Synopsis
Human granulocytic anaplasmosis (HGA), a deer tick transmitted rickettsial infection caused by Anaplasma phagocytophilum, is a common cause of undifferentiated fever in the Northeast and Upper Midwest U.S. Patients are often initially diagnosed with a mild viral infection, and illness readily resolves in most cases. However, as many as 3% may develop life threatening complications and nearly 1% die from the infection. A history of tick bite and a high degree of clinical suspicion thus warrant consideration for doxycycline treatment in both adults and children, even in the absence of known tick bite, a negative blood film examination, or pending results of specific A. phagocytophilum diagnostic tests such as paired serology or PCR on acute phase blood. Antibody tests and titers should not be used to monitor active infection as detectable antibodies can remain present for years. Moreover, persistent infection has never been reported. While co-infections with Borrelia burgdorferi and Babesia microti occur, there is little evidence to suggest synergism of disease or a role for A. phagocytophilum in chronic illness. Preventive measures include avoiding tick-infested areas, use of tick repellents, and careful searches of skin to remove attached ticks; no vaccine is available.
Keywords: anaplasmosis, human, granulocytic, diagnosis, management
INTRODUCTION
Tick-borne infections have been recognized in the United States for more than a century. Following the description of the agent of Rocky Mountain spotted fever (RMSF) in 1906,1 several clinically important tick-associated human infectious syndromes have since been characterized.2-10 One tick-borne infection, human granulocytic anaplasmosis (HGA) caused by the rickettsia Anaplasma phagocytophilum, is transmitted by Ixodes scapularis ticks in the U.S. and can be sometimes confused with or complicates Lyme disease. A compilation of data published by the CDC and in Morbidity and Mortality Weekly Reports since HGA became nationally reportable includes at least 15,952 cases since 1995 (Figure 1). In fact, the incidence of HGA increased 12-fold between 2001 and 2011, and the disease can cause severe illness and occasionally death in otherwise healthy individuals. While many patients with competent immune systems resolve their illnesses spontaneously even without antibiotic treatment, most symptomatic patients benefit from specific antibiotic therapy. As with most rickettsial infections, poor outcomes can occur without early identification and specific treatment, usually with doxycycline. The major difficulty with HGA is that the early symptoms and signs are nonspecific, often mimicking a viral illness, and rapid sensitive tests for diagnosis early in infection are not widely available. Thus, it is difficult to arrive at a specific diagnosis early in the course of the illness when antibiotic therapy is most likely to be successful. This chapter will focus on current practice for the diagnosis and management of HGA.11,12
Figure 1.
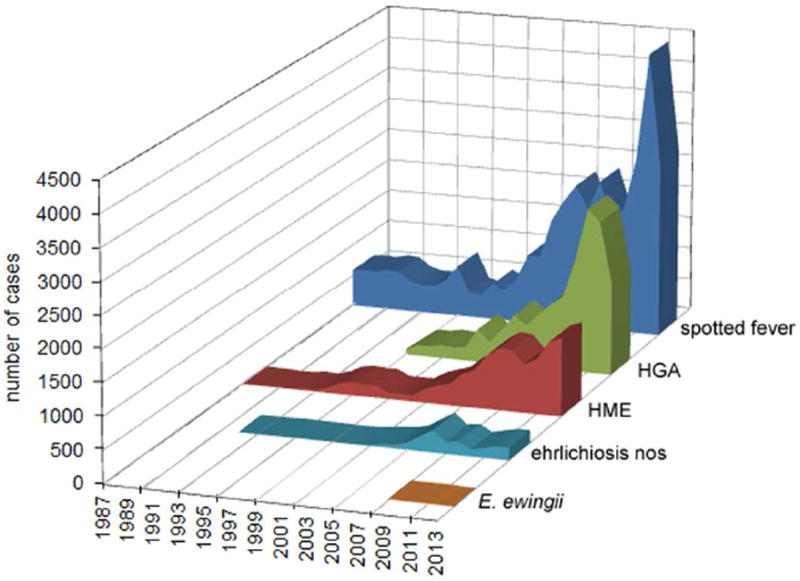
Reported cases of tick-borne rickettsial infections in the U.S., 1987-2013. The cumulative area plots demonstrate the overall burden of infection during this time interval. HGA – human granulocytic anaplasmosis; HME – human monocytic ehrlichiosis; spotted fever – spotted fever group rickettsioses, including Rocky Mountain spotted fever; nos – not otherwise specified. Data extracted from Morbidity and Mortality Weekly Reports, 1987-2014.
PATIENT HISTORY
Most cases of HGA develop in individuals exposed to or bitten by Ixodes ticks.13-16 Thus, a history of a tick bite or well established exposure to ticks is an important historical clue. Ticks in the Ixodes persulcatus-complex serve as competent vectors for multiple pathogens that can infect humans including A. phagocytophilum,11 Borrelia burgdorferi (the agent of Lyme borreliosis),17 Babesia microti (the agent of babesiosis),18 Ehrlichia muris-like agent,19 Borrelia miyamotoi,3 and Powassan virus 20Vector ticks in North American endemic habitats include Ixodes scapularis in the northeastern and upper Midwest regions of the USA, and I. pacificus along the northern Pacific coast. Despite its wide distribution, the majority of HGA cases are reported from the upper Midwest and northeastern United States, overlapping the endemic regions for Ixodes scapularis (the black-legged deer tick).21 Recent epidemiologic studies of tick habitats demonstrate that the endemic areas have expanded, and as such it is anticipated that so will the range where HGA will be found in humans.22-24 However, at least 25% of patients with proven HGA do not report exposure to ticks and thus, it should not be used as an absolute criterion for diagnosis or clinical suspicion.
Seroprevalence and incidence vary directly with age, suggesting that the principal risk factor for contracting HGA is duration of residence in endemic areas.25,26 Ixodes scapularis ticks, while widespread, transmit infections to human in a rather limited geographic range. Therefore, understanding the ranges of endemic risk is also a critical component for patient evaluation. Serosurveys among individuals bitten by ticks in the USA demonstrate A. phagocytophilum seroprevalence rates ranging between 8.9 and 36%.25 Bakken et al. reported a seroprevalence of 14.9% among healthy residents from northwestern Wisconsin who had no history of a recent tick bite.26 The highest annual US average HGA incidence rates between 2000 and 2007 were reported in Rhode Island (32.1 per million), Minnesota (30.2 per million), Connecticut (13.1 per million), Wisconsin (8.2 per million), New York State (7.7 per million) and Massachusetts (6.5 per million).21 However, HGA incidence rates exceeding 650 cases per million are also reported in some northwestern Wisconsin counties.14,21,27 HGA also occurs at a much lower incidence in Europe and Scandinavia and it is increasingly described in eastern parts of Asia, especially China, South Korea, and Japan.
