Abstract
Objective
Increased visceral adiposity has been closely linked to insulin resistance, endothelial dysfunction, and cardiometabolic disease in obesity, but pathophysiological mechanisms are poorly understood. We sought to investigate mechanisms of vascular insulin resistance by characterizing depot-specific insulin responses and gain evidence that altered functionality of transcription factor forkhead box protein O-1 (FOXO-1) may play an important role in obesity-related endothelial dysfunction.
Approach and Results
We intra-operatively collected paired subcutaneous and visceral adipose tissue samples from 56 severely obese (BMI 43±7 kg/m2) and 14 non-obese subjects during planned surgical operations, and characterized depot-specific insulin-mediated responses using western blot and quantitative immunofluorescence techniques. Insulin signaling via phosphorylation of FOXO-1 and consequent endothelial nitric oxide synthase (eNOS) stimulation was selectively impaired in the visceral compared to subcutaneous adipose tissue and endothelial cells of obese subjects. In contrast, tissue actions of insulin were preserved in non-obese individuals. Pharmacological antagonism with AS184256 and biological silencing using siRNA-mediated FOXO-1 knockdown reversed insulin resistance and restored eNOS activation in the obese.
Conclusions
We observed profound endothelial insulin resistance in the visceral adipose tissue of obese humans which improved with FOXO-1 inhibition. FOXO-1 modulation may represent a novel therapeutic target to diminish vascular insulin resistance. Additionally, characterization of endothelial insulin resistance in the adipose microenvironment may provide clues to mechanisms of systemic disease in human obesity.
Keywords: Endothelial nitric oxide synthase, FOXO-1, insulin, obesity
Introduction
Obesity and its associated cardiometabolic complications have developed into major health care problems worldwide1–3. Regional adiposity with central accumulation of ectopic visceral fat, in particular, has been closely associated with insulin resistance, endothelial dysfunction, and cardiovascular disease4–10. Although insulin resistance generally implies diminished actions of insulin in mediating glucose uptake, transport, and storage, insulin also exerts important physiological actions upon the vasculature that regulate metabolism and blood flow via activation of endothelial nitric oxide synthase (eNOS) and endothelial nitric oxide (NO) production11, 12. Impaired insulin signaling in the vasculature has been shown to promote vascular inflammation, vasoconstriction, and progression of atherosclerotic plaques12–18. While little is known about mechanisms of vascular insulin resistance, experimental models suggest that transcription factor forkhead box O-1 (FOXO-1) may be a potential key mediator involved in the pathogenic process. Experimental studies show that FOXO-1 downregulates eNOS protein expression19, and conversely endothelial ablation of FOXO-1 blunts atherosclerosis in animal models15. However, the role of FOXO-1 in the human vasculature and its regulation of eNOS bioaction in adipose tissue and obesity-related disease are completely unknown. In this study, we aimed to characterize the function of FOXO-1 in the pathophysiology of endothelial insulin resistance in human obesity, examine adipose depot-specific responses, and differentiate findings in obese and non-obese individuals.
Materials and Methods
Materials and methods are available in the online-only Data Supplement.
Results
Study population
A total of 56 obese (BMI 43 ±7 kg/m2) and 14 non-obese (BMI 25.7±2 kg/m2) subjects were recruited. As displayed in table 1, obese individuals had higher BMI, waist circumference, plasma insulin, HOMA, HbA1c, hs-CRP, and cardiac risk factors.
Table 1.
Clinical characteristic
| Parameter | Obese (n=56) |
Non-obese (n=14) |
p-value |
|---|---|---|---|
| Age (yrs) | 42 ± 12 | 35 ± 10 | 0.051 |
| Female (%) | 86 | 50 | <0.05 |
| BMI (kg/m2) | 43 ± 7 | 25.7 ± 2 | <0.0001 |
| Waist circumference (cm) | 117 ± 12 | 74 ± 8 | <0.0001 |
| Weight (kg) | 120 ± 18 | 74 ± 8 | <0.0001 |
| Insulin (mIU/mL) | 17.5 ± 19 | 7.2 ± 4 | <0.05 |
| Glucose (mg/dL) | 108 ± 77 | 89 ± 13 | 0.37 |
| HbA1C (%) | 6.2 ± 1.8 | 5.5 ± 1 | <0.05 |
| HOMA-IR | 4.6 ± 8.4 | 1.6 ± 1 | <0.0001 |
| hsCRP (mg/dL) | 11.6 ± 8.6 | 1.4 ± 1 | <0.0001 |
| Triglycerides (mg/dL) | 117 ± 78 | 95 ± 44 | 0.09 |
| HDL-C (mg/dL) | 46 ± 11 | 52 ± 16 | 0.23 |
| LDL-C (mg/dL) | 116 ± 26 | 115 ± 33 | 0.81 |
| Systolic BP (mmHg) | 126 ± 14 | 123 ± 13 | 0.40 |
| Diastolic BP (mmHg) | 73 ± 14 | 72 ± 11 | 0.81 |
| Diabetes mellitus (%) | 27 | 0 | <0.05 |
| Hypertension (%) | 39 | 7 | <0.05 |
| Hypercholesterolemia (%) | 20 | 14 | 0.50 |
Data are mean ± SD.
