Abstract
A major problem for the rapidly growing population of older adults (age 65 and older) is age-related declines in vision, which have been associated with increased risk of falls and vehicle crashes. Research suggests that the increased risk is associated with declines in contrast sensitivity and acuity. We examined whether perceptual learning could be used to improve age-related declines in contrast sensitivity. Older and younger adults were trained over seven days using a forced-choice orientation discrimination task with stimuli that varied in contrast with multiple levels of additive noise. The results indicate that older individuals, following training, performed as well as pre-trained college-aged participants. Improvements transferred to an untrained orientation, and were not associated with changes in retinal illuminance. Improvements in far acuity in younger individuals and near acuity in older individuals were also found. These findings indicate that behavioral interventions can greatly improve visual performance for older adults.
Keywords: Aging, Visual Perception, Perception, Adult Development
Introduction
The population of the United States is rapidly aging (Wiener & Tilly, 2002). The US estimates that between 2000 and 2050 the number of older individuals (over age 65) will increase 135% whereas the population under age 65 will increase only 32% (Wiener & Tilly, 2002). Individuals living to an advanced age (age 85 and older)—a group likely to need health and long-term care services—will increase by a staggering 350%. In raw numbers these percentages translate to an estimated increase of 47 million individuals aged 65 and older (Wiener & Tilly, 2002). An important issue will be to develop strategies to address the health needs of this ever-growing sector of the population.
It is well documented in the literature that aging results in significant declines in cognition. These declines include changes in executive function, fluid intelligence and working memory, inhibitory control and attention, and language processing (See Craik & Salthouse, 2007 for a detailed review). In addition to cognitive declines, there are significant age-related declines in vision and visual processing that influence the health and well-being of an aging population. These declines have been identified as a major factor in the incidence of falls among the elderly (Lord, Smith, & Menant, 2010). In addition, age-related declines in vision have been associated with increased motor-vehicle crash risk (Owsley et al., 1998; Owsley, Stalvey, Wells, & Sloane, 1999) with the crash risk of drivers over the age of 75 exceeding novice young drivers (Evans, 2004). The types of crashes that occur change with driver age: older drivers have an increase in collisions with another moving vehicle and a decrease in single-vehicle or speed-related crashes (Langford & Koppel, 2006), suggesting specific declines in visual function with age.
Given the health outcomes of age-related declines in vision and important question is what aspects of vision and visual processing decline with age? A substantial corpus of research (see Owsley, 2011 and Andersen, 2012 for reviews) has shown age-related declines in vision for a vast range of visual function including contrast sensitivity (Richards, 1977), orientation discrimination (Betts, Sekuler, & Bennett, 2007), visual acuity (Sekuler, Owsley & Hutman, 1982), motion perception (Bennett, Sekuler, & Sekuler, 2007), form perception (Roudaia, Bennett, & Sekuler, 2008), and optic flow (Atchley & Andersen, 1998). While these age-related declines in vision could be due to optical, retinal, cortical, or pathological changes, there is substantial evidence suggesting a cortical locus for declines in visual function (Spear, 1993). Specifically, studies have suggested that these changes in vision may be due to decreased cortical inhibition in visual cortex (Schmolesky, Wang, Pu, & Leventhal, 2000).
Of all the age-related declines in vision and visual processing discussed above, declines in contrast sensitivity is one of the most pronounced. Age-related declines in contrast sensitivity have significant impacts on visual function, including the ability to detect and resolve detail (e.g., Owsley, Sekuler & Siemsen, 1983), process motion information important for balance (Sundermier, Woollacott, Jensen, & Moore, 1996), or process information when driving (Liutkevičienė, Cebatorienė, Liutkevičienė, Jašinskas, & Zaliūnienė, 2013). Declines in contrast sensitivity has been found to be related to the likelihood of falls among older adults (Lord, Clark & Webster, 1991). Contrast sensitivity declines are most apparent at high spatial frequencies, and while it has been suggested that this decrease in contrast sensitivity is primarily due to optical factors, the decline in contrast sensitivity for moving stimuli has been suggested to have a larger neural component (Burton, Owsley, & Sloane, 1993). Given these declines, are there any interventions for improving contrast sensitivity among the elderly? In the present study, we examined whether behavioral training, or perceptual learning, can be used to improve age-related declines in contrast sensitivity.
Perceptual learning refers to improved visual performance due to repeated exposure to stimuli – usually at or near threshold. A central issue in perceptual learning is whether the improved performance is specific to the trained stimuli (specificity) or transfers to non-trained stimuli. A number of perceptual learning studies have shown that perceptual improvements are specific to the trained stimuli and do not transfer to other stimuli or tasks. Specificity has been shown for orientation (Karni & Sagi, 1991), task difficulty (Ahissar & Hochstein, 1997), presence or absence of external noise (Dosher & Lu, 2005), location in the visual field (Schoups, Vogels, & Orban, 1995), as well as a combination of these factors (DeLoss, Watanabe, & Andersen, 2014). This specificity remains an important issue to examine if perceptual learning paradigms are to be used to counteract age-related declines in vision. For these reasons, it was important to examine specificity for the trained stimuli, as well as the possibility of transfer to other real-world tasks.