Despite the strong association with tick bite, HGA can be acquired through alternate exposures to A. phagocytophilum. Horowitz described HGA in a woman during pregnancy.28 The infant developed HGA eight days after being borne, and the authors argued that transplacental transmission A. phagocytophilum occurred. Several butchers from northwestern Wisconsin acquired HGA after butchering large quantities of white tail deer carcasses during hunting season[36].29 None of the butchers had noted a preceding tick-bite, raising the query as to whether they acquired HGA by direct exposure to infected deer blood through skin cuts, through inhalation of aerosolized blood, or through infected blood splashed directly on mucous membranes. A cluster of HGA cases associated with a severely hemorrhagic febrile illness occurred after nosocomial exposure in a Chinese hospital, ostensibly associated with exposure to the index patient’s blood or respiratory secretions.30 A. phagocytophilum remains viable and infectious in refrigerated, stored blood for as long as 18 days.31 A seroprevalence study of 992 blood-donors from Connecticut and Wisconsin demonstrated that 0.4 to 0.9% of the blood donors have A. phagocytophilum antibodies.32 There is no U.S. mandate to screen blood products for A. phagocytophilum infection, and at least 8 cases of HGA acquired via transfusions of infected leuko-reduced blood products have been reported since 2007.33
Beyond A. phagocytophilum, Ixodes ticks are often coinfected with other human pathogens.34,35 Coinfections also occur frequently in the white-footed mouse (Peromyscus leucopus),36,37 and serological investigations and prospective studies of humans who had Lyme borreliosis demonstrate that between 1 and 9 % of individuals living in Wisconsin,38 or western Sweden39 have serologic evidence of prior A. phagocytophilum infection. In a subsequent surveillance study of HGA in Wisconsin, 7 of 142 patients (5%) had erythema migrans and serologic evidence of recent B. burgdorferi infection,27 and both pathogens have been recovered in culture from coinfected individuals on several occasions.40 Thus, sequential or simultaneous infections caused by multiple human pathogens could occur after one or multiple tick-bites, and physicians who diagnose and treat patients with HGA must always consider the possibility of co-infection with other tick-borne agents.
Presentations and Complications
Patients with HGA frequently present with a nonspecific febrile illness. The clinical range of HGA spans from asymptomatic infection to fatal disease and there is a direct correlation between patient age and/or comorbid illnesses and the severity.41 Most symptomatic patients report exposure to ticks one to two weeks before the onset of illness and often complain of fever/sweating/rigors, headache, myalgia, and arthralgia (Table 1), although the physical examination is often otherwise unrevealing. HGA can be severe with 36% of patients requiring hospitalization and 3% with life-threatening complications;21 in one report, up to 17% of hospitalized patients required admission to an intensive care unit.14 Even though many patients present with severe headache or stiff neck prompting lumbar puncture, spinal fluid analysis is usually unremarkable.42 Only a single patient with defined meningitis had A. phagocytophilum documented in the CSF.,43 Among 2,040 cases reported to the CDC between 2000-2007, five (0.2%) had reports of meningitis or encephalitis.21
Table 1.
Published signs, symptoms, and key laboratory abnormalities (%) reported among laboratory-confirmed human granulocytotropic anaplasmosis (HGA) in the USA, Europe, and in Asia (N = 68 to 794 across features).
| Frequency of complaint | Symptom, Sign, or Laboratory Abnormality (number patients evaluated) | Median % (IQR) |
|---|---|---|
| Common | Fever (794) | 100 (90-100) |
| Malaise (391) | 97 (90-98) | |
| Headache (648) | 82 (64-93) | |
| Myalgia (789) | 76 (67-87) | |
| Arthralgia (661) | 56 (27-69) | |
| Elevated serum ALT or AST (397) | 83 (63-98) | |
| Thrombocytopenia (566) | 75 (61-91) | |
| Leukopenia (566) | 55 (47-71) | |
|
| ||
| Less common | Stiff neck (64) | 45 (34-48) |
| Nausea (521) | 39 (35-49) | |
| Cough (523) | 29 (20-30) | |
| Elevated serum creatinine (199) | 49 (25-71) | |
| Anemia (198) | 28 (6-44) | |
|
| ||
| Uncommon | Diarrhea (317) | 21 (13-28) |
| Vomiting (312) | 20 (19-29) | |
| Confusion (470) | 17 (17-18) | |
| Rash* (489) | 6 (3-10) | |
Erythema migrans where described
Serious opportunistic infections can occur in immunocompromised patients during the course of HGA, and fatal cases of herpes simplex esophagitis, Candida albicans pneumonitis/esophagitis, and invasive pulmonary aspergillosis are described.14,44,45 Even though the case fatality rate is ≤1%, significant complications can occur, including septic or toxic shock-like syndrome, acute respiratory distress syndrome, invasive opportunistic infections with both viral and fungal agents, rhabdomyolysis, pancarditis, acute renal failure, hemorrhage, and neurologic diseases such as brachial plexopathy, demyelinating polyneuropathy, and acute transient sensorineural hearing loss.21,45-48
Most patients present with nonspecific changes in routine hematological and chemistry blood tests. Permutations of leukopenia, a left shift (sometimes reaching 50% or even higher), thrombocytopenia, and mild to moderate elevation of hepatic transaminase activities are present in the majority of patients and provide suggestive clues to the diagnosis.41,49 Although both leukopenia and thrombocytopenia are present in many patients at the initial presentation, these abnormalities usually normalize by the end of the second week. Thus, normal WBC and platelet concentrations should not dissuade medical providers from including HGA in the differential diagnosis if the patient reports illness for more than 1 week. In contrast, patients who present with nonspecific fever of less than 7 days duration and either leukocytosis or thrombocytosis have a low probability of having HGA.49
Diagnostic Testing and Imaging
HGA can be laboratory-confirmed at the point of care by examination of a Wright- or Giemsa-stained peripheral blood smear during the early stage of infection.41,49,50 At least 20%, and in some studies up to 100% of patients present with morulae in the cytoplasm of peripheral blood neutrophils in the first week of illness.15,50,51 PCR amplification of A. phagocytophilum–specific DNA from acute phase blood14,15,52 or isolation of A. phagocytophilum in HL-60 promyelocytic leukemia cell cultures inoculated with acute phase blood13,41,53 can also confirm the diagnosis during the early stage of infection, but these test modalities are available in only a limited number of public health and commercial reference laboratories. Acute and convalescent serologic testing using an indirect fluorescent antibody method for A. phagocytophilum IgG with demonstration of four-fold change or seroconversion is the most sensitive confirmatory laboratory test, and has been used most commonly to confirm HGA.25,38,54-56 Specific IgM tests are only reactive during the first 40 days after infection, and are less sensitive than those that detect IgG antibodies, even during this early interval.57 Once a patient becomes seroreactive, antibodies can persist for months or years in the absence of any clinical or laboratory-based evidence for ongoing infection; thus, reductions in antibody titers cannot be used as monitors of effective treatment.25,55 Patients infected by A. phagocytophilum will often develop antibodies that concurrently react with Ehrlichia chaffeensis, the causative agent of human monocytic ehrlichiosis. In some regions, the ticks that transmit these bacteria are both abundant. Thus, the definitive diagnosis must include antibody titers for both pathogens.
