Summary
Recent studies have shown that ultra-large complexes (ULCs) of platelet factor 4 (PF4) and heparin (H) play an essential role in the pathogenesis of Heparin-Induced Thrombocytopenia (HIT), an immune-mediated disorder caused by PF4/H antibodies. Because antigenic PF4/H ULCs assemble through non-specific electrostatic interactions, we reasoned that disruption of charge-based interactions can modulate the immune response to antigen. We tested a minimally anticoagulant compound (2-O, 3-O desulfated heparin or ODSH) with preserved charge to disrupt PF4/H complex formation and immunogenicity. We show that ODSH disrupts complexes when added to pre-formed PF4/H ULCs and prevents ULC formation when incubated simultaneously with PF4 and UFH. In other studies, we show that excess ODSH reduces HIT antibody (Ab) binding in immunoassays and that PF4/ODSH complexes do not cross-react with HIT Abs. When ODSH and UFH are mixed at equimolar concentrations, we show that there is a negligible effect on amount of protamine required for heparin neutralization and reduced immunogenicity of PF4/UFH in the presence of ODSH. Taken together, these studies suggest that ODSH can be used concurrently with UFH to disrupt PF4/H charge interactions and provides a novel strategy to reduce antibody mediated complications in HIT.
Keywords: Platelet Factor 4, PF4, ODSH, heparin and HIT, immunogenicity
Introduction
Unfractionated heparin (UFH, or heparin) is the anticoagulant of choice for interventional procedures and surgical procedures (cardiopulmonary bypass or CPB), where rapid and reversible anticoagulation is highly desirable. Despite the advent of new anticoagulant agents in the past decade, none have been able to supplant heparin’s unique pharmacologic niche. Alternative anticoagulant therapies, such as low-molecular weight heparins (LMWHs), fondaparinux and danaparoid and the direct thrombin inhibitors (DTIs) have a number of shortcomings, including long half-lives (LMWHs, fondaparinux or danapaorid), narrow therapeutic window (DTIs), and/or lack of reversibility (1–3). Aptamers, synthetic stable RNA molecules capable of binding protein targets, hold promise for indications such as CPB (4, 5); however, these drugs are in the early stages of clinical development and have yet to be studied in large clinical populations.
The therapeutic benefits of heparin are offset by its well-recognized immune complications. Heparin and heparin-derivates are highly sensitizing drugs and are associated with development of antibodies (Abs) to complexes of platelet factor 4 (PF4) and heparin. PF4/H antibodies occur in ~8–17% of general medical and surgical patients exposed to heparin, 2–8% of patients treated with LMWH and 1–2% of patients treated with fondaparinux (6, 7). A subset of these seropositive patients succumbs to life and limb-threatening complications of Heparin-Induced Thrombocytopenia (HIT) (6). In addition to the clinically devastating complications of HIT caused by PF4/H Abs, the high frequency of asymptomatic PF4/H Abs in heparinized patients is clinically problematic. Currently there are no sensitive biomarkers of antibody pathogenicity, and in many clinical centers without hematologic expertise, PF4/H seropositivity is often misdiagnosed as clinical HIT (8, 9). Additionally, findings of PF4/H antibodies in medically ill patients with other causes of thrombocytopenia (infection, drugs or intravascular devices) often confounds diagnostic evaluation, contributes to an overdiagnosis of HIT (8, 9) and leads to unnecessary exposure to potent DTI’s.
Currently, there are no therapeutic strategies directed to reducing the sensitizing properties of heparin or disruption of HIT antibody binding sites. The rationale for such an approach is based on studies by Krauel and colleagues, who showed that danaparoid, a mixture of glycosaminoglycans with anticoagulant activity, may be therapeutic in HIT through disruption of PF4/H antigenic complexes (10). In these studies, the authors showed that therapeutic doses of danaparoid displaced PF4 from platelet binding sites, reduced PF4/H complex size and inhibited HIT antibody binding to antigenic complexes in the presence of drug (10). While danaparoid was effective in disrupting antigenic complex formation, it was not an effective substitute for heparin, due to its modest anticoagulant effect {anti-Factor Xa (FXa) =14 U mg−1}, long half-life (24.5 ± 9.6 h) and lack of reversibility (11, 12).
Recently, a chemically modified heparin molecule with minimal anticoagulant effect, 2-O, 3-O, desulfated heparin (ODSH, Paringenix Inc. Weston, FL) was developed to preserve the well-known anti-inflammatory properties of heparin without its anticoagulant effect (13). ODSH is chemically derived from heparin through selective desulfation of the 2-oxygen (2-O) position on α-L-idouronic acid (2-sulfate) and the 3-O position at D-glucosamine-N-sulfate (3,6-disulfate) to form 2-O,3-O desulfated heparin (13, 14). Desulfation of heparin at the 3-O site reduces its binding affinity to antithrombin by ~20,000 fold (15) and, therefore, markedly attenuates its anticoagulant function. Selective desulfation of UFH at the 2-O, 3-O sites, however, has been shown not to significantly alter other physical properties shared with UFH, including its MW 8,000–14,000 Da, half-life ranging (30–60 minutes), reversibility and charge-dependent interactions with a variety of cationic proteins, including human leukocyte elastase and cathepsin G (13, 14). Because ODSH has preserved charge interactions but not the anticoagulant activity of heparin, we asked if ODSH can interfere with PF4/H complex formation and thereby reduce HIT antibody binding and/or modulate the immunogenicity of PF4/H complexes.
Materials and Methods
Murine PF4, Heparin and ODSH
Unless specified, reagents were purchased from Sigma Aldrich (St. Louis, MO). Recombinant murine PF4 (mPF4) or human PF4 (hPF4) was prepared from bacteria as previously described (16–18). For MW calculations, PF4 was estimated at 7.8k Da for monomer and 31.2 kDa per tetramer (17); UFH (100 or 1000 Units/mL; Heplock; Elkins-Sinn Inc, Cherry Hill, NJ) was estimated to have a specific activity at 140 U/mg and mean MW of 15 kDa (17, 19); ODSH (Paringenix, Weston, Florida) was estimated at an average MW= 11 kDa. Because UFH was the only heparin (H) compound tested in this manuscript, from here in, UFH will be used interchangeably with heparin (H). To facilitate comparisons of UFH and ODSH in a clinical context, UFH concentrations will be expressed in U/mL and µg/mL and ODSH concentrations will be expressed as µg/mL in the remainder of the manuscript.
Thrombin generation assay (chromogenic endpoint)
Activity of UFH or ODSH was determined colorimetrically using bovine antithrombin (AT, both kindly provided by Dr. Walter Kisiel, University of New Mexico) according to previously described methods (20). Briefly, UFH (0–100 U/ml or 0–714 µg/mL) or ODSH (0–500 µg/ml) was incubated in buffer (50 mM Tris, 150 mM NaCl and 0.5% bovine serum albumin) with AT (10 µg/mL) for 3 minutes, followed by addition of IIa (1.5 µg/mL) for 2 minutes. Activity of residual thrombin was measured through endpoint substrate conversion of S2238 (1.2 mg/mL, Diapharma Group, Inc, West Chester, Ohio) at 405 nm in a Spectra Plus 384 Plate reader (MDS technologies, Sunnyvale, CA). Positive (pos) control included wells containing only IIa with S-2238 substrate (no AT) and negative control (neg) consisted of a reaction which included IIa, AT, 0.2 U/mL UFH and S2238, leading to maximum inhibition of thrombin generation. Residual thrombin activity was calculated as follows:
Neutralization of UFH or ODSH by protamine (PRT) was performed through modification of the thrombin generation assay. UFH (0.5 U/mL or 3.6 µg/mL) alone or with ODSH (2.6, 5.2 and 10.4 µg/mL) was incubated with increasing amounts of protamine (PRT; 50–250 µg/mL, PRT MW: 5.1 kDa) and residual thrombin was measured using conditions described above. For all thrombin generation assays, the inhibitory concentration leading to 50% residual thrombin (IC50) was calculated.