In the past decade, a number of studies have examined perceptual learning in older individuals (Andersen, Ni, Bower, & Watanabe, 2010; Bower & Andersen, 2011; Bower, Watanabe, & Andersen, 2013; DeLoss et al., 2014). Only one of these studies found complete specificity to an untrained retinal location (Andersen et al., 2010), while numerous other studies found partial to complete transfer to an untrained task, orientation, or stimulus (Bower & Andersen, 2011; Bower et al., 2013; DeLoss et al., 2014). This decreased specificity may be due to decreased neural inhibition, as well as decreased orientation and direction neuronal selectivity (Schmolesky et al., 2000). Due to this decreased selectivity, broader ranges of neurons may be recruited during training, allowing for transfer not typically seen in younger adults. Although these age-related decreases in selectivity may make fine discrimination more difficult, it may result in a greater benefit from perceptual learning because of decreased specificity and greater generality for trained tasks and stimuli.
The present study assessed whether perceptual learning could be used as a possible intervention to counteract age-related declines in contrast sensitivity. Younger and older observers performed an orientation discrimination task using sine-wave gratings that varied in contrast. We assessed whether training resulted in improved performance for targets at a specific orientation, transferred to targets at an untrained orientation, or transferred to other tasks (e.g. near and far acuity). While previous studies have used contrast training paradigms to improve vision in middle-aged individuals (Polat, 2009), to our knowledge this is the first study to examine the use of perceptual learning to improve vision in adults 65 years of age and older. In addition, it is possible that improved performance could be due to changes in retinal illuminance – the amount of light reaching the retina. To examine this issue pupil diameter was measured to examine retinal illuminance. If improved performance is due to increased light reaching the retina (pupil dilation) then retinal illuminance should be correlated with improvements in performance due to training.
Methods
Subjects
Sixteen older individuals from the surrounding community (8 male and 8 female) and sixteen younger individuals from the University of California, Riverside (8 male and 8 female) participated in the experiment. The sample size was determined based on pilot studies and previous research demonstrating an effect of training in older and younger participants (Andersen et al., 2010; Bower & Andersen, 2011; Bower et al., 2013; DeLoss et al., 2014). All observers were paid for their participation in the experiment. Younger and older participants were reimbursed at a rate of $10 per hour. Older individuals were reimbursed an additional $5 per hour to cover transportation costs to the campus. The sample size for each age group was comparable to other studies investigating the effects of perceptual learning. They were naïve concerning the purpose of the study and all had normal or corrected-to-normal visual acuity. All subjects were screened using an array of cognitive and perceptual tests. Demographic information of the participants is presented in Table 1. One-way analyses of variance were conducted and no significant differences were found on any measure prior to training (F < = 2.5, p > 0.05). Participants were also pre-screened for eye disease and neurological disorders.
Table 1.
Means and standard deviations of participant demographics and results from cognitive and perceptual tests.
| Younger | Older | |||
|---|---|---|---|---|
|
|
||||
| Variable | Mean | SD | Mean | SD |
| Age (years) | 22.43 | 1.16 | 71.23 | 5.85 |
| Education (years) | 15 | 0.88 | 15.15 | 2.34 |
| Log Contrast Sensitivity | 1.37 | 0.43 | 1.29 | 0.19 |
| LogMAR Visual Acuity* | −0.02 | 0.09 | 0.09 | 0.12 |
| Digit Span Forward | 10.79 | 1.72 | 9.85 | 1.63 |
| Digit Span Backward | 7.71 | 1.94 | 6.23 | 2.39 |
| WAIS – Matrix Reasoning* | 20.29 | 2.67 | 16.92 | 4.19 |
Note:
Contrast sensitivity measured using the Pelli Robson Test (Pelli, Robson & Wilkins, 1988).
Difference between groups is significant at p < 0.05.
Apparatus
Stimuli were presented on a 21″ CRT monitor (Viewsonic P225F) at a resolution of 1024×768 with a refresh rate of 100Hz (non-interlaced). The monitor was driven by a Dell Vostro 430 equipped with an Intel Core i5 750 processor using the Windows XP (Service Pack 3) operating system. The mean luminance value of the monitor was 53.82 cd/m2. An NVIDIA GeForce GTS 240 graphics card was used along with a Bits ++ system (Cambridge Research Systems). This allowed the system to achieve 14-bit grayscale (16,384 grayscale levels). Custom experimental software was written in MATLAB (The Mathworks, Inc., version 7.8.0.347); the Psychophysics Toolbox extensions were also utilized (Brainard, 1997; Pelli, 1997). The monitor was calibrated using a ColorCal2 colorimeter (Cambridge Research Systems). Gamma correction was performed through linearization of the color lookup table.
Stimuli and Procedures
The experiment consisted of 1.5 hours per day of testing/training over seven days. Participants were required to complete the study within three weeks of their first testing session. The monitor was viewed at a distance of 94 centimeters. Head position was stabilized with the use of an EyeLink 1000 Tower Mount (SR Research) and stimuli were viewed binocularly. Any corrective lenses or contacts normally worn by the participants were allowed during the experiment. All stimuli were viewed through a plano-convex glass collimation lens (45.7 cm diameter) with a 19% magnification factor to minimize any age-related differences in accommodative focus. The size of the stimuli was corrected to account for this magnification factor. The experiment was run in a darkened room and the only light source in the room during the experiment was the monitor. Stimuli during the experiment were Gabor patches presented at 1.5 cycles/deg visual angle, 0.65 deg standard deviation of the Gaussian mask, and the phase of the Gabor was randomized +/−180 degrees on each trial.
