Abstract
BACKGROUND
Early hypertension control reduces the risk of cardiovascular complications among patients with diabetes mellitus. There is a need to improve hypertension management among patients with diabetes mellitus.
OBJECTiVE
We aimed to evaluate rates and associations of hypertension diagnosis and treatment among patients with diabetes mellitus and incident hypertension.
DESIGN
This was a 4-year retrospective analysis of electronic health records.
PARTICIPANTS
Adults ≥18 years old (n = 771) with diabetes mellitus, who met criteria for incident hypertension and received primary care at a large, Midwestern academic group practice from 2008 to 2011 were included
MAIN MEASURES
Cut-points of 130/80 and 140/90 mmHg were used to identify incident cases of hypertension. Kaplan-Meier analysis estimated the probability of receiving: 1) an initial hypertension diagnosis and 2) antihypertensive medication at specific time points. Cox proportional-hazard frailty models (HR; 95 % CI) were fit to identify associations of time to hypertension diagnosis and treatment.
KEY RESULTS
Among patients with diabetes mellitus who met clinical criteria for hypertension, 41 % received a diagnosis and 37 % received medication using the 130/80 mmHg cut-point. At the 140/90 mmHg cut-point, 52 % received a diagnosis and 49 % received medication. Atrial fibrillation (HR 2.18; 1.21–4.67) was associated with faster diagnosis rates; peripheral vascular disease (HR 0.18; 0.04–0.74) and fewer primary care visits (HR 0.93; 0.88–0.98) were associated with slower diagnosis rates. Atrial fibrillation (HR 3.07; 1.39–6.74) and ischemic heart disease/congestive heart failure (HR 2.16; 1.24–3.76) were associated with faster treatment rates; peripheral vascular disease (HR 0.16; 0.04–0.64) and fewer visits (HR 0.93; 0.88–0.98) predicted slower medication initiation. Diagnosis and treatment of incident hypertension were similar using cut-points of 130/80 and 140/90 mmHg.
CONCLUSIONS
Among patients with diabetes mellitus, even using a cut-point of 140/90 mmHg, approximately 50 % remained undiagnosed and untreated for hypertension. Future interventions should target patients with multiple comorbidities to improve hypertension and diabetes clinical care.
KEY WORDS: hypertension, diabetes mellitus, diagnosis, electronic health records
INTRODUCTION
Macrovascular and microvascular complications are major causes of morbidity and mortality in patients with diabetes mellitus.1 Hypertension is common in patients with diabetes mellitus and is a risk factor for accelerating the progression of such complications.2 Blood pressure control following the onset of hypertension in patients with diabetes is associated with lower rates of microvascular complications1,2; blood pressure control within 6 months is associated with a significant decrease in major cardiovascular events.2 This underscores the importance of early recognition and timely hypertension control in patients with diabetes. Despite the importance of controlling blood pressure in patients with diabetes mellitus, there is evidence that blood pressure control is suboptimal among U.S. patients with diabetes.2,3
A better understanding of factors associated with delays in diagnosis and treatment of hypertension among patients with diabetes could inform the development of interventions to improve the clinical care of diabetes. We therefore aimed to identify associations with an initial hypertension diagnosis and antihypertensive medication initiation among individuals with diabetes mellitus.
METHODS
Sample
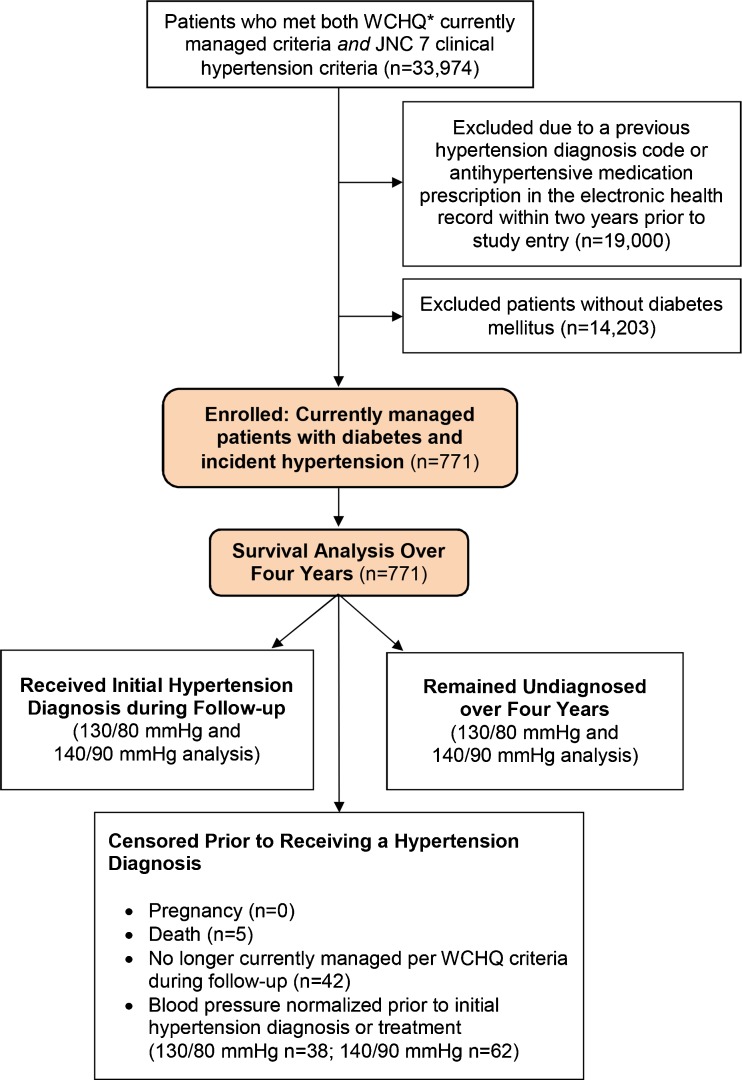
This study was approved by the University of Wisconsin-Madison Health Sciences Institutional Review Board with a waiver of consent. This secondary retrospective cohort analysis used electronic health record data from a large, Midwestern, multi-disciplinary academic group practice. To construct the sample (Fig. 1), we identified all patients ≥18 years old who met criteria from the Wisconsin Collaborative for Healthcare Quality (WCHQ)4,5 for being “currently managed” in the healthcare system between 1 January 2008 and 31 December 2011. Per WCHQ criteria, patients had to have ≥ 2 billable office encounters in an outpatient, non-urgent, primary care setting, or one primary care and one office encounter in an urgent care setting, in the 3 years prior to study enrollment, with at least one visit in the prior 2 years.6 Electronic health records were assessed for the date a patient met the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) clinical blood pressure criteria for a new diagnosis of hypertension7 (incident hypertension) without receiving a previous diagnosis or treatment for hypertension. JNC 7 criteria were the established U.S. hypertension guidelines during the reporting period. A patient met blood pressure eligibility criteria based on electronic health record data if: a) ≥ 3 outpatient blood pressure measurements from three separate dates, ≥ 30 days apart, but within a two-year span, were elevated (systolic blood pressure ≥ 130 mmHg or diastolic blood pressure ≥ 80 mmHg) according to JNC 7 criteria; or b) there were two elevated blood pressures8,9 (systolic blood pressure ≥ 160 mmHg or diastolic blood pressure ≥ 100 mmHg), ≥ 30 days apart within a 2-year period. If more than one blood pressure was taken at a visit, the average was used.10 Hospital and emergency department blood pressures were excluded.
