Abstract
Objective
The effects of coronary perivascular adipose tissue (PVAT) on vasomotor tone are influenced by an obese phenotype and are distinct from other adipose tissue depots. The purpose of this investigation was to examine the effects of lean and obese coronary PVAT on end-effector mechanisms of coronary vasodilation and to identify potential factors involved.
Approach and Results
Hematoxylin and eosin staining revealed similarities in coronary perivascular adipocyte size between lean and obese Ossabaw swine. Isometric tension studies of isolated coronary arteries from Ossabaw swine revealed that factors derived from lean and obese coronary PVAT attenuated vasodilation to adenosine. Lean coronary PVAT inhibited KCa and KV7, but not KATP channel mediated dilation in lean arteries. In the absence of PVAT, vasodilation to KCa and KV7 channel activation was impaired in obese arteries relative to lean arteries. Obese PVAT had no effect on KCa or KV7 channel mediated dilation in obese arteries. In contrast, obese PVAT inhibited KATP channel mediated dilation in both lean and obese arteries. The differential effects of obese versus lean PVAT were not associated with changes in either coronary KV7 or KATP channel expression. Incubation with calpastatin attenuated coronary vasodilation to adenosine in lean but not obese arteries.
Conclusions
These findings indicate that lean and obese coronary PVAT attenuates vasodilation via inhibitory effects on vascular smooth muscle K+ channels and that alterations in specific factors such as calpastatin are capable of contributing to the initiation and/or progression of smooth muscle dysfunction in obesity.
Keywords: adipose tissue, coronary, obesity, vasodilation
Introduction
Perivascular adipose tissue (PVAT) surrounds large arteries throughout the body and is capable of producing adipokines that act directly upon the adjacent vasculature.1;2 PVAT-derived factors have been shown to stimulate chemotaxis, inflammation, and endothelial dysfunction, thereby implicating local PVAT signaling in the initiation and progression of vascular disease.3-8 Data from recent studies investigating vascular responses to PVAT-derived factors suggest that the endothelial and smooth muscle effects of these substances are highly dependent on anatomic location of the adipose/vascular depot and the underlying disease state of the subjects from which the tissues were obtained.2 Such discrepant phenotypic effects of PVAT are not surprising given marked differences in protein expression and secretion profiles of adipose tissue depots from lean versus obese subjects.9-15 However, current understanding of how these alterations influence mechanisms of vascular function remains limited.
Differences in the vascular effects of PVAT-derived factors are evident when comparing peripheral (non-cardiac) versus coronary-cardiac PVAT. In particular, aortic,16;17 mesenteric,18;19 and internal thoracic artery20;21 PVAT have been shown to significantly diminish contractile responses to a variety of agonists. This vasodilator or “anti-contractile” effect is attributed to production of adipose-derived relaxing factor(s) (ADRF(s)) that promote endothelial dependent and/or independent vasodilation via activation of voltage-dependent KV7 channels,18 BKCa channels,21;22 and Kir channels.23 In contrast, factors released from coronary PVAT have been shown to attenuate endothelial-dependent dilation5;7 and potentiate coronary artery contractions.12 These deleterious effects of coronary PVAT are augmented in the setting of obesity and are directly associated with marked alterations in the coronary PVAT proteome and the functional expression of coronary K+ and Ca2+ channels.12;24;25 Specifically, our laboratory has recently demonstrated that the endogenous calpain inhibitor calpastatin26;27 is significantly elevated in the secreted protein expression profile of obese coronary PVAT and is sufficient to dose-dependently augment coronary artery contractions in the absence of PVAT.12 Obesity has also been found to diminish the contribution of end-effector K+ channels to coronary vasodilator responses.28 These channels include voltage-dependent (KV), Ca2+-activated (KCa), and ATP-sensitive (KATP) channels, which regulate smooth muscle membrane potential and participate in the regulation of coronary vascular resistance.29 However, the extent to which coronary PVAT-derived factors modulate the role of these channels has not been investigated.
Accordingly, the purpose of this investigation was to delineate the effects of lean and obese coronary PVAT on end-effector mechanisms of coronary vasodilation and to identify potential PVAT-derived factors involved. Studies were specifically designed to test the hypothesis that lean and obese PVAT differentially attenuate KCa, KV, and KATP channel mediated vasodilation in the coronary circulation and that calpastatin contributes to these effects. Findings from this investigation add to growing evidence supporting a role for PVAT in the pathogenesis of vascular dysfunction in obesity-induced coronary disease.
Materials and Methods
Materials and methods are available in the online-only data supplement.
Results
Phenotype of Lean and Obese Ossabaw Swine
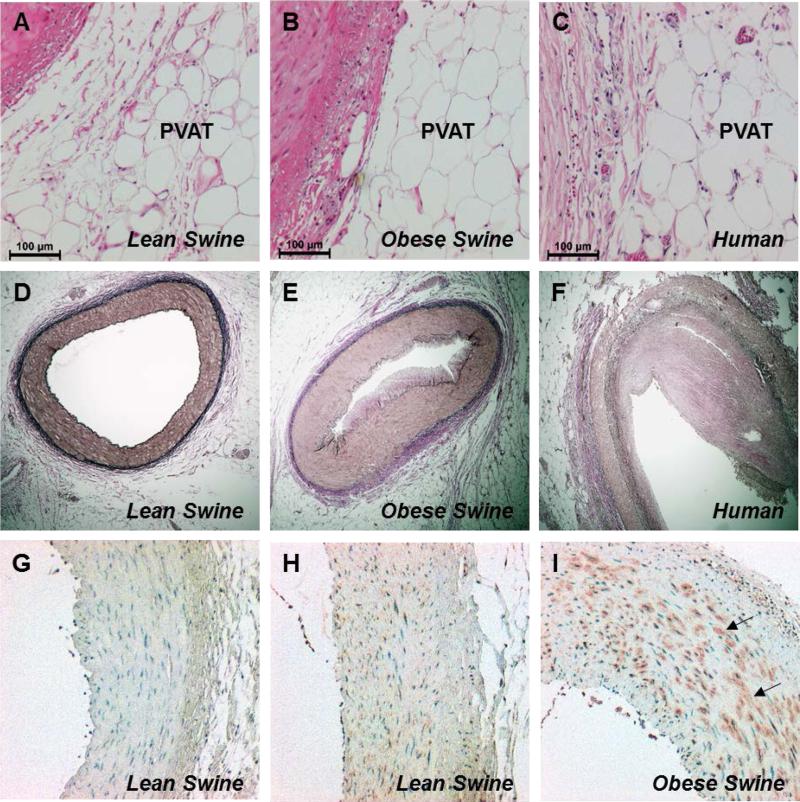
Compared to their lean counterparts, obese swine exhibited significant increases in body weight, fasting glucose, total cholesterol, and triglycerides (Table 1). Histopathological analyses to examine the morphology of perivascular adipocytes were performed on sections of coronary arteries with the adjacent PVAT intact. Hematoxylin and eosin staining revealed apparent similarities in perivascular adipocyte size between lean (Figure 1A) and obese (Figure 1B) swine. Specifically, adipocyte diameter averaged 70 ± 1 μm in lean and 67 ± 2 μm in obese swine (P = 0.24). These values are consistent with measures of coronary perivascular adipocyte diameter (Figure 1C, average = 66 ± 2 μm, n =2) from human subjects with evidence of coronary artery disease (Figure 1F). Verhoeff-van Gieson elastin stain demonstrated the presence of atheroma formation in obese (Figure 1E) compared to lean (Figure 1D) swine. These data are consistent with findings from other studies from our investigative team which documented ~15-20% stenosis of major coronary arteries (using intravascular ultrasound) in obese Ossabaw swine.30-32 Immunostaining for CD163, a marker for cells of the monocyte/macrophage lineage,33 revealed prominent staining in the medial layer of obese arteries (Figure 1I) with only modest staining evident in lean arteries (Figure 1H) relative to isotype control (Figure 1G). These findings are consistent with prior reports of inflammation in coronary arteries from obese swine.34
TABLE 1.