Task Practice
On the first day of the study, before the experiment began all participants were given a 30 trial practice session to familiarize them with the task. These practice trials were presented without noise. Participants completed 15 trials using a standard that was 45 degrees clockwise off vertical and 15 trials using a standard that was 45 degree counter-clockwise off vertical. At the beginning of each trial participants were shown a fixation point in the center of the display. To attract attention, the fixation point alternated from black to white every 400 milliseconds (ms) for 1600 ms (Betts, Taylor, Sekuler, & Bennett, 2005). Participants were then presented the standard orientation for 100 ms. A second fixation point then alternated black and white every 300 ms for 1200 ms. Participants were then shown the target stimulus for 100 ms. During task familiarization the target was rotated either 25 degrees clockwise or 25 degrees counter-clockwise way from the standard orientation. The screen was then blanked to the uniform mid-gray value indicating that the participant should make their response. The participant’s task was to judge whether the target stimulus was rotated clockwise or counterclockwise compared to standard orientation (Figure 1). Responses were made using the left and right arrow keys on the keyboard. Audio feedback was provided on each trial indicating whether the participant was correct. Participants were then prompted to “Press any key to continue.” to begin the next trial. During the familiarization task participants were also intentionally instructed to get at least one trial incorrect to familiarize them with the auditory feedback provided on incorrect trials.
Figure 1.
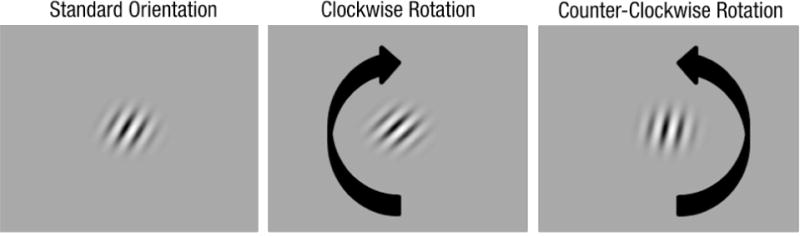
An example of the task used in the study, demonstrating a 15° clockwise and counterclockwise rotation of the 25° clockwise standard orientation.
Testing
Testing of orientation discrimination thresholds occurred during the first and last days (days 1 and 7) of the experiment. Participants were tested at five external noise levels. The Gabors were embedded in additive Gaussian noise in four of the five testing blocks. The first block consisted of a no-noise condition with the standard deviation of the additive Gaussian noise set to zero, the standard deviation of the Gaussian distribution was then increased by 0.084375 in each successive block for a maximum standard deviation of the Gaussian distribution of 0.3375 in block 5. Standard orientations were either clockwise 25 degrees or counterclockwise 25 degrees off vertical (see Figure 2 for an example of the stimuli). These standards were counterbalanced across subjects for testing order as well as for their assigned training orientation. These two standards were chosen based on previous research demonstrating that improvements in orientation sensitivity degrade approximately 40 degrees away from the trained orientation (Matthews, Liu, & Qian, 2001). These two specific standard orientations were chosen, as they are 50 degrees offset from one another. During days 1 and 7, pre-training Michelson contrast thresholds for the trained and untrained orientation standards were assessed at the five noise levels using QUEST (Watson & Pelli, 1983). QUEST was initialized with a criterion level of 0.75, β = 1.4, δ = 0.025, and γ = 0.5. On testing days, the guessed contrast values for QUEST for each successive noise level were 10%, 15%. 20%, 25% and 30% contrast, with a standard deviation of 5% contrast. Participants completed 60 trials at each noise level during testing. All stimuli were viewed through a circular annulus with a radius of 8 degrees visual angle that was placed against the surface of the monitor. The annulus was used to remove any edge cues that may be used in the orientation discrimination task. The background between trials and during inter-stimulus-intervals (ISI) consisted of the mid-gray value of the monitor (for no noise blocks) or additive Gaussian noise with the mean set at the mid-gray level of the monitor and a standard deviation that matched the additive Gaussian noise (for blocks with external noise). Trials progressed in the same fashion as described in the familiarization task, though the standard orientations were 25 degrees clockwise or 25 counter-clockwise off vertical as previously described, and the contrast of the Gabor on each trial was determined by QUEST. The orientation offset was fixed at 15 degrees on all trials. Post-training thresholds for the trained and untrained orientations were measured on day 7 using the same procedure as that used on day 1. Pupil size was also measured on each trial during testing days using an Eyelink 1000 configured with a tower mount and was used to derive retinal illuminance values.
Figure 2.

An example of the stimuli used in the experiment, the top row demonstrates the 25° clockwise standard orientation, and the bottom row demonstrates the 25° counter-clockwise standard orientation. The top row is displayed at 75% contrast; the bottom row is displayed at 25% contrast. The five noise levels used in the study are shown in the figure in increasing order from left to right.
