Abstract
Cell-mediated gene therapy may treat bone fragility disorders. Dermal fibroblasts (DFb) may be an alternative cell source to stem cells for orthopedic gene therapy because of their rapid cell yield and excellent plasticity with bone morphogenetic protein-2 (BMP2) gene transduction. Autologous DFb or BMP2-expressing autologous DFb were administered in twelve rabbits by two delivery routes; a transcortical intra-medullar infusion into tibiae and delayed intra-osseous injection into femoral drill defects. Both delivery methods of DFb-BMP2 resulted in a successful cell engraftment, increased bone volume, bone mineral density, improved trabecular bone microarchitecture, greater bone defect filling, external callus formation, and trabecular surface area, compared to non-transduced DFb or no cells. Cell engraftment within trabecular bone and bone marrow tissue was most efficiently achieved by intra-osseous injection of DFb-BMP2. Our results suggested that BMP2-expressing autologous DFb have enhanced efficiency of engraftment in target bones resulting in a measurable biologic response by the bone of improved bone mineral density and bone microarchitecture. These results support that autologous implantation of DFb-BMP2 warrants further study on animal models of bone fragility disorders such as osteogenesis imperfecta and osteoporosis to potentially enhance bone quality, particularly along with other gene modification of these diseases.
Keywords: Autologous implantation, Dermal fibroblasts, Bone morphogenetic protein-2, Intra-osseous cell delivery, Intra-medullar cell delivery
INTRODUCTION
Bone fragility disorders are challenging orthopedic conditions, such as osteogenesis imperfecta (OI) and osteoporosis, resulting in severe disability with frequent painful fractures.1,2 The bone fragility in OI is most often the result of a mutation in one of two genes that encode the alpha-chains of type I collagen, COL1A1 and COL1A2, leading to abnormal bone formation and turnover.1 Osteoporosis is a condition characterized by imbalanced bone formation and resorption resulting in decreased bone density and strength.2 Systemic application of osteoclast inhibitors have been a primary treatment option for bone fragility disorders with modest therapeutic responses, but may be associated with respiratory distress and abnormal bone development.3,4 Also, osteoclast inhibitors are known to cross the placenta and may pose risk to a developing fetus.5
Cell therapy has potential for the treatment of bone fragility disorders.6 Correction of disease-causing COL1A1 and COL1A2 gene mutations has been achieved in mesenchymal stem cells (MSC) isolated from OI patients to synthesize normal type-I collagen.7,8 In addition, over-expression of the normal COL1A1 gene in MSCs from OI patients could partially rescue the dominant-negative mutations.9 Therefore, such genetically-renovated cells can be locally implanted back to the patients’ long bones to restore normal bone matrix and normalize tissue function if engraftment can be achieved and sustained.10 On the other hand, local application of autologous or allogenic MSC alone has shown some improvement in bone formation, trabecular thickness, osseous microstructure, and bone strength in the mouse and rabbit models of osteoporosis.11,12 Engineering of MSC with BMP2 may produce a more robust effect than MSC alone in osteoporosis.
Despite the extensive research on use of stem cells for cell therapy, other cell sources, such as dermal fibroblasts (DFb) may be an alternative candidate due to their excellent plasticity and reprogramming capabilities to iPSCs.14 There are many advantages of using DFb for cell therapy, in contrast to stem cells, including the relatively less painful harvest technique, low risk of donor site infection or morbidity, less fastidious culture procedure, as well as rapid and high cell yield.15–17 DFb have shown to have a differentiation ability into bone-forming cells by the transduction of osteogenic genes such as bone morphogenetic protein-2 (BMP2),15–19 and such genetically-modified DFbs have been autologously transplanted to achieve sufficient cell engraftment, promote bone formation, and increase mineral density in rodent15,17 and equine models.18,19
Successful cell therapy application for bone fragility disorders may be dependent on both optimal cell engraftment in target bones and secretion of osteogenic growth factors from the engrafted cells affecting host progenitors.10 An initial work demonstrated that MSC infused into mouse femurs were sufficiently engrafted and contributed to bone formation.20 In a large animal model, delayed DFb injection into granulation tissue at a bone injury site has shown to be an effective cell delivery strategy for BMP2-transduced DFb with much lower engraftment of non-transduced DFb cells.18,19 Also, the previous work showed that the secreted BMP2 from the genetically-modified cells may be a primary contributor on the efficacy of cell-mediated BMP2 therapy to affect host progenitor cells and induce bone formation.19
The objectives of this study were to prove efficacy of autologous implantation of BMP2-expressing DFb by intra-osseous or intra-medullar delivery to improve the cell engraftment rate, bone mineral density, and microarchitecture. We hypothesized that BMP2-expressing DFb would be successfully engrafted in target bone in order to improve bone mineral density and microarchitecture by both intra-osseous and intra-medullar cell delivery methods, and that BMP2-expressing DFb would have superior engraftment than non-transduced DFb.
MATERIALS AND METHODS
Experimental design
All procedure were approved by the Institutional Animal Care and Use Committee of The Ohio State University. By using 12 rabbits, intra-osseous injection and intra-medullar infusion of BMP2-expressing autologous DFb were compared to non-transduced DFb (Fig 1A, 1B, 1C). Cell labeling and comparable osteogenic differentiation of MSC and DFb were validated in vitro prior to in vivo studies. At 56 days after the DFb/DFb-BMP2 administration, cell engraftment was assessed by PCR and immunohistochemical staining, as well as the bone density and structure assessed by μCT and histomorphometry.
Figure 1.
