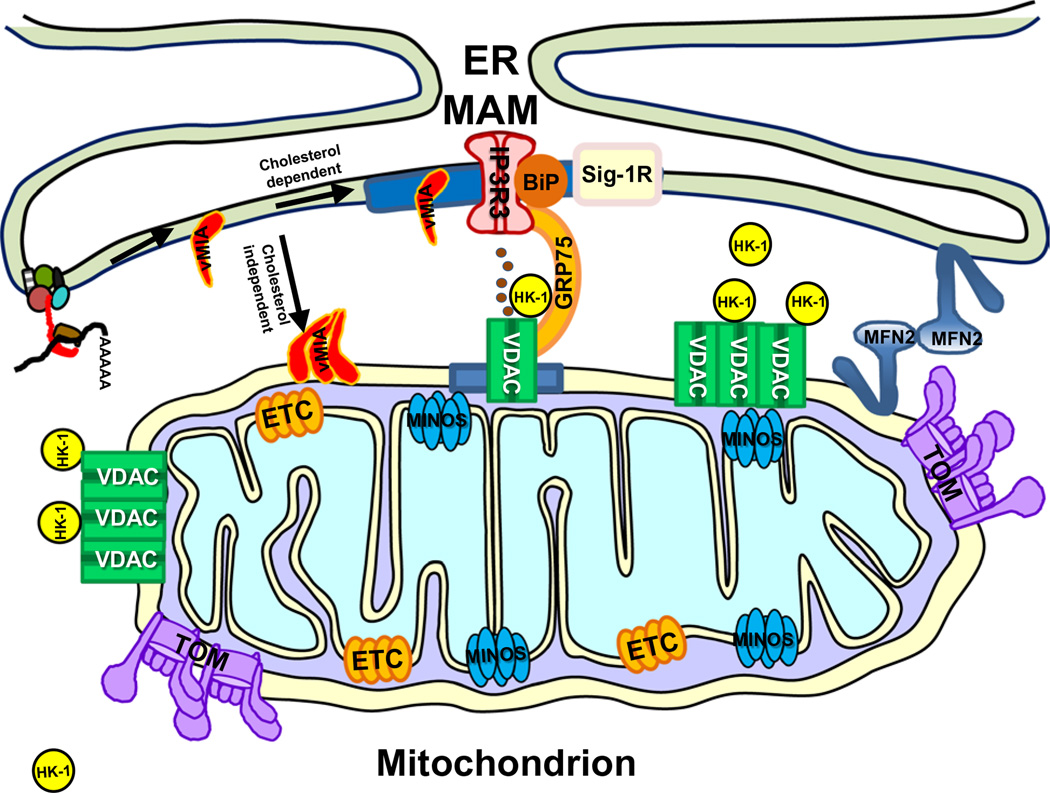
Fig. 2.
Schematic of vMIA trafficking and clustered distribution with respect to endogenous mitochondrial protein clusters. Shown is the translation of vMIA RNA at the ER membrane and its cholesterol dependent association with MAM lipid rafts (blue box), in close proximity with Sig-1R [5, 39]. MAM tether proteins (Mfn2) and components of the MAM calcium signaling complex, including IP3R3, its chaperone BiP, GRP75, and VDAC are shown. vMIA uses a cholesterol-independent mechanism to translocate to the OMM [5], where it is organized in clusters [93]. Other mitochondrial proteins including VDAC and associated hexokinase 1 (HK-1) [95, 96], and components of the TOM complex [94], electron transport chain (ETC) [95], as well as the MINOS complex [100] are also organized in clusters in mitochondrial membranes
The vast majority of endogenous mitochondrial proteins are encoded in the nucleus, synthesized in the cytosol, and trafficked to the mitochondria. The mitochondrial translocase of the OMM (TOM) complex serves as the gatekeeper for import of most mitochondrial proteins [28]. TOM is involved in translocation of mitochondrial protein precursors, particularly those with NH2-terminal mitochondrial targeting signals, for example, Tom20 and Tom70 [29]. Tom20 has a moderately hydrophobic leader sequence with proximal basic residues just downstream of its leader sequence [30]. Upon insertion into the OMM, most Tom20 residues are exposed in the cytosol. The intracellular sorting signals of vMIA contain analogously positioned moderately hydrophobic signal and downstream basic residues similar to those of Tom20 [2]. Nonetheless, vMIA mitochondrial targeting signal retargets a vMIA-Tom20 chimera to the ER and OMM [2].
The role of trafficking for MAM and OMM localization of vMIA is not unique to this viral protein. Hepatitis C virus (HCV) protein NS3/4A serine protease that cleaves activated mitochondrial antiviral signaling (MAVS) protein is associated with MAM, through membrane-targeting domains within NS4A and the amphipathic α helix of NS3 [20]. While dual trafficking of cellular proteins to the ER and to mitochondria is often competitive [31], sequential ER to mitochondrial trafficking of cellular proteins has also been found. For example, an unidentified cellular glycoprotein [32] associates with mitochondrial complexes I and V in the inner mitochondrial membrane (IMM), as well as apoptosis inducing factor (AIF), acyl-CoA:diacylglycerol acyltransferase 2 (DGAT2), retinol dehydrogenase, and retinol-binding protein traffic from the ER to mitochondria [33–35]. Mitochondrial AIF is synthesized in the ER, packaged into MAM transport vesicles, and transported to mitochondria before fusion with OMM [33].
The MAM is enriched in cholesterol-containing lipid microdomains (Fig. 2) [36, 37]. These microdomains are required for proper MAM trafficking of cellular proteins including sigma 1 receptor (Sig-1R) and erlins, which chaperone and facilitate degradation, respectively, of the inositol 1,4,5-trisphosphate receptor (IP3R) [36–38]. In the MAM lipid microdomains, vMIA localizes in close proximity of Sig-1R as demonstrated by fluorescence resonance energy transfer (FRET) between these two proteins [5, 39]. vMIA association with MAM lipid microdomains is cholesterol dependent; however, this is not needed for its trafficking to the OMM (Figs. 1, 2) [5]. These findings indicate that a series of lipid and protein interactions are employed to ensure proper trafficking of proteins to the MAM.
HCMV infection reorganizes the MAM and mitochondria through vMIA
Mitochondrial activities are modulated by calcium (Ca2+) signaling [10, 15, 16]. Under physiological conditions, ER chaperones, including BiP, calnexin, and calreticulin store Ca2+ in the ER [40]. Cellular Ca2+ homeostasis by ER and mitochondria involves Ca2+ uptake and release by ER and mitochondria such that Ca2+ is efficiently transported between these two organelles [41]. Cytosolic Ca2+ is taken up by the ER through the action of sarcoplasmic or endoplasmic reticulum Ca2+-ATPase (SERCA) and ryanodine receptor (RyR) [41]. The ER then releases Ca2+ in the context of mitochondria by the use of inositol 1,4,5-trisphosphate receptor (IP3R). IP3R is enriched at the MAM, where its activity is augmented by its MAM chaperone, Sig-1R, a ligand-operated chaperone that stabilizes IP3R and thus regulates Ca2+ influx from the ER to mitochondria [42]. Constitutive low level IP3R-mediated Ca2+ release is essential for efficient mitochondrial respiration and maintenance of normal cellular bioenergetics [16]. But, continued and increased mitochondrial Ca2+ drives the adaptive metabolic phase of early ER stress [18].