Phenotypic Characteristics of Lean and Obese Ossabaw Swine
| Lean | Obese | |
|---|---|---|
| Body weight (kg) | 62 ± 6 | 100 ± 5* |
| Heart weight (g) | 182 ± 16 | 222 ± 15 |
| Mean arterial pressure (mmHg) | 102 ± 9 | 107 ± 5 |
| Glucose (mg/dL) | 154 ± 14 | 232 ± 21* |
| Insulin (μU/mL) | 12 ± 1 | 14 ± 3 |
| Total cholesterol (mg/dL) | 74 ± 4 | 340 ± 61* |
| Triglycerides (mg/dL) | 46 ± 5 | 78 ± 14* |
Values are mean ± SE for a subset of lean (n=10) and obese (n=10) swine.
P<0.05 vs. lean
Figure 1.
Representative images of immunohistochemical analyses of coronary arteries and associated PVAT obtained from human (n=2), and lean and obese swine (n=4, each group). Hematoxylin and eosin-stained sections (10X) illustrated similarities in perivascular adipocyte morphology between humans and swine (A-C). Verhoeff-van Gieson stained sections (4X) showed evidence of atheroma formation in human (F) and obese (E) compared to lean (D) arteries. CD163 staining (10X) indicated a marked increase in macrophages in obese (I, arrows) compared to lean (H) arteries relative to isotype control (G).
Lean and Obese PVAT Attenuate Coronary Vasodilation
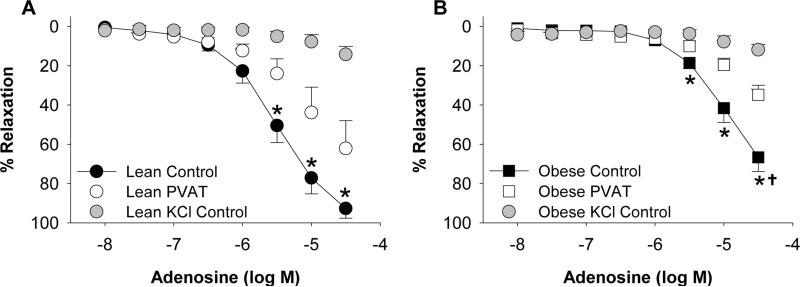
To initially examine the effects of PVAT on vasodilation, coronary arteries cleaned of surrounding PVAT from lean and obese swine were incubated with or without a known quantity of coronary PVAT (0.3 g) from the same animal for 30 min (Figure II in the online-only supplement). Arteries were then pre-constricted with the thromboxane A2 mimetic U46619 (1 μM). Active tension development of control arteries to U46619 (1 μM) in the absence of PVAT averaged 9.01 ± 0.41 g in lean and 10.20 ± 0.61 g in obese arteries (P = 0.09). In arteries treated with PVAT, active tension development averaged 9.61 ± 0.42 g in lean and 9.88 ± 0.53 g in obese arteries (P = 0.68). Vasodilation to adenosine (30 μM) was reduced ~25% in obese (Figure 2B) compared to lean (Figure 2A) arteries in the absence of PVAT (P < 0.001). The presence of PVAT significantly attenuated adenosine relaxation at concentrations >3 μM in arteries from both lean and obese swine. Although maximal responses to adenosine were lower in obese arteries, the overall degree of PVAT inhibition on maximal adenosine-induced dilation (30 μM) was similar in lean (~31%) and obese (~32%) arteries (Figure 2A vs. Figure 2B). Preconstriction with KCl (60 mM), which averaged 7.19 ± 0.22 g in lean and 8.27 ± 0.90 g in obese arteries (P = 0.36), essentially abolished dilation to adenosine in both lean and obese arteries. Additional experiments in endothelium denuded coronary arteries demonstrated that adenosine-induced dilation was unaffected by removal of the endothelium in both control (P = 0.94) and PVAT treated (P = 0.99) arteries. Denudation was confirmed in these studies by <15% relaxation to bradykinin (1μM).
Figure 2.
Coronary PVAT attenuates adenosine induced vasodilation. In control arteries cleaned of PVAT (n=6 each group), maximal vasodilation to adenosine was reduced ~25% in lean (A) compared to obese (B) arteries. The presence of PVAT from the same animal (n=6 each group) impaired dilation to a similar extent and constriction with KCl (n=3 each group) abolished adenosine dilation in lean and obese arteries. *P<0.05, PVAT vs. control. †P<0.001, lean vs. obese control.