Figure 1.
Study sample: enrollment and analysis. *WCHQ: Wisconsin Collaborative for Healthcare Quality.
Each patient meeting blood pressure eligibility and currently managed criteria received an “index date” (the first date both criteria were met). A 365-day period prior to this index date was the “baseline period” to assess patients’ comorbidities and healthcare utilization. Patients were included if they met Hebert’s criteria for a diabetes mellitus diagnosis in the baseline period (ICD-9 codes: 250.00–250.93 [diabetes and complications], 357.2 [polyneuropathy in diabetes], 362.0–362.02 [diabetic retinopathy], 366.41 [diabetic cataract]).11 All of the patients were diagnosed with diabetes by screening lab tests. Screening methodologies for diabetes (e.g., HgbA1C, fasting plasma glucose, etc.) within this healthcare system have been previously published.12 Patients continued to accrue time in the study from the index date until they achieved study outcomes (hypertension diagnosis and/or antihypertensive medication initiation), censoring, or the study ended. Patients were censored if they died (censored day of death; 0.6 %), were no longer currently managed (censored at the end of the calendar year; 5.4 %), or achieved hypertension control prior to diagnosis or treatment, defined as three consecutive normal blood pressures on three separate dates (130/80 mmHg 5.2 % [n = 38]; 140/90 mmHg 8.1 % [n = 62]).
Prior to enrollment, patients were excluded if they had a previous diagnosis of hypertension based on Tu’s ICD-9 criteria (401.x [essential hypertension], 402.x [hypertensive heart disease], 403.x [hypertensive renal disease], 404.x [hypertensive heart and renal disease], 405.x [secondary hypertension]),13 or had an antihypertensive prescription in the electronic health record. Patients who were pregnant during the study were excluded 1 year before, during, and 1 year following pregnancy using a modified Manson approach (0 %).14 The final sample was 771 currently managed patients with diabetes and incident hypertension (Fig. 1).
Primary Outcome Variables
This study evaluated two primary outcomes: 1) time (days) from index date to the initial hypertension diagnosis, and 2) time (days) from enrollment to antihypertensive medication initiation. Days to diagnosis and treatment were converted into months. During the study period, 130/80 mmHg was the hypertension diagnosis and treatment threshold for patients with diabetes (JNC 7).7 However, our analysis also included 140/90 mmHg to reflect clinical trials and guideline updates.1,15,16 An initial hypertension diagnosis was defined as the date of the first outpatient electronic health record documentation of an ICD-9 code for hypertension per the Tu criteria13 or ICD-9 code 796.2 (“elevated blood pressure without a diagnosis of hypertension”).17 Medication initiation was defined by the first outpatient electronic health record documentation of an antihypertensive prescription as defined by Multum LexiconTM Plus (Cerner Multum, Inc., Denver, CO), a database of all prescription and drug products in the U.S.
Explanatory Variables
The selection of explanatory variables was guided by the concept of clinical inertia (delays in diagnosis and treatment).18–20 According to previous studies, competing comorbidities, in addition to multiple patient, healthcare system, and provider factors, contribute to clinical inertia.19,21 Baseline patient-related variables included: age, gender, marital status, race/ethnicity, tobacco use, socioeconomic status (defined as ever having Medicaid), body mass index, and severity of blood pressure elevation at study entry (categorized as 130–159/80–99 mmHg or ≥ 160/100 mmHg). Patient comorbidities were defined with ICD-9 codes using validated algorithms. The Centers for Medicare and Medicaid Services’ Chronic Condition Warehouse algorithm was used to identify ischemic heart disease.22 An established algorithm was used to identify stroke/transient ischemic attack.23 Elixhauser algorithms24 were utilized to identify depression, other neurologic disorders, collagen vascular disease, and solid tumors (without metastasis); other published algorithms were used to identify hyperlipidemia,25 chronic kidney disease,26 eye disease,27 congestive heart failure,25 peripheral vascular disease,27 and dementia.28 Due to low prevalence, comorbidities were categorized into the following categories for analysis: microvascular disease (chronic kidney disease and/or eye disease), non-cardiac cerebrovascular complications (stroke/transient ischemic attack and/or other neurologic conditions), ischemic heart disease/congestive heart failure (congestive heart failure and/or ischemic heart disease), and other comorbidities (dementia, collagen vascular disease, and/or malignancy).
The number of primary care, specialty, and urgent care visits were assessed at the baseline time point. Primary care visits included Family Medicine/Family Practice, Internal Medicine, Obstetrics/Gynecology, and Pediatrics/Adolescent Medicine physicians (faculty, residents, fellows), nurse practitioners, or physician assistants.29,30 Patients were assigned a primary care provider based on patterns of outpatient face-to-face Evaluation & Management visits to physicians in the group as reported in the electronic health record billing records.6 Providers’ specialties, ages, and genders were acquired from the provider group’s human resources office and/or the American Medical Association 2011 Masterfile data.
Statistical Analysis
The data set was constructed using SAS 9.1.3 (SAS Institute, Inc., Cary, NC) and subsequently analyzed using Stata 13.1 (Stata-Corp, College Station, TX). Initial analyses summarized categorical variables as percentages and continuous variables using means and standard deviations. Kaplan-Meier survival curves evaluated the probability of obtaining a diagnosis and receiving treatment as a function of time since meeting enrollment criteria. Multivariate Cox proportional-hazards regression analyses were conducted using shared frailty models to incorporate a random effect for each physician and account for within-physician correlation.31,32 Adjusted hazard ratios and 95 % confidence intervals were obtained for receiving an initial hypertension diagnosis and hypertension treatment. Differences were considered significant at p < 0.05.
