Abstract
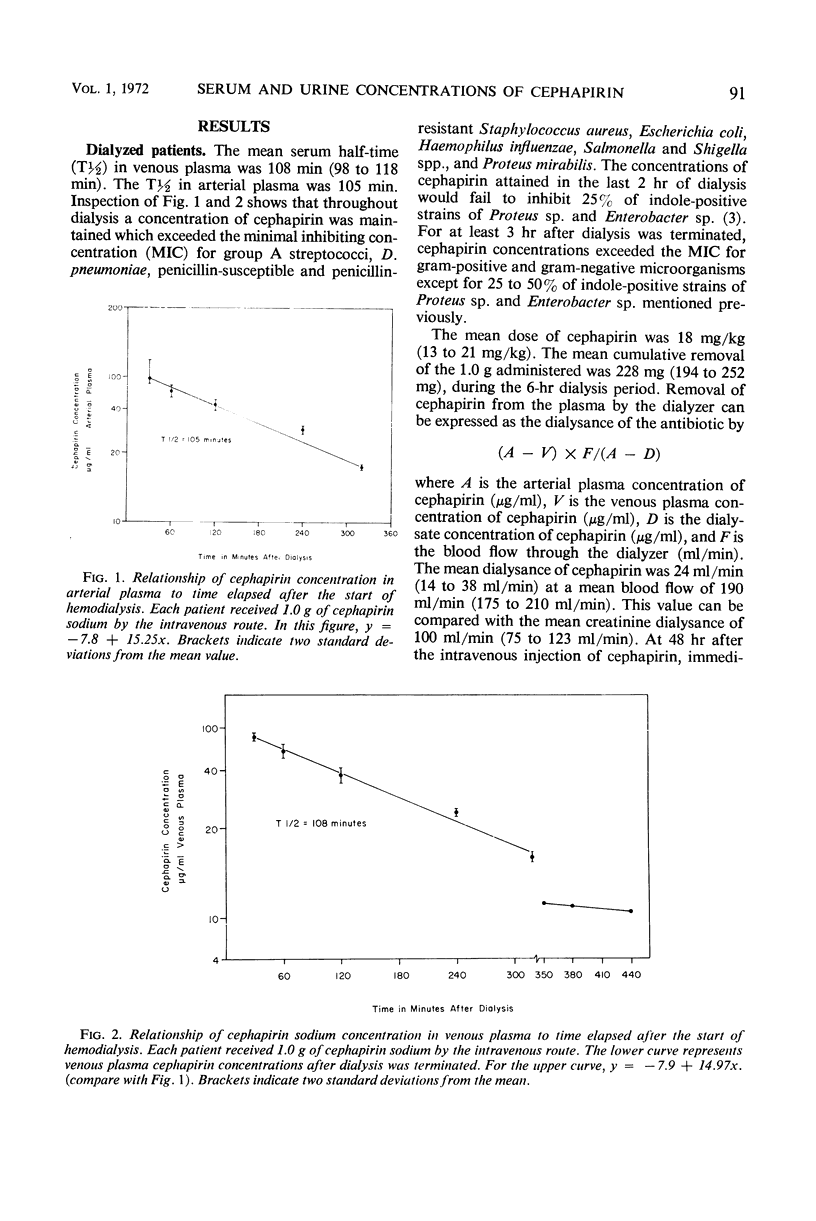
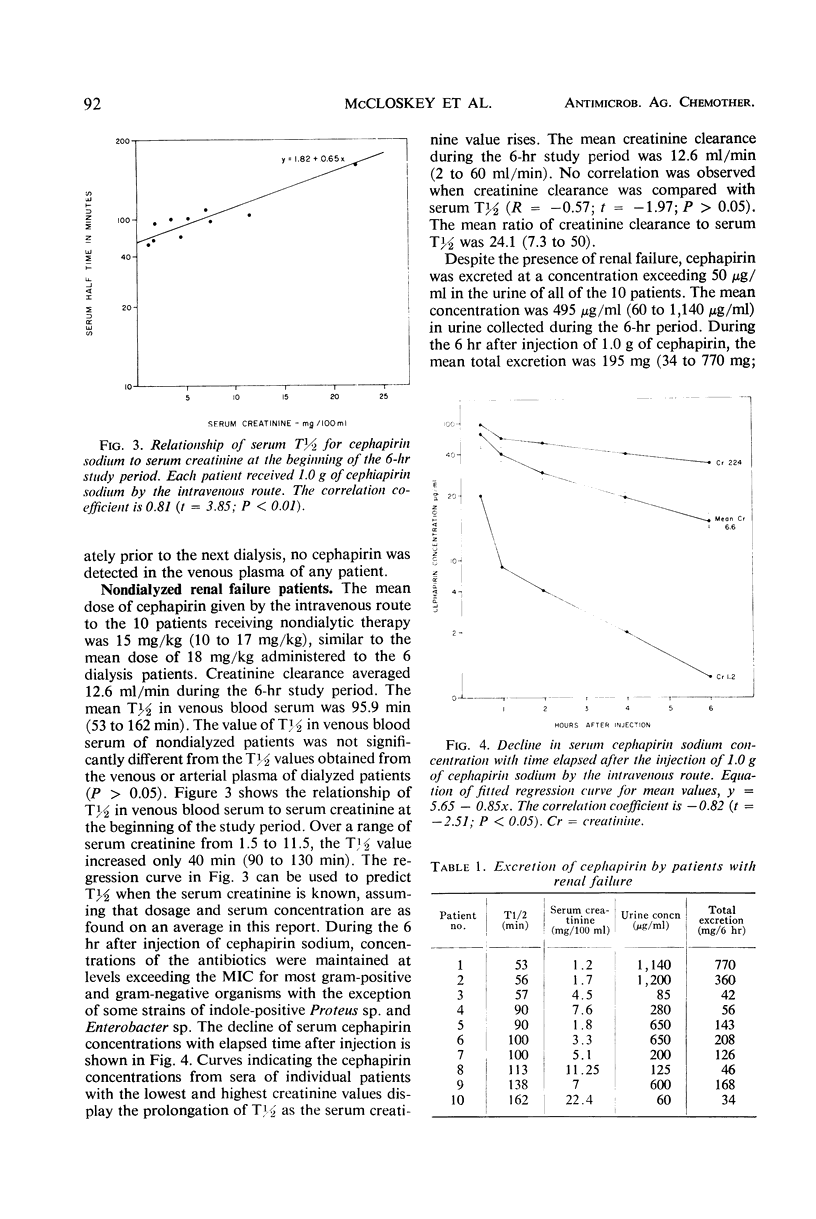
Six patients undergoing chronic hemodialysis and 10 patients with chronic renal insufficiency hospitalized for nondialytic therapy received 1.0 g of cephapirin sodium by the intravenous route. The concentrations of cephapirin in arterial and venous plasma, dialysate, venous blood, and urine were measured during the ensuing 6 hr. The serum half-life of cephapirin was 105 to 108 min for the dialyzed patients and 95.9 min for the nondialyzed patients. Dialysis removed 22.8% of the administered dose. Nondialyzed patients excreted 19.5% of the administered dose in the urine. The concentration of cephapirin in the urine of all nondialyzed patients exceeded 50 μg/ml. The recovery of cephapirin in the urine collected for 6 hr after injection was from 34 to 770 mg (mean 195 mg). To maintain a concentration of cephapirin in the blood and urine which exceeds the minimal inhibitory concentration for most gram-positive and gram-negative microorganisms, nondialyzed patients should receive 15 to 18 mg of cephapirin per kg every 12 hr. Dialyzed patients should receive the same dose just prior to dialysis and every 12 hr thereafter.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benner E. J. Renal damage associated with prolonged administration of ampicillin, cephaloridine, and cephalothin. Antimicrob Agents Chemother (Bethesda) 1969;9:417–420. [PubMed] [Google Scholar]
- Bergan T., Midtvedt T., Erikssen J. Human pharmacokinetics of cephalexin. Pharmacology. 1970;4(5):264–272. doi: 10.1159/000136146. [DOI] [PubMed] [Google Scholar]
