Abstract
Store-operated calcium entry is a central mechanism in cellular calcium signalling and in maintaining cellular calcium balance. This review traces the history of research on store-operated calcium entry, the discovery of STIM and ORAI as central players in calcium entry, and the role of STIM and ORAI in biology and human disease. It describes current knowledge of the basic mechanism of STIM-ORAI signalling and of the varied mechanisms by which STIM-ORAI signalling can be modulated.
Introduction
Store-operated calcium entry
The idea of store-operated calcium entry developed from studies of calcium signalling in the 1970s and 1980s. The field built on a considerable body of unsung work, but its progress can be traced in a few prominent papers [1–4]. Several conclusions had been firmly established by the early 1980s— that cells possess internal calcium stores, that calcium is released from internal stores in response to certain physiological agonists, that this mobilization of calcium leads to calcium efflux from the cell, and that therefore, over time, external calcium is needed to refill cellular calcium stores. Importantly, it was recognized that cells can be primed for uptake of calcium by the same physiological agonist that releases calcium from internal stores, and that continuing occupancy of the receptor is not needed for calcium uptake. The demonstration that the second messenger inositol 1,4,5-trisphosphate (IP3) releases calcium from ER stores [5] delineated the initial part of the calcium signalling pathway, from receptor through phospholipase C through production of IP3 and calcium release. Revisiting the earlier experiments with this pathway in mind, and with use of the newly available calcium indicator Fura-2, established that enhanced calcium uptake is independent not only of receptor occupancy but also of residual IP3, until internal calcium stores are refilled [6]. The accumulated evidence set the stage for wide acceptance of a model that sensing of store content controls a plasma membrane calcium influx mechanism [7,8].
CRAC current
The proposed store-operated calcium entry mechanism rapidly gained experimental support with the electrophysiological demonstration of the calcium release-activated calcium (CRAC) current in mast cells and T cells [9–11]. Further work reinforced the direct connection of CRAC current to Fcε receptor or T cell receptor engagement and to ER calcium store depletion [12–16]. The CRAC current is characterized inter alia by its responsiveness to store depletion, its small whole-cell current densities, its very small single-channel current, and its extreme selectivity for calcium under physiological conditions. CRAC current is not restricted to mast cells and T cells, though it was more readily detected there, in part because the cells have fewer interfering currents and in part because of dedicated experimentation. A current with the same characteristics has since been detected in many cell types. It is worth noting specifically the identification of CRAC current in Drosophila S2 cells [17] because of their role in RNA interference (RNAi) screens described below. Despite the distinctive electrophysiological fingerprint, the basis of the classical CRAC current in STIM and ORAI proteins was not identified for many years.
Less selective channels
This review focuses on the calcium-selective STIM-ORAI-dependent CRAC current. However, the STIM-ORAI pathway is not the only source of calcium for refilling of ER stores. It has long been recognized that other less selective calcium channels are activated upon depletion of ER calcium stores in some cell types [18], and particular attention has been given to the possible role of TRPC channels. This has been a controversial area, since it is clear that TRPC channels are not directly controlled by store depletion under many conditions of stimulation [19]. Nonetheless, there is now evidence that STIM controls the activation of certain TRPC channels [20,21] and that STIM-ORAI signalling controls insertion of TRPC1 channels into the plasma membrane in salivary gland cells [22]. The contribution of TRPC channels to store-operated calcium entry has been reviewed recently in [23].
Discovery of STIM and ORAI
RNAi screens
RNAi technology opened the way to the next major advances in the field. In 2005, two RNAi screens in Drosophila S2 cells and human HeLa cells focused on subsets of candidate genes and, using the cytoplasmic calcium signal as a readout, discovered the essential role of STIM proteins in store-operated calcium entry [24,25]. RNAi technology had developed at that time to the point that Drosophila cells could be used in genome-wide RNAi screening. In 2006, three genome-wide screens in Drosophila S2 cells, with either calcium entry or sustained signalling to the calcium-dependent transcription factor NFAT as readout, identified ORAI proteins as essential to store-operated calcium entry [26–28]. Drosophila has only a single Orai protein, whereas humans have three, and the NFAT-based screen in Drosophila cells was supported by parallel genetic mapping to human ORAI1 of a SCID mutation that impairs calcium influx in T cells [26].
Required for CRAC current
CRAC current had been defined originally in T cells and mast cells, and therefore it is important that several reports verified that STIM1 and ORAI1 were essential contributors to the classical CRAC current in T cells and mast cells. RNAi-mediated knockdown of STIM1 reduced store-operated calcium entry and CRAC current in human Jurkat T cells [24,25], and Stim1 deficiency impaired calcium influx in mouse mast cells and CRAC current in mouse T cells [29,30]. In the latter cases, calcium influx was restored by expression of Stim1 [29,30]. The strongest evidence linking ORAI1 to CRAC current was the absence of CRAC current in T cells of human SCID patients homozygous for the ORAI1(R91W) mutation, and restoration of the current by expression of wildtype ORAI1 in those cells [26]. Likewise, differentiated Orai1−/− T cells and Orai1−/− mast cells from mice had greatly reduced CRAC currents compared to wildtype controls [31,32], and normal T cell calcium influx was restored by expressing Orai1 [32]. Consistent with the broad expression of CRAC current, calcium signalling was also affected by Stim1 or Orai1 deficiency in other cell types in mice [32–36]. The fact that not all the cell types that have CRAC current showed a detectable impairment attests to the redundancy of STIM and ORAI proteins or to compensation by other calcium channels.
Pathology
Additional evidence on the role of STIM-ORAI calcium signalling in immunity has accumulated from a few human kindreds where STIM1 or ORAI1 loss-of-function mutations cause immunodeficiency [reviewed in 37]. In one specific instance, a human patient with STIM1-related immunodeficiency also exhibited a detectable impairment of platelet function [38]. Other heritable alterations in STIM1 or ORAI1 present as dominant, gain-of-function mutations. The first reported dominant mutation in STIM or ORAI— aside from mutations intentionally engineered into the proteins— was in a line of mice derived after chemical mutagenesis with N-ethyl-N-nitrosourea [39]. The heterozygous mice exhibited abnormal platelet function, interpreted as due to a partial constitutive activation of Stim1. Subsequently, various dominant mutations in human STIM1 and human ORAI1 have been shown to cause tubular aggregate myopathy, with one family having the same dominant STIM1 D84G mutation identified in the mice [40–43], and a separate dominant STIM1 R304W mutation has been shown to underlie Stormorken syndrome [41,44,45].
STIM and ORAI proteins
Description: STIM proteins
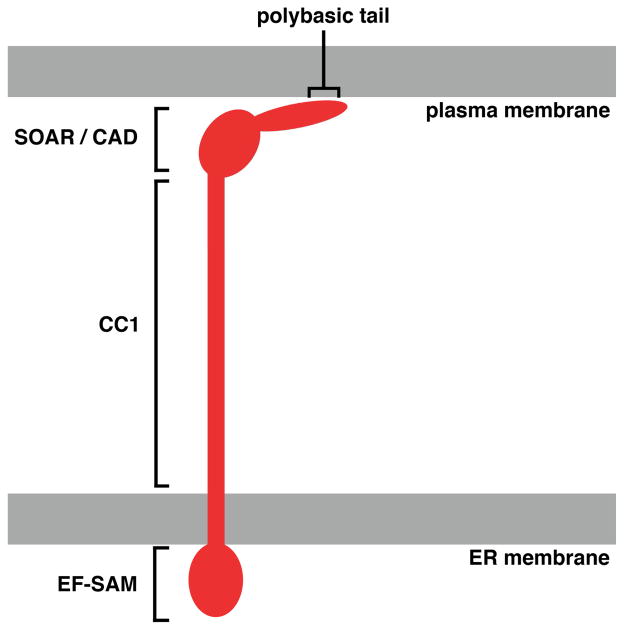
There are two human STIM proteins, STIM1 and STIM2 [reviewed in 46]. Both are predominantly located in the ER, though a minor amount of STIM1 is expressed at the cell surface [25,47–49]. ER-localized STIM controls CRAC channel gating [25,50–53]. STIM1 and STIM2 are similar in overall architecture, with an N-terminal domain in the ER lumen, a single transmembrane segment anchoring the protein in the ER, and a C-terminal cytoplasmic domain. Key subregions of the cytoplasmic domain— CC1, SOAR/CAD, and a polybasic segment at the C-terminus— are indicated in [FIGURE 1] and discussed later. The isolated recombinant STIM1 cytoplasmic domain is a dimer [54], as are its functional fragments that include the SOAR/CAD domain [55–58], and full-length STIM is believed to exist as a dimer in unstimulated cells [46,59]. Some insight has been obtained into the structure of specific STIM regions. NMR structures have been reported for the isolated ER-luminal domains of both STIM1 and STIM2 with calcium bound to an EF-hand [60,61]. A crystal structure of the SOAR/CAD fragment of the STIM1 cytoplasmic domain— which was defined as the minimal fragment able to activate the ORAI channel [56,62,63]— and an NMR structure of a separate overlapping fragment represent two different conformations of this part of the STIM protein [58,64].
FIGURE 1.
Cartoon view of STIM1 in an extended conformation, bridging the distance from ER to plasma membrane. For clarity, a single STIM monomer is shown, but the active extended form in cells is oligomeric. Functional regions of STIM discussed in the text are the calcium-sensing EF-SAM domain in the ER lumen, the cytoplasmic CC1 region that both stabilizes inactive STIM and transmits the activating conformational change upon ER calcium store depletion, the SOAR/CAD domain that recruits and gates ORAI channels, and the polybasic tail that interacts with plasma membrane phosphoinositides.
