Abstract
Elevated uric acid levels have recently been found to be associated with slower disease progression in Parkinson’s disease, Huntington’s disease, multiple system atrophy, and mild cognitive impairment. The aim of this study is to determine whether serum uric acid levels predict survival in amyotrophic lateral sclerosis (ALS). A total of 251 people with ALS enrolled in two multicenter clinical trials were included in our analysis. The main outcome measure was survival time, which was calculated as time to death, tracheostomy, or permanent assistive ventilation, with any event considered a survival endpoint. Cox proportional hazards models were used to estimate the hazard ratio (HR) of reaching a survival endpoint according to baseline uric acid levels after adjusting for markers of disease severity (FVC, total ALSFRS-R score, time since symptom onset, diagnostic delay, BMI, bulbar vs. spinal onset, age, and riluzole use). There was a dose-dependent survival advantage in men, but not women, with higher baseline uric acid levels (logrank test: p = 0.018 for men, p = 0.81 for women). There was a 39% reduction in risk of death during the study for men with each 1 mg/dl increase in uric acid levels (adjusted HR: 0.61, 95% CI 0.39–0.96, p = 0.03). This is the first study to demonstrate that serum uric acid is associated with prolonged survival in ALS, after adjusting for markers of disease severity. Similar to previous reports in Parkinson’s disease, this association was seen in male subjects only.
Keywords: Uric acid, Urate, Amyotrophic lateral sclerosis, Predictor, Survival
Introduction
The potential role of uric acid in neurodegeneration has been an area of interest ever since high serum urate concentrations were linked to a reduced risk of developing Parkinson’s disease [1–4]. Uric acid levels were also found to be predictors of clinical and radiographic progression in men with Parkinson’s disease, with higher levels associated with slower rates of decline [5, 6]. Furthermore, higher uric acid levels have been correlated with slower clinical progression in Huntington’s disease, multiple system atrophy, and mild cognitive impairment [7–10]. While the mechanisms underlying these epidemiological observations are not clear, it has been speculated that uric acid may have a protective influence on neuronal cell death because of its antioxidant properties [11–13]. Thus, research efforts are underway to test the safety of elevating serum uric acid concentrations in Parkinson’s disease as a novel potential therapy [clinical trial NCT00833690].
The role of uric acid in amyotrophic lateral sclerosis (ALS) has been less well studied. Two prior studies reported that serum urate concentrations were lower in subjects with ALS compared to a convenience sample of healthy controls [14, 15]. Zoccolella also reported an association between low uric acid levels and longer disease duration, which they hypothesized to be due to malnutrition, but they did not adjust for BMI in their analyses [14, 16]. No prior study has tested whether the positive correlation between uric acid levels and disease progression seen in men with Parkinson’s disease would be duplicated in ALS. We therefore tested whether uric acid levels would predict disease progression and survival in a large cohort of ALS clinical trial participants.
Methods
Subjects
Partners Healthcare Institutional Review Board and Northeast ALS Consortium (NEALS) approval for use of blood samples from all studies was obtained. The study population consisted of participants in two clinical research databases: the trial of celecoxib in ALS and the trial of arimoclomol in ALS [17, 18]. Subjects were followed at multiple ALS centers of the NEALS between 2001 and 2006. All subjects met El Escorial World Federation of Neurology criteria for the diagnosis of definite or probable ALS at the time of blood sampling [19]. Subjects on thiazide diuretics (16) or allupurinol (0) were excluded from our analyses. At the time of this study, baseline serum samples were available for 251 subjects from the two clinical databases combined. Four- and 8-month follow-up samples were also available for a total of 177 and 148 subjects. Complete data on medical history, physical examination, neurologic examination, and forced vital capacity (FVC) were available for all participants. ALS Functional Rating Scale-Revised scores were also assessed. Survival times were calculated as time to death, tracheostomy, or permanent assistive ventilation, with any event considered a survival endpoint. Permanent ventilation was defined as invasive or non-invasive ventilation use for more than 22 h/day for 14 consecutive days. Subjects were followed for a mean of 267.2 ± 131.9 days.
