Abstract
Transient receptor potential (TRP) proteins are cation channels that comprise a superfamily of molecular sensors that enable animals to detect a wide variety of environmental stimuli. This versatility enables vertebrate and invertebrate TRP channels to function in a diversity of senses, ranging from vision to taste, smell, touch, hearing, proprioception and thermosensation. Moreover, many individual TRP channels are activated through a surprising range of sensory stimuli. The multitasking nature of TRP channels raises the question as to whether seemingly disparate activators gate TRPs through common strategies. In this regard, a recent major advance is the discovery that a phospholipase C (PLC)-dependent signaling cascade activates the TRP channels in Drosophila photoreceptor cells through generation of force in the lipid-bilayer. The premise of this review is that mechanical force is a unifying, common strategy for gating TRP channels. In addition to several TRP channels that function in mechanosensation and are gated by force applied to the cells, changes in temperature and in the concentration of lipophilic second messengers through stimulation of signaling cascades, cause architectural modifications of the cell membrane, which in turn activate TRP channels through mechanical force. Consequently, TRPs are capable of functioning as stretch-activated channels, even in cases in which the stimuli that initiate the signaling cascades are not mechanical. We propose that most TRPs are actually mechanosensitive channels (MSCs), which undergo conformational changes in response to tension imposed on the lipid bilayer, resulting in channel gating.
Introduction
The founding member of the TRP family of cation channels was identified nearly 20 years ago in Drosophila [1,2]. We now know that this group of proteins is conserved from worms to humans [3], and consists of between 13 and 28 members depending on the species [4,5,6]. The TRPs are subdivided into seven subfamilies based on sequence homology (TRPC, TRPV, TRPM, TRPA, TRPN, TRPML and TRPP) [7], and include the common features of six transmembrane segments and permeability to cations. TRPs serve as sensors for a broad spectrum of stimuli, including light [2,8,9], odors [10,11,12], tastants [13,14], acids [15,16], temperature [17,18], gravity [19], auditory stimuli [20,21], as well as light and noxious mechanical forces [22,23,24]. These channels not only promote the perception of the external environment, they allow individual cells in animals to sample and respond to internal stimuli. One example is TRPP2 (Polycystin2), which is proposed to sense fluid flow in renal tubules [25]. Mutations in the genes encodingTRPP2 and a related protein with 11 predicted transmembrane segments, TRPP1 (Polycystin1), are the major causes of autosomal dominant polycystic kidney disease [26,27,28].
The stimuli that activate TRPs do so either through multistep signaling cascades, or through a single step, that does not depend on production of second messengers. Examples of multistep mechanisms are the cascades in fly photoreceptor cells and mammalian intrinsically photosensitive retinal ganglion cells, which are initiated by light-activation of rhodopsins [29,30]. These classical G-protein coupled receptors (GPCRs) engage heterotrimeric G-proteins that stimulate PLC, which in turn activate TRPC channels [2,8,9,31,32]. Similar signaling cascades function in a variety of other cell types such as mammalian taste receptor cells [13]. In contrast to these multistep mechanisms, TRP channels also appear to be gated in a single step by changes in force, binding of chemicals and shifts in temperature [4,5,6,33,34].
Strikingly, many individual TRP channels are activated through a range of stimuli. A notable illustration of this polymodal feature of TRPs is activation of TRPV1 by capsaicin (the active ingredient of chili peppers) [17], allyl isothiocyanate (AITC, component of mustard and wasabi that is responsible for their pungent taste) [35], resiniferatoxin (a toxin found in a cactus-type plant) [36], noxious heat [17], acidic pH [17], and N-acyl amide [37].
How do TRP channels respond to such broad arrays of stimuli? A breakthrough in our understanding of the mechanisms underlying channel gating emerged from the recent high-resolution structure of TRPV1, which was solved by cryo-electron microscopy [38,39]. Using peptide toxin and small vanilloid agonists, it now appears that opening of the channel pore results from a dual gate that involves structural changes in the outer pore domain and lower gate. Not all stimuli may contribute to channel opening through precisely the same biophysical mechanism, since different stimuli may act on one or the other portions of the dual gate, and then the effects are coupled allosterically. The dual-gating of TRPV1 explains why various chemical agonists promote channel gating. However, without obtaining “snapshots” of the channel at different temperatures, we still do not know how TRPV1 is thermally activated.