Lean Coronary PVAT Inhibits KCa and KV7 Channels
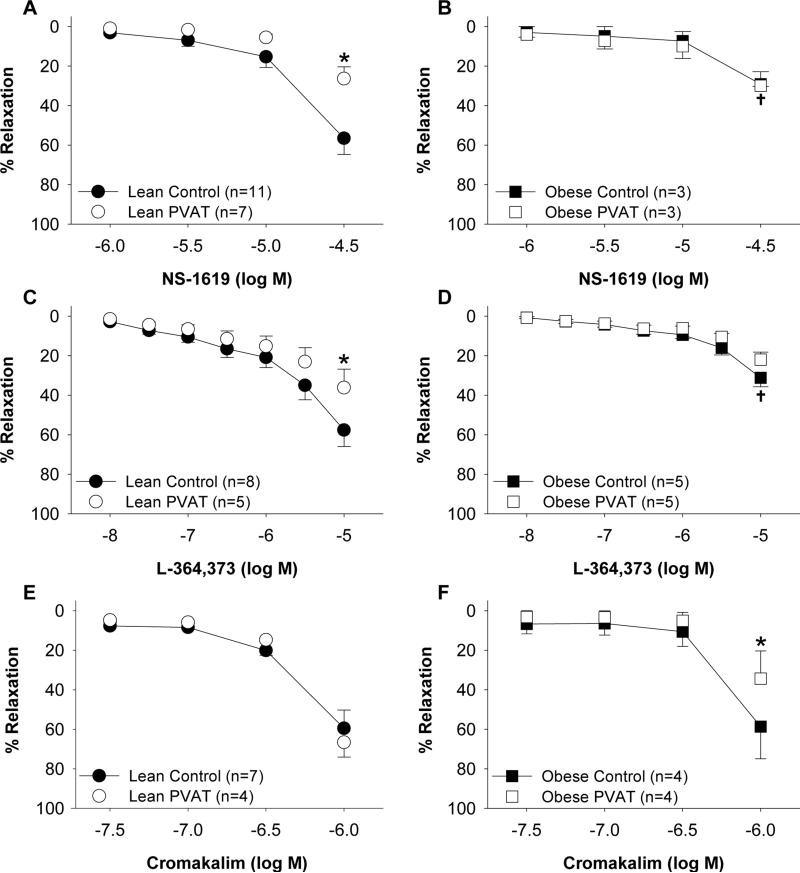
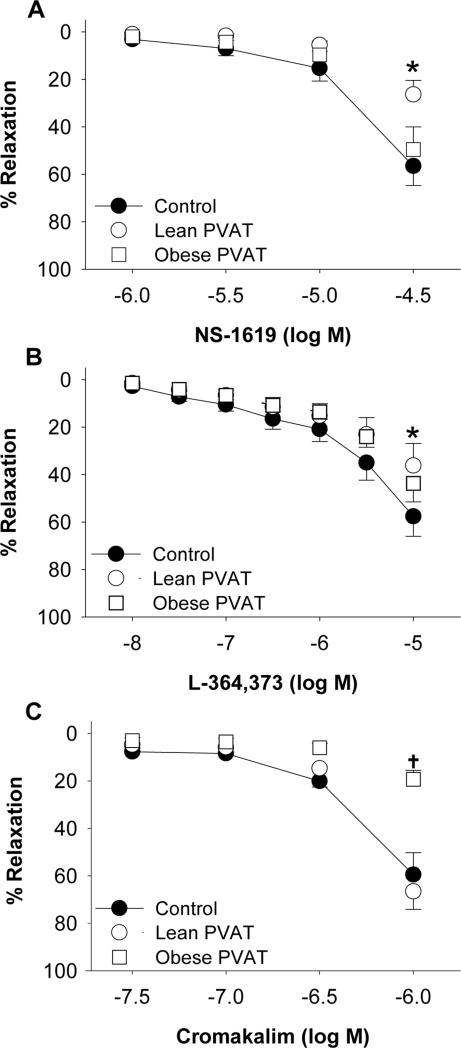
The contribution of KCa channels to coronary vasodilation in lean and obese hearts was examined by comparing responses to the KCa channel agonist NS1619 (1 μM – 30 μM). Overall responses to NS-1619 (30 μM) were reduced ~30% in obese compared to lean control arteries in the absence of PVAT (P = 0.01; Figure 3A vs. Figure 3B). Compared to control responses, the addition of PVAT attenuated dilator responses to the NS-1619 (30 μM) by ~30% in lean arteries (P < 0.001; Figure 3A). In contrast, NS-1619 mediated dilation was unaffected by the addition of PVAT in obese arteries (P = 0.90; Figure 3B).
Figure 3.
Effect of coronary PVAT on K+ channel mediated dilation is altered in obesity. Arteries were incubated in the absence (control) or presence of PVAT from the same animal. PVAT attenuated vasodilation to the KCa channel agonist NS-1619 in lean (A) but not obese (B) arteries. Dilation to the KV7 channel agonist L-364,373 was reduced in the presence of PVAT in lean (C) but not obese (D) arteries. In the absence of PVAT, dilation to NS-1619 and L-364,373 was impaired in obese (B, D) relative to lean (A, C) arteries. KATP channel mediated dilation to cromakalim was unaffected by PVAT in lean (E) arteries but was impaired by PVAT in obese (F) arteries. *P<0.05, PVAT vs. control. †P<0.05, lean vs. obese control.