Biochemical procedures
Serum samples were obtained at the time of enrollment, and then aliquoted and frozen at −80°C until time of use. Samples were run on an Architect Ci8200 System™ using uric acid kits following the manufacturer’s instructions (Abbott Laboratories, Abbott Park, IL). Results were corrected for measurement variance by adjusting for differences in control samples that were randomly inserted between subject samples to detect changes in batch measurements.
Statistical analysis
All analyses were performed using SAS statistical software version 9.2 (SAS Institute, Cary, NC). All uric acid values were corrected for measurement error as above. Uric acid levels were normally distributed within sex (reference range: 3.5–7.2 mg/dl for males and 2.6–6 mg/dl for females). Uric acid levels were stratified into quartiles based on the distribution of the study results. Due to the extreme differences in the distribution of uric acid levels between men and women, sex-specific quartiles were used: for males <4.8, 4.8–5.4, 5.41–6.35, >6.35 mg/dl; for females <3.4, 3.4–3.9, 3.91–4.8, >4.8 mg/dl.
Survival was analyzed using the Kaplan–Meier method using the logrank test. Multivariate survival analysis was performed using Cox proportional hazard models for each gender. The following covariates assessed at the time of enrollment were included using a stepwise selection rule with a retention criterion of p < 0.1: uric acid level, age, FVC, total ALSFRS-R score, body mass index (BMI) calculated as weight (kg)/height (m)2, site of symptom onset (bulbar vs. limb), time since symptom onset, diagnostic delay (as defined by time since symptom onset and diagnosis [20, 21], and riluzole use. The length of time that samples were stored at −80°C prior to processing was also included as a covariate. All variables were treated as continuous, except for gender, bulbar onset, and riluzole use, which were dichotomized. Predictors of baseline uric acid levels were determined using a multivariate linear regression model which included the same covariates as in the Cox proportional hazard models above. For change over time in FVC and ALSFRS-R scores, we used a random effects model for longitudinal data including BMI as a covariate.
Results
At total of 251 subjects from two studies were included in our analysis (Table 1). As expected, uric acid levels were higher in men (mean 5.67 ± 1.34 mg/dl) compared to women (mean 4.25 ± 1.32 mg/dl) and were normally distributed within each sex (data not shown). In our multivariate model of baseline uric acid levels (Supplementary Table 1), disease duration and bulbar onset did not predict uric acid levels, after adjusting for BMI and male gender. Interestingly, longer time between symptom onset and diagnosis (suggesting a slower disease progression) was associated with higher levels of uric acid. Longer duration of storage at −80°C was also associated with lower uric acid levels. For subjects with repeated measurements, serum uric acid levels remained essentially unchanged during the follow-up time of 8 months. The mean uric acid level in 251 subjects at baseline was 5.15 ± 1.49 mg/dl, in 177 subjects at month 4 was 5.37 ± 2.73 mg/dl and in 148 subjects at month 8 was 4.96 ± 1.67 mg/dl (p = 0.44 for the fixed effect of time on uric acid levels).
Table 1.
