Abstract
Purpose:
Medical imaging techniques such as magnetic resonance imaging and magnetic particle imaging (MPI) utilize time-varying magnetic fields that are subject to magnetostimulation limits, which often limit the speed of the imaging process. Various human-subject experiments have studied the amplitude and frequency dependence of these thresholds for gradient or homogeneous magnetic fields. Another contributing factor was shown to be number of cycles in a magnetic pulse, where the thresholds decreased with longer pulses. The latter result was demonstrated on two subjects only, at a single frequency of 1.27 kHz. Hence, whether the observed effect was due to the number of cycles or due to the pulse duration was not specified. In addition, a gradient-type field was utilized; hence, whether the same phenomenon applies to homogeneous magnetic fields remained unknown. Here, the authors investigate the pulse duration dependence of magnetostimulation limits for a 20-fold range of frequencies using homogeneous magnetic fields, such as the ones used for the drive field in MPI.
Methods:
Magnetostimulation thresholds were measured in the arms of six healthy subjects (age: 27 ± 5 yr). Each experiment comprised testing the thresholds at eight different pulse durations between 2 and 125 ms at a single frequency, which took approximately 30–40 min/subject. A total of 34 experiments were performed at three different frequencies: 1.2, 5.7, and 25.5 kHz. A solenoid coil providing homogeneous magnetic field was used to induce stimulation, and the field amplitude was measured in real time. A pre-emphasis based pulse shaping method was employed to accurately control the pulse durations. Subjects reported stimulation via a mouse click whenever they felt a twitching/tingling sensation. A sigmoid function was fitted to the subject responses to find the threshold at a specific frequency and duration, and the whole procedure was repeated at all relevant frequencies and pulse durations.
Results:
The magnetostimulation limits decreased with increasing pulse duration (Tpulse). For Tpulse < 18 ms, the thresholds were significantly higher than at the longest pulse durations (p < 0.01, paired Wilcoxon signed-rank test). The normalized magnetostimulation threshold (BNorm) vs duration curve at all three frequencies agreed almost identically, indicating that the observed effect is independent of the operating frequency. At the shortest pulse duration (Tpulse ≈ 2 ms), the thresholds were approximately 24% higher than at the asymptotes. The thresholds decreased to within 4% of their asymptotic values for Tpulse > 20 ms. These trends were well characterized (R2 = 0.78) by a stretched exponential function given by , where the fitted parameters were α = 0.44, β = 4.32, and γ = 0.60.
Conclusions:
This work shows for the first time that the magnetostimulation thresholds decrease with increasing pulse duration, and that this effect is independent of the operating frequency. Normalized threshold vs duration trends are almost identical for a 20-fold range of frequencies: the thresholds are significantly higher at short pulse durations and settle to within 4% of their asymptotic values for durations longer than 20 ms. These results emphasize the importance of matching the human-subject experiments to the imaging conditions of a particular setup. Knowing the dependence of the safety limits to all contributing factors is critical for increasing the time-efficiency of imaging systems that utilize time-varying magnetic fields.
Keywords: magnetostimulation threshold, peripheral nerve stimulation, pulse duration, magnetic particle imaging, magnetic resonance imaging
1. INTRODUCTION
Time-varying magnetic fields in medical imaging are subject to two main safety concerns: magnetostimulation limits and specific absorption rate (SAR) limits.1–3 In magnetic resonance imaging (MRI), for example, the maximum slew rate of the magnetic field gradients has to abide by magnetostimulation thresholds above which the subjects can experience uncomfortable peripheral nerve stimulation (PNS).1,3 The radiofrequency (RF) fields in MRI, on the other hand, operate at a much higher frequency range (usually 64–128 MHz) and are limited by tissue heating, commonly referred to as SAR.2 These safety limits determine the imaging speed and are partly responsible for MRI being slower than other imaging modalities such as CT, x-ray, or ultrasound. A recently introduced medical imaging modality called magnetic particle imaging (MPI) also utilizes time-varying magnetic fields, albeit at different frequencies. MPI images the spatial distribution of superparamagnetic tracers in vivo, with no background tissue signal, making it ideal for angiographic imaging applications.4–7 Although the frequencies and the spatial distributions of the magnetic fields used in MPI are different than those in MRI, they are also subject to both magnetostimulation and SAR limits.8,9 Specifically, for the drive field in MPI, magnetostimulation was shown to be the dominant safety concern for frequencies up to 50 kHz,1,9 potentially extending up to 150 kHz.10,11
Knowing the safety limits and their dependence on all contributing factors is crucial for fast imaging. In MPI, both the scanning rate and the signal-to-noise ratio (SNR) scale with the amplitude and the frequency of the drive field.5,9 In theory, by operating at the point where the product of the frequency and the allowed field amplitude is maximized, one can simultaneously improve the image quality and shorten the scan times. With this goal, we recently investigated the effect of frequency on magnetostimulation limits. Our study revealed that the magnetostimulation limits for the drive field in MPI decrease hyperbolically as a function of frequency, converging to a constant asymptote.9 These human-subject experiments were performed using homogeneous magnetic fields applied in the arm and leg in the frequency range of 0.5–50 kHz. Our work showed, for the first time, that magnetostimulation thresholds do not have large subject-to-subject variation and that stimulation thresholds inversely correlate with body part diameter. While this latter finding was predicted in theory, performing the experiments with a large variation in body part diameter (i.e., arm vs leg) was the key to demonstrating this effect experimentally. Importantly, our results confirmed that subject-to-subject variation seen in MRI magnetostimulation studies could be influenced by instrument setup and by patient positioning within the MRI scanner.12,13 Furthermore, due to the hyperbolic relationship between magnetostimulation thresholds and frequency, a larger intersubject variation is seen at lower frequencies. Therefore, the fact that the gradient field of MRI operates at relatively lower frequencies (∼1 kHz) can also contribute to larger intersubject variation.