High Ca2+ microdomains between ER and mitochondria are facilitated by the close apposition of the ER and OMM, which enables efficient transport of Ca2+ ions from ER to mitochondria without increasing bulk cytosolic Ca2+ levels (represented in Fig. 2) [10]. For the calcium to be transported into mitochondria, the Ca2+ ions need to pass across the outer and inner mitochondrial membranes (OMM and IMM). The voltage-dependent anion-selective channel (VDAC) facilitates the high OMM permeability for Ca2+. However, the mitochondrial calcium uniporter (MCU) in the IMM facilitates Ca2+ entry into the mitochondrial matrix [43]. Opening of the MCU channel is regulated by an EF hand protein mitochondrial calcium uptake 1 (MICU1), which is known to bind MCU channel and supports cooperative activation of the mitochondrial uniporter at high cytosolic Ca2+ concentrations [44, 45]. Finally, the mitochondrion is also capable of releasing calcium, which is facilitated by the leucine zipper-EF-hand containing transmembrane protein 1 (LETM1). LETM1 is an electrogenic Ca2+/H+ antiporter, distinct from MCU that is responsible for Ca2+ uptake in mitochondria when mitochondrial Ca2+ is low [46]. HCMV infection increases the abundances of MAM Ca2+ signaling complex components including the IMM Ca2+ uniporter complex—MCU (7.7 ± 0.8-fold) and MICU1 (9.8 ± 1.5-fold), as well as the mitochondrial pump LetM1 (12.5 ± 2.5 fold) and the ER pump SERCA (3.3 ± 0.7 fold) [4, 6]. VDAC (7.1 ± 0.8-fold), cytosolic GRP75 (23.9 ± 5.4-fold), and the Sig-1R chaperone are also increased in the MAM of HCMV infected cells at late times [4, 6]. vMIA causes efflux of ER Ca2+ stores [47].
MAM plays important roles in responding to cellular stress particularly mediated by Ca2+ release from the ER stores. Although mitochondria mobilized close to ER initially provide a protective effect during early ER stress responses [18], mitochondrial Ca2+ overload can induce mitochondrial-mediated apoptosis [19]. The later phases of ER stress lead to tightening of ER–mitochondrial contacts and induction of apoptosis [8]. ER stress in obesity increases MAM formation and resulting mitochondrial Ca2+ overload, which in turn increases mitochondrial reactive oxygen species production and mitochondrial dysfunction [48].
HCMV infection reprograms host cells to increased aerobic glycolysis and anaplerotic use of the TCA cycle [49, 50]. Affecting MAM function may enable HCMV to reprogram cellular metabolism. HCMV infection changes the MAM proteome to control multiple cellular functions particularly blocking ER stress responses, altering metabolism, and inhibiting mitochondria-mediated apoptosis [4, 6, 51]. Global changes in MAM-associated machineries affecting translation and ER translocation, Ca2+ signaling, metabolism, and mitochondria-mediated apoptosis suggest that HCMV infection induces a restructuring of ER–mitochondrial contacts by late times of infection. This restructuring predictably increases cell survival and affects bioenergetics. Further, the increased abundance of IMM, intermembrane space, and matrix proteins in the MAM fraction suggests that HCMV infection increases the stability or expands ER–mitochondrial contacts by late times of infection.
Notably, ER and mitochondrial stress response proteins including GRP78, GRP75, hsp10, and hsp60 are among the most augmented proteins in the MAM and in the total cell fractions at late times of HCMV infection [6]. In contrast, different MAM proteins (calreticulin, calnexin, and PACS-2) and cytosolic glycolytic enzymes appear to be relocalized from other sub-cellular compartments to the MAM suggesting the importance of their increased abundance in the MAM during HCMV infection. Consistent with its ability to blunt ER stress responses, HCMV infection blocks relocation of the MAM stress sensor Sig-1R [42] to bulk ER [6].
Aside from calcium homeostasis, essential lipids synthesized in the ER are transferred between ER and mitochondria at the MAM. Lipid synthetic enzymes including phosphatidylserine synthase types 1 and 2 (PSS-1, PSS-2), DGAT2, fatty acid CoA ligase 4 (FACL-4), ceramide synthase, and shingolipid-specific glycotransferases are enriched in MAM, facilitating delivery of their membrane-bound products to mitochondria [17, 34, 52, 53]. Conversely, phosphatidylethanolamine (PE) is transferred back from the OMM to the ER where it is modified by ER enzymes to make phosphatidylcholine [54].
Innate immune responses use pattern recognition receptors, such as retinoic acid-inducible gene I (RIG-I), to provide an important defense against virus pathogens [55]. RIG-I is an essential cytosolic receptor that recognizes short viral dsRNAs or 5′ triphosphorylated blunt ends of RNA, an interaction that has been visualized by ground state depletion (GSD) superresolution imaging [56–58]. RNA binding induces a conformational change in RIG-I that exposes caspase recruitment domains (CARDs) and thereby allows its binding of ATP, unanchored lysine 63 (K63)-linked polyubiquitin chains as well as short dsRNAs [59, 60]. The resulting activated RIG-I tetramers serve as a platform to recruit the mitochondrial antiviral protein, MAVS [61]. Recruited MAVS, in turn, polymerizes and activates transcription factors IRF3 and NFκB, which then translocate to the nucleus and induce transcription of type I interferon and interferon response genes. MAVS protein on the OMM can bind (possibly indirectly) to the ER-localized stimulator of interferon genes (STING) at the MAM and, thereby, augment interferon responses to DNA viruses [62–64]. MAVS and STING binding is amplified by the close apposition of the ER and OMM at the MAM [21]. vMIA increases mitochondrial biogenesis [65] and causes mitochondrial fragmentation in transfected cells and during infection [21, 24, 39, 66–68]. Through this, vMIA disrupts interactions between MAVS and STING and thereby downstream signaling against HCMV infection [21].
It is currently thought that vMIA-induced mitochondrial fragmentation is mediated by Bax, Mfn2, and the inorganic phosphate carrier [66–68]. Aside from MAVS, vMIA binds and relocalizes another cell defense protein, viperin, from the ER to mitochondria. At mitochondria, viperin increases lipogenesis during HCMV infection [27]. Similarly, vMIA causes retargeting of Bax from the cytosol onto the OMM and MAM and blocks Bax-mediated permeabilization of the OMM [24–26]. Bax relocalized by vMIA to the MAM is degraded by the action of the proteasome, facilitating the antiapoptotic function attributed to vMIA [51]. Blocking the ER to mitochondrial trafficking of vMIA compromises the antiapoptotic function of vMIA, and its mitochondrial targeting signal (residues 1–35) is invariant in 27 primary clinical HCMV strains studied [69]. Thus, vMIA affects multiple MAM and mitochondria functions by its subcellular trafficking.