Experimental models (A), protocol (B), and assignment table (C) of the in vivo studies. Twelve rabbits received intra-medullar infusion and intra-osseous infusion of autologous dermal fibroblasts (DFb) transduced by FV vector carrying green florescent protein (GFP) genes, subsequently non-transduced or transduced by Adenoviral (Ad) vectors encoding morphogenetic protein-2 (BMP2) genes, as illustrated. *One of 12 rabbits (DFb-BMP2 injection/infusion in one hindlimb and saline injection/infusion in the contralateral limb) died at day 2 with unknown reason and was not included in any outcome assessments.
In vitro osteogenic differentiation and cell labeling of DFb
Recombinant foamy viral (FV) vectors with green fluorescent protein (GFP) gene (FV-GFP) were produced by calcium phosphate transfection of 293T cells with four plasmids (i.e., pΔϕFPF, pCiGSΔPsi, pCiPS, and pCiES), and purified by filtration and ultracentrifugation, as described before.21 The infectious titer of FV vectors was determined by transducing HT1080 cells with flow cytometry.21 Recombinant adenoviral (Ad) vectors with the cytomegalovirus promoter carrying human bone morphogenetic protein-2 (BMP2) gene (Ad-BMP2) or GFP gene (Ad-GFP) were generated to confirm gene expression and osteogenic differentiation in MSC and DFb. Ad vectors were propagated in Ad293 cells, and purified by filtration (AdEasy™ Virus Purification kit), as described previously.18,19,22 The infectious titers of the Ad-BMP2/GFP were determined by use of a commercially available kit (BD Clontech™ Adeno-X Rapid Titer Kit), and expression of transgenes was verified in cell culture.18,19,22
Dermal tissues harvested from the back region by skin punch biopsy and cancellous bone harvested from the iliac crest by curetting using six healthy juvenile New Zealand White rabbits (6-month-old) were used to isolate DFb and MSC, as described before.18,19 The monolayer cultures of DFb and MSC in 48-well plates were transfected with Ad-BMP2 or Ad-GFP at 10, 50, 100, 150, and 200 moi (multiplicities of infection). The transduction efficiency of GFP genes was quantified by fluorescent microscopy at days 2, and BMP2 protein concentration measured by ELISA (R&D Systems, Mineapolis, MN) at days 0, 2, 7, and 14. Osteogenic differentiation of DFb and MSC was quantified at days 7 and 14 by counting the number of von Kossa-positive mineralized bony nodules per well.18,19 The monolayer cultures of DFb in 25-cm2 flasks were transfected with FV-GFP at 2 moi, and cultured for 7 days as it became 90–100% confluent. Then, the DFb was trypsinized, transduction efficiency of GFP genes quantified by flow cytometry, and GFP-positive cells sorted, re-seeded in flasks, and cultured for 5 days. Same steps were repeated for the second cell-sorting. These 3 passages of DFb (i.e., non-sorted, single-sorted, and double-sorted) were re-seeded 48-well plates, and GFP gene transduction was quantified by fluorescent microscopy at days 1, 7, 14, 21, and 28.
Intra-osseous injection compared to intra-medullar infusion of BMP2-expressing DFb
Twelve rabbits were used to compare cell engraftment and bone formation of intra-osseous injection to intra-medullar infusion and of BMP2-expressing DFb to non-transduced DFb (Fig 1A, 1B, 1C). In each rabbit, the skin punch biopsy and distal femur drilling (2 drill holes [2.38-mm diameter, 10-mm depth] per femoral condyle at 5-mm and 15-mm proximal from the knee joint) were performed at day -28 under general anesthesia, and the dermal tissues were digested to isolate DFb.18,19 Autologous DFb were cultured and expanded, transduced by FV-GFP (2 moi) at day -21, processed for cell-sorting at day -14, and transduced by Ad-BMP2 (200 moi) at day -2. The rabbits were re-anesthetized at day 0, and one randomly selected limb received an intra-osseous cell injection (1×108 of DFb [n=6] or DFb-BMP2 [n=6]) in the distal femur (into the 2 drill holes filled with granulation tissue, 5×107 cells per hole) and an intra-medullar cell infusion (1×108 of DFb [n=6] or DFb-BMP2[n=6]) in the proximal tibia by passing a tube through the unicortical diaphyseal drill hole (2.38-mm diameter). The contralateral limb of each rabbit was used uninjected (n=6) or saline injection/infusion (n=6). All cell injections/infusions were made in 1 mL total volume with PBS containing 1×108 autologous DFb/DFb-BMP2. The distal femur and proximal tibia in the same limb always received the same treatment. At day 2, one of 12 rabbits (DFb-BMP2 injection/infusion in one hindlimb and saline injection/infusion in the contralateral limb) died for an unknown reason and was not included in the outcome assessments. At day 56, the 11 rabbits were euthanized, and both hindlimbs were harvested and scanned by μCT (Inveon, Siemens, Knoxville, TN, USA). The cortical bone, cancellous bone, drill hole filling tissue, and external callus regions of the distal femurs and proximal tibiae (areas within 3-cm from knee joint) were evaluated for bone volume (mm3), bone mineral density (mg/ml), trabecular bone surface area (mm2) and trabecular thickness (μm).19,23 The fixed and decalcified femurs and tibiae were paraffin-embedded, sectioned at 5-μm, and stained with Masson’s Trichrome. The five representative sites on cortical bone regions were evaluated for epiphyseal cortical thickness (mm) and subchondral bone thickness (mm). The five representative sites on trabecular bone regions were evaluated for %bone area, fractal dimension,24 inter-connectivity index,25 and Euler number.26 The five representative sites on drill defect regions were evaluated for the %porosity and %maturity.19
Evaluation of DFb engraftment and biodistribution
The paraffin-embedded femurs and tibiae were sectioned, and immuno-histochemical staining was performed (Benchmark XT, Ventana Medical Systems, Inc., Tucson, AZ) using anti-GFP antibody (Abcam Inc., Cambridge, MA). The five representative sites on each section were viewed by microscopy to count the number of positive cells (per mm2) in each of cortical bone, trabecular bone, bone marrow, and defect filling tissue regions. To determine the cell engraftment, RNA was extracted from distal femurs, proximal tibiae, and proximal radius (reference tissue), and transgene expression of GFP was quantified by RT-PCR (ABI Prism 7000, PE Applied Biosystems, Foster City, CA) and expressed as fold changes relative to the reference tissue and rabbit β-actin gene expression.22 For the six rabbits treated with DFb-BMP2, genomic DNA was extracted from thoracic/abdominal organs (i.e., liver, heart, lung, spleen, kidney), regional lymph-nodes (i.e., inguinal and popliteal lymph-nodes) in both hindlimbs, and distal femur and proximal tibiae of both hindlimbs. Quantitative PCR was performed to detect the CMV sequences and quantify as the copy number per 100 ng DNA sample, as described before.22
Statistical analysis
Repeated-measure analysis of variance (ANOVA) (SAS Institute Inc., Cary, NC) was used to evaluate the effects of DFb engraftment with the post-test multiple comparisons between the treatment groups using Proc Mixed statistical models for continuous outcomes and Genmod statistical models for categorical outcomes. Repeated variables were considered to be nested within horse, and the distribution of data was assessed by use of a subset of normality tests.18,19 Significance level was set at p<0.05 for all analyses.