Description: ORAI proteins
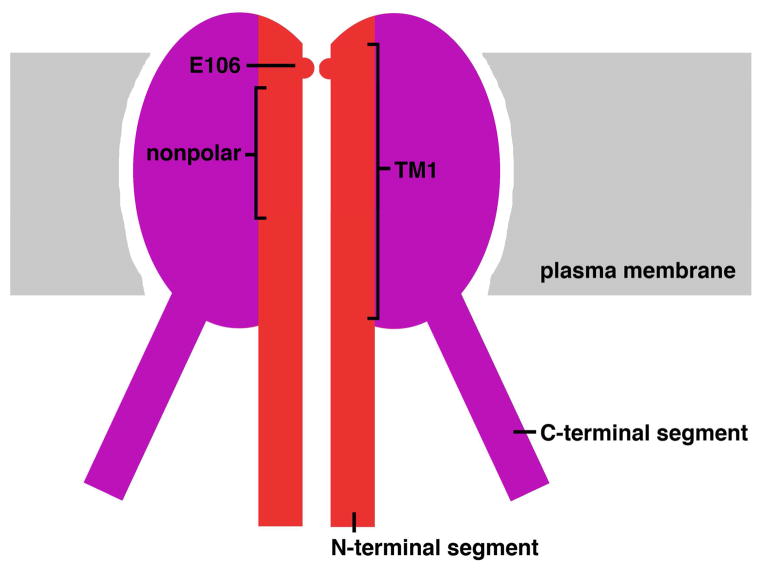
There are three human ORAI proteins, ORAI1–3 [reviewed in 65–68]. Each ORAI monomer has four transmembrane helices, with 81–87% pairwise sequence identity between human ORAI-family proteins in the transmembrane portions, and complete sequence identity in the functionally critical transmembrane helix 1 (TM1) [FIGURE 2]. The cytoplasmic N- and C-terminal portions of the human ORAI proteins [27,69] also exhibit considerable sequence conservation in the segments concerned with direct STIM-ORAI interaction. The basis of STIM-ORAI signalling is discussed in more detail below. A crystal structure of the closed Drosophila Orai channel shows that it is hexameric [70]. The close similarity between the transmembrane sequences of Drosophila Orai and human ORAI1 proteins suggests that the human CRAC channel also assembles as a hexamer, although there has been some support for the view that it is a tetramer [reviewed in 71].
FIGURE 2.
Cartoon view of the ORAI1 channel. Only two subunits of the hexameric channel are depicted. Labelled are the pore-lining transmembrane helices (TM1), the E106 residues that form a calcium-binding site, the nonpolar region of the pore that constitutes the main barrier to ion flux, and the C- and N-terminal cytoplasmic segments that interact with STIM proteins.
Cell biology
The cell biology of STIM-ORAI signalling is straightforward. Upon calcium store depletion, STIM moves within the ER to ER-plasma membrane junctions [25,48,50–52,72]. Targeting of STIM to these sites, observed as ‘puncta’ by light microscopy, is independent of ORAI [25,48,50,52,62]. STIM then rapidly recruits ORAI to these ER-plasma membrane junctions [52,73,74], where the two proteins are in close contact as indicated by STIM-ORAI FRET using appropriate labels [75–78]. The development of CRAC current lags behind the movement of STIM by a few seconds when examined at high time resolution [50], probably reflecting the additional slow step of recruiting ORAI channels. The relocalization of STIM and ORAI correlates in time with CRAC current and in space with calcium influx [25,50,73]. Overexpression of human STIM and ORAI in mammalian cells or of Drosophila Stim and Orai in insect cells results in large CRAC currents upon store depletion [28,51,79,80], indicating that only STIM and ORAI are limiting for CRAC current.
Definitive functions of STIM
The established functions of STIM proteins are sensing calcium in the ER lumen and communicating store depletion to other proteins, including ORAI, in the plasma membrane. A simple demonstration of calcium sensing is that D76A, E87Q, and other replacements in the calcium-binding STIM1 EF-hand that lower its affinity for calcium result in a movement of STIM to ER-plasma membrane junctions resembling that elicited by store depletion and in constitutive calcium influx [25,48,51,81]. On more detailed investigation, STIM relocalization to puncta displays a steep dependence on ER calcium concentration, occurring in the concentration range 100–300 μM for STIM1 and 200–400 μM for STIM2 [82,83]. The evidence that STIM1 communicates a signal to ORAI is equally compelling. The earliest detectable effect of store depletion on STIM1 is a conformational change or ‘oligomerization’ [72,75] that correlates closely with CRAC current [82]. Artificial oligomerization of STIM1 also elicits STIM1 movement to ER-plasma membrane junctions and gating of CRAC channels [82]. Expression of the STIM1 cytoplasmic domain, physically uncoupled from ER and from control by ER-luminal calcium, activates endogenous CRAC current constitutively in Jurkat T cells, RBL cells, and Stim1−/− mouse T cells [20,54,55,84]. The overall conclusion is that STIM1 is a calcium-regulated activator of the CRAC channel. STIM2 functions in a similar way to STIM1, with the differences that STIM2 is particularly sensitive to changes in calcium concentration near basal ER calcium levels [83], and that STIM2 is less effective than STIM1 in activating calcium influx [85,86]. Its cellular functions are to regulate basal cytoplasmic and ER calcium levels and to contribute in some cases to receptor-initiated signalling [30,83,87,88].
Definitive functions of ORAI
ORAI proteins are the monomeric channel subunits of the CRAC channel and other calcium channels. This characterization was first supported by the finding that an E106D replacement in the ORAI1 pore, or a corresponding replacement in the Drosophila Orai pore, alters ion selectivity [89–91]. It gained further strong support from the demonstration that ORAI1 expressed in the yeast S cerevisiae and isolated in sealed membrane vesicles can be gated by recombinant STIM C terminus to release calcium from the vesicles [54]. S cerevisiae has no ER-based calcium signalling or STIM-ORAI signalling, and hence the implication is that no other dedicated mammalian plasma membrane protein is required for channel function. Later a purified Drosophila Orai(V174A) channel, corresponding to the human ORAI1(V102A) mutant that conducts both sodium and calcium constitutively, was reconstituted into liposomes and shown to support sodium efflux from the liposomes [70]. Finally, purified human ORAI1 has been reconstituted into liposomes and supports calcium efflux when gated with recombinant STIM1 C terminus [92]. This latter experiment is the definitive demonstration that channel function needs only ORAI1 and gating by STIM1. All three ORAI proteins can function as STIM-operated channels when coexpressed with STIM1 in mammalian cells [51,93–96]. Native ORAI3 homomeric channels that are gated by STIM have been described [97,98], although it is not clear that they represent a major component of calcium signalling in most cells. Native ORAI1/ORAI3 heteromeric channels, gated by arachidonic acid or leukotriene C4, may be widespread [99–104]. STIM1 is required for function of the arachidonic acid/leukotriene-gated channels, but they are not store-operated. The physiological function of ORAI2 remains unclear. A systematic analysis of cytoplasmic calcium regulation in resting cells detected a contribution of ORAI2 to ER calcium leak and attributed it to nascent ORAI2 channels in the ER destined for the plasma membrane [105]. This need not reflect the major role of ORAI2 channels in cells, since the experiments were designed to examine calcium balance in resting cells and calcium influx after treatment with thapsigargin, and hence it is possible that the normal physiological stimulus for ORAI2 was not provided.
STIM activation
Calcium sensing
Structures of the calcium-bound forms of STIM1 and STIM2 luminal domains have been reported [60,61]. Each consists of a pair of EF-hands, only one of which binds calcium, and a sterile alpha motif (SAM) domain tightly interacting with the broad nonpolar surface presented by the ‘open’ EF-hands. The relative affinities of the STIM1 and STIM2 EF-SAM domains for calcium correlate with the relative responsiveness of STIM1 and STIM2 in cells to ER calcium store depletion [61]. The isolated STIM1 and STIM2 EF-SAM domains undergo a substantial loss of secondary structure, exposure of nonpolar surface, and aggregation upon dissociation of calcium [106–108]; and, partly for this reason, no structure of a calcium-free EF-SAM domain has been obtained thus far. The isolated EF-SAM domains of the two proteins do not behave identically, in that loss of secondary structure and aggregation are appreciably slower in the case of STIM2 [107]. Aggregation may be an artifact of detaching the EF-SAM domain from its linkage to the ER, and is probably not a direct correlate of the conformational change and oligomerization observed in cells, since other work suggests that physiological oligomerization is largely due to the cytoplasmic domain of STIM [55,59,109]. Besides protein aggregates, dimers of the STIM1 EF-SAM domain are observed in the absence of calcium [60,106], and under certain conditions are the predominant form [110]. STIM luminal domain dimers may be significant for the activating conformational change described below.
Conformational change
The similarity between the STIM C-terminal polybasic segment and PIP2-binding segments in other proteins led to an early proposal that STIM targeting to ER-plasma membrane junctions is driven by STIM oligomerization and a consequent increased avidity for plasma membrane polyphosphoinositides [72]. The notion that STIM proteins can bind polyphosphoinositides proved correct [111–114], and increased avidity probably promotes STIM targeting to ER-plasma membrane junctions at native levels of STIM and ORAI. However, accumulating evidence also indicated that the first step in activation is a conformational change [57,115]. The replacements L248S or L251S in the STIM1 CC1 region led to a conformational change or physical extension of the cytoplasmic domain fragment STIM1(233-474) and resulted in more effective interaction with ORAI1 [57]. Lesser, but still appreciable, changes were observed with certain single-residue replacements within the SOAR/CAD domain, or with the combined replacements 318EEELE322 > AAALA within CC1 [57]. The latter engineered STIM1, termed E4A [115], calls attention to a part of the STIM1 linear sequence where the Y316A replacement also causes constitutive activation [116] and the R304W mutation causes Stormorken syndrome [41,44,45]. The variety of replacements that reduce the stability of the STIM1 inactive state points to extensive changes in buried surface in the transition from resting to active STIM, but not necessarily to strong intramolecular interactions. Both the L251S and E4A replacements lead to a specific conformational change measurable in the STIM C-terminal cytoplasmic domain, STIM1(233-685), as a physical extension from 3–4 nm to >9 nm [113]. Analysis of wildtype, L251S, and related engineered STIM1 proteins provided the connection between ER-luminal calcium sensing and physiological activation of STIM1— STIM1 luminal domain dimerization buries a surface containing L251, and thereby releases the polybasic tail of STIM1 to interact with plasma membrane lipids, releases the SOAR/CAD domain to interact with ORAI, and changes the preferred conformation of the STIM1 cytoplasmic domain to extended [113]. Additional critical evidence on intramolecular interactions within the STIM1 cytoplasmic domain, from FRET assays in cells [59], has permitted a more detailed interpretation. The inactive conformation of STIM is maintained principally through a CC1α1-CC3 interaction, which both retains the functional regions of STIM1 near the ER and away from their targets in plasma membrane and masks the regions needed for STIM oligomerization [59,113]. ER calcium store depletion initiates activation by the dimerization of paired luminal domains, which favors burial of the CC1α1 surface that engages CC3 in the inactive conformation, releasing the regions that will interact with the plasma membrane and ORAI and priming the STIM cytoplasmic region for further oligomerization through CC3-CC3 interactions [59,113].