The minimal presumptive diagnostic criteria for HGA are unexplained fever and non-specific symptoms such as headache, generalized myalgias, and rigors accompanied by suggestive changes in routine laboratory tests.11,12,14,41,56 Probable and laboratory-confirmed HGA require a nonspecific febrile illness and laboratory confirmation by PCR, culture of blood, and/or detection of specific A. phagocytophilum antibodies in serum (Table 2). Patients who present with an acute clinical illness compatible with HGA should always be considered for specific antibiotic treatment.12,14,41,56 HGA is a reportable illness and all confirmed cases must be reported to the CDC or to the local state health department in the state where the diagnosis was made. The tabular list of International Classification of Diseases (ICD-9) has categorized HGA under the subheading tick-borne rickettsioses/other ehrlichiosis with the numerical code 082.49 (ICD-10 code A77.49).58 It is important to remember that bites by infected Ixodes ticks may lead to simultaneous infections caused by multiple pathogens. The reported frequency of Lyme disease and HGA coinfection varies between 2 to 11.7%,59 and patients who have a positive blood culture for A. phagocytophilum and-or a four-fold rise in antibody titer to at least 640 have significantly more symptoms in total than patients with early Lyme disease defined by the presence of erythema migrans only.60
Table 2.
| Case definition | Laboratory test result |
|---|---|
| Supportive HGA | Morulae present in peripheral blood smear neutrophilsa or |
| Single serum A. phagocytophilum IgG titer by IFAb ≥ 640 | |
|
| |
| Confirmed HGA | A. phagocytophilum IFA IgG seroconversion d or |
| Positive A. phagocytophilum PCRc of blood or | |
| Isolation of A. phagocytophilum from bloode or | |
| A. phagocytophilum antigen present in tissue sample by immunohistochemistry | |
Definitions are dependent upon a presentation with manifestations clinically-consistent with HGA.
Light microscopy of Wright stained peripheral acute phase blood;
Indirect immunofluorescent antibody test with A. phagocytophilum antigen;
Polymerase chain reaction with specific A. phagocytophilum primers;
Fourfold or greater change in serum antibody titer;
Isolation of A. phagocytophilum from blood incubated in HL-60 human promyelocytic cell line.
Imaging studies play little additional roles in the specific diagnosis of HGA but can be very useful for evaluation of the extent of disease and specific organ/tissue involvement.
DIFFERENTIAL DIAGNOSIS
Owing to the undifferentiated presentation of HGA, the differential diagnosis can be vast. With the common manifestations of fever, headache, myalgia and malaise, viral syndromes such as enterovirus infection, Epstein-Barr virus infection, human herpes virus-6 infection, human parvovirus B19 infection, viral hepatitis, West Nile fever and Chickungunya fever should be included on the list of differential diagnoses. Other tick-borne infections including Lyme disease, Borrelia miyamotoi infection, babesiosis, Ehrlichia muris-like agent infection, Powassan virus infection, and B. hermsii relapsing fever must also be kept in mind. Acute bacterial infections to consider include disseminated gonococcal infection, endocarditis, meningococcemia, Mycoplasma pneumoniae infection, group A streptococcal post-infectious syndrome, secondary syphilis, septic shock syndromes, and typhoid fever (Table 3). Inflammatory illnesses of a possible infectious or non-infectious origin include allergic drug reactions, idiopathic thrombocytopenic purpura, immune complex-mediated illnesses, Kawasaki disease, thrombotic thrombocytopenic purpura, and hemophagocytic and macrophage activation syndromes.
Table 3.
Differential Diagnosis of Human Granulocytic Anaplasmosis.
| Exposure type | Viral syndromes | Bacterial agents/syndromes | Parasitic agents/syndromes | Non-infectious syndromes |
|---|---|---|---|---|
| History of vector exposure | Powassan virus disease/tick-borne encephalitis | Lyme disease | Babesiosis | |
| West Nile virus disease | B. miyamotoi infection | Malaria | ||
| Dengue virus fever | B. hermsii infection | |||
| Colorado tick fever | E. chaffeensis infection | |||
| Heartland virus fever | E. ewingii infection | |||
| Severe fever with thrombocytopenia virus infection | E. muris-like agent infection | |||
| Chickungunya virus disease | Rocky Mountain spotted fever | |||
| Murine typhus | ||||
| African tick-bite fever | ||||
| Scrub typhus | ||||
| Bartonellosis | ||||
| Tularemia | ||||
| Leptospirosis | ||||
|
| ||||
| No vector exposure | EBV infection | Acute bacterial endocarditis | Kawasaki syndrome | |
| Human herpes virus-6 infection | Secondary syphilis | ITP | ||
| Parvovirus B19 infection | N. gonorrhea sepsis | TTP | ||
| Viral hepatitis A, B, C | N. meningitidis sepsis | Hemophagocytic syndrome | ||
| Enterovirus infection | Group A Streptococcal infection | Immune-complex illness | ||
| Hantaan virus infection | Leptospirosis | Allergic drug reaction | ||
| Typhoid fever | Acute leukemia | |||
| Lymphoma | ||||
Typically, patients with HGA present during the warm seasons when ticks are known to be active, and up to 75% will report tick bite or exposure to ticks in known tick-infested regions. Other vector-borne zoonoses should be considered for the patient who has had recent documented insect or arthropod-bites, including babesiosis,, Colorado tick fever, human monocytic ehrlichiosis, leptospirosis, Lyme disease, murine typhus, Q fever, rat-bite fever, Rocky Mountain spotted fever and tularemia. In travelers, the list could be expanded to include dengue fever, malaria, leptospirosis, and tick-borne encephalitis. Occasionally the major manifestation will be reflected in hematologic laboratory abnormalities when the differential diagnosis should also include malignancies such as leukemia and lymphoma, especially when intracytoplasmic structures such as Auer rods may be identified and confused with morulae.
TREATMENT
In vitro investigations indicate that A. phagocytophilum is uniformly susceptible to the tetracycline antibiotics.61-64 Doxycycline hyclate has traditionally been the agent of choice because of its good patient tolerance and favorable pharmacokinetic properties compared with other tetracycline derivatives. For the most part, HGA is a mild illness, but there is a known direct relationship between serious infection, including cases with a fatal outcome, and patient variables such as advanced age, ongoing immunosuppressive therapy, predisposing chronic inflammatory illnesses or underlying malignant diseases.42,51 Because of the potential for serious or even fatal infection it is therefore recommended that all patients with suspected or documented HGA should undergo treatment with oral or intravenous doxycycline hyclate in the absence of specific contraindications to tetracycline drugs (Table 4).
