Abstract
Schistosomiasis is a serious parasitic disease in humans, which can lead to liver fibrosis and death. Accumulating evidence indicated that targeting the deregulated microRNAs could mitigate disease outcomes. Here, we showed that progressive hepatic schistosomiasis caused elevation of miR-21 and efficient and sustained inhibition of miR-21 by using highly hepatic tropic adeno-associated virus serotype 8 (rAAV8) protected mice against the lethal schistosome infection through the attenuation of hepatic fibrosis. We demonstrated an additive role of IL-13 and TGF-β1 in up-regulating the miR-21 expression in the hepatic stellate cells (HSCs) by activation of the SMAD proteins. Further, the down-regulation of miR-21 in the HSCs reversed hepatic fibrosis by enhancing SMAD7 expression, thus repressing TGF-β1/Smad and IL-13/Smad pathways.
Conclusion
Our study revealed the mechanism of IL-13-mediated schistosomiasis hepatic fibrosis by up-regulation of miR-21 and highlights the potential of rAAV8-mediated miR-21 inhibition as a therapeutic intervention for hepatic fibrotic diseases, such as schistosomiasis.
Keywords: microRNA, schistosomiasis, hepatic fibrosis, SMAD signaling, gene therapy
Schistosomiasis is a serious parasitic disease throughout the world's tropical regions, affecting over 200 million people.1 The life cycle of Schistosoma japonicum (S. japonicum) is complex. Post-mating, female schistosoma located in the mesenteric veins of the large intestine produce numerous eggs that can be transported via portal veins to the liver. Here, the larval miracidia within the mature eggs secrete toxins that elicit host immune responses including granulomatous inflammation and fibrotic reactions. Liver fibrosis results in portal hypertension and variceal bleeding, and is the primary cause of mortality from schistosomiasis.1
Hepatic fibrosis is a common outcome of chronic liver injuries and occurs when hepatic stellate cells (HSCs) are activated to become proliferative, contractile, and fibrogenic myofibroblasts.2 Activated HSCs secrete excess extracellular matrix (ECM), which is deposited in the liver resulting in fibrosis. HSCs also play an important role in linking hepatic inflammation to fibrogenesis.3
The deregulation of miRNAs is associated with many human diseases.4 Recent investigations revealed that over-expression of miR-21 have been observed in numerous cancers and fibrotic diseases.5-8 Other studies provide further evidence that miRNAs can mediate the outcomes of host-pathogen interactions. For example, miR-155 has an anti-HIV-1 effect by targeting HIV-1 dependency factors,9 while miR-122 appears to regulate replication of the hepatitis C virus.10 These findings have generated much interest in targeting deregulated miRNAs to mitigate human diseases.
As schistosome egg-induced hepatic granulomatous and fibrotic process is complex, we hypothesized that host miRNAs are involved in the pathogenesis of hepatic schistosomiasis. We tested this hypothesis in a well-studied murine model of human schistosomiasis by miRNA profiling and by using a recently developed recombinant adeno-associated virus (rAAV)-based approach for potent and sustained miRNA inhibition in the liver.11 We report that a number of miRNAs are deregulated in the progression of hepatic schistosomiasis and that rAAV-mediated down-regulation of one of these miRNAs (miR-21) provided protection against lethal schistosome infection in a mouse model.
Materials and Methods
Mice and parasite infections
Six-week-old male BABL/c mice were purchased from the experimental animal center of Second Military Medicine University. For infections, mice were exposed percutaneously to 16-30 S. japonicum cercariae which were shed from the lab-infected snails (Oncomelaniahupensis), obtained from the National Institute of Parasitic Disease, Chinese Center for Disease Control and Prevention. All procedures performed on animals within this study were conducted in accordance with and by approval of Second Military Medical University.
Other Materials and Methods
A detailed description of methodologies (miRNA microarray, RT-PCR, western blot, in situ hybridization, primary cell isolation and culture, construction of plasmids and vectors, etc.) used in this study can be found in the Supporting Materials and Methods.
Statistics
Results are reported as mean ± s.d. and compared among groups using two-tailed Student's t- test, one-way ANOVA or Kaplan-Meier method. Data were considered statistically significant for P values less than 0.05.
Results
Identification of altered miRNA expression profiles
To assess miRNA expression profiles in the liver of mice infected with cercaria of S. japonicum, we analyzed the liver samples collected at various time points following the infection by using miRCURYTM locked nucleic acid (LNA) miRNA arrays. We showed significant differences in the miRNA expression profiles of the samples from day 42 onwards but not the early-stage infections (Supporting Fig. 1A). Compared with normal livers, 25 of up-regulated and 9 of down-regulated miRNAs were found in the mice infected with S. japonicum for 70 days (Supporting Fig. 1B). The expression levels of a selected subset of the miRNAs from miRNA array analysis were verified by Real-time PCR (RT-PCR), and the results revealed strong concordance between the two methods (Supporting Fig. 1C).
rAAV8-anti-miR-21-TuD mediated down-regulation of miR-21
To investigate if those up-regulated miRNAs were involved in the progression of hepatic schistosomiasis, we selected four up-regulated miRNAs (i.e. miR-21, miR-223, miR-146a and miR-146b) as the primary candidates for their sustained inhibition using highly hepatic tropic rAAV8 vectors expressing Tough Decoy RNAs (TuDs). 11 The potency and specificity of the four TuD constructs were validated for inhibition of the corresponding miRNAs in HEK293 cells (Supporting Result and Supporting Fig. 2; miR-21 as an example).