Sensitivity Analysis
A sensitivity analysis was conducted to account for the potential influence of lifestyle counseling prior to a hypertension diagnosis or antihypertensive medication. Cox proportional-hazards models were performed with combined outcome variables that included patients whose blood pressure normalized prior to receiving a hypertension diagnosis or antihypertensive medication.
RESULTS
Descriptive Data
A total of 771 patients met inclusion criteria (Fig. 1). There were a total of 396 providers (15 nurse practitioners and 381 physicians). Table 1 summarizes patient demographic, comorbidity, visits, and healthcare provider characteristics. Patients had a mean (standard deviation) of 15 (13) months of follow-up. Overall, 49 % of patients were male, 14 % had a history of Medicaid, 69 % were obese, and 83 % had stage 1 hypertension.3 Many chronic conditions, including dyslipidemia, atrial fibrillation, and ischemic heart disease/congestive heart failure, were more common in ≥ 60-year olds than in younger age groups.
Table 1.
Baseline Demographics*
| Total population n = 771 | Received hypertension diagnosis n = 315 | Received antihypertensive medication n = 286 | |
|---|---|---|---|
| Patient characteristics | |||
| Age, m (SD) | 54 (14) | 56 (14) | 56 (14) |
| Female | 396 (51) | 153 (49) | 130 (45) |
| Race/ethnicity | |||
| White | 643 (83) | 260 (83) | 235 (82) |
| Non-white† | 128 (17) | 55 (17) | 51 (18) |
| Marital status | |||
| Married/Partnered | 440 (57) | 186 (59) | 179 (63) |
| Unmarried | 331 (43) | 129 (41) | 107 (37) |
| Medicaid, ever‡ | 110 (14) | 43 (14) | 40 (14) |
| Tobacco use category | |||
| Current tobacco use | 349 (45) | 143 (45) | 133 (47) |
| Non-smoker/former tobacco use | 422 (55) | 172 (55) | 153 (54) |
| Body mass index, kg/m2, m (SD) | 34 (8.3) | 34 (8.0) | 34 (8.5) |
| Primary spoken language | |||
| English | 712 (92) | 299 (95) | 273 (95) |
| Other | 59 (7.7) | 16 (5.1) | 13 (4.6) |
| Stage of hypertension§ | |||
| Stage 1 | 639 (83) | 245 (78) | 224 (78) |
| Stage 2 | 132 (17) | 70 (22) | 62 (22) |
| Comorbidities | |||
| Microvascular complications of diabetes‖ | 49 (6.4) | 17 (5.4) | 16 (5.6) |
| Baseline dyslipidemia | 377 (49) | 154 (49) | 141 (49) |
| Atrial fibrillation | 11 (1.4) | 6 (1.9) | 7 (2.5) |
| Cerebrovascular disease with or without complications¶ | 19 (2.5) | 5 (1.6) | 6 (2.1) |
| Peripheral vascular disease | 31 (4.0) | 7 (2.2) | 6 (2.1) |
| Depression | 75 (9.7) | 26 (8.3) | 28 (9.8) |
| Ischemic heart disease# | 31 (4.0) | 14 (4.4) | 16 (5.6) |
| Low prevalence indicator** | 52 (6.7) | 16 (5.1) | 19 (6.6) |
| Baseline ambulatory visit counts, annual, m (SD) | |||
| Primary care visits | 3.6 (3.1) | 3.1 (2.5) | 3.1 (2.6) |
| Specialty care visits | 2.8 (3.3) | 2.3 (2.8) | 2.2 (3.0) |
| Urgent care visits | 0.53 (1.1) | 0.39 (0.89) | 0.35 (0.82) |
| Provider characteristics†† | |||
| Female | 337 (44) | 143 (45) | 125 (44) |
| Specialty providing majority of ambulatory care | |||
| Internal medicine | 318 (41) | 131 (42) | 114 (40) |
| Family medicine | 375 (49) | 154 (49) | 141 (49) |
| Other‡‡ | 78 (10) | 30 (9.5) | 31 (11) |
| Age, m (SD) | 47 (10) | 46 (10) | 46 (10) |
*Values represent numerators and percents unless otherwise specified. Diagnosis and treatment is based on 130/80 mmHg threshold
†Non-white ethnicities: African American (8.0 %); Hispanic/Latino (3.1 %); Asian (2.0 %); Native Hawaiian/Pacific Islander (0.9 %); American Indian/Alaska Native (0.1 %); Unknown (2.5 %)
‡On Medicaid during the baseline or study period
§Stage of hypertension is defined using JNC-7 criteria
‖An indicator variable was created for microvascular disease that consists of the presence of chronic kidney disease and/or eye disease
¶An indicator variable was created for cerebrovascular disease for the presence of any of the following co-morbidities: stroke, transient ischemic attack, or other neurologic condition
#An indicator variable was created for ischemic heart disease that consists of the presence of congestive heart failure and/or ischemic heart disease
**Due to low prevalence, an indicator variable was created for the presence of any of the following comorbidities: dementia, collagen vascular disease, malignancy
†† AMA is the source for the raw physician data (provider ages only); statistics, tables or tabulations were prepared by User-Customer (PI: H. Johnson) using AMA Masterfile data
‡‡ Other specialties: Pediatrics; obstetrics/gynecology; geriatrics