Adipose depot-specific insulin-mediated eNOS activation
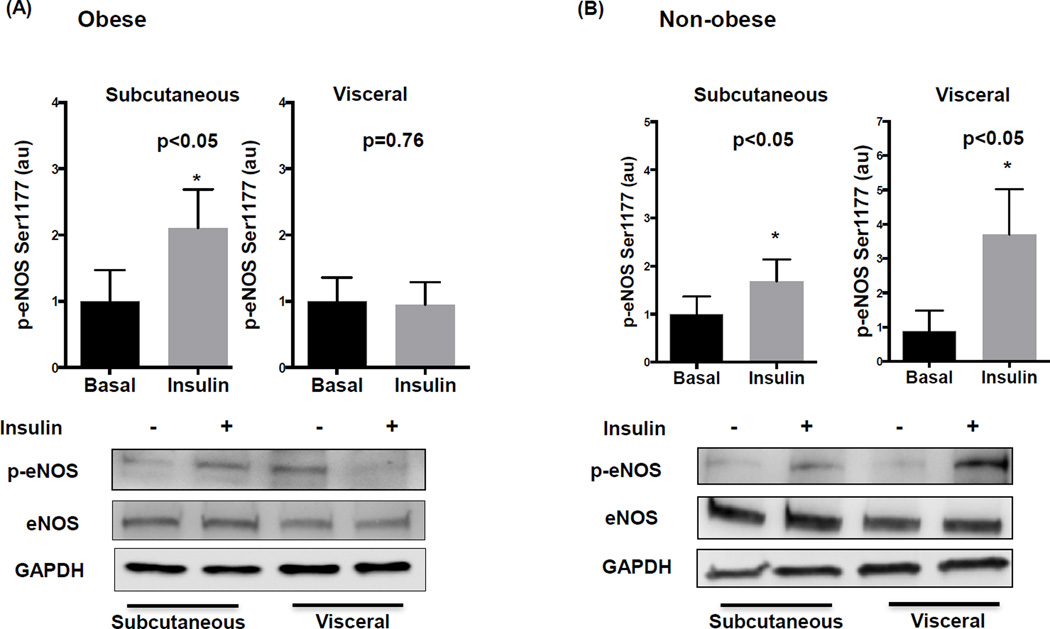
As shown in figure 1A, insulin stimulation significantly increased eNOS phosphorylation at activation site serine 1177 in the subcutaneous fat of obese subjects which was blunted in the visceral depot. In contrast, p-eNOS increased significantly in both visceral and subcutaneous tissue in non-obese individuals (figure 1B), and the same pattern was evident for p-AKT expression (supplemental figure I). No inter-depot difference in total basal eNOS protein expression was observed. Basal phosphorylation of eNOS was significantly higher in the visceral adipose depot from obese compared to non-obese individuals (p<0.05) but we found no difference in their subcutaneous depot (p=0.15). Additionally, insulin stimulation did not promote differential activation of eNOS at alternative site serine 633 which has been shown to be a protein kinase-A (PKA) site20 (supplemental figure II).
Figure 1. Adipose depot comparison of insulin mediated eNOS activation in obese and non-obese subjects.
A) Insulin significantly increased eNOS phosphorylation at serine 1177 in subcutaneous fat (n=8, p<0.05) but had no effect in visceral fat (n=11, p=0.76) in obese subjects. Representative adipose tissue western blots with and without insulin stimulation quantified for phosphorylated eNOS at serine1177, total eNOS, and GAPDH are displayed. B) Insulin significantly induced p-eNOS in both the subcutaneous and visceral depots in non-obese subjects (n=7, p<0.05). Data are presented as arbitrary units (au), indexed to 1 as the basal condition. Data presented as mean ± SEM. GAPDH= control band.
Isolated endothelial cell responses
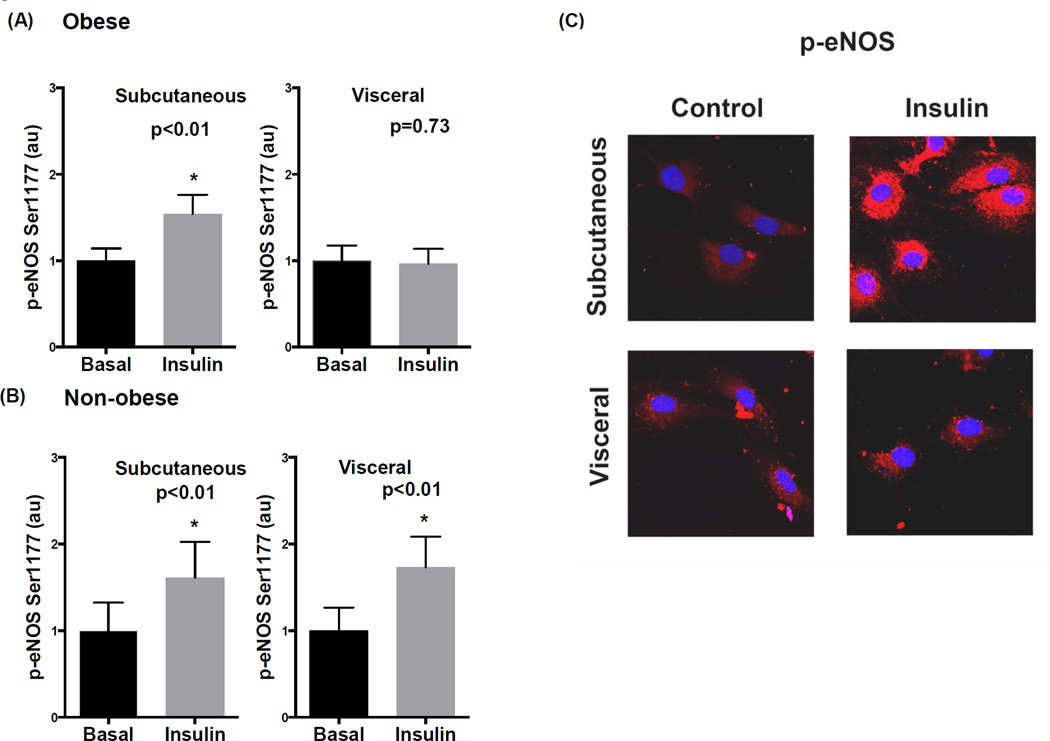
Consistent with whole adipose tissue findings, endothelial cells isolated from fat tissues of obese subjects displayed impaired p-eNOS to insulin in the visceral compared to subcutaneous depot as displayed in figure 2A. This corresponded with reduced nitric oxide production in endothelial cells from the visceral compared to subcutaneous depot (supplemental figure IIIA–B). Conversely, eNOS activation was preserved in both depots in non-obese subjects (figure 2B). Representative quantitative immunofluorescence images of adipose endothelial cells from obese subjects are displayed in figure 2C illustrating cellular insulin resistance in visceral fat. Total eNOS expression was similar in endothelial cells from both depots (supplemental figure IVA).
Figure 2. Quantitative immunofluorescence for p-eNOS in isolated adipose endothelial cells.
A) Insulin significantly increased eNOS phosphorylation at serine 1177 in endothelial cells isolated from subcutaneous fat (n=9, p<0.01) which was blunted in the visceral depot of obese subjects (n=10, p=0.73). B) Insulin significantly induced p-eNOS in both the subcutaneous and visceral depots in non-obese subjects (n=6, p<0.01). C) Representative immunofluorescence images of isolated endothelial cells demonstrating impaired p-eNOS to insulin in visceral fat. Red color = p-eNOS. Blue color = DAPI. Data are presented as arbitrary units (au), indexed to 1 as the basal condition. Data presented as mean ± SEM.
