Abstract
Introduction
Recent studies have provided conflicting data regarding the role of dyslipidemia in amyotrophic lateral sclerosis (ALS). The aim of this study was to determine whether cholesterol level are an independent predictor of survival in ALS.
Methods
Cholesterol levels were measured in 427 ALS subjects from three clinical trial databases.
Results
The LDL/HDL ratio did not decrease over time, despite significant declines in body mass index (BMI), forced vital capacity (FVC), and ALSFRS-R. After adjusting for BMI, FVC, and age, the lipid ratio was not associated with survival. There was a “U”-shaped association between BMI and mortality, with the highest survival at 30–35 kg/m2. The adjusted hazard ratio for the linear association between BMI and survival was 0.860 (95% CI 0.80–0.93, P = 0.0001).
Conclusions
We found that dyslipidemia is not an independent predictor of survival in ALS. BMI is an independent prognostic factor for survival after adjusting for markers of disease severity.
Keywords: ALS, BMI, cholesterol, HDL, LDL, lipid, obesity, survival
Abnormal energy metabolism in amyotrophic lateral sclerosis (ALS) is an active area of research (reviewed by Dupuis et al.1). ALS patients lose weight as the disease progresses due to both dysphagia with decreased food intake and hypermetabolism, that is, increased energy expenditure. Recently, Dupuis et al. suggested that the abnormal energy balance in ALS is characterized by dyslipidemia and that the lipid ratio might be used as a separate prognostic marker.2 Subsequent studies regarding the role of lipid metabolism in ALS provided conflicting results. Chio et al. found no independent association between lipid levels and survival.3 Most recently, Dorst et al. reported prolonged life expectancy in ALS patients with hypercholesterolemia based on data from a German database.4 However, the association with hyperlipidemia was not significant after adjusting for body mass index (BMI) and age at onset.
The aim of this study was to test whether cholesterol levels independently predict survival in a large series of ALS subjects. We hypothesized that cholesterol levels would change over time with disease progression and weight loss. Therefore, cholesterol levels were measured in 427 ALS subjects and they were measured repeatedly in a subset of subjects from three clinical trial databases.
METHODS
Patients
Approval for use of blood samples from all studies was obtained from the Partners Healthcare Institutional Review Board and the Northeast ALS Consortium (NEALS). The study population consisted of participants in three clinical trial databases: the Trial of Celecoxib in ALS; the Trial of Topiramate in ALS; and a single-center prospective cohort study.5,6 The single-center cohort study consisted of 128 patients with sporadic or familial ALS enrolled at Massachusetts General Hospital between April 1998 and April 2005. Subjects in the Celecoxib and Topiramate studies were followed at multiple ALS centers between 1998 and 2004. All subjects met El Escorial World Federation of Neurology criteria for the diagnosis of definite or probable ALS at the time of blood sampling.7 Subjects treated with lipid-lowering agents were excluded from our analyses. Baseline blood samples were available for 427 subjects from the three clinical databases combined. Four- or 8-month follow-up samples were also available for a total of 188 subjects (Table 1). Survival times were calculated as time to death, tracheostomy, or permanent assistive ventilation, with either event considered a survival endpoint. Permanent ventilation was defined as invasive or non-invasive ventilation use for >22 hours/day for 14 consecutive days. Patients were followed clinically for a mean period of 335 ± 251 days with a median follow-up of 392 days (Table 1).
Table 1.