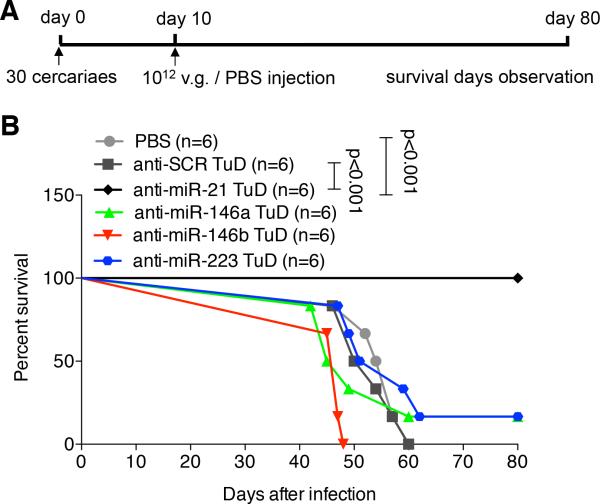
To examine their potential roles in ameliorating schistosomiasis in vivo, mice were first exposed to a lethal dose of S. japonicum cercaria and then intravenously injected with either rAAV8-anti-miR-TuD vectors or the scramble vector or PBS control at 10 days post-infection (Fig. 1A). We found that a single dose of rAAV8-anti-miR-21-TuD protected infected mice from the lethal effect of schistosomiasis. All the mice (n=6) receiving rAAV8-anti-miR-21-TuD survived to the end of the study duration (i.e. 80 days; Fig. 1B, p < 0.001). In contrast, all the mice receiving other rAAV8-anti-miRNA-TuD vectors (3 vector groups, n=6 per group) or the control vector expressing anti-scramble-TuD (n=6) or PBS (n=6) died within 60 days post-infection except for that one mouse each that was treated with rAAV8-anti-miR-146a-TuD and rAAV8-anti-miR-223-TuD respectively, survived to the end of the study duration (Fig. 1B).
Fig. 1.
Down-regulation of miR-21 protected mice from lethal schistosome infection. (A) Time schedule for parasite infection and intravenous injections of various virus constructs or PBS. Mice were infected with 30 S. japonicum cercariae at day 0 and treated with various vectors at a dose of 1×1012 virus genomes, or PBS at day 10 post-infection. The animals were subjected to an 80-day survival study. (B) Kaplan Meier Survival curves were plotted for all groups as indicated.
Attenuation of hepatic fibrosis by the down-regulation of miR-21
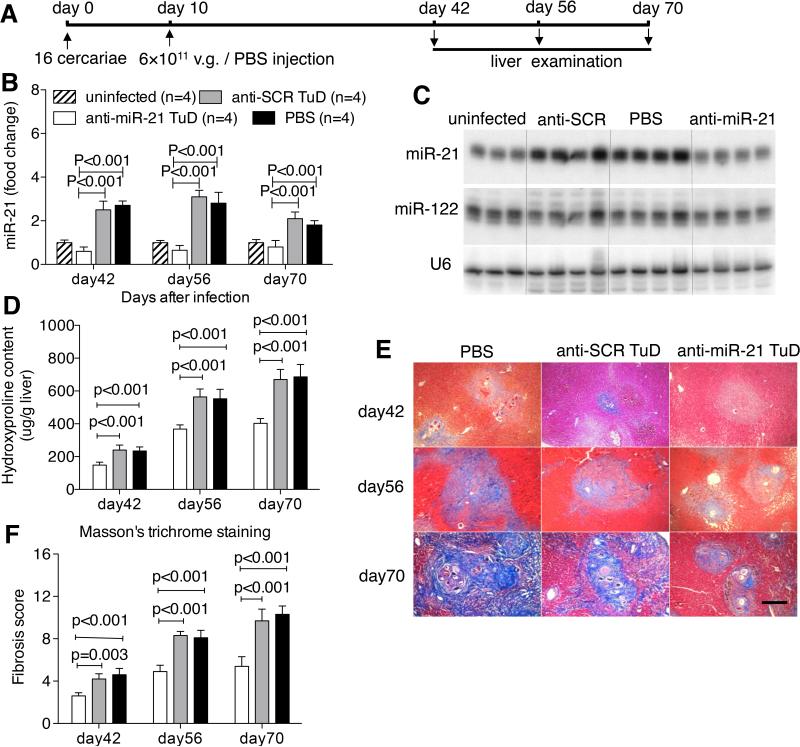
Next, we investigated if the rAAV8-anti-miR-21-TuD-mediated intervention is indeed through effective and specific inhibition of miR-21 activity that in turn attenuates the pathological progression of the disease. To this end, mice were exposed to a mild dose of parasites that would lead to a slower disease progression and prolonged survival and then administered with the rAAV8-anti-miR-21-TuD (Fig. 2A). Our data revealed that the level of miR-21 in the anti-miR-21-TuD treated group was significantly lower than the control groups at all three post-infection time points (Fig. 2B and 2C). To examine possible off-target miRNA inhibition of anti-miR-21-TuD, we also analyzed the levels of hepatic miR-122 that is the most abundant miRNA in liver. Our data confirmed significant reduction of hepatic miR-21 but not miR-122 in the mice receiving rAAV8-anti-miR-21-TuD vector (Fig. 2C and Supporting Fig. 3A). We also quantified the miR-21 levels in the other organs including heart, kidney, lung, pancreas, spleen, brain, and muscle, and found no significant differences of the miR-21 expression among the three groups infected by parasites (Supporting Fig. 4), which could reflect the highly hepatic tropism of AAV8. We assessed hepatic hydroxyproline content and examined liver histopathology. Inhibition of miR-21 in the anti-miR-21-TuD treated animals significantly reduced the hydroxyproline content in their livers by 38.3 ± 8.3%, 34.8 ± 4.4% and 39.9 ± 4.5% at the 3 time points as compared to the control groups (Fig. 2D). Masson's trichrome staining of liver sections showed significantly reduced collagen deposition in the treated mice (Fig. 2E and 2F). Interestingly, the hepatic granulomas in the anti-miR-21-TuD group were also significantly shrunk as compared to those in the control groups (Supporting Fig. 3B and 3C). However, there were no significant differences of the recovered worm and egg burden among these groups, indicating the rAAV-delivered TuD treatments had no effect on the parasite development, reproduction and survivals (Supporting Fig. 3D and 3E). Besides, comparison of liver indexes and transduced vector genomes in the liver showed no significant differences among all study groups (Supporting Fig. 3F and 3G).