Training
Training occurred on days 2 through 6 using the same stimuli used in the testing phase. During training, all participants completed five blocks, one at each noise level. These consisted of 150 trials per block. Participants were allowed to take a short break after each block. During each training day, QUEST was run using the same parameters as during the testing days with one modification. The contrast threshold guess for QUEST for each participant used the threshold derived on the previous day of testing (for day 2) or training (for days 3 through 6). Using this method, the participants were constantly trained at their 75% correct threshold during each training day. This allowed training to be constantly optimized for any improvement that occurred between or within testing or training sessions. Training for all subjects resulted in 750 training trials per day, for a total of 3750 training trials over the course of the experiment.
Results
Threshold Analysis
A 2 (day) by 5 (noise) by 2 (age) by 2 (trained vs. untrained standard) mixed repeated-measures analysis of variance was conducted on the thresholds obtained from the testing days. Two older individuals and three younger individuals had to be removed from the analysis, as QUEST was not able to converge on a stable threshold estimate in at least one of the conditions pre or post training. Results indicated a significant effect of day, F(1,25) = 30.696, p < .001, ηp2 = .551, indicating a significant improvement in thresholds post-training. A significant day by age interaction was also found, F(1,25) = 6.583, ηp2 = .208, indicating greater learning by the older group as shown in Figure 3. Indeed, there was a significant difference between older and younger adults prior to training, F(1,25) = 8.314, p = .008, ηp2 = .250. However, after training the effect of age was no longer significant, F(1,25) = 3.996, p = 0.057, ηp2 = .138, suggesting similar levels of performance for older and younger subjects following training. There was also a significant effect of noise, F(4,100) = 198.463, p < .001, ηp2 = .888. As expected, as the level of external noise increased, so did the contrast threshold. A significant noise by age interaction was also found, F(4,100) = 4.209, p = .035, ηp2.= .144. Older individuals showed decreased tolerance to external noise as exhibited by higher thresholds in the high noise conditions, pre and post training. There was also a significant day by noise by age effect, F(4,100) = 2.506, p = .047, ηp2 = .091. Older participants exhibited greater reductions in thresholds in the higher noise cases as compared to younger participants.
Figure 3.
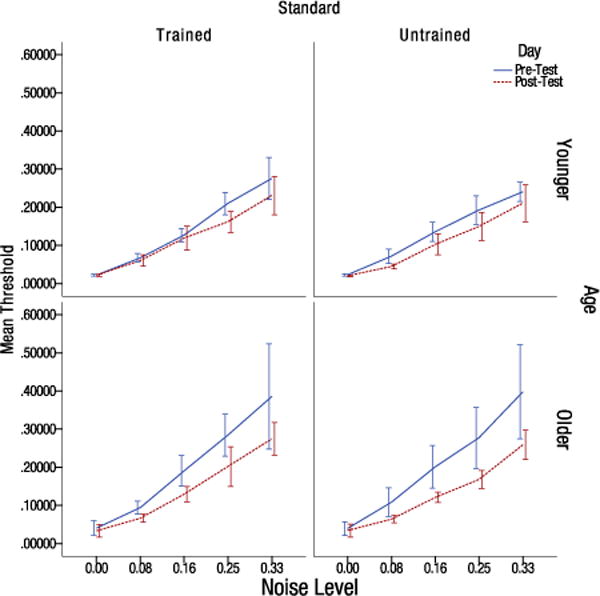
Pre-test and post-test Michelson contrast thresholds for older and younger individuals split by the trained and untrained orientation at the five noise levels (values indicate the standard deviation of the Gaussian distribution). Error bars indicate the 95% confidence interval.
A possible factor that might account for the learning is that improvements in performance are a result of repeated testing with the stimuli and practice with the task. To examine this issue we analyzed the change in threshold between days 1 and 2. The main effect of day was not significant for younger subjects, F(1,13) = 2.04, p = 0.177, ηp2 = .135, or for older subjects, F(1,12) = 3.23, p = 0.098, ηp2 = .212, suggesting that the improved performance over the full range of training was not the result of repeated testing with the stimuli or task practice.
Retinal Illuminance Analysis
To examine whether a change in pupil size was correlated with the change in threshold. Pupil size measurements were taken during each stimulus presentation, which were then converted into retinal illuminance (Trolands). Percentage change scores from pre-test to post-tests for retinal illuminance and contrast thresholds were calculated. Any change greater than +/− 2.5 standard deviations was excluded from the analysis. All reported p-values are one tailed as it was hypothesized that letting in more light would cause an improvement in performance for low contrast stimuli, particularly in older individuals who have decreased retinal illuminance. Overall, there was a significant correlation between the percentage change in threshold and percentage change in retinal illuminance with training, r(224) = −.134, p = 0.019. However, further analysis indicated that this was driven primarily by younger participants. When the data was split by age group, younger individuals continued to show a significant correlation, r(122) = −.228, p = .004, while older individuals showed no significant correlation, r(102) = −0.059, p = .276, as shown in Figure 4.
Figure 4.