RESULTS
Osteogenic differentiation and cell labeling of DFb
The DFb and MSC showed comparable gene transduction (Fig 2A; left) and protein production (Fig 2A; right) at high dosage (200 moi), whereas DFb was significantly less permissive (P<0.02) to Ad-vector than MSC at lower dosages (50, 100, 150 moi). For this reason, the high dosage (200 moi) was selected for Ad-BMP2 in subsequent experiments. When Ad-BMP2 was applied at high dosage (200 moi), the DFb and MSC showed comparable osteogenic differentiation with equivalent number of mineralized nodules at 7 and 14 days after gene transduction (Fig 2B).
Figure 2.
Gene transduction by Adenoviral (Ad) vector carrying green fluorescent protein (GFP) genes was significantly greater (*P<0.02) in MSC compared to dermal fibroblasts (DFb) at 50, 100, and 150 moi (multiplicities of infection), but it was comparable between MSC and DFb at 200 moi (A; left), and the bone morphogenetic protein-2 (BMP2) production measured by ELISA was significantly greater (#P<0.04) in MSC with 100 or 200 moi Ad-BMP2 or DFb with 200 moi Ad-BMP2 compared to DFb with 100 moi Ad-BMP2 (A; right). When 200 moi Ad-BMP2 was applied, DFb and MSC showed comparable level of mineralized nodule formation (Von Kossa staining) at days 7 and 14 (B). The cell-sorting procedures of DFb with FV-GFP transduction using flow-cytometry were facilitated to increase the proportion of GFP labeled cells (C; left), and the GFP gene expression sustained similar level up to 28 days after the cell-sortings (C; right). Data were expressed as mean+s.e.m. NS: there were no significant differences among treatment groups.
Cell labeling of DFb with FV-GFP as successfully achieved within 7 days after FV-GFP gene transduction, but it was relatively low transduction efficiency (13±2%). Fluorocytometric cell-sorting was facilitated to increase the proportion of labeled cells by removing GFP-negative cells, and the %transductions were improved to 78±7% by the first cell-sorting, 93±5% by the second cell-sorting (Fig 2C; left), and sustained up to 28 days after the cell-sorting (Fig 2C; right).
Evaluation of BMP-2 on intra-osseous and intra-medullar DFb injection/infusion
On the quantitative μCT (Fig 3A), the distal femur trabecular bone with intra-osseous DFb-BMP2 injection had a significantly greater (P<0.03) bone volume (Fig 3C; upper left), bone density (Fig 3C; upper right), and bone surface area (Fig 3C; lower left), compared to no-injection, saline, or DFb group. Also, an intra-osseous DFb-BMP2 injection induced a significantly greater (P<0.02) external callus formation (Fig 3D; left) and drill hole filling (Fig 3E; left). The proximal tibia trabecular bone with intra-medullar DFb-BMP2 infusion had a significantly greater (P<0.01) bone volume (Fig 3C; upper left), bone surface area (Fig 3C; lower left), and trabecular thickness (Fig 3C; lower right), compared to saline or DFb group. The cortical bone in the distal femur and proximal tibia did not show any improvement in bone volume (Fig 3B; left) or bone density (Fig 3B; right) by intra-osseous or intra-medullar DFb-BMP2 cell therapy.
Figure 3.
Micro-computed tomographic (μCT) images (A) and quantitative analysis of efficacy of dermal fibroblast (DFb)-mediated bone morphogenetic protein-2 (BMP2) gene therapy in limbs ofrabbits after intra-osseous injection and intra-medullar infusion of DFb or DFb-BMP2 (Fig 1B, 1D, and 1E). In cortical bone regions of distal femur and proximal tibiae, bone volume (B; left) and density (B; right) were not different among treatments. In trabecular bone regions of distal femur and proximal tibiae, bone volume (C; upper left), bone density (C; upper right), bone surface area (C; lower left), and trabecular thickness (C; lower right) were significantly greater (P<0.03) in DF-BMP2 injected/infused bone tissues compared to other 3 groups. In external callus regions of distal femur, bone volume (D; left) was significantly greater (P<0.02) in DF-BMP2 injected/infused bone tissues compared to other 3 groups, although bone density (D; right) was not different among treatments. In drill defect regions of distal femur, bone volume (E; left) was significantly greater (P<0.01) in DF-BMP2 injected/infused bone tissue compared to the other 3 groups, although bone density (E; right) was not different among treatments. Data were expressed as mean+s.e.m. abcDifferent letters differ significantly (P<0.05). NS: there were no significant differences among treatment groups.