Relocalization
Relocalization of STIM to ER-plasma membrane junctions is the visible consequence of conformational change. The relocalization proceeds in parallel with further oligomerization of STIM through the SOAR/CAD domain [55,59], which will support STIM targeting to the plasma membrane [72]. The ease of STIM1 activation in the absence of a stimulus, on STIM overexpression, suggests that STIM is poised for the activating conformational change even at rest. Indeed, one interpretation of the distance measurements made with STIM1 cytoplasmic domain is that STIM monomers are constantly sampling the extended conformation with low probability [113]. In this case, STIM1 overexpression may drive oligomerization by increasing the probability that STIM1 dimers in which both monomers are coincidentally transiently active will encounter another active STIM1 dimer before reverting to the inactive state. Relocalization to the ER-plasma membrane junction is a local process that occurs by diffusion of STIM in the ER membrane [72,117]. The effectiveness of local store depletion in activating STIM-ORAI signalling has important implications for physiological signalling. STIM is targeted both to plasma membrane lipids and to ORAI— in the absence of ORAI or at low levels of ORAI relative to STIM, the STIM polybasic tail is required for relocalization to puncta [62,72], presumably reflecting the interaction of the polybasic tail with PIP2 [111–114]. Single-molecule tracking shows that, at low levels of ORAI, diffusing STIM1 is effectively trapped by this interaction with the plasma membrane [118]. Formation of STIM puncta is further stabilized by interaction of STIM with ORAI [62,118].
ORAI activation
Recruitment to ER-plasma membrane junctions
ORAI1 is recruited upon calcium store depletion into clusters that overlap STIM1 puncta [50,52,73]. ORAI moves to these sites by diffusion in the plasma membrane and becomes ‘trapped’ reversibly by its interaction with STIM [118,119]. An intact ORAI C terminus is required for STIM-dependent relocalization of ORAI [62,74,75]. The physical basis of ORAI recruitment has been directly demonstrated in the binding of an ORAI1 C-terminal peptide to STIM1 [54,62,75], and the role of the binding interaction in ORAI recruitment has been substantiated by the effects of point mutations in this region of ORAI [75,76,120]. The NMR structure of a STIM fragment-ORAI C terminal peptide complex has two ORAI C-terminal peptides binding across a STIM fragment dimer in a way that explains the effects of the previously reported point mutations [64]. Comparison of that STIM-ORAI peptide NMR structure with the earlier SOAR/CAD domain crystal structure [58] indicates that the STIM1 SOAR/CAD domain helices— freed by the activating conformational change in STIM1— rearrange to a conformation that can engage the ORAI C-terminal helices [59,64].
Gating
ORAI clustering in itself is not sufficient for gating. Channels missing the N-terminal cytoplasmic portion of ORAI1 are recruited into clusters in cells but fail to conduct current [74,75]. Extensive mapping of the N-terminal region necessary for gating in ORAI1 and ORAI3 has implicated the segments ORAI1(74-91) and ORAI3(48-65) [56,74,75,121–123]. The role of the STIM-ORAI N terminus interaction in gating in cells has been well documented [92,124–126]. There is further evidence that STIM binding to the ORAI C terminus orients STIM for productive binding to the N terminus [124]. The basis for the gating interaction has been demonstrated in direct binding of recombinant STIM1 to the expressed ORAI1 N-terminal fragment or to a synthetic ORAI N-terminal peptide [54,62,92]. The STIM-dependent gating conformational change has been recapitulated in vitro with purified STIM1 and ORAI1, and— consistent with the experiments in cells— the gating conformational change in vitro is prevented by mutations that impair STIM1-ORAI1 peptide binding and is competed by synthetic ORAI N-terminal peptide [92]. The effects of a series of STIM1(F394X) replacements on STIM1-ORAI1 interaction and gating may suggest that the ORAI N-terminal peptides occupy part of a composite site on STIM1 adjacent to the pocket occupied by ORAI C-terminal peptides [86], but the structure of the STIM complex with ORAI N-terminal peptide remains to be determined, and the STIM-ORAI stoichiometry in a productive interaction is unknown. The literature on ORAI recruitment and gating has been reviewed in detail [127,128].
Conductance pathway
The pore calcium-binding site was first defined electrophysiologically based on the effects of mutations at E106 in human ORAI1 or corresponding mutations in Drosophila Orai [89–91,129,130]. The picture of the pore was refined by targeted introduction of cysteine residues, and examination of either block of the ORAI1 current by cadmium [131] or oxidative crosslinking of the introduced cysteines [132]. The conclusion from these studies was that the ORAI1 pore is lined along its entire length by the conserved TM1 helices, in agreement with the packing of TM1 helices later observed in the Drosophila Orai structure [70]. In brief, the conductance pathway consists of an external vestibule, the calcium-binding site at E106, a nonpolar region lined by the section of the TM1 helices immediately internal to the calcium-binding site, and a more flexible inner region of the TM1 helices [70,131,132]. The state-dependent accessibility of the engineered cysteine in ORAI1(G98C/E106D) indicated the presence of an external gate near V102 [133], and this conclusion has been strengthened by the findings that the nonpolar region of the pore containing V102 constitutes a barrier to ion permeation and that gating of purified ORAI1 by recombinant STIM1 induces movement near V102 and E106 [92]. The latter finding may explain the previously noted close coupling of gating and ion selectivity [129,133]. Since STIM must bind the cytoplasmic regions ORAI1(66-91) to initiate gating, these segments may function in parallel as an internal gate [70,134], although a barrier function for this part of the ORAI1 channel has not yet been demonstrated.
STIM-ORAI modulation
Membrane potential
Although ORAI channel gating is indifferent to membrane potential, the inward calcium current through open channels shows a steep dependence on membrane potential under physiological conditions. In experimental studies of calcium influx into intact cells, depolarization with elevated potassium is sometimes used to remove this variable. A practical implication is that calcium influx through ORAI channels can be modulated by physiological activation of nonselective cation channels, such as TRPM4 channels [135,136], or by closure of potassium channels [137]. This feature of ORAI channels has raised the possibility of therapeutic use of potassium channel inhibitors [138–141].
Calcium
Calcium-dependent negative feedback was noted in the earliest electrophysiological studies of the CRAC channel [12]. This negative feedback is generally parsed into two forms, fast calcium-dependent inactivation occurring on a time course of tens to hundreds of milliseconds, and slow calcium-dependent inactivation, on a time course tens to hundreds of seconds [142–145]. In fast inactivation, calcium entering through a CRAC channel has a highly local action, negatively regulating the same channel [142]. Fast inactivation depends on calmodulin and on a segment of STIM cytoplasmic domain adjacent to, but not part of, the SOAR/CAD domain that is essential for channel gating [146]. The presence or absence of fast calcium-dependent inactivation has also been shown to depend on the relative levels of STIM and ORAI expressed in a cell [147]. Slow calcium-dependent inactivation reflects both refilling of ER calcium stores and other processes [143,144]. The first insights into one such process have come with the discovery that the STIM-associated protein SARAF facilitates slow calcium-dependent inactivation [148,149]
pH
ORAI1 channels are inhibited by mildly acidic pH, with very substantial block of the current at pH 6.0–6.5 [150–152]. Conversely, some increase in current is seen with mild alkalinization. This sensitivity to pH changes in the physiological range may alter calcium signalling at tissue sites where pH is lowered, for example in inflammation, ischemia, or the tumor microenvironment, or in the normal functioning of the kidney. In an interesting sidelight, one distinguishing feature of the heteromeric ORAI1/ORAI3 ARC channel is that it is substantially less sensitive than the CRAC channel to a reduction in pH from 7.4 to 6.7 [99].
Phosphorylation
STIM1 was shown to be a phosphoprotein long before its role in store-operated calcium entry was defined. Only a few studies have defined phosphorylation sites by mass spectrometry of endogenous STIM1, however; instead, phosphorylation sites and physiological outcomes have been investigated primarily through analysis of cells expressing mutant STIM1 with various nonphosphorylatable serine-to-alanine or phosphomimetic serine-to-glutamate substitutions. The best-characterized sites are present at the C-terminus of STIM1, occur in a Ser/Thr-Pro sequence context, and are phosphorylated by proline-directed kinases such as ERK1/2, p38 MAPK, and mitotic kinases [153; reviewed in 154,155]. STIM1 is phosphorylated during store depletion by thapsigargin as well as in response to physiological stimulation, and this is generally thought to be an inhibitory modification. Reported physiological outcomes include the suppression of store-operated calcium entry by phospho-STIM1, most notably during mitosis; and dissociation of phospho-STIM1 from the plus-end microtubule tracking protein EB1, a process that appears to be required for exclusion of endoplasmic reticulum membranes from the mitotic spindle and for cell migration [reviewed in 154,155]. One study also suggested that tyrosine phosphorylation of STIM1 in platelets was important for store-operated calcium entry, but the conclusions relied mainly on the use of Src-family kinase inhibitors and the tyrosine phosphorylation sites on STIM1 have not yet been identified [156].
A recent study showed that STIM1 with an alanine substitution at a specific threonine residue, Thr389, was unable to activate arachidonic acid-activated “ARC” channels – heteromultimers of ORAI1 and ORAI3 in the plasma membrane – while remaining capable of activating ORAI1 CRAC channels that are responsive to store depletion [157]. Thr389 lies in a consensus sequence for phosphorylation by the cyclic AMP-activated protein kinase A (PKA), and overexpression of a phosphomimetic version, STIM1 T389E, permitted (although it did not increase) arachidonic acid-activated ARC channel activity while diminishing CRAC channel activity. These are intriguing data, which should be extended by determining (i) the mechanism by which Thr389-phosphorylated STIM1 selectively activates ARC channels and (ii) the actual phosphorylation status of STIM1 in resting, store-depleted and arachidonic acid-activated cells and in cells in which cAMP levels are pharmacologically elevated. ARC and CRAC channels are activated by plasma membrane-localised and ER-resident STIM1 respectively, and the authors provide evidence for an interesting mechanism that involves the reversible phosphorylation of plasma membrane STIM1 via anchoring of PKA and its counter-enzyme, the phosphatase calcineurin, to the A kinase-anchor protein AKAP79 at the plasma membrane [157].
The protein kinase C (PKC) family member, PKCβ1, was shown to phosphorylate two N-terminal residues (S27 and S30) of ORAI1 in a calcium-dependent manner, based on the fact that serine-to-alanine substitutions at these positions diminished the phosphorylation of recombinant ORAI1 fragments by PKCβ1 in vitro as well the phosphorylation level of full-length ORAI1 in cells [158]. As observed for STIM1 C-terminal phosphorylations, ORAI1 phosphorylation at these N-terminal residues also appears to be an inhibitory modification, based on the fact that PKC inhibition, RNAi-mediated depletion of PKCβ1, and overexpression of the ORAI1 S27,30A mutant in cells depleted of endogenous ORAI1 all increase store-operated calcium entry [158; reviewed in 159]. It is likely, though not yet proven formally, that these inhibitory phosphorylations of STIM1 and ORAI1 represent normal inhibitory feedback mechanisms that operate in physiologically stimulated cells.