Baseline clinical and demographic patient characteristics.
| Celecoxib study | Single-center cohort study | Topiramate study | Total | |
|---|---|---|---|---|
| Baseline | 217 | 53 | 157 | 427 |
| Month Invalid expression xmlXPathEval: evaluation failed |
||||
|
| ||||
| Overall | ||||
| Mean follow-up (SD) (days) | 272.65 (104.86) | 773.56 (626.66) | 371.98 (139.02) | 335.08 (251.24) |
| Median follow-up (days) | 393 | 574 | 366 | 392 |
| Deceased (%) | 15.63 | 35.29 | 24.20 | 19.18 |
| Male (%) | 63.65 | 55.88 | 66.88 | 63.62 |
| Mean age (SD) | 53.91 (13.06) | 51.39 (13.73) | 56.50 (12.68) | 54.15 (13.10) |
| Bulbar (%) | 18.83 | 29.41 | 21.66 | 20.37 |
| Riluzole (%) | 68.36 | 82.35 | 70.70 | 70.11 |
| Supplementary vitamin E (%) | 72.88 | 72.06 | 76.43 | 73.54 |
Bulbar = bulbar-onset ALS; SD = Standard Deviation.
Biochemical Procedures
The Trial of Celecoxib in ALS and the single-center prospective cohort study provided serum samples, whereas the Trial of Topiramate in ALS provided plasma samples. Samples were aliquoted and frozen at −80°C until time of use. Samples were run on an Architect Ci8200 system using the total cholesterol, Ultra HDL, and Multigent Direct LDL kits, following the manufacturer’s instructions (all Abbott Laboratories, Abbott Park, Illinois). Because samples were collected at random times and were not fasting, we measured direct LDL rather than calculated LDL. Results were corrected for measurement variance using a set of control samples that were randomly inserted between patient samples to detect changes in batch measurements.
Statistical Analysis
All analyses were performed using SAS statistical software, version 9.2 (SAS Institute, Inc., Cary, North Carolina). BMI was calculated as: weight (kg) / height2 (m). Results were adjusted for type of sample collected (serum or plasma). Total cholesterol, LDL, and HDL were normally distributed.
Change over time was analyzed using random effects models for longitudinal data. Survival was analyzed graphically and with the Kaplan–Meier method using the log-rank test. The following variables were stratified into quartiles based on the distribution of the study results: total cholesterol (≤191, 191.01–216, 216.01–250, or >250 mg/dl); LDL (≤84, 84.01–108, 108.01–132, or >132 mg/ dl); HDL (≤38, 38.01–46.50, 46.51–56, or >56 mg/dl); and LDL/HDL ratio (≤1.82, 1.83–2.27, 2.28–2.95, or >2.95). BMI was stratified according to World Health Organization (WHO) criteria: underweight, <18.5; normal weight, 18.5–24.99; overweight, 25–29.99; obese class I, 30–34.99; obese class II, 35–39.99; or obese class III, >40. Subjects with BMI >3 standard deviations from the mean (BMI >56, N = 3) were excluded on the assumption that these values may have been due to measurement error (recorded weight or height in English rather than metric units). In 1 case with repeated measurements, BMI was recalculated based on 4-month evaluation.
Multivariate survival analysis was performed using Cox proportional hazard models including the following covariates assessed at the time of enrollment: gender; age at time of enrollment; forced vital capacity (FVC); total ALS Functional Rating Scale—revised (ALSFRS-R) score; BMI; site of onset (bulbar vs. spinal); time since symptom onset; diagnostic delay, as defined by time since symptom onset and diagnosis8,9; sample type (plasma versus serum); history of cardiovascular disease; Topiramate or Celecoxib treatment assignment; vitamin E supplementation; and riluzole use. The length of time that samples were frozen prior to processing was included as a covariate. Feeding tube placement (N = 3) was not included, because, due to the small number of subjects, the variable did not satisfy the proportional hazards assumption. The linear relationship between BMI and survival was tested by including the quadratic term BMI2 as a covariate.
RESULTS
A total of 427 subjects from three studies were included in our analysis (Table 1). There were no significant differences in baseline demographics among the three included studies. The single-center prospective cohort was followed significantly longer than the other studies and therefore had a higher rate of mortality.
We hypothesized that cholesterol levels would change with disease progression over time, specifically that LDL levels would decrease and HDL levels would increase due to weight loss and declining FVC.2,10,11 As shown in Table 2, cholesterol levels were essentially unchanged despite a predictable but small decline in BMI and a strong decline in FVC and ALSFRS-R.