On the histomorphometric analysis (Fig 4A), the distal femur trabecular bone with intra-osseous DFb-BMP2 injection had a significantly greater (P<0.04) bone area (Fig 4C; upper left), fractal dimension (Fig 4C; upper right), inter-connectivity index (Fig 4C; lower left), and Euler number (Fig 4C; lower right), compared to no-injection, saline, or DFb group. Also, an intra-osseous DFb-BMP2 injection induced a significantly (P<0.02) less porosity (Fig 4D; left) and greater bone maturity (Fig 4D; right) in the drill hole filling tissue. The proximal tibia trabecular bone with intra-medullar DFb-BMP2 infusion had a significantly greater bone area (Fig 4C; upper left), inter-connectivity index (Fig 4C; lower left), and Euler number (Fig 4C; lower right), compared to no-injection, saline, or DFb group. The cortical bone in distal femur and proximal tibia did not show any improvement in epiphyseal thickness (Fig 4B; left) or subchondral bone thickness (Fig 4B; right) by intra-osseous or intra-medullar DFb-BMP2 cell therapy.
Figure 4.
Histomorphometric evaluation of the distal femurs (A; left) and proximal tibiae (A; right) for the efficacy of dermal fibroblast (DFb)-mediated bone morphogenetic protein-2 (BMP2) gene therapy in limbs of rabbits after intra-osseous injection and intra-medullar infusion of DFb or DFb-BMP2 (Fig 1B, 1D, and 1E). In cortical bone regions of distal femur and proximal tibiae, epiphyseal cortical thickness (B; left) and subchondral bone thickness (B; right) were not different among treatments. In trabecular bone regions of distal femur and proximal tibiae, bone area (C; upper left), fractal dimension (C; upper right), inter-connectivity index (C; lower left), and Euler number (C; lower right) were significantly greater (P<0.04) in DF-BMP2 injected/infused bone tissues compared to other 3 groups. In drill defect regions of distal femur, porosity (D; left) was significantly less (P<0.007) and maturity (D; right) was significantly greater (P<0.02) in DF-BMP2 injected/infused bone tissues compared to other 3 groups. Data were expressed as mean+s.e.m. abcDifferent letters differ significantly (P<0.05). NS: there were no significant differences among treatment groups.
Cell engraftment and biodistribution of BMP2-expressing autologous DFb
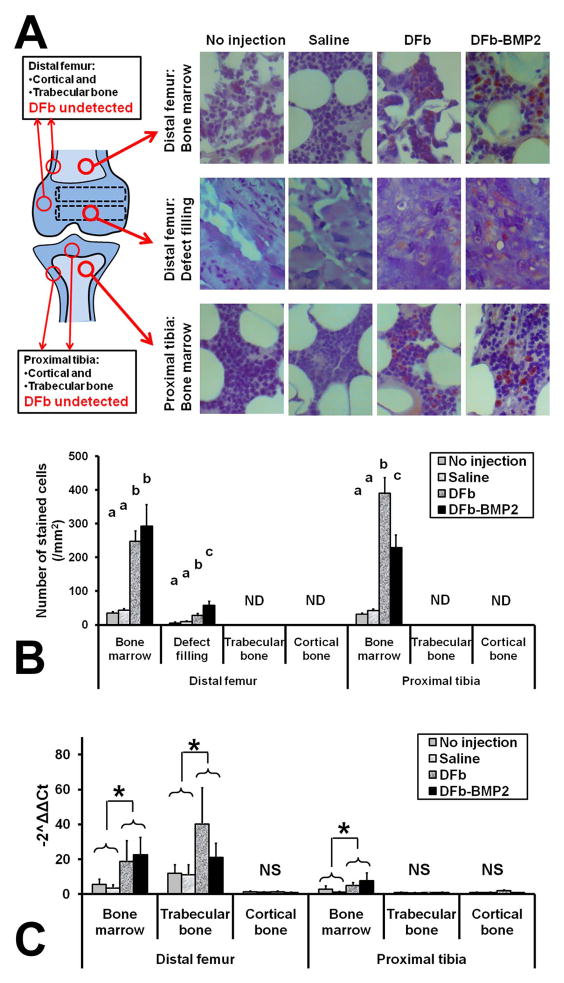
On the immunohistochemical staining of GFP, successful DFb/DFb-BMP2 engraftment by intra-osseous injection was demonstrated within the drill defect filling tissue in trabecular bone and bone marrow in the distal femurs (Fig 5A) with significantly greater (P<0.003) number of GFP-positive cells compared to the no-injection or saline groups (Fig 5B). In defect filling tissue of distal femurs, the number of GFP-positive cells was significantly greater in DFb-BMP2 group compared to the DFb group (Fig 5B). Also, successful DFb/DFb-BMP2 engraftment by intra-medullar infusion was demonstrated within the bone marrow tissue in the proximal tibiae (Fig 5A) with significantly greater (P<0.001) number of GFP-positive cells compared to the no-injection or saline groups (Fig 5B) and similar numbers of engrafted cells as by intra-osseous injection. In the bone marrow tissues of the proximal tibiae, the number of GFP-positive cells was significantly less (P<0.04) in the DFb-BMP2 group compared to the DFb group (Fig 5B).
Figure 5.