Table 4.
| Antibiotic drug | Patient age (years) | Antibiotic dose | Duration (days) |
|---|---|---|---|
| Doxycycline hyclate | ≤ 8 | 2.2 mg/kg 2 times daily IVa or POb | 4 - 5c |
| > 8 | 100 mg 2 times daily IV or PO | 10 - 14d | |
|
| |||
| Tetracycline HCl | > 8 | 500 mg 4 times daily PO | 10 - 14 |
|
| |||
| Rifampin | Pediatrice | 20 mg/kg/d (max. 600 mg) in 2 divided doses PO | 5 - 7f |
| Adultg | 300 mg 2 times daily PO | 5 - 7 | |
Intravenous administration;
Oral administration,
Until fever has resolved and three additional days;
14 days recommended if suspected co-incubating B. burgdorferi infection;
Individuals aged 16 years or less;
Short duration since therapy not directed towards co-incubating B. burgdorferi infection;
Individuals aged 18 years or older.
The recommended therapy for adults is doxycycline 100 mg given orally at 12 hour intervals.42,65 Children older than 8 years should also be treated with doxycycline given in divided doses with dosage adjusted to the patient’s weight (4.4 mg/kg/24 hours, maximum dose 100 mg).65,66 Doxycycline is also the drug of choice for children who are seriously ill regardless of age.66 Doxycycline therapy typically leads to clinical improvement in 24-48 hours.14,41,65,66 Thus, patients who fail to respond to treatment within this time frame should be re-evaluated for alternative diagnoses and treatment.
The optimal duration of doxycycline therapy has not been established. Patients who have been treated for 7 to 10 days resolve their infection completely, and relapse or chronic infection has never been reported, even for those patients who were never treated with an active antibiotic. However, adult patients who are considered at risk for co-infection with B. burgdorferi should continue doxycycline therapy for a full 14 days. A shorter course of doxycycline (5 to 7 days) has been advocated for pediatric age-group patients because of the potential risk for adverse effects (dental staining) seen occasionally in young children.65-67
Rifamycins also have excellent in vitro activity against A. phagocytophilum.61-63 A few pregnant women and pediatric patients have been treated successfully with rifampin.68-70 Thus, patients who have HGA and who are unsuited for tetracycline treatment due to a history of drug allergy or pregnancy, and children younger than 8 years of age who are not seriously ill should be considered for rifampin therapy. Studies with levofloxacin demonstrate some activity in vitro.61-63 However, at least one patient with HGA who received a 13 day course of levofloxacin initially had a clinical response only to relapse when the regimen was discontinued suggesting that fluoroquinolones should not be used.71
PROGNOSIS AND LONG TERM OUTCOME
More than 15,952 patients were diagnosed with HGA and reported to state and federal health-agencies since 1994. Only 8 patients are known from published literature to have died during the active phase of HGA, although a CDC review of national surveillance systems between 2000 and 2007 identified 11 fatalities.14,21,44,45 While the case fatality rate for HGA has been estimated at 1.2% among those 20-39 years of age, the overall case fatality rate is likely between 0.2% and 1.2%.21 Published case-report series indicate that HGA most often is a mild, self-limited illness that resolves even without antibiotic treatment.14,15,55,72-74
Patients who are treated with doxycycline or rifampin typically resolve fever and most of their physical complaints within 24 to 48 hours. A small number of patients who are diagnosed with HGA do not receive any antibiotic therapy or they receive ineffective antibiotic therapy, but nearly all these patients make a complete recovery within 60 days.14 PCR analysis of serial blood samples collected from untreated patients during convalescence indicates that bacteremia can persist for up to 30 days.29,75-77 There are no published reports of patients with active clinical illness persisting beyond two months, although a single longitudinal study in Wisconsin reported significantly more recurrent or continuous fevers, chills, fatigue and sweats within 1 year after infection.73 Thus, the long term prognosis appears to be favorable and patients are expected to make a complete recovery. There is currently no published clinical evidence to suggest that untreated HGA evolves into a chronic illness in humans, as persistently elevated antibody titers should be interpreted as evidence of past infection rather than proof of an ongoing unresolved infectious process.
IMMUNITY AND REINFECTION
Most patients acquire HGA in the geographical region where they live, work or recreate.14-16,21,72,73 It is therefore reasonable to assume that those individuals remain at risk for future bites by infected Ixodes ticks and potential HGA reinfection. Nevertheless, conclusive evidence of A. phagocytophilum infection occurring more than once is exceedingly rare.78 Passive administration of A. phagocytophilum antibodies partially protects laboratory animals in murine models of HGA against infection; thus, it is likely that patients who develop high antibody titers to A. phagocytophilum are equally protected against reinfection after subsequent tick-bites. Serum A. phagocytophilum antibody titers remain elevated for a median of 12-18 months after HGA has resolved.38 However, some infected human patients maintain elevated A. phagocytophilum antibody titers for as long as 3 years after infection.16,25,55 Whether previous infections of humans leads to immunologic memory and a subsequent anamnestic protective immune response upon re-challenge with A. phagocytophilum is unknown.
Horses that are convalescent from A. phagocytophilum infection develop immunity and resistance to experimental challenge 8 weeks after infection.79 However, laboratory mice that were actively immunized with lysates of purified A. phagocytophilum were only partially protected against challenges with A. phagocytophilum.80 The incomplete protection by both immunization with heat-inactivated bacteria and passive antibody administration suggests that protective immunity requires more than the presence of antibodies.
PREVENTION
Avoidance of tick bites and prompt removal of attached ticks remain the best disease prevention strategy. Individuals who are exposed in tick habitats should wear protective clothing, including long-sleeved shirts, long-legged pants, socks wrapped outside the pant legs and close-toed shoes to make it harder for ticks to reach bare skin and attach (bite). Light-colored pants could make it easier to see and remove crawling ticks. Tick repellents such as DEET (N,N-diethyl-m-toluamide) are available for application to exposed skin and clothing, and alternative repellents such as picaridin are becoming more readily available. These agents should be considered for use by individuals whose occupation or recreation exposes to tick habitats where the risk of being bitten is high. Permethrin cannot be applied directly to skin, but can be applied to clothing before the clothing is worn and is considered an excellent choice for significant exposure risks. No vaccines that prevent human or veterinary granulocytic anaplasmosis are currently available.
Persons who spend time in tick-endemic areas should inspect themselves frequently for ticks. All attached ticks should be removed by gently grasping the tick with tweezers or forceps close to the skin and slowly pulling straight out with constant traction. Routine disinfection of the bite wound with isopropyl alcohol or tincture of iodine reduces the risk of contamination of the bite site with skin bacteria. Studies have shown that a period of 4 to 24 hours or more may be necessary before A. phagocytophilum becomes biologically “activated” and successful transmission of infective organisms from the tick to the host takes place.81 Thus, the longer an infected tick is permitted to feed, the more likely the bite will result in infection. Therefore, prompt and complete removal of attached ticks is indicated to minimize the risk of infection. The potential value of prophylactic doxycycline administration has never been tested in prospective, randomized trials.