Superresolution fluorescence imaging techniques for virus studies
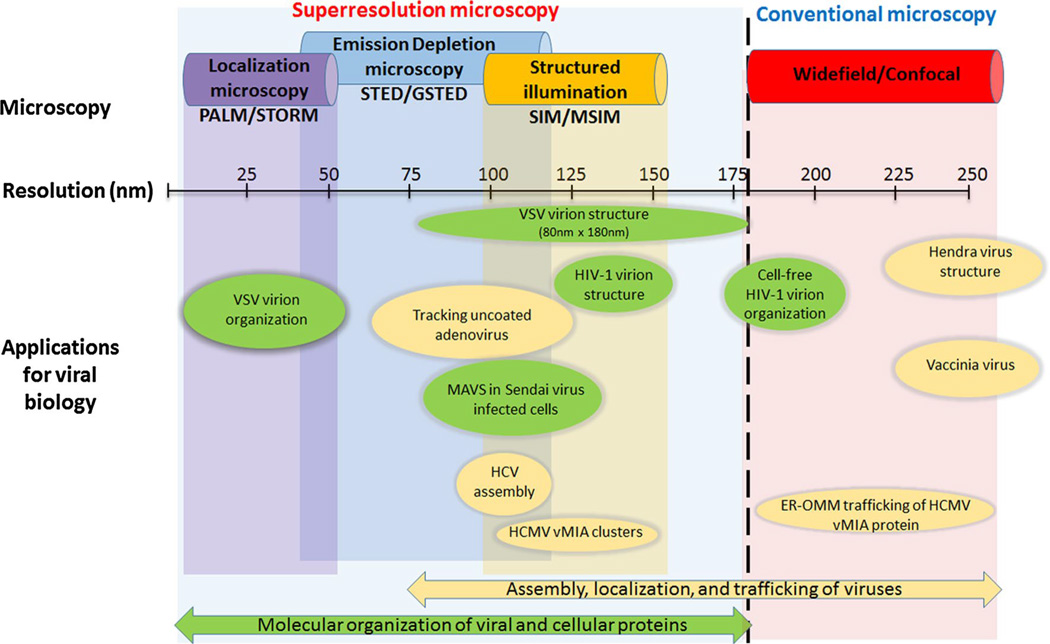
Given their small size (generally ≤100 nm), viruses imaged with conventional fluorescent microscopy are observed as spots often twice their actual size (Fig. 3). Thus, imaging of virions and viral protein biology with superresolution methods, which can peer beyond the diffraction barrier (~200 nm), has begun in earnest. The techniques used for these experiments typically fall into one of three categories, structured illumination microscopy (SIM) [70], stimulated emission depletion (STED) microscopy [71], or one of the numerous localization microscopy (LM) techniques. The best resolutions from each of these methods vary with LM reaching 10–20 nm, while STED is generally cited as ~35– 40 nm (although 75–80 nm is often more practical) [72, 73], and SIM is usually reported between 100 and 140 nm. It would be convenient to simply use LM since it provides the best resolution, but each of these offers advantages and disadvantages which make them appropriate for different specimens and different experiments (Fig. 3).
Fig. 3.
Application of superresolution imaging to study viral assembly and trafficking. This schematic highlights the suitability of the superresolution and conventional imaging approaches for studying assembly, localization, uncoating, and trafficking of a variety of viruses. While the applications have been broadly categorized into studies relevant to viral organization (green) and localization/trafficking (yellow), the studies on which this schematic is based upon [76, 78, 79, 81, 83, 85, 87, 93] illustrate that depending upon the question, more than one of these imaging methodologies should be used
Rather than simply list generic or hypothetical uses of the superresolution techniques, we concentrate on examples to demonstrate what can be learned from subjecting virus-related samples to superresolution imaging techniques. When applied to studying virus biology, superresolution imaging techniques are generally trying to answer questions falling into three categories: (1) sub-virus structure and localization, (2) virus assembly and release, and (3) clustering of viral and cell components during infection and replication. Obviously, overlap is expected between these categories, and often, the information derived during these imaging experiments is similar. Reviews published elsewhere [74, 75] describe studies, which focus on superresolution in virus structure, assembly, and release experiments. Here, we concentrate on virus protein trafficking and localization to intracellular components to demonstrate the progress being made as a result of the superresolution imaging revolution.
Subviral structure localization
With superresolution optical methods now able to resolve structures or localize molecules on a scale smaller than a virus particle, a natural progression was to use these methods to study virus structure. Optical superresolution still lacks the resolution of EM, which is often used in virus structure studies, but the fluorescence techniques have the advantage of higher throughput during sample preparation and can include multiple labels. For instance, STED was used to monitor and quantify the redistribution of the HIV envelope protein (Env), which correlated with viral entry efficiency [76]. Env clusters were observed previously at particle and T cell contacts using electron tomography [77], and STED allowed the study of these sub-particle structures in numbers high enough for in-depth statistical analysis.
Gated STED (GSTED) was used to determine the relative localizations of adenovirus DNA (vDNA) and capsid protein [78]. The signals from the two markers localized well with different relative sizes. The capsid was found to be ~120 nm (FWHM determined from Gaussian fits of line profiles), while the vDNA produced a FWHM of ~80 nm. These were in good agreement with capsid sizes determined from structural studies after accounting for the additional size of the antibody labels. These images also showed that free cytoplasmic vDNA (not associated with capsid) covered an area ~25 % larger than capsid-associated vDNA suggesting compaction of the vDNA once inside the capsid. Conventional images show these as simple diffraction-limited spots, and little subviral details can be discerned.
Cluster analysis using LM data offers another approach to viewing relative structural alterations in virions. For instance, analyses using dSTORM on matrix and capsid proteins during HIV-1 infection found a significant increase (236 %) in the cluster sizes of capsid proteins when compared with the matrix protein [79]. Using a photoactivated LM (PALM) approach with FlAsH tagged IN, an enzyme involved in the integration of the HIV DNA into the host genome, the observed structural differences correlated with the location of the complex in the cytoplasm or in the nucleus [80]. The data showed a wide range of cluster sizes but suggested that this enzyme remained in intact capsids within the cytoplasm, which had a mean FWHM of ~100 nm. Upon entering the nucleus, the cluster size showed a decrease to a mean FWHM of ~30 nm suggesting a large structural modification in passage through the nuclear pores.
The localization of Hendra virus (HeV) proteins was imaged [81] using both GSD [82] and SIM. In good agreement with EM studies, these determined the HeV particles inside cells were ~300 nm in diameter. Both GSD and SIM indicate that the M matrix protein is more closely associated with the ribonucleoprotein complex (RNP) containing the RNA genome than with the virus envelope. A similar assessment of the sub-virion localization of the polymerase complex subunits, L and P, in the vesicular stomatitis virus (VSV) [83] indicated the proteins were located toward one end of the asymmetrically shaped virus and occupied ~50 nm of a 150 nm cavity inside the virion.
While SIM generally provides less resolution than STED or LM techniques, even it has been useful in the study of some virion structures. For example, 3D-SIM imaging was performed on vaccinia virus infected cells [84]. Fluorescent protein-tagged virus components, A3 (core protein) and B5 (integral membrane protein), provided markers for the virus particle lumen and the envelope surrounding the core, respectively. With these, the outer shell of the virus was distinguished from the core and showed that the normally diffraction-limited spots of virus particle formation could be monitored at subviral resolutions. These studies set the stage for monitoring another virus protein, A36, at these resolutions [85]. A36 is a viral transmembrane protein located in the outer membrane of the double membrane particle and has a large cytoplasmic domain. This domain mediates interactions with a number of intracellular proteins including cytoskeletal structures. Since the hollow sphere was easily resolved, SIM monitored the redistribution of A36 and A36 mutants along the periphery during virus assembly and release. It was noted that specific mutants in A36 known to decrease virus release did not undergo the normal redistribution when the particle was located in extracellular space.
Clustering of viral and cell components during infection and replication
In many studies, the interest is less in the virus particle structure and more in the relative localization of virus particles or viral proteins with intracellular components. Important to note here is that these superresolution techniques are limited just as conventional microscopy in that they cannot provide certainty concerning colocalization of two components. However, they can allow inferences about localization within a much smaller specified area or volume. For example, despite the compact cellular environment, STED imaging of HIV-1 virions located in dendritic cells showed little colocalization with most intracellular components except actin [86]. The particles colocalized at immunological synapses when co-cultured with CD4+ T cells and were used to establish the functional relevance of these synapses before further study using higher-resolution electron microscopy (EM) techniques.
Another example of virus components located to specific structures inside cells includes studies of the HCV, which during part of its life cycle localizes its capsid protein core to lipid droplets associated with ER membranes. Colocalization studies using conventional microscopy of envelope proteins with the core protein on these droplets proved to be inconclusive since the droplets are diffraction-limited spot sized [87]. Using a LM technique (dSTORM), the relative localizations of core and an envelope protein E2 were imaged on small lipid droplets (0.2–0.5 µm) located within 1–2 µm from the plasma membrane [87]. Although both proteins could be found associated with the droplets, dSTORM images revealed very little colocalization or mixing of the proteins with each other in their respective punctate regions. Similar concerns about the precise localization of their protein of interest prompted use of the added resolution of SIM to conclusively determine that a flavivirus cell penetrating protein (CPP) localized mainly to the membrane of endosomes as opposed to being located in the lumen as observed by conventional microscopy [88].
