Abstract
Background
Skeletal muscle loss (sarcopenia) is a major clinical complication in alcoholic cirrhosis with no effective therapy. Skeletal muscle autophagic proteolysis and myostatin expression (inhibitor of protein synthesis) are increased in cirrhosis and believed to contribute to anabolic resistance. A prospective study was performed to determine the mechanisms of sarcopenia in alcoholic cirrhosis and potential reversal by leucine.
Methods
In 6 well-compensated, stable alcoholic cirrhotic patients and 8 controls, serial vastus lateralis muscle biopsies were obtained before and 7h after a single oral BCAA mixture enriched with leucine (BCAA/LEU). Primed-constant infusion of L-[ring-2H5]-phenylalanine was used to quantify whole body protein breakdown (WbPB) and muscle protein fractional synthesis rate (FSR) using liquid chromatography/mass spectrometry. Muscle expression of myostatin, mTOR targets, autophagy markers, protein ubiquitination and intracellular amino acid deficiency sensor, general control of nutrition 2 (GCN2) were quantified by immunoblots and leucine transporter (SLC7A5) and glutamine exchanger (SLC38A2) by real time PCR.
Results
Following oral administration, plasma BCAA concentrations showed a similar increase in cirrhosis and controls. Skeletal muscle FSR was 9.63±0.36%/h in controls and 9.05±0.68%/h in cirrhotics (p=0.54). Elevated WbPB in cirrhosis was reduced with BCAA/LEU (p=0.01). Fasting skeletal muscle molecular markers showed increased myostatin expression, impaired mTOR signaling and increased autophagy in cirrhosis compared to controls (p<0.01). BCAA/LEU did not alter myostatin expression but mTOR signaling, autophagy measures and GCN2 activation were consistently reversed in cirrhotic muscle (p<0.01). SLC7A5 expression was higher in basal state in cirrhosis than controls (p<0.05) but increased with BCAA/LEU only in controls (p<0.001).
Conclusions
We demonstrate that impaired mTOR1 signaling and increased autophagy in skeletal muscle of alcoholic cirrhosis patients is acutely reversed by BCAA/LEU.
Introduction
Loss of skeletal muscle mass or sarcopenia is the major component of malnutrition in cirrhosis and occurs in the majority of patients (1). Sarcopenia reduces survival, quality of life and post-liver transplant outcomes in patients with cirrhosis (2–4). Despite widespread recognition of the clinical significance of sarcopenia in cirrhosis, there are no effective therapies because there is limited understanding of the mechanisms of sarcopenia and cirrhosis is believed to be a state of anabolic resistance (5, 6). Sarcopenia occurs due to either an increase in proteolysis, a reduction in protein synthesis or a combination of the two. Past studies using tracer methodology in patients with cirrhosis to quantify the rate of whole body protein breakdown (WbPB) have yielded conflicting results. In patients with cirrhosis WbPB is either lower or not different compared to that in controls (7, 8). Estimated rates of whole body protein synthesis in cirrhosis have been reported to be lower than in controls (9–11). Concerns about complications from muscle biopsies in cirrhosis have precluded direct quantification of skeletal muscle protein synthesis by measuring incorporation of labeled amino acids into muscle protein. Recent molecular studies in muscle biopsies from patients with cirrhosis and controls have shown increased skeletal muscle expression and nearly fourfold higher plasma concentrations of myostatin in cirrhosis (12, 13). Myostatin is a TGFβ superfamily member that inhibits protein synthesis via impaired mTOR signaling (14). We have also reported increased autophagy markers in the skeletal muscle from cirrhotic patients (15, 16).
Hyperammonemia is a consistent abnormality in cirrhosis due to impaired ureagenesis and portosystemic shunting. Skeletal muscle hyperammonemia in cirrhosis induces transcriptional up-regulation of myostatin and increases autophagy, both of which contribute to sarcopenia (12, 15). Since impaired mTOR signaling decreases protein synthesis as well as increased autophagy, activation of mTOR is a potential approach to reverse impaired muscle protein synthesis in cirrhosis (17, 18). Leucine, a potent direct activator of mTOR, increases muscle protein synthesis and inhibits autophagy (18, 19). Plasma and skeletal muscle concentrations of leucine and other branched chain amino acids (BCAA) are decreased in cirrhosis (20, 21). Previous studies on BCAA supplementation in cirrhosis have shown changes in blood concentrations of amino acids and ammonia and that muscle metabolizes ammonia but the effects on skeletal muscle mass or molecular signaling responses were not reported (5, 22, 23). This may be because skeletal muscle metabolic and molecular responses were not directly evaluated or because skeletal muscle ammonia disposal places an increased demand for leucine that was not provided in these studies. It is also not known if leucine can overcome the anabolic resistance in cirrhosis due to myostatin mediated impaired protein synthesis. Finally, lack of muscle specific beneficial response to BCAA supplementation may be due to decreased intracellular transport of leucine that has also not been evaluated. We therefore hypothesized that cirrhotic patients need a much higher dose of leucine to stimulate mTOR because the carbon skeleton from leucine provides a pathway for skeletal muscle ammonia detoxification (22, 24). The present prospective study is the first of its kind to quantify the acute skeletal muscle molecular and whole body metabolic responses to a leucine enriched BCAA supplement in cirrhosis and control subjects.
Patients and Methods
The study was performed in 14 adult healthy controls (n=8) and patients with alcoholic cirrhosis (n=6). Alcoholic cirrhotics had been abstinent for at least 6 months and were in stable clinical condition defined as Child’s score of ≤7, no gastrointestinal bleeding for at least 3 months, no clinical, microbiological or laboratory evidence of infection, renal failure, encephalopathy, malignancy, diabetes mellitus, comorbidities including heart failure or pulmonary disease, use of medications that affect protein turnover including corticosteroids and β-blockers. Healthy control subjects matched for body composition were also included. Subjects were deemed to be healthy on the basis of clinical history, physical examination and standard laboratory tests including a complete metabolic panel and urine examination.
Body composition was measured by dual energy X-ray absorptiometry (DEXA) [GE Lunar iDEXA,] and the EnCORE software version 11.0 used for analysis of the images. Written informed consent was obtained from each subject before participation in the study, which was approved by the Institutional Review Board and the Clinical Research Unit (CRU) of the Cleveland Clinic, Cleveland.
Design
All subjects who qualified for the study were instructed to consume a controlled protein intake of 0.8g/kg/day and refrain from unusual or excessive physical activity for 3 days prior to the study. After an overnight fast, the fourth day (study day) subjects were admitted to the CRU at the Cleveland Clinic at 7:00AM. On the study day, vital signs were documented, 18G polyethylene catheter was inserted into the antecubital vein of one arm for the infusion of isoosmolar solution contained L-[ring-2H5]-phenylalanine (98% isotopic enrichment) (Cambridge Isotope Laboratories, Andover, MA) prepared on the same day by dissolving the stable isotope in sterile 0.5N saline. The contralateral hand was placed in a heating blanket, and second 18G polyethylene catheter inserted in the dorsal vein to draw arterialized blood samples into EDTA coated vials (as described earlier) (25). After basal blood and urine samples were obtained, muscle biopsies were obtained from the lateral portion of the vastus lateralis (~15–20cm. above the knee) using a 5 mm. Bergstrom biopsy needle (Depuy, Warsaw, IN). Under sterile precautions, the area was marked and 2% lidocaine was injected for local anesthesia. Approximately 150 mg of muscle tissue was obtained during each biopsy. After removing any visible fat and connective tissue, muscle was blotted dry to remove any blood and immediately frozen in liquid nitrogen before being stored at −80°C.