SUMMARY
Patients who present with non-specific fever after exposure to ticks should be evaluated by clinical examination and routine laboratory testing to determine if the illness is potentially a tick-borne infection. Laboratory abnormalities such as leukopenia with relative granulocytosis and a left shift, thrombocytopenia, and mild increases in serum hepatic transaminase activities warrant consideration for treatment with doxycycline. These patients should also undergo specific laboratory testing to confirm the diagnosis of HGA.
Sensitive and specific laboratory tests that provide rapid diagnostic confirmation are generally not available in the acute care setting.11,41 Thus, patients with suspected HGA should begin empiric antibiotic treatment as soon as blood samples have been collected for confirmatory laboratory testing. Acute phase serum samples should be paired with convalescent serum to detect seroconversion in those instances, especially when blood-smear microscopy, PCR, or cell culture testing are either unavailable or inconclusive.
Figure 2.
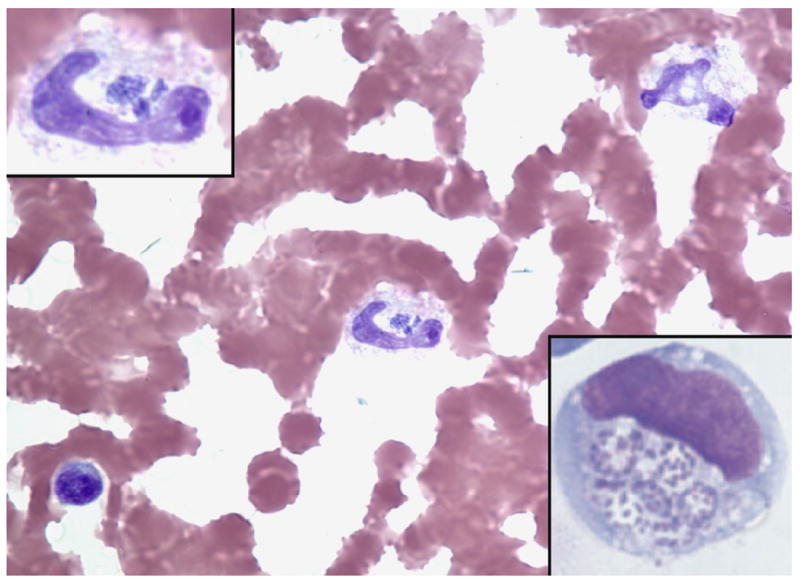
Anaplasma phagocytophilum-infected band neutrophil in human peripheral blood (Wright stain, original magnification × 260). The top left inset shows the same band neutrophil and demonstrates several morulae with a stippled basophilic appearance corresponding to individual bacteria (magnification × 520). The bottom right insert shows A. phagocytophilum cultivated in vitro in the human promyelocytic leukemia cell line HL-60. Here, the individual basophilic bacteria are easily visualized within vacuoles of the infected cell (LeukoStat stain; magnification × 520).
Key Points.
The clinical presentation of human granulocytic anaplasmosis is an acute febrile nonspecific viral-like illness. Leukopenia, thrombocytopenia and elevated hepatic transaminases are commonly seen early in disease.
A history of a tick bite or exposure is important, but its absence and lack of a diagnostic test result should not mitigate clinical consideration.
Early treatment with doxycycline for adults and children (including those younger than 8 years of age) should be instituted on clinical suspicion alone.
Although hospitalization occurs in 36%, and life threatening disease occurs in 3% of cases; the case fatality rate is low (0.6%) and most patients resolve infections without complications.
Persistent infection has not been demonstrated, and evidence to support a role in chronic illness is lacking. Acute coinfections with other tick-transmitted pathogens can occur.
Acknowledgments
This work was supported by NIH R01AI44102
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ricketts HT. The study of “Rocky Mountain spotted fever“ (tick fever) by means of animal inoculations. J Am Med Assoc. 1906;47:1–10. [Google Scholar]
- 2.Pritt BS, McFadden JD, Stromdahl E, et al. Emergence of a novel Ehrlichia sp. agent pathogenic for humans in the Midwestern United States. 6th International Meeting on Rickettsiae and Rickettsial Diseases; Heraklion, Crete, Greece: Hellenic Society for Infectious Diseases; 2011. Abstract no P075. [Google Scholar]
- 3.Platonov AE, Karan LS, Kolyasnikova NM, et al. Humans infected with relapsing fever spirochete Borrelia miyamotoi, Russia. Emerg Infect Dis. 2011;17:1816–23. doi: 10.3201/eid1710.101474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steere AC, Malawista SE, Snydman DR, et al. Lyme arthritis: an epidemic of oligoarticular arthritis in children and adults in three connecticut communities. Arthritis and rheumatism. 1977;20:7–17. doi: 10.1002/art.1780200102. [DOI] [PubMed] [Google Scholar]
- 5.Maeda K, Markowitz N, Hawley RC, Ristic M, Cox D, McDade JE. Human infection with Ehrlichia canis, a leukocytic rickettsia. N Engl J Med. 1987;316:853–6. doi: 10.1056/NEJM198704023161406. [DOI] [PubMed] [Google Scholar]
- 6.Healy GR, Speilman A, Gleason N. Human babesiosis: reservoir in infection on Nantucket Island. Science. 1976;192:479–80. doi: 10.1126/science.769166. [DOI] [PubMed] [Google Scholar]
- 7.Buller RS, Arens M, Hmiel SP, et al. Ehrlichia ewingii, a newly recognized agent of human ehrlichiosis. N Engl J Med. 1999;341:148–55. doi: 10.1056/NEJM199907153410303. [DOI] [PubMed] [Google Scholar]
- 8.Bakken JS, Dumler JS, Chen SM, Eckman MR, Van Etta LL, Walker DH. Human granulocytic ehrlichiosis in the upper Midwest United States. A new species emerging? JAMA. 1994;272:212–8. [PubMed] [Google Scholar]
- 9.Burgdorfer W. Arthropod-borne spirochetoses: a historical perspective. Eur J Clin Microbiol Infect Dis. 2001;20:1–5. doi: 10.1007/s100960000415. [DOI] [PubMed] [Google Scholar]
- 10.McMullan LK, Folk SM, Kelly AJ, et al. A new phlebovirus associated with severe febrile illness in Missouri. N Engl J Med. 2012;367:834–41. doi: 10.1056/NEJMoa1203378. [DOI] [PubMed] [Google Scholar]
- 11.Chapman AS, Bakken JS, Folk SM, et al. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever, ehrlichioses, and anaplasmosis--United States: a practical guide for physicians and other health-care and public health professionals. MMWR Recomm Rep. 2006;55:1–27. [PubMed] [Google Scholar]
- 12.Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1089–134. doi: 10.1086/508667. [DOI] [PubMed] [Google Scholar]
- 13.Goodman JL, Nelson C, Vitale B, et al. Direct cultivation of the causative agent of human granulocytic ehrlichiosis. N Engl J Med. 1996;334:209–15. doi: 10.1056/NEJM199601253340401. [DOI] [PubMed] [Google Scholar]