Incident Hypertension Diagnosis Rates
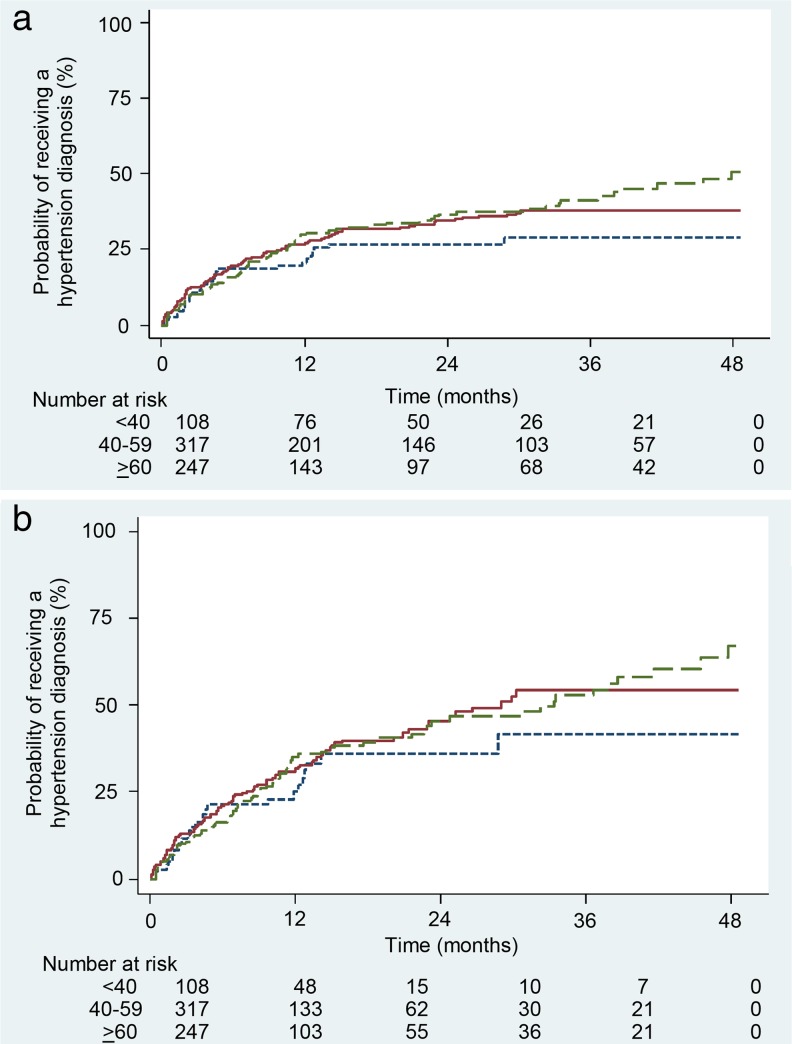
Using the 130/80 mmHg diagnosis threshold, 315 (41 %) of patients with diabetes and incident hypertension received a hypertension diagnosis (Fig. 2a). Adults < 40 years old had the lowest (33 %) diagnosis rate, compared to 41 % of those 40–59 years old and 44 % of those ≥ 60 years old. Rates of diagnosis were highest during the initial 6 months after meeting hypertension criteria. Among those who received a diagnosis, the median (interquartile range) time to diagnosis was 1.9 (0–8.5) months and mean (standard deviation) time to diagnosis was 5.8 (8.8) months. Using the 140/90 mmHg diagnosis threshold (Fig. 2b), 398 (52 %) of patients received a hypertension diagnosis and the differences in diagnosis rates were similar across age groups.
Figure 2.
a Kaplan-Meier curve of time to hypertension diagnosis among patients with diabetes and incident hypertension (≥ 130/80 mmHg)  . b Kaplan-Meier curve of time to hypertension diagnosis among patients with diabetes and incident hypertension (≥ 140/90 mmHg)
. b Kaplan-Meier curve of time to hypertension diagnosis among patients with diabetes and incident hypertension (≥ 140/90 mmHg)  .
.
Associations with an Initial Hypertension Diagnosis
After adjusting for patient and provider factors, using the 130/80 mmHg diagnosis threshold (Table 2), age was not associated with hypertension diagnosis rates. A baseline diagnosis of atrial fibrillation (HR 2.18; 1.21–4.67) was associated with a faster diagnosis rate. Slower diagnosis rates were associated with peripheral vascular disease (HR 0.18; 0.04–0.74) and fewer primary care visits (HR 0.93; 0.88–0.98). For 140/90 mmHg (Table 3), similarly, peripheral vascular disease was associated with a slower diagnosis. However, Medicaid use was associated with a faster diagnosis rate (HR 1.62; 1.07–2.45). Provider characteristics were not associated with an initial hypertension diagnosis for 130/80 mmHg or 140/90 mmHg.
Table 2.
Adjusted HRs and 95 % CIs of Independent Associations of an Initial Hypertension Diagnosis (≥130/80 mmHg) and Antihypertensive Medication Initiation Among Individuals with Diabetes (n = 771)
| Patient characteristics | ||||
|---|---|---|---|---|
| Diagnosis | Treatment | |||
| Adjusted HR (95 % CI) | p value | Adjusted HR (95 % CI) | p value | |
| Age | ||||
| Age <40 years | – | – | ||
| Age 40–59 years | 1.38 (0.89–2.15) | 0.15 | 1.37 (0.89–2.11) | 0.15 |
| Age ≥60 years | 1.58 (0.98–2.55) | 0.06 | 1.32 (0.83–2.10) | 0.25 |
| Male | 0.92 (0.69–1.22) | 0.56 | 1.28 (0.97–1.68) | 0.08 |
| Race/ethnicity | ||||
| White | – | – | ||
| Non-white* | 1.19 (0.81–1.75) | 0.38 | 1.17 (0.80–1.70) | 0.42 |
| Tobacco use category | ||||
| Current tobacco use | – | – | ||
| Non-smoker/former tobacco use | 1.07 (0.75–1.54) | 0.70 | 1.11 (0.79–1.56) | 0.53 |
| Medicaid, ever† | 1.35 (0.90–2.03) | 0.15 | 1.29 (0.86–1.93) | 0.21 |
| Stage 2 hypertension‡ | 1.25 (0.88–1.79) | 0.22 | 1.19 (0.85–1.68) | 0.31 |
| Comorbidities | ||||
| Microvascular complications of diabetes§ | 1.12 (0.66–1.89) | 0.68 | 0.97 (0.57–1.66) | 0.91 |
| Baseline dyslipidemia | 0.92 (0.69–1.22) | 0.55 | 0.99 (0.76–1.30) | 0.94 |
| Atrial fibrillation | 2.18 (1.21–4.67) | 0.03 | 3.07 (1.39–6.74) | 0.005 |
| Peripheral vascular disease | 0.18 (0.04–0.74) | 0.02 | 0.16 (0.04–0.64) | 0.01 |
| Ischemic heart disease‖ | 1.30 (0.68–2.48) | 0.42 | 2.16 (1.24–3.76) | 0.007 |
| Low prevalence Indicator¶ | 0.79 (0.43–1.45) | 0.45 | 1.37 (0.82–2.30) | 0.23 |
| Baseline ambulatory visit counts, annual | ||||
| Primary care visits | 0.93 (0.88–0.98) | 0.01 | 0.93 (0.88–0.98) | 0.01 |
*Non-White ethnicities: African American (8.0 %); Hispanic/Latino (3.1 %); Asian (2.0 %); Native Hawaiian/Pacific Islander (0.9 %); American Indian/Alaska Native (0.1 %); Unknown (2.5 %)
†On Medicaid during the baseline or study period
‡Stage of hypertension is defined using JNC-7 criteria
§An indicator variable was created for microvascular disease that consists of the presence of chronic kidney disease and/or eye disease
‖An indicator variable was created for ischemic heart disease that consists of the presence of congestive heart failure and/or ischemic heart disease
¶Due to low prevalence, an indicator variable was created for the presence of any of the following comorbidities: dementia, collagen vascular disease, malignancy
Bold denotes p < 0.05
Table 3.