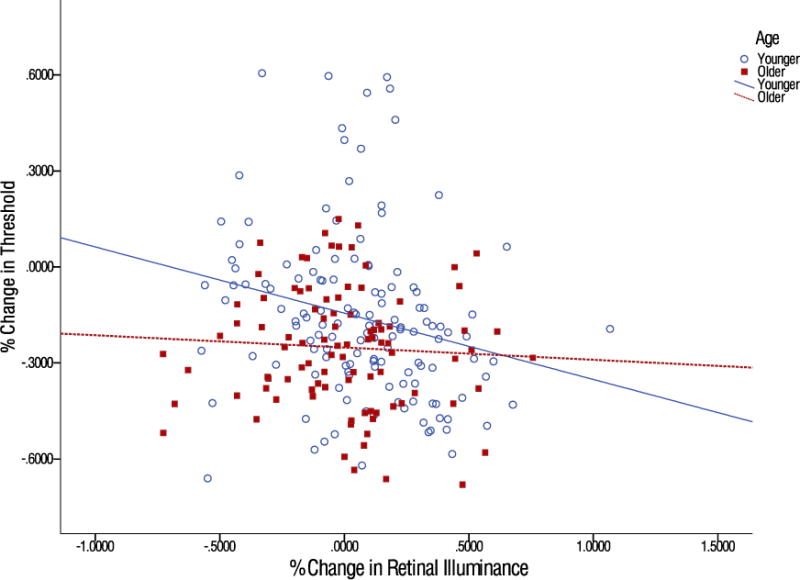
Scatterplot of the percentage change in retinal illuminance and the correlation for older and younger individuals with the percentage change in threshold. Each point represents a single measurement at each of the five noise levels. The lines represent the best-fit linear trend for each age group.
Previous research has shown that pupil size can alter the retinal modulation transfer function (Van Nes & Bouman, 1967) which might enhance low and high spatial frequencies of the external noise and subsequently affect learning. To examine this issue we correlated pre-test and post-test the change in pupil size with the change in contrast thresholds. The correlation was significant for younger adults, r(122) = −0.36, p < .001, as well as for older adults, r(102) = −0.18, p = .035. However, this accounts for only 13% of the variance in the improvements seen in younger participants and only 3.2% of the variance in older participants. These findings suggest that any changes in the spatial frequency characteristics of the external noise due to changes in pupil size are likely only a small factor in the overall learning present in younger individuals and explain very little of the overall effect of learning in older subjects.
Contrast Sensitivity and Acuity Analysis
Near, far acuity, and contrast sensitivity measurements were made pre and post-training, all acuity measurements are reported in LogMAR units. Far acuity measurements were made using the 3 meter 2000 Series Revised ETDRS Chart 2(Precision Vision) at a distance of 3 meters. Near acuity measurements were made using the 2000 series New ETDRS Chart 3 at a distance of 40 cm. Contrast sensitivity was measured using the Pelli-Robson Contrast Sensitivity Chart. All measures report one-tailed p-values. Corrections for multiple comparisons were made using A single contrast sensitivity measurement for one younger participant was lost and is missing from the analysis.
While neither younger nor older individuals demonstrated any significant improvement in contrast sensitivity, and t(12) = −1.389, p = .190, d = −0.385, t(12) = −1.477, p = .0825, d = −0.410, respectively (Figure 5). To adjust for multiple comparisons in analyzing the acuity data, the Benjamini-Hochberg procedure was used to control the false discovery rate (Benjamini & Hochberg, 1995). Older individuals showed a significant improvement in near acuity, t(12) = 2.217, pFDR = .047, d = 0.615. While no significant change in near acuity was found in younger individuals, t(13) = .265, pFDR = .398, d = 0.071, (Figure 6). Similarly, older individuals showed no significant change in far acuity, t(12) = .503, pFDR = .398, d = 0.140. While a significant improvement in far acuity was found for younger individuals, t(13) = 2.592, pFDR = .044, d = 0.693 (Figure 7).
Figure 5.
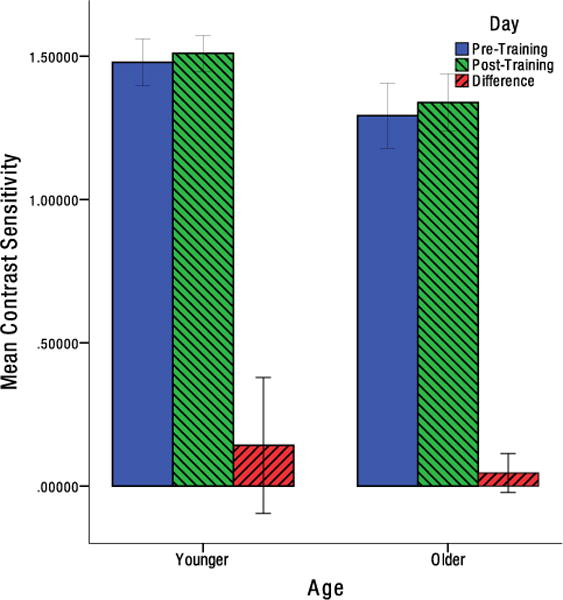
Mean change in contrast sensitivity from pre-test to post-test for older and younger individuals for log contrast. Error bars indicate the 95% confidence interval.
Figure 6.