Immunohistochemical (IHC) evaluation (A,B) and RT-PCR analysis (C) for the efficacy of dermal fibroblast (DFb)-mediated bone morphogenetic protein-2 (BMP2) gene therapy in limbs of rabbits after intra-osseous injection and intra-medullar infusion of DFb or DFb-BMP2 (Fig 1B, 1D, and 1E). In the bone marrow or defect filling tissues of distal femurs, the number of GFP-positive cells was significantly greater (P<0.003) in DFb or DF-BMP2 injected/infused bone tissues compared to no-injection or saline groups. In the bone marrow tissues of proximal tibiae, the number of GFP-positive cells was significantly greater (P<0.001) in DFb or DF-BMP2 injected/infused bone tissues compared to no-injection or saline groups. In the bone marrow or trabecular bone tissues of distal femurs, the GFP-gene expression was significantly greater (P<0.04) in DFb and DF-BMP2 injected/infused bone tissues compared to no-injection and saline groups. In the bone marrow tissues of proximal tibiae, the GFP-gene expression was significantly greater (P<0.04) in DFb and DF-BMP2 injected/infused bone tissues compared to no-injection and saline groups.
On the RT-PCR analyses of gfp genes, successful DFb/DFb-BMP2 engraftment by intra-osseous injection was demonstrated within the trabecular bone and bone marrow in the distal femurs with significantly greater (P<0.04) gfp gene expression compared to the no-injection and saline groups (Fig 5C). Also, successful DFb/DFb-BMP2 engraftment by intra-medullar infusion was demonstrated within the bone marrow tissue in the proximal tibiae (Fig 5C). However, DFb/DFb-BMP2 were not engrafted in cortical bone tissues in either distal femurs or proximal tibiae (Fig 5C).
On the quantitative PCR of tissue samples from the 6 rabbits who received DFb-BMP2, the CMV promoter sequences were detected in various locations of the hindlimbs treated with DFb-BMP2 including distal femurs, proximal tibiae, and popliteal regional lymph nodes (Table 1). The CMV promoter sequences were not detected in any organs evaluated and any tissues in the contralateral hindlimbs (Table 1).
Table 1.
Quantitative PCR detection of CMV promoter for evaluation of biodistribution of dermal fibroblasts (DFb) transduced with bone morphogenetic protein-2 (BMP2) genes using adenoviral vectors with CMV promoters. In these rabbits, one randomly assigned hindlimb received intra-osseous injection of 1×108 DFb-BMP2 in distal femur, intra-medullar infusion of 1×108 DFb-BMP2 in proximal tibia (Fig 1B, 1D, and 1E). The contralateral hindlimbs were assigned for no-injection or saline groups. In the hindlimbs treated with DFb-BMP2, the CMV promoter sequences were detected in distal femurs, proximal tibiae, and popliteal lymph nodes. The CMV promoter sequences were not detected in any thoracic/abdominal organs evaluated and any tissues in the contralateral hindlimbs. Detection limit was <50 copies per 100ng DNA.
| Sample tissue | Number of samples with CMV detected | Copy number of CMV per 100ng DNA (Mean ± SEM) | ||
|---|---|---|---|---|
| DFb-BMP2-treated hindlimb | Distal femur | Bone marrow | 6/6 | 1.2×104 ± 1.2×103 |
| Trabecular bone | 6/6 | 2.9×105 ± 7.9×104 | ||
| Cortical bone | 1/6 | 2.7×102 | ||
| Proximal tibia | Bone marrow | 6/6 | 7.2×104 ± 7.9×104 | |
| Trabecular bone | 5/6 | 4.9×102 ± 4.8×101 | ||
| Cortical bone | 0/6 | |||
| Vastus lateralis muscle | 0/6 | |||
| Extensor digitrum longus muscle | 0/6 | |||
| Popliteal lymph node | 3/6 | 2.6×102 ± 3.1×101 | ||
| Inguinal lymph node | 0/6 | |||
| Contralateral hindlimb | Distal femur | Bone marrow | 0/6 | |
| Trabecular bone | 0/6 | |||
| Cortical bone | 0/6 | |||
| Proximal tibia | Bone marrow | 0/6 | ||
| Trabecular bone | 0/6 | |||
| Cortical bone | 0/6 | |||
| Vastus lateralis muscle | 0/6 | |||
| Extensor digitrum longus muscle | 0/6 | |||
| Popliteal lymph node | 0/6 | |||
| Inguinal lymph node | 0/6 | |||
| Thoracic or abdominal organs | Heart | 0/6 | ||
| Lung | 0/6 | |||
| Liver | 0/6 | |||
| Spleen | 0/6 | |||
| Kidney | 0/6 | |||
| Ovary | 0/4 | |||
| Testis | 0/2 | |||
DISCUSSION
Autologous implantation of DFb genetically-modified to secrete BMP2 using single local applications such as an intra-osseous injection or intra-medullar infusion can improve bone mineral density and trabecular microarchitecture in this rabbit long bone model. In our study, the DFb-BMP2-injected distal femur had greater bone volume, mineral density, drill defect filling, and trabecular microarchitecture with higher interconnectivity and fractality compared to no injection, saline, and DFb alone groups (Fig 3 and 4). Also, the DFb-BMP2-infused proximal tibia had greater bone volume and trabecular microarchitecture compared to saline and DFb alone groups. Improvement in these mineral density and microarchitecture parameters should reflect an increased physical strength of bone tissues.23–26 Therefore, both intra-osseous and intra-medullar delivery methods can be utilized to efficiently engraft the BMP2-expressing DFb to increase mineral density and strengthen bones for the treatments of bone fragility disorders. By using rodent and equine bones, autologous DFb with osteogenic genes have already been successfully engrafted to induce bone formation and improve mineral density.15,17–19 However, our study is first to compare the effects of two different cell delivery routes (i.e., intra-osseous injection and intra-medullar infusion) utilizing either BMP2-expressing or non-transduced cells. Certainly, future studies are warranted to determine if the autologous implantation of BMP2-expressing DFb can achieve the functional improvement of the treated bones (e.g., physical strength and stiffness) in animal models of OI and osteoporosis. Also, it is of great interest to reprogram the DFb from OI patients into stem cells (iPSCs), inactivate their mutant COL1A1/COL1A2 genes, and deliver them by intra-osseous or intra-medullar methods, because fibroblast-derived stem cells might produce similar results of MSC-derived iPSCs as shown in previous works of OI cell therapy.8
In general, cell-mediated gene therapy can provide two therapeutic modalities; (i) genetically-charged cells for engraftment, and (ii) growth factors secreted from the cells for paracrine stimulus; yet, it has been speculated which one has a primary treatment effect. In our study, improvements of bone mineral density and trabecular microarchitecture were evident in the proximal tibiae with intra-medullar DFb-BMP2 delivery, even though the intra-medullary infused DFb were not proven to be engrafted within the trabecular bone tissue. In addition, the intra-osseous or intra-medullar injection/infusion of non-transduced DFb did not significantly alter the bone density or microarchitecture. These results may suggest that the secreted BMP2 from the transduced DFb affecting endosteal lining cells were sufficient to commence bone turnover and mineralization in trabecular bone tissue. Also, authors’ previous work demonstrated that the efficacy of DFb-BMP2 could be primarily due to the secreted BMP2 affecting endogenous progenitors on the endosteal lining to modulate the formation/resorption of trabecular bone.19 Hence, osteogenic growth factors secreted from the injected/infused cells might be a primary contributor to improve bone mineral density and microarchitecture, at least in the cell therapy strategy demonstrated in our study. If so, it is probable that the type of cell used for delivery of the BMP2 gene would not make a significant difference in improving bone formation and density.