Adjusted HRs and 95 % CIs of Independent Associations of an Initial Hypertension Diagnosis (≥140/90 mmHg) and Antihypertensive Medication Initiation Among Individuals with Diabetes (n = 771)
| Patient characteristics | ||||
|---|---|---|---|---|
| Diagnosis | Treatment | |||
| Adjusted HR (95 % CI) | P value | Adjusted HR (95 % CI) | p value | |
| Age | ||||
| Age < 40 years | – | – | ||
| Age 40–59 years | 1.35 (0.86–2.11) | 0.19 | 1.22 (0.79–1.89) | 0.38 |
| Age ≥ 60 years | 1.42 (0.87–2.32) | 0.16 | 1.08 (0.67–1.74) | 0.77 |
| Male | 0.82 (0.62–1.09) | 0.17 | 1.10 (0.84–1.46) | 0.49 |
| Race/ethnicity | ||||
| White | – | – | ||
| Non-white* | 1.28 (0.87–1.90) | 0.21 | 1.27 (0.86–1.88) | 0.22 |
| Tobacco use | ||||
| Current tobacco use | – | – | ||
| Non-smoker/former tobacco use | 1.04 (0.72–1.51) | 0.82 | 1.16 (0.82–1.65) | 0.40 |
| Medicaid, ever† | 1.62 (1.07–2.45) | 0.02 | 1.43 (0.94–2.16) | 0.10 |
| Stage 2 hypertension‡ | 1.15 (0.80–1.66) | 0.44 | 0.98 (0.68–1.40) | 0.91 |
| Comorbidities | ||||
| Microvascular complications of diabetes§ | 1.26 (0.74–2.15) | 0.39 | 0.94 (0.54–1.63) | 0.82 |
| Baseline dyslipidemia | 1.01 (0.76–1.34) | 0.96 | 1.10 (0.83–1.45) | 0.52 |
| Atrial fibrillation | 2.22 (0.85–5.85) | 0.11 | 2.86 (1.26–6.48) | 0.01 |
| Peripheral vascular disease | 0.23 (0.06–0.95) | 0.04 | 0.19 (0.05–0.79) | 0.02 |
| Ischemic heart disease‖ | 1.48 (0.76–2.87) | 0.25 | 2.77 (1.52–5.05) | 0.001 |
| Low prevalence indicator¶ | 0.79 (0.42–1.47) | 0.46 | 1.38 (0.81–2.34) | 0.23 |
| Baseline ambulatory visit counts, annual | ||||
| Primary care visits | 0.99 (0.94–1.05) | 0.81 | 0.99 (0.94–1.04) | 0.73 |
*Non-White ethnicities: African American (8.0 %); Hispanic/Latino (3.1 %); Asian (2.0 %); Native Hawaiian/Pacific Islander (0.9 %); American Indian/Alaska Native (0.1 %); Unknown (2.5 %)
†On Medicaid during the baseline or study period
‡Stage of hypertension is defined using JNC-7 criteria
§An indicator variable was created for microvascular disease that consists of the presence of chronic kidney disease and/or eye disease
‖An indicator variable was created for ischemic heart disease that consists of the presence of congestive heart failure and/or ischemic heart disease
¶Due to low prevalence, an indicator variable was created for the presence of any of the following comorbidities: dementia, collagen vascular disease, malignancy
Bold denotes p < 0.05
Antihypertensive Medication Initiation Rates
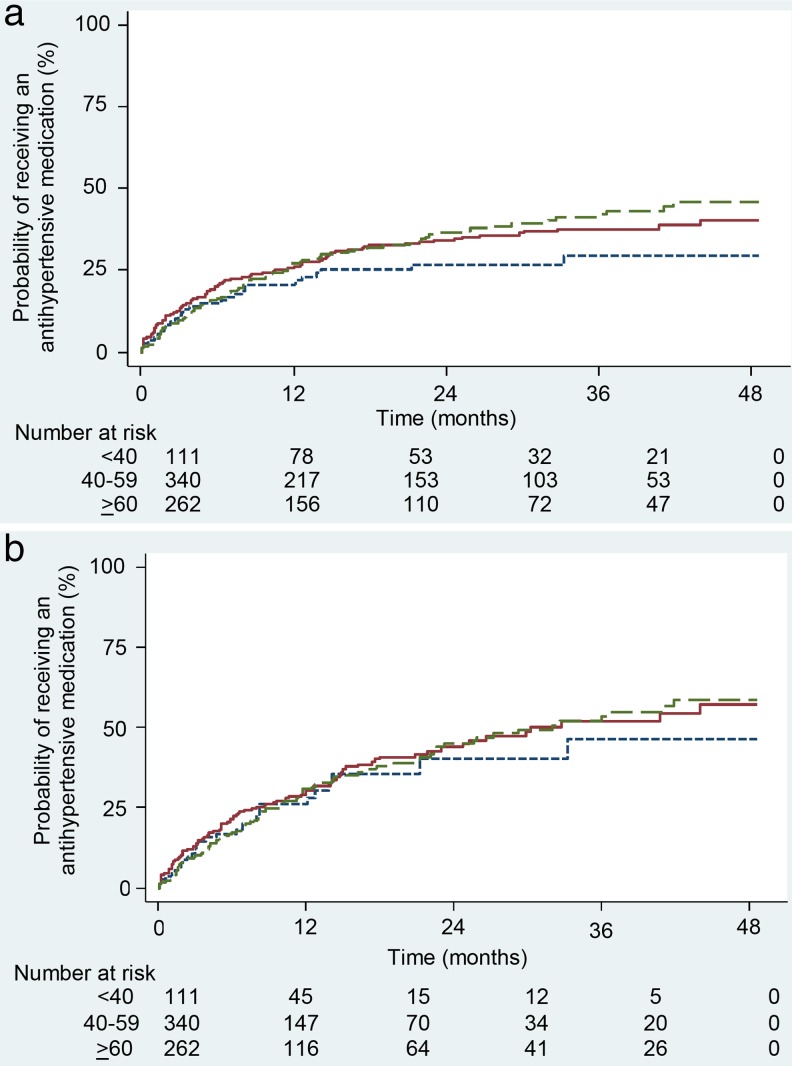
For 130/80 mmHg, an initial antihypertensive medication was prescribed in 286 (37 %) patients (Fig. 3a). Adults < 40 years of age had the lowest rate of antihypertensive medication initiation (31 %), compared to 37 % of 40–59-year olds and 40 % of ≥ 60-year olds. Rates of medication initiation were highest in the initial 6 months. Among patients who received an antihypertensive medication, the median (interquartile range) time to treatment was 3.3 (0.2–10.0) months and the mean (standard deviation) time to treatment was 6.9 (9.0) months. Using the 140/90 mmHg threshold (Fig. 3b), 377 (49 %) received antihypertensive medication and < 40-year olds continued to have the slowest medication initiation rate.