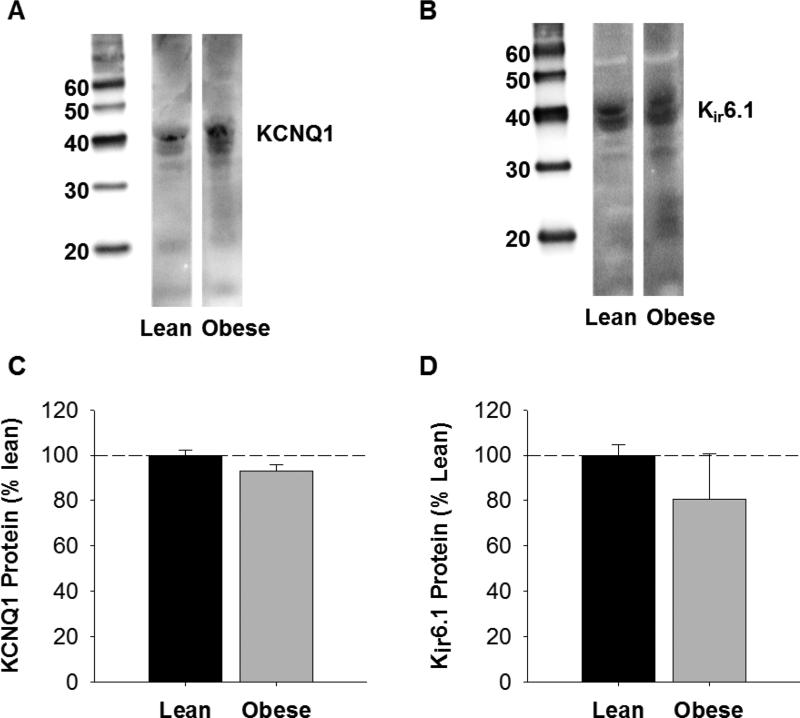
To investigate the role of KV7 channels in coronary vasodilation, responses to the KV7 channel agonist L-364,373 (10 nM – 10 μM) were examined in lean and obese arteries. Dilation to L-364,373 was significantly attenuated at doses >3 μM in obese compared to lean control arteries in the absence of PVAT (P < 0.05; Figure 3C vs. Figure 3D). The presence of PVAT attenuated L-364,373 mediated dilation (10 μM) by ~20% in lean arteries (P = 0.02; Figure 3C) but had no effect in obese arteries (P = 0.98; Figure 3D). Western blot analyses of Kv7 channel (KCNQ1) protein indicated that the abundance of KCNQ1 was not significantly different in lean versus obese coronary arteries (P = 0.11; Figure 4A and 4C). Abundance of β-actin was not significantly different in lean versus obese arteries (P = 0.91), indicating equal protein loading between samples.
Figure 4.
Western blot analysis of coronary artery KCNQ1 and Kir6.1 channel expression. Representative blots of KCNQ1 (A) and Kir6.1 (B) channel expression in lean and obese arteries. Expression levels of both KCNQ1 (C) and Kir6.1 (D) were unaffected by an obese phenotype (P=0.11 and P=0.40, respectively). Average data (n=3 for each group) are expressed as % protein observed in lean swine.
Obese coronary PVAT Inhibits KATP Channels
Studies to investigate the effect of coronary PVAT on KATP channel mediated dilation were performed by comparing responses to the KATP channel agonist cromakalim (30 nM – 1 μM) in lean and obese arteries. Control responses to cromakalim (1 μM) were not significantly different in lean versus obese arteries in the absence of PVAT (P = 0.90; Figure 3E and 3F). The presence of coronary PVAT from the same animal had no effect on dilation to cromakalim (1 μM) in lean arteries (P = 0.57; Figure 3E). In contrast, PVAT significantly attenuated dilation to cromakalim in obese arteries (P = 0.02; Figure 3F). Western blot analyses show that abundance of KATP channel pore forming unit (Kir 6.1) protein was not different in obese compared to lean arteries (P = 0.40; Figure 4B and 4D). Abundance of β-actin was not significantly different in lean versus obese arteries (P = 0.34), indicating equal protein loading between samples.
Differential Effects of Lean versus Obese Coronary PVAT
In order to evaluate the specific effects of lean versus obese PVAT on K+ channel function, independent of differences in coronary artery responsiveness, control coronary arteries (cleaned of PVAT) from lean swine were incubated with known quantities of PVAT (0.3 g) from either lean or obese swine sacrificed on the same day. In contrast to the inhibitory effects of lean PVAT, obese PVAT had no effect on relaxation to NS-1619 (30 μM) or L-364,373 (10 μM) in lean arteries (P = 0.40; Figure 5A and P = 0.10; Figure 5B). Alternatively, obese PVAT significantly attenuated relaxation to cromakalim (1 μM) (P < 0.001; Figure 5C) while lean PVAT had no effect (P = 0.57).
Figure 5.
Effects of lean versus obese PVAT on coronary K+ channel mediated dilation in lean arteries. For comparison purposes, lean control and lean PVAT responses are re-plotted from Figure 2A, 2C and 2E. Obese PVAT had no effect on vasodilation of lean arteries to the KCa channel agonist NS-1619 (n=4 [A]) or the KV7 channel agonist L-364,373 (n=3 [B]). Dilation of lean arteries to the KATP channel agonist cromakalim was attenuated by obese but not lean PVAT (n=3 [C]). *P<0.05 lean PVAT vs. control. †P<0.05, obese PVAT vs. control.
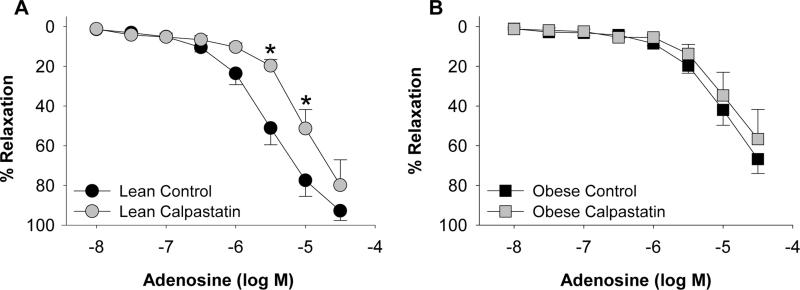
Based on previous findings,12 additional proof-of-principle studies were performed to investigate the effects of calpastatin (10 μM) on coronary vasodilation. Incubation with calpastatin significantly attenuated vasodilation to adenosine (from 3 μM to 10 μM) in lean arteries cleaned of PVAT (P = 0.001; Figure 6A). In contrast, exposure to calpastatin had no effect on adenosine dilation in obese arteries cleaned of PVAT (P = 0.30; Figure 6B).
Figure 6.
Effect of calpastatin on coronary artery vasodilation to adenosine. Incubation with calpastatin (10 μM) attenuated adenosine dilation in lean (n=5 each group [A]) but not obese (n=5 [B]) arteries. *P<0.05 vs. control.