Fig. 2.
Down-regulation of miR-21 attenuated schistosomiasis-induced hepatic fibrosis in mice. (A) Time schedule for parasite infection and intravenous injections of virus constructs or PBS and sample withdrawal. Mice were infected with 16 S. japonicum cercariae at day 0 or remained uninfected. Infected mice received various vectors at a dose of 6×1011 virus genomes or PBS at day 10 post-infection. Liver samples were collected at the indicated time points. (B) RT-PCR analysis of expression levels of miR-21 in the liver samples. (C) Northern blots analysis of miR-21, miR-122, and U6 in total RNA from liver of mice after 56-day infection. Three or four biological replicates are shown. (D) Collagen content of the livers determined as hydroxyproline content. (E) Masson's trichrome staining of collagen in the liver sections. Scale bar, 200 μm. (F) Fibrosis scores measured from Masson's trichrome staining liver sections.
rAAV8-anti-miR-21-TuD mediated reduction of hepatocellular damage
Mortality from murine schistosomiasis is also associated with other factors such as the Th1/Th2 responsive cytokines, hepatic and intestinal histopathology as well as sepsis.12 The results from a separate experiment revealed that mice treated with rAAV8-anti-miR-21-TuD showed significant reduction in circulating levels of alanine transaminase (ALT), suggesting the treatment alleviated hepatocellular damage (Supporting Fig. 5A). Conversely, there were no significant differences in serum lipopolysaccharides (LPS) levels among the groups, indicating that down-regulation of miR-21 in liver did not relieve the damage to the intestinal mucosal barrier (Supporting Fig. 5B). Furthermore, lack of significant differences in the levels of IFN-γ and IL-4 among the groups suggested that down-regulation of miR-21 did not alter Th1/Th2 responses to schistosome parasite infection in mice (Supporting Fig. 5C and 5D). Consistent with previous studies,13 IFN-γ levels in all groups decreased after day 35, while IL-4 was non-detectable in all groups at day 35 but dramatically elevated at later time points. This observation was verified by analysis of the intracellular IFN-γ and IL-4 production in the CD4+ T cells isolated from the spleens of study animals (Supporting Fig. 5E, 5F and 5G). In addition, we detected the expression level of TNF-α, IL-5 and IL-10 in the liver tissues, and found no significant differences among all study groups (Supporting Fig. 6).
Therapeutic potential of rAAV8-anti-miR-21-TuD mediated inhibition of miR-21
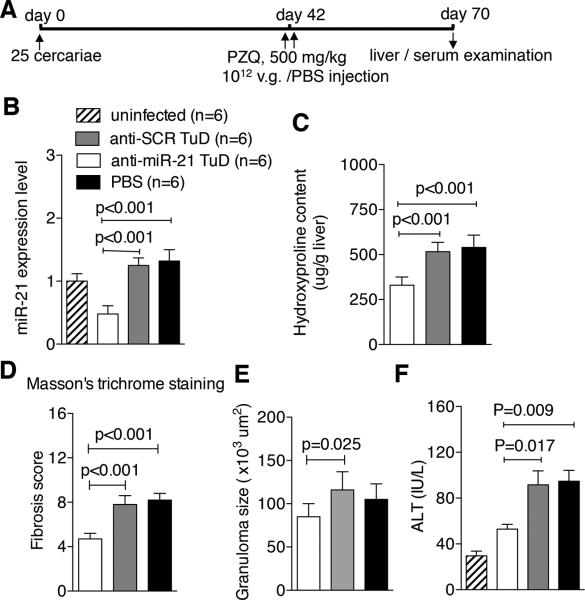
To investigate if the down-regulation of miR-21 can reverse the egg-inducing hepatic fibrosis, mice were infected with a moderate dose of the parasites. At 42 days post-infection, when hepatic fibrosis and egg granulomas became evident, mice were treated with praziquantel. Following administration of praziquantel, mice were injected either with rAAV8 vectors or PBS, and then necropsied at 70 days post-infection (Fig. 3A). Again, the expression of miR-21 was significantly depleted in the rAAV8-anti-miR-21-TuD treated mice (Fig. 3B). Importantly, the rAAV8-anti-miR-21-TuD treatment diminished hydroxyproline content by 36.0 ± 4.1% and collagen expression by 39.7 ± 6.9%, as compared with the controls (Fig. 3C, 3D and Supporting Fig. 7A). Further, such a treatment also reduced the size of egg granulomas by 27.1 ± 13.0% (Fig. 3E and Supporting Fig. 7A) and the ALT level in serum by 44.1 ± 19.1% (Fig. 3F). However, comparison of liver indexes and egg burdens showed no significant differences among all study groups (Supporting Fig. 7B and 7C).