Baseline clinical and demographic patient characteristics
| Characteristics | Celecoxib study (n = 222) | Arimoclomol study (n = 29) | Males (n = 158) | Females (n = 93) | Overall (n = 251) |
|---|---|---|---|---|---|
| Men, no. (%) | 140 (63.1) | 18 (62.1) | – | – | 158 (62.9) |
| Age, mean (SD) (years) | 54.1 (12.7) | 53.3 (11.0) | 53.0 (12.0) | 55.6 (13.3) | 53.9 (12.5) |
| BMI, mean (SD) | 27.0 (9.2) | 25.5 (3.9) | 27.1 (4.8) | 26.3 (4.1) | 26.8 (8.8) |
| Deceased, no. (%) | 42 (18.9) | 0 (0) | 22 (13.9) | 20 (21.5) | 42 (16.7) |
| Bulbar, no. (%) | 39 (17.6) | 2 (6.9) | 25 (15.8) | 16 (17.2) | 41 (16.3) |
| Riluzole, no. (%) | 142 (64.0) | 13 (44.8) | 93 (58.9) | 55 (59.1) | 148 (59.0) |
| FVC, mean (SD) | 87.3 (15.9) | 86.0 (19.0) | 88.2 (16.2) | 85.0 (16.0) | 87.1 (16.3) |
| Total ALSFRS-R score, mean (SD) | 42.8 (5.7) | 42.4 (6.0) | 43.5 (5.7) | 41.6 (5.6) | 42.8 (5.8) |
| Follow-up period, mean (SD) (days) | 288.2 (125.5) | 107.4 (32.7) | 268 (128.9) | 265 (137.6) | 267.2 (131.9) |
| Time from diagnosis, mean (SD) (days) | 297.5 (295.7) | 274.9 (248.7) | 291.3 (287.3) | 294.0 (290.3) | 294.9 (290.3) |
| Time from symptom onset, mean (SD) (days) | 682.9 (432.8) | 589.4 (308.5) | 669.5 (423.9) | 671.2 (417.0) | 672.1 (420.9) |
| Diagnostic delay, mean (SD) (days) | 385.4 (310.0) | 314.5 (242.2) | 378.2 (306.5) | 377.2 (300.8) | 377.2 (303.4) |
The arimoclomol study experienced no deaths, in part due to the inclusion criteria and in part due to the short duration of follow-up in that study ALSFRS-R ALS functional rating scale revised total score, BMI body mass index, Bulbar bulbar onset ALS, FVC forced vital capacity percent of predicted, no. number, SD standard deviation
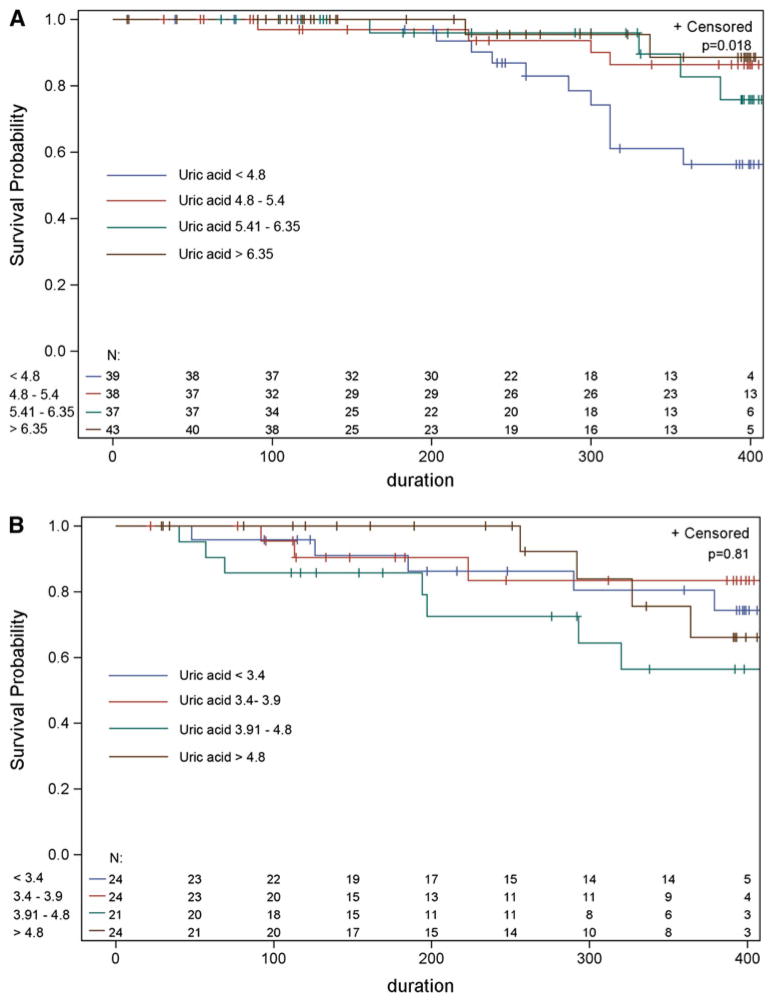
Because of the higher levels of uric acid in men, survival analysis was performed based on sex-specific quartiles, as in the Parkinson’s literature [5, 6]. There was a dose-dependent survival advantage with each unit increase in baseline uric acid levels in men, but not women (logrank test: p = 0.018 for men, p = 0.81 for women, Fig. 1). A Cox proportional hazards model was then used to adjust for markers of disease severity (age at enrollment, FVC, total ALSFRS-R score, time since symptom onset, diagnostic delay, BMI, bulbar vs. spinal onset, and riluzole use). In men, but not in women, there was a 39% reduction in risk of death during the study with each 1 mg/dl increase in uric acid levels (Table 2a, b; HR in men = 0.61, 95% CI 0.39–0.96, p = 0.03). Supplementary Table 2 shows the Cox proportional hazards model forcing age, BMI, bulbar onset, duration of sample storage and total ALSFRS-R into the model; the hazard ratio for uric acid levels was still highly significant (HR = 0.59, 95% CI 0.36–0.97, p = 0.039). We also performed a sensitivity analysis restricting the analysis to the 139 men who were part of the trial of celecoxib in ALS, and again the logrank test was highly significant (p = 0.019).