Table 2.
Change over time in BMI and cholesterol levels.
| Baseline* (N = 188) | Month Invalid expression xmlXPathEval: evaluation failed time (in months) |
P-value | |||
|---|---|---|---|---|---|
|
|
|||||
| BMI | 26.5 (4.34) | 26.63 (4.9) | 25.74 (4.29) | −0.073 | 0.0006* |
| FVC | 88.13 (17.81) | 77.83 (19.85) | 72.24 (22.41) | −2.31 | 8.7E-31* |
| ALSFRS-R | 42.63 (6.27) | 38.98 (7.46) | 36.76 (8.24) | −0.93 | 1.1E-36* |
| Total cholesterol | 230.24 (43.49) | 226.48 (46.04) | 228.13 (49.60) | −0.32 | 0.38 |
| LDL | 127.67 (31.72) | 123.88 (34.13) | 125.48 (35.47) | −0.30 | 0.28 |
| HDL | 52.06 (12.19) | 49.81 (11.2) | 50.10 (12.17) | −0.22 | 0.0055* |
| LDL/HDL ratio | 2.42 (0.92) | 2.60 (0.86) | 2.64 (0.93) | 0.0057 | 0.32 |
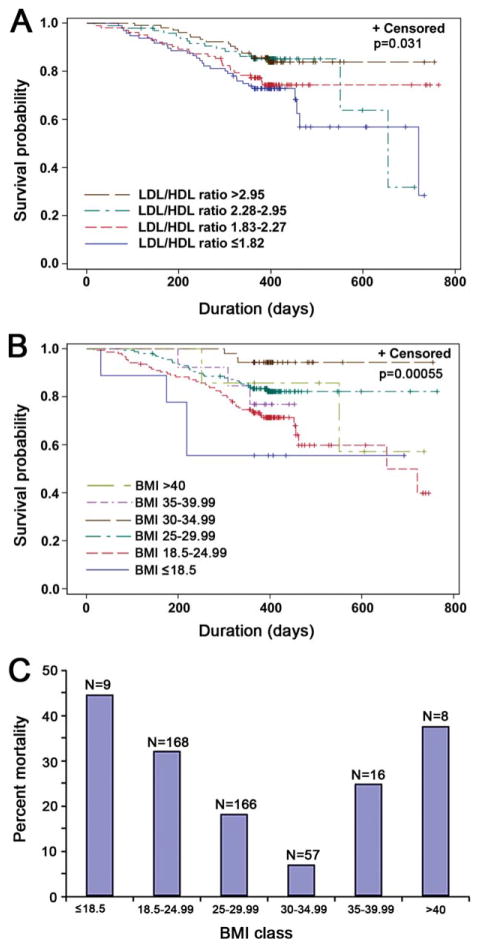
Next, when a survival analysis was performed dividing subjects into LDL/HDL ratios <2.99 or >2.99, there was a trend toward significance (log rank: P = 0.075; see Supplementary Material Fig. S1). More interestingly, when the LDL/HDL ratio was stratified into quartiles, the log-rank test for equality over strata was significant (P = 0.031; Fig. 1A). However, as shown in Table 3, there were significant differences in BMI, ALSFRS-R, and age across the quartiles and, after adjusting for each of these variables, the lipid ratio variable (LDL/HDL ratio) was no longer significantly associated with survival.
FIGURE 1.