Discussion
This investigation was designed to delineate the effects of lean and obese coronary PVAT on end-effector mechanisms of coronary vasodilation and to identify potential factors involved. The major new findings of this study are: (1) diameters of adipocytes in epicardial coronary PVAT are similar in lean and obese swine (2) factors derived from lean and obese coronary PVAT attenuate vasodilation in response to adenosine; (3) lean coronary PVAT inhibits KCa and KV7 channel mediated dilation but has no effect on KATP channel mediated dilation in lean arteries; (4) coronary vasodilation to KCa and KV7 channel activation is impaired in obese relative to lean arteries in the absence of PVAT; (5) obese PVAT has no effect on KCa or KV7 channel activation in obese arteries; (6) obese PVAT inhibits KATP channel mediated vasodilation in both lean and obese coronary arteries; (7) calpastatin attenuates coronary vasodilation to adenosine in lean but not obese arteries. These findings provide novel evidence that lean and obese PVAT-derived factors attenuate coronary vasodilation via differential inhibition of K+ channels and implicate a mechanistic link between alterations in PVAT-derived factors, such as calpastatin, and diminished functional expression of coronary K+ channels in the setting of obesity.
Although the ability of PVAT to produce transferrable factors that influence the vasculature is well established, current understanding regarding the nature of this effect in specific adipose tissue depots remains rather limited. While the majority of studies on peripheral (non-cardiac) PVAT support the production of ADRF(s) and an overall “anti-contractile” effect,17-22;35-38 recent data indicate that coronary PVAT is unique relative to these other adipose depots both in its expression profile and effects on the vasculature.6;10;12 In particular, factors derived from coronary PVAT have been found to attenuate endothelial-dependent dilation and potentiate coronary artery contractions, especially in the setting of obesity.5-7;12 Other studies in lean or hypercholesterolemic swine show little/no anti-contractile effect of coronary PVAT in response to endothelin-1, angiotensin II, or the thromboxane A2 mimetic U46619.6;39;40 Results from the present study further support the distinct vascular effects of coronary PVAT in that lean and obese coronary PVAT significantly attenuate coronary vasodilator responses to adenosine. This impaired dilator response is directly related to effects of PVAT-derived factors on smooth muscle K+ channels, as adenosine-induced dilation was unaffected by endothelial denudation and was essentially abolished by pre-constriction with KCl (Figure 2A and 2B). Inhibitory effects of PVAT on K+ channels have significant (patho)physiologic implications as prior studies have clearly demonstrated the contribution of KV and KATP channels to the regulation of coronary microvascular tone29;41 and KCa channels in endothelial-dependent dilation.42;43
The present findings provide novel evidence that lean and obese PVAT have distinct inhibitory effects on specific K+ channel subtypes in lean and obese coronary arteries. Specifically, factors derived from lean coronary PVAT impair KCa and KV7 channel mediated dilation, while factors derived from obese coronary PVAT attenuate KATP channel mediated dilation (Figure 3). The lack of effect of obese coronary PVAT on KCa and KV7 channels was observed in both obese (Figure 3) and lean (Figure 5) coronary arteries and is thus not related to intrinsic differences in smooth muscle phenotype of lean versus obese swine. Therefore, the “cross-over” studies in which lean arteries (i.e. with normal vascular smooth muscle function) were incubated with lean and obese PVAT, strongly support that lean and obese PVAT-derived factors differentially affect KCa, KV7, and KATP channels. This distinction is important as we found that coronary vasodilation in response to KCa and KV7 channel agonists is attenuated in obese relative to lean arteries in the absence of PVAT (Figure 3). These data are consistent with prior work from our laboratory and others which demonstrated the functional down-regulation of BKCa and KV channels in the coronary circulation24;25;44-46 and suggest the potential for PVAT-derived factors to contribute to the initiation and progression of coronary vascular dysfunction in the setting of obesity.
There are several potential mechanisms that could contribute to the effects of PVAT-derived factors on coronary K+ channels. In particular, it does not appear that differences in K+ channel expression levels are responsible for the divergent effects of PVAT, as Western analyses revealed similar levels of KV7 (KCNQ1) and KATP (Kir6.1) channels in coronary arteries of lean and obese swine (Figure 4). Previous studies from our group also found augmented expression of BKCa channel subunits in coronary arteries of obese swine.25 However, it is possible that expression of other channel subtypes and/or subunits could be altered in the setting of obesity. The potential for direct effects of PVAT-derived factors on specific coronary K+ channels is supported by prior evidence that NS-1619, L-364,373, and cromakalim directly open KCa, KV, and KATP channels (i.e. without activating transmembrane signaling pathways), as these agonists have been shown to bind to sites on channel subunits and increase the open probability of excised membrane patches.47-49 Additionally, cellular signaling pathways also influence the response of these K+ channels to their respective agonists. For example, ischemic stimuli and Rho kinase signaling influence the response of KCa channels to NS-1619, 50;51 while the effects of L-364,373 on KV7 channels may intersect with ERK signaling52 and PKC alters the KATP channel response to cromakalim.53 Therefore, it is possible that cellular signaling, including post-translational modifications such as phosphorylation, influences the response of vascular smooth muscle K+ channel activation in the presence of PVAT.54 Such effects are in line with previous studies from our laboratory which demonstrated that coronary PVAT influences both PKC and Rho kinase signaling.6;12 Whether coronary PVAT-derived factors interact with K+ channels directly and/or influence their function indirectly through intracellular signaling pathways warrants further investigation.
Identification of the precise factors responsible for the vascular effects of coronary PVAT remains a daunting task. Recent studies by the Weintraub laboratory have established that adipocytes from human coronary PVAT have a distinct phenotype and exhibit elevated expression of pro-inflammatory genes and genes associated with angiogenesis, coagulation, and vascular morphology.9;10;55;56 Evidence of macrophage infiltration (Figure 1I) and atheroma formation (Figure 1E) in obese arteries support a potential role for inflammatory cross-talk between PVAT and the artery wall in the pathogenesis of atherosclerosis. A previous global proteomic assessment revealed the up-regulation of proteins associated with cellular growth, proliferation, and movement in obese versus lean coronary PVAT from swine.12 These differences appear to be independent of gross changes in morphology, as similarities in adipocyte diameter were found between lean and obese PVAT (Figure 1A and 1B). Of particular interest is the endogenous calpain inhibitor, calpastatin, which we have shown to be significantly elevated in supernatant of obese coronary PVAT and to dose-dependently augment coronary artery contractions.12 Findings from the current investigation further support that calpastatin is capable of mimicking the effects of coronary PVAT in that it acts to impair smooth muscle dilation in response to adenosine in lean coronary arteries (Figure 6A). The loss of this effect in obese coronary arteries (Figure 6B) is consistent with the lack of effect of obese PVAT on KCa and KV7 channel mediated dilation in obese arteries (Figure 3B and 3D), and suggests that chronic local exposure of the coronary circulation to factors such as calpastatin could contribute to the impairment of smooth muscle function in the setting of obesity.