Insulin effects on FOXO-1
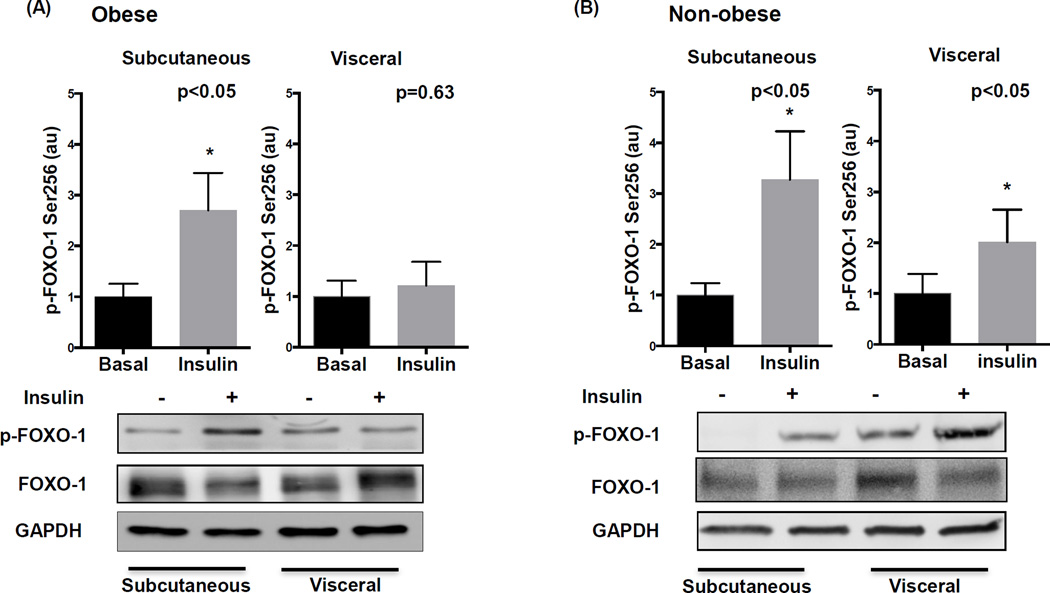
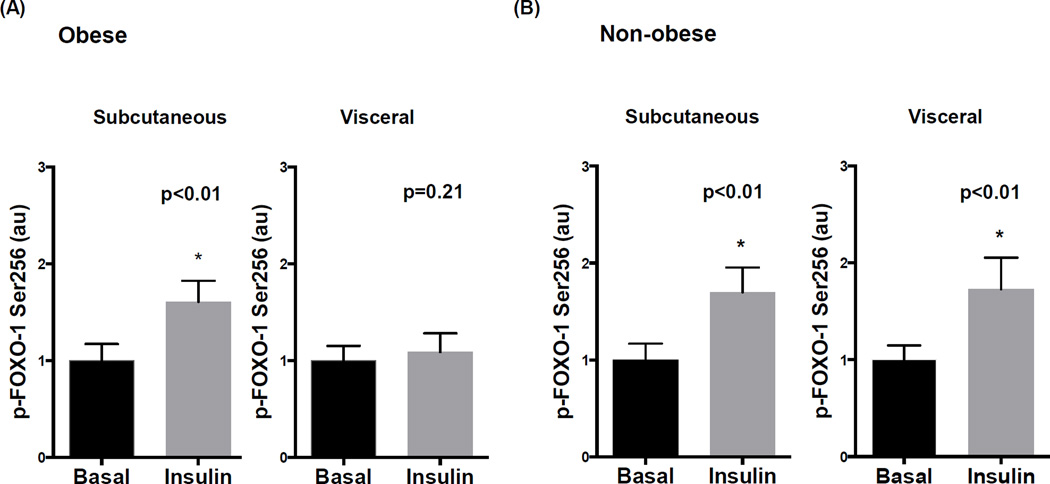
Impairment in phosphorylation and inactivation of FOXO-1 at Serine 256 is implicated as a potential mechanism of insulin resistance in experimental models. In obese subjects, insulin induced FOXO-1 phosphorylation in subcutaneous fat which was blunted in the visceral depot (figure 3A), while total FOXO-1 protein was similar in both regions (supplemental figure IVB). In non-obese subjects, p-FOXO-1 to insulin was preserved in all depots (Figure 3B). Consistent with above data, we observed selective impairment in FOXO-1 signaling in endothelial cells isolated from visceral fat in only the obese (Figures 4A–B).
Figure 3. Adipose depot comparison of insulin mediated FOXO-1 phosphorylation in obese and non-obese subjects.
A) Insulin significantly increased FOXO-1 phosphorylation at serine 256 in subcutaneous fat (n=12, p<0.05) but had no effect in the visceral fat of obese subjects (n=13, p=0.63). Representative adipose tissue western blots with and without insulin stimulation quantified for phosphorylated FOXO-1 at serine 256, total FOXO-1, and GAPDH are displayed. B) Insulin significantly induced p-FOXO-1 in both the subcutaneous and visceral depots in non-obese subjects (n=7, P<0.05). Data are presented as arbitrary units (au), indexed to 1 as the basal condition. Data presented as mean ± SEM. GAPDH= control band.
Figure 4. Quantitative immunofluorescence for p-FOXO-1 in isolated adipose endothelial cells.
A) Insulin significantly increased FOXO-1 phosphorylation at serine 256 in endothelial cells isolated from subcutaneous fat (n=11, p<0.01) which was blunted in the visceral depot of obese subjects (n=11, p=0.21). B) Insulin significantly induced p-FOXO-1 in both the subcutaneous and visceral depots in non-obese subjects (n=6, p<0.01). Data are presented as arbitrary units (au), indexed to 1 as the basal condition. Data presented as mean ± SEM.