Oxidative modifications
In the DT40 chicken B cell line as well as in mouse embryonic fibroblasts, induction of oxidative stress by treatment with hydrogen peroxide, or depletion of glutathione with an inhibitor of gamma-glutamyl synthetase (the rate-limiting enzyme for glutathione synthesis), resulted in an increased influx of extracellular calcium that was dependent on STIM1-ORAI1 signalling since it was not observed in cells lacking STIM1 [160]. Under these conditions, STIM1 became modified by S-glutathionylation at cysteine-56. Notably (and surprisingly), an alanine substitution at Cys-56 mimicked the effect of oxidative stress. Because Cys-56 is located just N-terminal to the ER-luminal EF hand, the authors suggest that both S-glutathionylation and alanine substitution decrease the affinity of STIM1 for calcium in the ER lumen, thus mimicking the effect of store depletion. In fact, both oxidative stress and the C56A mutation caused STIM1 to reorganize into puncta and colocalize with ORAI1 at ER-plasma membrane junctions, thus explaining the enhancement of basal and stimulated calcium influx.
In contrast, acute induction of oxidative stress by preincubation with hydrogen peroxide was shown to decrease thapsigargin-induced STIM1-ORAI1 signalling in transfected HEK cells as well as in Jurkat T cells [161]. STIM1-ORAI3 signalling was far less affected, and the authors traced the difference to the presence of a cysteine residue (Cys-195) near the C-terminal end of the third transmembrane segment in ORAI1 that is absent in ORAI3. It remains to be determined whether the opposing effects of oxidative stress on STIM1 and ORAI1 signalling are observed simultaneously in the same cell types, and if so, to what extent they balance each other out under different stimulation conditions.
Protein-protein interactions
This topic has been comprehensively covered in recent reviews [reviewed in 159,162], and so only a few selected interactors will be described briefly here. (i) CRACR2A was identified, through affinity purification of overexpressed FLAG-tagged ORAI1, as an EF hand-containing protein that associated with ORAI1 following ER Ca2+ store depletion and potentiated store-operated Ca2+ entry [163]. CRACR2A interacts with the same ORAI N-terminal peptide segment as does STIM1, and also itself binds STIM1. Depletion of CRACR2A diminished store-operated Ca2+ entry, whereas overexpression of an EF-hand mutant of CRACR2A incapable of binding Ca2+ caused STIM1 to form puncta and enhanced Ca2+ influx [163]. The authors provide evidence suggesting that CRACR2A stabilizes ORAI1-STIM1 complexes at low intracellular Ca2+ concentrations, thus transiently enhancing STIM-ORAI interaction, but dissociates from the ORAI1-STIM1 complex at high Ca2+ concentrations. (ii) SPCA2, a secretory pathway Ca2+-ATPase, regulates the sequestration of Ca2+ in the Golgi lumen and is overexpressed in many breast cancers [164]. Depletion of SPCA2 in cell lines decreased, whereas overexpression of SPCA2 increased, basal intracellular concentrations of Ca2+, which correlated strongly with cell proliferation. The authors showed that a fraction of plasma membrane-localized SPCA2 bound directly to ORAI1 and mediated Ca2+ entry in a manner independent of STIM1 and ER Ca2+ store depletion. The exact mechanism by which this occurs remains to be elucidated. (iii) SARAF (TMEM66), a single-pass transmembrane protein in the ER membrane, colocalizes with STIM at ER-plasma membrane junctions and inhibits STIM-ORAI communication [148]. Depletion of SARAF increased, whereas SARAF overexpression decreased, basal Ca2+ levels, and store-operated Ca2+ entry after store depletion. Electrophysiological studies in cells depleted of or overexpressing SARAF point to an involvement in slow Ca2+-dependent inactivation.
Membrane nanodomains
A new and enticing element in STIM-ORAI signalling is the involvement of plasma membrane nanodomains. One thread of this story emerged in the observation that delivery of phosphatidylinositol 5-phosphatase to ‘ordered lipid regions’ inhibited STIM-ORAI signalling, whereas delivery of the phosphatase to ‘disordered lipid regions’ did not [165]. At a minimum, this result— paired with the divergent effects of overexpressing the PI4P 5-kinases Iβ and Iγ on STIM1-ORAI1 association in response to store depletion— implicated local phosphoinositide levels in controlling STIM-ORAI interaction and function. A second thread is that rearrangement of septin scaffold proteins and PIP2 at ER-plasma membrane junctions is an early consequence of store depletion and is necessary for efficient STIM-ORAI interaction [166]. The specific roles of septins and PIP2 at ER-plasma membrane junctions have not been sorted out, nor have other proteins and lipids at the junctions been examined in the same way, so the appropriate tentative conclusion is that the observed rearrangements may be an indication of a broader local reorganization of proteins and lipids. The most recent piece of this developing story is evidence that physically distinct nanodomains control productive STIM-ORAI interaction and the slow calcium-dependent inactivation involving SARAF [149].
Conclusion
This review has focused on the process of store-operated calcium entry. It has introduced STIM and ORAI proteins as a concrete basis for this process, and explored the cellular and biochemical mechanisms through which STIM and ORAI sense and respond to ER calcium store depletion, and some of the ways that STIM-ORAI signalling is modulated. Inevitably, a number of important papers have not found a place in this brief narrative, but the papers cited will serve as a solid framework for further reading in this area.
Acknowledgments
Work in the authors’ laboratories on STIM-ORAI signalling, including preparation of this review, is supported by US National Institutes of Health grants AI 084167, AI 040127, and GM 110397.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.van Breemen C, Farinas BR, Gerba P, McNaughton ED. Excitation-contraction coupling in rabbit aorta studied by the lanthanum method for measuring cellular calcium flux. Circ Res. 1972;30:44–54. doi: 10.1161/01.res.30.1.44. [DOI] [PubMed] [Google Scholar]
- 2.Putney JW., Jr Muscarinic, alpha-adrenergic and peptide receptors regulate the same calcium influx sites in the parotid gland. J Physiol. 1977;268:139–149. doi: 10.1113/jphysiol.1977.sp011851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parod RJ, Putney JW., Jr The role of calcium in the receptor mediated control of potassium permeability in the rat lacrimal gland. J Physiol. 1978;281:371–381. doi: 10.1113/jphysiol.1978.sp012428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casteels R, Droogmans G. Exchange characteristics of the noradrenaline-sensitive calcium store in vascular smooth muscle cells of rabbit ear artery. J Physiol. 1981;317:263–279. doi: 10.1113/jphysiol.1981.sp013824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Streb H, Irvine RF, Berridge MJ, Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol 1,4,5-trisphosphate. Nature. 1983;306:67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- 6.Takemura H, Putney JW., Jr Capacitative calcium entry in parotid acinar cells. Biochem J. 1989;258:409–412. doi: 10.1042/bj2580409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Putney JW., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 8.Putney JW., Jr Capacitative calcium entry revisited. Cell Calcium. 1990;11:611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- 9.Penner R, Matthews G, Neher E. Regulation of calcium influx by second messengers in rat mast cells. Nature. 1988;334:499–504. doi: 10.1038/334499a0. [DOI] [PubMed] [Google Scholar]
- 10.Matthews G, Penner E, Neher E. Second messenger-activated calcium influx in rat peritoneal mast cells. J Physiol. 1989;418:105–130. doi: 10.1113/jphysiol.1989.sp017830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis RS, Cahalan MD. Mitogen-induced oscillations of cytosolic Ca2+ and transmembrane Ca2+ current in human leukemic T cells. Cell Regul. 1989;1:99–112. doi: 10.1091/mbc.1.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355:353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- 13.Hoth M, Penner R. Calcium release-activated calcium current in rat mast cells. J Physiol. 1993;465:359–386. doi: 10.1113/jphysiol.1993.sp019681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zweifach A, Lewis RS. Mitogen-regulated Ca2+ current of T lymphocytes is activated by depletion of intracellular Ca2+ stores. Proc Natl Acad Sci USA. 1993;90:6295–6299. doi: 10.1073/pnas.90.13.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Partiseti M, Le Deist F, Hivroz C, Fischer A, Korn H, Choquet D. The calcium current activated by T cell receptor and store depletion in human lymphocytes is absent in a primary immunodeficiency. J Biol Chem. 1994;269:32327–32335. [PubMed] [Google Scholar]