Fig. 1.
Kaplan–Meier survival curves stratified by gender and uric acid levels. Censored patients are indicated by crosses on the corresponding survival curve. Survival curves have been truncated at 400 days (when less than five subjects were at risk). a Male subjects: blue line uric acid <4.8 mg/dl; red line uric acid 4.8–5.4 mg/dl; green line uric acid 5.41–6.35 mg/dl; brown line uric acid >6.35 mg/dl (logrank p = 0.018). b Female subjects: blue line uric acid <3.4 mg/dl; red line uric acid 3.4–3.9 mg/dl; green line uric acid 3.91–4.8 mg/dl; brown line uric acid >4.8 mg/dl (logrank p = 0.81). N total number at risk by uric acid quartile
Table 2.
Final proportional hazards model for survival in 156 male (a) and 92 female (b) subjects with complete follow-up data
| Parameter | HR (95% CI) | p value |
|---|---|---|
| (a) Males | ||
| Serum uric acid level (mg/dl) | 0.61 (0.39–0.96) | 0.03 |
| Age (years) | 1.04 (0.99–1.08) | 0.09 |
| FVC (%) | 0.97 (0.94–0.99) | 0.03 |
| Time from symptom onset (months) | 0.95 (0.91–0.99) | 0.02 |
| (b) Females | ||
| Age (years) | 1.10 (1.04–1.16) | <0.001 |
| BMI | 0.84 (0.72–0.98) | 0.02 |
| ALSFRS-R | 0.85 (0.77–0.93) | <0.001 |
| Time from diagnosis (months) | 0.94 (0.88–0.99) | 0.04 |
CI confidence interval, FVC forced vital capacity-percent of predicted, HR hazard ratio, ALSFRS-R ALS functional rating scale revised, BMI body mass index
Using a random effects model to analyze the change in FVC and ALSFRS-R over time, we found a non-significant trend towards slower progression in ALSFRS-R in males with higher baseline uric acid levels (p value for the interaction between uric acid and time = 0.08, Supplementary Tables 3, 4).
Discussion
Our findings suggest that higher baseline serum uric acid levels are associated with increased survival in men using a large ALS cohort. The fact that this association was seen in men only is not surprising, as this has been previously reported in Parkinson’s disease [5, 6]. The graphical analysis appears to suggest a threshold effect with improved survival seen in subjects with uric acid levels greater than 4.8 mg/dl. This may explain why uric acid did not determine survival in women (in our study, only 22% of women had uric acid levels greater than 4.8 mg/dl). Further, a biological interaction between gender and uric acid might explain the stronger association between uric acid levels and survival in men.