Effect of pharmacological and biological FOXO-1 inhibition
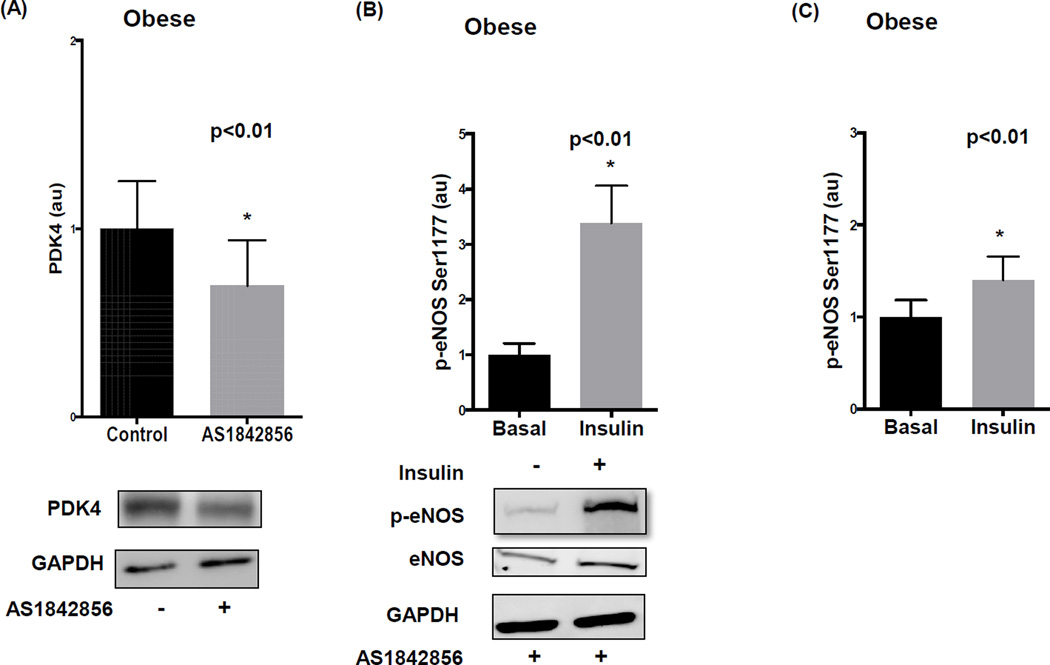
We examined whether FOXO-1 antagonism augments eNOS in visceral fat using specific inhibitor AS1842856. To confirm drug bioaction, we demonstrated diminished PDK4 expression, a known downstream target of FOXO-121, within 24 hours of pharmacological treatment (p<0.01) (figure 5A). We observed upregulation of basal eNOS expression (supplemental figure VA) and markedly improved (>3-fold) insulin-mediated p-eNOS in visceral fat in the presence of AS1842856 as displayed in figure 5B. When we examined the specificity of this effect for endothelial cells we observed significant improvement in insulin sensitivity (Figure 5C) that was also associated with increased basal eNOS protein (supplemental figure VB). Further supporting the functional relevance of the observed changes, AS184256 treatment increased insulin-mediated NO production by endothelial cells from the visceral depot (supplemental figure IIIB) but had no significant effect on subcutaneous endothelial cells where insulin responses were already relatively preserved (supplemental figure IIIA). Moreover, FOXO-1 inhibition had no significant effect on p-eNOS in the subcutaneous adipose depot of the obese (supplemental figure VI).
Figure 5. Pharmacological inhibition of FOXO-1 in the visceral adipose tissue of obese individuals.
A) FOXO-1 inhibitor AS1842856 significantly reduced PDK4 protein expression in visceral fat quantified by western blot analysis (n=7, p<0.01). B) Insulin-mediated activation of eNOS in the visceral adipose tissue of obese subjects significantly increased after 24 hours of AS1842856 exposure (n=7, p<0.01) C) Similarly, insulin-mediated activation of eNOS in isolated endothelial cells significantly increased with AS1842856 treatment quantified by immunofluorescence (n=6, p<0.01). Data are presented as arbitrary units (au), indexed to 1 as the basal condition. Data presented as mean ± SEM. GAPDH= control band.
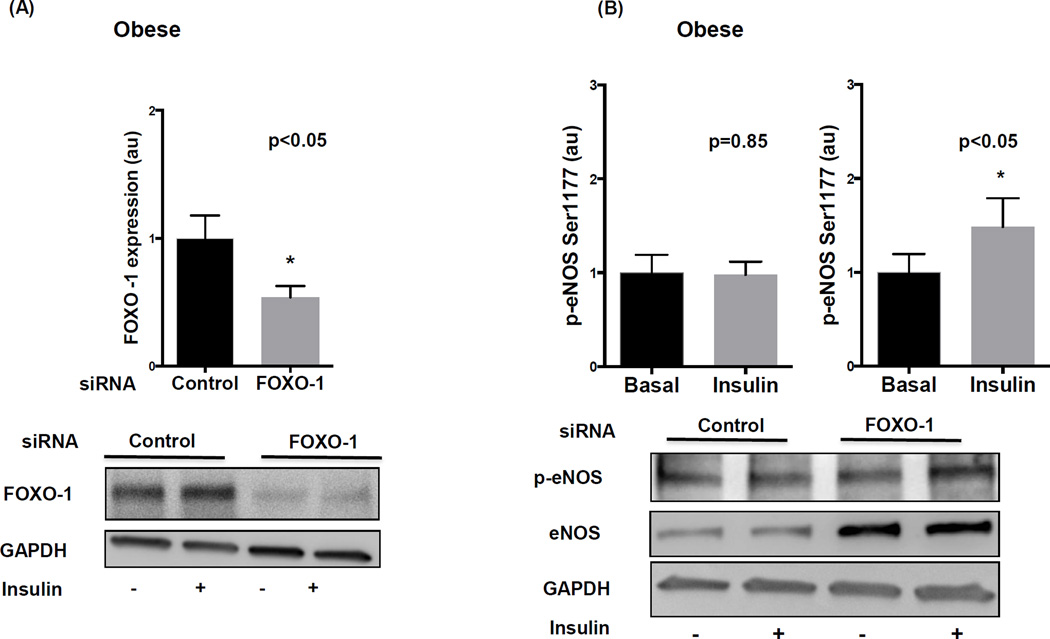
To exclude non-specific off-target drug effects, we utilized complementary siRNA methods to selectively diminish FOXO-1 activity in visceral fat which produced the desired decline in FOXO-1 compared to control as shown in figure 6A. This was associated with significant increase in basal eNOS (supplemental figure VC) and insulin-induced p-eNOS at Ser 1177 (figure 6B), in similar magnitude to that observed with drug treatment. Based on our cumulative findings, a proposed schematic summary diagram of impaired insulin signaling in visceral obesity is displayed in figure 7. Lastly, we observed a significant positive correlation between p-eNOS expression in visceral adipose endothelial cells and brachial arterial endothelium-dependent flow-mediated vasodilation prompting speculation for a pathophysiological adipose-systemic connection (supplemental figure VII).
Figure 6. siRNA mediated knockdown of FOXO-1 in the visceral adipose depot of obese individuals.
A) FOXO-1 siRNA significantly reduced FOXO-1 protein expression in the visceral fat of obese individuals compared to control siRNA (n=7, p<0.05). B) siRNA mediated knockdown of FOXO-1 restored activation of eNOS in response to insulin treatment (n=9, p<0.05) compared to control siRNA (n=9, p=0.85). Data represent western blot analyses quantified as arbitrary units (au), indexed to 1 as the basal condition. Data presented as mean ± SEM. GAPDH= control band.