- 16.Zhang L, McCloskey MA. Immunoglobulin E receptor-activated calcium conductance in rat mast cells. J Physiol. 1995;483:59–66. doi: 10.1113/jphysiol.1995.sp020567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yeromin AV, Roos J, Stauderman KA, Cahalan MD. A store-operated calcium channel in Drosophila S2 cells. J Gen Physiol. 2004;123:167–182. doi: 10.1085/jgp.200308982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 19.DeHaven WI, Jones BF, Petranka JG, Smyth JT, Tomita T, Bird GS, Putney JW., Jr TRPC channels function independently of STIM1 and Orai1. J Physiol. 2009;587:2275–2298. doi: 10.1113/jphysiol.2009.170431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang GN, Zeng W, Kim JY, Yuan JP, Han L, Muallem S, Worley PF. STIM1 carboxyl-terminus activates native SOC, Icrac and TRPC1 channels. Nat Cell Biol. 2006;8:1003–1010. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- 21.Asanov A, Sampieri A, Moreno C, Pacheco J, Salgado A, Sherry R, Vaca L. Combined single channel and single molecule detection identifies subunit composition of STIM1-activated transient receptor potential canonical (TRPC) channels. Cell Calcium. 2015;57:1–13. doi: 10.1016/j.ceca.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 22.Cheng KT, Liu X, Ong HL, Swaim W, Ambudkar IS. Local Ca2+ entry via Orai1 regulates plasma membrane recruitment of TRPC1 and controls cytosolic Ca2+ signals required for specific cell functions. PLoS Biol. 2011;9(3):e1001025. doi: 10.1371/journal.pbio.1001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng KT, Ong HL, Liu X, Ambudkar IS. Contribution and regulation of TRPC channels in store-operated Ca2+ entry. Curr Top Membr. 2013;71:149–179. doi: 10.1016/B978-0-12-407870-3.00007-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Veliçelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+ -store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 27.Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet JP. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang SL, Yeromin AV, Zhang XH, Yu Y, Safrina O, Penna A, Roos J, Stauderman KA, Cahalan MD. Genome-wide RNAi screen of Ca2+ influx identifies genes that regulate Ca2+ release-activated Ca2+ channel activity. Proc Natl Acad Sci USA. 2006;103:9357–9362. doi: 10.1073/pnas.0603161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baba Y, Nishida K, Fujii Y, Hirano T, Hikida M, Kurosaki T. Essential function for the calcium sensor STIM1 in mast cell activation and anaphylactic responses. Nat Immunol. 2008;9:81–88. doi: 10.1038/ni1546. [DOI] [PubMed] [Google Scholar]
- 30.Oh-hora M, Yamashita M, Hogan PG, Sharma S, Lamperti E, Chung W, Prakriya M, Feske S, Rao A. Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nat Immunol. 2008;9:432–443. doi: 10.1038/ni1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vig M, DeHaven WI, Bird GS, Billingsley JM, Wang H, Rao PE, Hutchings AB, Jouvin MH, Putney JW, Kinet JP. Defective mast cell effector functions in mice lacking the CRACM1 pore subunit of store-operated calcium release-activated calcium channels. Nat Immunol. 2008;9:89–96. doi: 10.1038/ni1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gwack Y, Srikanth S, Oh-hora M, Hogan PG, Lamperti ED, Yamashita M, Gelinas C, Neems DS, Sasaki Y, Feske S, Prakriya M, Rajewsky K, Rao A. Hair loss and defective T- and B-cell function in mice lacking ORAI1. Mol Cell Biol. 2008;28:5209–5222. doi: 10.1128/MCB.00360-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stiber J, Hawkins A, Zhang ZS, Wang S, Burch J, Graham V, Ward CC, Seth M, Finch E, Malouf N, Williams RS, Eu JP, Rosenberg P. STIM1 signalling controls store-operated calcium entry required for development and contractile function in skeletal muscle. Nat Cell Biol. 2008;10:688–697. doi: 10.1038/ncb1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Varga-Szabo D, Braun A, Kleinschnitz C, Bender M, Pleines I, Pham M, Renné T, Stoll G, Nieswandt B. The calcium sensor STIM1 is an essential mediator of arterial thrombosis and ischemic brain infarction. J Exp Med. 2008;205:1583–1591. doi: 10.1084/jem.20080302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bergmeier W, Oh-hora M, McCarl CA, Roden RC, Bray PF, Feske S. R93W mutation in Orai1 causes impaired calcium influx in platelets. Blood. 2009;113:675–678. doi: 10.1182/blood-2008-08-174516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Braun A, Varga-Szabo D, Kleinschnitz C, Pleines I, Bender M, Austinat M, Bösl M, Stoll G, Nieswandt B. Orai1 (CRACM1) is the platelet SOC channel and essential for pathological thrombus formation. Blood. 2009;113:2056–2063. doi: 10.1182/blood-2008-07-171611. [DOI] [PubMed] [Google Scholar]
- 37.Feske S. Immunodeficiency due to defects in store-operated calcium entry. Ann N Y Acad Sci. 2011;1238:74–90. doi: 10.1111/j.1749-6632.2011.06240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakamura L, Sandrock-Lang K, Speckmann C, Vraetz T, Bührlen M, Ehl S, Heemskerk JW, Zieger B. Platelet secretion defect in a patient with stromal interaction molecule 1 deficiency. Blood. 2013;122:3696–3698. doi: 10.1182/blood-2013-08-522037. [DOI] [PubMed] [Google Scholar]
- 39.Grosse J, Braun A, Varga-Szabo, Beyersdorf N, Schneider B, Zeitlmann L, Hanke P, Schropp P, Mühlstedt S, Zorn C, Huber M, Schmittwolf C, Jagla W, Yu P, Kerkau T, Schulze H, Nehls M, Nieswandt B. An EF hand mutation in Stim1 causes premature platelet activation and bleeding in mice. J Clin Invest. 2007;117:3540–3550. doi: 10.1172/JCI32312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Böhm J, Chevessier F, Maues De Paula A, Koch C, Attarian S, Feger C, Hantaï D, Laforêt P, Ghorab K, Vallat JM, Fardeau M, Figarella-Branger D, Pouget J, Romero NB, Koch M, Ebel C, Levy N, Krahn M, Eymard B, Bartoli M, Laporte J. Constitutive activation of the calcium sensor STIM1 causes tubular-aggregate myopathy. Am J Hum Genet. 2013;92:271–278. doi: 10.1016/j.ajhg.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nesin V, Wiley G, Kousi M, Ong EC, Lehmann T, Nicholl DJ, Suri M, Shahrizaila N, Katsanis N, Gaffney PM, Wierenga KJ, Tsiokas L. Activating mutations in STIM1 and ORAI1 cause overlapping syndromes of tubular myopathy and congenital miosis. Proc Natl Acad Sci USA. 2014;111:4197–4204. doi: 10.1073/pnas.1312520111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hedberg C, Niceta M, Fattori F, Lindvall B, Ciolfi A, D’Amico A, Tasca G, Petrini S, Tulinius M, Tartaglia M, Oldfors A, Bertini E. Childhood onset tubular aggregate myopathy associated with de novo STIM1 mutations. J Neurol. 2014;261:870–876. doi: 10.1007/s00415-014-7287-x. [DOI] [PubMed] [Google Scholar]
- 43.Endo Y, Noguchi S, Hara Y, Hayashi YK, Motomura K, Miyatake S, Murakami N, Tanaka S, Yamashita S, Kizu R, Bamba M, Goto YI, Matsumoto N, Nonaka I, Nishino I. Dominant mutations in ORAI1 cause tubular aggregate myopathy with hypocalcemia via constitutive activation of store-operated Ca2+ channels. Hum Mol Genet. 2015;24:637–648. doi: 10.1093/hmg/ddu477. [DOI] [PubMed] [Google Scholar]
- 44.Misceo D, Holmgren A, Louch WE, Holme PA, Mizobuchi M, Morales RJ, Maues De Paula A, Stray-Pedersen A, Lyle R, Dalhus B, Christensen G, Stormorken H, Tjønnfjord GE, Frengen E. A dominant STIM1 mutation causes Stormorken syndrome. Hum Mutat. 2014;35:556–564. doi: 10.1002/humu.22544. [DOI] [PubMed] [Google Scholar]
- 45.Morin G, Bruechle NO, Singh AR, Knopp C, Jedraszak G, Elbracht M, Brémond-Gignac D, Hartmann K, Sevestre H, Deutz P, Hérent D, Nürnberg P, Roméo B, Konrad K, Mathieu-Dramard M, Oldenburg J, Bourges-Petit E, Shen Y, Zerres K, Ouadid-Ahidouch H, Rochette J. Gain-of-function in STIM1 (P.R304W) is associated with Stormorken syndrome. Hum Mutat. 2014;35:1221–1232. doi: 10.1002/humu.22621. [DOI] [PubMed] [Google Scholar]
- 46.Soboloff J, Rothberg BS, Madesh M, Gill DL. STIM proteins: dynamic calcium signal transducers. Nat Rev Mol Cell Biol. 2012;13:549–565. doi: 10.1038/nrm3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Manji SS, Parker NJ, Williams RT, van Stekelenburg L, Pearson RB, Dziadek M, Smith PJ. STIM1: a novel phosphoprotein located at the cell surface. Biochim Biophys Acta. 2000;1481:147–155. doi: 10.1016/s0167-4838(00)00105-9. [DOI] [PubMed] [Google Scholar]
- 48.Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, Stauderman KA, Cahalan MD. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soboloff J, Spassova MA, Hewavitharana T, He LP, Xu W, Johnstone LS, Dziadek MA, Gill DL. STIM2 is an inhibitor of STIM1-mediated store-operated Ca2+ entry. Curr Biol. 2006;16:1465–1470. doi: 10.1016/j.cub.2006.05.051. [DOI] [PubMed] [Google Scholar]
- 50.Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J Cell Biol. 2006;174:803–813. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mercer JC, DeHaven WI, Smyth JT, Wedel B, Boyles RR, Bird GS, Putney JW., Jr Large store-operated calcium selective currents due to co-expression of Orai1 or Orai2 with the intracellular calcium sensor, Stim1. J Biol Chem. 2006;281:24979–24990. doi: 10.1074/jbc.M604589200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu P, Lu J, Li Z, Yu X, Chen L, Xu T. Aggregation of STIM1 underneath the plasma membrane induces clustering of Orai1. Biochem Biophys Res Commun. 2006;350:969–976. doi: 10.1016/j.bbrc.2006.09.134. [DOI] [PubMed] [Google Scholar]