An important confounding effect that must be considered in magnetostimulation experiments is the duration of the pulse envelope. A seminal work on magnetostimulation by Budinger et al. showed that, for gradient-type magnetic fields at 1.27 kHz, the magnetostimulation limits decrease as the number of oscillations in the magnetic pulse increases, and stay constant for pulses containing more than 16 oscillations (i.e., corresponding to 13 ms pulse duration at 1.27 kHz).14 These experiments were conducted on two subjects only and at a single frequency; hence, one cannot deduce whether the observed effect was due to the number of oscillations or the pulse duration. Whether the thresholds of homogeneous magnetic fields also decrease with increasing pulse durations remains another open question.
In this work, we investigate the pulse duration dependence of magnetostimulation limits for homogeneous magnetic fields, such as the ones used for the drive field in MPI. We perform human subject experiments using an arm stimulator coil, with experiments performed at eight different pulse durations ranging between 2 and 125 ms, applied at three different frequencies. The frequencies are chosen as 1.2, 5.7, and 25.5 kHz: a 20-fold range of frequencies, where one of the frequencies matches the one tested by Budinger et al. We show, with a total of 34 experiments performed on six subjects, that the magnetostimulation limits decrease as a function of pulse duration and settle to within 4% of their asymptotic values for durations longer than 20 ms. The normalized magnetostimulation curves from all three tested frequencies agree almost identically, which demonstrates for the first time that the effect of pulse duration is independent of the operating frequency. While the primary motivation of this work is the safety limits for MPI, our results equally apply to any technique that utilizes homogeneous magnetic fields in the kHz frequency range.
2. METHODS
This study was approved by the Committee for Protection of Human Subjects at University of California, Berkeley. We designed and conducted human-subject experiments to determine the pulse duration dependence of magnetostimulation thresholds. “Threshold” was defined as the peak-to-peak (pp) magnetic field amplitude at which the PNS sensations first become noticeable. The thresholds were tested on the forearms of the subjects. The subjects did not experience any pain; they described the PNS sensation as a gentle twitching, tingling, numbing, or tapping sensation.
2.A. Magnetic field stimulation setup
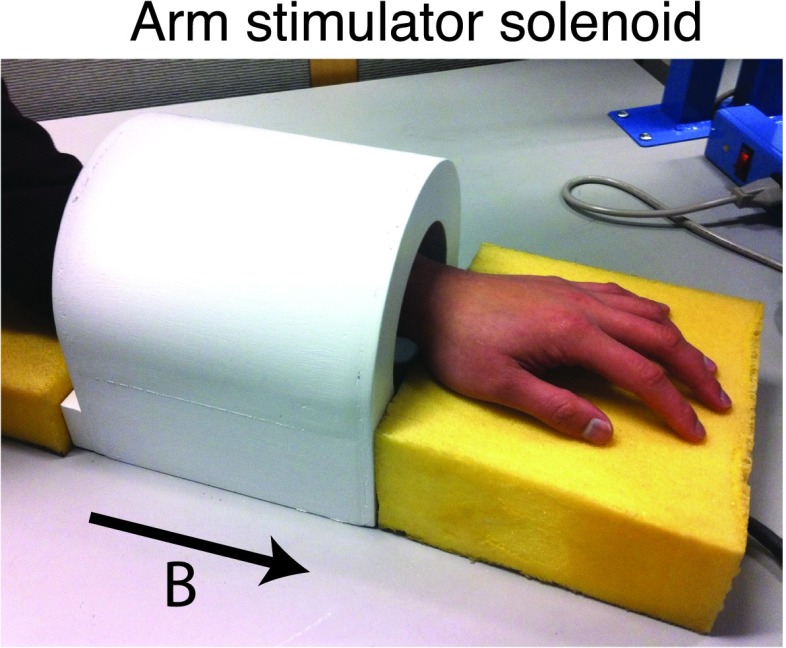
A solenoidal coil was constructed to test the magnetostimulation thresholds in the forearm.9 This solenoid, shown in Fig. 1(a), had a free bore size of 11 cm in diameter and 17 cm in length, and was designed to generate high-amplitude homogeneous magnetic fields. The measured magnetic field amplitude was 410 μT/A at the center, and the measured homogeneity was greater than 95% in a 7 cm-long region down the axis of the coil.
FIG. 1.
Magnetostimulation limits were tested in the forearm using a solenoidal coil with 95% homogeneity in a 7 cm-long region. The solenoid created an axial magnetic field (direction shown with the arrow). The measured magnetic field amplitude at the center of the solenoid was 410 μT/A.
Duration dependence of magnetostimulation thresholds was tested at three different frequencies: 1.2, 5.7, and 25.5 kHz. At the power limit of the amplifier (AE Techron 7782, AE Techron, Elkhart, IN), the magnetic fields measured at these frequencies at the center of the solenoid were 200, 140, and 100 mT-pp, respectively. At each frequency, eight different pulse lengths ranging between 2 and 125 ms were utilized. The amplitude, frequency, and duration of each pulse were controlled via a data acquisition module (NI USB-6259, National Instruments, Austin, TX). A Rogowski AC current probe (PEM Ltd., Nottingham, UK) was utilized to record the AC current running through the solenoid in real time. As the field amplitude at the center of the coil is known to be 410 μT/A, recording the current is equivalent to recording the magnetic field in real time.