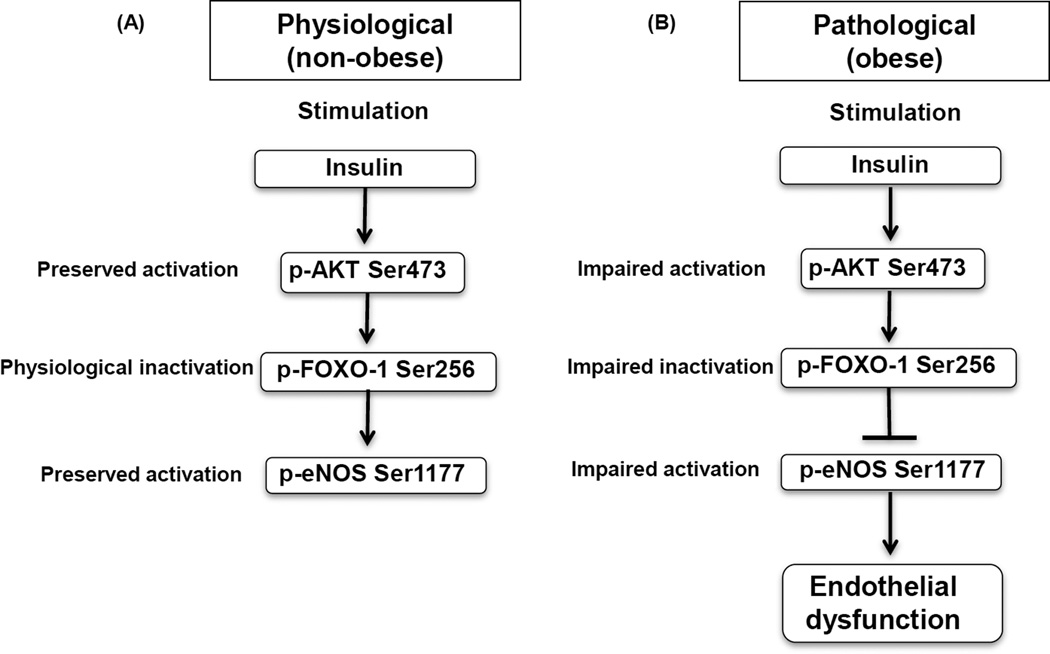
Figure 7. Proposed diagram of impaired insulin signaling in visceral obesity.
A) Under physiological conditions, insulin stimulates AKT activation which in turn inactivates FOXO-1 via phosphorylation, lifting an inhibitory effect of FOXO-1 on eNOS B) In pathological conditions, impaired insulin signaling and blunted eNOS activation promote endothelial dysfunction.
Discussion
We describe, for the first time, evidence of profound endothelial insulin resistance in the visceral adipose tissue of obese humans. We demonstrate diminished agonist-mediated activation of endothelial NOS, representing a key early pathogenic step in the development of endothelial dysfunction and atherogenesis, which was associated with impaired FOXO-1 signaling. Both targeted pharmacological inhibition and biological siRNA-mediated knockdown of FOXO-1 restored insulin sensitivity in human visceral adipose tissue. Our findings suggest that FOXO-1 modulation may represent a novel mechanism and potential therapeutic target to diminish vascular insulin resistance in obesity.
In addition to a wide range of metabolic actions, insulin modulates vascular function in part via stimulation of vascular nitric oxide production that controls vasodilation and arterial pressure14. Insulin regulates blood flow through activation of eNOS in vascular cells by binding to the IRS-1 receptor with subsequent Akt-mediated phosphorylation and activation of eNOS at Ser1177. Obese individuals with insulin resistance exhibit endothelial dysfunction22, and functional impairment in this pathway at the level of the endothelium is implicated in mechanisms of cardiovascular disease23. In animals, endothelium-specific deletion of the insulin receptor impairs eNOS bioavailability, promotes atherogenesis, and links to whole-body insulin resistance, hypertension, and ischemia24, 25,26–28. Recent work from our laboratory demonstrated impaired insulin-stimulated eNOS phosphorylation, inflammation, and vasodilator dysfunction of endothelial cells isolated from the vascular wall of obese diabetics29. Compelling evidence is thus mounting that support a mechanistic link between vascular insulin resistance and arterial disease, and suggests that improving vascular insulin sensitivity may represent a therapeutic target.
Clinical data consistently link degree of visceral adiposity burden to cardiometabolic risk, presumably owing to forced ectopic expansion and maladaptive remodeling of visceral fat, which leads to dysregulated release of pro-atherogenic adipocytokines that are implicated in the pathogenesis of systemic disease31. In contrast, clinical ramifications of subcutaneous expansion have been mixed with some reports even suggesting it as a favorable metabolic sink32, 33. Published data support the widely accepted notion that the visceral adipose tissue microenvironment, compared to subcutaneous fat, exhibits pro-inflammatory, pro-oxidant, and anti-angiogenic properties associated with severe endothelial dysfunction34–36. Our novel finding of preserved insulin sensitivity in the visceral fat of non-obese subjects was remarkable, and suggests that a pathogenic phenotype is not necessarily inherent to all visceral fat per se, but develops as a consequence of obesity. While very few clinical studies have been able to specifically examine tissue pathophysiology given the difficult nature of obtaining invasive intra-abdominal biopsies from healthy lean subjects, at least one study reported normal microvasculature in the visceral fat of lean individuals which exhibited dysfunction only in obese conditions as in our findings37.