Correlation between lipid levels, BMI, and survival in ALS. Kaplan–Meier survival curves of 427 ALS patients stratified according to their LDL/HDL ratio (A) or BMI (B). Censored patients are indicated by crosses on the corresponding survival curve. The survival curves were truncated at 764 days (the maximum time of follow-up in the Topiramate study). (A) Blue line: LDL/HDL ratio ≤1.82; red line: LDL/HDL ratio 1.83–2.27; green line: LDL/HDL ratio 2.28–2.95; brown line: LDL/ HDL ratio >2.95 (log rank: P = 0.031). (B) Blue line: BMI ≤18.5; red line: BMI 18.5–24.99; green line: BMI 25–29.99; brown line: BMI 30–34.99; purple line: BMI 35–39.99, light green line: BMI >40 (log rank, P = 0.00055). (C) Bar graph of percent mortality by BMI class show U-shaped relationship between unadjusted all-cause mortality and BMI. The nadir for all-cause mortality was seen at BMI = 30–35. N = total number at risk by BMI class. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Table 3.
Baseline disease severity measures by quartile of LDL/HDL ratio.
| LDL/HDL ratio <1.82 | LDL/HDL ratio 1.82–2.27 | LDL/HDL ratio 2.28–2.95 | LDL/HDL ratio >2.95 | ANOVA P-value | |
|---|---|---|---|---|---|
| Number of subjects | 106 | 108 | 105 | 108 | |
| Total ALSFRS-R | 33.46 (8.51) | 36.17 (9.42) | 37.51 (9.06) | 38.66 (7.92) | 0.0002 |
| FVC | 82.56 (18.67) | 84.35 (22.86) | 88.39 (20.04) | 86.19 (16.97) | 0.2 |
| BMI | 24.71 (5.35) | 26.67 (5.27) | 29.08 (13.22) | 29.34 (17.70) | 0.01 |
| Age | 57.72 (12.38) | 55.71 (14.47) | 53.81 (11.27) | 51.71 (11.78) | 0.004 |
Data expressed as mean and standard deviation (SD). LDL, low-density lipoprotein; HDL, high-density lipoprotein; ANOVA, analysis of variance; ALSFRS-R, ALS Functional Rating Scale—revised; FVC, functional vital capacity (percent of predicted); BMI, body mass index. P-values calculated by ANOVA across all quartiles.
Our final multivariate proportional hazards model using stepwise selection did not include any cholesterol variables (Table 4; in Supplementary Material, Table S1, the lipid ratio and ALSFRS-R have been forced into the model). Higher BMI, higher FVC, and younger age were associated with improved survival (Fig. 1B and data not shown), and each of these variables was co-linear with the lipid ratio.
Table 4.
Final proportional hazards model for survival in all 427 ALS subjects.
| Hazard ratio | 95% CI | P-value | |
|---|---|---|---|
| Age | 1.03 | (1.01–1.05) | 0.0009 |
| Cardiovascular history | 2.27 | (1.39–3.69) | 0.0009 |
| Time from symptom onset | 0.99 | (0.99–0.99) | 0.0001 |
| BMI | 0.86 | (0.80–0.93) | 0.0001 |
| BMI2 | 1.001 | (1.000–1.001) | 0.02 |
| FVC | 0.97 | (0.96–0.98) | 0.000007 |
Age, BMI (body mass index), FVC (forced vital capacity), and time since symptom onset are included as continuous variables, whereas cardiovascular disease history is dichotomous. Hazard ratios were calculated using a Cox proportional hazards model with stepwise selection. Interpreting the results, subjects with a reported history of cardiovascular disease were twice as likely to die during the study, whereas there was a 14% reduction in the risk of death during the study with each increasing unit of BMI, with a reduction in survival at the highest levels of BMI.
BMI was significantly associated with survival even after adjusting for disease severity, including ALSFRS-R, FVC, time since symptom onset, time since diagnosis, and bulbar onset, with a dose-dependent increase in survival for each unit of BMI (adjusted hazard ratio 0.86, confidence interval 0.80–0.93, P = 0.0001; Fig. 1B and Table 4). The second-order term (BMI2) was also significant, showing that there is a U-shape to the survival curve, with the maximum survival time seen in subjects with BMI 30–35 (WHO obese class I) (Fig. 1C). A sensitivity analysis was performed, excluding subjects from the Trial of Topiramate in ALS given the potential side effect of weight loss of this medication, which indicated that the differences across strata were still significant (log rank: P = 0.001).