PF4 filter binding assay
We examined the binding of PF4 to UFH and ODSH using a filter-trapping method previously described for PF4 interactions with heparin-like molecules (21). In this experiment, 35S-labeled UFH (1 µL; approximately 10,000 cpm) was incubated with a fixed amount of PF4 (17 µg/mL) to form complexes. After complex formation, increasing amounts of unlabeled UFH (0–3.33 U/mL or 0–24 µg/mL) or ODSH (0–13.3 µg/mL) diluted in reaction buffer (50 mM Tris, 130 mM NaCl, pH 7.3) was added and incubated for 30 min at 37°C to displace 35S-UFH. The mixture was then spotted onto a nitrocellulose membrane, which binds to protein nonspecifically, permitting the capture of PF4 and 35S-UFH complexes. The membrane wells were then excised and the bound radioactivity was determined with a scintillation counter.
UV Absorbance
Studies of light transmission/absorbance were performed as previously described (17, 22) to assay for effects of UFH or ODSH on the spectral properties of PF4. In brief, mPF4 (100 µg/ml) was mixed with increasing concentrations of UFH (0–50 U/mL or 0–357 µg/mL) and ODSH (0–71 µg/ml) in H2O and incubated for 30 minutes. After incubation, A280nm was recorded using a Spectra Max Plus 384 Plate reader (MDS technologies, Sunnyvale, CA). The results were analyzed using SoftMax Pro. V. 5.3.
Zeta potential
Zeta potential (ζ-potential), which is related to the surface charge of particles in solution, was measured as previously described (17, 22). For determination of surface charge, mPF4 (100 µg/ml) was incubated with increasing concentrations of UFH (0–50 U/mL or 0–357 µg/mL) or ODSH (0–71 µg/ml) in H2O and measurements were recorded using a Zetasizer (Malvern Instruments, Worcestershire, United Kingdom). The ζ-potential was calculated using the Henry equation and analyzed using the accompanying Zetasizer software (Malvern).
Photon Correlation Spectroscopy
To examine the effects of UFH or ODSH on disruption of pre-formed PF4/H or PF4/ODSH complexes, photon correlation spectroscopy (PCS) was performed. PCS or dynamic light scattering is a technique used for sizing submicron particles (range 3 nm – 5 µm) (23). To perform this assay particles in solution are illuminated with a laser beam and particle motion is analyzed as time-dependent fluctuations in light intensity. Because the motion of a particle in solution is inversely related to its size, measurements provide estimates of particle size distribution. For the studies shown, PCS was performed using a Zetasizer Nano ZS (Malvern, Worcestshire, UK) with a fixed 173° scattering angle and external fiber angle, and a 633-nm helium-neon laser. Data were analyzed using the associated Zetasizer software (Dispersion Technology Software 4.2, Malvern).
To determine effects of UFH or ODSH on disruption of preformed multimolecular complexes, mPF4/ODSH or mPF4/H complexes were formed in H2O at molar ratios leading to ULCs (as determined by ζ-potential). After 15 minutes allowing for complex formation, increasing amounts of UFH (0–10 U/mL or 0–71 µg/mL) or ODSH (0–200 µg/mL) was added to solutions of mPF4/H (50 µg/mL PF4 and 1.25 U/mL or 9 µg/mL UFH in H2O) or PF4/ODSH (50 µg/mL PF4 and 10 µg/mL ODSH in H2O) followed by a 15 minute incubation. After final incubation, complex size was determined by PCS. Size of PF4/H ULCs was also assessed by incubating mPF4 (50 µg/mL) and UFH (1.25 U/mL or 9 µg/mL) concurrently with increasing amounts of ODSH (0–200 µg/mL) for 30 minutes.
Patient samples and hPF4/H ELISA
Plasma from HIT patients or normal plasma was obtained using informed consent under an IRB approved protocol (IRB Registry # Pro00012901). To determine the effect of increasing amounts of ODSH on HIT antibody binding in a commercial HIT ELISA (PF4 Enhanced®, Genetics Technology Institute, GTI, Waukesha, WI), HIT antibodies were incubated with increasing amounts of ODSH (0.5 µg/mL–10 µg/mL) in manufacturer’s diluent buffer and binding of HIT antibodies to wells was determined per manufacturer’s instructions.
To determine if HIT antibodies recognized complexes of human PF4/ODSH, hPF4 was mixed with various amounts of ODSH (0.4–3.2 µg/mL) in buffer (phosphate buffered solution, PBS) and incubated in 96-well microtiter wells overnight. Coated plates were washed and wells were serially incubated with HIT antibodies {1:100 dilution in Tris buffered saline (TBS) containing 0.5%Tween}, secondary antibody (goat anti-human IgG/A/M; 1:100 dilution polyclonal Goat Anti-mouse IgG-γ chain, Sigma). Binding was determined colorimetrically using HRP/TMBZ (KPL, Inc., Gaithersburg, Maryland) and measured in a plate reader (SpectraMax).
Murine Immunization Model
We tested the immunogenicity of mixtures of PF4/H ± ODSH using a previously described immunization model (24). Mice (n=20 mice/cohort) were injected via retro-orbital plexus according to our standard immunization protocol with mPF4 (100 µg/mL)/H (5 U/mL or 36 µg/mL) ± ODSH (26.2 µg/mL) in a final volume of 100 µL containing HBSS daily for five days. Blood samples for ELISA were collected in anesthetized mice from the retro-orbital blood plexus in acid-dextrose citrate solution (ACD formula A, Baxter Healthcare Corporation, Deerfield IL) at baseline and at weekly intervals for four weeks after the start of immunizations. All studies were performed with the approval of the Institutional Animal Care & Use Committee at Duke University.
Results
Inhibition of thrombin generation by UFH and ODSH
Recent studies have established that ODSH has low affinity for AT (Kd = 339 µM or 4000 µg/ml) as compared to UFH (Kd=1.56 µM or 22 µg/ml for UFH) (13). In published studies, serial batches of ODSH showed consistently reduced United States Pharmacopeia (USP) (7 ± 0.3 U of anticoagulant activity/mg), anti-Xa (1.9 ± 0.1 U/mg), and anti-IIa (1.2 ± 0.1 U/mg) activities relative to UFH (165–190 U/mg activity for all 3 assays) (13). To confirm that ODSH had reduced anticoagulant function, but preserved charge-dependent properties, we performed a thrombin (IIa) generation assay and a PF4-binding assay, to respectively examine the anticoagulant and charge-dependent interactions of ODSH.