Figure 3.
a Kaplan-Meier curve of time to antihypertensive medication initiation among patients with diabetes and incident hypertension (≥130/80 mmHg)  . b Kaplan-Meier curve of time to antihypertensive medication initiation among patients with diabetes and incident hypertension (≥140/90 mmHg)
. b Kaplan-Meier curve of time to antihypertensive medication initiation among patients with diabetes and incident hypertension (≥140/90 mmHg)  .
.
Associations with Time to Antihypertensive Medication Initiation
Using the cut-point of 130/80 mmHg (Table 2), factors associated with faster medication initiation included atrial fibrillation (HR 3.07; 1.39–6.74) and ischemic heart disease/congestive heart failure (HR 2.16; 1.24–3.76). Patients with peripheral vascular disease (HR 0.16; 0.04–0.64) and fewer primary care visits (HR 0.93; 0.88–0.98) had significantly slower medication initiation rates. While not statistically significant, a trend suggests that those ≥ 60 years of age may have received antihypertensive medication at a faster rate than adults < 40 years of age. Similarly, for 140/90 mmHg (Table 3), atrial fibrillation and ischemic heart disease were associated with faster medication initiation rates; peripheral vascular disease was associated with slower rates. Provider characteristics were not associated with antihypertensive medication initiation for 130/80 mmHg or 140/90 mmHg.
Sensitivity Analysis
Cox proportional-hazards models were performed with combined outcome variables that included patients whose blood pressure normalized prior to receiving a hypertension diagnosis or antihypertensive medication. Overall, the, associations remained consistent with the initial models. Using the cut-point of 130/80 mmHg, peripheral vascular disease (HR 0.28; 0.10–0.76) was associated with slower hypertension diagnosis rates. Atrial fibrillation (HR 2.7; 1.3–5.6) and ischemic heart disease/congestive heart failure (HR 2.2; 1.3–3.6) were associated with faster medication initiation; peripheral vascular disease (HR 0.31; 0.14–0.82) was associated with slower medication initiation. Findings were similar for 140/90 mmHg.
DISCUSSION
In a cohort of patients seen in a single health system, we observed that delays in time to diagnosis and treatment of hypertension were common among patients with diabetes. Even with the higher cut-point (140/90 mmHg), approximately 50 % of patients with diabetes mellitus remained undiagnosed and untreated for hypertension. Our seminal findings highlight important associations contributing to clinical inertia (delays) in the hypertension management of patients with diabetes.18–20
Prior literature supports our main findings. A study in Spain evaluated delays in hypertension diagnosis among patients with diabetes mellitus.3 Although generalizability to a U.S. population is limited due to healthcare system differences, similarly, patient-level factors (comorbidities) were the primary associations with hypertension diagnosis and treatment rates. In addition, several studies described delays in hypertension diagnosis and treatment in a U.S. population not limited to patients with diabetes.10,33–35 Similar to those studies, we demonstrated that fewer primary care visits in the baseline period were associated with slower diagnosis and treatment rates, emphasizing the importance of timely hypertension follow-up.
Unfortunately, among patients with diabetes, comorbid conditions can negatively impact patient care, given the competing demands, and they contribute to clinical inertia in hypertension management.18–20 Conditions with similar pathophysiology and treatments (concordant conditions) may cue providers to offer synergistic care for the conditions with overlapping care goals.36,37 This may explain improved hypertension diagnosis and treatment rates in our study for patients with diabetes and atrial fibrillation or ischemic heart disease/congestive heart failure. In contrast, discordant conditions may promote clinical inertia; however, there is conflicting data. In one study, patients were less likely to have antihypertensive treatment intensified for uncontrolled hypertension with each additional discordant condition.38 In another study, patients with higher clinical complexity, including discordant conditions, were more likely to receive higher quality diabetes care and achieve hypertension control.39 In our study, peripheral vascular disease, a presumably concordant condition, was associated with decreased rates of hypertension diagnosis and treatment. Significantly increased mortality associated with advanced peripheral vascular disease may explain these findings, since guideline-concordant care may not have the same benefit-to-risk ratio in patients with advanced comorbidities.39
Given the management complexity of diabetes with other comorbid conditions, our data further supports the idea that future interventions should consider patients with comorbidities as a target group. Guidelines recommend a team-based approach to diabetes management,1 to address complex comorbidities and increase the achievement of diabetes-related treatment goals.40,41 However, tailored strategies such as clinical decision support are needed to assist providers and patients with complex comorbidity management.42
One of the limitations of this study is the use of data from a single healthcare system, potentially limiting the generalizability. However, this healthcare system is one of the ten largest physician practices in the United States, with primary care clinics in both urban and rural settings. Misclassification of hypertension or other comorbidities is a concern; however, the use of established algorithms decreases this risk. Diabetes screening was based on established guidelines at the time of the study. Our study addressed prevalent diabetes; therefore, we did not assess time from a diabetes diagnosis. However, the length of follow-up should reflect hypertension management in this population. Our exclusion criteria (previous antihypertensive medication) decreased the prevalence of atrial fibrillation and congestive heart failure compared to previous observational studies.43,44 Although this may limit the generalizability, our rates reflect a population of patients with diabetes and incident hypertension, and highlight the importance of a timely response to newly elevated blood pressures. Occasionally, patients are started on antihypertensive medication (e.g., ACE-inhibitor) due to a diagnosis of diabetes and not hypertension. However, in our analysis, only 9.3 % of patients received an antihypertensive medication without a diagnosis of hypertension. Additionally, patients were censored if blood pressures normalized prior to a hypertension diagnosis or antihypertensive initiation. Therefore, patients only accrued time if they had continuously elevated blood pressures. Lastly, some patients may have been initially treated with lifestyle modifications resulting in blood pressure normalization. To address this concern, sensitivity analyses were performed and demonstrated similar results to our initial models: complex comorbidities and visit frequency remained important associations with hypertension diagnosis and treatment. Unfortunately, ambulatory blood pressure data was not available; future studies would be beneficial to evaluate for white coat and masked hypertension in this population.