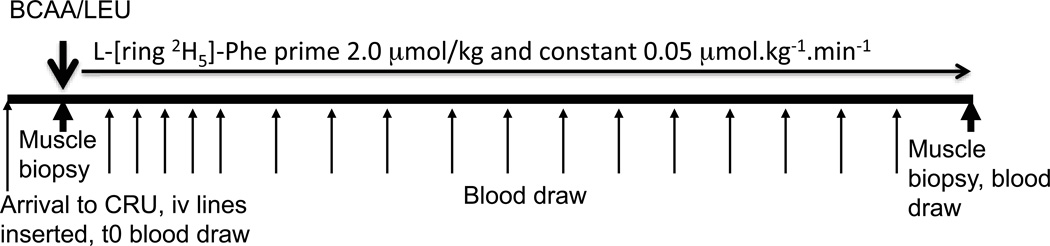
An oral mixture of BCAA/LEU (7.5 g L-leucine, 3.75 g L-isoleucine, 3.75 g L-valine) in granular form (Solvil, Vitaflo USA Inc; Alexandria, VA) was dissolved in 200 ml of non-caffeinated/non-caloric soft drink was administered over 2–3 minutes orally. This mixture was used because leucine enriched BCAA have been reported to assist with skeletal muscle ammonia disposal (22, 26) and thereby improve anabolic resistance. A primed (2.0 µmol/kg) constant (0.05 µmol.kg−1.min−1) infusion of L-[ring-2H5]-phenylalanine was started simultaneously and maintained for 420 minutes. The experimental protocol is shown in Figure 1. Blood samples were drawn at selected time points as shown in Figure 1. At 420 minutes, a second muscle biopsy was performed in the contralateral leg in order to determine the incorporation of the isotope tracer into muscle protein. Amino acid concentrations and L-[ring-2H5]-phenylalanine enrichment were quantified at different time points in the plasma samples.
Figure 1.
Experimental design of the study. Subjects ingested 15 g of leucine enriched branched chain amino acid mixture (BCAA/LEU) containing 7.5g L-leucine, 3.75 gm each of L-isoleucine and L-valine at 0 min. Mixed muscle protein fractional synthesis rate was determined from muscle biopsy samples collected before and after BCAA/LEU ingestion. WbPB was calculated from the dilution of dilution of L-[ring 2H5]-phenylalanine in plasma.
Amino acid measurements
After deproteination using 2% sulfosalicylic acid, free amino acids in plasma were separated by ion exchange chromatography and quantified using known standards. The details are provided in the supplementary methods section (SI).
Sample preparation and liquid chromatography/mass spectrometry/mass spectrometry (LC-MS/MS) analyses for enrichment of L-[ring-2H5]-phenylalanine
Plasma was deproteinized using trichloroacetic acid, centrifuged and plasma tracer L-[ring-2H5]-phenylalanine/tracee (endogenous, unlabeled phenylalanine) were analyzed by LC-MS/MS. Muscle biopsies were processed to quantify enrichment of L-[ring-2H5]-phenylalanine in the cytoplasmic precursor and bound to muscle protein after hydrolysis as described by us earlier (27). The tracer/tracee ratio (TTR) of phenylalanine was determined using an LC-ESI-MS system following 9-fluorenylmethoxy carbonyl (Fmoc) derivatization. The details of the procedure are described in the supplementary methods section (SI).
Skeletal muscle expression of molecular regulators of protein synthesis and breakdown
For immunoblots, total protein was extracted from skeletal muscle and quantified as described by us previously (15). Skeletal muscle expression of myostatin (Abcam, Cambridge, MA), phospho-p70 S6 kinase Thr389, phospho-S6 Ser240/244 and phospho-4EBP1 Thr37/46 (Cell Signaling, Danvers, MA) were quantified by immunoblots using protocols standardized in our laboratory (12, 15). Lipidation of LC3, ATG 5,7 expression, P62 degradation and Beclin1 overexpression were used as readouts for autophagy as described by us previously (15, 16). Total RNA was extracted, reverse transcribed to cDNA and expression of mRNA for leucine transporter SLC7A5/LAT1, glutamine exchanger SLC38A2, autophagy genes and critical proteasome component, MuRF1 were quantified using real time PCR on a Stratagene Mx3000P (Stratagene, LaJolla, CA) using a SYBR protocol on a fluorescence temperature cycler using methods described by us earlier (15). Relative differences were normalized to the expression of β-actin. The primer sequences, gene identification, and product size are shown in supplementary table 1. Real time PCR products were then separated by gel electrophoresis to confirm specific product presence and size.
Calculations
WbPB was calculated from the isotope enrichment values of L-[ring-2H5]-phenylalanine in plasma (28–30) using the tracer/tracee ratio (TTR) in arterialized plasma.
Whole body protein breakdown (Ra of phenylalanine)= Infusion rate/ TTR
Fractional synthesis rates were calculated using the formula:
Where
ΔEp = increment in the muscle protein-bound phenylalanine molar percent excess (MPE) between two biopsies
Eb = average arterial phenylalanine MPE during the isotopic steady state between the two biopsies
T = time interval (min) between the biopsies
Statistical Analyses
All data were presented as mean ± standard error unless stated otherwise. Qualitative variables were compared using the Chi square test. Quantitative variables were compared using the Student’s ‘t’ test for data that was normally distributed and the Mann-Whitney test for skewed data. For serial measurements, paired tests were employed. Amino acid concentrations were compared at multiple time points using ANOVA with Bonferroni post hoc analyses.
Results
The clinical and demographic features of controls and patients with cirrhosis are shown in table 1. The relatively low MELD and Child’s scores were consistent with our inclusion criteria of well-compensated cirrhosis patients. Body composition was similar in the 2 groups. None of the subjects had any complications related to the serial muscle biopsies. Furthermore, despite a small but non-significant increase in blood ammonia concentration (supplementary table 2) over time in the cirrhotic patients, none of the patients showed evidence of change in mental status.
Table 1.
Clinical, Demographic and body composition characteristics
| CONTROLS | CIRRHOSIS | |
|---|---|---|
| Number | 8 | 6 |
| Age | 45.0±3.86(27.2–59.3) | 54.08±1.91(45.8–59.1) |
| Gender (M:F) | 4:4 | 5:1 |
| Serum ALT | 16.71±1.87(8–24) | 42.67±9.70(20–92)** |
| Serum creatinine (mg/dl) | 0.90±0.09(.53–1.15) | 0.87±0.09(.57–1.25)* |
| International Normalized Ratio (INR) | 0.97±0.02(.9–1) | 1.10±0.02(1–1.2)** |
| Serum Albumin | 4.47±0.3(4.1–4.9) | 4.20±0.12(4–4.8) |
| Child Score | 5.17.±0.15 | |
| MELD Score | 6.21±.23 | |
| Body Mass Index (BMI) | 26.75±1.18(23–32.6) | 24.78±.81(22.1–27.5) |
| Body Fat, % | 34.63±2.05(25.2–41.7) | 33.92±4.07(19.9–44.5) |
| Lean Body Mass (LBM), Kg | 48.49±4.12(35.72–72.58 | 47.68±3.93(35.86–61.91) |
| Legs fat mass, kg | 8.63±0.66(5.32–11.17) | 7.54±0.66(5.13–9.75) |
| Lean Legs Mass (LLM), Kg | 16.74±1.43(11.68–23.91) | 15.71±1.21(12.07–17.25) |
| Trunk Fat mass, Kg | 12.88±1.16(9.13–18.39) | 13.80±2.46(4.48–22.28) |
| Trunk Lean Mass, Kg | 22.40±1.93(16.59–34.54) | 22.98±2.25(16.71–30.64) |
All values mean±SEM;
p<0.05,
p<0.01 compared to controls.