2.B. Adjusting pulse duration
Ideally, one would like to use magnetic pulses with constant amplitudes (i.e., flat envelopes) when testing the effects of pulse duration on stimulation thresholds. However, the LC resonant inductive/capacitive components of the magnetostimulation setup result in nonzero ramp up/down times and a bullet-shaped pulse envelope, as shown in Fig. 2. Especially at short durations (e.g., 2 ms pulse shown in Fig. 2), the envelope may not even reach a plateau due to long ramp/up down durations. Hence, to accurately test the effects of pulse durations on magnetostimulation limits, the ramp/up down durations need to be minimized. In this work, we have employed a pre-emphasis based pulse-shaping method, where the pulse that is sent to the power amplifier was preadjusted. Accordingly, to reduce the ramp-up time, the envelope amplitude was increased for the first couple of cycles in a pulse. Likewise, the ramp-down time was reduced by utilizing higher amplitude with 180° phase offset for the last couple of cycles in a pulse. This pre-emphasized pulse was then sent to the power amplifier, and the rest of the magnetostimulation setup. Example pulses with different pulse durations at 25.5 kHz are given in Fig. 2, with and without pulse shaping, where the plots show the actual measured magnetic fields. After applying the pulse shaping method, the ramp-up/down times are minimized and the pulse envelopes are flattened. The effective duration of a pulse is then measured as the duration of the flat portion of its envelope. As seen in Fig. 2, the improvement due to pulse shaping is especially prominent at short pulse durations.
FIG. 2.
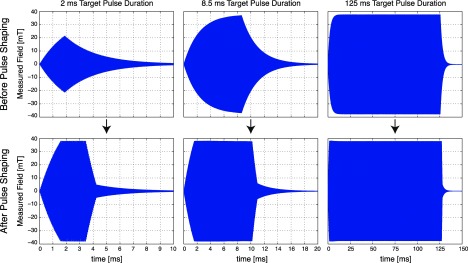
Accurately controlling magnetic pulse duration is of critical importance for determining its effects on magnetostimulation thresholds. Here, we utilized a pulse shaping method based on pre-emphasis, which counteracts the inductive/capacitive effects in the setup. (Top) Without pulse shaping, the nonzero ramp-up/down durations of the magnetic stimulator setup results in a bullet-shaped pulse envelope. This effect is more problematic at short pulse lengths, and is relatively less significant at long pulse durations. For example, at 2 ms target pulse duration, the pulse does not even reach its intended peak amplitude. (Bottom) Pulse shaping via pre-emphasis significantly improves the pulse envelope by reducing the ramp-up/down durations. The improvement is especially significant at shorter pulse durations. After the pulse shaping method, the effective duration of a pulse is measured as the flat portion of its envelope. All plots show the magnetic field amplitudes generated at 25.5 kHz, measured in real time. The individual cycles in the pulses cannot be resolved in the figure due to relatively high frequency.
2.C. Human-subject experiments
A total of six healthy subjects were recruited and screened for magnetic safety considerations for the presence of metal in their body (e.g., pacemakers, aneurysm clips, and metallic implants). The mean and standard deviations for the age, weight, and height of the recruited subjects were 27 ± 5 yr, 76 ± 7 kg, and 179 ± 4 cm, respectively. All six subjects were male.
Each experiment consisted of testing the magnetostimulation thresholds of a subject at a single frequency at eight different pulse lengths, which took approximately 30–40 min. Depending on availability, some subjects were tested at multiple frequencies, while others were tested only at a single frequency. In addition, some subjects were tested more than once at some frequencies. The total numbers of experiments performed at each frequency were as follows: 12 experiments at 1.2 kHz, 12 experiments at 5.7 kHz, and 10 experiments at 25.5 kHz. Furthermore, to accurately determine the effects of pulse duration on a single subject’s stimulation thresholds, one of the subjects (Subject #4) was tested three times at each frequency. The number of experiments for each subject at each frequency of interest is summarized in Table I.
TABLE I.
Number of experiments performed for each subject at each frequency of interest. A total of six healthy subjects were recruited. Depending on their availability, some subjects were tested at multiple frequencies, while others were tested only at a single frequency. Each experiment lasted approximately 30–40 min, and consisted of testing the magnetostimulation thresholds of a single subject at a single frequency at eight different pulse lengths.
| Number of experiments | |||
|---|---|---|---|
| Subject No. | 1.2 kHz | 5.7 kHz | 25.5 kHz |
| 1 | 3 | 3 | |
| 2 | 3 | ||
| 3 | 3 | 3 | |
| 4 | 3 | 3 | 3 |
| 5 | 3 | ||
| 6 | 3 | 3 | 1 |
| Total | 12 | 12 | 10 |
During the experiment, the subject was seated in a chair with forearm inside the solenoid. Earplugs and over-the-head earmuffs were utilized to isolate any audio feedback from the solenoid that could induce false positives for stimulation.15,16 The magnetic pulses were spaced at 2-s intervals, which gave the subjects enough time to report a stimulation sensation via a “mouse click.” The amplitude of the next pulse was adjusted depending on the stimulation feedback from the subject.