Mechanisms underlying these associations remain relatively ill-defined from a molecular perspective. In this study, we focused on FOXO-1 because no prior study has examined its role in the pathogenesis of insulin resistance in vascular cells of obese humans. In experimental models, FOXO-1 has been shown to regulate eNOS by reducing its mRNA and protein expression via transcriptional repression15, 19, 38. Mimicking a hyperglycemic insulin-resistant state via exposure of cultured cells to high glucose activates FOXO-1 and blunts eNOS39. Animal models also suggest that endothelium-specific deletion of FOXO-1 delays progression of atherosclerosis and its knockdown improves eNOS bioavailability15. In our present study, we newly demonstrate that FOXO-1 expressed in human adipose tissue plays a significant role in mediating endothelial insulin resistance. To gain evidence for potential therapeutic modulation, we demonstrated that FOXO-1 inhibition using pharmacological antagonism improved eNOS protein expression and insulin-mediated phosphorylation at serine 1177, the commonly reported major index of eNOS activation, suggesting effects at both pre and post-translational levels, while no effect was seen on serine 633 phosphorylation20, 30. We affirmed our findings with complementary methodology using selective siRNA-mediated FOXO-1 knockdown which confirmed negative regulation of eNOS by FOXO-1. Moreover, increased p-eNOS following FOXO-1 antagonism was associated with functional rise in nitric oxide production by vascular endothelial cells. We observed hyper-phosphorylation of eNOS under basal conditions in the visceral depot of obese subjects which has been described previously under disease conditions including obesity and likely represents a compensatory mechanism29. We acknowledge that regulation of FOXO-1 on p-eNOS responses is likely complex and additional studies are required to further probe these regulatory pathways relevant to disease mechanisms in humans. Collectively, our findings suggest that dysfunctional FOXO-1 signaling plays a role in insulin resistant states and its antagonism may have a beneficial effect on vascular biology, although no approved drug is yet currently available for clinical investigation.
While our findings may be largely specific to visceral adipose tissue, we observed an interestingly significant correlation between endothelial phenotype in visceral fat and systemic arterial endothelium-dependent vasodilation, which in turn has been shown to correlate with coronary responses and predict future cardiovascular events40. We have also previously shown agonist-induced p-eNOS in adipose endothelial cells to be a reliable functional and quantifiable cellular readout linked to vasomotor properties of human arterioles41. As such, pathophysiological mechanisms learned from the adipose microenvironment may provide valuable translational clues to systemic disease, as responses in the adipose vasculature have been shown to correlate with cardiac risk factors and systemic arterial function42.
We emphasize the clinical relevance of elucidating molecular mechanisms of vascular insulin resistance in obesity particularly in relation to weight loss strategies to improve cardiovascular outcomes. Sustained weight loss with medical/dietary intervention is difficult and to date bariatric surgery stands alone as the sole durable weight reduction treatment shown to decrease long-term (> 10-year) cardiovascular mortality43. While specific mechanisms of risk reduction are unknown, improved survival has been linked primarily to plasma insulin as the key biomarker of clinical response43. We have similarly recently shown that improved endothelial function with weight loss was directly tied to insulin sensitivity44. We thus affirm that preservation of insulin signaling may be a key homeostatic mechanism of blood vessels that develops perturbations in obesity, and elucidating disease mechanisms whose origins may lie within the visceral milieu may be highly clinically significant.
There are several limitations to our study. The experimental design was limited to a surgical population undergoing planned operations and bariatric subjects were severely obese (class III), thus findings may not be applicable to the general population or lesser degrees of obesity. However, this was counterbalanced by our ability to directly study properties of human visceral adipose tissue which would otherwise be impossible. While we performed experiments on specimens immediately following surgical biopsy, technical applications may not exactly recapitulate the in vivo physiological environment. We examined inter-depot responses but did not specifically compare BMI categories. Most participants in the study were women which reflects the general clinical practice nationally and sex differences in populations that seek weight loss treatments44, 45. We focused on serine 1177 phosphorylation site as the primary signal for eNOS stimulation however alternative activation sites may have additional roles. Lastly, the extent to which local insulin resistance in fat contributes to vascular dysfunction and cardiovascular disease systemically in obese states remains unclear.
In conclusion, we demonstrate the presence of endothelial insulin resistance in the visceral fat of obese subjects which was reversible with FOXO-1 antagonism. FOXO-1 modulation may represent a novel therapeutic target to diminish vascular insulin resistance. With clinical data consistently linking visceral adiposity burden to cardiovascular risk, characterization of cellular derangements in the adipose microenvironment may provide clues to mechanisms of systemic disease.
Supplementary Material
Significance.
Obesity is a mounting healthcare problem and is associated with cardiometabolic complications. In particular, regional accumulation of visceral fat has been associated with endothelial dysfunction, insulin resistance and cardiovascular dysfunction. In this study we used a novel approach to understand endothelial insulin resistance in human obesity comparing subcutaneous and visceral fat and isolated endothelial cells from the same obese individuals as well as comparing visceral fat and endothelial cells from obese to non-obese subjects. We observe presence of endothelial insulin resistance in visceral fat and endothelial cells of obese subjects which was reversed with FOXO-1 antagonism. FOXO-1 modulation may represent a novel therapeutic target to diminish vascular insulin resistance.
Acknowledgements
None.
Sources of funding
Dr. Gokce is supported by National Institutes of Health (NIH) grants HL081587, HL114675, and HL126141. Dr. Karki is supported by NIH grant T32 HL07224.
Abbreviations and Acronyms
- AKT
protein kinase B
- BMI
body mass index
- eNOS
endothelial nitric oxide synthase
- FOXO-1
forkhead box O-1
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HbA1C
hemoglobin A1C
- HOMA-IR
homeostasis model assessment of insulin resistance
- hs-CRP
high-sensitivity C-reactive protein
- PDK4
pyruvate dehydrogenase kinase-4
- siRNA
small interfering ribonucleic acid
Footnotes
Disclosures
None.