Since hijacking of a cell by a virus often leads to dramatic morphological changes in intracellular organelles, superresolution offers new insight through finer mapping of these alterations. For example, RNA viruses often induce the formation of punctate structures in the cytoplasm which have been described as “X-bodies” or replication “factories,” “organelles,” or “complexes.” Using 3D-SIM, Linnik and colleagues [89] followed up on previous confocal microscopy studies of potato virus X (PVX) induced X-bodies to more finely map the relative localizations of PVX proteins necessary for virus movement. The coat protein (CP), triple gene block (TGB) proteins, TGB1, TGB2, and TGB3 were imaged together or separately using intracellular markers. Their studies found that the granular patterns exhibited by TGB2 and TGB3 were actually labeling a fine reticulum of remodeled ER membranes. Although these structures appeared to be contiguous granules by confocal microscopy, SIM indicated they had “donut-shaped” loops with ~300 nm outer diameters and ~125 nm inner diameter. 3D-SIM also made clearer the morphological alterations in cell organelles such as the Golgi apparatus. In PVX-infected cells, the trans-Golgi network (TGN) rearranged into donut-like structures distinct in size from the TGB2 and TGB3 labeled structures, whereas they appeared as punctate granules by confocal microscopy.
Indeed, it is often the presence of punctate signals with a size of the diffraction limit, which drives the need for a superresolution view. Simply determining the sizes and shapes of clusters or domains is generally the first step in better characterizing diffraction-limited spots by superresolution, but these data offer insight into the density and distribution of proteins at very small scales. For instance, the MAVS protein polymerizes and forms helical filaments during RNA virus infection. Using SIM, it was found in rod-shaped clusters on the mitochondria [90] and was noted to be ≤100 nm in diameter since the resolving power of SIM is ~100 nm. Clustered MAVS had a mean length of ~400 nm with maximum observed length of ~800 nm. These clusters are easily seen with diffraction-limited microscopy, but such detailed length measurements could not be made using conventional imaging nor could an upper limit of 100 nm be placed on the maximum diameter. Importantly, these polymerized MAVS helical fibers serve as the activation platform for downstream interferon signaling.
Even smaller domains were noted when imaging DCSIGN, a C-type lectin which involved in catching and internalizing pathogens. STED microscopy of the dendritic cell side of a virus entry point found clustering of DC-SIGN on scales missed by conventional imaging [91]. Importantly, STED offered the capability to monitor the clustering on mutant forms of DC-SIGN. By doing so, the key regions of the molecule necessary for clustering and subsequent pathogen internalization were narrowed to its extracellular neck region. This molecule’s behavior was further explored in experiments using LM (dSTORM) which also found that deletion of cytoplasmic domain resulted in no clustering [92]. Remarkably, these experiments also revealed that the microdomains were actually nano-sized domains in which the smallest clusters contained only 4–8 DC-SIGN molecules.
Superresolution imaging of vMIA and mitochondrial proteins
At the MAM, the ER membrane and OMM are spaced at 10–30 nm [8, 9]. Because of the close proximity of ER and mitochondrial membranes as well as OMM and IMM at the MAM, electron tomography has provided the best MAM imaging [8, 9]. While tomography provides excellent high resolution (<20 nm), the approach is limited by fixation and dehydration, which can distort membrane structures.
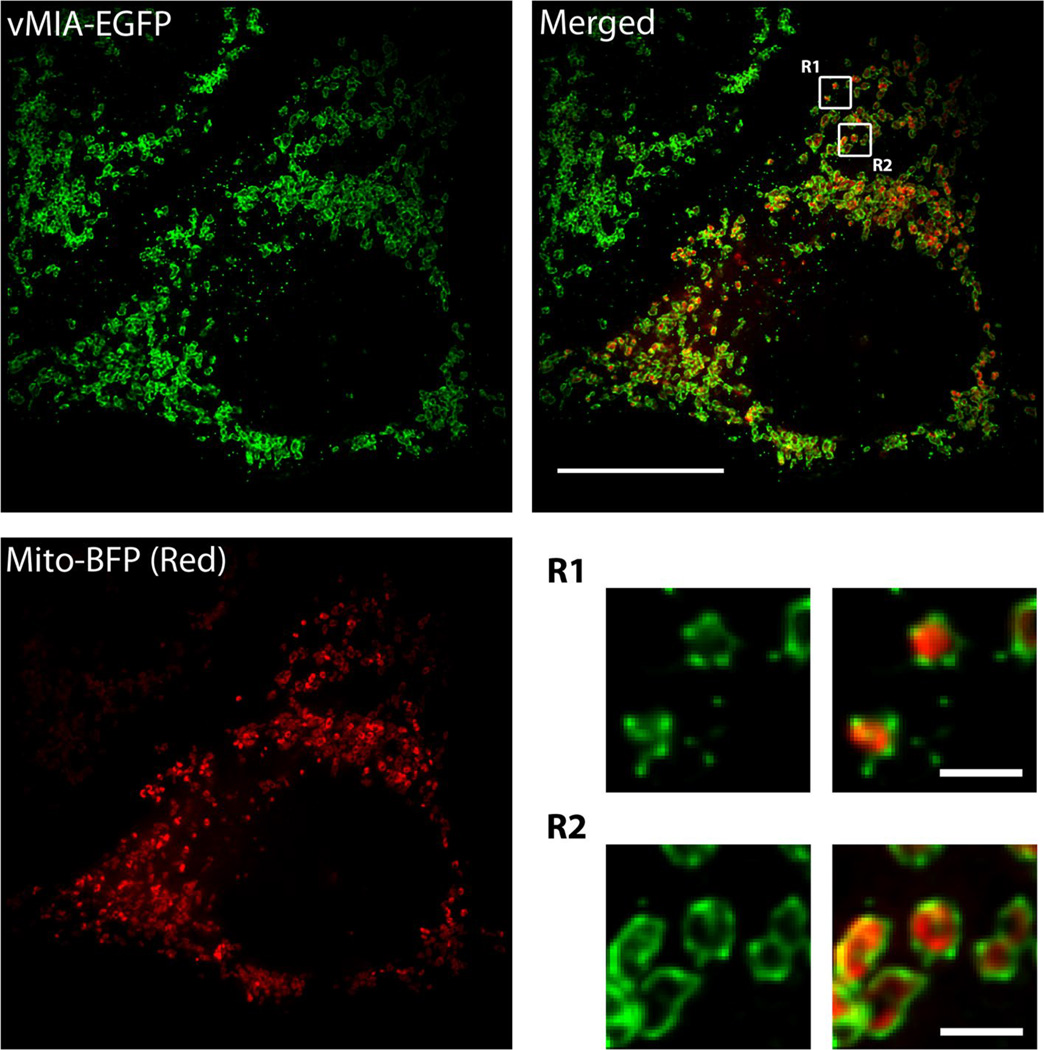
Superresolution imaging can provide novel information on sub-cellular and sub-organellar distribution of proteins. To improve imaging of vMIA location, we used a combination of deconvolved confocal imaging and three superresolution approaches, gated STED, MSIM, and PALM [93]. Deconvolution of confocal microscopy image resolved vMIA at the MAM, away from mitochondrial matrix [93]. However, deconvolved confocal imaging falls short of resolving the sub-mitochondrial location (IMM versus OMM) or the physical organization of vMIA (diffused versus clustered). Superresolution imaging combined with confocal imaging localized the vMIA clusters away from the mitochondrial matrix. Superresolution imaging using MSIM, GSTED, and PALM each detected existence of vMIA in clusters, while multicolor MSIM additionally established the OMM localization of the vMIA clusters away from the mitochondrial intermembrane space and mitochondrial matrix (Fig. 4) [93]. Moreover, the combined use of superresolution approaches consistently established that vMIA at the OMM exists in clusters of ~100–150 nm.