Our analysis was subject to several limitations. First, samples had been kept in storage at −80°C for up to 10 years, which raises the question of possible sample denaturation. Fortunately, these samples had not been subjected to any prior freeze–thaw cycle, which reduces the possibility of sample degradation. We included the duration of storage as a covariate in all of our analyses and this variable did not affect the results. On the other hand, performing all measurements in the same laboratory, at the same time, and under identical conditions, minimized the risk of measurement bias. Second, although this was the largest study to date to examine uric acid levels in ALS patients, the number of deaths during the studies was low due to the short duration of follow-up. Confirmatory studies will be required to confirm the association, to test whether these results are applicable beyond a clinical trials population, and to test whether uric acid levels are of clinical utility in predicting survival. Third, we were surprised to find that uric acid levels did not change over time, despite an average of weight loss in subjects of 0.3 kg/month. A smaller recent study also found no change in the mean levels of uric acid in 46 subjects over 10 months (mean 4.78 ± 1.3 vs. 4.92 ± 1.1 mg/dl although the two groups were not the same) [15]. In our study, there was a trend towards slower ALSFRS-R decline in men with higher baseline uric acid levels, which did not reach significance (p = 0.08).
Our results are similar to recent reports of an association between serum uric acid concentrations and clinical progression in other neurological disorders including Parkinson’s disease, Huntington’s disease, multiple system atrophy and mild cognitive impairment [5–9]. Combined, these results suggest that uric acid may be involved in the pathophysiology of neuronal cell death, possibly serving a neuroprotective role, or it may serve as an indirect marker of the neurodegenerative processes that underlie these conditions.
In our multivariate analysis, we were able to adjust for known confounders of uric acid levels including gender, BMI, and duration of sample storage. However, residual confounding may have occurred because of unmeasured genetic [22], dietary (such as dairy and alcohol intake [23]) or other environmental factors, which may be associated with both uric acid levels and survival. Thus, uric acid levels may be an additional marker of disease severity, not otherwise captured by our standard outcome measurements, or may be an independent predictor of survival.
Supplementary Material
Acknowledgments
We would like to thank Patrick Sluss, Ph.D., and the Clinical Laboratory Research Core at Massachusetts General Hospital for performing the biochemical analyses and for their excellent technical support. We thank David Schoenfeld, Ph.D. and Alberto Ascherio, M.D., Dr. P.H. for helpful discussions about data analysis and interpretation. We thank Swati Aggarwal, M.D. for helping with study design. We would also like to thank the Massachusetts General Hospital Neurology Clinical Trials Unit and the members of the Northeast ALS Consortium (NEALS) for allowing access to the blood samples and clinical data that were used in this study. Finally, we thank patients and their families for participating in these clinical trials and for contributing to clinical research. Drs. Wills and Paganoni had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The study was supported by the Muscular Dystrophy Association and the Digiovanni ALS research fund.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00415-012-6440-7) contains supplementary material, which is available to authorized users.
Conflicts of interest The authors declare that they have no conflicts of interest.
Contributor Information
Sabrina Paganoni, Neurology Clinical Trials Unit, Massachusetts General Hospital, Harvard Medical School, Charlestown Navy Yard, Building 149,149 13th Street, 2nd Floor, Room 2274, Charlestown, MA 02129, USA.
May Zhang, Neurology Clinical Trials Unit, Massachusetts General Hospital, Harvard Medical School, Charlestown Navy Yard, Building 149,149 13th Street, 2nd Floor, Room 2274, Charlestown, MA 02129, USA.
Alejandro Quiroz Zárate, Department of Biostatistics, Harvard School of Public Health, Boston, MA, USA.
Matthew Jaffa, Neurology Clinical Trials Unit, Massachusetts General Hospital, Harvard Medical School, Charlestown Navy Yard, Building 149,149 13th Street, 2nd Floor, Room 2274, Charlestown, MA 02129, USA.