References
- 1.Flegal KMCM, Kit BK, Ogden CL. prevalence of obesity and trends in the distribution of body mass index among US adults. Journal of the American Medical Association. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 2.Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. 2011;378:815–825. doi: 10.1016/S0140-6736(11)60814-3. [DOI] [PubMed] [Google Scholar]
- 3.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, Hu FB, Hubbard VS, Jakicic JM, Kushner RF, Loria CM, Millen BE, Nonas CA, Pi-Sunyer FX, Stevens J, Stevens VJ, Wadden TA, Wolfe BM, Yanovski SZ American College of Cardiology/American Heart Association Task Force on Practice G and Obesity S. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63:2985–3023. doi: 10.1016/j.jacc.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 4.de Jongh RT, Serne EH, RG IJ, de Vries G, Stehouwer CD. Impaired microvascular function in obesity: implications for obesity-associated microangiopathy, hypertension, and insulin resistance. Circulation. 2004;109:2529–2535. doi: 10.1161/01.CIR.0000129772.26647.6F. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura K, Fuster JJ, Walsh K. Adipokines: a link between obesity and cardiovascular disease. J Cardiol. 2014;63:250–259. doi: 10.1016/j.jjcc.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coutinho T, Goel K, de Sa DC, Kragelund C, Kanaya AM, Zeller M, Park JS, Kober L, Torp-Pedersen C, Cottin Y, Lorgis L, Lee SH, Kim YJ, Thomas R, Roger VL, Somers VK, Lopez-Jimenez F. Central Obesity and Survival in Subjects With Coronary Artery Disease A Systematic Review of the Literature and Collaborative Analysis With Individual Subject Data. J Am Coll Cardiol. 2011;57:1877–1886. doi: 10.1016/j.jacc.2010.11.058. [DOI] [PubMed] [Google Scholar]
- 7.Britton KA, Fox CS. Ectopic fat depots and cardiovascular disease. Circulation. 2011;124:e837–e841. doi: 10.1161/CIRCULATIONAHA.111.077602. [DOI] [PubMed] [Google Scholar]
- 8.Despres JP. Body fat distribution and risk of cardiovascular disease: an update. Circulation. 2012;126:1301–1313. doi: 10.1161/CIRCULATIONAHA.111.067264. [DOI] [PubMed] [Google Scholar]
- 9.Neeland IJ, Turer AT, Ayers CR, Powell-Wiley TM, Vega GL, Farzaneh-Far R, Grundy SM, Khera A, McGuire DK, de Lemos JA. Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. JAMA. 2012;308:1150–1159. doi: 10.1001/2012.jama.11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah RV, Murthy VL, Abbasi SA, Blankstein R, Kwong RY, Goldfine AB, Jerosch-Herold M, Lima JA, Ding J, Allison MA. Visceral Adiposity and the Risk of Metabolic Syndrome Across Body Mass Index: The MESA Study. JACC Cardiovasc Imaging. 2014;7:1221–1235. doi: 10.1016/j.jcmg.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muniyappa R, Montagnani M, Koh KK, Quon MJ. Cardiovascular actions of insulin. Endocr Rev. 2007;28:463–491. doi: 10.1210/er.2007-0006. [DOI] [PubMed] [Google Scholar]
- 12.Kearney MT, Duncan ER, Kahn M, Wheatcroft SB. Insulin resistance and endothelial cell dysfunction: studies in mammalian models. Exp Physiol. 2008;93:158–163. doi: 10.1113/expphysiol.2007.039172. [DOI] [PubMed] [Google Scholar]
- 13.Qiang L, Tsuchiya K, Kim-Muller JY, Lin HV, Welch C, Accili D. Increased atherosclerosis and endothelial dysfunction in mice bearing constitutively deacetylated alleles of Foxo1 gene. J Biol Chem. 2012;287:13944–13951. doi: 10.1074/jbc.M111.332767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hornstra JM, Serne EH, Eringa EC, Wijnker MC, de Boer MP, Yudkin JS, Smulders YM. Insulin's microvascular vasodilatory effects are inversely related to peripheral vascular resistance in overweight, but insulin-sensitive subjects. Obesity. 2013;21:2557–2561. doi: 10.1002/oby.20406. [DOI] [PubMed] [Google Scholar]
- 15.Tsuchiya K, Tanaka J, Shuiqing Y, Welch CL, DePinho RA, Tabas I, Tall AR, Goldberg IJ, Accili D. FoxOs integrate pleiotropic actions of insulin in vascular endothelium to protect mice from atherosclerosis. Cell metabolism. 2012;15:372–381. doi: 10.1016/j.cmet.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gregor MF, Hotamisligil GS. Inflammatory Mechanisms in Obesity. Annual Review of Immunology, Vol 29. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 17.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–880. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 18.Alvehus M, Buren J, Sjostrom M, Goedecke J, Olsson T. The human visceral fat depot has a unique inflammatory profile. Obesity. 2010;18:879–883. doi: 10.1038/oby.2010.22. [DOI] [PubMed] [Google Scholar]
- 19.Potente M, Urbich C, Sasaki K, Hofmann WK, Heeschen C, Aicher A, Kollipara R, DePinho RA, Zeiher AM, Dimmeler S. Involvement of Foxo transcription factors in angiogenesis and postnatal neovascularization. J Clin Invest. 2005;115:2382–2392. doi: 10.1172/JCI23126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boo YC, Hwang J, Sykes M, Michell BJ, Kemp BE, Lum H, Jo H. Shear stress stimulates phosphorylation of eNOS at SeR635) by a protein kinase A-dependent mechanism. Am J Physiol-Heart C. 2002;283:H1819–H1828. doi: 10.1152/ajpheart.00214.2002. [DOI] [PubMed] [Google Scholar]
- 21.Piao L, Sidhu VK, Fang YH, Ryan JJ, Parikh KS, Hong Z, Toth PT, Morrow E, Kutty S, Lopaschuk GD, Archer SL. FOXO1-mediated upregulation of pyruvate dehydrogenase kinase-4 (PDK4) decreases glucose oxidation and impairs right ventricular function in pulmonary hypertension: therapeutic benefits of dichloroacetate. J Mol Med (Berl) 2013;91:333–346. doi: 10.1007/s00109-012-0982-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arcaro G, Cretti A, Balzano S, Lechi A, Muggeo M, Bonora E, Bonadonna RC. Insulin causes endothelial dysfunction in humans: sites and mechanisms. Circulation. 2002;105:576–582. doi: 10.1161/hc0502.103333. [DOI] [PubMed] [Google Scholar]