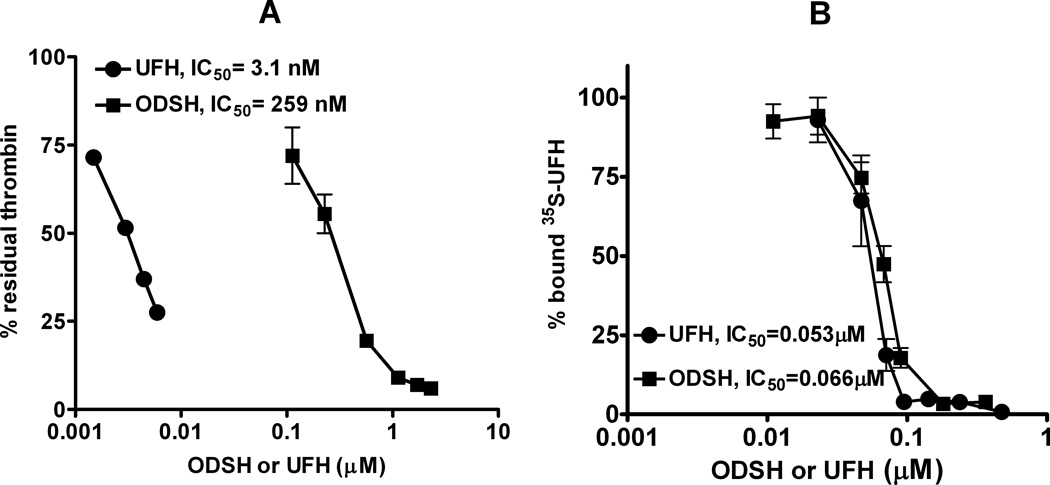
As shown in Figure 1A, incubation of UFH with AT, IIa and substrate (S2238) leads to dose dependent neutralization of IIa. The amount of UFH required for inhibiting 50% of thrombin (IC50) was 3.1 nM (0.0066 U/mL or 0.047 µg/mL). In keeping with previous observations (13, 14), ODSH has markedly reduced anti-IIa activity, as indicated by an 90-fold lower IC50 (259 nM or 2.85 µg/mL) as compared to UFH. The combined anticoagulant effects of ODSH and UFH were also examined. As shown in Supplemental Figure S1, increasing amounts of ODSH added to a fixed concentration of UFH (6 ng/mL or 0.5 nM) had minimal anticoagulant effect on thrombin generation. At ODSH concentrations that were ~50–60 fold in molar excess (30 nM ODSH) enhanced the anticoagulant effect of UFH by 13%. Indeed, an ODSH concentration of 940 ng/mL (85 nM) was needed to accelerate UFH’s anticoagulant effect by 50%. These data confirm that ODSH has a weak, but dose-dependent anticoagulant effect, when combined with UFH.
Figure 1. Comparison of ODSH and UFH with respect to thrombin generation and PF4 binding.
(A) Thrombin generation assay. UFH or ODSH were incubated with antithrombin, thrombin and chromogenic substrate in buffer containing 50mM Tris, 150mM NaCl and 0.5% bovine serum albumin, pH 7.4. The amount of UFH or ODSH required to inhibit 50% of thrombin was calculated. Each data point represents mean ± SD of duplicate wells and is representative of three independent experiments. (B) Competition of labeled H (35S) with unlabeled UFH or ODSH in reaction buffer containing 50 mM Tris and 130 mM NaCl, pH 7.3. Binding was assayed as bound 35S. Each data point corresponds to mean ± SD of duplicate wells and is representative of two independent experiments.
To determine if ODSH retains sufficient charge to interact with PF4, we examined binding of ODSH to PF4 in a competitive binding assay using radiolabeled (35S) UFH. As shown in Figure 1B, UFH and ODSH have similar binding affinities for PF4 as indicated by IC50’s for displacing bound 35S-UFH (UFH=0.053 µM or 0.8 µg/mL v. ODSH=0.066 µM or 0.73 µg/mL). Taken together, these studies suggest that although ODSH has minimal anticoagulant activity, its charge-dependent interactions with PF4 are well-preserved.
Biophysical interactions of ODSH and PF4
Recent studies of PF4/H complex formation have shown that UFH, a negatively charged carbohydrate, dramatically lowers the surface charge of PF4, a positively charged protein and facilitates complex formation through charge interactions (17). PF4/H ULC formation can be readily monitored through changes in light-scattering properties (measured as changes in light absorbance/transmission) and/or changes in the surface charge of PF4 (measured as ζ-potential).
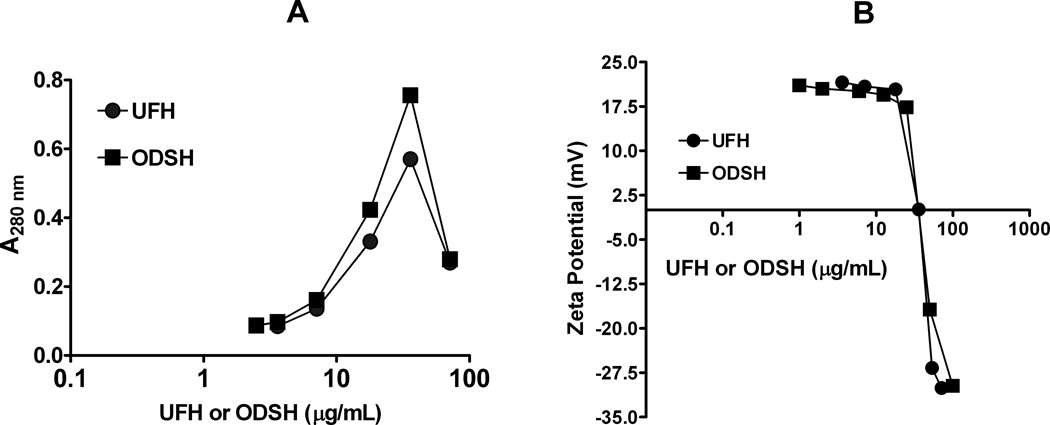
To determine if ODSH alters the biophysical properties of PF4 in a manner analogous to UFH and leads to PF4/ODSH complex formation, we incubated PF4 (100 µg/mL) with increasing amounts of ODSH (0–71 µg/mL) or UFH (0–10 U/mL or 0–71 µg/mL) and measured light absorbance (A280nm) and ζ-potential in H2O. As shown in Figure 2, when PF4 was mixed with increasing amounts of ODSH or UFH, we noted similar dose-dependent changes in light absorbance and ζ-potential. As shown in Figure 2A, when increasing amounts of ODSH or UFH are added to PF4 (100 µg/mL) in H2O, light absorbance peaks for ODSH and UFH at 36 µg/mL (3.3 µM for ODSH and 5 U/mL or 2.4 µM for UFH). These findings were comparable to changes in PF4’s ζ-potential and the amount of UFH or ODSH needed to neutralize the charge of 100 µg/mL PF4. As shown in Figure 2B, ODSH and UFH neutralize PF4’s surface charge at ~36 µg/mL (PF4:ODSH molar ratio~ 1:1). These studies indicate that, as with UFH, ODSH interactions with PF4 are stoichiometric, charge-dependent and result in formation of PF4/ODSH ULCs at comparable molar ratios of ~1:1 in vitro.