- 53.Baba Y, Hayashi K, Fujii Y, Mizushima A, Watarai H, Wakamori M, Numaga T, Mori Y, Iino M, Hikida M, Kurosaki T. Coupling of STIM1 to store-operated Ca2+ entry through its constitutive and inducible movement in the endoplasmic reticulum. Proc Natl Acad Sci USA. 2006;103:16704–16709. doi: 10.1073/pnas.0608358103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou Y, Meraner P, Kwon HT, Machnes D, Oh-hora M, Zimmer J, Huang Y, Stura A, Rao A, Hogan PG. STIM1 gates the store-operated calcium channel ORAI1 in vitro. Nat Struct Mol Biol. 2010;17:112–116. doi: 10.1038/nsmb.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Muik M, Fahrner M, Derler I, Schindl R, Bergsmann J, Frischauf I, Groschner K, Romanin C. A cytosolic homomerization and a modulatory domain within STIM1 C terminus determine coupling to ORAI1 channels. J Biol Chem. 2009;284:8421–8426. doi: 10.1074/jbc.C800229200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yuan JP, Zeng W, Dorwart MR, Choi YJ, Worley PF, Muallem S. SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nat Cell Biol. 2009;11:337–343. doi: 10.1038/ncb1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muik M, Fahrner M, Schindl R, Stathopulos P, Frischauf I, Derler I, Plenk P, Lackner B, Groschner K, Ikura M, Romanin C. STIM1 couples to ORAI1 via an intramolecular transition into an extended conformation. EMBO J. 2011;30:1678–1689. doi: 10.1038/emboj.2011.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang X, Jin H, Cai X, Li S, Shen Y. Structural and mechanistic insights into the activation of Stromal interaction molecule 1 (STIM1) Proc Natl Acad Sci USA. 2012;109:5657–5662. doi: 10.1073/pnas.1118947109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fahrner M, Muik M, Schindl R, Butorac C, Stathopulos P, Zheng L, Jardin I, Ikura M, Romanin C. A coiled-coil clamp controls both conformation and clustering of stromal interaction molecule 1 (STIM1) J Biol Chem. 2014;289:33231–33244. doi: 10.1074/jbc.M114.610022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stathopulos PB, Zheng L, Li GY, Plevin MJ, Ikura M. Structural and mechanistic insights into STIM1-mediated initiation of store-operated calcium entry. Cell. 2008;135:110–122. doi: 10.1016/j.cell.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 61.Zheng L, Stathopulos PB, Schindl R, Li GY, Romanin C, Ikura M. Auto-inhibitory role of the EF-SAM domain of STIM proteins in store-operated calcium entry. Proc Natl Acad Sci USA. 2011;108:1337–1342. doi: 10.1073/pnas.1015125108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Park CY, Hoover PJ, Mullins FM, Bachhawat P, Covington ED, Raunser S, Walz T, Garcia KC, Dolmetsch RE, Lewis RS. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell. 2009;136:876–890. doi: 10.1016/j.cell.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kawasaki T, Lange I, Feske S. A minimal regulatory domain in the C terminus of STIM1 binds to and activates ORAI1 CRAC channels. Biochem Biophys Res Commun. 2009;385:49–54. doi: 10.1016/j.bbrc.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stathopulos PB, Schindl R, Fahrner M, Zheng L, Gasmi-Seabrook GM, Muik M, Romanin C, Ikura M. STIM1/Orai1 coiled-coil interplay in the regulation of store-operated calcium entry. Nat Commun. 2013;4:2963. doi: 10.1038/ncomms3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hogan PG, Lewis RL, Rao A. Molecular basis of calcium signalling in lymphocytes: STIM and ORAI. Annu Rev Immunol. 2010;28:491–533. doi: 10.1146/annurev.immunol.021908.132550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hogan PG. STIM1-ORAI1 store-operated calcium channels. Chapter 6.24. In: Montal M, Egelman EH, editors. Comprehensive Biophysics, volume 6, Channel Proteins. Academic Press; Oxford: 2012. pp. 223–233. [Google Scholar]
- 67.Prakriya M. Store-operated Orai channels: structure and function. Curr Top Membr. 2013;71:1–32. doi: 10.1016/B978-0-12-407870-3.00001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hoth M, Niemeyer BA. The neglected CRAC proteins: Orai2, Orai3, and STIM2. Curr Top Membr. 2013;71:237–271. doi: 10.1016/B978-0-12-407870-3.00010-X. [DOI] [PubMed] [Google Scholar]
- 69.Gwack Y, Srikanth S, Feske S, Cruz-Guilloty F, Oh-hora M, Neems DS, Hogan PG, Rao A. Biochemical and functional characterization of Orai proteins. J Biol Chem. 2007;282:16232–16243. doi: 10.1074/jbc.M609630200. [DOI] [PubMed] [Google Scholar]
- 70.Hou X, Pedi L, Diver MM, Long SB. Crystal structure of the calcium release-activated calcium channel Orai. Science. 2012;338:1308–1313. doi: 10.1126/science.1228757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thompson JL, Shuttleworth TJ. How many Orai’s does it take to make a CRAC channel? Sci Rep. 2013;3:1961. doi: 10.1038/srep01961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liou J, Fivaz M, Inoue T, Meyer T. Live-cell imaging reveals sequential oligomerization and local plasma membrane targeting of stromal interaction molecule 1 after Ca2+ store depletion. Proc Natl Acad Sci USA. 2007;104:9301–9306. doi: 10.1073/pnas.0702866104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Luik RM, Wu MM, Buchanan J, Lewis RS. The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J Cell Biol. 2006;174:815–825. doi: 10.1083/jcb.200604015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li Z, Lu J, Xu P, Xie X, Chen L, Xu T. Mapping the interacting domains of STIM1 and Orai1 in Ca2+ release-activated Ca2+ channel activation. J Biol Chem. 2007;282:29448–29456. doi: 10.1074/jbc.M703573200. [DOI] [PubMed] [Google Scholar]
- 75.Muik M, Frischauf I, Derler I, Fahrner M, Bergsmann J, Eder P, Schindl R, Hesch C, Polzinger B, Fritsch R, Kahr H, Madl J, Gruber H, Groschner K, Romanin C. Dynamic coupling of the putative coiled-coil domain of ORAI1 with STIM1 mediates ORAI1 channel activation. J Biol Chem. 2008;283:8014–8022. doi: 10.1074/jbc.M708898200. [DOI] [PubMed] [Google Scholar]
- 76.Navarro-Borelly L, Somasundaram A, Yamashita M, Ren D, Miller RJ, Prakriya M. STIM1-Orai1 interactions and Orai1 conformational changes revealed by live-cell FRET microscopy. J Physiol. 2008;586:5383–5401. doi: 10.1113/jphysiol.2008.162503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barr VA, Bernot KM, Srikanth S, Gwack Y, Balagopalan L, Regan CK, Helman DJ, Sommers CL, Oh-hora M, Rao A, Samelson LE. Dynamic movement of the calcium sensor STIM1 and the calcium channel Orai1 in activated T-cells: puncta and distal caps. Mol Biol Cell. 2008;19:2802–2817. doi: 10.1091/mbc.E08-02-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Calloway N, Vig M, Kinet JP, Holowka D, Baird B. Molecular clustering of STIM1 with Orai1/CRACM1 at the plasma membrane depends dynamically on depletion of Ca2+ stores and on electrostatic interactions. Mol Biol Cell. 2009;20:389–399. doi: 10.1091/mbc.E07-11-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Peinelt C, Vig M, Koomoa DL, Beck A, Nadler MJS, Koblan-Huberson M, Lis A, Fleig A, Penner R, Kinet JP. Amplification of CRAC current by STIM1 and CRACM1 (Orai1) Nat Cell Biol. 2006;8:771–773. doi: 10.1038/ncb1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Soboloff J, Spassova MA, Tang XD, Hewavitharana T, Xu W, Gill DL. Orai1 and STIM reconstitute store-operated calcium channel function. J Biol Chem. 2006;281:20661–20665. doi: 10.1074/jbc.C600126200. [DOI] [PubMed] [Google Scholar]
- 81.Spassova MA, Soboloff J, He LP, Xu W, Dziadek MA, Gill DL. STIM1 has a plasma membrane role in the activation of store-operated Ca2+ channels. Proc Natl Acad Sci USA. 2006;103:4040–4045. doi: 10.1073/pnas.0510050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Luik RM, Wang B, Prakriya M, Wu MM, Lewis RS. Oligomerization of STIM1 couples ER calcium depletion to CRAC channel activation. Nature. 2008;454:538–542. doi: 10.1038/nature07065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brandman O, Liou J, Park WS, Meyer T. STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell. 2007;131:1327–1339. doi: 10.1016/j.cell.2007.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang SL, Kozak JA, Jiang W, Yeromin AV, Chen J, Yu Y, Penna A, Shen W, Chi V, Cahalan MD. Store-dependent and -independent modes regulating Ca2+ release-activated Ca2+ channel activity of human Orai1 and Orai2. J Biol Chem. 2008;283:17662–17771. doi: 10.1074/jbc.M801536200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bird GS, Hwang SY, Smyth JT, Fukushima M, Boyles RR, Putney JW., Jr STIM1 is a calcium sensor specialized for digital signaling. Curr Biol. 2009;19:1724–1729. doi: 10.1016/j.cub.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang X, Wang Y, Zhou Y, Hendron E, Mancarella S, Andrake MD, Rothberg BS, Soboloff J, Gill DL. Distinct Orai-coupling domains in STIM1 and STIM2 define the Orai-activating site. Nat Commun. 2014;5:3183. doi: 10.1038/ncomms4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kar P, Nelson C, Parekh AB. CRAC channels drive digital activation and provide analog control and synergy to Ca2+-dependent gene regulation. Curr Biol. 2012;22:242–247. doi: 10.1016/j.cub.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 88.Thiel M, Lis A, Penner R. STIM2 drives Ca2+ oscillations through store-operated Ca2+ entry caused by mild store depletion. J Physiol. 2013;591:1433–1445. doi: 10.1113/jphysiol.2012.245399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 90.Yeromin AV, Zhang SL, Jiang W, Yu Y, Safrina O, Cahalan MD. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–229. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vig M, Beck A, Billingsley JM, Lis A, Parvez S, Peinelt C, Koomoa DL, Soboloff J, Gill DL, Fleig A, Kinet JP, Penner R. CRACM1 multimers form the ion-selective pore of the CRAC channel. Curr Biol. 2006;16:2073–2079. doi: 10.1016/j.cub.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gudlur A, Quintana A, Zhou Y, Hirve N, Mahapatra S, Hogan PG. STIM1 triggers a gating rearrangement at the extracellular mouth of the ORAI1 channel. Nat Commun. 2014;5:5164. doi: 10.1038/ncomms6164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lis A, Peinelt C, Beck A, Parvez S, Monteilh-Soller M, Fleig A, Penner R. CRACM1, CRACM2, and CRACM3 are store-operated Ca2+ channels with distinct functional properties. Curr Biol. 2007;17:794–800. doi: 10.1016/j.cub.2007.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.DeHaven WI, Smyth JT, Boyles RR, Putney JW., Jr Calcium inhibition and calcium potentiation of Orai1, Orai2, and Orai3 calcium release-activated calcium channels. J Biol Chem. 2007;282:17548–17556. doi: 10.1074/jbc.M611374200. [DOI] [PubMed] [Google Scholar]
- 95.Schindl R, Bergsmann J, Frischauf I, Derler I, Fahrner M, Muik M, Fritsch R, Groschner K, Romanin C. 2-aminoethoxydiphenyl borate alters selectivity of Orai3 channels by increasing their pore size. J Biol Chem. 2008;283:20261–20267. doi: 10.1074/jbc.M803101200. [DOI] [PubMed] [Google Scholar]