Trabecular bone tissue or medullar cavity of long bone could be considered as a potential cell engraftment site in orthopedic cell therapy, in preference to systemic administration. Our study demonstrated the successful engraftment of DFb/DFb-BMP2 within trabecular bone when the cells were directly injected into the granulation tissue filled in surgical created drill hole defects (Fig 5). Undetectable level of cell engraftment in trabecular bone tissue of proximal tibia where DFb/DFb-BMP2 were infused intra-medullarly (Fig 5) may indicate that these cells cannot directly migrate or be embedded into existing trabeculae. Likewise, cortical bone tissues were not engrafted by DFb/DFb-BMP2 in either delivery methods (Fig 5). Therefore, if BMP2-expressing DFb were applied to bone fragility disorders, the cells may need to be injected into repair tissue at the fracture site or granulation tissue in surgically created bone defects after curettage of unhealthy bone.
Systemic biodistribution of locally administered DFb-BMP2 was not evident in this study due to no detection of CMV-promoter in distant organs or adjacent muscles, with an exception of regional popliteal lymph nodes (Table 1 and 2). Authors’ previous works have shown that delayed DFb injection into firm granulation tissue can contain the locally injected cells.18,19 For the same reason, the intra-osseous cell injection in this study, which was a delayed injection of DFb-BMP2 into the drill hole defects of distal femurs filled with granulation tissue, could minimize cell leakage. An encouraging finding in this study was that the intra-medullar cell infusion did not cause systemic distribution of DFb-BMP2 (Table 1). This is in agreement with another study reporting that the bone marrow MSCs infused directly into the medullar cavity can settle at the delivery site and very few MSCs leak and migrate elsewhere.27 One potential explanation for the cell containment within marrow space is that environmental factors existing in the medullar cavity might be helpful for the infused cells to osteogenically-commit and persist within the bone. In the previous study,28 adipose-derived MSCs were infused into the medullar cavity, retrieved and re-cultured, and systemically transplanted into a different animal. Such an exposure of adipose MSCs to bone microenvironment enhanced the homing, migration, and survival of MSCs in the recipient bones; therefore, authors concluded that the medullar cavity may contain certain signaling molecules giving cells ‘osteoblast-like properties’ so that the committed MSCs returned to and persisted in the bone tissues when they were re-injected systemically in the second time.28 Similar mechanism has been speculated in bone-metastasized breast tumor cells which possess a homing effect by expression of osteoblast-specific genes.29 For these reasons, it is possible that, in our study, infusing DFb into medullar cavity could confer the cells with the osteoblastic properties and ability to persist within the bone.
The in vitro experiments in our study demonstrated that MSC and DFb can have comparable transduction efficiency, BMP protein synthesis, and osteogenic capacity when the Ad-GFP/BMP2 were applied at high dosage (200 moi). Therefore, DFb may be considered as an alternative cell vector for cell-mediated gene therapy for bone fragility disorders, at least for the purpose of increasing the bone mineral density. Use of rapidly growing DFb may have greater therapeutic and practical benefits, as opposed to MSC, for future clinical application of cell therapy by improving time- and cost-effectiveness. In our study, expansion of MSC into 100 million cells required an average time length of 104 days (range: 63 to 147 days) between tissue harvest and cell infusion, whereas the expansion of DFb into 100 million cells were easily achieved in an average of 25 days (range: 21 to 28 days) after the tissue harvest.
To authors’ knowledge, this is the first study to demonstrate that the autologous implantation of DFb-BMP2 via an intra-osseous/medullar cell delivery can increase bone mineral density. However, a number of issues need to be addressed before this strategy is assessed for clinical use. First, relative efficacy of two cell delivery methods (intra-osseous injection and intra-medullar infusion) would be best studied by applying both methods in the same bone simultaneously using the cells with different marker genes. The study presented here was preliminary work and designed to confirm an efficacy, feasibility, and successful cell engraftment by the different cell delivery strategies. Second, further work would be necessary to determine if systemically administered DFb-BMP2 can exploit a homing effect to self-recruit into certain part of osseous tissues with active bone turnover, although systemic application of osteogenic gene-induced cells may cause of ectopic mineralized foci.30 Third, simultaneous application of cell-mediated gene therapy and anti-resorptive therapy should be tested for a potential synergetic effect to more effectively treat bone fragility disorders. Fourth, the safety aspects of using viral vectors (eg, tumorigenicity of the transfected cells, innate immune reaction against the residual viral capsids/genomes) must be cautiously assessed prior to the clinical application.