- 14.Bakken JS, Krueth J, Wilson-Nordskog C, Tilden RL, Asanovich K, Dumler JS. Clinical and laboratory characteristics of human granulocytic ehrlichiosis. JAMA. 1996;275:199–205. [PubMed] [Google Scholar]
- 15.Aguero-Rosenfeld ME, Horowitz HW, Wormser GP, et al. Human granulocytic ehrlichiosis: a case series from a medical center in New York State. Ann Intern Med. 1996;125:904–8. doi: 10.7326/0003-4819-125-11-199612010-00006. [DOI] [PubMed] [Google Scholar]
- 16.Horowitz HW, Aguero-Rosenfeld ME, McKenna DF, et al. Clinical and laboratory spectrum of culture-proven human granulocytic ehrlichiosis: comparison with culture-negative cases. Clin Infect Dis. 1998;27:1314–7. doi: 10.1086/515000. [DOI] [PubMed] [Google Scholar]
- 17.Steere AC. Lyme disease. N Engl J Med. 2001;345:115–25. doi: 10.1056/NEJM200107123450207. [DOI] [PubMed] [Google Scholar]
- 18.Krause PJ. Babesiosis. Med Clin North Am. 2002;86:361–73. doi: 10.1016/s0025-7125(03)00092-0. [DOI] [PubMed] [Google Scholar]
- 19.Pritt BS, Sloan LM, Johnson DK, et al. Emergence of a new pathogenic Ehrlichia species, Wisconsin and Minnesota, 2009. N Engl J Med. 2011;365:422–9. doi: 10.1056/NEJMoa1010493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McLean DM, Donohue WL. Powassan virus: isolation of virus from a fatal case of encephalitis. Can Med Assoc J. 1959;80:708–11. [PMC free article] [PubMed] [Google Scholar]
- 21.Dahlgren FS, Mandel EJ, Krebs JW, Massung RF, McQuiston JH. Increasing incidence of Ehrlichia chaffeensis and Anaplasma phagocytophilum in the United States, 2000-2007. Am J Trop Med Hyg. 2011;85:124–31. doi: 10.4269/ajtmh.2011.10-0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diuk-Wasser MA, Hoen AG, Cislo P, et al. Human risk of infection with Borrelia burgdorferi, the Lyme disease agent, in eastern United States. Am J Trop Med Hyg. 2012;86:320–7. doi: 10.4269/ajtmh.2012.11-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koffi JK, Leighton PA, Pelcat Y, et al. Passive surveillance for I. scapularis ticks: enhanced analysis for early detection of emerging Lyme disease risk. Journal of medical entomology. 2012;49:400–9. doi: 10.1603/me11210. [DOI] [PubMed] [Google Scholar]
- 24.Hao Q, Geng Z, Hou XX, et al. Seroepidemiological Investigation of Lyme Disease and Human Granulocytic Anaplasmosis among People Living in Forest Areas of Eight Provinces in China. Biomed Environ Sci. 2013;26:185–9. doi: 10.3967/0895-3988.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Aguero-Rosenfeld ME, Donnarumma L, Zentmaier L, et al. Seroprevalence of antibodies that react with Anaplasma phagocytophila, the agent of human granulocytic ehrlichiosis, in different populations in Westchester County, New York. J Clin Microbiol. 2002;40:2612–5. doi: 10.1128/JCM.40.7.2612-2615.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bakken JS, Goellner P, Van Etten M, et al. Seroprevalence of human granulocytic ehrlichiosis among permanent residents of northwestern Wisconsin. Clin Infect Dis. 1998;27:1491–6. doi: 10.1086/515048. [DOI] [PubMed] [Google Scholar]
- 27.Belongia EA, Gale CM, Reed KD, et al. Population-based incidence of human granulocytic ehrlichiosis in northwestern Wisconsin, 1997-1999. J Infect Dis. 2001;184:1470–4. doi: 10.1086/324517. [DOI] [PubMed] [Google Scholar]
- 28.Horowitz HW, Kilchevsky E, Haber S, et al. Perinatal transmission of the agent of human granulocytic ehrlichiosis. N Engl J Med. 1998;339:375–8. doi: 10.1056/NEJM199808063390604. [DOI] [PubMed] [Google Scholar]
- 29.Bakken JS, Krueth JK, Lund T, Malkovitch D, Asanovich K, Dumler JS. Exposure to deer blood may be a cause of human granulocytic ehrlichiosis. Clin Infect Dis. 1996;23:198. doi: 10.1093/clinids/23.1.198. [DOI] [PubMed] [Google Scholar]
- 30.Zhang L, Liu Y, Ni D, et al. Nosocomial transmission of human granulocytic anaplasmosis in China. JAMA. 2008;300:2263–70. doi: 10.1001/jama.2008.626. [DOI] [PubMed] [Google Scholar]
- 31.Kalantarpour F, Chowdhury I, Wormser GP, Aguero-Rosenfeld ME. Survival of the human granulocytic ehrlichiosis agent under refrigeration conditions. J Clin Microbiol. 2000;38:2398–9. doi: 10.1128/jcm.38.6.2398-2399.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leiby DA, Chung AP, Cable RG, et al. Relationship between tick bites and the seroprevalence of Babesia microti and Anaplasma phagocytophila (previously Ehrlichia sp.) in blood donors. Transfusion. 2002;42:1585–91. doi: 10.1046/j.1537-2995.2002.00251.x. [DOI] [PubMed] [Google Scholar]
- 33.Townsend RL, Moritz ED, Fialkow LB, Berardi V, Stramer SL. Probable transfusion-transmission of Anaplasma phagocytophilum by leukoreduced platelets. Transfusion. 2014 doi: 10.1111/trf.12675. [DOI] [PubMed] [Google Scholar]
- 34.Telford SR, 3rd, Dawson JE, Katavolos P, Warner CK, Kolbert CP, Persing DH. Perpetuation of the agent of human granulocytic ehrlichiosis in a deer tick-rodent cycle. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:6209–14. doi: 10.1073/pnas.93.12.6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Varde S, Beckley J, Schwartz I. Prevalence of tick-borne pathogens in Ixodes scapularis in a rural New Jersey County. Emerg Infect Dis. 1998;4:97–9. doi: 10.3201/eid0401.980113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anderson JF. The natural history of ticks. The Medical clinics of North America. 2002;86:205–18. doi: 10.1016/s0025-7125(03)00083-x. [DOI] [PubMed] [Google Scholar]