Figure 2. Changes in light absorbance (A) and surface charge (B) of PF4 with increasing amounts of UFH or ODSH.
PF4 (100µg/ml) was incubated with increasing amounts of UFH or ODSH in H2O and changes in absorbance (A) and zeta potential (B) were measured. Each data point corresponds to an individual measurement and is representative of three independent experiments.
Disruption of PF4/UFH complexes in the presence of UFH and ODSH
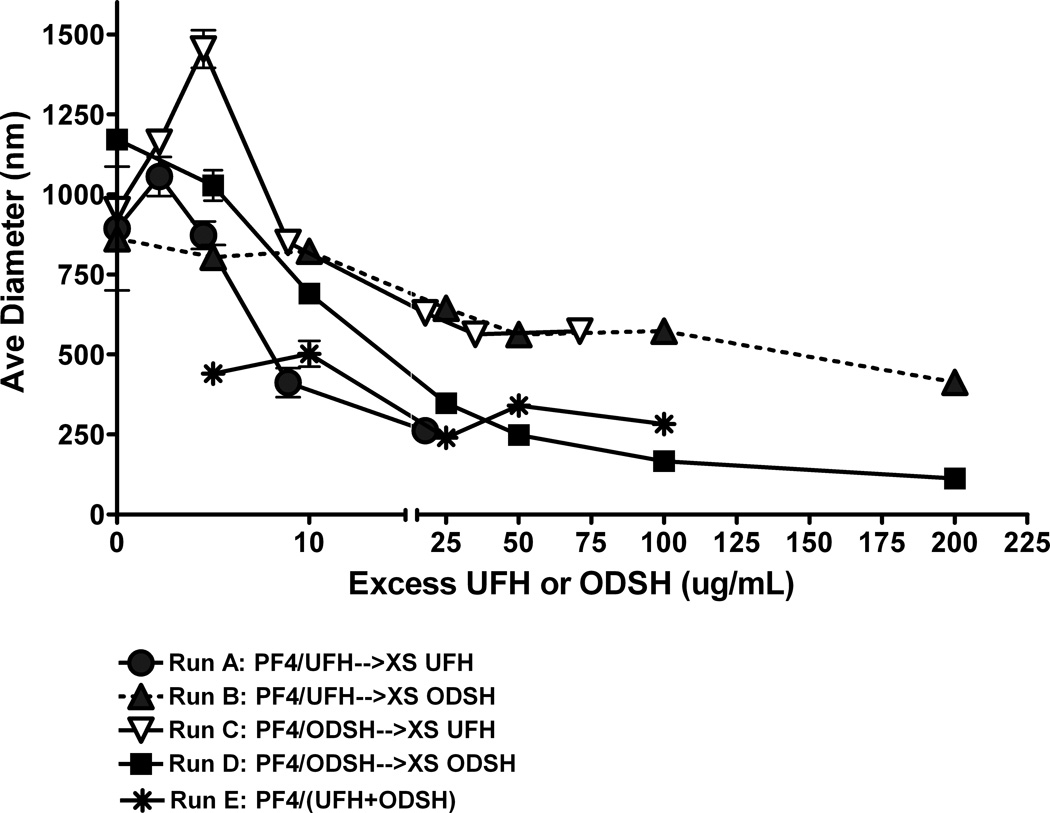
Because ODSH exerts minimal anticoagulant activity and yet retains charge-based interactions with PF4, we hypothesized that ODSH could interfere with PF4/H ULC formation. To examine effects of ODSH on PF4/H ULC formation, we incubated PF4 (50 µg/mL) with concentrations of UFH (1.25 U/mL or 9 µg/mL) or ODSH (10 µg/mL) to form measurable ULCs. After complexes stabilized for 15 minutes, pre-formed PF4/H or PF4/ODSH complexes were incubated with increasing amounts of UFH or ODSH as described in methods and complex size was measured using PCS. In other experiments, PF4 (50 µg/mL) was incubated concurrently with UFH (1.25 U/mL or 9 µg/mL) and increasing amounts of ODSH to examine effects co-incubation of carbohydrates on ULC assembly. As shown in Figure 3, we noted that when PF4/H or PF4/ODSH complexes are pre-formed and subsequently incubated with the other carbohydrate (ODSH added to preformed complexes of PF4/H or vice versa), there is only a partial dissolution of complexes as assessed by PCS. However, when UFH or ODSH is added to PF4 containing the same carbohydrate (i.e., increasing ODSH is added to complexes of PF4/ODSH or vice versa), complex size is markedly reduced with excess carbohydrate. When PF4 is incubated with UFH concurrently with increasing amounts of ODSH, complex formation is markedly inhibited at concentrations of ODSH that were not associated with complex disruption in sequential dosing experiments. These studies suggest that UFH or ODSH is only partially effective in reducing pre-formed complexes containing a different carbohydrate; however, if ODSH is concurrently incubated with UFH and PF4, complex formation is markedly impaired, which is likely due to a charge imbalance, caused by an excess amount of negative charges.
Figure 3. Disruption of PF4/H or PF4/ODSH complexes by excess UFH or ODSH.
PF4/UFH or PF4/ODSH complexes were formed in H20 as described in methods and size measured by PCS. In Runs A–D, complexes were first formed for 15 minutes and subsequently incubated with increasing amounts of UFH or ODSH as described for another 15 minutes and size measured by PCS. In Run E, PF4 was mixed concurrently with UFH and ODSH and complex size measured after 30 minutes. Each data point corresponds to an individual measurement and is representative of two independent experiments.