DISCUSSION
The role of cholesterol metabolism in amyotrophic lateral sclerosis (ALS) has been an area of interest since Dupuis et al. suggested that dyslipidemia might be a protective factor in ALS patients.2 In their series, Dupuis et al. reported that a high LDL/HDL ratio (>2.99) was associated with a significant survival advantage, as compared with a ratio of <2.99. However, they did not adjust their results for BMI or weight loss.
We hypothesized that this survival advantage might be due to changes in lipid levels over the course of the disease, and therefore we measured lipids repeatedly over time. Surprisingly, there was no significant change in total cholesterol, LDL levels, or LDL/HDL ratio over 8 months despite a significant decrease in both BMI and FVC. This is despite the fact that our baseline data did show a positive association between the LDL/HDL ratio and ALSFRS-R scores, in support of our original hypothesis. The reasons for this lack of decline could be that our observation period was not long enough or that our sample size was not large enough to detect subtle changes in lipid levels over time.
One possible criticism of our study is that samples were stored at −80°C for up to 10 years prior to use, which raises the question of sample denaturation. However, all samples were stored for similar lengths of time; therefore, there was no differential bias. In addition, the duration of storage was included as a covariate and was not significant in any of our analyses. Because lipid measurements were performed in the same laboratory at the same time under identical conditions, the risk of measurement bias was minimized.
Our unadjusted log-rank test confirmed a positive association between dyslipidemia and survival, in agreement with the studies by Dupuis et al. and Dorst et al.2,4 However, when our results were adjusted for BMI, FVC, and age using a proportional hazards model, the lipid ratio was no longer an independent predictor of survival. Similar to our study, Dorst et al. found that age and BMI were significant confounders of cholesterol levels in their multivariate analysis.4
Interestingly, we found a much stronger association between BMI and survival (adjusted hazard ratio 0.86, P < 0.0001) when compared with findings by Dorst et al.4 The positive correlation between BMI and survival in our cohort was not limited to subjects with abnormally low BMI (i.e., malnourished patients) as described elsewhere.12–17 One possible explanation might be that BMI is simply a marker of disease severity. However, in our cohort, BMI was an independent prognostic factor, even after adjusting for measures of disease severity, including FVC, total ALSFRS-R score, bulbar onset, riluzole use, and duration of disease (time since symptom onset and time since diagnosis). An alternative explanation might be that subjects who were normal weight or underweight are more hypermetabolic than obese subjects, leading to a more rapid decline in BMI.18 However, in our study, there was no difference in the rate of weight loss across the BMI strata (data not shown). Our study was limited by the fact that we did not have pre-morbid weights, nor were we able to measure the degree of weight loss prior to study enrollment.
In our cohort, there was a U-shaped relationship between survival and BMI. The nadir for mortality was seen in subjects who were mildly clinically obese (BMI 30–35, WHO obese class I). One possible explanation for the increased mortality in subjects with morbid obesity (BMI >35) is that this small number of subjects (N = 24) had a high frequency (46%) of cardiovascular disease (also a significant predictor of mortality in our multivariate model). Interestingly, the increase in mortality did not appear to be directly due to cardiovascular events, because, of the 38 total subjects reporting a history of cardiovascular disease, only 3 died from cardiovascular events (stroke or myocardial infarction).
Increased survival from increased nutrition and body mass is consistent with studies in the superoxide dismutase 1 (SOD1) mutant mouse model of ALS, which showed that a hypercaloric diet leads to increased body weight and survival.19,20 The improved survival associated with mild obesity seen in our cohort may have been due to higher baseline energy reserves, differences in the degree of hypermetabolism among subjects, increased cholesterol levels ameliorating abnormal lipid metabolism,21 or differences in pre-morbid physical activity, as hypothesized by Scarmeas et al.22 These findings have possible implications for nutritional counseling and raise the question of whether artificially increasing BMI in ALS patients through hyperalimentation might improve survival (see clinical trial NCT00983983 at www.clinicaltrials.gov).