- 96.Lee KP, Yuan JP, Zeng W, So I, Worley PF, Muallem S. Molecular determinants of fast Ca2+-dependent inactivation and gating of the Orai channels. Proc Natl Acad Sci USA. 2009;106:14687–14692. doi: 10.1073/pnas.0904664106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Motiani RK, Abdullaev IF, Trebak M. A novel native store-operated calcium channel encoded by Orai3: selective requirement of Orai3 versus Orai1 in estrogen receptor-positive versus estrogen receptor-negative breast cancer cells. J Biol Chem. 2010;285:19173–19183. doi: 10.1074/jbc.M110.102582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Motiani RK, Zhang X, Harmon KE, Keller RS, Matrougui K, Bennett JA, Trebak M. Orai3 is an estrogen receptor α-regulated Ca2+ channel that promotes tumorigenesis. FASEB J. 2013;27:63–75. doi: 10.1096/fj.12-213801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mignen O, Shuttleworth TJ. IARC, a novel arachidonate-regulated, noncapacitative Ca2+ entry channel. J Biol Chem. 2000;275:9114–9119. doi: 10.1074/jbc.275.13.9114. [DOI] [PubMed] [Google Scholar]
- 100.Mignen O, Thompson JL, Shuttleworth TJ. Both Orai1 and Orai3 are essential components of the arachidonate-regulated Ca2+-selective (ARC) channels. J Physiol. 2008;586:185–195. doi: 10.1113/jphysiol.2007.146258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Thompson JL, Mignen O, Shuttleworth TJ. The ARC channel—an endogenous store-independent Orai channel. Curr Top Membr. 2013;71:125–148. doi: 10.1016/B978-0-12-407870-3.00006-8. [DOI] [PubMed] [Google Scholar]
- 102.González-Cobos JC, Zhang X, Zhang W, Ruhle B, Motiani RK, Schindl R, Muik M, Spinelli AM, Bisaillon JM, Shinde AV, Fahrner M, Singer HA, Matrougui K, Barroso M, Romanin C, Trebak M. Store-independent Orai1/3 channels activated by intracrine leukotrieneC4: role in neointimal hyperplasia. Circ Res. 2013;112:1013–1025. doi: 10.1161/CIRCRESAHA.111.300220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang X, González-Cobos JC, Schindl R, Muik M, Ruhle B, Motiani RK, Bisaillon JM, Zhang W, Fahrner M, Barroso M, Matrougui K, Romanin C, Trebak M. Mechanisms of STIM1 activation of store-independent leukotriene C4-regulated Ca2+ channels. Mol Cell Biol. 2013;33:3715–3723. doi: 10.1128/MCB.00554-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang X, Zhang W, González-Cobos JC, Jardin I, Romanin C, Matrougui K, Trebak M. Complex role of STIM1 in the activation of store-independent Orai1/3 channels. J Gen Physiol. 2014;143:345–359. doi: 10.1085/jgp.201311084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bandara S, Malmersjö S, Meyer T. Regulators of calcium homeostasis identified by inference of kinetic model parameters from live single cells perturbed by siRNA. Sci Signal. 2013 Jul 9;6(283):ra56. doi: 10.1126/scisignal.2003649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stathopulos PB, Li GY, Plevin MJ, Ames JB, Ikura M. Stored Ca2+ depletion-induced oligomerization of stromal interaction molecule 1 (STIM1) via the EF-SAM region. An initiation mechanism for capacitative Ca2+ entry. J Biol Chem. 2006;281:35855–35862. doi: 10.1074/jbc.M608247200. [DOI] [PubMed] [Google Scholar]
- 107.Zheng L, Stathopulos PB, Li GY, Ikura M. Biophysical characterization of the EF-hand and SAM domain containing Ca2+ sensory region of STIM1 and STIM2. Biochem Biophys Res Commun. 2008;369:240–246. doi: 10.1016/j.bbrc.2007.12.129. [DOI] [PubMed] [Google Scholar]
- 108.Stathopulos PB, Zheng L, Ikura M. Stromal interaction molecule (STIM) 1 and STIM2 calcium sensing regions exhibit distinct unfolding and oligomerization kinetics. J Biol Chem. 2009;284:728–732. doi: 10.1074/jbc.C800178200. [DOI] [PubMed] [Google Scholar]
- 109.Covington ED, Wu MM, Lewis RS. Essential role for the CRAC activation domain in store-dependent oligomerization of STIM1. Mol Biol Cell. 2010;21:1897–1907. doi: 10.1091/mbc.E10-02-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Furukawa Y, Teraguchi S, Ikegami T, Dagliyan O, Jin L, Hall D, Dokholyan NV, Namba K, Akira S, Kurosaki T, Baba Y, Standley DM. Intrinsic disorder mediates cooperative signal transduction in STIM1. J Mol Biol. 2014;426:2082–2097. doi: 10.1016/j.jmb.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 111.Walsh CM, Chvanov M, Haynes LP, Petersen OH, Tepikin AV, Burgoyne RD. Role of phosphoinositides in STIM1 dynamics and store-operated calcium entry. Biochem J. 2009;425:159–168. doi: 10.1042/BJ20090884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ercan E, Momburg F, Engel U, Temmerman K, Nickel W, Seedorf M. A conserved, lipid-mediated sorting mechanism of yeast Ist2 and mammalian STIM proteins to the peripheral ER. Traffic. 2009;10:1802–1818. doi: 10.1111/j.1600-0854.2009.00995.x. [DOI] [PubMed] [Google Scholar]
- 113.Zhou Y, Srinivasan P, Razavi S, Seymour S, Meraner P, Gudlur A, Stathopulos P, Ikura M, Rao A, Hogan PG. Initial activation STIM1, the regulator of store-operated calcium entry. Nat Struct Mol Biol. 2013;20:973–981. doi: 10.1038/nsmb.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bhardwaj R, Müller HM, Nickel W, Seedorf M. Oligomerization and Ca2+/calmodulin control binding of the ER Ca2+ sensors STIM1 and STIM2 to plasma membrane lipids. Biosci Rep. 2013;33:art:e00077. doi: 10.1042/BSR20130089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Korzeniowski MK, Manjarrés IM, Várnai P, Balla T. Activation of STIM1-Orai1 involves an intramolecular switching mechanism. Sci Signal. 2010;3(148):ra82. doi: 10.1126/scisignal.2001122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yu J, Zhang H, Zhang M, Deng Y, Wang H, Lu J, Xu T, Xu P. An aromatic amino acid in the coiled-coil 1 domain plays a crucial role in the auto-inhibitory mechanism of STIM1. Biochem J. 2013;454:401–409. doi: 10.1042/BJ20130292. [DOI] [PubMed] [Google Scholar]
- 117.Ong HL, Liu X, Tsaneva-Atanasova K, Singh BB, Bandyopadhyay BC, Swaim WD, Russell JT, Hegde RS, Sherman A, Ambudkar IS. Relocalization of STIM1 for activation of store-operated Ca2+ entry is determined by the depletion of subplasma membrane endoplasmic reticulum Ca2+ store. J Biol Chem. 2007;282:12176–12185. doi: 10.1074/jbc.M609435200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wu MM, Covington ED, Lewis RS. Single-molecule analysis of diffusion and trapping of STIM1 and Orai1 at endoplasmic reticulum-plasma membrane junctions. Mol Biol Cell. 2014;25:3672–3685. doi: 10.1091/mbc.E14-06-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Madl J, Weghuber J, Fritsch R, Derler I, Fahrner M, Frischauf I, Lackner B, Romanin C, Schütz GJ. Resting state Orai1 diffuses as homotetramer in the plasma membrane of live mammalian cells. J Biol Chem. 2010;285:41135–41142. doi: 10.1074/jbc.M110.177881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Frischauf I, Muik M, Derler I, Bergsmann J, Fahrner M, Schindl R, Groschner K, Romanin C. Molecular determinants of the coupling between STIM1 and Orai channels: differential activation of Orai1-3 channels by a STIM1 coiled-coil mutant. J Biol Chem. 2009;284:21696–21706. doi: 10.1074/jbc.M109.018408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lis A, Zierler S, Peinelt C, Fleig A, Penner R. A single lysine in the N-terminal region of store-operated channels is critical for STIM1-mediated gating. J Gen Physiol. 2010;136:673–686. doi: 10.1085/jgp.201010484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bergsmann J, Derler I, Muik M, Frischauf I, Fahrner M, Pollheimer P, Schwarzinger C, Gruber HJ, Groschner K, Romanin C. Molecular determinants within N terminus of Orai3 protein that control channel activation and gating. J Biol Chem. 2011;286:31565–31575. doi: 10.1074/jbc.M111.227546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fukushima W, Tomita T, Janoshazi A, Putney JW., Jr Alternative translation initiation gives rise to two isoforms of Orai1 with distinct plasma membrane mobilities. J Cell Sci. 2012;125:4354–4361. doi: 10.1242/jcs.104919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.McNally BA, Somasundaram A, Jairaman A, Yamashita M, Prakriya M. The C- and N-terminal STIM1 binding sites on Orai1 are required for both trapping and gating CRAC channels. J Physiol. 2013;591:2833–2850. doi: 10.1113/jphysiol.2012.250456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Derler I, Plenk P, Fahrner M, Muik M, Jardin I, Schindl R, Gruber HJ, Groschner K, Romanin C. The extended transmembrane Orai1 N-terminal (ETON) region combines binding interface and gate for activation by STIM1. J Biol Chem. 2013;288:29025–29034. doi: 10.1074/jbc.M113.501510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zheng H, Zhou MH, Hu C, Kuo E, Peng X, Hu J, Kuo L, Zhang SL. Differential roles of the C and N termini of Orai1 protein in interacting with stromal interaction molecule 1 (STIM1) for Ca2+ release-activated Ca2+ (CRAC) channel activation. J Biol Chem. 2013;288:11263–11272. doi: 10.1074/jbc.M113.450254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gudlur A, Zhou Y, Hogan PG. STIM-ORAI interactions that control the CRAC channel. In: Prakriya M, editor. Current Topics in Membranes. Vol. 71. Burlington: Academic Press; 2013. pp. 33–58. [DOI] [PubMed] [Google Scholar]