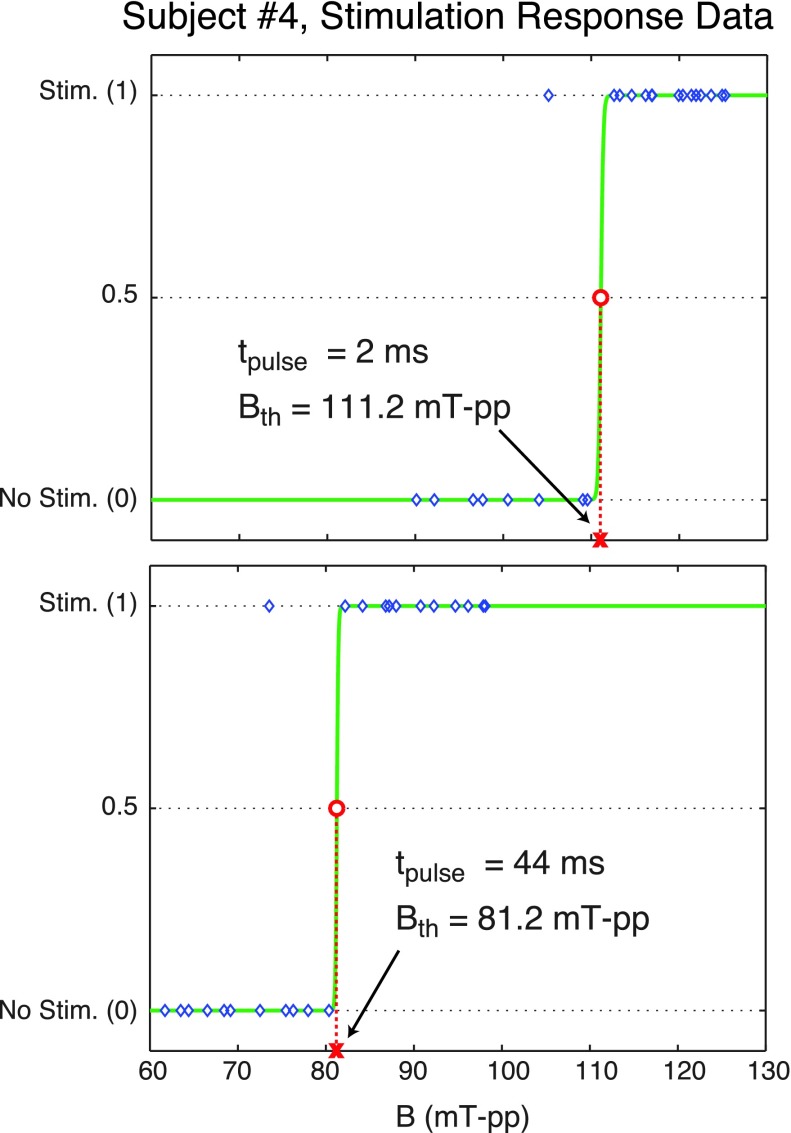
At a given pulse duration and frequency, first the pulse amplitude was slowly increased to determine an approximate threshold level, Bapp. Then, a secondary test was performed to more accurately determine the threshold in a window of ±20% of Bapp with a step size of 2% of Bapp. Within this window, the order in which amplitudes were tested was chosen randomly to prevent a potential bias to the ordering of the amplitudes.9 Subject responses (i.e., stimulation or no stimulation) were recorded after each pulse, and the magnetostimulation threshold was determined based on subject responses. Given in Fig. 3 are example stimulation response data for Subject #4 for two different pulse durations at 5.7 kHz. Here, “1” denotes that the subject reported stimulation via a mouse click, and “0” denotes no reported stimulation.
FIG. 3.
Stimulation response data from Subject #4 for two different pulse lengths at 5.7 kHz. The blue diamonds depict the subject responses at tested pulse amplitudes (stimulation = 1, no stimulation = 0), the solid green curves are the fitted sigmoid functions, and the red circles are the estimated stimulation thresholds, Bth. In this example, the threshold was significantly lower at longer pulse duration: 111.2 mT-pp at 2 ms pulse length vs 81.2 mT-pp at 44 ms pulse length.
This two-step test (i.e., determining the approximate threshold level followed by the adjusted-window test) was repeated at all eight pulse durations, while the frequency was kept constant throughout the experiment. The order in which the pulse durations were tested was randomized during each test. This randomization was crucial to avoid any bias that may stem from the subject getting tired and/or feeling numb toward the end of the experiment. After the completion of the experiment, the subject was asked to describe the stimulation sensation.
2.D. Data analysis
As described in our previous work,9 we model the subject stimulation responses as a probabilistic event with a cumulative distribution function (CDF) given as
| (1) |
Here, B is the peak-to-peak magnetic field strength. This sigmoid function equals zero at low field strengths (i.e., no stimulation), and approaches one at high field strengths (i.e., stimulation felt 100% of the time). The transition width between these two states is governed by the parameter W. Bth is the 50% crossing of the CDF, and we define this level as the magnetostimulation threshold. For the experiments performed in this work, the fitted sigmoids typically featured very sharp transitions (i.e., small W values).
After the subject responses were recorded, the ones from the adjusted-window test (i.e., the first step of the two-step test) were fitted to the sigmoid function in Eq. (1) through a Levenberg–Marquardt nonlinear least-squares regression that simultaneously estimates Bth and W. Figure 3 provides example stimulation response data from Subject #4 along with the fitted Bth values. The responses in this figure feature sharp transitions from “no stimulation” state to “stimulation” state, with only a few outlier data points. In some cases, however, subject responses may be inconsistent when the applied field amplitude is in the vicinity of the stimulation threshold. The sigmoid curve can accurately model those cases by allowing a softer transition region (i.e., increased W).