Blood phenylalanine enrichment and amino acid concentrations
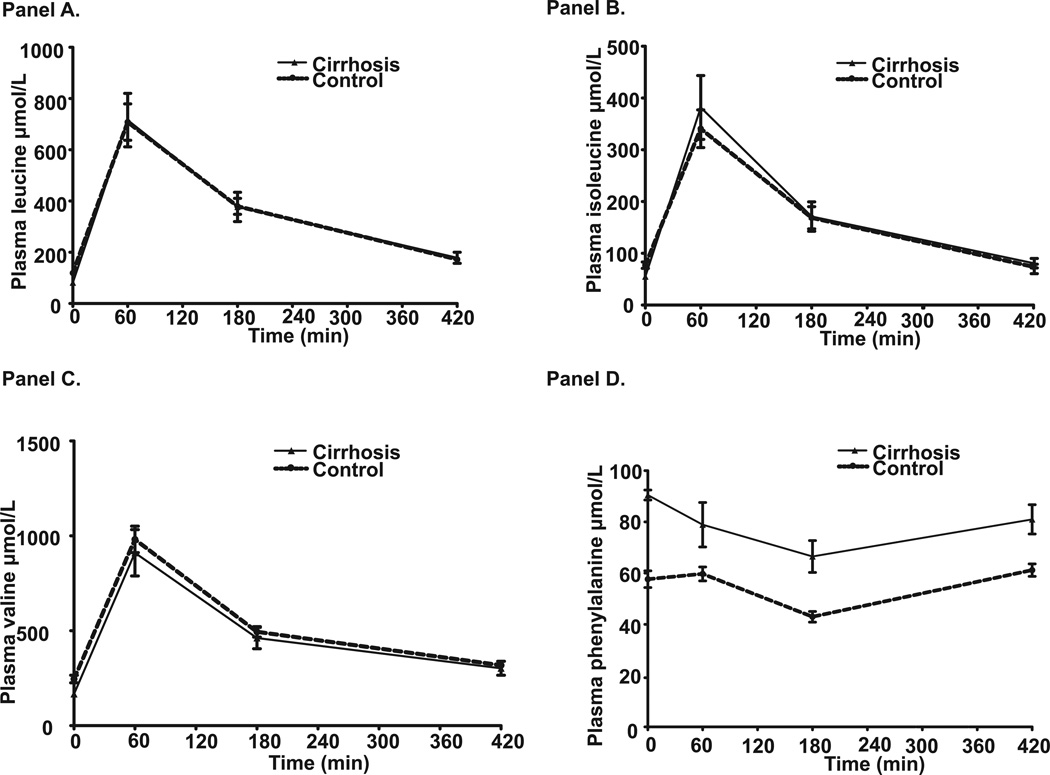
Following the BCAA/LEU ingestion, mean L-[ring-2H5]-phenylalanine enrichment of phenylalanine (t/T) in the cirrhotics was 0.095±0.024 and in the controls was 0.106±0.024 (p>0.1). Phenylalanine enrichment during the course of the study in the 2 groups is shown in supplementary figure 1 and isotopic steady state was achieved within 120 minutes. Plasma amino acid profiles at selected time intervals are shown in supplementary table 2. Plasma leucine, isoleucine and valine were significantly lower in the cirrhotics (p<0.05) while phenylalanine and tyrosine were higher (p<0.05) in cirrhotics as compared to controls at time 0 (basal state). Interestingly there was a rapid increase and subsequent decrease in blood leucine, isoleucine and valine concentrations, which were similar over time in both groups (Figure 2A, B and C). In contrast, phenylalanine (Figure 2D) and other essential amino acids (supplementary table 2) concentrations decreased over time but reached basal state at 420 minutes.
Figure 2.
Plasma amino acid concentration (µmol.l-1) in the basal state and at different time points after ingestion of leucine enriched branched chain amino acid mixture showed lower leucine (Panel A), isoleucine (Panel B) and valine (Panel C) at baseline in cirrhotics compared to controls. Subsequently, the concentrations of branched chain amino acids increased and then decreased in controls and cirrhotics in a similar pattern demonstrating normal absorption in cirrhotics. In contrast, plasma concentrations of phenylalanine (Panel D) decreased in both controls and cirrhotics indicating increased protein synthesis driving the reduction in plasma concentrations of amino acids that were not supplemented.
Muscle protein fractional synthesis rates
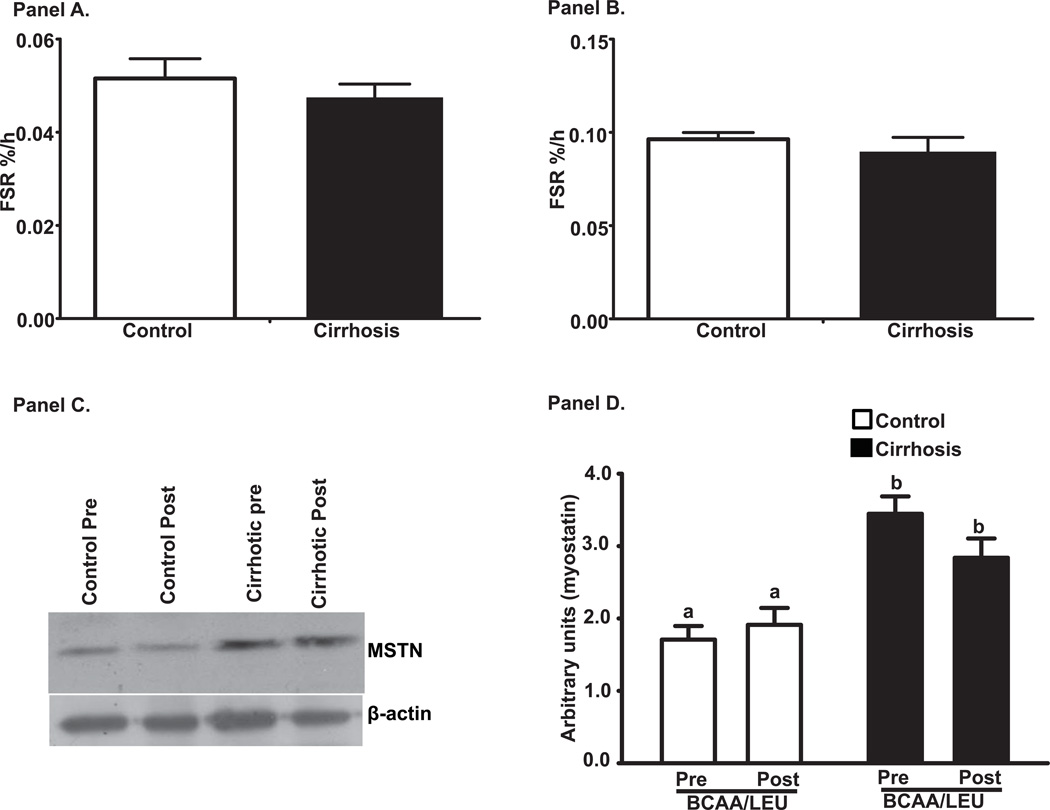
Figure 3 shows the mixed muscle protein FSR for controls and cirrhotics in response to BCAA/LEU. There was no significant difference (p>0.1) in the 2 groups when either the plasma (Figure 3A) or the intracellular precursor pool (Figure 3B) was used for these calculations. Molecular studies showed that expression of myostatin was significantly higher (p<0.001) in cirrhotics compared to controls but did not decrease in response to BCAA/LEU (Figure 3C, D).
Figure 3.