- 23.Mather KJ, Steinberg HO, Baron AD. Insulin Resistance in the Vasculature. J Clin Invest. 2014;123:2. doi: 10.1172/JCI67166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duncan ER, Crossey PA, Walker S, Anilkumar N, Poston L, Douglas G, Ezzat VA, Wheatcroft SB, Shah AM, Kearney MT. Effect of endothelium-specific insulin resistance on endothelial function in vivo. Diabetes. 2008;57:3307–3314. doi: 10.2337/db07-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vicent D, Ilany J, Kondo T, Naruse K, Fisher SJ, Kisanuki YY, Bursell S, Yanagisawa M, King GL, Kahn CR. The role of endothelial insulin signaling in the regulation of vascular tone and insulin resistance. J Clin Invest. 2003;111:1373–1380. doi: 10.1172/JCI15211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atochin DN, Wang A, Liu VW, Critchlow JD, Dantas AP, Looft-Wilson R, Murata T, Salomone S, Shin HK, Ayata C, Moskowitz MA, Michel T, Sessa WC, Huang PL. The phosphorylation state of eNOS modulates vascular reactivity and outcome of cerebral ischemia in vivo. J Clin Invest. 2007;117:1961–1967. doi: 10.1172/JCI29877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kashiwagi S, Atochin DN, Li Q, Schleicher M, Pong T, Sessa WC, Huang PL. eNOS phosphorylation on serine 1176 affects insulin sensitivity and adiposity. Biochem Biophys Res Commun. 2013;431:284–290. doi: 10.1016/j.bbrc.2012.12.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rask-Madsen C, King GL. Mechanisms of Disease: endothelial dysfunction in insulin resistance and diabetes. Nat Clin Pract Endocrinol Metab. 2007;3:46–56. doi: 10.1038/ncpendmet0366. [DOI] [PubMed] [Google Scholar]
- 29.Tabit CE, Shenouda SM, Holbrook M, Fetterman JL, Kiani S, Frame AA, Kluge MA, Held A, Dohadwala MM, Gokce N, Farb MG, Rosenzweig J, Ruderman N, Vita JA, Hamburg NM. Protein kinase C-beta contributes to impaired endothelial insulin signaling in humans with diabetes mellitus. Circulation. 2013;127:86–95. doi: 10.1161/CIRCULATIONAHA.112.127514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ritchie SA, Kohlhaas CF, Boyd AR, Yalla KC, Walsh K, Connell JM, Salt IP. Insulin-stimulated phosphorylation of endothelial nitric oxide synthase at serine-615 contributes to nitric oxide synthesis. Biochem J. 2010;426:85–90. doi: 10.1042/BJ20091580. [DOI] [PubMed] [Google Scholar]
- 31.Tchernof A, Despres JP. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013;93:359–404. doi: 10.1152/physrev.00033.2011. [DOI] [PubMed] [Google Scholar]
- 32.Porter SA, Massaro JM, Hoffmann U, Vasan RS, O'Donnel CJ, Fox CS. Abdominal subcutaneous adipose tissue: a protective fat depot? Diabetes Care. 2009;32:1068–1075. doi: 10.2337/dc08-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McLaughlin T, Lamendola C, Liu A, Abbasi F. Preferential fat deposition in subcutaneous versus visceral depots is associated with insulin sensitivity. J Clin Endocrinol Metab. 2011;96:E1756–E1760. doi: 10.1210/jc.2011-0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ngo DT, Farb MG, Kikuchi R, Karki S, Tiwari S, Bigornia SJ, Bates DO, LaValley MP, Hamburg NM, Vita JA, Hess DT, Walsh K, Gokce N. Antiangiogenic actions of vascular endothelial growth factor-A165b, an inhibitory isoform of vascular endothelial growth factor-A, in human obesity. Circulation. 2014;130:1072–1080. doi: 10.1161/CIRCULATIONAHA.113.008171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bigornia SJ, Farb MG, Mott MM, Hess DT, Carmine B, Fiscale A, Joseph L, Apovian CM, Gokce N. Relation of depot-specific adipose inflammation to insulin resistance in human obesity. Nutr Diabetes. 2012;2:e30. doi: 10.1038/nutd.2012.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu XJ, Gauthier MS, Hess DT, Apovian CM, Cacicedo JM, Gokce N, Farb M, Valentine RJ, Ruderman NB. Insulin sensitive and resistant obesity in humans: AMPK activity, oxidative stress, and depot-specific changes in gene expression in adipose tissue. J Lipid Res. 2012;53:792–801. doi: 10.1194/jlr.P022905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Virdis A, Santini F, Colucci R, Duranti E, Salvetti G, Rugani I, Segnani C, Anselmino M, Bernardini N, Blandizzi C, Salvetti A, Pinchera A, Taddei S. Vascular generation of tumor necrosis factor-alpha reduces nitric oxide availability in small arteries from visceral fat of obese patients. J Am Coll Cardiol. 2011;58:238–247. doi: 10.1016/j.jacc.2011.01.050. [DOI] [PubMed] [Google Scholar]
- 38.Dharaneeswaran H, Abid MR, Yuan L, Dupuis D, Beeler DL, Spokes K, Janes L, Sciuto T, Kang PM, Jaminet SC, Dvorak A, Grant MA, Regan E, Aird WC. FoxO1-Mediated Activation of Akt Plays a Critical Role in Vascular Homeostasis. Circ Res. 2014 doi: 10.1161/CIRCRESAHA.115.303227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanaka J, Qiang L, Banks AS, Welch CL, Matsumoto M, Kitamura T, Ido-Kitamura Y, DePinho RA, Accili D. Foxo1 links hyperglycemia to LDL oxidation and endothelial nitric oxide synthase dysfunction in vascular endothelial cells. Diabetes. 2009;58:2344–2354. doi: 10.2337/db09-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gokce N. Clinical assessment of endothelial function: ready for prime time? Circ Cardiovasc Imaging. 2011;4:348–350. doi: 10.1161/CIRCIMAGING.111.966218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farb MG, Tiwari S, Karki S, Ngo DT, Carmine B, Hess DT, Zuriaga MA, Walsh K, Fetterman JL, Hamburg NM, Vita JA, Apovian CM, Gokce N. Cyclooxygenase inhibition improves endothelial vasomotor dysfunction of visceral adipose arterioles in human obesity. Obesity. 2013 doi: 10.1002/oby.20505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dharmashankar K, Welsh A, Wang J, Kizhakekuttu TJ, Ying R, Gutterman DD, Widlansky ME. Nitric oxide synthase-dependent vasodilation of human subcutaneous arterioles correlates with noninvasive measurements of endothelial function. Am J Hypertens. 2012;25:528–534. doi: 10.1038/ajh.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sjostrom L, Peltonen M, Jacobson P, Sjostrom CD, Karason K, Wedel H, Ahlin S, Anveden A, Bengtsson C, Bergmark G, Bouchard C, Carlsson B, Dahlgren S, Karlsson J, Lindroos AK, Lonroth H, Narbro K, Naslund I, Olbers T, Svensson PA, Carlsson LM. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307:56–65. doi: 10.1001/jama.2011.1914. [DOI] [PubMed] [Google Scholar]
- 44.Bigornia SJ, Farb MG, Tiwari S, Karki S, Hamburg NM, Vita JA, Hess DT, Lavalley MP, Apovian CM, Gokce N. Insulin status and vascular responses to weight loss in obesity. J Am Coll Cardiol. 2013 doi: 10.1016/j.jacc.2013.07.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Longitudinal Assessment of Bariatric Surgery C. Flum DR, Belle SH, King WC, Wahed AS, Berk P, Chapman W, Pories W, Courcoulas A, McCloskey C, Mitchell J, Patterson E, Pomp A, Staten MA, Yanovski SZ, Thirlby R, Wolfe B. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med. 2009;361:445–454. doi: 10.1056/NEJMoa0901836. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.