Several limitations and issues should also be discussed. In our study, the efficiency of Ad-transduction was only determined by manual counting of GFP-positive cells under fluorescent microscope (Figure 2A). Use of flow-cytometric analysis might produce more accurate assessment of transduction efficiency. In addition, although the injected/infused DFb were incorporated into bone or marrow tissue in our results (Figure 5), other investigators showed that Ad vector-transduced cells were rapidly eliminated in 7–10 days by T cells that directed against the transgene product and adenoviral antigens.31,32 Two potential explanations for our findings are; (1) intra-osseous or intra-medullar environments may have a limited number of T cells, and, therefore, Ad-transduced DFb were not completely cleared and incorporated into bone/marrow tissues, (2) Ad-transduced DFb might be cleared by T cells, and only the small portion of DFb that were not transduced by Ad-vectors could remain within bone/marrow tissues, since the GFP expression detected by immune-staining (Figure 5A and 5B) and PCR (Figure 5C) were transduced by Foamy-vectors. Moreover, in our study, the fibroblast phenotypic markers were not compared among the DFb harvested from each rabbit, and whether the GFP insertion altered the growth parameters or the phenotype was not confirmed. These factors might affect the differences in cell engraftments and osteogenic capacities between animals. Furthermore, our study utilized only one cell type (DFb). It is our interest to compare the osteoinductive capabilities of DFb against other cell type such as mesenchymal stem cells using this experimental model in future, even though previous work demonstrated that osteoinduction by ex-vivo BMP2 gene delivery would not be different between stem cells and skin fibroblasts.33
Acknowledgments
We thank Dr David Russell and Dr Ken Ohmine from University of Washington, School of Medicine, Division of Hematology for the generous use of vectors. We thank Dr Seth Jump, Dr Kelly Santangelo, Dr Maria Menendez, Dr Stephanie Lewis, Michelle Carlton, Jeanne Green, and Alan Fletcher for technical support, and Tim Vojt for illustrations. This study was supported by Osteogenesis Imperfecta Foundation Michael Geisman Fellowship, National Institute of Arthritis and Musculoskeletal and Skin Diseases, K08 AR049201 and NIH R01 AR048328-06.
Footnotes
All authors have no conflict of interest to declare.
References
- 1.Rauch F, Glorieux FH. Osteogenesis imperfecta. Lancet. 2004;363:1377–1385. doi: 10.1016/S0140-6736(04)16051-0. [DOI] [PubMed] [Google Scholar]
- 2.Rossini M, Di Munno O, Gatti D, et al. Optimising bisphosphonate treatment outcomes in postmenopausal osteoporosis: review and Italian experience. Clin Exp Rheumatol. 2011;29:728–735. [PubMed] [Google Scholar]
- 3.Munns CF, Rauch F, Mier RJ, et al. Respiratory distress with pamidronate treatment in infants with severe osteogenesis imperfect. Bone. 2004;35:231–234. doi: 10.1016/j.bone.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Pizones J, Plotkin H, Parra-Garcia JI, et al. Bone healing in children with osteogenesis imperfecta treated with bisphosphonates. J Pediatr Orthop. 2005;25:332–335. doi: 10.1097/01.bpo.0000152940.10487.c9. [DOI] [PubMed] [Google Scholar]
- 5.Chan B, Zacharin M. Maternal and infant outcome after pamidronate treatment of polyostotic fibrous dysplasia and osteogenesis imperfecta before conception: a report of four cases. J Clin Endocrinol Metab. 2006;91:2017–2020. doi: 10.1210/jc.2005-2548. [DOI] [PubMed] [Google Scholar]
- 6.Jethva R, Otsuru S, Dominici M, et al. Cell therapy for disorders of bone. Cytotherapy. 2009;11:3–17. doi: 10.1080/14653240902753477. [DOI] [PubMed] [Google Scholar]
- 7.Chamberlain JR, Schwarze U, Wang PR, et al. Gene targeting in stem cells from individuals with osteogenesis imperfecta. Science. 2004;303:1198–1201. doi: 10.1126/science.1088757. [DOI] [PubMed] [Google Scholar]
- 8.Chamberlain JR, Deyle DR, Schwarze U, et al. Gene targeting of mutant COL1A2 alleles in mesenchymal stem cells from individuals with osteogenesis imperfecta. Mol Ther. 2008;16:187–193. doi: 10.1038/sj.mt.6300339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pochampally RR, Horwitz EM, DiGirolamo CM, et al. Correction of a mineralization defect by overexpression of a wild-type cDNA for COL1A1 in marrow stromal cells (MSCs) from a patient with osteogenesis imperfecta: a strategy for rescuing mutations that produce dominant-negative protein defects. Gene Ther. 2005;12:1119–1125. doi: 10.1038/sj.gt.3302514. [DOI] [PubMed] [Google Scholar]
- 10.Niyibizi C, Li F. Potential implications of cell therapy for osteogenesis imperfecta. Int J Clin Rheumtol. 2009;4:57–66. doi: 10.2217/17584272.4.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Z, Goh J, Das De S, et al. Efficacy of bone marrow-derived stem cells in strengthening osteoporotic bone in a rabbit model. Tissue Eng. 2006;12:1753–1761. doi: 10.1089/ten.2006.12.1753. [DOI] [PubMed] [Google Scholar]