Effects of ODSH on HIT antibody binding
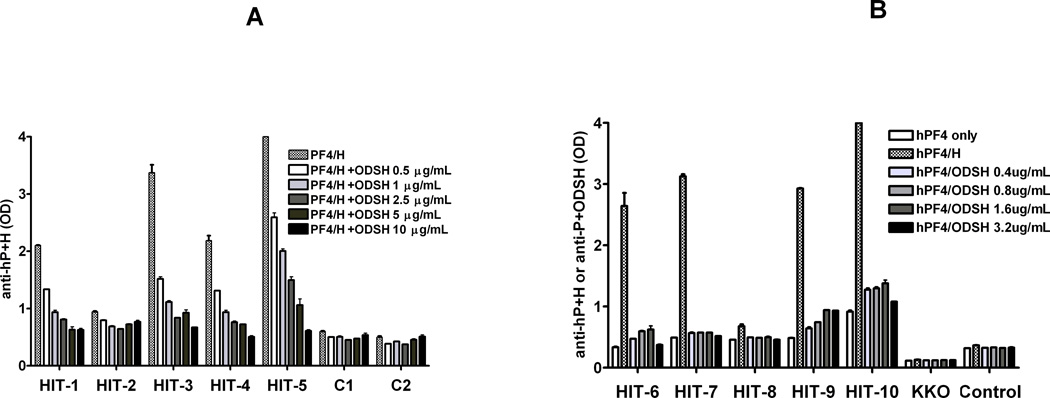
To determine if ODSH can modulate binding of HIT antibodies to PF4/H ULCs, we performed a HIT ELISA in the absence or presence of increasing amounts of ODSH (0–10 µg/mL). As shown in Figure 4A, plasma from 5 patients with HIT (HIT 1–5) or two control subjects (C1–2) was tested by a commercial HIT ELISA. As shown in Figure 4A, binding of HIT antibodies was reduced proportionally with increasing amounts of ODSH. With low doses of ODSH (0.5 µg/mL), HIT Ab binding was reduced by 17–55%. When HIT Abs were incubated in the presence of higher concentrations of ODSH (10 µg/mL), Ab binding was significantly reduced in 4/5 patients by 70–86%. To determine if HIT antibodies cross-react with PF4/ODSH complexes, we developed a PF4/ODSH ELISA by incubating PF4 (10 µg/mL) with a variable amounts of ODSH (0.4–3.2 µg/mL) to yield PF4/ODSH complexes containing molar ratios of 9:1, 4:1, 2:1, and 1:1 (ODSH concentrations of 0.4, 0.8, 1.6 and 3.2µg/mL respectively). Wells coated with PF4/H served as a positive control. As shown in Figure 4B, HIT antibodies showed increased binding to PF4/H complexes, but did not bind to PF4 alone or PF4/ODSH complexes formed at various molar ratios. Reduced binding of HIT antibodies to PF4/ODSH complexes was not due to increased “elution” or loss of PF4 in wells coated with PF4/ODSH. When the amount of unbound PF4 was measured in wells incubated with PF4/UFH or PF4/ODSH, amounts of unbound PF4 were comparable (Supplemental Data, Figure S2) as was binding of anti-PF4 polyclonal antibodies to wells coated with PF4/UFH or PF4/ODSH (data not shown). Taken together, these studies indicate that small concentrations of ODSH (0.5–10 µg/mL) are sufficient to reduce HIT antibody binding (by >30–50%) and that HIT antibodies do not cross-react with PF4/ODSH complexes.
Figure 4. Binding of HIT antibodies.
(A) Effects of excess ODSH on HIT antibody binding to PF4 antigenic complexes. HIT antibodies (1–5) or control subjects (1–2) were tested in a commercial HIT ELISA in the presence of increasing amounts of ODSH (0.5–10 µg/mL) in diluent buffer supplied by the manufacturer. Each data point corresponds to mean ± SD of duplicate wells and is representative of three independent experiments (B) Cross-reactivity of HIT antibodies with PF4/ODSH complexes. HIT antibodies (6–10), control subject and KKO, a murine HIT-like monoclonal antibody, were incubated with wells coated with human PF4 and increasing amounts of ODSH (0.4–3.2 µg/mL) and binding was compared to wells coated with huPF4/UFH. Each data point corresponds to mean ± SD of duplicate wells and is representative of two independent experiments.
Effects of combined UFH/ODSH on protamine reversal
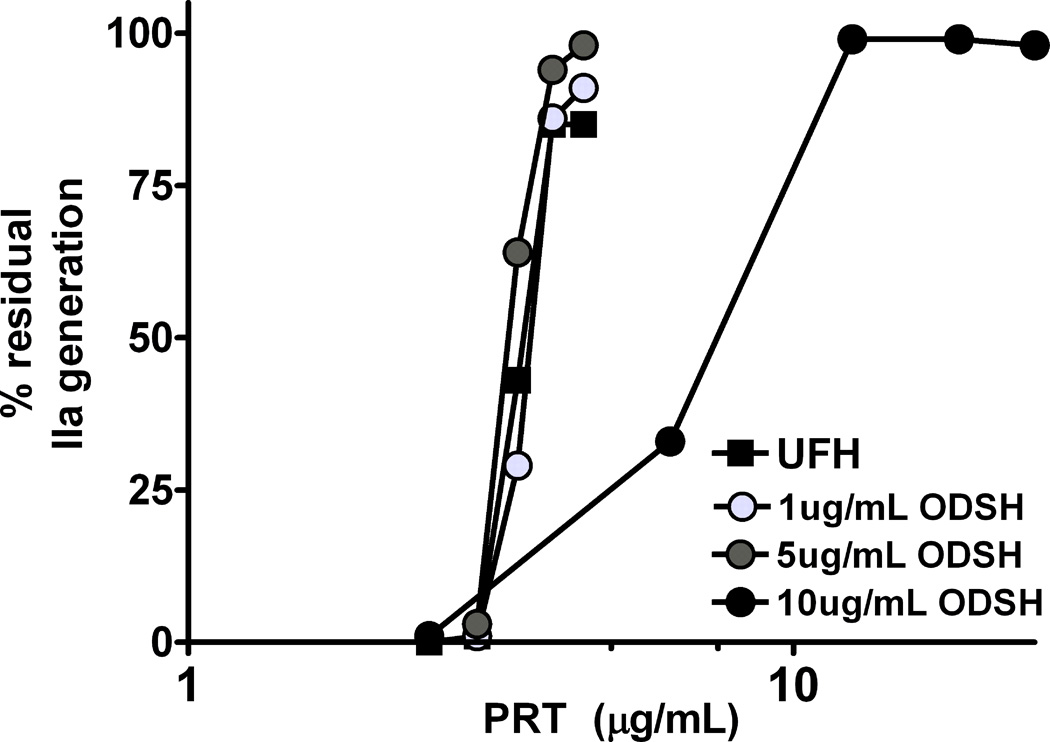
Preserved electrostatic interactions of ODSH with PF4 suggest that ODSH may show similar interactions with other positively charged proteins, such as protamine (PRT). PRT is the only commercial agent approved by the Food and Drug Administration (FDA) for reversing UFH after CPB. To determine if the combined use of ODSH and UFH would require higher doses of PRT for anticoagulant reversal, we performed a protamine neutralization assay using UFH alone (0.5 U/mL or 3.6 µg/mL) or UFH with increasing amounts of ODSH (2.6, 5.2 and 10.4 µg/mL) to yield UFH:ODSH molar ratios of 1:1, 1:2, and 1:4. As shown in Figure 5, in the absence of ODSH, the IC50 for PRT neutralization of UFH was 4 µg/mL. When ODSH was added to UFH at equimolar or two-fold excess (1:1 or 1:2), the IC50 for PRT neutralization was comparable to UFH alone (4.3 and 5.2 µg/mL respectively). When ODSH was in molar excess of UFH by 4-fold, higher amounts of PRT were required to neutralize the two compounds (IC50, PRT=8.4 µg/mL). These studies indicate that when ODSH is combined with UFH at equimolar or two-fold molar excess, PRT dosing requirements are not significantly altered.
Figure 5. Protamine neutralization of UFH or UFH+ODSH.
UFH (0.5 U/mL) was incubated with or without increasing concentrations of ODSH (2.6, 5.2 and 10.4 µg/mL) and a protamine (PRT) neutralization assay was performed as described in methods. The amount of PRT required to inhibit 50% of thrombin generation was calculated as IC50. Each data point corresponds to mean ± SD of duplicate wells and is representative of two independent experiments.