Fig. 3.
Down-regulation of miR-21 partially reversed schistosomiasis-mediated hepatic fibrosis. (A) Time schedule for parasite infection and intravenous injections of virus constructs or PBS and sample withdrawal. Mice were infected with 25 S. japonicum cercariae or remained uninfected. The infected mice were treated with praziquantel to kill the parasites, and then received either the different vectors at a dose of 1×1012 virus genomes or PBS at day 42 post-infection. Liver and serum samples of the mice were collected at day 70 post-infection. (B) RT-PCR analysis of expression levels of miR-21 in the liver samples. (C, D) Collagen content of the livers was measured as (C) hydroxyproline content and (D) fibrosis score determined from Masson's trichrome staining liver sections (Supporting Fig. 6A). (E) Mean area of granuloma measured from Mayer's H&E staining liver sections (Supporting Fig. 6A) using a calibrated measuring eyepiece. (F) Levels of serum ALT.
The IL-13-mediated up-regulation of miR-21 expression in HSCs
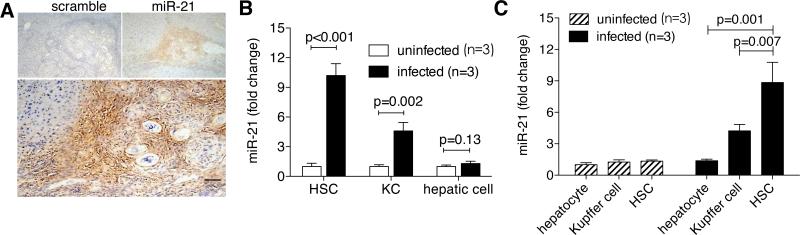
To identify the specific hepatic cell types involved in the up-regulation of miR-21 expression, we used in situ hybridization to detect miR-21 expression in mouse liver sections. The result showed that the hybridization signals were primarily restricted to the small interstitial cells in periphery of egg granulomas (Fig. 4A). Furthermore, the up-regulation of miR-21 was primarily observed in the isolated HSCs (10.2 ± 1.2 folds, p< 0.001) and Kupffer cells (4.6 ± 0.9 folds, p=0.002), but not in hepatocytes of the infected mice (p = 0.13) (Fig. 4B). We also analyzed the relative expression of miR-21 in different hepatic cell types by qPCR and found no significant difference of the expression in HSCs, Kupffer cells and hepatocytes of uninfected livers, while in infected livers, miR-21 expression level was significantly up-regulated in HSC relative to that in Kupffer cells (209%) and hepatocytes (646%) (Fig. 4C).
Fig. 4.
MiR-21 was primarily located in the activated HSCs. (A) In situ hybridization of miR-21 in liver sections from infected mice after 56-day infection. Scale bar, 200 μm. (B) RT-PCR analysis of miR-21 expression levels in HSCs, hepatocytes, and Kupffer cells isolated from livers of three to five uninfected or infected mice after 56-day infection. (C) Hepatocytes, Kupffer cells (KC), HSCs were isolated from livers of uninfected or infected mice (56 days post-infection), and relative miR-21 expression in comparison with hepatocytes of uninfected mice was determined by qPCR.