- 128.Shim AH, Tirado-Lee L, Prakriya M. Structural and functional mechanisms of CRAC channel regulation. J Mol Biol. 2015;427:77–93. doi: 10.1016/j.jmb.2014.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yamashita M, Navarro-Borelly L, McNally BA, Prakriya M. Orai1 mutations alter ion permeation and Ca2+-dependent fast inactivation of CRAC channels: evidence for coupling of permeation and gating. J Gen Physiol. 2007;130:525–540. doi: 10.1085/jgp.200709872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Spassova MA, Hewavitharana T, Fandino RA, Kaya A, Tanaka J, Gill DL. Voltage gating at the selectivity filter of the Ca2+ release-activated Ca2+ channel induced by mutation of the Orai1 protein. J Biol Chem. 2008;283:14938–14945. doi: 10.1074/jbc.M702208200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.McNally BA, Yamashita M, Engh A, Prakriya M. Structural determinants of ion permeation in CRAC channels. Proc Natl Acad Sci USA. 2009;106:22516–22521. doi: 10.1073/pnas.0909574106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhou Y, Ramachandran S, Oh-hora M, Rao A, Hogan PG. Pore architecture of the ORAI1 store-operated calcium channel. Proc Natl Acad Sci USA. 2010;107:4896–4901. doi: 10.1073/pnas.1001169107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.McNally BA, Somasundaram A, Yamashita M, Prakriya M. Gated regulation of CRAC channel ion selectivity by STIM1. Nature. 2012;482:241–245. doi: 10.1038/nature10752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang SL, Yeromin AV, Hu J, Amcheslavsky A, Zheng H, Cahalan MD. Mutations in Orai1 transmembrane segment 1 cause STIM1-independent activation of Orai1 channels at glycine 98 and channel closure at arginine 91. Proc Natl Acad Sci USA. 2011;108:17838–17843. doi: 10.1073/pnas.1114821108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Launay P, Cheng H, Srivatsan S, Penner R, Fleig A, Kinet JP. TRPM4 regulates calcium oscillations after T cell activation. Science. 2004;306:1374–1377. doi: 10.1126/science.1098845. [DOI] [PubMed] [Google Scholar]
- 136.Vennekens R, Olausson J, Meissner M, Bloch W, Mathar I, Philipp SE, Schmitz F, Weissgerber P, Nilius B, Flockerzi V, Freichel M. Increased IgE-dependent mast cell activation and anaphylactic responses in mice lacking the calcium-activated nonselective cation channel TRPM4. Nat Immunol. 2007;8:312–20. doi: 10.1038/ni1441. [DOI] [PubMed] [Google Scholar]
- 137.DeCoursey TE, Chandy KG, Gupta S, Cahalan MD. Voltage-gated K+ channels in human T lymphocytes: a role in mitogenesis? Nature. 1984;307:465–468. doi: 10.1038/307465a0. [DOI] [PubMed] [Google Scholar]
- 138.Beeton C, Wulff H, Barbaria J, Clot-Faybesse O, Pennington M, Bernard D, Cahalan MD, Chandy KG, Béraud E. Selective blockade of T lymphocyte K+ channels ameliorates experimental autoimmune encephalomyelitis, a model for multiple sclerosis. Proc Natl Acad Sci USA. 2001;98:13942–13947. doi: 10.1073/pnas.241497298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chandy KG, Wulff H, Beeton C, Pennington M, Gutman GA, Cahalan MD. K+ channels as targets for specific immunomodulation. Trends Pharmacol Sci. 2004;25:280–289. doi: 10.1016/j.tips.2004.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Matheu MP, Beeton C, Garcia A, Chi V, Rangaraju S, Safrina O, Monaghan K, Uemura MI, Li D, Pal S, de la Maza LM, Monuki E, Flügel A, Pennington MW, Parker I, Chandy KG, Cahalan MD. Imaging of effector memory T cells during a delayed-type hypersensitivity reaction and suppression by Kv1.3 channel block. Immunity. 2008;29:602–614. doi: 10.1016/j.immuni.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lam J, Wulff H. The lymphocyte potassium channels Kv1.3 and KCa3.1 as targets for immunosuppression. Drug Dev Res. 2011;72:573–584. doi: 10.1002/ddr.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zweifach A, Lewis RS. Rapid inactivation of depletion-activated calcium current (ICRAC) due to local calcium feedback. J Gen Physiol. 1995;105:209–226. doi: 10.1085/jgp.105.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zweifach A, Lewis RS. Slow calcium-dependent inactivation of depletion-activated calcium current. Store-dependent and -independent mechanisms. J Biol Chem. 1995;270:14445–14451. doi: 10.1074/jbc.270.24.14445. [DOI] [PubMed] [Google Scholar]
- 144.Parekh AB. Slow feedback inhibition of calcium release-activated calcium current by calcium entry. J Biol Chem. 1998;273:14925–14932. doi: 10.1074/jbc.273.24.14925. [DOI] [PubMed] [Google Scholar]
- 145.Fierro L, Parekh AB. Fast calcium-dependent inactivation of calcium release-activated calcium current (CRAC) in RBL-1 cells. J Membr Biol. 1999;168:9–17. doi: 10.1007/s002329900493. [DOI] [PubMed] [Google Scholar]
- 146.Mullins FM, Park CY, Dolmetsch RE, Lewis RS. STIM1 and calmodulin interact with Orai1 to induce Ca2+-dependent inactivation of CRAC channels. Proc Natl Acad Sci USA. 2009;106:15495–15500. doi: 10.1073/pnas.0906781106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Scrimgeour N, Litjens T, Ma L, Barritt GJ, Rychkov GY. Properties of Orai1 mediated store-operated current depend on the expression levels of STIM1 and Orai1 proteins. J Physiol. 2009;587:2903–2918. doi: 10.1113/jphysiol.2009.170662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Palty R, Raveh A, Kaminsky I, Meller R, Reuveny E. SARAF inactivates the store operated calcium entry machinery to prevent excess calcium refilling. Cell. 2012;149:425–438. doi: 10.1016/j.cell.2012.01.055. [DOI] [PubMed] [Google Scholar]
- 149.Maléth J, Choi S, Muallem S, Ahuja M. Translocation between PI(4,5)P2-poor and PI(4,5)P2-rich microdomains during store depletion determines STIM1 conformation and Orai1 gating. Nat Commun 2014. 2014;5:5843. doi: 10.1038/ncomms6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Malayev A, Nelson DJ. Extracellular pH modulates the Ca2+ current activated by depletion of intracellular Ca2+ stores in human macrophages. J Membr Biol. 1995;146:101–111. doi: 10.1007/BF00232684. [DOI] [PubMed] [Google Scholar]
- 151.Scrimgeour NR, Wilson DP, Rychkov GY. Glu106 in the Orai1 pore contributes to fast Ca2+-dependent inactivation and pH dependence of Ca2+ release-activated Ca2+ (CRAC) current. Biochem J. 2012;441:743–753. doi: 10.1042/BJ20110558. [DOI] [PubMed] [Google Scholar]
- 152.Beck A, Fleig A, Penner R, Peinelt C. Regulation of endogenous and heterologous Ca2+ release-activated Ca2+ currents by pH. Cell Calcium. 2014;56:235–243. doi: 10.1016/j.ceca.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sundivakkam PC, Natarajan V, Malik AB, Tiruppathi C. Store-operated Ca2+ entry (SOCE) induced by protease-activated receptor-1 mediates STIM1 protein phosphorylation to inhibit SOCE in endothelial cells through AMP-activated protein kinase and p38β mitogen-activated protein kinase. J Biol Chem. 2013;288:17030–17041. doi: 10.1074/jbc.M112.411272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Putney JW. Alternative forms of the store-operated calcium entry mediators, STIM1 and Orai1. Curr Top Membr. 2013;71:109–123. doi: 10.1016/B978-0-12-407870-3.00005-6. [DOI] [PubMed] [Google Scholar]
- 155.Pozo-Guisado E, Martin-Romero FJ. The regulation of STIM1 by phosphorylation. Commun Integr Biol. 2013;6:e26283. doi: 10.4161/cib.26283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lopez E, Jardin I, Berna-Erro A, Bermejo N, Salido GM, Sage SO, Rosado JA, Redondo PC. STIM1 tyrosine-phosphorylation is required for STIM1-Orai1 association in human platelets. Cell Signal. 2012;24:1315–1322. doi: 10.1016/j.cellsig.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 157.Thompson JL, Shuttleworth TJ. AKAP79-mediated PKA-phosphorylation of STIM1 determines selective activation of ARC channels, a store-independent Orai channel. J Physiol. 2014;593:559–572. doi: 10.1113/jphysiol.2014.284182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kawasaki T, Ueyama T, Lange I, Feske S, Saito N. Protein kinase C-induced phosphorylation of Orai1 regulates the intracellular Ca2+ level via the store-operated Ca2+ channel. J Biol Chem. 2010;285:25720–25730. doi: 10.1074/jbc.M109.022996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Shaw PJ, Qu B, Hoth M, Feske S. Molecular regulation of CRAC channels and their role in lymphocyte function. Cell Mol Life Sci. 2013;70:2637–2656. doi: 10.1007/s00018-012-1175-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hawkins BJ, Irrinki KM, Mallilankaraman K, Lien YC, Wang Y, Bhanumathy CD, Subbiah R, Ritchie MF, Soboloff J, Baba Y, Kurosaki T, Joseph SK, Gill DL, Madesh M. S-glutathionylation activates STIM1 and alters mitochondrial homeostasis. J Cell Biol. 2010;190:391–405. doi: 10.1083/jcb.201004152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bogeski I, Kummerow C, Al-Ansary D, Schwarz EC, Koehler R, Kozai D, Takahashi N, Peinelt C, Griesemer D, Bozem M, Mori Y, Hoth M, Niemeyer BA. Differential redox regulation of ORAI ion channels: a mechanism to tune cellular calcium signaling. Sci Signal. 2010;3:ra24. doi: 10.1126/scisignal.2000672. [DOI] [PubMed] [Google Scholar]
- 162.Srikanth S, Gwack Y. Molecular regulation of the pore component of CRAC channels, Orai1. Curr Top Membr. 2013;71:181–207. doi: 10.1016/B978-0-12-407870-3.00008-1. [DOI] [PubMed] [Google Scholar]
- 163.Srikanth S, Jung HJ, Kim KD, Souda P, Whitelegge J, Gwack Y. A novel EF-hand protein, CRACR2A, is a cytosolic Ca2+ sensor that stabilizes CRAC channels in T cells. Nat Cell Biol. 2010;12:436–446. doi: 10.1038/ncb2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Feng M, Grice DM, Faddy HM, Nguyen N, Leitch S, Wang Y, Muend S, Kenny PA, Sukumar S, Roberts-Thomson SJ, Monteith GR, Rao R. Store-independent activation of Orai1 by SPCA2 in mammary tumors. Cell. 2010;143:84–98. doi: 10.1016/j.cell.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Calloway N, Owens T, Corwith K, Rodgers W, Holowka D, Baird B. Stimulated association of STIM1 and Orai1 is regulated by the balance of PtdIns(4,5)P2 between distinct membrane pools. J Cell Sci. 2011;124:2602–2610. doi: 10.1242/jcs.084178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sharma S, Quintana A, Findlay GM, Mettlen M, Baust B, Jain M, Nilsson R, Rao A, Hogan PG. An siRNA screen for NFAT activation identifies septins as coordinators of store-operated Ca2+ entry. Nature. 2013;499:238–242. doi: 10.1038/nature12229. [DOI] [PMC free article] [PubMed] [Google Scholar]