Effects of combined UFH/ODSH on mPF4/H antibody formation
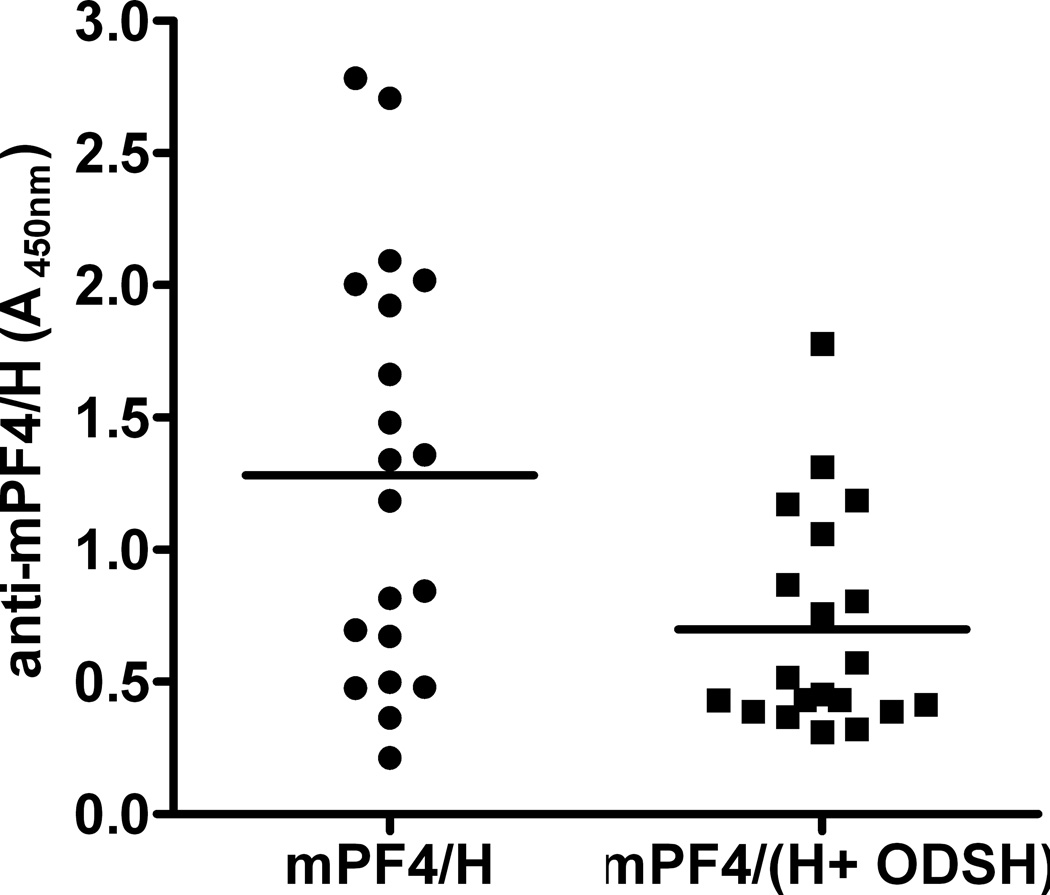
We have previously shown that mPF4/H complexes are highly immunogenic in vivo and that mice injected with solutions of mPF4/H complexes over a course of 5 days develop antibodies to mPF4/H (18, 24). Using this murine model, our studies indicate that ODSH is less immunogenic than an equimolar amount of UFH (Supplemental Data, Figure S3). To determine if ODSH can be combined with UFH for clinical use and would lead to reduced immunogenicity of mPF4/H complexes, mice were immunized with solutions mPF4 (100 µg/mL) in the presence of 5 U/mL UFH (35.7 µg/mL or 2.4 µM) alone or combined with ODSH (26.2 µg/mL, 2.4 µM, UFH:ODSH 1:1 molar ratio) according to our immunization protocol. As shown in Figure 6, antigenic complexes containing UFH were more immunogenic (mean anti-PF4/H A450nm ± SD: 1.281± 0.782) than complexes containing equimolar amounts UFH and ODSH (mean anti-PF4/(H +ODSH): A450nm ± SD: 0.699 ± 0.409; p<0.0054 by two-tailed unpaired t-test). Consistent with human HIT Abs, PF4/H antibodies from mice injected with mPF4/H bound poorly to wells coated with mPF4/ODSH complexes (Supplemental Data, Figure S4). These studies, as well as those shown in Figure 3, suggest that ODSH interferes with PF4/H complex formation and not only modulates the reactivity of HIT antibody binding to antigen, but also interferes with the immunogenicity of PF4/H complexes.
Figure 6. Immunization with mPF4/H in the presence or absence of ODSH.
mPF4 was mixed with H (5 U/mL) or H with equimolar amounts of ODSH (26.2 µg/mL) and complexes were injected into animals daily for five days. Significant differences were seen in mice injected with mPF4/H v. mPF4 (H+ODSH) (p<0.005 by two-tailed unpaired student t-test). Each data point corresponds to average of duplicate measurements and is representative of two independent experiments.
Discussion
In this report, we show that a heparin derivative, ODSH, with minimal anticoagulant activity but preserved charge interactions, disrupts PF4/H multimolecular complex assembly, reduces HIT antibody binding and interferes with the in vivo immunogenicity of mPF4/H complexes. We also show that addition of ODSH at equimolar to two-fold molar excess of UFH has minimal effects on anticoagulation and does not significantly influence the amount of PRT needed for heparin reversal. These findings provide important in vitro and in vivo rationale for assessing the effects of combined ODSH and UFH in selected HIT patient populations where DTIs may be contraindicated or have a limited role.
ODSH was developed as a pharmacologic agent to replicate the diverse anti-inflammatory effects of heparin without its attendant hemorrhagic risks. As shown in recent studies, loss of 2-O and 3-O sulfated residues in ODSH interferes with anticoagulant effect, but does not significantly modulate the anti-inflammatory properties of this heparinoid (13). ODSH and heparin are functionally similar with respect to inhibition of complement activation, leukocyte adhesion and interactions of advanced glycation end-products (AGEs) with their receptors (RAGE)(14). Anti-inflammatory effects of ODSH occur at IC50 values comparable to that of UFH (0.2–4 µg/mL). The shared anti-inflammatory properties of ODSH and heparin are presumably mediated by charge-dependent interactions, as biologic responses of ODSH and UFH are quantitatively similar in studies using synthetic sulfated compounds (25) and/or protamine (26). By contrast, the anticoagulant effect of ODSH requires much higher concentrations, and is not apparent until steady state levels of 30–60 µg/mL are achieved, corresponding to aPTT values of ~45 seconds. In keeping with these observations, our studies show that ODSH, a desulfated heparin, has diminished anticoagulant activity (>90-fold reduction in anticoagulant activity as compared to UFH, Figure 1A), but retains charged interactions with PF4. ODSH shows similar binding affinity to PF4, as shown by a competitive binding assay (Figure 1B) and displays similar biophysical interactions with PF4, as indicated by light absorbance and ζ-potential (Figures 2 A& B).