In conclusion, at the 130/80 mmHg threshold, the majority of patients with diabetes and incident hypertension remained undiagnosed (59 %) and untreated (63 %) for hypertension. Analysis at 140/90 mmHg demonstrated that approximately 50 % of patients remained undiagnosed and untreated. Interventions targeting complex patients with multiple comorbidities are needed to improve hypertension diagnosis and treatment rates in patients with diabetes.
Acknowledgements
Contributors: The authors gratefully acknowledge Katie Ronk, BS, for data preparation, and Jamie LaMantia, BS, and Colleen Brown, BA, for manuscript preparation.
Funders: Research reported in this manuscript was supported by the UW Health Innovation Program and the Clinical and Translational Science Award (CTSA) program, previously through the National Center for Research Resources (NCRR) under award number UL1RR025011, and now by the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health under award number U54TR000021. Margaret Wallace and Elizabeth Magnan are supported by a National Research Service Award (T32HP10010) from the Health Resources and Services Administration to the University of Wisconsin Department of Family Medicine. Heather Johnson is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K23HL112907, and also by the University of Wisconsin Centennial Scholars Program of the University of Wisconsin School of Medicine and Public Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Additional funding for this project was provided by the University of Wisconsin School of Medicine and Public Health from The Wisconsin Partnership Program.
Prior Presentations: Some of the findings reported in the study were presented by Margaret Wallace in abstract form at the 2013 Wisconsin Health Improvement and Research Partnership Forum on 12 September 2013.
Conflicts of Interest
E. Magnan and H. Johnson have clinical appointments with the academic group practice that has a financial interest in delivering care to the general population from which study subjects were drawn. For the remaining authors, no conflicts were declared.
REFERENCES
- 1.American Diabetes Association Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(1):S14–80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 2.Parati G, Bilo G, Ochoa JE. Benefits of tight blood pressure control in diabetic patients with hypertension: importance of early and sustained implementation of effective treatment strategies. Diabetes Care. 2011;34(2):S297–303. doi: 10.2337/dc11-s243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Burgos-Lunar C, Del Cura-González I, Salinero-Fort MA, Gómez-Campelo P, Pérez de Isla L, Jiménez-García R. Delayed diagnosis of hypertension in diabetic patients monitored in primary care. Rev Esp Cardiol. 2013;66:700–6. [DOI] [PubMed]
- 4.Hatahet MA, Bowhan J, Clough EA. Wisconsin Collaborative for Healthcare Quality (WCHQ): lessons learned. WMJ. 2004;103:45–8. [PubMed] [Google Scholar]
- 5.Sheehy A, Pandhi N, Coursin DB, et al. Minority status and diabetes screening in an ambulatory population. Diabetes Care. 2011;34:1289–94. doi: 10.2337/dc10-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thorpe CT, Flood GE, Kraft SA, Everett CM, Smith MA. Effect of patient selection method on provider group performance estimates. Med Care. 2011;49:780–5. doi: 10.1097/MLR.0b013e31821b3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 8.Myers MG, Tobe SW, McKay DW, et al. New algorithm for the diagnosis of hypertension. Am J Hypertens. 2005;18:1369–74. doi: 10.1016/j.amjhyper.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 9.Schmittdiel J, Selby JV, Swain B, et al. Missed opportunities in cardiovascular disease prevention?: low rates of hypertension recognition for women at medicine and obstetrics-gynecology clinics. Hypertension. 2011;57:717–22. doi: 10.1161/HYPERTENSIONAHA.110.168195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson HM, Thorpe CT, Bartels CM, et al. Undiagnosed hypertension among young adults with regular primary care use. J Hypertens. 2014;32:65–74. doi: 10.1097/HJH.0000000000000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hebert PL, Geiss LS, Tierney EF, Engelgau MM, Yawn BP, McBean AM. Identifying persons with diabetes using Medicare claims data. Am J Med Qual. 1999;14:270–7. doi: 10.1177/106286069901400607. [DOI] [PubMed] [Google Scholar]
- 12.Sheehy AM, Flood GE, Tuan WJ, Liou JI, Coursin DB, Smith MA. Analysis of guidelines for screening diabetes mellitus in an ambulatory population. Mayo Clin Proc. 2010;85:27–35. doi: 10.4065/mcp.2009.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tu K, Chen Z, Lipscombe LL, Canadian Hypertension Education Program Outcomes Research Taskforce Prevalence and incidence of hypertension from 1995 to 2005: a population-based study. CMAJ. 2008;178:1429–35. doi: 10.1503/cmaj.071283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manson JM, McFarland B, Weiss S. Use of an automated database to evaluate markers for early detection of pregnancy. Am J Epidemiol. 2001;154:180–7. doi: 10.1093/aje/154.2.180. [DOI] [PubMed] [Google Scholar]
- 15.Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–85. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–20. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 17.Hansen ML, Gunn PW, Kaelber DC. Underdiagnosis of hypertension in children and adolescents. JAMA. 2007;298:874–9. doi: 10.1001/jama.298.8.874. [DOI] [PubMed] [Google Scholar]
- 18.Parchman ML, Pugh JA, Romero RL, Bowers KW. Competing demands or clinical inertia: the case of elevated glycosylated hemoglobin. Ann Fam Med. 2007;5:196–201. doi: 10.1370/afm.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Connor PJ, Sperl-Hillen JM, Johnson PE, Rush WA, Blitz G. Clinical Inertia and Outpatient Medical Errors. In: Henriksen K, Battles JB, Marks ES, Lewin DI, editors. Advances in Patient Safety: From Research to Implementation (Volume 2: Concepts and Methodology) Rockville: Agency of Healthcare Research and Quality (US); 2005. pp. 293–308. [Google Scholar]
- 20.Huebschmann AG, Mizrahi T, Soenksen A, Beaty BL, Denberg TD. Reducing clinical inertia in hypertension treatment: a pragmatic randomized controlled trial. J Clin Hypertens (Greenwich). 2012;14:322–9. doi: 10.1111/j.1751-7176.2012.00607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gil-Guillén V, Orozco-Beltrán D, Peréz RP, et al. Clinical inertia in diagnosis and treatment of hypertension in primary care: quantification and associated factors. Blood Press. 2010;19:3–10. doi: 10.3109/08037050903350762. [DOI] [PubMed] [Google Scholar]
- 22.Buccaneer Computer Systems & Services, Inc. Chronic Condition Data Warehouse Reference List. Available at: www.ccwdata.org/web/guest/condition-categories. Accessed January 12, 2015.