Fig. 4.
Visualization of vMIA clustering on the OMM by MSIM imaging. HeLa cells were transfected with vectors expressing vMIA-EGFP (green) and mitochondrial marker Mito-BFP (pseudocolored red) using Lipofectamine 2000. Transfected cells were fixed with 4 % paraformaldehyde and imaged using MSIM as described [93]. Zoom of the regions R1 and 2 on the merged image show the clustered distribution of vMIA-EGFP at the OMM. The scale bars represent 15 µm on the merged full-size image and 1 µm on the zoomed images
Multiple mitochondrial proteins located at the OMM and the IMM have been found using superresolution imaging to be organized in clusters. Tom20 and Tom22, components of the TOM complex, are clustered at the OMM [94]. Tom20 clusters (30–40 nm) are larger than a single TOM complex (diameter of ~14.5 nm). Importantly, the distribution of Tom20 clusters differs depending upon functional requirements of mitochondria. Further, the density of Tom20 clusters is higher in metabolically active cells [94].
When examined by STED imaging of Percoll-purified mitochondria from murine, heart showed that VDAC type 1 (VDAC1) protein formed clusters in four size distributions of ~22, ~33, ~55, and ~83 nm at the OMM [95]. Other investigators found that the human VDAC isoforms (VDAC1–3), which have high degrees of primary sequence homology, had different sub-mitochondrial distributions [96]. Human VDAC1 and VDAC2 isoforms are distributed in clusters in the OMM and colocalize in relatively large clusters of 300–900 nm, which were frequently composed of several smaller clusters [96]. In contrast, human VDAC3 is more uniformly distributed on the OMM. However, when co-expressed with the other VDAC isoforms, some colocalization of VDAC3 with VDAC1 and 2 was induced. VDAC binds to cytosolic hexokinase-1 (HK-1) [97]. Although VDAC1 is known to bind HK-1, VDAC1 and VDAC2 showed only partial colocalization with HK-1, whereas VDAC3 colocalized more with HK-1 (Fig. 2) [96].
Mitochondrial cristae shape determines the assembly and stability of the respiratory chain supercomplexes and thereby respiratory efficiency [98]. Cytochrome c oxidase subunit 2, complex IV of the electron transport chain (ETC), is distributed in clusters of ~20 and 28 nm (Fig. 2) [95]. Mitochondrial inner membrane organizing system (MINOS) is critical for the maintenance of cristae morphology and has been proposed to play a central part in ER–mitochondrial organizing network the controls mitochondrial membrane architecture and biogenesis [99]. Using STED imaging of antibody labeled proteins, three MINOS subunits, mitofilin, MINOS1, and CHCHD3, were found to form clusters of ~85 nm, in which the MINOS subunits were highly colocalized [100]. Intriguingly, all three MINOS subunits formed an ordered arrangement at the rim of mitochondria in human fibroblasts, Vero and HeLa cells. However, the MINOS complex did not form spatially extended superstructures in human fibroblasts [100].
Summary and outlook
Superresolution imaging has the clear potential to provide valuable insight into the nanoscale organization of viral machineries, which provide essential replicative processes. Case in point is the detection of non-uniform distribution of vMIA at the OMM. This distribution was not detectable by conventional microscopy because of its diffraction limited resolution. Further, this technology allows virologists to study how viruses alter cellular organelles to establish replication compartments and virus assembly. In a few years, superresolution imaging techniques of all varieties will take their place beside wide-field, confocal, and multiphoton microscopy as standard laboratory tools rather than exotic new toys available to only a few. While many of the current developments in superresolution imaging have been focused on improving static spatial resolution, there is increasing focus in doing so together with real-time imaging of live cells. With such developments, studies of virus biology are likely to accelerate and nagging questions about the behavior of a favorite virus inside a cell that investigators have pondered for decades may finally be answered.
Acknowledgments
This work was partially funded by the NSF Grant (MCB 1244509) to ACP and JKJ. The confocal microscopy imaging was supported by a core Grant (1P30HD40677) to the Children’s Intellectual and Developmental Disabilities Research Center. This work was also supported by the Intramural Research Program of the National Institutes of Health including the National Institute of Biomedical Imaging and Bioengineering. We thank Kristen Rainey for assisting with imaging and analysis. We thank Andrew York and Hari Shroff for sharing MSIM analysis and deconvolution software. We thank Harald Hess for the use of the PALM analysis software, PeakSelector.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Contributor Information
Anamaris M. Colberg-Poley, Email: acolberg-poley@childrensnational.org, Center for Genetic Medicine Research, Children’s National Health System, 111 Michigan Ave, NW, Washington, DC 20010, USA; Department of Integrative Systems Biology, George Washington University School of Medicine and Health Sciences, Washington, DC 20037, USA; Departments of Pediatrics and of Biochemistry and Molecular Medicine, George Washington University School of Medicine and Health Sciences, Washington, DC 20037, USA.
George H. Patterson, Email: pattersg@mail.nih.gov, Section on Biophotonics, National Institute of Biomedical Imaging and Bioengineering, National Institutes of Health, Bethesda, MD 20892, USA.
Kyle Salka, Center for Genetic Medicine Research, Children’s National Health System, 111 Michigan Ave, NW, Washington, DC 20010, USA.
Shivaprasad Bhuvanendran, Center for Genetic Medicine Research, Children’s National Health System, 111 Michigan Ave, NW, Washington, DC 20010, USA.
David Yang, George Washington University School of Medicine and Health Sciences, Washington, DC 20037, USA.
Jyoti K. Jaiswal, Email: jkjaiswal@childrensnational.org, Center for Genetic Medicine Research, Children’s National Health System, 111 Michigan Ave, NW, Washington, DC 20010, USA; Department of Integrative Systems Biology, George Washington University School of Medicine and Health Sciences, Washington, DC 20037, USA; Department of Pediatrics, George Washington University School of Medicine and Health Sciences, Washington, DC 20037, USA.