Our studies suggest that ODSH may not be an optimal therapeutic alternative in HIT if administered as a single agent, because of its weak anticoagulant effect, intact PF4 binding, and propensity for PF4/ODSH complex formation (Figure 1). Indeed, our studies suggest that ODSH may be more therapeutically effective if administered alongside UFH. Concurrent administration of ODSH and UFH would allow for the beneficial effect of UFH, while minimizing its immunogenicity due to impaired PF4/H ULC formation. Studies in Figure 3 indicate that while ODSH can effectively disrupt pre-formed PF4/H complexes, ODSH is much more effective at preventing complex formation when it is incubated simultaneously with UFH. These observations suggest that complex formation is more sensitive to the balance of electrostatic forces than complex dissociation. Additionally, subtle differences in the profiles of complex dissociation between ODSH and UFH are noted in cross-competition studies shown in Figure 3. Although studies in Figure 1B suggest that UFH and ODSH have comparable affinity for PF4, it appears that complexes formed by one carbohydrate are less dissociable by the other. In Figure 3 we noted that if excess ODSH is added to PF4/H or UFH is added to PF4/ODSH, residual complex size is higher than that seen when the same carbohydrate is added in excess (e.g. excess UFH is added to PF4/H). We speculate that PF4 binding sites for UFH and ODSH likely differ due to variations in charge composition.
ODSH is also effective in reducing HIT antibody binding to nascent and/or pre-formed PF4/H complexes. Rao et. al. showed that ODSH did not support platelet activation in the presence of HIT antibodies when tested over a wide range of concentrations (0.75–100 µg/mL) in a14C-serotonin release assay (SRA) (13). In these SRA studies, ODSH was shown to inhibit the platelet activating effects of UFH at concentrations as low as 25 µg/mL(13). In recent studies of ODSH, Krauel and colleagues (27) showed that ODSH is also more effective than UFH at displacing bound PF4 and PF4/H complexes from platelets, thereby reducing HIT antibody binding and downstream effects on platelet activation. Similar to these observations, we demonstrate that binding of HIT antibodies in a commercial ELISA is inhibited 37% on average by small doses of ODSH (0.5 µg/ml, Figure 4). When ODSH is added at higher doses (2.5–10 µg/mL), HIT antibody binding to antigen was reduced by 60–67%. We also show that PF4/H antibodies from patients or murine derived PF4/H antibodies display minimal cross-reactivity with PF4/ODSH complexes. When PF4/ODSH ULCs are formed at various molar ratios (PF4:ODSH molar ratios ranging from 9:1, 4:1, 1.6:1, and 1:1), there is minimal binding of HIT antibodies as compared to binding of HIT antibodies to wells coated with PF4/H complexes(Figure 4B and Figure S4). Taken together, these studies, alongside observations by Krauel et. al (27) and Rao et. al., suggest that ODSH interferes with several critical aspects of the antigen-antibody interactions in HIT, including displacement of PF4 from cellular targets, disruption of PF4/H complex formation, and reduction of HIT antibody binding to antigenic PF4/H complexes.
Because ODSH could be potentially used with UFH in settings such as CPB, we examined the effects of combining ODSH and UFH on protamine (PRT) neutralization. As shown in Figure 5, we demonstrate that there is no significant increase in PRT dose required for equimolar amounts ODSH and UFH (0.24 µM). With increasing amounts of ODSH (2- or 4-fold molar excess of UFH), there was gradual increase in the amounts of PRT needed for heparin neutralization. Similar findings were noted by Krauel and colleagues (27) on the minimal effects of 2–4 fold molar excess of ODSH on the aPTT. In studies by Krauel et. al., and in our supplemental data (Figure S1) there was no prolongation of the aPTT or anti-Xa when ODSH was added to UFH at equimolar concentrations (2 µg/mL). ODSH at 2–4 fold molar excess of UFH prolonged the aPTT by 2.5 and 6.8 seconds with negligible effect on the anti-Xa levels (27). In the thrombin generation assay shown in Figure S1, we demonstrate that the weak anticoagulant effects of ODSH are not apparent until ODSH is >20-fold molar excess.
In our studies, we also show that equimolar ratios of ODSH and UFH profoundly alter the immunogenicity of PF4/H complexes in vivo. As shown in Figure 6, when PF4 is mixed with UFH (5 U/mL or 36 µg/mL) in the absence or presence of equimolar amounts of ODSH, there is a marked reduction in the immunogenicity of injected PF4/H complexes. In additional studies, we show that PF4/ODSH complexes are less immunogenic than equimolar amounts of PF4/UFH in our murine model (Figure S3), suggesting that while charge interactions appear to be preserved with regard to interactions with PF4, there are subtle changes in the charge content of PF4/ODSH complexes as compared to PF4/UFH that render the former complexes less immunogenic in vivo and less reactive with pre-formed HIT antibodies. Moreover, our studies suggest that if ODSH is co-administered with equimolar amounts of UFH, or in slight molar excess (two-fold), there is significant reduction in antibody binding and immunogenicity without increasing anticoagulant effect or the amount of PRT needed for heparin neutralization.
While our studies provide a rationale and initial dosing strategies for use of ODSH, we recognize several limitations that could impact the feasibility of future studies in human subjects. Our studies, with the exception of murine studies, were primarily performed in vitro using buffer systems, using defined concentrations of PF4, UFH and ODSH with limited amounts of other plasma constituents. It is possible that ODSH interactions with PF4 and UFH could be significantly altered by other heparin binding proteins that are present in plasma. It is also possible that ODSH may not significantly attenuate and, in certain instances, could potentially exacerbate PF4/H complex formation. While enhanced PF4/H or PF4/H/ODSH complex formation remains a theoretical concern, our studies suggest that concomitant dosing of UFH and ODSH will alter the charge balance of PF4/H complex formation at onset and thus attenuate the immunogenicity of PF4/H or PF4/ODSH complexes.
It is also important to recognize the limitations of our murine model in extending findings to human disease. Our experimental model utilizes high doses of mPF4, UFH and ODSH, concentrations of which may or may not be achieved in circulation, even for subjects undergoing CPB. For this reason, we recommend that dosing strategies of ODSH for reducing HIT antibody binding or immunogenicity should be based on molar ratios relative to UFH, rather than absolute concentrations of drug.
Despite these limitations, our studies provide a rationale for examining the role of ODSH and PF4/H complex disruption as a viable therapeutic strategy for reducing the immune complications of HIT while retaining the beneficial therapeutic effects of the drug. Future studies in pre-clinical models, such as use of a rodent model of cardiopulmonary bypass (28, 29), can be designed to optimize the safety and efficacy of the dosing regimens identified in this manuscript.
Supplementary Material
Acknowledgements
This study was supported by the National Institutes of Health HL081395 (GMA), American Heart Association Grant-in-Aid (GMA), Paringenix Inc. (GMA) and USEPA STAR award and RD83241301(MRW), HL094463 (JL), AI050050 (JL) and AI074775 (JL) and Coagulation Research Trust Fund of the Medical Foundation of North Carolina (DMM).
Conflict of Interest Disclosures
GMA has received research funding support from Paringenix, Inc. PMQD and SM are employees of Paringenix, Inc.
Footnotes
Presented in part at the 52nd American Society of Hematology Annual Meeting and Exposition, December 6th, 2010, Orlando, FL.
Author Statement
All authors had full access to data and contributed to drafting of the manuscript.