It is important to recognize that although a differential effect of lean versus obese PVAT on vascular function was demonstrated in lean, healthy arteries, the effect of lean PVAT on obese arteries was not examined in this investigation. Additionally, findings of the present study were produced following short-term (~30 min) exposure to PVAT ex vivo. Thus, a critical question remains as to whether chronic exposure of the coronary vasculature to the PVAT milieu directly contributes to the altered functional expression of K+ channels in the setting of obesity. We propose that as the severity of obesity and other cardiovascular risk factors (insulin resistance, hypercholesterolemia, hypertension) progresses, changes in the secretion profile of coronary PVAT adversely influences the function and expression of coronary ion channels. However, the extent to which phenotypic alterations in coronary PVAT causally contribute to mechanistic alterations in the obese coronary circulation merits further study.
In summary, the current findings demonstrate that although coronary perivascular adipocytes from lean and obese swine share similar morphology, lean and obese PVAT-derived factors impair vasodilation via differential inhibition of vascular smooth muscle K+ channels. Specifically, our data are the first to demonstrate that lean coronary PVAT attenuates KCa and KV7 channel mediated dilation, while obese coronary PVAT impairs KATP channel mediated dilation. These results further support the paradigm of distinct “outside to inside” signaling influences of coronary PVAT and that alterations in specific factors such as calpastatin are capable of contributing to the initiation and/or progression of smooth muscle dysfunction in the setting of obesity.
Supplementary Material
Significance.
Coronary perivascular adipose tissue (PVAT) normally surrounds the major coronary arteries of the heart. Evidence is mounting to support the potential for factors derived from coronary PVAT to influence the pathogenesis of coronary vascular disease. In particular, recent studies indicate that coronary PVAT-derived factors initiate/potentiate contraction of vascular smooth muscle, a property distinct from other adipose tissue depots, and that this effect is significantly augmented in the setting of obesity. However, the effects of coronary PVAT on vasodilation have not been clearly defined. Results from this investigation indicate that coronary PVAT attenuates dilation via inhibitory effects on vascular K+ channels and that the mechanisms and factors involved in mediating these effects are markedly altered in the setting of obesity. These findings provide new insights into the unique vasoactive properties of coronary PVAT and the potential role of PVAT-derived factors in obesity-induced coronary disease.
Acknowledgements
None
Sources of Funding
This publication was made possible in part by the Indiana University Health – Indiana University School of Medicine Strategic Research Initiative (CECARE); HL117620 (Tune-Mather); TL1 TR000162 (Noblet; Sassoon); 13POST1681001813 (Goodwill)
Nonstandard Abbreviations and Acronyms
- PVAT
perivascular adipose tissue
Footnotes
Disclosures
None
Reference List
- 1.Boydens C, Maenhaut N, Pauwels B, Decaluwe K, Van d V. Adipose tissue as regulator of vascular tone. Curr Hypertens Rep. 2012;14:270–278. doi: 10.1007/s11906-012-0259-6. [DOI] [PubMed] [Google Scholar]
- 2.Owen M, Noblet J, Sassoon D, Conteh A, Goodwill A, Tune J. Perivascular adipose tissue and coronary disease. Arterioscler Thromb Vasc Biol. 2014;34:1643–1649. doi: 10.1161/ATVBAHA.114.303033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henrichot E, Juge-Aubry CE, Pernin A, et al. Production of chemokines by perivascular adipose tissue: a role in the pathogenesis of atherosclerosis? Artriosclerosis Thrombosis & Vascular Biology. 2005;25:2594–2599. doi: 10.1161/01.ATV.0000188508.40052.35. [DOI] [PubMed] [Google Scholar]
- 4.Juge-Aubry CE, Henrichot E, Meier CA. Adipose tissue: a regulator of inflammation. Best Pract Res Clin Endocrinol Metab. 2005;19:547–566. doi: 10.1016/j.beem.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Payne GA, Bohlen HG, Dincer UD, Borbouse L, Tune JD. Periadventitial adipose tissue impairs coronary endothelial function via PKC-beta-dependent phosphorylation of nitric oxide synthase. Am J Physiol Heart Circ Physiol. 2009;297:H460–H465. doi: 10.1152/ajpheart.00116.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Payne GA, Borbouse L, Kumar S, et al. Epicardial perivascular adipose-derived leptin exacerbates coronary endothelial dysfunction in metabolic syndrome via a protein kinase C-beta pathway. Arterioscler Thromb Vasc Biol. 2010;30:1711–1717. doi: 10.1161/ATVBAHA.110.210070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Payne GA, Borbouse L, Bratz IN, et al. Endogenous adipose-derived factors diminish coronary endothelial function via inhibition of nitric oxide synthase. Microcirculation. 2008;15:417–426. doi: 10.1080/10739680701858447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verhagen SN, Visseren FL. Perivascular adipose tissue as a cause of atherosclerosis. Atherosclerosis. 2011;214:3–10. doi: 10.1016/j.atherosclerosis.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 9.Chatterjee TK, Stoll LL, Denning GM, et al. Proinflammatory phenotype of perivascular adipocytes: influence of high-fat feeding. Circ Res. 2009;104:541–549. doi: 10.1161/CIRCRESAHA.108.182998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chatterjee T, Aronow B, Tong W, et al. Human coronary artery perivascular adipocytes overexpress genes responsible for regulating vascular morphology, inflammation, and hemostasis. Physiol Genomics. 2013;45:697–709. doi: 10.1152/physiolgenomics.00042.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baker AR, Silva NF, Quinn DW, et al. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc Diabetol. 2006;5:1. doi: 10.1186/1475-2840-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Owen MK, Witzmann FA, McKenney ML, et al. Perivascular Adipose Tissue Potentiates Contraction of Coronary Vascular Smooth Muscle: Influence of Obesity. Circulation. 2013;128:9–18. doi: 10.1161/CIRCULATIONAHA.112.001238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng KH, Chu CS, Lee KT, et al. Adipocytokines and proinflammatory mediators from abdominal and epicardial adipose tissue in patients with coronary artery disease. Int J Obes (Lond) 2008;32:268–274. doi: 10.1038/sj.ijo.0803726. [DOI] [PubMed] [Google Scholar]