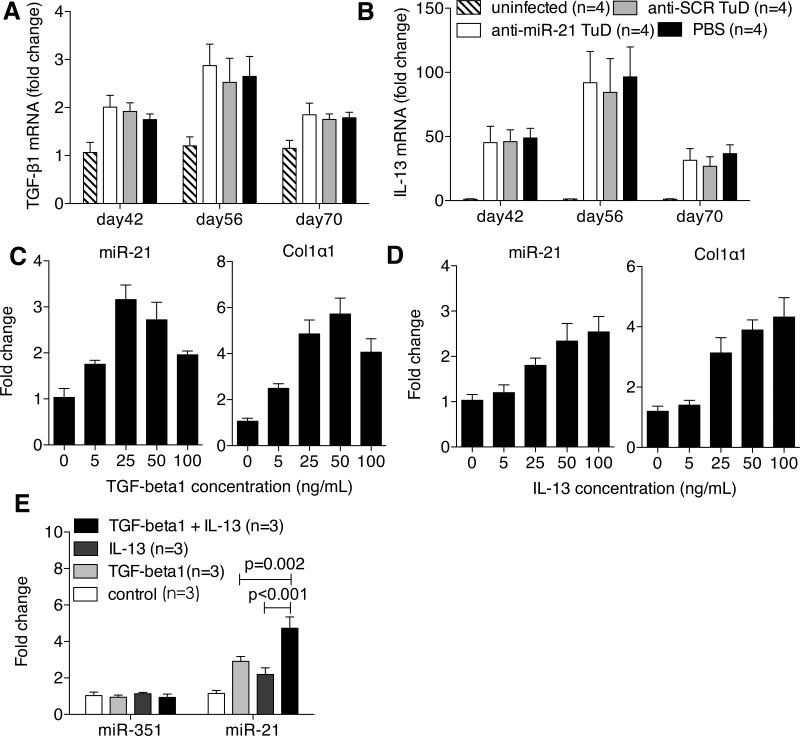
Both TGF-β1 and IL-13 have been identified as major mediators to promote schistosomiasis hepatic fibrosis by activating HSCs.14,15 We demonstrated that the expressions of the two cytokines, particularly of IL-13, were significantly up-regulated in the schistosome infected livers (Fig. 5A and 5B). Moreover, the up-regulation of their expressions seemed to be concurrent with the elevation of miR-21 peaked at day 56 after infection. However, the anti-miR-21-TuD intervention did not alter the expressions of both TGF-β1 and IL-13, indicating that the miR-21 should not involve with regulation of both TGF-β1 and IL-13 expressions.
Fig. 5.
The additive role of the TGF-β1 and IL-13 in the schistosomiasis hepatic fibrosis through up-regulation of miR-21expression. (A, B) RT-PCR analysis of expression levels of TGF-β1 (A) and IL-13 (B) in the infected livers from the 4 groups described in Fig. 2A. (C, D) MiR-21 or Col1α1 expression levels in HSCs after TGF-β1 or IL-13 stimulation. Primary mouse HSCs were treated at day 3 after seeding with 0, 5, 25, 50, 100 ng/mL TGF-β1 or IL-13 for 24 hours. Total RNA was extracted for RT-PCR analysis. (E) Primary mouse HSCs were treated at day 3 after seeding with 25 ng/mL TGF-β1, 50 ng/mL IL-13 or the mixture of 25 ng/mL TGF-β1 and 50 ng/mL IL-13 for 24 hours, respectively. Total RNA was extracted for RT-PCR analysis of miR-21 expression levels.
The TGF-β1/Smad pathway has been shown to induce fibrosis by up-regulating miR-21 expression,6,7 but the mechanism of IL-13-induced hepatic fibrogenesis in schistosomiasis is largely unclear. The fact that miR-21 inhibition effectively attenuated the fibrosis prompted us to ask whether the IL-13-mediated schistosomiasis haptic fibrosis is also through elevating miR-21 expression.We isolated the primary HSCs and stimulated the cells with recombinant IL-13 and TGF-β1, respectively. We found that the TGF-β1 induced both miR-21 and collagen expression as expected (Fig. 5C); but, importantly, addition of IL-13 also resulted in significant increases in both miR-21 and collagen expression in a dose-dependent manner (Fig. 5D). In addition, the combined stimulations of the TGF-β1 and IL-13 led to an additive increase in the miR-21 expression (Fig. 5E). These data indicated that besides TGF-β1, the IL-13 also significantly up-regulated the miR-21 expression in the HSCs and their additive roles in modulating miR-21 expression.
To verify the observation that the down-regulation of miR-21 by the anti-miR-21-TuD intervention did not affect the expression of both TGF-β1 and IL-13 in vivo, we transfected a HSC cell line with either miR-21 mimics or inhibitors (anti-miR-21) in vitro. We found that the transfection with either miR-21 mimics or the inhibitor altered the miR-21 level and production of collagen, but did not affect the expression level of TGF-β1 (Supporting Fig. 8). In addition, we carried out another experiment with a DC cell line that can produce TGF-β1 by induction of SEA (soluble egg antigens of S. japonicum). We showed that the SEA stimulated the DC line to elevate expression of TGF-β1 (Supporting Fig. 8, NC+SEA and Mock+SEA) as compared to that in the control (blank). However, transfection of the DC line with either miR-21 mimics or inhibitor did not affect the expression of TGF-β1 (miR-21+SEA and anti-miR-21+SEA groups), indicating the miR-21 level did not affect production of the TGF-β1 in the cell lines.