Fractional synthesis rates (%/h) of mixed muscle protein in response to 15g leucine enriched branched chain amino acid mixture (7.5 g L-leucine, 3.75 g L-isoleucine; 3.75 g L-valine). Calculations were done with the plasma (Panel A) and intracellular (Panel B) precursor pools and showed no difference between cirrhosis and controls. Panel C: Representative immunoblots of myostatin expression in skeletal muscle from cirrhotics (n=6) and controls (n=6). Panel D: Densitometry of these blots showed that myostatin expression was significantly higher in cirrhotics compared to controls in the postabsorptive (basal) state and there was no change in response to BCAA/LEU administration. a–b p<0.001. Same alphabets indicate no significant differences. Pre: refers to before the BCAA/LEU (basal/postabsorptive phase) and Post refers to after the BCAA/LEU (supplement).
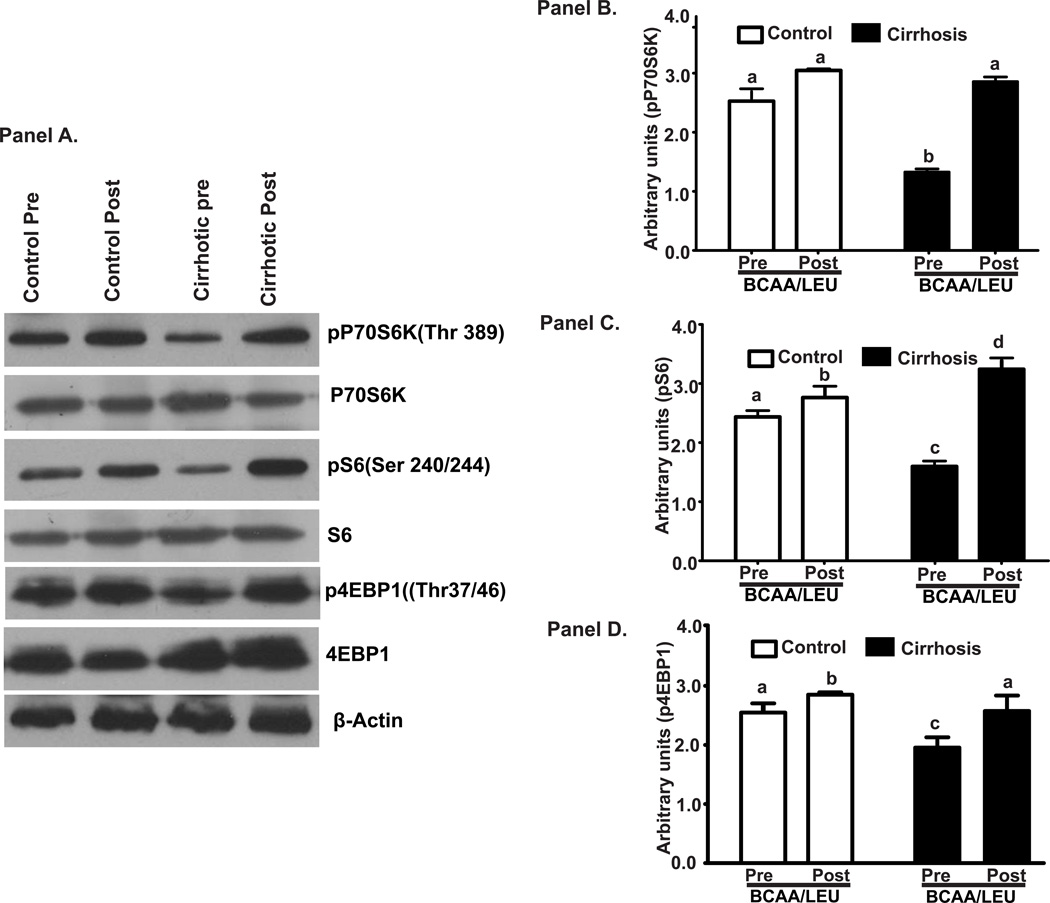
Critical leucine responsive signaling molecules and mTOR1 targets p70s6k, s6 protein and 4EBP1 (Figure 4A, B, C, D) showed impaired mTOR1 activation in the skeletal muscle of cirrhotics at baseline but responded to BCAA/LEU. In contrast, in controls, the mTOR1 activation by leucine did not increase to the same extent as in cirrhotics.
Figure 4.
Panel A: Representative immunoblots of the signaling molecules regulating protein synthesis and mTOR1 activation that included phosphorylation of p70s6k, s6 protein and 4EBP1 in skeletal muscle from cirrhotics and controls. Panels B-D: Densitometry of these blots showed impaired signaling in cirrhotic muscle in the basal/postabsorptive state that increased significantly in both cirrhotics (n=6) and controls (n=6) after BCAA/LEU administration. a–c, b–c, a–d, c–d p<0.001; a–b, b–d,p<0.05. Same alphabets indicate no significant differences. Pre: refers to before the BCAA/LEU (basal/postabsorptive phase) and Post refers to after the BCAA/LEU (supplement).
Whole body protein Ra and muscle proteolysis markers
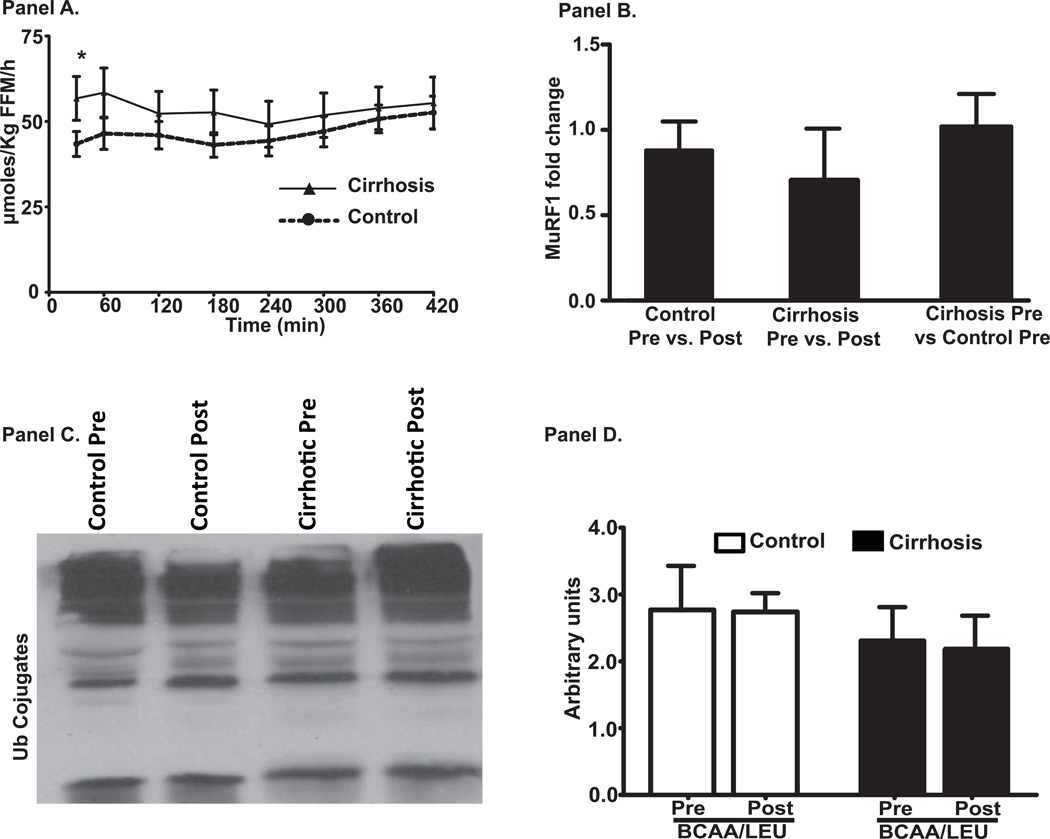
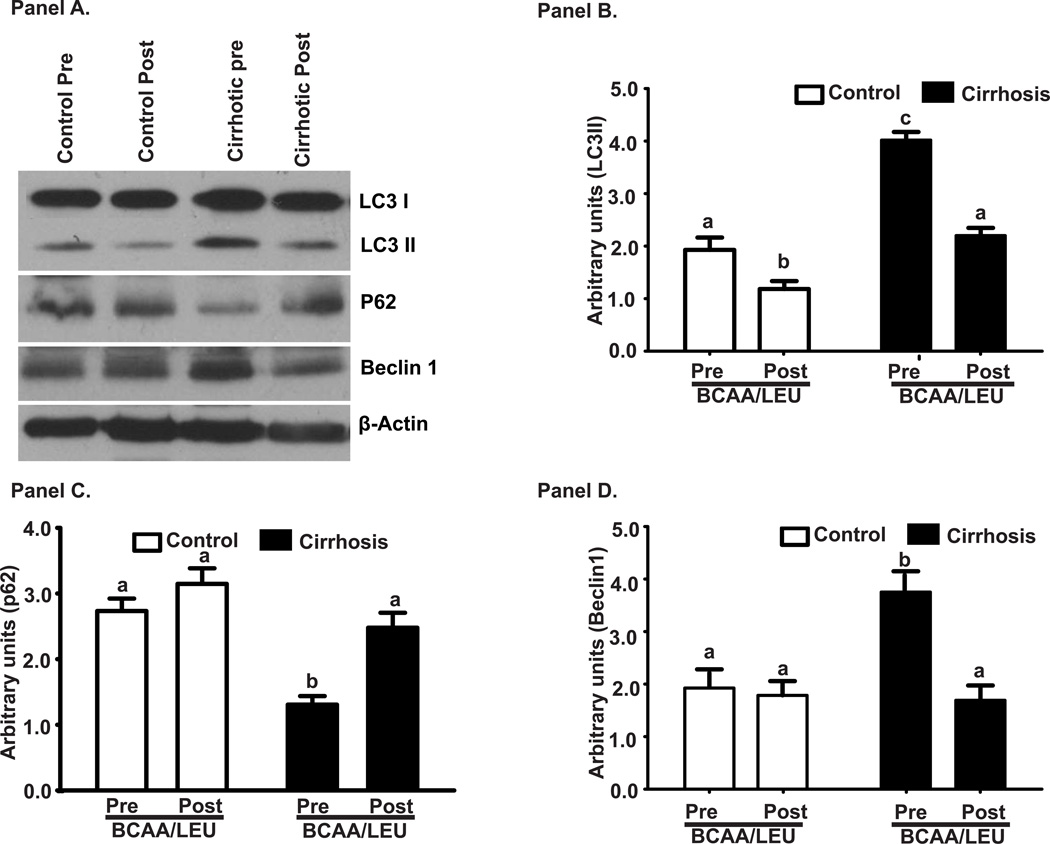
Whole body Ra of phenylalanine, a measure of WbPB was higher (p<0.01) in cirrhotics than controls at baseline (Figure 5A). In response to BCAA/LEU, whole body Ra in cirrhotics was suppressed to nearly control values. In the skeletal muscle, the 2 major proteolysis pathway components were studied. Critical proteasome component MuRF1 mRNA and ubiquitinated protein immunoblots were unaltered in the cirrhotic muscle compared to controls and did not respond to BCAA/LEU (Figure 5B, C, D). In contrast, expression of