- Chisholm D. R., Leitner F., Misiek M., Wright G. E., Price K. E. Laboratory studies with a new cephalosporanic acid derivative. Antimicrob Agents Chemother (Bethesda) 1969;9:244–246. [PubMed] [Google Scholar]
- Gordon R. C., Barrett F. F., Clark D. J., Yow M. D. Laboratory and pharmacologic studies of BL-P-1322 (cephapirin sodium) in children. Curr Ther Res Clin Exp. 1971 Jun;13(6):398–406. [PubMed] [Google Scholar]
- Hoehn M. M., Murphy H. W., Pugh C. T., Davis N. E. Paper chromatographic techniques for the determination of cephalothin and desacetylcephalothin in body fluids. Appl Microbiol. 1970 Nov;20(5):734–736. doi: 10.1128/am.20.5.734-736.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunin C. M., Atuk N. Excretion of cephaloridine and cephalothin in patients with renal impairment. N Engl J Med. 1966 Mar 24;274(12):654–656. doi: 10.1056/NEJM196603242741205. [DOI] [PubMed] [Google Scholar]
- McCloskey R. V., Becker G. G. Evaluation of the Cutler-Orme method for administration of kanamycin during renal failure. Antimicrob Agents Chemother (Bethesda) 1970;10:161–164. [PubMed] [Google Scholar]
- Venuto R. C., Plaut M. E. Cephalothin handling in patients undergoing hemodialysis. Antimicrob Agents Chemother (Bethesda) 1970;10:50–52. [PubMed] [Google Scholar]
- Wick W. E., Wright W. E., Kuder H. V. Cephaloglycin and its biologically active metabolite desacetylcephaloglycin. Appl Microbiol. 1971 Mar;21(3):426–434. doi: 10.1128/am.21.3.426-434.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]