Acknowledgments
The authors thank Patrick Sluss, PhD, and the staff of the Clinical Laboratory Research Core at Massachusetts General Hospital for performing the biochemical analyses and also for their excellent technical support. We also thank those in the Massachusetts General Hospital Neurology Clinical Trials Unit and the members of the Northeast ALS Consortium (NEALS) for allowing us access to the blood samples and clinical data that were used in this study. Finally, we thank the patients and their families for participating in these clinical trials and for contributing to clinical research. This study was supported by the Muscular Dystrophy Association and the Digiovanni ALS Research Fund.
Abbreviations
- ALS
amyotrophic lateral sclerosis
- ALSFRS-R
ALS Functional Rating Scale—revised
- BMI
body mass index
- FVC
functional vital capacity
- HDL
high-density lipoprotein
- HR
hazard ratio
- LDL
low-density lipoprotein
- NEALS
Northeast ALS Consortium
- SOD1
superoxide dismutase 1
- WHO
World Health Organization
References
- 1.Dupuis L, Pradat PF, Ludolph AC, Loeffler JP. Energy metabolism in amyotrophic lateral sclerosis. Lancet Neurol. 2011;10:75–82. doi: 10.1016/S1474-4422(10)70224-6. [DOI] [PubMed] [Google Scholar]
- 2.Dupuis L, Corcia P, Fergani A, Gonzalez De Aguilar JL, Bonnefont-Rousselot D, Bittar R, et al. Dyslipidemia is a protective factor in amyotrophic lateral sclerosis. Neurology. 2008;70:1004–1009. doi: 10.1212/01.wnl.0000285080.70324.27. [DOI] [PubMed] [Google Scholar]
- 3.Chio A, Calvo A, Ilardi A, Cavallo E, Moglia C, Mutani R, et al. Lower serum lipid levels are related to respiratory impairment in patients with ALS. Neurology. 2009;73:1681–1685. doi: 10.1212/WNL.0b013e3181c1df1e. [DOI] [PubMed] [Google Scholar]
- 4.Dorst J, Kuhnlein P, Hendrich C, Kassubek J, Sperfeld AD, Ludolph AC. Patients with elevated triglyceride and cholesterol serum levels have a prolonged survival in amyotrophic lateral sclerosis. J Neurol. 2011;258:613–617. doi: 10.1007/s00415-010-5805-z. [DOI] [PubMed] [Google Scholar]
- 5.Cudkowicz ME, Shefner JM, Schoenfeld DA, Zhang H, Andreasson KI, Rothstein JD, et al. Trial of celecoxib in amyotrophic lateral sclerosis. Ann Neurol. 2006;60:22–31. doi: 10.1002/ana.20903. [DOI] [PubMed] [Google Scholar]
- 6.Cudkowicz ME, Shefner JM, Schoenfeld DA, Brown RH, Jr, Johnson H, Qureshi M, et al. A randomized, placebo-controlled trial of topiramate in amyotrophic lateral sclerosis. Neurology. 2003;61:456–464. doi: 10.1212/wnl.61.4.456. [DOI] [PubMed] [Google Scholar]
- 7.Brooks BR, Miller RG, Swash M, Munsat TL World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- 8.Ganesalingam J, Stahl D, Wijesekera L, Galtrey C, Shaw CE, Leigh PN, et al. Latent cluster analysis of ALS phenotypes identifies prognostically differing groups. PLoS One. 2009;4:e7107. doi: 10.1371/journal.pone.0007107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zoccolella S, Beghi E, Palagano G, Fraddosio A, Samarelli V, Lamberti P, et al. Predictors of delay in the diagnosis and clinical trial entry of amyotrophic lateral sclerosis patients: a population-based study. J Neurol Sci. 2006;250:45–49. doi: 10.1016/j.jns.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 10.Tisi GM, Conrique A, Barrett-Connor E, Grundy SM. Increased high density lipoprotein cholesterol in obstructive pulmonary disease (predominant emphysematous type) Metabolism. 1981;30:340–346. doi: 10.1016/0026-0495(81)90113-x. [DOI] [PubMed] [Google Scholar]