autophagy markers, LC3 lipidation, P62 degradation and Beclin1 overexpression were significantly increased (p<0.01) in cirrhotic patients compared to controls at baseline and were reversed by BCAA/LEU (Figure 6A–D). These were accompanied by similar changes in the mRNA of ATG5, ATG7, LC3 and Beclin1 (Supplementary Figure 2). The expression of P62 mRNA was significantly lower (p<0.001) after leucine supplementation.
Figure 5.
Panel A: Whole body phenylalanine Ra (measure of proteolysis) showed higher rate of proteolysis in the basal state in cirrhosis compared to controls (*p<0.01). In response to leucine enriched branched chain amino acids, whole body phenylalanine Ra showed a significant decrease in cirrhotics but not in controls. The whole body phenylalanine Ra reached basal values at 7 h after BCAA/LEU ingestion. Panel B: Relative quantification of critical proteasome component, MuRF1 mRNA in cirrhosis and controls showed unaltered expression in cirrhotics compared to controls in the basal/postabsorptive phase and no significant change following BCAA/LEU. Panel C: Representative immunoblots of ubiquitinated proteins in cirrhotics and controls before and after BCAA/LEU (n=6 in each group). Panel D: Densitometry of the blots were similar in both cirrhotics and controls in the basal/postabsorptive phase and their expression did not change in response to BCAA/LEU. Pre: refers to before the BCAA/LEU (basal/postabsorptive phase) and Post refers to after the BCAA/LEU (supplement).
Figure 6.
The expression of autophagy markers, LC3 lipidation, P62 degradation and Beclin1 overexpression were significantly increased (p<0.01) in cirrhotic patients compared to controls at baseline and were reversed by BCAA/LEU
Response to Leucine enriched branched chain amino acid supplementation
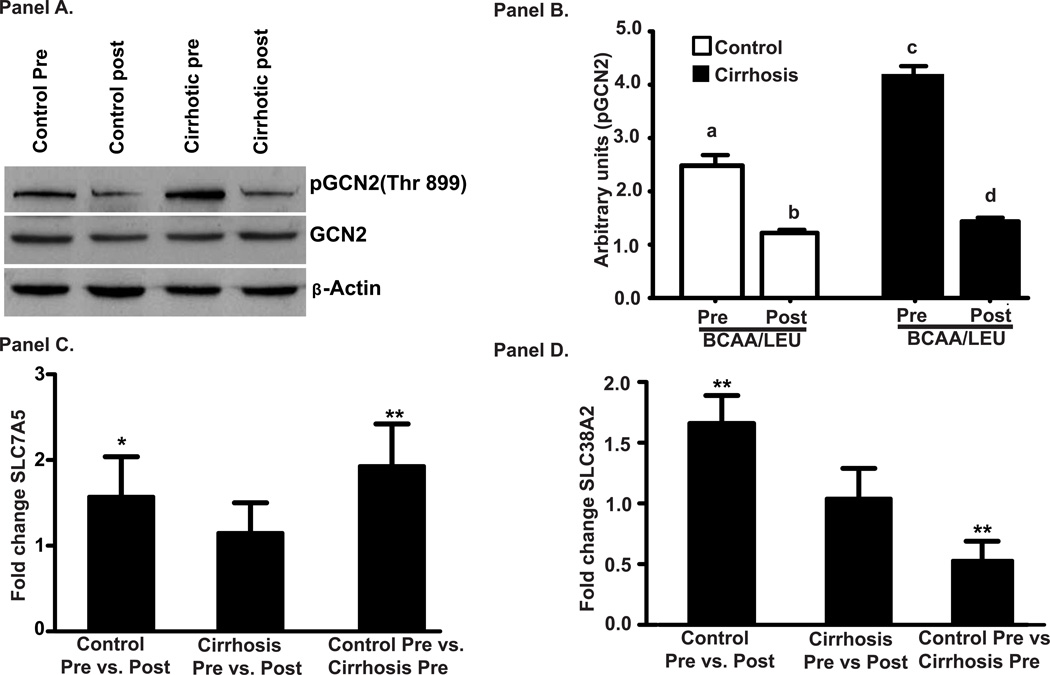
Phosphorylation and activation of GCN2, a critical upstream regulator of mTOR1 activation and a sensor of intracellular amino acid deficiency, was increased (p<0.001) in the muscle of cirrhotic patients compared to controls and was reversed in response to BCAA/LEU (Figure 7A, B). Leucine transporter SLC7A5 mRNA was significantly higher (p<0.01) in cirrhotic patients compared to controls in the basal state and its expression increased (p<0.05) only in controls (Figure 7C). In contrast, expression of glutamine exchanger, SLC38A2 mRNA, was significantly lower (p<0.01) in cirrhotic patients compared to controls in the basal state while its expression in response to BCAA/LEU increased (p<0.01) only in controls (Figure 7D).