The IL-13-mediated up-regulation of miR-21 through the SMAD signaling
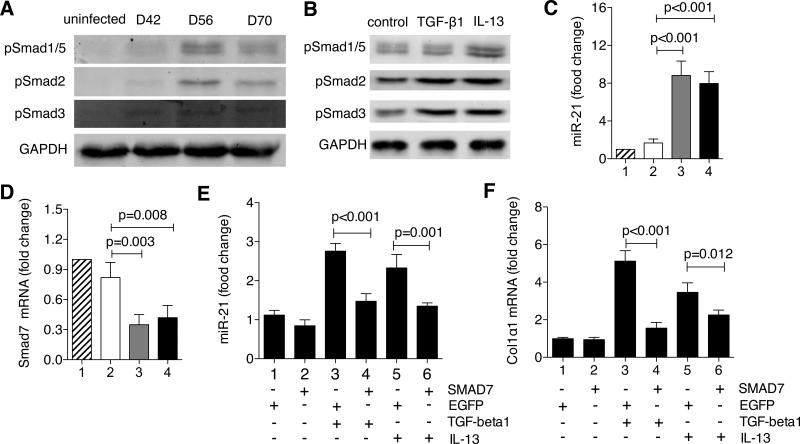
The TGF-β1/SMAD pathway has been shown to up-regulate miR-21 expression through a post-transcriptional regulation in which the activation of SMAD proteins by phosphorylation is required.16 We asked whether the up-regulation of miR-21 upon schistosome infection is also dependent on the activation of SMAD proteins in HSCs. Therefore, we assayed the SMAD proteins in the isolated HSCs of the infected mice by western blot, and found that SMAD proteins 1/5, 2 and 3 were all phosphorylated at all the three time points after infection, which signifies activation of SMAD proteins, and the intensity of p-SMAD proteins peaked at 56 days after infection, well correlated with the trend of miR-21 expression (Fig. 6A). To identify specific molecules that activate the SMAD proteins, we stimulated the mouse primary HSCs with recombinant TGF-β1 and IL-13, respectively, and found that the TGF-β1 activated the SMAD2 and 3 proteins while IL-13 activated all the SMAD proteins tested including SMAD1/5 (Fig. 6B). These data suggested that the activation of SMAD proteins by IL-13, in addition to TGF-β, also trigger the up-regulation of miR-21 upon schistosome infection.
Fig. 6.
The TGF-β1- and IL-13-mediated up-regulation of miR-21 involves the SMAD pathway. (A) Phosphorylation-mediated activations of SMAD proteins were determined by western blot for HSCs isolated from the livers of three to five uninfected or infected mice at various time points. (B) Primary mouse HSCs were treated at day 3 after seeding with 25 ng/mL TGF-β1 or 50 ng/mL IL-13 for 24 hours. Total protein was extracted and analyzed for phospho-SMAD proteins by western blot. (C, D) HSCs were isolated from the livers of three to five mice from the four groups aforementioned after 56-day infection, respectively. Total RNA was extracted for RT-PCR analysis of (C) miR-21 and (D) SMAD7 expression levels. (E, F) Primary mouse HSCs were transfected with either EGFP or SMAD7 plasmids at day 2 after seeding and then treated with 25 ng/mL TGF-β1 or 50 ng/mL IL-13 at day 3 for 24 hours. Total RNA was extracted for RT-PCR analysis of (E) miR-21 and (F) Col1α1 expression levels.
SMAD7 is a major inhibitory regulator of SMAD signaling, but also a target of miR-21.6 We further postulated that the elevated miR-21 activates SMAD proteins by inhibiting SMAD7 in HSCs, resulting in the increased expression of collagens. To this end, we quantified the transcripts of miR-21, SMAD7 and collagens in the isolated HSCs of the infected mice. As expected, significantly elevated miR-21 expression was observed in the infected mice without anti-miR-21-TuD treatment (Fig. 6C, group 3 and 4), resulting in dramatically decreased SMAD 7 expression (Fig. 6D, group 3 and 4 and Supporting Fig. 9B) and thereby increased collagen expression such as collagen I and III (Supporting Fig. 9A, group 3 and 4). In contrast, the anti-miR-21-TuD intervention generated the opposite results as observed in the uninfected group (Fig. 6C, 6D, and Supporting Fig. 9A, 9B; group 1 and 2).
To further study the roles of SMAD7 in the schistosomiasis hepatic fibrosis, we transfected the isolated mouse HSCs with a plasmid expressing SMAD7. The overexpression of SMAD7 significantly decreased the TGF-β1- induced miR-21 and collagen expressions (Fig. 6E and 6F). More importantly, the overexpression also significantly reduced the IL-13-induced miR-21 and collagen expression (Fig. 6E and 6F), further demonstrating that IL-13-mediated hepatic fibrosis be through the up-regulation of miR-21.
Discussion
It is well known that the regulation and function of miRNAs is both organ and cell-type specific. The HSCs are the main source of myofibroblasts in the liver. Our data showed that up-regulation of miR-21 primarily occurred in the HSCs during hepatic fibrosis, which is different from other fibrotic organs such as the fibrotic lung in the myofibroblasts6 and the fibrotic kidney in distal tubular epithelial cells.7,8 Moreover, the regulators of schistosomiasis hepatic fibrosis are far more complicated than expected. Several persuasive infection studies with TGF-β knockout mice demonstrated that TGF-β1 should play a modest role in the pathogenesis of schistosomiasis, while the IL-13 is a critical pro-fibrotic factor in schistosomiasis associated liver fibrogenesis and its profibrogenic effect is TGF-β independent. 15,17 IL-13 canonically signals through activating JAK/STAT6 pathway to exert its effects, but it is controversial whether JAK/STAT6 pathway mediates IL-13-dependent fibrogenesis.18 Recently, Liu et al found that IL-13 activated TGF-β-independent SMAD signaling, and Smad1 and Smad2, rather than Smad3, are key mediators of IL-13–dependent CTGF expression in HSCs via the Erk-MAPK pathway rather than the JAK/STAT6 pathway,18 which indicated that IL-13 exerts its profibrogenic effect in HSCs via SMAD pathway. Here we showed that TGF-β1 and IL-13 additively activated HSCs to produce collagens through up-regulation of the same miRNA (i.e. miR-21) by activation of distinct SMAD proteins, and down-regulation of miR-21 expression by rAAV8-anti-miR-21-TuD in the liver repressed IL-13- and TGF-β1-induced hepatic fibrosis via inhibition of SMAD signaling but not affecting the expression of both TGF-β1 and IL-13. This finding provided substantial basis for developing a miR-21-targeted therapeutic strategy to limit the progression of hepatic fibrosis by feedback regulation of both TGF-β1 and IL-13 pathways.