- 11.Brownell KD, Stunkard AJ. Differential changes in plasma high-density lipoprotein-cholesterol levels in obese men and women during weight reduction. Arch Intern Med. 1981;141:1142–1146. [PubMed] [Google Scholar]
- 12.Desport JC, Preux PM, Magy L, Boirie Y, Vallat JM, Beaufrere B, et al. Factors correlated with hypermetabolism in patients with amyotrophic lateral sclerosis. Am J Clin Nutr. 2001;74:328–334. doi: 10.1093/ajcn/74.3.328. [DOI] [PubMed] [Google Scholar]
- 13.Desport JC, Preux PM, Truong CT, Courat L, Vallat JM, Couratier P. Nutritional assessment and survival in ALS patients. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:91–96. doi: 10.1080/14660820050515386. [DOI] [PubMed] [Google Scholar]
- 14.Desport JC, Preux PM, Truong TC, Vallat JM, Sautereau D, Couratier P. Nutritional status is a prognostic factor for survival in ALS patients. Neurology. 1999;53:1059–1063. doi: 10.1212/wnl.53.5.1059. [DOI] [PubMed] [Google Scholar]
- 15.Desport JC, Torny F, Lacoste M, Preux PM, Couratier P. Hypermetabolism in ALS: correlations with clinical and paraclinical parameters. Neurodegener Dis. 2005;2:202–207. doi: 10.1159/000089626. [DOI] [PubMed] [Google Scholar]
- 16.Kasarskis EJ, Berryman S, Vanderleest JG, Schneider AR, McClain CJ. Nutritional status of patients with amyotrophic lateral sclerosis: relation to the proximity of death. Am J Clin Nutr. 1996;63:130–137. doi: 10.1093/ajcn/63.1.130. [DOI] [PubMed] [Google Scholar]
- 17.Bouteloup C, Desport JC, Clavelou P, Guy N, Derumeaux-Burel H, Ferrier A, et al. Hypermetabolism in ALS patients: an early and persistent phenomenon. J Neurol. 2009;256:1236–1242. doi: 10.1007/s00415-009-5100-z. [DOI] [PubMed] [Google Scholar]
- 18.Jawaid A, Murthy SB, Wilson AM, Qureshi SU, Amro MJ, Wheaton M, et al. A decrease in body mass index is associated with faster progression of motor symptoms and shorter survival in ALS. Amyotroph Lateral Scler. 2010;11:542–548. doi: 10.3109/17482968.2010.482592. [DOI] [PubMed] [Google Scholar]
- 19.Zhao Z, Lange DJ, Voustianiouk A, MacGrogan D, Ho L, Suh J, et al. A ketogenic diet as a potential novel therapeutic intervention in amyotrophic lateral sclerosis. BMC Neurosci. 2006;7:29. doi: 10.1186/1471-2202-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mattson MP, Cutler RG, Camandola S. Energy intake and amyotrophic lateral sclerosis. Neuromol Med. 2007;9:17–20. doi: 10.1385/nmm:9:1:17. [DOI] [PubMed] [Google Scholar]
- 21.Fergani A, Oudart H, Gonzalez De Aguilar JL, Fricker B, Rene F, Hocquette JF, et al. Increased peripheral lipid clearance in an animal model of amyotrophic lateral sclerosis. J Lipid Res. 2007;48:1571–1580. doi: 10.1194/jlr.M700017-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scarmeas N, Shih T, Stern Y, Ottman R, Rowland LP. Premorbid weight, body mass, and varsity athletics in ALS. Neurology. 2002;59:773–775. doi: 10.1212/wnl.59.5.773. [DOI] [PubMed] [Google Scholar]