References
- 1.Mavinakere MS, Williamson CD, Goldmacher VS, Colberg-Poley AM. Processing of human cytomegalovirus UL37 mutant glycoproteins in the endoplasmic reticulum lumen prior to mitochondrial importation. J Virol. 2006;80(14):6771–6783. doi: 10.1128/JVI.00492-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williamson CD, Colberg-Poley AM. Intracellular sorting signals for sequential trafficking of human cytomegalovirus UL37 proteins to the endoplasmic reticulum and mitochondria. J Virol. 2010;84:6400–6409. doi: 10.1128/JVI.00556-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bozidis P, Williamson CD, Colberg-Poley AM. Mitochondrial and secretory human cytomegalovirus UL37 proteins traffic into mitochondrion-associated membranes of human cells. J Virol. 2008;82(6):2715–2726. doi: 10.1128/JVI.02456-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bozidis P, Williamson CD, Wong DS, Colberg-Poley AM. Trafficking of UL37 proteins into mitochondrion-associated membranes during permissive human cytomegalovirus infection. J Virol. 2010;84(15):7898–7903. doi: 10.1128/JVI.00885-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williamson CD, Zhang A, Colberg-Poley AM. The human cytomegalovirus protein UL37 exon 1 associates with internal lipid rafts. J Virol. 2011;85(5):2100–2111. doi: 10.1128/JVI.01830-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang A, Williamson CD, Wong DS, et al. Quantitative proteomic analyses of human cytomegalovirus-induced restructuring of endoplasmic reticulum–mitochondrial contacts at late times of infection. Mol Cell Proteomics. 2011;10(M111):009936. doi: 10.1074/mcp.M111.009936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mavinakere MS, Colberg-Poley AM. Dual targeting of the human cytomegalovirus UL37 exon 1 protein during permissive infection. J Gen Virol. 2004;85(Pt 2):323–329. doi: 10.1099/vir.0.19589-0. [DOI] [PubMed] [Google Scholar]
- 8.Csordas G, Renken C, Varnai P, et al. Structural and functional features and significance of the physical linkage between ER and mitochondria. J Cell Biol. 2006;174(7):915–921. doi: 10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedman JR, Lackner LL, West M, DiBenedetto JR, Nunnari J, Voeltz GK. ER tubules mark sites of mitochondrial division. Science. 2011;334(6054):358–362. doi: 10.1126/science.1207385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szabadkai G, Bianchi K, Varnai P, et al. Chaperonemediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J Cell Biol. 2006;175(6):901–911. doi: 10.1083/jcb.200608073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456(7222):605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- 12.Friedman JR, Webster BM, Mastronarde DN, Verhey KJ, Voeltz GK. ER sliding dynamics and ER-mitochondrial contacts occur on acetylated microtubules. J Cell Biol. 2010;190(3):363–375. doi: 10.1083/jcb.200911024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cerqua C, Anesti V, Pyakurel A, et al. Trichoplein/mitostatin regulates endoplasmic reticulum–mitochondria juxtaposition. EMBO Rep. 2010;11(11):854–860. doi: 10.1038/embor.2010.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simmen T, Aslan JE, Blagoveshchenskaya AD, et al. PACS-2 controls endoplasmic reticulum-mitochondria communication and Bid-mediated apoptosis. EMBO J. 2005;24(4):717–729. doi: 10.1038/sj.emboj.7600559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayashi T, Rizzuto R, Hajnoczky G, Su TP. MAM: more than just a housekeeper. Trends Cell Biol. 2009;19:81–88. doi: 10.1016/j.tcb.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cardenas C, Miller RA, Smith I, et al. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell. 2010;142(2):270–283. doi: 10.1016/j.cell.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stone SJ, Vance JE. Phosphatidylserine synthase-1 and -2 are localized to mitochondria-associated membranes. J Biol Chem. 2000;275(44):34534–34540. doi: 10.1074/jbc.M002865200. [DOI] [PubMed] [Google Scholar]
- 18.Bravo R, Vicencio JM, Parra V, et al. Increased ER–mitochondrial coupling promotes mitochondrial respiration and bioenergetics during early phases of ER stress. J Cell Sci. 2011;124(Pt 13):2143–2152. doi: 10.1242/jcs.080762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hajnoczky G, Csordas G, Das S, et al. Mitochondrial calcium signalling and cell death: approaches for assessing the role of mitochondrial Ca2+ uptake in apoptosis. Cell Calcium. 2006;40(5–6):553–560. doi: 10.1016/j.ceca.2006.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horner SM, Park HS, Gale M., Jr Control of innate immune signaling and membrane targeting by the Hepatitis C virus NS3/4A protease are governed by the NS3 helix alpha0. J Virol. 2012;86(6):3112–3120. doi: 10.1128/JVI.06727-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castanier C, Garcin D, Vazquez A, Arnoult D. Mitochondrial dynamics regulate the RIG-I-like receptor antiviral pathway. EMBO Rep. 2010;11(2):133–138. doi: 10.1038/embor.2009.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reboredo M, Greaves RF, Hahn G. Human cytomegalovirus proteins encoded by UL37 exon 1 protect infected fibroblasts against virus-induced apoptosis and are required for efficient virus replication. J Gen Virol. 2004;85(Pt 12):3555–3567. doi: 10.1099/vir.0.80379-0. [DOI] [PubMed] [Google Scholar]
- 23.Goldmacher VS, Bartle LM, Skaletskaya A, et al. A cytomegalovirus- encoded mitochondria-localized inhibitor of apoptosis structurally unrelated to Bcl-2. Proc Natl Acad Sci USA. 1999;96(22):12536–12541. doi: 10.1073/pnas.96.22.12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poncet D, Pauleau AL, Szabadkai G, et al. Cytopathic effects of the cytomegalovirus-encoded apoptosis inhibitory protein vMIA. J Cell Biol. 2006;174(7):985–996. doi: 10.1083/jcb.200604069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma J, Edlich F, Bermejo GA, Norris KL, Youle RJ, Tjandra N. Structural mechanism of Bax inhibition by cytomegalovirus protein vMIA. Proc Natl Acad Sci USA. 2012;109(51):20901–20906. doi: 10.1073/pnas.1217094110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pauleau AL, Larochette N, Giordanetto F, et al. Structurefunction analysis of the interaction between Bax and the cytomegalovirus- encoded protein vMIA. Oncogene. 2007;26(50):7067–7080. doi: 10.1038/sj.onc.1210511. [DOI] [PubMed] [Google Scholar]
- 27.Seo JY, Yaneva R, Hinson ER, Cresswell P. Human cytomegalovirus directly induces the antiviral protein viperin to enhance infectivity. Science. 2011;332(6033):1093–1097. doi: 10.1126/science.1202007. [DOI] [PubMed] [Google Scholar]
- 28.Dukanovic J, Rapaport D. Multiple pathways in the integration of proteins into the mitochondrial outer membrane. Biochim Biophys Acta. 2011;1808(3):971–980. doi: 10.1016/j.bbamem.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 29.Rapaport D. Finding the right organelle. Targeting signals in mitochondrial outer-membrane proteins. EMBO Rep. 2003;4(10):948–952. doi: 10.1038/sj.embor.embor937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanaji S, Iwahashi J, Kida Y, Sakaguchi M, Mihara K. Characterization of the signal that directs Tom20 to the mitochondrial outer membrane. J Cell Biol. 2000;151(2):277–288. doi: 10.1083/jcb.151.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyazaki E, Kida Y, Mihara K, Sakaguchi M. Switching the sorting mode of membrane proteins from cotranslational endoplasmic reticulum targeting to posttranslational mitochondrial import. Mol Biol Cell. 2005;16(4):1788–1799. doi: 10.1091/mbc.E04-08-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chandra NC, Spiro MJ, Spiro RG. Identification of a glycoprotein from rat liver mitochondrial inner membrane and demonstration of its origin in the endoplasmic reticulum. J Biol Chem. 1998;273(31):19715–19721. doi: 10.1074/jbc.273.31.19715. [DOI] [PubMed] [Google Scholar]
- 33.Chiang SF, Huang CY, Lin TY, Chiou SH, Chow KC. An alternative import pathway of AIF to the mitochondria. Int J Mol Med. 2012;29(3):365–372. doi: 10.3892/ijmm.2011.849. [DOI] [PubMed] [Google Scholar]