- 37.Stafford KC, 3rd, Massung RF, Magnarelli LA, Ijdo JW, Anderson JF. Infection with agents of human granulocytic ehrlichiosis, lyme disease, and babesiosis in wild white-footed mice (Peromyscus leucopus) in Connecticut. J Clin Microbiol. 1999;37:2887–92. doi: 10.1128/jcm.37.9.2887-2892.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitchell PD, Reed KD, Hofkes JM. Immunoserologic evidence of coinfection with Borrelia burgdorferi, Babesia microti, and human granulocytic Ehrlichia species in residents of Wisconsin and Minnesota. J Clin Microbiol. 1996;34:724–7. doi: 10.1128/jcm.34.3.724-727.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dumler JS, Dotevall L, Gustafson R, Granstrom M. A population-based seroepidemiologic study of human granulocytic ehrlichiosis and Lyme borreliosis on the west coast of Sweden. J Infect Dis. 1997;175:720–2. doi: 10.1093/infdis/175.3.720. [DOI] [PubMed] [Google Scholar]
- 40.De Martino SJ, Carlyon JA, Fikrig E. Coinfection with Borrelia burgdorferi and the agent of human granulocytic ehrlichiosis. N Engl J Med. 2001;345:150–1. doi: 10.1056/NEJM200107123450218. [DOI] [PubMed] [Google Scholar]
- 41.Bakken JS, Dumler JS. Clinical diagnosis and treatment of human granulocytotropic anaplasmosis. Ann N Y Acad Sci. 2006;1078:236–47. doi: 10.1196/annals.1374.042. [DOI] [PubMed] [Google Scholar]
- 42.Bakken JS, Dumler JS. Human granulocytic ehrlichiosis. Clin Infect Dis. 2000;31:554–60. doi: 10.1086/313948. [DOI] [PubMed] [Google Scholar]
- 43.Lee FS, Chu FK, Tackley M, Wu AD, Atri A, Wessels MR. Human granulocytic ehrlichiosis presenting as facial diplegia in a 42-year-old woman. Clin Infect Dis. 2000;31:1288–91. doi: 10.1086/317466. [DOI] [PubMed] [Google Scholar]
- 44.Hardalo CJ, Quagliarello V, Dumler JS. Human granulocytic ehrlichiosis in Connecticut: report of a fatal case. Clin Infect Dis. 1995;21:910–4. doi: 10.1093/clinids/21.4.910. [DOI] [PubMed] [Google Scholar]
- 45.Jahangir A, Kolbert C, Edwards W, Mitchell P, Dumler JS, Persing DH. Fatal pancarditis associated with human granulocytic Ehrlichiosis in a 44-year-old man. Clin Infect Dis. 1998;27:1424–7. doi: 10.1086/515014. [DOI] [PubMed] [Google Scholar]
- 46.Bakken JS, Erlemeyer SA, Kanoff RJ, Silvestrini TC, 2nd, Goodwin DD, Dumler JS. Demyelinating polyneuropathy associated with human granulocytic ehrlichiosis. Clin Infect Dis. 1998;27:1323–4. [PubMed] [Google Scholar]
- 47.Horowitz HW, Marks SJ, Weintraub M, Dumler JS. Brachial plexopathy associated with human granulocytic ehrlichiosis. Neurology. 1996;46:1026–9. doi: 10.1212/wnl.46.4.1026. [DOI] [PubMed] [Google Scholar]
- 48.Lepidi H, Bunnell JE, Martin ME, Madigan JE, Stuen S, Dumler JS. Comparative pathology, and immunohistology associated with clinical illness after Ehrlichia phagocytophila-group infections. Am J Trop Med Hyg. 2000;62:29–37. doi: 10.4269/ajtmh.2000.62.29. [DOI] [PubMed] [Google Scholar]
- 49.Bakken JS, Aguero-Rosenfeld ME, Tilden RL, et al. Serial measurements of hematologic counts during the active phase of human granulocytic ehrlichiosis. Clin Infect Dis. 2001;32:862–70. doi: 10.1086/319350. [DOI] [PubMed] [Google Scholar]
- 50.Rand JV, Tarasen AJ, Kumar J, Homan SM, Tobin E. Intracytoplasmic granulocytic morulae counts on confirmed cases of ehrlichiosis/anaplasmosis in the Northeast. Am J Clin Pathol. 2014;141:683–6. doi: 10.1309/AJCP6Q2BOKYALDYZ. [DOI] [PubMed] [Google Scholar]
- 51.Dumler JS, Madigan JE, Pusterla N, Bakken JS. Ehrlichioses in humans: epidemiology, clinical presentation, diagnosis, and treatment. Clin Infect Dis. 2007;45(Suppl 1):S45–51. doi: 10.1086/518146. [DOI] [PubMed] [Google Scholar]
- 52.Massung RF, Slater KG. Comparison of PCR assays for detection of the agent of human granulocytic ehrlichiosis, Anaplasma phagocytophilum. J Clin Microbiol. 2003;41:717–22. doi: 10.1128/JCM.41.2.717-722.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dumler JS, Choi KS, Garcia-Garcia JC, et al. Human granulocytic anaplasmosis and Anaplasma phagocytophilum. Emerg Infect Dis. 2005;11:1828–34. doi: 10.3201/eid1112.050898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aguero-Rosenfeld ME. Diagnosis of human granulocytic ehrlichiosis: state of the art. Vector borne and zoonotic diseases (Larchmont, NY) 2002;2:233–9. doi: 10.1089/153036602321653815. [DOI] [PubMed] [Google Scholar]
- 55.Bakken JS, Haller I, Riddell D, Walls JJ, Dumler JS. The serological response of patients infected with the agent of human granulocytic ehrlichiosis. Clin Infect Dis. 2002;34:22–7. doi: 10.1086/323811. [DOI] [PubMed] [Google Scholar]
- 56.Belongia EA, Reed KD, Mitchell PD, et al. Tickborne infections as a cause of nonspecific febrile illness in Wisconsin. Clin Infect Dis. 2001;32:1434–9. doi: 10.1086/320160. [DOI] [PubMed] [Google Scholar]
- 57.Walls JJ, Aguero-Rosenfeld M, Bakken JS, et al. Inter- and intralaboratory comparison of Ehrlichia equi and human granulocytic ehrlichiosis (HGE) agent strains for serodiagnosis of HGE by the immunofluorescent-antibody test. J Clin Microbiol. 1999;37:2968–673. doi: 10.1128/jcm.37.9.2968-2973.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. [September 19, 2014];ICD10Data.com. 2014 2014, at http://www.icd10data.com/ICD10CM/Codes/A00-B99/A75-A79/A77-/A77.49.)