- 23.Goldstein LB. Accuracy of ICD-9-CM coding for the identification of patients with acute ischemic stroke: effect of modifier codes. Stroke. 1998;29:1602–4. doi: 10.1161/01.STR.29.8.1602. [DOI] [PubMed] [Google Scholar]
- 24.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Borzecki AM, Wong AT, Hickey EC, Ash AS, Berlowitz DR. Identifying hypertension-related comorbidities from administrative data: what’s the optimal approach? Am J Med Qual. 2004;19:201–6. doi: 10.1177/106286060401900504. [DOI] [PubMed] [Google Scholar]
- 26.Foley RN, Murray AM, Li S, et al. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol. 2005;16:489–95. doi: 10.1681/ASN.2004030203. [DOI] [PubMed] [Google Scholar]
- 27.Newton KM, Wagner EH, Ramsey SD, et al. The use of automated data to identify complications and comorbidities of diabetes: a validation study. J Clin Epidemiol. 1999;52:199–207. doi: 10.1016/S0895-4356(98)00161-9. [DOI] [PubMed] [Google Scholar]
- 28.Taylor DH, Jr, Ostbye T, Langa KM, Weir D, Plassman BL. The accuracy of Medicare claims as an epidemiological tool: the case of dementia revisited. J Alzheimers Dis. 2009;17:807–15. doi: 10.3233/JAD-2009-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Druss BG, Marcus SC, Olfson M, Tanielian T, Pincus HA. Trends in care by nonphysician clinicians in the United States. N Engl J Med. 2003;348:130–7. doi: 10.1056/NEJMsa020993. [DOI] [PubMed] [Google Scholar]
- 30.Pham HH, Schrag D, Hargraves JL, Bach PB. Delivery of preventive services to older adults by primary care physicians. JAMA. 2005;294:473–81. doi: 10.1001/jama.294.4.473. [DOI] [PubMed] [Google Scholar]
- 31.Gutierrez RG, StataCorp Parametric frailty and shared frailty survival models. The Stata Journal. 2002;2:22–44. [Google Scholar]
- 32.McGilchrist CA, Aisbett CW. Regression with frailty in survival analysis. Biometrics. 1991;47:461–6. doi: 10.2307/2532138. [DOI] [PubMed] [Google Scholar]
- 33.Brooks EL, Preis SR, Hwang SJ, et al. Health insurance and cardiovascular disease risk factors. Am Med J. 2010;123:741–7. doi: 10.1016/j.amjmed.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson HM, Thorpe CT, Bartels CM, et al. Antihypertensive medication initiation among young adults with regular primary care use. J Gen Intern Med. 2014;29:723–31. doi: 10.1007/s11606-014-2790-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nguyen QC, Waddell EN, Thomas JC, Huston SL, Kerker BD, Gwynn RC. Awareness, treatment, and control of hypertension and hypercholesterolemia among insured residents of New York City, 2004. Prev Chronic Dis. 2011;8:A109. [PMC free article] [PubMed] [Google Scholar]
- 36.Pentakota SR, Rajan M, Fincke BG, et al. Does diabetes care differ by type of chronic comorbidity?: An evaluation of the Piette and Kerr framework. Diabetes Care. 2012;35:1285–92. doi: 10.2337/dc11-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piette JD, Kerr EA. The impact of comorbid chronic conditions on diabetes care. Diabetes Care. 2006;29:725–31. doi: 10.2337/diacare.29.03.06.dc05-2078. [DOI] [PubMed] [Google Scholar]
- 38.Turner BJ, Hollenbeak CS, Weiner M, Ten Have T, Tang SS. Effect of unrelated comorbid conditions on hypertension management. Ann Intern Med. 2008;148:578–86. doi: 10.7326/0003-4819-148-8-200804150-00002. [DOI] [PubMed] [Google Scholar]
- 39.Woodard LD, Urech T, Landrum CR, Wang D, Petersen LA. Impact of comorbidity type on measures of quality for diabetes care. Med Care. 2011;49:605–10. doi: 10.1097/MLR.0b013e31820f0ed0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leal S, Glover JJ, Herrier RN, Felix A. Improving quality of care in diabetes through a comprehensive pharmacist-based disease management program. Diabetes Care. 2004;27:2983–4. doi: 10.2337/diacare.27.12.2983. [DOI] [PubMed] [Google Scholar]
- 41.Machado M, Bajcar J, Guzzo GC, Einarson TR. Sensitivity of patient outcomes to pharmacist interventions. Part I: systematic review and meta-analysis in diabetes management. Ann Pharmacother. 2007;41:1569–82. doi: 10.1345/aph.1K151. [DOI] [PubMed] [Google Scholar]
- 42.Vogeli C, Shields AE, Lee TA, et al. Multiple chronic conditions: prevalence, health consequences, and implications for quality, care management, and costs. J Gen Intern Med. 2007;22(3):391–5. doi: 10.1007/s11606-007-0322-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nichols GA, Hillier TA, Erbey JR, Brown JB. Congestive heart failure in type 2 diabetes: prevalence, incidence, and risk factors. Diabetes Care. 2001;24:1614–9. doi: 10.2337/diacare.24.9.1614. [DOI] [PubMed] [Google Scholar]
- 44.Nichols GA, Reinier K, Chugh SS. Independent contribution of diabetes to increased prevalence and incidence of atrial fibrillation. Diabetes Care. 2009;32:1851–6. doi: 10.2337/dc09-0939. [DOI] [PMC free article] [PubMed] [Google Scholar]