The nonlinear fitting procedure was performed at all relevant frequencies and pulse lengths separately, and the magnetostimulation thresholds were determined. Next, statistical analyses were performed: paired Wilcoxon signed-rank test was utilized to compare the distribution of stimulation thresholds at different pulse durations.
3. RESULTS
Figure 4 shows the magnetostimulation thresholds as a function of pulse duration for a single subject (Subject #4). For each frequency, the plotted curve denotes the average of three experiments. As seen in Fig. 4(a), at all frequencies tested, the thresholds decrease with increasing pulse duration. The rate of change is more significant at shorter pulse durations (i.e., for Tpulse < 20 ms), and the stimulation thresholds asymptotically converge to a constant value. Figure 4(a) also shows that for fixed pulse duration, the thresholds are lower at higher frequencies. This result is in agreement with our earlier findings, which demonstrated that magnetostimulation thresholds monotonically decrease with increasing frequency.9
FIG. 4.
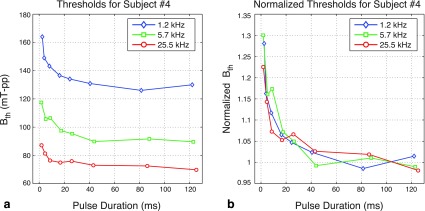
Magnetostimulation thresholds as a function of pulse duration for a single subject (Subject #4). The plotted curves denote the average of three tests at each frequency. (a) The thresholds decrease with increasing pulse duration, and asymptotically converge to a constant value. For fixed pulse duration, the thresholds are lower at higher frequencies. (b) Normalized magnetostimulation thresholds exhibit the same trend as a function of pulse duration, independent of the operating frequency. Here, the values for each data set were normalized by the average of the thresholds at the two longest pulse durations.
When each data set is normalized by its asymptote at long pulse durations, the curves from all three frequencies line up as shown in Fig. 4(b). Here, the average of the thresholds at the two longest pulse durations (Tpulse ≈ 85 ms and Tpulse ≈ 125 ms) was taken as the asymptote. Figure 4(b) demonstrates that the normalized magnetostimulation thresholds exhibit the same trend as a function of pulse duration, independent of the operating frequency.
Figure 5(a) shows the magnetostimulation thresholds as a function of pulse duration for all six subjects. A total of 12 experiments were performed at 1.2 kHz, 12 experiments at 5.7 kHz, and 10 experiments at 25.5 kHz. For each frequency, the plotted curve shows the mean value of the magnetostimulation thresholds, and the error bars denote the standard deviations. For fixed pulse duration, the thresholds are lower at higher frequencies, which is in agreement with our earlier findings.9 For all three frequencies, the thresholds decrease with increasing pulse duration. At the four shortest pulse durations (Tpulse < 18 ms), the thresholds were significantly higher than at the longest pulse durations (p < 0.01, paired Wilcoxon signed-rank test, performed separately at each frequency).
FIG. 5.
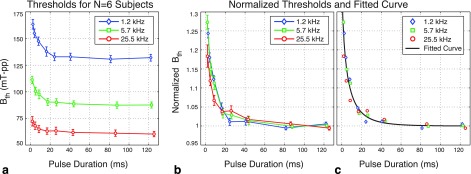
Magnetostimulation thresholds are consistent as a function of pulse duration for all six subjects. (a) The mean thresholds and the standard deviations for each pulse duration and frequency are shown. For fixed pulse duration, the thresholds are lower at higher frequencies. (b) Normalized magnetostimulation thresholds at different frequencies agree nicely, implying that the effect of pulse duration on stimulation thresholds is independent of frequency. Here, the values for each data set were normalized by the average of the thresholds at the two longest pulse durations. The error bars denote the mean and standard deviation values for the normalized thresholds at each pulse duration and frequency. (c) The magnetostimulation thresholds decrease with increasing pulse duration and stabilize for durations longer than approximately 20 ms (within 5% of the asymptotic value). These data trends are well characterized by a stretched exponential function, shown with the solid black line overlaid on top of the mean normalized thresholds.
The effect of pulse duration is better seen when we look at the normalized magnetostimulation thresholds, as shown in Fig. 5(b). Here, the data set from each experiment was normalized by the threshold values at the two longest pulse durations (i.e., the average of the thresholds at Tpulse ≈ 85 ms and Tpulse ≈ 125 ms). The error bars denote the mean and standard deviations for the normalized data sets among all experiments. The key observation from Fig. 5(b) is that the curves from different frequencies agree almost identically, implying that the effect of pulse duration is independent of the operating frequency. The magnetostimulation thresholds decrease with increasing pulse duration, and settle to within 4% of their asymptotic values for durations longer than 20 ms. At the shortest pulse duration tested in this work (Tpulse ≈ 2 ms), the thresholds are approximately 24% higher than at the asymptotes.