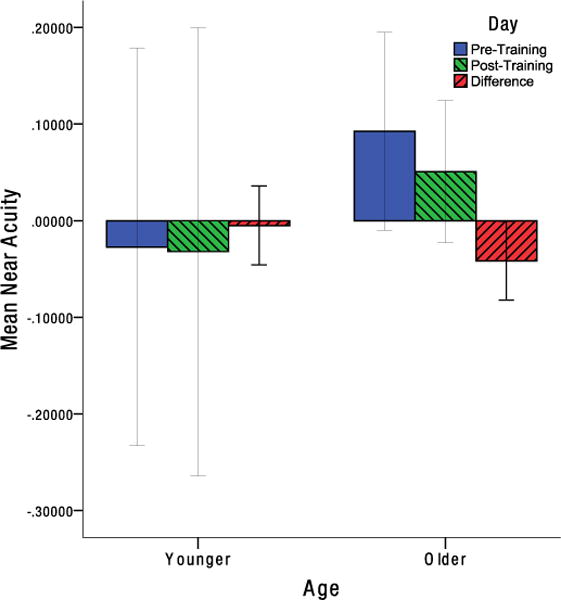
Mean change in near acuity from pre-test to post-test for older and younger individuals in LogMAR units. Error bars indicate the 95% confidence interval.
Figure 7.
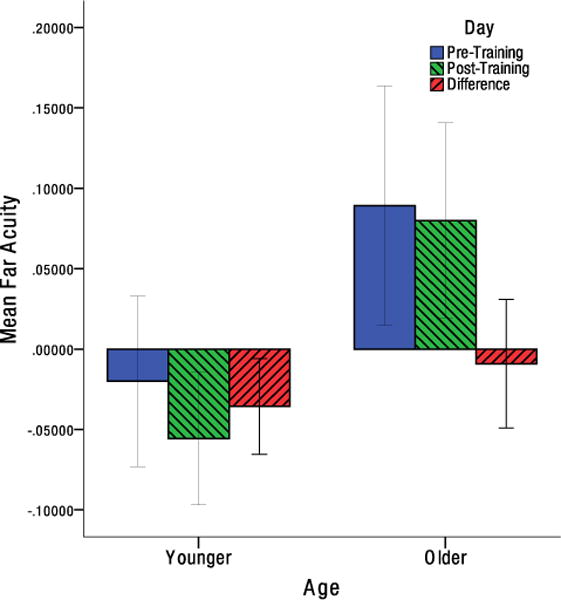
Mean change in far acuity from pre-test to post-test for older and younger individuals in LogMAR units. Error bars indicate the 95% confidence interval.
Overall, the results of the present study indicate a number of important findings. First, contrast sensitivity can be improved through behavioral training in both younger and older individuals. The performance improvements for older adults are striking. Consider contrast sensitivity thresholds for the high noise condition – the most difficult condition for both age groups. Prior to training, younger observers (mean threshold of 0.25) had significantly lower thresholds than older observers (mean threshold of 0.39) for the high noise condition, F(1,25) = 6.341, p < .01, ηp2 = .201. However, an analysis comparing the performance for the high noise condition for pre-training younger adults (mean threshold of 0.25) and post-training older adults (mean threshold of 0.26) was not significant, F(1,25) = 0.152, p > .05, ηp2 = .006. The change in the pattern of results is due to improved performance for older adults, as indicated by a significant effect of training for the high noise condition, pre-training and post-training thresholds of 0.39 and 0.26 respectively, F(1,12) = 10.215, p < .01, ηp2 = .460.
Discussion
The present study examined the use of perceptual learning to improve the ability to see low contrast stimuli by older adults. A major finding of the present study is that five days of training for older adults resulted in performance that was not statistically different from younger adults prior to training. This finding indicates that perceptual learning can be used to counteract age-related declines in contrast sensitivity. These improvements are the result of changes in sensory processing and not due to optical efficiency of the eye.
Although the magnitude of learning was considerable for processing the contrast of the trained stimuli, this learning did not to transfer to a standard contrast sensitivity chart. This may be due to the low fidelity of a chart-based test, in which small improvements in contrast sensitivity may not be detectable. Future research that measures the entire contrast sensitivity function will be needed to determine whether there are any fine changes in contrast sensitivity to untrained stimuli.
A second important finding was that both younger and older individuals showed significant improvement in acuity with perceptual learning training to improve contrast sensitivity. Interestingly, the improvements in acuity were associated with the range of acuity most problematic for each age group. Younger individuals tend to have near optimal near acuity, as a result of a strong and fast accommodative response (Koretz, Kaufman, Neider, & Goeckner, 1989), and only showed a significant change in far acuity. Older individuals suffer from losses in near acuity, due to significant declines in the accommodative response (Koretz et al., 1989). The improved performance in near acuity for older adults might be due to changes in improvements in the accommodative response. Interestingly, previous research has found improved acuity due to perceptual learning with middle-aged adults (median age of 51) when declines in accommodative focus or presbyopia occur (Polat et al., 2012). However, unlike the present study, which used five days of training, the training for middle aged adults occurred 3 times a week for 3 months over 36 training sessions (Polat et al., 2012). An important issue for future research would be to measure the accommodative focus response of older adults, over the age of 65, in response to training in contrast sensitivity.