- 14.Langheim S, Dreas L, Veschini L, et al. Increased expression and secretion of resistin in epicardial adipose tissue of patients with acute coronary syndrome. Am J Physiol Heart Circ Physiol. 2010;298:H746–H753. doi: 10.1152/ajpheart.00617.2009. [DOI] [PubMed] [Google Scholar]
- 15.McKenney M, Schultz K, Boyd J, Byrd J, Alloosh M. Epicaridial adipose excision slows the progression of porcine coronary atherosclerosis. J Cardiothorac Surg. 2014;9 doi: 10.1186/1749-8090-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dubrovska G, Verlohren S, Luft FC, Gollasch M. Mechanisms of ADRF release from rat aortic adventitial adipose tissue. Am J Physiol Heart Circ Physiol. 2004;286:H1107–H1113. doi: 10.1152/ajpheart.00656.2003. [DOI] [PubMed] [Google Scholar]
- 17.Lohn M, Dubrovska G, Lauterbach B, Luft FC, Gollasch M, Sharma AM. Periadventitial fat releases a vascular relaxing factor. FASEB J. 2002;16:1057–1063. doi: 10.1096/fj.02-0024com. [DOI] [PubMed] [Google Scholar]
- 18.Schleifenbaum J, Kohn C, Voblova N, et al. Systemic peripheral artery relaxation by KCNQ channel openers and hydrogen sulfide. J Hypertens. 2010;28:1875–1882. doi: 10.1097/HJH.0b013e32833c20d5. [DOI] [PubMed] [Google Scholar]
- 19.Verlohren S, Dubrovska G, Tsang SY, et al. Visceral periadventitial adipose tissue regulates arterial tone of mesenteric arteries. Hypertension. 2004;44:271–276. doi: 10.1161/01.HYP.0000140058.28994.ec. [DOI] [PubMed] [Google Scholar]
- 20.Gao YJ, Zeng ZH, Teoh K, et al. Perivascular adipose tissue modulates vascular function in the human internal thoracic artery. J Thorac Cardiovasc Surg. 2005;130:1130–1136. doi: 10.1016/j.jtcvs.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 21.Malinowski M, Deja MA, Golba KS, Roleder T, Biernat J, Wos S. Perivascular tissue of internal thoracic artery releases potent nitric oxide and prostacyclin-independent anticontractile factor. Eur J Cardiothorac Surg. 2008;33:225–231. doi: 10.1016/j.ejcts.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 22.Lynch F, Withers S, Yao Z, et al. Perivascular adipose tissue-derived adiponectin activates BK(Ca) channels to induce anticontractile responses. Am J Physiol Heart Circ Physiol. 2013;304:H786–H795. doi: 10.1152/ajpheart.00697.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao YJ, Lu C, Su LY, Sharma AM, Lee RM. Modulation of vascular function by perivascular adipose tissue: the role of endothelium and hydrogen peroxide. Br J Pharmacol. 2007;151:323–331. doi: 10.1038/sj.bjp.0707228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berwick ZC, Dick GM, Moberly SP, Kohr MC, Sturek M, Tune JD. Contribution of voltage-dependent K(+) channels to metabolic control of coronary blood flow. J Mol Cell Cardiol. 2012;52:912–919. doi: 10.1016/j.yjmcc.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borbouse L, Dick GM, Asano S, et al. Impaired function of coronary BK(Ca) channels in metabolic syndrome. Am J Physiol Heart Circ Physiol. 2009;297:H1629–H1637. doi: 10.1152/ajpheart.00466.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Minobe E, Asmara H, Saud ZA, Kameyama M. Calpastatin domain L is a partial agonist of the calmodulin-binding site for channel activation in Cav1.2 Ca2+ channels. J Biol Chem. 2011;286:39013–39022. doi: 10.1074/jbc.M111.242248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Letavernier E, Perez J, Bellocq A, et al. Targeting the calpain/calpastatin system as a new strategy to prevent cardiovascular remodeling in angiotensin II-induced hypertension. Circ Res. 2008;102:720–728. doi: 10.1161/CIRCRESAHA.107.160077. [DOI] [PubMed] [Google Scholar]
- 28.Berwick Z, Dick G, Tune J. Heart of the matter: coronary dysfunction in metabolic syndrome. J Mol Cell Cardiol. 2012;52:848–856. doi: 10.1016/j.yjmcc.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dick GM, Tune JD. Role of potassium channels in coronary vasodilation. Exp Biol Med (Maywood ) 2010;235:10–22. doi: 10.1258/ebm.2009.009201. [DOI] [PubMed] [Google Scholar]
- 30.Dyson MC, Alloosh M, Vuchetich JP, Mokelke EA, Sturek M. Components of metabolic syndrome and coronary artery disease in femal Ossabaw swine fed excess atherogenic diet. Comp Med. 2006;56:35–45. [PubMed] [Google Scholar]
- 31.Edwards JM, Neeb ZP, Alloosh MA, et al. Exercise training decreases store-operated Ca2+entry associated with metabolic syndrome and coronary atherosclerosis. Cardiovasc Res. 2010;85:631–640. doi: 10.1093/cvr/cvp308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neeb Z, Edwards J, Aloosh M, Long X, Mokelke E, Sturek M. Metabolic syndrome and coronary artery disease in Ossabaw comapred with Yucatan swine. Comparative Medicine. 2010;60:300–315. [PMC free article] [PubMed] [Google Scholar]
- 33.Lau S, Chu P, Weiss L. A specific marker of macrophages in paraffin-embedded tissue samples. Hematopathology. 2004;122:794–801. doi: 10.1309/QHD6-YFN8-1KQX-UUH6. [DOI] [PubMed] [Google Scholar]
- 34.Turk J, Carroll J, Laughlin H, et al. C-reactive protein correlates with macrophage accumulation in coronary arteries of hypercholesterolemic pigs. J Appl Physiol. 2003;95:1301–1304. doi: 10.1152/japplphysiol.00342.2003. [DOI] [PubMed] [Google Scholar]