To model the observed decrease in magnetostimulation thresholds with increasing pulse duration, numerous commonly used decay functions were fitted to the data points. The functions that were tried included exponential functions, polynomials, Gaussian functions, power functions (e.g., axb + c), Hill functions (e.g., a/[b + xc]), and stretched exponentials. According to these curve-fitting tests, the data points were best characterized with the fewest number of parameters using a stretched exponential function (also known as a Weibull function). Shown in Fig. 5(c) in solid black line, the fitted function can be denoted as
| (2) |
Here, Tpulse is the pulse duration in ms and BNorm is the normalized magnetostimulation threshold, such that BNorm = 1 at the asymptote (i.e., as Tpulse goes to infinity). The fitted parameters resulting from nonlinear least-squares regression were α = 0.44, β = 4.32, and γ = 0.60, providing a goodness-of-fit of R2 = 0.78. According to the fitted curve [solid black line in Fig. 5(c)], the threshold is 24% higher than the asymptote at Tpulse = 2 ms, and it reaches to within 4% of the asymptote at Tpulse = 20 ms.
The subjects described the stimulation sensation at shorter pulse durations as harder to detect, and at longer pulse durations as more intense. To test whether this phenomenon was reflected in the experimental data, we performed statistical tests on the ratio of W to Bth, which can be interpreted as the normalized transition width of the sigmoid curve in Eq. (1). A larger W/Bth ratio denotes a softer transition from “no stimulation” to “stimulation” state, corresponding to higher inconsistency in subject responses around the threshold level. Accordingly, the statistical analysis showed that the normalized transition widths were significantly higher at the two shortest pulse durations (Tpulse < 6 ms) than at the longest pulse durations (p < 0.01, paired Wilcoxon signed-rank test), consistent with the subjects’ descriptions stated above.
4. DISCUSSION
The drive field frequency and amplitude are the key drivers of SNR and imaging speed in MPI. To increase the image quality for a fixed scan time, it is desirable to image at the highest drive field amplitude and slew rate. The key to maximizing the slew rate is to understand the human safety limits on the magnetic field amplitude and frequency of the drive field. In our previous work, we investigated the frequency dependence of the magnetostimulation limits for the drive field, with the goal of choosing the optimum frequency. However, we observed that the reliability of our data was strongly correlated with our experimental setup, specifically the duration of the pulse applied to the subject. The goal of this work is to further investigate this finding and to determine how the pulse duration affects magnetostimulation limits.
Our experiments have revealed for the first time that the magnetostimulation thresholds decrease as a function of pulse duration, and that the relative effect is independent of the operating frequency. In other words, it is harder to induce nerve stimulation using a shorter pulse. This effect is especially more significant when the pulse duration is shorter than 20 ms. A related previous work by Budinger et al. tested the effects of number of cycles on magnetostimulation thresholds, where the experiments were performed on two subjects at a single frequency of 1.27 kHz.14 The result was that the thresholds stayed nearly constant when the number of cycles was greater than 16, corresponding to 13 ms pulse duration. This agrees with the results given here: our human-subjects experiments at 1.2 kHz reach an average of within 4% of the asymptote at the closest tested pulse duration of 16 ms (average of 12 experiments). Similarly, the fitted function in Fig. 5(c) is within 6% of the asymptotic value at 13 ms. However, when the experiment is performed at a single frequency as in Ref. 14, it is not possible to distinguish whether the determining factor is the number of cycles or the pulse duration. Here, by performing human-subject experiments at three different frequencies, we have shown conclusively that the determining factor is the pulse duration. The normalized magnetostimulation curves agree nicely over a 20-fold range of frequencies (1.2–25.5 kHz), indicating that the duration dependence of the magnetostimulation thresholds is independent of the operating frequency.
To model the decay in magnetostimulation thresholds as a function of pulse duration, a stretched exponential function was utilized. While this model provided the best fit to the data points (R2 = 0.78) among the many decay functions that we tested, the goodness-of-fit is affected by the normalization step. Here, the data set from each experiment was normalized by the average of the thresholds at the two longest pulse durations, with the assumption that these thresholds were close to the asymptotic values and that they were measured accurately. Any inaccuracy in these measurements affects the entire normalized curve. For example, if the data sets are normalized by the thresholds at the longest pulse duration alone (i.e., Tpulse ≈ 125 ms), the goodness-of-fit drops to R2 = 0.73. In theory, testing the subjects at additional long pulse durations would improve the accuracy of the normalization step. However, this would require lengthening the already long experiments (30–40 min), which may in turn lead to increasingly inconsistent reporting of the stimulation threshold as the subjects get more tired. We observed that when experiments exceed 45 min, subjects occasionally fell asleep and missed the first detectable levels of PNS, only waking up to stronger stimulation sensations. Here, the tested pulse durations were chosen to capture the overall effect of the duration on magnetostimulation limits, while keeping the experimental sessions at reasonable lengths.
The magnetic pulses in this experiment were spaced at 2-s intervals, which was long enough for the subjects to report a PNS sensation via a mouse click, but short enough to keep the session length to around 30–40 min. It remains a future work to show, for fixed pulse duration, how short the intervals can get before the stimulation thresholds start to go down again, i.e., how the duty cycle affects the magnetostimulation thresholds. Currently in MPI, the drive field remains on throughout imaging, where the scan time varies from a couple of seconds to minutes. Hence, MPI currently operates at 100% duty cycle and at long pulse duration regime. In the end, once all the contributing factors to magnetostimulation limits are known, the optimum scanning scheme should minimize the scan time needed to cover a region of interest while keeping the SNR of the image high (i.e., maximize the SNR efficiency).