Hong Yu, Neurology Clinical Trials Unit, Massachusetts General Hospital, Harvard Medical School, Charlestown Navy Yard, Building 149,149 13th Street, 2nd Floor, Room 2274, Charlestown, MA 02129, USA.
Merit E. Cudkowicz, Neurology Clinical Trials Unit, Massachusetts General Hospital, Harvard Medical School, Charlestown Navy Yard, Building 149,149 13th Street, 2nd Floor, Room 2274, Charlestown, MA 02129, USA
Anne-Marie Wills, Email: awills@partners.org, Neurology Clinical Trials Unit, Massachusetts General Hospital, Harvard Medical School, Charlestown Navy Yard, Building 149,149 13th Street, 2nd Floor, Room 2274, Charlestown, MA 02129, USA.
References
- 1.Weisskopf MG, O’Reilly E, Chen H, Schwarzschild MA, Ascherio A. Plasma urate and risk of Parkinson’s disease. Am J Epidemiol. 2007;166:561–567. doi: 10.1093/aje/kwm127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Vera M, Rahman MM, Rankin J, Kopec J, Gao X, Choi H. Gout and the risk of Parkinson’s disease: a cohort study. Arthritis Rheum. 2008;59(11):1549–1554. doi: 10.1002/art.24193. [DOI] [PubMed] [Google Scholar]
- 3.De Lau LM, Koudstaal PJ, Hofman A, Breteler MM. Serum uric acid levels and the risk of Parkinson disease. Ann Neurol. 2005;58:797–800. doi: 10.1002/ana.20663. [DOI] [PubMed] [Google Scholar]
- 4.Alonso A, Rodriguez LA, Logroscino G, Hernan MA. Gout and risk of Parkinson disease: a prospective study. Neurology. 2007;69:1696–1700. doi: 10.1212/01.wnl.0000279518.10072.df. [DOI] [PubMed] [Google Scholar]
- 5.Ascherio A, LeWitt PA, Xu K, Eberly S, Watts A, Matson WR, Marras C, Kieburtz K, Rudolph A, Bogdanov MB, Schwid SR, Tennis M, Tanner CM, Beal MF, Lang AE, Oakes D, Fahn S, Shoulson I, Schwarzschild MA Parkinson Study Group DATA-TOP Investigators. Urate as a predictor of the rate of clinical decline in Parkinson disease. Arch Neurol. 2009;66:1460–1468. doi: 10.1001/archneurol.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwarzschild MA, Schwid SR, Marek K, Watts A, Lang AE, Oakes D, Shoulson I, Ascherio A, Hyson C, Gorbold E, Rudolph A, Kieburtz K, Fahn S, Gauger L, Goetz C, Seibyl J, Forrest M, Ondrasik J Parkinson Study Group PRECEPT Investigators. Serum urate as a predictor of clinical and radiographic progression in Parkinson disease. Arch Neurol. 2008;65:716–723. doi: 10.1001/archneur.2008.65.6.nct70003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Auinger P, Kieburtz K, McDermott MP. The relationship between uric acid levels and Huntington’s disease progression. Mov Disord. 2010;25:224–228. doi: 10.1002/mds.22907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Euser SM, Hofman A, Westendorp RG, Breteler MM. Serum uric acid and cognitive function and dementia. Brain. 2009;132:377–382. doi: 10.1093/brain/awn316. [DOI] [PubMed] [Google Scholar]
- 9.Irizarry MC, Raman R, Schwarzschild MA, Becerra LM, Thomas RG, Peterson RC, Ascherio A, Aisen PS. Plasma urate and progression of mild cognitive impairment. Neurodegener Dis. 2009;6:23–28. doi: 10.1159/000170883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JE, Song SK, Sohn YH, Lee PH. Uric acid as a potential disease modifier in patients with multiple system atrophy. Mov Disord. 2011;26:1533–1536. doi: 10.1002/mds.23556. [DOI] [PubMed] [Google Scholar]