Figure 7.
Panel A: Representative immunoblots of intracellular amino acid sensor general control of nutrition derepressed 2 (GCN2) from skeletal muscle protein from cirrhotics (n=6) and controls (n=6). Panel B: Densitometry of these blots showed significantly greater phosphorylation GCN2 in the skeletal muscle of cirrhotics compared to controls in the basal/postabsorptive phase and decreased in response to leucine enriched branched chain amino acid supplementation in both cirrhotic and controls muscle. a–b, a–c, a–d, bc, p<0.001; b–d p<0.05. Panel C: Relative quantification of leucine transporter, SLC7A5 mRNA showed increased expression of SLC7A5 in controls in response to BCAA/LEU (* p<0.05) but not in cirrhotics. However, expression of leucine transporter, SLC7A5 expression in the basal/postabsorptive state was higher (p<0.01) in cirrhotic muscle compared to controls (** p<0.01). Panel D: Glutamine exchanger, SLC38A2 mRNA was significantly increased in control subjects (p<0.01) following BCAA/LEU (** p<0.001) but did not in cirrhotics in response to the supplement. In contrast, glutamine exchanger, SLC38A2 expression was significantly lower (p<0.001) in cirrhotics in the basal/postabsorptive state compared to that in controls. Pre: refers to before the BCAA/LEU (basal/postabsorptive phase) and Post: refers to after the BCAA/LEU (supplement).
Discussion
The present study is the first of its kind to directly quantify muscle protein synthesis rate in mixed muscle protein and identify the molecular perturbations responsible for sarcopenia in cirrhotic patients compared to controls. A single dose of BCAA/LEU supplement not only activated molecular pathways that stimulate protein synthesis but also resulted in similar fractional synthesis rates of mixed muscle protein in cirrhosis and controls. Interestingly, autophagy rather than proteasome mediated skeletal muscle proteolysis contributes to the observed decrease WbPB in response to the BCAA/LEU intake. Absorption and plasma concentrations of BCAA in cirrhosis and controls were similar. Our novel observations provide a compelling rationale for the use of leucine enriched amino acid supplements to stimulate mixed muscle protein synthesis to reverse sarcopenia of cirrhosis.
Sarcopenia in cirrhosis is recognized as a major yet understudied complication in cirrhosis with no effective therapies (5). Previous metabolic studies have quantified the rates of whole body proteolysis and indirectly quantified protein synthesis rates in cirrhosis (7, 31). These data are believed to reflect skeletal muscle protein turnover and synthesis since muscle constitutes approximately 50–60% of protein stores in the mammalian system. The data are conflicting because precise quantification of muscle protein synthesis and identifying the perturbations in regulatory signaling pathways require muscle biopsies. Muscle biopsies have rarely been performed in cirrhosis patients and serial biopsies necessary to quantify tracer incorporation have not been performed due to the perceived risks related to coagulopathy, thrombocytopenia and platelet dysfunction. The present studies demonstrate the safety and feasibility of serial muscle biopsies in well-compensated patients with cirrhosis.
Our metabolic and molecular data show that BCAA/LEU was able to reverse the impaired signaling pathways responsible for protein synthesis and result in similar FSR as that of controls. Of the 3 amino acids administered, leucine is believed to have a potent effect on directly activating mTOR1 either via its effect on Rag proteins on the lysosomal membrane or on the leucine amino acyl transferase (32, 33). Our data are consistent with previous reports that leucine directly activates mTOR1 and increases protein synthesis quantified by incorporation of labeled amino acid (28, 34, 35). Interestingly, elevated myostatin expression was unaltered demonstrating that the direct activation of mTOR by leucine was able to reverse the molecular mechanism of anabolic resistance and resultant protein synthesis.
It is important that studies on protein synthesis be interpreted in the context of proteolysis. Our data show that WbPB was higher in cirrhotic patients than controls and is consistent with previous reports by others in stable patients with cirrhosis (36). This has been interpreted to reflect increased skeletal muscle protein breakdown (8, 10, 36). Proteasomal degradation and autophagy are the major skeletal muscle proteolysis pathways (6). Using molecular tools we demonstrated that autophagy rather than the ubiquitin-proteasome system is responsible for muscle protein breakdown in cirrhosis. These data are consistent with our previous reports that skeletal muscle autophagy markers are increased while those of the proteasome pathway are unaltered in cirrhosis (15, 16). In response to BCAA/LEU, autophagy markers were reduced but the critical proteasome gene MuRF1 and ubiquitination of proteins were not altered. This is consistent with our previous data that ammonia is a potent activator of skeletal muscle autophagy and leucine promotes muscle ammonia disposal with reduction in autophagy in the muscle. The expression pattern of the autophagy genes was similar that of the respective proteins except that of P62 that had an unexpectedly lower expression after leucine administration. This may be due to transcriptional or post transcriptional modifications following the BCAA/LEU and needs to be evaluated further. Our molecular studies on muscle tissue suggest that BCAA/LEU suppresses WbPB by reduction in muscle autophagy. Even though the contribution of other tissues to the whole body tracer dilution was not studied, our complementary metabolic and molecular data provide the first evidence that elevated muscle autophagy and whole body proteolysis in cirrhosis are responsive to nutrient supplementation with BCAA/LEU. We believe that the high dose of leucine is responsible for the reduction in autophagy because leucine inhibits autophagy via direct activation of mTOR1 (37). This is consistent with observations in the present study as well as by others that leucine supplementation activates skeletal muscle mTOR1 (18).
BCAA/LEU resulted in lowering of the molecular markers of autophagy without any significant effect on proteasome component or ubiquitination of skeletal muscle protein and normalized WbPB. Our novel observations suggest that skeletal muscle leucine deficiency increases muscle autophagy as well as WbPB and supplementing leucine reverses this phenomenon. Consistently, GCN2, a measure of intracellular amino acid deficiency (uncharged t-RNA) was activated in the basal or post-absorptive (fasting) state in cirrhosis and this was reversed by BCAA/LEU. GCN2 inhibits mTOR1 and this in turn activates autophagy, a potential mechanism to replenish intracellular nutrient deficiency (38). It is possible that autophagy is increased in cirrhosis due to other mechanisms (15). However, reduction in phosphoGCN2 with reversal of mTOR1 activity and autophagy markers by BCAA/LEU suggests that leucine deficiency mediates the skeletal muscle molecular and metabolic perturbations because isoleucine and valine have not been reported to alter mTOR signaling or autophagy.
Our observations are interesting because previous data on the use of BCAA in hepatic encephalopathy have not reported beneficial effects on muscle mass (5). However, skeletal muscle mass, protein synthesis or alterations in signaling pathways regulating protein synthesis and breakdown were not evaluated in the studies reported to date. Furthermore, this is the first study to have used a large dose of leucine that directly activates mTOR1, bypassing protein kinase B/Akt, the canonical upstream activator of mTOR. The metabolic demand for BCAA is increased in cirrhosis to provide anaplerotic input to compensate for the cataplerotic loss of alpha-ketoglutarate to detoxify ammonia (22, 24, 26). Our data complement previous reports that BCAA deficiency in cirrhosis is due an increased skeletal muscle metabolic demand and a much larger dose of leucine is therefore required for mTOR1 activation(26). An alternative explanation could be that the sensitivity of mTOR1 to leucine is reduced in cirrhosis but has not been evaluated. Other upstream regulators of mTOR including AMP kinase and SIRT1 were not evaluated in this study because more muscle tissue would have been needed than could be safely obtained in this study and should form part of future studies. The present studies lay the foundation for examining the therapeutic potential for the use of leucine supplementation and the mechanisms of sarcopenia in cirrhosis.