- 12.Takada K, Inaba M, Ichioka N, et al. Treatment of senile osteoporosis in SAMP6 mice by intra-bone marrow injection of allogeneic bone marrow cells. Stem Cells. 2006;24:399–405. doi: 10.1634/stemcells.2005-0068. [DOI] [PubMed] [Google Scholar]
- 13.Lien CY, Chih-Yuan Ho K, Lee OK, et al. Restoration of bone mass and strength in glucocorticoid-treated mice by systemic transplantation of CXCR4 and cbfa-1 co-expressing mesenchymal stem cells. J Bone Miner Res. 2009;24:837–848. doi: 10.1359/jbmr.081257. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 15.Rutherford RB, Moalli M, Franceschi RT, et al. Bone morphogenetic protein-transduced human fibroblasts convert to osteoblasts and form bone in vivo. Tissue Eng. 2002;8:441–452. doi: 10.1089/107632702760184709. [DOI] [PubMed] [Google Scholar]
- 16.Hirata K, Mizuno A, Yamaguchi A. Transplantation of skin fibroblasts expressing BMP-2 contributes to the healing of critical-sized bone defects. J Bone Miner Metab. 2007;25:6–11. doi: 10.1007/s00774-006-0721-0. [DOI] [PubMed] [Google Scholar]
- 17.Lattanzi W, Parrilla C, Fetoni A, et al. Ex vivo-transduced autologous skin fibroblasts expressing human Lim mineralization protein-3 efficiently form new bone in animal models. Gene Ther. 2008;15:1330–1343. doi: 10.1038/gt.2008.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishihara A, Zekas LJ, Litsky AS, et al. Dermal fibroblast-mediated BMP2 therapy to accelerate bone healing in an equine osteotomy model. J Orthop Res. 2009;28:403–411. doi: 10.1002/jor.20978. [DOI] [PubMed] [Google Scholar]
- 19.Ishihara A, Zekas LJ, Weisbrode SE, et al. Comparative efficacy of dermal fibroblast-mediated and direct adenoviral bone morphogenetic protein-2 gene therapy for bone regeneration in an equine rib model. Gene Ther. 2010;17:733–744. doi: 10.1038/gt.2010.13. [DOI] [PubMed] [Google Scholar]
- 20.Li F, Wang X, Niyibizi C. Bone marrow stromal cells contribute to bone formation following infusion into femoral cavities of a mouse model of osteogenesis imperfecta. Bone. 2010;47:546–555. doi: 10.1016/j.bone.2010.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trobridge GD, Allen J, Peterson L, et al. Foamy and lentiviral vectors transduce canine long-term repopulating cells at similar efficiency. Hum Gene Ther. 2009;20:519–523. doi: 10.1089/hum.2008.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishihara A, Shields KM, Litsky AS, et al. Osteogenic gene regulation and relative acceleration of healing by adenoviral-mediated transfer of human BMP-2 or -6 in equine osteotomy and ostectomy models. J Orthop Res. 2008;26:764–771. doi: 10.1002/jor.20585. [DOI] [PubMed] [Google Scholar]
- 23.Bouxsein ML, Boyd SK, Christiansen BA, et al. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25:1468–1486. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- 24.Weinstein RS, Majumdar S. Fractal geometry and vertebral compression fractures. J Bone Miner Res. 1994;9:1797–1802. doi: 10.1002/jbmr.5650091117. [DOI] [PubMed] [Google Scholar]
- 25.Dalle Carbonare L, Valenti MT, Bertoldo F, et al. Bone microarchitecture evaluated by histomorphometry. Micron. 2005;36:609–616. doi: 10.1016/j.micron.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 26.Chappard D, Legrand E, Pascaretti C, et al. Comparison of eight histomorphometric methods for measuring trabecular bone architecture by image analysis on histological sections. Microsc Res Tech. 1999;45:303–312. doi: 10.1002/(SICI)1097-0029(19990515/01)45:4/5<303::AID-JEMT14>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 27.Li Q, Hisha H, Yasumizu R, et al. Analyses of very early hemopoietic regeneration after bone marrow transplantation: comparison of intravenous and intrabone marrow routes. Stem Cells. 2007;25:1186–1194. doi: 10.1634/stemcells.2006-0354. [DOI] [PubMed] [Google Scholar]
- 28.Liao X, Li F, Wang X, et al. Distribution of murine adipose-derived mesenchymal stem cells in vivo following transplantation in developing mice. Stem Cells Dev. 2008;17:303–314. doi: 10.1089/scd.2007.0086. [DOI] [PubMed] [Google Scholar]
- 29.Yin JJ, Pollock CB, Kelly K. Mechanisms of cancer metastasis to the bone. Cell Res. 2005;15:57–62. doi: 10.1038/sj.cr.7290266. [DOI] [PubMed] [Google Scholar]
- 30.Byers BA, Guldberg RE, García AJ. Synergy between genetic and tissue engineering: Runx2 overexpression and in vitro construct development enhance in vivo mineralization. Tissue Eng. 2004;10:1757–1766. doi: 10.1089/ten.2004.10.1757. [DOI] [PubMed] [Google Scholar]
- 31.Yang Y, Li Q, Ertl HC, et al. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J Virol. 1995;69:2004–2015. doi: 10.1128/jvi.69.4.2004-2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang Y, Jooss KU, Su Q, et al. Immune responses to viral antigens versus transgene product in the elimination of recombinant adenovirus-infected hepatocytes in vivo. Gene Ther. 1996;3:137–144. [PubMed] [Google Scholar]
- 33.Gugala Z, Olmsted-Davis EA, Gannon FH, et al. Osteoinduction by ex vivo adenovirus-mediated BMP2 delivery is independent of cell type. Gene Ther. 2003;10:1289–1296. doi: 10.1038/sj.gt.3302006. [DOI] [PubMed] [Google Scholar]