The power specification of our amplifier was a constraining factor while choosing subjects, especially at short pulse durations where higher instantaneous power is needed to induce stimulation. Among the subjects that were prescreened and tested at 2 ms pulse duration, our setup could induce nerve stimulation only among the male subjects (results not shown). Considering that the male subjects had bigger forearms than the female subjects, this result is consistent with our previous work where we have shown a strong inverse correlation between magnetostimulation thresholds and body part size.9 It should be noted that there was no significant variation with body part size for chronaxie time (a measure of the time required to depolarize the nerves),9 which implies that the body part size only has a scaling effect on the magnetostimulation thresholds. Hence, while all six subjects in this work were chosen from the male population, we expect the female population to display the same threshold vs duration trends.
The goal of this work was to better understand the factors that determine the magnetostimulation limits in MPI via experiments performed on the forearm, which has reduced hardware requirements when compared to experiments in the torso. Note that our earlier work, with experiments performed on the arm and the leg of the human subjects, was able to predict that the magnetostimulation limits in the torso would be around 14 mT-pp for axially applied magnetic fields with frequencies above 25 kHz (high frequency asymptote).9 Later, a direct study on the torso (i.e., for whole body imaging) determined the magnetostimulation limits to lie within the range 8.8–15.2 mT-pp for the torso (for axial fields with frequencies between 24 and 162 kHz).10 Hence, accurately controlled experiments as the ones in this work shed light onto the magnetostimulation limits in general, whichever part of the body the application may target. In addition, as discussed above, the chronaxie time did not show a significant variation with body part size.9 Likewise, our preliminary work that compared axial and transverse magnetostimulation limits showed comparable chronaxie times (for N = 3 subjects), independent of the applied field direction.17 Therefore, while the absolute level of thresholds can vary for different body parts and field orientations, we expect to see similar duration dependence, which remains to be shown experimentally.
Our previous work demonstrated that the magnetostimulation thresholds decrease with increasing frequency, and the hyperbolic relation between frequency and threshold is valid for frequencies extending to 50 kHz.9 However, these results were in contrast to a recent work where the magnetostimulation thresholds were 60% higher at 59 kHz than at 25 kHz.18 The results presented here help to clarify that the reason behind this seeming inconsistency could be the difference in the pulse durations used in each work: our experiments in Ref. 9 used long pulses (25–30 ms) with flat envelopes, such that the pulses were practically in the asymptotic regime of the “duration vs threshold” curve. On the other hand, the experiments in Ref. 18 utilized brief pulses of approximately 12–20 cycles with exponentially decaying envelopes, and because the number of cycles was kept constant instead of the pulse duration, the pulses got shorter with increasing frequency (0.5–0.8 ms pulse duration at 25 kHz vs 0.2–0.3 ms at 59 kHz). The exponentially decaying envelope may have further shortened the “effective” pulse durations. Hence, the reason behind the measured 60% higher threshold at 59 kHz could be the shorter pulse durations used at higher frequencies. Note that our experiments here do not cover pulse durations shorter than 2 ms. Therefore, further studies are needed to experimentally verify this conclusion. It is important to emphasize that the experiments in Ref. 18 were concerning the gradient magnetic field in MRI, where brief pulses are typically utilized. Hence, the results of that work provide important insight into the potential of utilizing higher frequency gradient systems for MRI. Similarly, the goal of our previous work in Ref. 9 was to understand how frequency affects the magnetostimulation thresholds for MPI, where magnetic pulses with high duty cycles and long durations are utilized. Accordingly, once the effect of pulse duration is taken into account, the two studies are consistent with each other and with the results provided in this work.
5. CONCLUSION
In this work, we have shown for the first time that the magnetostimulation thresholds decrease with increasing pulse duration and that this effect is independent of the operating frequency. The normalized threshold vs duration curves display almost identical trends for a 20-fold range of frequencies (from 1.2 to 25.5 kHz). The thresholds at 2 ms pulse duration are approximately 24% higher than at long pulse durations, and they settle to within 4% of their asymptotic values for durations longer than 20 ms. These results underline the importance of matching the human-subject experiments to the imaging conditions of a particular setup. The drive field in MPI typically utilizes long pulse durations with high duty cycle, whereas MRI uses much shorter pulses and reduced duty cycle. Knowing all the contributing factors of the safety limits is critical for increasing the time-efficiency of these imaging systems.
ACKNOWLEDGMENTS
The authors would like to thank George Z. Zhang, David Chang, Wisely Yang, Bo Zheng, and Kuan Lu for their assistance in the stimulation setup, and to Tolga Cukur for discussions on statistical data analysis. This work was supported in part by a Siebel Stem Cell Institute Postdoctoral Fellowship, CIRM Tools and Technology Grant No. RT2-01893, a UC Discovery grant, and Grant No. 1R01EB013689 from the National Institute of Biomedical Imaging and Bioengineering. This work was supported in part by the Scientific and Technological Research Council of Turkey (TUBITAK) through a TUBITAK 3501 Grant (No. 114E167) and a TUBITAK 2232 Grant No. (113C012), and by the European Commission through an FP7 Marie Curie Career Integration Grant No. (PCIG13-GA-2013-618834). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of CIRM, any other agency of the State of California, the National Institute of Biomedical Imaging and Bioengineering, the National Institutes of Health, TUBITAK, or the European Commission.