Despite a number of studies on amino acid supplementation in cirrhosis, whether cellular uptake of leucine is altered in the cirrhotic muscle is not known. We report for the first time that the leucine transporter, SLC7A5 expression was increased in patients with cirrhosis compared with controls. However, in response to BCAA/LEU, the expression of SLC7A5 increased only in the controls and is similar to that reported by others (39). These data complement our observations that intracellular amino acid concentration does increase as observed by reduction in phospho-GCN2 as well as evidence of leucine signaling via mTOR1 activation. Consistent with our observations on direct molecular and kinetic measures that reflect increased muscle protein synthesis, plasma concentrations of other essential amino acids decreased. This was interpreted to be due to the stimulation of protein synthesis that increases the utilization of these essential amino acids that were not included in the supplement and suggest that other essential amino acids also need to be replaced when leucine is used to stimulate protein synthesis. Interestingly, others have reported that plasma phenylalanine concentrations did not change in response to a single dose of BCAA in cirrhosis(23). This supports our interpretation that the large dose of supplemental leucine is able to overcome the skeletal muscle anabolic resistance in cirrhosis, provide sufficient leucine to meet the anaplerotic demand for muscle ammonia detoxification, decrease autophagy and stimulate protein synthesis. This interpretation is supported by a lower increase in blood ammonia with the BCAA/LEU supplementation in our study than was reported by others with BCAA alone(23).
Even though our data provide strong evidence of the response of the signaling pathways regulating protein synthesis and autophagy, protein synthesis rate in the basal state prior to BCAA/LEU was not studied. This would have complemented our molecular studies by showing that in addition to the reversal of signaling abnormalities by BCAA/LEU, there was an increase in mixed muscle protein FSR in response to the intervention. Responses at different time points after BCAA/LEU were also not studied. All these studies are important but would have required additional muscle biopsies. As mentioned earlier, the feasibility of serial muscle biopsies in cirrhosis has not been established till the conclusion of the present study but should form part of future studies. Another potential limitation was that BCAA/LEU resulted in a reduction in other essential amino acids (EAA), potentially limiting the maximal protein synthesis response suggesting that future studies should include EAA rather than BCAA with a leucine supplement to activate mTOR1. Other limitations include the absence of a group of cirrhotics and controls administered an isonitrogenous, non-essential or balanced amino acids to determine if a nitrogen load may induce these beneficial effect. Others have however reported that a large dose of leucine significantly enhances protein synthesis in physiological states and our data on the molecular and metabolic responses in cirrhosis are consistent with these reports (35, 40). Notwithstanding these limitations, the present studies provide the first direct evidence of potential molecular mechanisms of sarcopenia in cirrhosis, and lay the foundation for novel mechanistic therapies.
Supplementary Material
Acknowledgments
Study was funded in part by a Pilot Project to SD from NIH grant P20 AA017837 (PI: Nagy)
References
- 1.Roongpisuthipong C, Sobhonslidsuk A, Nantiruj K, Songchitsomboon S. Nutritional assessment in various stages of liver cirrhosis. Nutrition. 2001;17:761–765. doi: 10.1016/s0899-9007(01)00626-8. [DOI] [PubMed] [Google Scholar]
- 2.Montano-Loza AJ, Meza-Junco J, Prado CM, Lieffers JR, Baracos VE, Bain VG, Sawyer MB. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol. 2012;10:166–173. 173 e161. doi: 10.1016/j.cgh.2011.08.028. [DOI] [PubMed] [Google Scholar]
- 3.Kalaitzakis E, Simren M, Olsson R, Henfridsson P, Hugosson I, Bengtsson M, Bjornsson E. Gastrointestinal symptoms in patients with liver cirrhosis: associations with nutritional status and health-related quality of life. Scand J Gastroenterol. 2006;41:1464–1472. doi: 10.1080/00365520600825117. [DOI] [PubMed] [Google Scholar]
- 4.Englesbe MJ, Patel SP, He K, Lynch RJ, Schaubel DE, Harbaugh C, Holcombe SA, et al. Sarcopenia and mortality after liver transplantation. J Am Coll Surg. 2010;211:271–278. doi: 10.1016/j.jamcollsurg.2010.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dasarathy S. Consilience in sarcopenia of cirrhosis. J Cachexia Sarcopenia Muscle. 2012;3:225–237. doi: 10.1007/s13539-012-0069-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Periyalwar P, Dasarathy S. Malnutrition in cirrhosis: contribution and consequences of sarcopenia on metabolic and clinical responses. Clin Liver Dis. 2012;16:95–131. doi: 10.1016/j.cld.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCullough AJ, Mullen KD, Kalhan SC. Defective nonoxidative leucine degradation and endogenous leucine flux in cirrhosis during an amino acid infusion. Hepatology. 1998;28:1357–1364. doi: 10.1002/hep.510280526. [DOI] [PubMed] [Google Scholar]
- 8.Tessari P, Inchiostro S, Barazzoni R, Zanetti M, Orlando R, Biolo G, Sergi G, et al. Fasting and postprandial phenylalanine and leucine kinetics in liver cirrhosis. Am J Physiol. 1994;267:E140–E149. doi: 10.1152/ajpendo.1994.267.1.E140. [DOI] [PubMed] [Google Scholar]
- 9.Tessari P, Barazzoni R, Kiwanuka E, Davanzo G, De Pergola G, Orlando R, Vettore M, et al. Impairment of albumin and whole body postprandial protein synthesis in compensated liver cirrhosis. Am J Physiol Endocrinol Metab. 2002;282:E304–E311. doi: 10.1152/ajpendo.00333.2001. [DOI] [PubMed] [Google Scholar]
- 10.Tessari P, Kiwanuka E, Vettore M, Barazzoni R, Zanetti M, Cecchet D, Orlando R. Phenylalanine and tyrosine kinetics in compensated liver cirrhosis: effects of meal ingestion. Am J Physiol Gastrointest Liver Physiol. 2008;295:G598–G604. doi: 10.1152/ajpgi.00355.2007. [DOI] [PubMed] [Google Scholar]
- 11.Morrison WL, Bouchier IA, Gibson JN, Rennie MJ. Skeletal muscle and whole-body protein turnover in cirrhosis. Clin Sci (Lond) 1990;78:613–619. doi: 10.1042/cs0780613. [DOI] [PubMed] [Google Scholar]
- 12.Qiu J, Thapaliya S, Runkana A, Yang Y, Tsien C, Mohan ML, Narayanan A, et al. Hyperammonemia in cirrhosis induces transcriptional regulation of myostatin by an NF-kappaB-mediated mechanism. Proc Natl Acad Sci U S A. 2013;110:18162–18167. doi: 10.1073/pnas.1317049110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia PS, Cabbabe A, Kambadur R, Nicholas G, Csete M. Brief-reports: elevated myostatin levels in patients with liver disease: a potential contributor to skeletal muscle wasting. Anesth Analg. 2010;111:707–709. doi: 10.1213/ANE.0b013e3181eac1c9. [DOI] [PubMed] [Google Scholar]
- 14.Trendelenburg AU, Meyer A, Rohner D, Boyle J, Hatakeyama S, Glass DJ. Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am J Physiol Cell Physiol. 2009;296:C1258–C1270. doi: 10.1152/ajpcell.00105.2009. [DOI] [PubMed] [Google Scholar]