A third finding was that both age groups showed significant transfer to the untrained orientation. This transfer was more pronounced at higher noise levels. This is likely due to the primary limiting factor for discrimination in high noise cases being the external noise itself, and not the contrast of the stimulus. This also suggests that filtering of external noise is not specific to the trained stimulus and may be a more general process that can transfer to a variety of tasks. One other possible explanation for the high degree of transfer is that the task used in the present study itself is a fairly low-precision task. At high levels of contrast with no noise, participants can perform the task consistently with near perfect accuracy, aside from any attentional lapses in their responses, as the task itself is a coarse orientation discrimination task with a constant 15° offset. Previous research has shown that high precision tasks tend to show greater specificity than that found when using a low precision task (Jeter, Dosher, Petrov, & Lu, 2009).
Finally, no significant correlation between a change in pupil size and improvement in the task was found for older individuals. However, a significant correlation was found in younger individuals, though this only accounted for approximately 5% of the variance. While it was proposed that older individuals may be compensating for a loss in retinal illuminance by dilating the pupils to allow more light to fall on the retina, the results from the retinal illuminance data do not support this hypothesis. Given that small amount of variance explained by retinal illuminance in younger individuals, it is unlikely that this was a primary factor in the improvements seen in the task or the improvements seen in far acuity. Finding a significant change in retinal illuminance for younger participants is an interesting result and additional research will be needed to replicate this finding and determine the impact of this effect on training and improved vision.
In summary, the results of the study indicate that perceptual learning may be a viable intervention for age-related declines in vision. The present study consisted of only five days of training, while many perceptual learning studies consist of much longer training periods, in some cases up to 30 days of training (Karni & Sagi, 1991; Levi, Polatt, & Polat, 1996). Given the short training period, the degree of improvement is quite impressive. Particularly in the case of the improvements found in near and far acuity which averaged 2–3 letters. Further study is needed to determine how much further improvement in near and far acuity may be obtained by older observers, as the current study did not consist of a long enough training period to determine whether or not asymptotic levels of performance were reached. However, the present findings are quite promising and provide further evidence of the plasticity of visual processing in advanced age.
References
- Ahissar M, Hochstein S. Task difficulty and the specificity of perceptual learning. Nature. 1997;387(6631):401–6. doi: 10.1038/387401a0. [DOI] [PubMed] [Google Scholar]
- Andersen GJ. Aging and vision: changes in function and performance from optics to perception. Wiley Interdisciplinary Reviews: Cognitive Science. 2012;3(3):403–10. doi: 10.1002/wcs.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen GJ, Ni R, Bower JD, Watanabe T. Perceptual learning, aging, and improved visual performance in early stages of visual processing. Journal of Vision. 2010;10(13) doi: 10.1167/10.13.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atchley P, Andersen GJ. The effect of age, retinal eccentricity, and speed on the detection of optic flow components. Psychology and Aging. 1998;13(2):297–308. doi: 10.1037/0882-7974.13.2.297. [DOI] [PubMed] [Google Scholar]
- Bennett PJ, Sekuler R, Sekuler AB. The effects of aging on motion detection and direction identification. Vision Res. 2007;47(6):799–809. doi: 10.1016/j.visres.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Betts LR, Sekuler AB, Bennett PJ. The effects of aging on orientation discrimination. Vision Research. 2007;47(13):1769–1780. doi: 10.1016/j.visres.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B. 1995;57(1):289–300. [Google Scholar]
- Betts LR, Sekuler AB, Bennett PJ. The effects of aging on orientation discrimination. Vision research. 2007;47(13):1769–80. doi: 10.1016/j.visres.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Betts LR, Taylor CP, Sekuler AB, Bennett PJ. Aging Reduces Center-Surround Antagonism in Visual Motion Processing. Neuron. 2005;45(3):361–6. doi: 10.1016/j.neuron.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Bower JD, Andersen GJ. Aging, perceptual learning, and changes in efficiency of motion processing. Vision research. 2011 doi: 10.1016/j.visres.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JD, Watanabe T, Andersen GJ. Perceptual Learning and Aging: Improved Performance for Low-Contrast Motion Discrimination. Frontiers in Psychology. 2013 Feb;4:1–7. doi: 10.3389/fpsyg.2013.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spatial Vision. 1997;10(4):433–6. doi: 10.1163/156856897X00357. [DOI] [PubMed] [Google Scholar]
- Burton KB, Owsley C, Sloane ME. Aging and neural spatial contrast sensitivity: photopic vision. Vision research. 1993;33(7):939–46. doi: 10.1016/0042-6989(93)90077-a. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Salthourse TA. The Handbook of Aging and Cognition. 3. Psychology Press; New York, NY: 2007. [Google Scholar]