- 11.Glantzounis GK, Tsimoyiannis EC, Kappas AM, Galaris DA. Uric acid and oxidative stress. Curr Pharm Des. 2005;11:4145–4151. doi: 10.2174/138161205774913255. [DOI] [PubMed] [Google Scholar]
- 12.Scott GS, Cuzzocrea S, Genovese T, Koprowski H, Hooper DC. Uric acid protects against secondary damage after spinal cord injury. Proc Natl Acad Sci USA. 2005;102:3483–3488. doi: 10.1073/pnas.0500307102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amaro S, Planas AM, Chamorro A. Uric acid administration in patients with acute stroke: a novel approach to neuroprotection. Expert Rev Neurother. 2008;8:259–270. doi: 10.1586/14737175.8.2.259. [DOI] [PubMed] [Google Scholar]
- 14.Zoccolella S, Simone IL, Capozzo R, Tortelli R, Leo A, D’Errico E, Logroscino G. An exploratory study of serum urate levels in patients with amyotrophic lateral sclerosis. J Neurol. 2011;258:238–243. doi: 10.1007/s00415-010-5735-9. [DOI] [PubMed] [Google Scholar]
- 15.Keizman D, Ish-Shalom M, Berliner S, Maimon N, Vered Y, Artamonov I, Tsehori J, Nefussy B, Drory VE. Low uric acid levels in serum of patients with ALS: further evidence for oxidative stress? J Neurol Sci. 2009;285:95–99. doi: 10.1016/j.jns.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Paganoni S, Deng J, Jaffa M, Cudkowicz ME, Wills AM. Body mass index, not dyslipidemia, is an independent predictor of survival in amyotrophic lateral sclerosis. Muscle Nerve. 2011;44:20–24. doi: 10.1002/mus.22114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cudkowicz ME, Shefner JM, Schoenfeld DA, Zhang H, Andreasson KI, Rothstein JD, Drachman DB. Trial of celecoxib in amyotrophic lateral sclerosis. Ann Neurol. 2006;60:22–31. doi: 10.1002/ana.20903. [DOI] [PubMed] [Google Scholar]
- 18.Cudkowicz ME, Shefner JM, Simpson E, Grasso D, Yu H, Zhang H, Shui A, Schoenfeld D, Brown RH, Wieland S, Barber JR Northeast ALS Consortium. Arimoclomol at dosages up to 300 mg/day is well tolerated and safe in amyotrophic lateral sclerosis. Muscle Nerve. 2008;38:837–844. doi: 10.1002/mus.21059. [DOI] [PubMed] [Google Scholar]
- 19.Brooks BR, Miller RG, Swash M, Munsat TL World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- 20.Ganesalingam J, Stahl D, Wijesekera L, Galtrey C, Shaw CE, Leigh PN, Al-Chalabi A. Latent cluster analysis of ALS phenotypes identifies prognostically differing groups. PLoS One. 2009;4:e7107. doi: 10.1371/journal.pone.0007107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zoccolella S, Beghi E, Palagano G, Fraddosio A, Samarelli V, Lamberti P, Lepore V, Serlenga L, Logroscino G. SLAP registry (2006) Predictors of delay in the diagnosis and clinical trial entry of amyotrophic lateral sclerosis patients: a population-based study. J Neurol Sci. 2006;250:45–49. doi: 10.1016/j.jns.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 22.Nath SD, Voruganti VS, Arar NH, Thameem F, Lopez-Alvarenga JC, Bauer R, Blangero J, MacCluer JW, Comuzzie AG, Abboud HE. Genome scan for determinants of serum uric acid variability. J Am Soc Nephrol. 2007;18:3156–3163. doi: 10.1681/ASN.2007040426. [DOI] [PubMed] [Google Scholar]
- 23.Gao X, Curhan G, Forman JP, Ascherio A, Choi HK. Vitamin C intake and serum uric acid concentration in men. J Rheumatol. 2008;35:1853–1858. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.