SMAD7 is a well-documented key antagonist of the SMAD signaling.19 Decreased expression of SMAD7 is always observed in the fibrotic tissue, while gene transfer of SMAD7 has been shown to be an effective method of blocking fibrogenesis. In addition, SMAD7 has been identified as a target gene of miR-21.6 Our data showed that SMAD-regulated collagen expression was significantly reduced by the overexpression of SMAD7, implying that the elevated expression of miR-21 enhanced the production of the collagens by targeting SMAD7 and thereby activation of the SMAD proteins. Thus, we proposed a positive feedback loop for regulation of the process of HSCs activation (Supporting Fig. 10): Infection with schistosome parasites elicits the up-regulation of TGF-β1 and IL-13 expression, which in turn increases the expression of miR-21 in HSCs through activation of SMAD 2 and 3 proteins by TGF-β1 and SMAD1/5, 2 and 3 by IL-13. This elevated expression of miR-21 then prompts TGF-β1/Smad and IL-13/Smad signaling to induce fibrogenic effects by relieving the inhibitory effect of SMAD7 in the Smad pathways. It is worthy pointing out that other signals and pathways that regulate the liver fibrosis through the mediators might also be involved in regulating this process.
MiRNAs have been identified as important regulators of gene expression and their deregulation is a common feature of a variety of human diseases. Therefore, targeting these regulators by gene delivery offers a new miRNA-directed therapeutic approach.20 It is possible that normalizing or correcting the deregulated expression of miRNAs could return a cell from a pathological state to its normal phenotype. As the primary cause of mortality in schistosomiasis is the formation of liver egg granulomas and secondary hepatic fibrosis, we set out to investigate the possibility to mitigate schistosomiasis-mediated liver fibrosis by down-regulating miR-21 expression. However, efficient, long-term and safe delivery of miR-21 inhibitor to the liver is the bottleneck of this strategy. To this end, we exploited recombinant adeno-associated virus (rAAV) as the vector platform for liver gene transfer. rAAV holds the promise for different in vivo gene transfer applications because of its broad tissue tropism, low immunogenicity, long-term episomal persistence, and encouraging track record of safety in a variety of preclinical and clinical studies.21 Particularly, AAV serotype 8-based rAAV has a strong liver tropism,22,23 and proven to be efficacious and safe for liver-directed clinical gene therapy of hemophilia B.24 Importantly, we have recently reported to use rAAV for efficient and long-term delivery of a tough RNA decoy (TuD) to deplete the most abundant hepatic miRNA, miR-122, and regulate cholesterol metabolism in mice.11 Therefore, we opted to use rAAV8 for liver-directed gene transfer of anti-miR-21-TuD to down-regulate miR-21 in the liver of mice with schistosomiasis. We demonstrated that a single administration of rAAV8-anti-miR-21-TuD provided a stable and efficient reduction of miR-21 expression, leading to an increase in expression of its target molecule and a corresponding phenotypic change. The rAAV8-mediated down-regulation of the single miRNA-21 in infected liver effectively prevented the progression of hepatic schistosomiasis and protected mice against the lethal effects of schistosomiasis infection. More importantly, the single administration of rAAV8-anti-miR-21-TuD to the infected mice reversed hepatic fibrosis and reduced the production of collagen and hydroxyproline contents, as well as the size of egg granulomas. Although praziquantel has proven to be an effective anthelmintic drug for destroying the parasite in schistosomiasis, there is no highly effective approach to mitigate hepatic fibrosis caused by schistosomiasis. Our data has clearly illustrated that the rAAV8-mediated miR-21 inhibition alleviates such a hepatic fibrosis. Thus, we may integrate the conventional schistosome parasite-specific anthelmintic drug treatment with miR-21-targeted hepatic fibrosis therapy as a novel combinatory drug-gene therapy to achieve better overall clinical outcomes in the patients with schistosomiasis. In addition, this study highlights the utility of the rAAV8-mediated regulation of miRNAs, particularly miR-21, as a method of gene therapy for the treatment of other human diseases. For example, as miR-21 over-expression has been reported to occur in numerous cancers,25 cardiovascular diseases,26 and in other pathophysiological conditions,5 the findings from our study may have profound implications for the treatment of those human diseases.
Supplementary Material
Acknowledgments
Financial Support
This study was supported by the National Natural Science Foundation of China (no. 81430051) and the National Basic Research Program (973 Program) (2007CB513100) in China, a grant from the National Institutes of Health to G.G. (UL1RR031982) and an internal grant from University of Massachusetts Medical School to GG.