- DeLoss DJ, Watanabe T, Andersen GJ. Optimization of perceptual learning: effects of task difficulty and external noise in older adults. Vision research. 2014;99:37–45. doi: 10.1016/j.visres.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosher BA, Lu ZL. Perceptual learning in clear displays optimizes perceptual expertise: learning the limiting process. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(14):5286–90. doi: 10.1073/pnas.0500492102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans L. Traffic Safety. Bloomfield Hills, MI: Science Serving Society; 2004. [Google Scholar]
- Jeter PE, Dosher BA, Petrov A, Lu ZL. Task precision at transfer determines specificity of perceptual learning. Journal of Vision. 2009;9(3) doi: 10.1167/9.3.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karni A, Sagi D. Where practice makes perfect in texture discrimination: evidence for primary visual cortex plasticity. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(11):4966–70. doi: 10.1073/pnas.88.11.4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koretz JF, Kaufman PL, Neider MW, Goeckner PA. Accommodation and presbyopia in the human eye—aging of the anterior segment. Vision Research. 1989;29(12):1685–92. doi: 10.1016/0042-6989(89)90150-8. [DOI] [PubMed] [Google Scholar]
- Langford J, Koppel S. Epidemiology of older driver crashes – Identifying older driver risk factors and exposure patterns. Transportation Research Part F: Traffic Psychology and Behaviour. 2006;9(5):309–21. doi: 10.1016/j.trf.2006.03.005. [DOI] [Google Scholar]
- Levi DM, Polatt URI, Polat U. Neural plasticity in adults with amblyopia. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(13):6830–4. doi: 10.1073/pnas.93.13.6830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liutkeviciene R, Cebatoriene D, Liutkeviciene G, Jašinskas V, Zaliuniene D. Associations between contrast sensitivity and aging. Medicina (Kaunas, Lithuania) 2013;49(6):273–7. [PubMed] [Google Scholar]
- Lord SR, Smith ST, Menant JC. Vision and falls in older people: risk factors and intervention strategies. Clinics in geriatric medicine. 2010;26(4):569–81. doi: 10.1016/j.cger.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Matthews N, Liu Z, Qian N. The effect of orientation learning on contrast sensitivity. Vision Research. 2001;41(4):463–71. doi: 10.1016/S0042-6989(00)00269-8. [DOI] [PubMed] [Google Scholar]
- Owsley C. Aging and vision. Vision research. 2011;51(13):1610–22. doi: 10.1016/j.visres.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owsley C, Ball K, McGwin G, Sloane ME, Roenker DL, White MF, Overley ET. Visual Processing Impairment and Risk of Motor Vehicle Crash Among Older Adults. JAMA: The Journal of the American Medical Association. 1998;279(14):1083–8. doi: 10.1001/jama.279.14.1083. [DOI] [PubMed] [Google Scholar]
- Owsley C, Stalvey B, Wells J, Sloane ME. Older drivers and cataract: driving habits and crash risk. The journals of gerontology. Series A, Biological sciences and medical sciences. 1999;54(4):M203–11. doi: 10.1093/gerona/54.4.m203. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spatial Vision. 1997;10(6):437–42. doi: 10.1163/156856897X00366. [DOI] [PubMed] [Google Scholar]
- Polat U. Making perceptual learning practical to improve visual functions. Vision Research. 2009;49(21):2566–73. doi: 10.1016/j.visres.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Polat U, Schor C, Tong JL, Zomet A, Lev M, Yehezkel O, Levi DM. Training the brain to overcome the effect of aging on the human eye. Scientific Reports. 2012;2:278. doi: 10.1038/srep00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards OW. Effects of luminance and contrast on visual acuity, ages 16 to 90 years. American Journal of Optometry and Physiological Optics. 1977;54(3):178–184. doi: 10.1097/00006324-197703000-00010. [DOI] [PubMed] [Google Scholar]
- Roudaia E, Bennett P, Sekuler A. The effect of aging on contour integration. Vision Research. 2008;48(28):2767–2774. doi: 10.1016/j.visres.2008.07.026. [DOI] [PubMed] [Google Scholar]
- Schmolesky MT, Wang Y, Pu M, Leventhal AG. Degradation of stimulus selectivity of visual cortical cells in senescent rhesus monkeys. Nature Neuroscience. 2000;3(4):384. doi: 10.1038/73957. doi:Article. [DOI] [PubMed] [Google Scholar]
- Sekuler R, Owsley C, Hutman L. Assessing spatial vision of older people. American Journal of Optometry and Physiological Optics. 1982;59(12):961–968. doi: 10.1097/00006324-198212000-00005. [DOI] [PubMed] [Google Scholar]
- Spear PD. Neural bases of visual deficits during aging. Vision Research. 1993;33(18):2589–609. doi: 10.1016/0042-6989(93)90218-L. [DOI] [PubMed] [Google Scholar]
- Sundermier L, Woollacott MH, Jensen JL, Moore S. Postural sensitivity to visual flow in aging adults with and without balance problems. Journal of Gerontology: Medical Sciences. 1996;51A:45–52. doi: 10.1093/gerona/51a.2.m45. [DOI] [PubMed] [Google Scholar]
- Van Ness FL, Bouman MA. Spatial modulation transfer in the human eye. Journal of the Optical Society of America. 1967;57:401–406. doi: 10.1364/josa.57.001082. [DOI] [PubMed] [Google Scholar]
- Watson AAB, Pelli DGD. QUEST: A Bayesian adaptive psychometric method. Attention, Perception, & Psychophysics. 1983;33(2):113–20. doi: 10.3758/bf03202828. [DOI] [PubMed] [Google Scholar]
- Wiener JM, Tilly J. Population ageing in the United States of America: implications for public programmes. International Journal of Epidemiology. 2002;31(4):776–81. doi: 10.1093/ije/31.4.776. [DOI] [PubMed] [Google Scholar]