- 15.Qiu J, Tsien C, Thapalaya S, Narayanan A, Weihl CC, Ching JK, Eghtesad B, et al. Hyperammonemia-mediated autophagy in skeletal muscle contributes to sarcopenia of cirrhosis. Am J Physiol Endocrinol Metab. 2012;303:E983–E993. doi: 10.1152/ajpendo.00183.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thapaliya S, Runkana A, McMullen MR, Nagy LE, McDonald C, Naga Prasad SV, Dasarathy S. Alcohol-induced autophagy contributes to loss in skeletal muscle mass. Autophagy. 2014;10:677–690. doi: 10.4161/auto.27918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jung CH, Jun CB, Ro SH, Kim YM, Otto NM, Cao J, Kundu M, et al. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell. 2009;20:1992–2003. doi: 10.1091/mbc.E08-12-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr. 2000;130:2413–2419. doi: 10.1093/jn/130.10.2413. [DOI] [PubMed] [Google Scholar]
- 19.Mordier S, Deval C, Bechet D, Tassa A, Ferrara M. Leucine limitation induces autophagy and activation of lysosome-dependent proteolysis in C2C12 myotubes through a mammalian target of rapamycin-independent signaling pathway. J Biol Chem. 2000;275:29900–29906. doi: 10.1074/jbc.M003633200. [DOI] [PubMed] [Google Scholar]
- 20.Kawaguchi T, Taniguchi E, Sata M. Effects of oral branched-chain amino acids on hepatic encephalopathy and outcome in patients with liver cirrhosis. Nutr Clin Pract. 2013;28:580–588. doi: 10.1177/0884533613496432. [DOI] [PubMed] [Google Scholar]
- 21.Iob V, Coon WW, Sloan M. Free amino acids in liver, plasma, and muscle of patients with cirrhosis of the liver. J Surg Res. 1967;7:41–43. doi: 10.1016/0022-4804(67)90008-x. [DOI] [PubMed] [Google Scholar]
- 22.Hayashi M, Ohnishi H, Kawade Y, Muto Y, Takahashi Y. Augmented utilization of branched-chain amino acids by skeletal muscle in decompensated liver cirrhosis in special relation to ammonia detoxication. Gastroenterol Jpn. 1981;16:64–70. doi: 10.1007/BF02820426. [DOI] [PubMed] [Google Scholar]
- 23.Dam G, Keiding S, Munk OL, Ott P, Buhl M, Vilstrup H, Bak LK, et al. Branched-chain amino acids increase arterial blood ammonia in spite of enhanced intrinsic muscle ammonia metabolism in patients with cirrhosis and healthy subjects. Am J Physiol Gastrointest Liver Physiol. 2011;301:G269–G277. doi: 10.1152/ajpgi.00062.2011. [DOI] [PubMed] [Google Scholar]
- 24.Schachter D, Sang JC. Regional differentiation in the rat aorta for a novel signaling pathway: leucine to glutamate. Am J Physiol. 1997;273:H1484–H1492. doi: 10.1152/ajpheart.1997.273.3.H1484. [DOI] [PubMed] [Google Scholar]
- 25.Dasarathy S, Kasumov T, Edmison JM, Gruca LL, Bennett C, Duenas C, Marczewski S, et al. Glycine and urea kinetics in nonalcoholic steatohepatitis in human: effect of intralipid infusion. Am J Physiol Gastrointest Liver Physiol. 2009;297:G567–G575. doi: 10.1152/ajpgi.00042.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holecek M. Branched-chain amino acids and ammonia metabolism in liver disease: therapeutic implications. Nutrition. 2013;29:1186–1191. doi: 10.1016/j.nut.2013.01.022. [DOI] [PubMed] [Google Scholar]
- 27.Dasarathy S, McCullough AJ, Muc S, Schneyer A, Bennett CD, Dodig M, Kalhan SC. Sarcopenia associated with portosystemic shunting is reversed by follistatin. J Hepatol. 2011;54:915–921. doi: 10.1016/j.jhep.2010.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luiking YC, Deutz NE, Memelink RG, Verlaan S, Wolfe RR. Postprandial muscle protein synthesis is higher after a high whey protein, leucine-enriched supplement than after a dairy-like product in healthy older people: a randomized controlled trial. Nutr J. 2014;13:9. doi: 10.1186/1475-2891-13-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Engelen MP, Com G, Anderson PJ, Deutz NE. New stable isotope method to measure protein digestibility and response to pancreatic enzyme intake in cystic fibrosis. Clin Nutr. 2013 doi: 10.1016/j.clnu.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jonker R, Deutz NE, Erbland ML, Anderson PJ, Engelen MP. Hydrolyzed casein and whey protein meals comparably stimulate net whole-body protein synthesis in COPD patients with nutritional depletion without an additional effect of leucine co-ingestion. Clin Nutr. 2014;33:211–220. doi: 10.1016/j.clnu.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mullen KD, Denne SC, McCullough AJ, Savin SM, Bruno D, Tavill AS, Kalhan SC. Leucine metabolism in stable cirrhosis. Hepatology. 1986;6:622–630. doi: 10.1002/hep.1840060412. [DOI] [PubMed] [Google Scholar]
- 32.Hong-Brown LQ, Brown CR, Kazi AA, Navaratnarajah M, Lang CH. Rag GTPases and AMPK/TSC2/Rheb mediate the differential regulation of mTORC1 signaling in response to alcohol and leucine. Am J Physiol Cell Physiol. 2012;302:C1557–C1565. doi: 10.1152/ajpcell.00407.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han JM, Jeong SJ, Park MC, Kim G, Kwon NH, Kim HK, Ha SH, et al. Leucyl-tRNA synthetase is an intracellular leucine sensor for the mTORC1-signaling pathway. Cell. 2012;149:410–424. doi: 10.1016/j.cell.2012.02.044. [DOI] [PubMed] [Google Scholar]
- 34.Deutz NE, Safar A, Schutzler S, Memelink R, Ferrando A, Spencer H, van Helvoort A, et al. Muscle protein synthesis in cancer patients can be stimulated with a specially formulated medical food. Clin Nutr. 2011;30:759–768. doi: 10.1016/j.clnu.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab. 2006;291:E381–E387. doi: 10.1152/ajpendo.00488.2005. [DOI] [PubMed] [Google Scholar]
- 36.Tessari P, Zanetti M, Barazzoni R, Biolo G, Orlando R, Vettore M, Inchiostro S, et al. Response of phenylalanine and leucine kinetics to branched chain-enriched amino acids and insulin in patients with cirrhosis. Gastroenterology. 1996;111:127–137. doi: 10.1053/gast.1996.v111.pm8698191. [DOI] [PubMed] [Google Scholar]
- 37.Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, Nyfeler B, Yang H, et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136:521–534. doi: 10.1016/j.cell.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chotechuang N, Azzout-Marniche D, Bos C, Chaumontet C, Gausseres N, Steiler T, Gaudichon C, et al. mTOR, AMPK, and GCN2 coordinate the adaptation of hepatic energy metabolic pathways in response to protein intake in the rat. Am J Physiol Endocrinol Metab. 2009;297:E1313–E1323. doi: 10.1152/ajpendo.91000.2008. [DOI] [PubMed] [Google Scholar]
- 39.Drummond MJ, Dickinson JM, Fry CS, Walker DK, Gundermann DM, Reidy PT, Timmerman KL, et al. Bed rest impairs skeletal muscle amino acid transporter expression, mTORC1 signaling, and protein synthesis in response to essential amino acids in older adults. Am J Physiol Endocrinol Metab. 2012;302:E1113–E1122. doi: 10.1152/ajpendo.00603.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rieu I, Balage M, Sornet C, Giraudet C, Pujos E, Grizard J, Mosoni L, et al. Leucine supplementation improves muscle protein synthesis in elderly men independently of hyperaminoacidaemia. J Physiol. 2006;575:305–315. doi: 10.1113/jphysiol.2006.110742. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.