REFERENCES
- 1.Reilly J. P., “Peripheral nerve stimulation by induced electric currents: Exposure to time-varying magnetic fields,” Med. Biol. Eng. Comput. 27(2), 101–110 (1989). 10.1007/bf02446217 [DOI] [PubMed] [Google Scholar]
- 2.Bottomley P. A. and Edelstein W. A., “Power deposition in whole-body NMR imaging,” Med. Phys. 8(4), 510–512 (1981). 10.1118/1.595000 [DOI] [PubMed] [Google Scholar]
- 3.Reilly J. P., “Maximum pulsed electromagnetic field limits based on peripheral nerve stimulation: Application to IEEE/ANSI C95.1 electromagnetic field standards,” IEEE Trans. Biomed. Eng. 45(1), 137–141 (1998). 10.1109/10.650371 [DOI] [PubMed] [Google Scholar]
- 4.Gleich B. and Weizenecker J., “Tomographic imaging using the nonlinear response of magnetic particles,” Nature 435(7046), 1214–1217 (2005). 10.1038/nature03808 [DOI] [PubMed] [Google Scholar]
- 5.Goodwill P. W. and Conolly S. M., “The X-space formulation of the magnetic particle imaging process: 1-D signal, resolution, bandwidth, SNR, SAR, and magnetostimulation,” IEEE Trans. Med. Imaging 29(11), 1851–1859 (2010). 10.1109/TMI.2010.2052284 [DOI] [PubMed] [Google Scholar]
- 6.Goodwill P. W., Saritas E. U., Croft L. R., Kim T. N., Krishnan K. M., Schaffer D. V., and Conolly S. M., “X-space MPI: Magnetic nanoparticles for safe medical imaging,” Adv. Mater. 24(28), 3870–3877 (2012). 10.1002/adma.201200221 [DOI] [PubMed] [Google Scholar]
- 7.Saritas E. U., Goodwill P. W., Croft L. R., Konkle J. J., Lu K., Zheng B., and Conolly S. M., “Magnetic particle imaging (MPI) for NMR and MRI researchers,” J. Magn. Reson. 229, 116–126 (2013). 10.1016/j.jmr.2012.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bohnert J., Gleich B., Weizenecker J., Borgert J., and Dössel O., “Optimizing coil currents for reduced SAR in magnetic particle imaging,” in World Congress on Medical Physics and Biomedical Engineering, September 7–12, 2009, Munich, Germany (Springer Berlin Heidelberg, Berlin, Heidelberg, 2010), pp. 249–252. 10.1007/978-3-642-03882-2_66 [DOI] [Google Scholar]
- 9.Saritas E. U., Goodwill P. W., Zhang G. Z., and Conolly S. M., “Magnetostimulation limits in magnetic particle imaging,” IEEE Trans. Med. Imaging 32(9), 1600–1610 (2013). 10.1109/TMI.2013.2260764 [DOI] [PubMed] [Google Scholar]
- 10.Schmale I. et al. , “Human PNS and SAR study in the frequency range from 24 to 162 kHz,” in 3rd International Workshop on Magnetic Particle Imaging (IEEE, Berkeley, CA, 2013). 10.1109/IWMPI.2013.6528346 [DOI] [Google Scholar]
- 11.Schmale I., Gleich B., Rahmer J., Bontus C., Schmidt J., and Borgert J., “MPI safety in the view of MRI safety standards,” IEEE Trans. Magnetics 51(2), 1–4 (2015). 10.1109/TMAG.2014.2322940 [DOI] [Google Scholar]
- 12.Geddes L. A., “Accuracy limitations of chronaxie values,” IEEE Trans. Biomed. Eng. 51(1), 176–181 (2004). 10.1109/TBME.2003.820340 [DOI] [PubMed] [Google Scholar]
- 13.Zhang B., Yen Y.-F., Chronik B. A., McKinnon G. C., Schaefer D. J., and Rutt B. K., “Peripheral nerve stimulation properties of head and body gradient coils of various sizes,” Magn. Reson. Med. 50(1), 50–58 (2003). 10.1002/mrm.10508 [DOI] [PubMed] [Google Scholar]
- 14.Budinger T. F., Fischer H., Hentschel D., Reinfelder H. E., and Schmitt F., “Physiological effects of fast oscillating magnetic field gradients,” J. Comput. Assisted Tomogr. 15(6), 909–914 (1991). 10.1097/00004728-199111000-00001 [DOI] [PubMed] [Google Scholar]
- 15.Harvey P. R. and Mansfield P., “Avoiding peripheral nerve stimulation: Gradient waveform criteria for optimum resolution in echo-planar imaging,” Magn. Reson. Med. 32(2), 236–241 (1994). 10.1002/mrm.1910320213 [DOI] [PubMed] [Google Scholar]
- 16.Chronik B. A. and Ramachandran M., “Simple anatomical measurements do not correlate significantly to individual peripheral nerve stimulation thresholds as measured in MRI gradient coils,” J. Magn. Reson. Imaging 17(6), 716–721 (2003). 10.1002/jmri.10300 [DOI] [PubMed] [Google Scholar]
- 17.Yu E., Saritas E. U., and Conolly S. M., “Comparison of magnetostimulation limits for axial and transverse drive fields in MPI,” in 3rd International Workshop on Magnetic Particle Imaging (IEEE, Berkeley, CA, 2013). 10.1109/IWMPI.2013.6528324 [DOI] [Google Scholar]
- 18.Weinberg I. N. et al. , “Increasing the oscillation frequency of strong magnetic fields above 101 kHz significantly raises peripheral nerve excitation thresholds,” Med. Phys. 39(5), 2578–2583 (2012). 10.1118/1.3702775 [DOI] [PMC free article] [PubMed] [Google Scholar]