- 34.Stone SJ, Levin MC, Zhou P, Han J, Walther TC, Farese RV., Jr The endoplasmic reticulum enzyme DGAT2 is found in mitochondria-associated membranes and has a mitochondrial targeting signal that promotes its association with mitochondria. J Biol Chem. 2009;284(8):5352–5361. doi: 10.1074/jbc.M805768200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang W, Napoli JL. The retinol dehydrogenase Rdh10 localizes to lipid droplets during acyl ester biosynthesis. J Biol Chem. 2013;288(1):589–597. doi: 10.1074/jbc.M112.402883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hayashi T, Fujimoto M. Detergent-resistant microdomains determine the localization of sigma-1 receptors to the endoplasmic reticulum–mitochondria junction. Mol Pharmacol. 2010;77(4):517–528. doi: 10.1124/mol.109.062539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Browman DT, Resek ME, Zajchowski LD, Robbins SM. Erlin-1 and erlin-2 are novel members of the prohibitin family of proteins that define lipid-raft-like domains of the ER. J Cell Sci. 2006;119(Pt 15):3149–3160. doi: 10.1242/jcs.03060. [DOI] [PubMed] [Google Scholar]
- 38.Pearce MM, Wang Y, Kelley GG, Wojcikiewicz RJ. SPFH2 mediates the endoplasmic reticulum-associated degradation of inositol 1,4,5-trisphosphate receptors and other substrates in mammalian cells. J Biol Chem. 2007;282(28):20104–20115. doi: 10.1074/jbc.M701862200. [DOI] [PubMed] [Google Scholar]
- 39.Colberg-Poley AM, Williamson CD. Intracellular sorting and trafficking of cytomegalovirus proteins during permissive infection. In: Reddehase MJ, editor. Cytomegaloviruses: from molecular pathogenesis to intervention, vol I. 2nd edn. Horizon, Norwich: Caister Academic Press; 2013. pp. 196–229. [Google Scholar]
- 40.Hendershot LM. The ER function BiP is a master regulator of ER function. Mt Sinai J Med. 2004;71(5):289–297. [PubMed] [Google Scholar]
- 41.Hajnoczky G, Booth D, Csordas G, et al. Reliance of ER-mitochondrial calcium signaling on mitochondrial EF-hand Ca2+ binding proteins: Miros, MICUs, LETM1 and solute carriers. Curr Opin Cell Biol. 2014;29:133–141. doi: 10.1016/j.ceb.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hayashi T, Su TP. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell. 2007;131(3):596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 43.De Stefani D, Raffaello A, Teardo E, Szabo I, Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476(7360):336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Csordas G, Golenar T, Seifert EL, et al. MICU1 controls both the threshold and cooperative activation of the mitochondrial Ca(2)(+) uniporter. Cell Metab. 2013;17(6):976–987. doi: 10.1016/j.cmet.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perocchi F, Gohil VM, Girgis HS, et al. MICU1 encodes a mitochondrial EF hand protein required for Ca(2+) uptake. Nature. 2010;467(7313):291–296. doi: 10.1038/nature09358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang D, Zhao L, Clapham DE. Genome-wide RNAi screen identifies Letm1 as a mitochondrial Ca2+/H+ antiporter. Science. 2009;326(5949):144–147. doi: 10.1126/science.1175145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharon-Friling R, Goodhouse J, Colberg-Poley AM, Shenk T. Human cytomegalovirus pUL37x1 induces the release of endoplasmic reticulum calcium stores. Proc Natl Acad Sci USA. 2006;103(50):19117–19122. doi: 10.1073/pnas.0609353103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arruda AP, Pers BM, Parlakgul G, Guney E, Inouye K, Hotamisligil GS. Chronic enrichment of hepatic endoplasmic reticulum–mitochondria contact leads to mitochondrial dysfunction in obesity. Nat Med. 2014;20(12):1427–1435. doi: 10.1038/nm.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chambers JW, Maguire TG, Alwine JC. Glutamine metabolism is essential for human cytomegalovirus infection. J Virol. 2010;84(4):1867–1873. doi: 10.1128/JVI.02123-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Munger J, Bajad SU, Coller HA, Shenk T, Rabinowitz JD. Dynamics of the cellular metabolome during human cytomegalovirus infection. PLoS Pathog. 2006;2(12):e132. doi: 10.1371/journal.ppat.0020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang A, Hildreth RL, Colberg-Poley AM. Human cytomegalovirus inhibits apoptosis by proteasome-mediated degradation of Bax at endoplasmic reticulum-mitochondrion contacts. J Virol. 2013;87(10):5657–5668. doi: 10.1128/JVI.00145-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bionda C, Portoukalian J, Schmitt D, Rodriguez-Lafrasse C, Ardail D. Subcellular compartmentalization of ceramide metabolism: MAM (mitochondria-associated membrane) and/ or mitochondria? Biochem J. 2004;382(Pt 2):527–533. doi: 10.1042/BJ20031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ardail D, Popa I, Bodennec J, Louisot P, Schmitt D, Portoukalian J. The mitochondria-associated endoplasmic-reticulum subcompartment (MAM fraction) of rat liver contains highly active sphingolipid-specific glycosyltransferases. Biochem J. 2003;371(Pt 3):1013–1019. doi: 10.1042/BJ20021834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Osman C, Voelker DR, Langer T. Making heads or tails of phospholipids in mitochondria. J Cell Biol. 2011;192(1):7–16. doi: 10.1083/jcb.201006159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reikine S, Nguyen JB, Modis Y. Pattern recognition and signaling mechanisms of RIG-I and MDA5. Front Immunol. 2014;5:342. doi: 10.3389/fimmu.2014.00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kato H, Takeuchi O, Sato S, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441(7089):101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 57.Hornung V, Ellegast J, Kim S, et al. 5′-Triphosphate RNA is the ligand for RIG-I. Science. 2006;314(5801):994–997. doi: 10.1126/science.1132505. [DOI] [PubMed] [Google Scholar]
- 58.Weber M, Gawanbacht A, Habjan M, et al. Incoming RNA virus nucleocapsids containing a 5′-triphosphorylated genome activate RIG-I and antiviral signaling. Cell Host Microbe. 2013;13(3):336–346. doi: 10.1016/j.chom.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Luo D, Ding SC, Vela A, Kohlway A, Lindenbach BD, Pyle AM. Structural insights into RNA recognition by RIG-I. Cell. 2011;147(2):409–422. doi: 10.1016/j.cell.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zeng W, Sun L, Jiang X, et al. Reconstitution of the RIG-I pathway reveals a signaling role of unanchored polyubiquitin chains in innate immunity. Cell. 2010;141(2):315–330. doi: 10.1016/j.cell.2010.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peisley A, Wu B, Xu H, Chen ZJ, Hur S. Structural basis for ubiquitin-mediated antiviral signal activation by RIG-I. Nature. 2014;509(7498):110–114. doi: 10.1038/nature13140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455(7213):674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhong B, Yang Y, Li S, et al. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity. 2008;29(4):538–550. doi: 10.1016/j.immuni.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 64.Ishikawa H, Barber GN. The STING pathway and regulation of innate immune signaling in response to DNA pathogens. Cell Mol Life Sci. 2011;68(7):1157–1165. doi: 10.1007/s00018-010-0605-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kaarbo M, Ager-Wick E, Osenbroch PO, et al. Human cytomegalovirus infection increases mitochondrial biogenesis. Mitochondrion. 2011;11(6):935–945. doi: 10.1016/j.mito.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 66.Norris KL, Youle RJ. Cytomegalovirus proteins vMIA and m38.5 link mitochondrial morphogenesis to Bcl-2 family proteins. J Virol. 2008;82(13):6232–6243. doi: 10.1128/JVI.02710-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McCormick AL, Smith VL, Chow D, Mocarski ES. Disruption of mitochondrial networks by the human cytomegalovirus UL37 gene product viral mitochondrion-localized inhibitor of apoptosis. J Virol. 2003;77(1):631–641. doi: 10.1128/JVI.77.1.631-641.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roumier T, Szabadkai G, Simoni AM, et al. HIV-1 protease inhibitors and cytomegalovirus vMIA induce mitochondrial fragmentation without triggering apoptosis. Cell Death Differ. 2006;13(2):348–351. doi: 10.1038/sj.cdd.4401750. [DOI] [PubMed] [Google Scholar]