- 59.Steere AC, McHugh G, Damle N, Sikand VK. Prospective study of serologic tests for lyme disease. Clin Infect Dis. 2008;47:188–95. doi: 10.1086/589242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wormser GP, Aguero-Rosenfeld ME, Cox ME, et al. Differences and similarities between culture-confirmed human granulocytic anaplasmosis and early lyme disease. J Clin Microbiol. 2013;51:954–8. doi: 10.1128/JCM.02929-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Horowitz HW, Hsieh TC, Aguero-Rosenfeld ME, et al. Antimicrobial susceptibility of Ehrlichia phagocytophila. Antimicrobial agents and chemotherapy. 2001;45:786–8. doi: 10.1128/AAC.45.3.786-788.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klein MB, Nelson CM, Goodman JL. Antibiotic susceptibility of the newly cultivated agent of human granulocytic ehrlichiosis: promising activity of quinolones and rifamycins. Antimicrobial agents and chemotherapy. 1997;41:76–9. doi: 10.1128/aac.41.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maurin M, Bakken JS, Dumler JS. Antibiotic susceptibilities of Anaplasma (Ehrlichia) phagocytophilum strains from various geographic areas in the United States. Antimicrobial agents and chemotherapy. 2003;47:413–5. doi: 10.1128/AAC.47.1.413-415.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Branger S, Rolain JM, Raoult D. Evaluation of antibiotic susceptibilities of Ehrlichia canis, Ehrlichia chaffeensis, and Anaplasma phagocytophilum by real-time PCR. Antimicrob Agents Chemother. 2004;48:4822–8. doi: 10.1128/AAC.48.12.4822-4828.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bakken JS, Dumler JS. Ehrlichia and Anaplasma species. In: Yu V, Weber R, Raoult D, editors. Antimicrobial Therapy and Vaccine. 2. New York, N.Y.: Apple Trees Production, L.L.C.; 2002. pp. 875–82. [Google Scholar]
- 66.Committee on Infectious Diseases AAoP. Ehrlichia and Anaplasma infections (human ehrlichiosis and anaplasmosis) In: Pickering LK, Baker CJ, Kimberlin DW, Long SS, editors. Red Book®: 2012 Report of the Committee on Infectious Diseases. 29. Elk Grove Village, IL: American Academy of Pediatrics; 2012. pp. 312–5. [Google Scholar]
- 67.Volovitz B, Shkap R, Amir J, Calderon S, Varsano I, Nussinovitch M. Absence of tooth staining with doxycycline treatment in young children. Clin Pediatr (Phila) 2007;46:121–6. doi: 10.1177/0009922806290026. [DOI] [PubMed] [Google Scholar]
- 68.Buitrago MI, Ijdo JW, Rinaudo P, et al. Human granulocytic ehrlichiosis during pregnancy treated successfully with rifampin. Clin Infect Dis. 1998;27:213–5. doi: 10.1086/517678. [DOI] [PubMed] [Google Scholar]
- 69.Elston DM. Perinatal transmission of human granulocytic ehrlichiosis. N Engl J Med. 1998;339:1941–2. [PubMed] [Google Scholar]
- 70.Krause PJ, Corrow CL, Bakken JS. Successful treatment of human granulocytic ehrlichiosis in children using rifampin. Pediatrics. 2003;112:e252–3. doi: 10.1542/peds.112.3.e252. [DOI] [PubMed] [Google Scholar]
- 71.Wormser GP, Filozov A, Telford SR, 3rd, et al. Dissociation between inhibition and killing by levofloxacin in human granulocytic anaplasmosis. Vec Borne Zoon Dis. 2006;6:388–94. doi: 10.1089/vbz.2006.6.388. [DOI] [PubMed] [Google Scholar]
- 72.Belongia EA, Reed KD, Mitchell PD, et al. Clinical and epidemiological features of early Lyme disease and human granulocytic ehrlichiosis in Wisconsin. Clin Infect Dis. 1999;29:1472–7. doi: 10.1086/313532. [DOI] [PubMed] [Google Scholar]
- 73.Ramsey AH, Belongia EA, Gale CM, Davis JP. Outcomes of treated human granulocytic ehrlichiosis cases. Emerg Infect Dis. 2002;8:398–401. doi: 10.3201/eid0804.010222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wallace BJ, Brady G, Ackman DM, et al. Human granulocytic ehrlichiosis in New York. Arch Intern Med. 1998;158:769–73. doi: 10.1001/archinte.158.7.769. [DOI] [PubMed] [Google Scholar]
- 75.Bjoersdorff A, Berglund J, Kristiansen BE, Soderstrom C, Eliasson I. Varying clinical picture and course of human granulocytic ehrlichiosis. Twelve Scandinavian cases of the new tick-borne zoonosis are presented. Lakartidningen. 1999;96:4200–4. [PubMed] [Google Scholar]
- 76.Schotthoefer AM, Meece JK, Ivacic LC, et al. Comparison of a real-time PCR method with serology and blood smear analysis for diagnosis of human anaplasmosis: importance of infection time course for optimal test utilization. J Clin Microbiol. 2013;51:2147–53. doi: 10.1128/JCM.00347-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dumler JS, Bakken JS. Human granulocytic ehrlichiosis in Wisconsin and Minnesota: a frequent infection with the potential for persistence. J Infect Dis. 1996;173:1027–30. doi: 10.1093/infdis/173.4.1027. [DOI] [PubMed] [Google Scholar]
- 78.Horowitz HW, Aguero-Rosenfeld M, Dumler JS, et al. Reinfection with the agent of human granulocytic ehrlichiosis. Ann Intern Med. 1998;129:461–3. doi: 10.7326/0003-4819-129-6-199809150-00007. [DOI] [PubMed] [Google Scholar]
- 79.Barlough JE, Madigan JE, DeRock E, Dumler JS, Bakken JS. Protection against Ehrlichia equi is conferred by prior infection with the human granulocytotropic Ehrlichia (HGE agent) J Clin Microbiol. 1995;33:3333–4. doi: 10.1128/jcm.33.12.3333-3334.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sun W, JW IJ, Telford SR, 3rd, et al. Immunization against the agent of human granulocytic ehrlichiosis in a murine model. J Clin Invest. 1997;100:3014–8. doi: 10.1172/JCI119855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Katavolos P, Armstrong PM, Dawson JE, Telford SR., 3rd Duration of tick attachment required for transmission of granulocytic ehrlichiosis. J Infect Dis. 1998;177:1422–5. doi: 10.1086/517829. [DOI] [PubMed] [Google Scholar]
- 82.Centers for Disease Control and Prevention; 2008. [September 19, 2014]. Ehrlichiosis and anaplasmosis 2008 case definition. 2014, at http://wwwn.cdc.gov/nndss/script/casedef.aspx?CondYrID=667&DatePub=1/1/2008.) [Google Scholar]