References
- 1.Frederiksen JW. Cardiopulmonary bypass in humans: bypassing unfractionated heparin. Ann Thorac Surg. 2000;70:1434–1443. doi: 10.1016/s0003-4975(00)01511-3. [DOI] [PubMed] [Google Scholar]
- 2.Greinacher A. The use of direct thrombin inhibitors in cardiovascular surgery in patients with heparin-induced thrombocytopenia. Semin Thromb Hemost. 2004;30:315–327. doi: 10.1055/s-2004-831044. [DOI] [PubMed] [Google Scholar]
- 3.Kurup V, Transue S, Wu Y, et al. Cardiac surgery in a patient with heparin-induced thrombocytopenia--cautions with use of the direct thrombin inhibitor, argatroban. Conn Med. 2006;70:245–250. [PubMed] [Google Scholar]
- 4.Nimjee SM, Rusconi CP, Harrington RA, et al. The potential of aptamers as anticoagulants. Trends Cardiovasc Med. 2005;15:41–45. doi: 10.1016/j.tcm.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Nimjee SM, Keys JR, Pitoc GA, et al. A novel antidote-controlled anticoagulant reduces thrombin generation and inflammation and improves cardiac function in cardiopulmonary bypass surgery. Mol Ther. 2006;14:408–415. doi: 10.1016/j.ymthe.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Arepally GM, Ortel TL. Heparin-Induced Thrombocytopenia. N Engl J Med. 2006;355:809–817. doi: 10.1056/NEJMcp052967. [DOI] [PubMed] [Google Scholar]
- 7.Warkentin TE, Cook RJ, Marder VJ, et al. Anti-platelet factor 4/heparin antibodies in orthopedic surgery patients receiving antithrombotic prophylaxis with fondaparinux or enoxaparin. Blood. 2005;106:3791–3796. doi: 10.1182/blood-2005-05-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuker A. Heparin-induced thrombocytopenia (HIT) in 2011: An epidemic of overdiagnosis. Thromb Haemost. 2011;106:993–994. doi: 10.1160/TH11-09-0677. [DOI] [PubMed] [Google Scholar]
- 9.Lo GK, Sigouin CS, Warkentin TE. What is the potential for overdiagnosis of heparin-induced thrombocytopenia? American J Hematol. 2007;82:1037–1043. doi: 10.1002/ajh.21032. [DOI] [PubMed] [Google Scholar]
- 10.Krauel K, Furll B, Warkentin TE, et al. Heparin-induced thrombocytopenia--therapeutic concentrations of danaparoid, unlike fondaparinux and direct thrombin inhibitors, inhibit formation of platelet factor 4-heparin complexes. J Thromb Haemost. 2008;6:2160–2167. doi: 10.1111/j.1538-7836.2008.03171.x. [DOI] [PubMed] [Google Scholar]
- 11.Meuleman DG. Orgaran (Org 10172): its pharmacological profile in experimental models. Haemostasis. 1992;22:58–65. doi: 10.1159/000216296. [DOI] [PubMed] [Google Scholar]
- 12.Danhof M, de Boer A, Magnani HN, et al. Pharmacokinetic considerations on Orgaran (Org 10172) therapy. Haemostasis. 1992;22:73–84. doi: 10.1159/000216298. [DOI] [PubMed] [Google Scholar]
- 13.Rao NV, Argyle B, Xu XY, et al. Low anticoagulant heparin targets multiple sites of inflammation, suppresses heparin-induced thrombocytopenia, and inhibits interaction of RAGE with its ligands. Am J Physiol Cell Physiol. 2010;299:C97–C110. doi: 10.1152/ajpcell.00009.2010. [DOI] [PubMed] [Google Scholar]
- 14.Fryer A, Huang YC, Rao G, et al. Selective O-desulfation produces nonanticoagulant heparin that retains pharmacological activity in the lung. J Pharmacol Exp Ther. 1997;282:208–219. [PubMed] [Google Scholar]
- 15.Atha DH, Lormeau JC, Petitou M, et al. Contribution of monosaccharide residues in heparin binding to antithrombin III. Biochemistr. 1985;24:6723–6729. doi: 10.1021/bi00344a063. [DOI] [PubMed] [Google Scholar]
- 16.Arepally GM, Kamei S, Park KS, et al. Characterization of a murine monoclonal antibody that mimics heparin-induced thrombocytopenia antibodies. Blood. 2000;95:1533–1540. [PubMed] [Google Scholar]
- 17.Suvarna S, Espinasse B, Qi R, et al. Determinants of PF4/heparin immunogeneicity. Blood. 2007;110:4253–4260. doi: 10.1182/blood-2007-08-105098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suvarna S, Rauova L, McCracken EK, et al. PF4/heparin complexes are T cell-dependent antigens. Blood. 2005;106:929–931. doi: 10.1182/blood-2004-12-4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rauova L, Poncz M, McKenzie SE, et al. Ultralarge complexes of PF4 and heparin are central to the pathogenesis of heparin-induced thrombocytopenia. Blood. 2005;105:131–138. doi: 10.1182/blood-2004-04-1544. [DOI] [PubMed] [Google Scholar]
- 20.Saggin L, Cazzola F, Corona G, et al. Neutralization of the antiheparin activity of platelet factor 4 by a monoclonal antibody. Thromb Haemost. 1992;6:137–143. [PubMed] [Google Scholar]
- 21.Stringer SE, Gallagher JT. Specific binding of the chemokin platelet factor 4 to heparan sulfate. J Biol Chem. 1997;272:20508–20514. doi: 10.1074/jbc.272.33.20508. [DOI] [PubMed] [Google Scholar]
- 22.Chudasama SL, Espinasse B, Hwang F, et al. Heparin modifies the immunogenicity of positively-charged proteins. Blood. 2010;116:6046–6053. doi: 10.1182/blood-2010-06-292938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson WW. Light scattering as a diagnostic for protein crystal growth--a practical approach. J Struct Biol. 2003;142:56–65. doi: 10.1016/s1047-8477(03)00038-8. [DOI] [PubMed] [Google Scholar]
- 24.Suvarna S, Qi R, Arepally GM. Optimization of a murine immunization model for study of PF4/heparin antibodies. J Thromb Haemost. 2009;7:857–864. doi: 10.1111/j.1538-7836.2009.03330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hopfner M, Alban S, Schumacher G, et al. Selectin-blocking semisynthetic sulfated polysaccharides as promising anti-inflammatory agents. J Pharm Pharmacol. 2003;55:697–706. doi: 10.1211/002235703765344621. [DOI] [PubMed] [Google Scholar]
- 26.Ferrer-Lopez P, Renesto P, Prevost MC, et al. Heparin inhibits neutrophil-induced platelet activation via cathepsin G. J Lab Clin Med. 1992;119:231–239. [PubMed] [Google Scholar]
- 27.Krauel K, Hackbarth C, Fürll B, et al. Heparin-induced thrombocytopenia: in vitro studies on the interaction of dabigatran, rivaroxaban, and low-sulfated heparin, with platelet factor 4 and anti-PF4/heparin antibodies. Blood. 2011 doi: 10.1182/blood-2011-05-353391. [DOI] [PubMed] [Google Scholar]
- 28.Grocott HP, Mackensen GB, Newman MF, et al. Neurological injury during cardiopulmonary bypass in the rat. Perfusion. 2001;16:75–81. doi: 10.1177/026765910101600111. [DOI] [PubMed] [Google Scholar]
- 29.Mackensen GB, Sato Y, Nellgard B, et al. Cardiopulmonary bypass induces neurologic and neurocognitive dysfunction in the rat. Anesthesiology. 2001;95:1485–1491. doi: 10.1097/00000542-200112000-00031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.