- 69.Hayajneh WA, Colberg-Poley AM, Skaletskaya A, et al. The sequence and antiapoptotic functional domains of the human cytomegalovirus UL37 exon 1 immediate early protein are conserved in multiple primary strains. Virology. 2001;279(1):233–240. doi: 10.1006/viro.2000.0726. [DOI] [PubMed] [Google Scholar]
- 70.Gustafsson MG. Surpassing the lateral resolution limit by a factor of two using structured illumination microscopy. J Microsc. 2000;198(Pt 2):82–87. doi: 10.1046/j.1365-2818.2000.00710.x. [DOI] [PubMed] [Google Scholar]
- 71.Dyba M, Hell SW. Focal spots of size lambda/23 open up far-field fluorescence microscopy at 33 nm axial resolution. Phys Rev Lett. 2002;88(16):163901. doi: 10.1103/PhysRevLett.88.163901. [DOI] [PubMed] [Google Scholar]
- 72.Betzig E, Patterson GH, Sougrat R, et al. Imaging intracellular fluorescent proteins at nanometer resolution. Science. 2006;313(5793):1642–1645. doi: 10.1126/science.1127344. [DOI] [PubMed] [Google Scholar]
- 73.Rust MJ, Bates M, Zhuang X. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM) Nat Methods. 2006;3(10):793–795. doi: 10.1038/nmeth929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Muller B, Heilemann M. Shedding new light on viruses: super-resolution microscopy for studying human immunodeficiency virus. Trends Microbiol. 2013;21(10):522–533. doi: 10.1016/j.tim.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 75.Grove J. Super-resolution microscopy: a virus’ eye view of the cell. Viruses. 2014;6(3):1365–1378. doi: 10.3390/v6031365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chojnacki J, Staudt T, Glass B, et al. Maturation-dependent HIV-1 surface protein redistribution revealed by fluorescence nanoscopy. Science. 2012;338(6106):524–528. doi: 10.1126/science.1226359. [DOI] [PubMed] [Google Scholar]
- 77.Sougrat R, Bartesaghi A, Lifson JD, et al. Electron tomography of the contact between T cells and SIV/HIV-1: implications for viral entry. PLoS Pathog. 2007;3(5):e63. doi: 10.1371/journal.ppat.0030063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang IH, Suomalainen M, Andriasyan V, et al. Tracking viral genomes in host cells at single-molecule resolution. Cell Host Microbe. 2013;14(4):468–480. doi: 10.1016/j.chom.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 79.Pereira CF, Rossy J, Owen DM, Mak J, Gaus K. HIV taken by STORM: super-resolution fluorescence microscopy of a viral infection. Virol J. 2012;9:84. doi: 10.1186/1743-422X-9-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lelek M, Di Nunzio F, Henriques R, Charneau P, Arhel N, Zimmer C. Superresolution imaging of HIV in infected cells with FlAsH-PALM. Proc Natl Acad Sci USA. 2012;109(22):8564–8569. doi: 10.1073/pnas.1013267109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Monaghan P, Green D, Pallister J, et al. Detailed morphological characterisation of Hendra virus infection of different cell types using super-resolution and conventional imaging. Virol J. 2014;11(1):200. doi: 10.1186/s12985-014-0200-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Folling J, Bossi M, Bock H, et al. Fluorescence nanoscopy by ground-state depletion and single-molecule return. Nat Methods. 2008;5(11):943–945. doi: 10.1038/nmeth.1257. [DOI] [PubMed] [Google Scholar]
- 83.Hodges J, Tang X, Landesman MB, et al. Asymmetric packaging of polymerases within vesicular stomatitis virus. Biochem Biophys Res Commun. 2013;440(2):271–276. doi: 10.1016/j.bbrc.2013.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Horsington J, Turnbull L, Whitchurch CB, Newsome TP. Sub-viral imaging of vaccinia virus using super-resolution microscopy. J Virol Methods. 2012;186(1–2):132–136. doi: 10.1016/j.jviromet.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 85.Horsington J, Lynn H, Turnbull L, et al. A36-dependent actin filament nucleation promotes release of vaccinia virus. PLoS Pathog. 2013;9(3):e1003239. doi: 10.1371/journal.ppat.1003239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Felts RL, Narayan K, Estes JD, et al. 3D visualization of HIV transfer at the virological synapse between dendritic cells and T cells. Proc Natl Acad Sci USA. 2010;107(30):13336–13341. doi: 10.1073/pnas.1003040107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Eggert D, Rosch K, Reimer R, Herker E. Visualization and analysis of hepatitis C virus structural proteins at lipid droplets by super-resolution microscopy. PLoS One. 2014;9(7):e102511. doi: 10.1371/journal.pone.0102511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chong MK, Chua AJ, Tan TT, Tan SH, Ng ML. Microscopy techniques in flavivirus research. Micron. 2014;59:33–43. doi: 10.1016/j.micron.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 89.Linnik O, Liesche J, Tilsner J, Oparka KJ. Unraveling the structure of viral replication complexes at super-resolution. Front Plant Sci. 2013;4:6. doi: 10.3389/fpls.2013.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xu H, He X, Zheng H, et al. Structural basis for the prion-like MAVS filaments in antiviral innate immunity. eLife. 2014;3:e01489. doi: 10.7554/eLife.01489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Manzo C, Torreno-Pina JA, Joosten B, et al. The neck region of the C-type lectin DC-SIGN regulates its surface spatiotemporal organization and virus-binding capacity on antigenpresenting cells. J Biol Chem. 2012;287(46):38946–38955. doi: 10.1074/jbc.M112.380121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu P, Wang X, Itano MS, et al. Low copy numbers of DC-SIGN in cell membrane microdomains: implications for structure and function. Traffic. 2014;15(2):179–196. doi: 10.1111/tra.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bhuvanendran S, Salka K, Rainey K, et al. Superresolution imaging of human cytomegalovirus vMIA localization in sub-mitochondrial compartments. Viruses. 2014;6(4):1612–1636. doi: 10.3390/v6041612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wurm CA, Neumann D, Lauterbach MA, et al. Nanoscale distribution of mitochondrial import receptor Tom20 is adjusted to cellular conditions and exhibits an inner-cellular gradient. Proc Natl Acad Sci USA. 2011;108(33):13546–13551. doi: 10.1073/pnas.1107553108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Singh H, Lu R, Rodriguez PF, et al. Visualization and quantification of cardiac mitochondrial protein clusters with STED microscopy. Mitochondrion. 2012;12(2):230–236. doi: 10.1016/j.mito.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Neumann D, Buckers J, Kastrup L, Hell SW, Jakobs S. Two-color STED microscopy reveals different degrees of colocalization between hexokinase-I and the three human VDAC isoforms. PMC Biophys. 2010;3(1):4. doi: 10.1186/1757-5036-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pastorino JG, Hoek JB. Regulation of hexokinase binding to VDAC. J Bioenerg Biomembr. 2008;40(3):171–182. doi: 10.1007/s10863-008-9148-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cogliati S, Frezza C, Soriano ME, et al. Mitochondrial cristae shape determines respiratory chain supercomplexes assembly and respiratory efficiency. Cell. 2013;155(1):160–171. doi: 10.1016/j.cell.2013.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.van der Laan M, Bohnert M, Wiedemann N, Pfanner N. Role of MINOS in mitochondrial membrane architecture and biogenesis. Trends Cell Biol. 2012;22(4):185–192. doi: 10.1016/j.tcb.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 100.Jans DC, Wurm CA, Riedel D, et al. STED superresolution microscopy reveals an array of MINOS clusters along human mitochondria. Proc Natl Acad Sci USA. 2013;110(22):8936–8941. doi: 10.1073/pnas.1301820110. [DOI] [PMC free article] [PubMed] [Google Scholar]