Abbreviations
- ECM
extracellular matrix
- HSCs
hepatic stellate cells
- LNA
locked nucleic acid
- miRNAs
microRNAs
- rAAV
recombinant adeno-associated virus
- RT-PCR
real-time PCR
- TGF
transforming growth factor
- TuD
Tough Decoy
References
- 1.Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet. 2006;368:1106–1118. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- 2.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, et al. TLR4 enhances TGF-β signaling and hepatic fibrosis. Nat Med. 2007;13:1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 4.Hwang HW, Mendell JT. MicroRNAs in cell proliferation, cell death, and tumorigenesis. Br J Cancer. 2006;7:297–304. doi: 10.1038/sj.bjc.6603023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krichevsky AM, Gabriely G. MiR-21: a small multi-faceted RNA. J Cell Mol Med. 2009;13:39–53. doi: 10.1111/j.1582-4934.2008.00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu G, Friggeri A, Yang Y, Milosevic J, Ding Q, Thannickal VJ, et al. MiR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J Exp Med. 2010;207:1589–1597. doi: 10.1084/jem.20100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zarjou A, Yang S, Abraham E, Agarwal A, Liu G. Identification of a microRNA signature in renal fibrosis: role of miR-21. Am J Physiol Renal Physiol. 2011;301:793–801. doi: 10.1152/ajprenal.00273.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signaling in fibroblasts. Nature. 2008;456:980–984. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- 9.Nathans R, Chu CY, Serquina AK, Lu CC, Cao H, Rana TM. Cellular microRNA and P bodies modulate host-HIV-1 interactions. Mol Cell. 2009;34:696–709. doi: 10.1016/j.molcel.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lanford RE, Hildebrandt-Eriksen ES, Petri A, Persson R, Lindow M, Munk ME, et al. Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science. 2010;327:198–201. doi: 10.1126/science.1178178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie J, Ameres SL, Friedline R, Hung JH, Zhang Y, Xie Q, et al. Long-term, efficient inhibition of microRNA function in mice using rAAV vectors. Nat Methods. 2012;9:403–409. doi: 10.1038/nmeth.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herbert DR, Hölscher C, Mohrs M, Arendse B, Schwegmann A, Radwanska M, et al. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity. 2004;20:623–635. doi: 10.1016/s1074-7613(04)00107-4. [DOI] [PubMed] [Google Scholar]
- 13.Pearce EJ, MacDonald AS. The immunobiology of schistosomiasis. Nat Rev Immunol. 2002;2:499–511. doi: 10.1038/nri843. [DOI] [PubMed] [Google Scholar]
- 14.Wahl SM, Frazier-Jessen M, Jin WW, Kopp JB, Sher A, Cheever AW. Cytokine regulation of schistosome-induced granuloma and fibrosis. Kidney Int. 1997;51:1370–1375. doi: 10.1038/ki.1997.187. [DOI] [PubMed] [Google Scholar]
- 15.Kaviratne M, Hesse M, Leusink M, Cheever AW, Davies SJ, McKerrow JH, et al. IL-13 activates a mechanism of tissue fibrosis that is completely TGF-beta independent. J Immunol. 2004;173:4020–4029. doi: 10.4049/jimmunol.173.6.4020. [DOI] [PubMed] [Google Scholar]
- 16.Davis BN, Hilyard AC, Lagna G, Hata A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature. 2008;454:56–61. doi: 10.1038/nature07086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herbert DR, Orekov T, Perkins C, Finkelman FD. IL-10 and TGF-beta redundantly protect against severe liver injury and mortality during acute schistosomiasis. J Immunol. 2008;181:7214–7220. doi: 10.4049/jimmunol.181.10.7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y, Meyer C, Müller A, Herweck F, Li Q, Müllenbach R, et al. IL-13 induces connective tissue growth factor in rat hepatic stellate cells via TGF-β-independent Smad signaling. J Immunol. 2011;187:2814–23. doi: 10.4049/jimmunol.1003260. [DOI] [PubMed] [Google Scholar]
- 19.Dooley S, Hamzavi J, Breitkopf K, Wiercinska E, Said HM, Lorenzen J, et al. SMAD7 prevents activation of hepatic stellate cells and liver fibrosis in rats. Gastroenterology. 2003;125:178–191. doi: 10.1016/s0016-5085(03)00666-8. [DOI] [PubMed] [Google Scholar]
- 20.Wurdinger T, Costa FF. Molecular therapy in the microRNA era. Pharmacogenomics J. 2007;7:297–304. doi: 10.1038/sj.tpj.6500429. [DOI] [PubMed] [Google Scholar]
- 21.Gao GP, Mueller C, Flotte TR. AAV as a gene therapy vector. In: Cucchiarini M, Madry H, editors. Regenerative therapy for the musculoskeletal system using recombinant adeno-associated viral vectors. Research Signpost; Kerala: 2010. pp. 1–21. [Google Scholar]
- 22.Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci U S A. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao GP, Lu Y, Sun X, Johnston J, Calcedo R, Grant R, et al. High-level transgene expression in nonhuman primate liver with novel adeno-associated virus serotypes containing self-complementary genomes. J Virol. 2006;80:6192–6194. doi: 10.1128/JVI.00526-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, Linch DC, et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med. 2011;365:2357–2365. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fleissner F, Jazbutyte V, Fiedler J, Gupta SK, Yin X, Xu Q, et al. Short communication: asymmetric dimethylarginine impairs angiogenic progenitor cell function in patients with coronary artery disease through a microRNA-21-dependent mechanism. Circ Res. 2010;107:138–143. doi: 10.1161/CIRCRESAHA.110.216770. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.