- 35.Agabiti-Rosei C, De Ciuceis C, Rossini C, et al. Anticontractile activity of perivascular fat in obese mice and the effect of long-term treatment with melatonin. J Hypertens. 2014;32:1264–1274. doi: 10.1097/HJH.0000000000000178. [DOI] [PubMed] [Google Scholar]
- 36.Withers S, Simpson L, Fattah S, Werner M, Heagerty A. cGMP-dependent protein kinase (PKG) mediates the anticontractile capacity of perivascular adipose tissue. Cardiovasc Res. 2014;101:130–137. doi: 10.1093/cvr/cvt229. [DOI] [PubMed] [Google Scholar]
- 37.Galvez B, de Castro J, Herold D, et al. Perivascular adipose tissue and mesenteric vascular function in spontaneously hypertensive rats. Arterioscler Thromb Vasc Biol. 2006;26:1297–1302. doi: 10.1161/01.ATV.0000220381.40739.dd. [DOI] [PubMed] [Google Scholar]
- 38.Lee RM, Lu C, Su LY, Gao YJ. Endothelium-dependent relaxation factor released by perivascular adipose tissue. J Hypertens. 2009;27:782–790. doi: 10.1097/HJH.0b013e328324ed86. [DOI] [PubMed] [Google Scholar]
- 39.Bunker AK, Laughlin MH. Influence of exercise and perivascular adipose tissue on coronary artery vasomotor function in a familial hypercholesterolemic porcine atherosclerosis model. J Appl Physiol. 2010;108:490–497. doi: 10.1152/japplphysiol.00999.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reifenberger MS, Turk JR, Newcomer SC, Booth FW, Laughlin MH. Perivascular fat alters reactivity of coronary artery: effects of diet and exercise. Med Sci Sports Exerc. 2007;39:2125–2134. doi: 10.1249/mss.0b013e318156e9df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berwick ZC, Dick GM, Tune JD. Heart of the matter: coronary dysfunction in metabolic syndrome. J Mol Cell Cardiol. 2012;52:848–856. doi: 10.1016/j.yjmcc.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miura H, Liu Y, Gutterman D. Human coronary arteriolar dilation to bradykinin depends on membrane hyperpolarization: contribution of nitric oxide and Ca2+-activated K+ channels. Circulation. 1999;99:3132–3138. doi: 10.1161/01.cir.99.24.3132. [DOI] [PubMed] [Google Scholar]
- 43.Miura H, Wachtel R, Liu Y, Loberiza F, Jr, Saito T, Miura M. Flow-induced dilation of human coronary arterioles: important role of Ca(2+)-activated K(+) channels. Circulation. 2001;103:1992–1998. doi: 10.1161/01.cir.103.15.1992. [DOI] [PubMed] [Google Scholar]
- 44.Lu T, Ye D, He T, Wang X, Wang H, Lee H. Imparied Ca2+-dependent activation of large-conductance Ca2+-activated K+ channels in the coronary artery smooth muscle cells of Zucker diabetic fatty rats. Biophys J. 2008;95:5165–5177. doi: 10.1529/biophysj.108.138339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mokelke E, Dietz N, Eckman D, Nelson M, Sturek M. Diabetic dyslipidemia and exercise affect coronary tone and differential regulation of conduit microvessel K+ current. Am J Physiol Heart Circ Physiol. 2005;288:H1223–H1241. doi: 10.1152/ajpheart.00732.2004. [DOI] [PubMed] [Google Scholar]
- 46.Yang Y, Jones A, Thomas T, Rubin L. Influence of sex, high-fat diet, and exercise training on potassium currents of swine coronary smooth muscle. Am J Physiol Heart Circ Physiol. 2007;293:H1553–H1563. doi: 10.1152/ajpheart.00151.2007. [DOI] [PubMed] [Google Scholar]
- 47.Sellers A, Ashford M. Activation of BKCa channels in acutely dissociated neurones from the rat ventromedial hypothalamus by NS1619. Br J Parmacol. 1994;113:659–661. doi: 10.1111/j.1476-5381.1994.tb17041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seebohm G, Pusch M, Chen J, Sanguinetti M. Parmacological activation of normal and arrhythmia-associated mutant KCNQ1 potassium channels. Circ Res. 2003;93:941–947. doi: 10.1161/01.RES.0000102866.67863.2B. [DOI] [PubMed] [Google Scholar]
- 49.D'hahan N, Jacquet H, Moreau C, Catty P, Vivaudou M. A transmembrane domain of hte sulfonylurea receptor mediates activation of ATP-sensitive K(+) channels by K(+) channel openers. Mol Pharmacol. 1999;56:308–315. doi: 10.1124/mol.56.2.308. [DOI] [PubMed] [Google Scholar]
- 50.Zuidema M, Yang Y, Wang M, et al. Antecedent hydrogen sulfide elicits an anti-inflammatory phenotype in postischemic murine small intestine: role of BK channels. Am J Physiol Heart Circ Physiol. 2010;299:H1554–H1567. doi: 10.1152/ajpheart.01229.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hu Y, Yang G, Xiao X, Liu L, Li T. Bkca opener, NS1619 pretreatment protects against shock-induced vascular hyporeactivity through PDZ-Rho GEF-RhoA-Rho kinase pathway in rats. J Trauma Acute Care Surg. 2014;76:394–401. doi: 10.1097/TA.0b013e3182aa2d98. [DOI] [PubMed] [Google Scholar]
- 52.Bardou O, Prive A, Migneault F, et al. K+ channels regulate ENaC expression via changes in promoter activity and control fluid clearance in alveolar epithelial cells. Biochem Biophys Acta. 2012;1818:1682–1690. doi: 10.1016/j.bbamem.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 53.Kubo M, Quayle J, Standen N. Angiotensin II inhibition of ATP-sensitive K+ currents in rat arterial smooth muscle cells through protein kinase C. J Physiol. 1997;503:489–496. doi: 10.1111/j.1469-7793.1997.489bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zavaritskaya O, Zhuravleva N, Schleifenbaum J, et al. Role of KCNQ channels in skeletal muscle arteries and periadventitial vascular dysfunction. Hypertension. 2013;61:151–159. doi: 10.1161/HYPERTENSIONAHA.112.197566. [DOI] [PubMed] [Google Scholar]
- 55.Chatterjee T, Idelman G, Blanco V, et al. Histone deacetylase 9 is a negative regulator of adipogenic differentiation. J Biol Chem. 2011;286:27836–27847. doi: 10.1074/jbc.M111.262964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Drolet R, Belanger C, Fortier M, et al. Fat depot-specific impact of visceral obesity on adipocyte adiponectin release in women. Obesity (Silver Spring) 2009;17:424–430. doi: 10.1038/oby.2008.555. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.