In this review, we posit that most TRPs are mechanosensitive channels (MSCs). In some instances, mechanical gating of TRP channels occurs by stimuli that activate TRPs through a single step, without employment of a signaling cascade. In other cases, even when the initial stimulus is not mechanical (e.g. light, chemicals or changes in temperature), TRPs are mechanically-gated through signaling cascades that alter the lipid composition of the membrane, change the conformation of the plasma membrane, and gate the TRPs through membrane stretch. Thus, even if activation of a TRP channel is coupled to a signaling cascade, and the initial stimulus is not mechanical force, the TRP can still be activated directly by force.
Direct Mechanical gating of TRP channels in one step
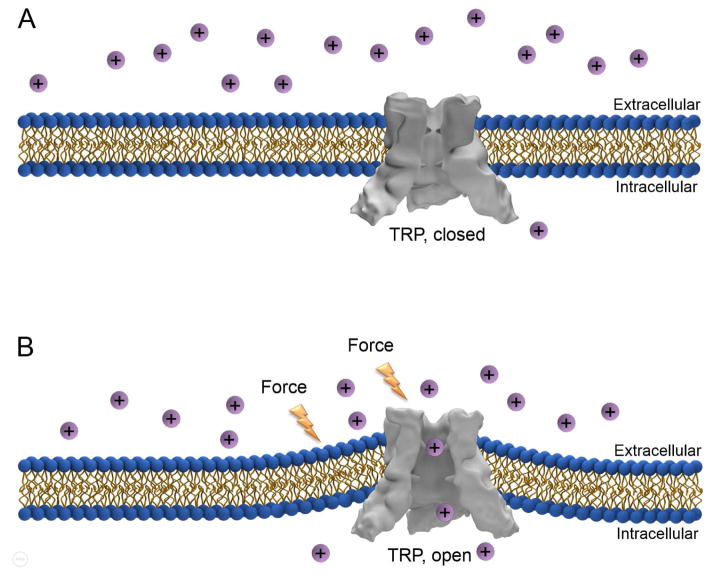
Most TRP channels are situated in the plasma membrane and as a result, force from an extracellular source could potentially modulate the open probability of the channels, without the contribution of a signaling cascade (Fig. 1). A mechanical stimulus could exert its influence on a TRP channel directly. Alternatively, mechanical force could alter the curvature of the lipid-bilayer, which in turn creates a tension that opens the channel. We also view this latter mechanism as direct mechanical activation, since the architectural modifications in the lipid bilayer provide the force that provokes opening of the channels.
Figure 1.
Model of direct force-activation of TRP channels without a signaling cascade. (A) The TRP channel is closed prior to the mechanical stimulus. (B) The TRP channel opens in response to application of external force to the plasma membrane and/or the TRP channel. No signaling cascade is involved in this process.
If a TRP is directly activated by force, without the contribution of a signal cascade, then the activation should be very rapid. Currently, the fastest known sensory signaling cascade is fly phototransduction. In Drosophila photoreceptor cells, some TRP and TRPL channel open within a couple of milliseconds of light exposure, and response is maximal within 10–20 milliseconds [40,41]. Thus, if mechanical activation occurs in less than ~2 millseconds, then it is likely to be independent of a signaling cascade.
The C. elegans TRPN channel, TRP-4 is required for force-induced conductance in the ciliated mechanosensory cephalic neuron, and is directly mechanically gated in vivo, as it is activated in less than one millisecond [42]. The founding TRPN channel, Drosophila NOMPC, is required for touch sensing in adult flies [24], and for detecting gentle touch in larvae [22]. Upon introduction into a heterologous cell expression system, NOMPC responds to mechanical stimuli within a 1–2 millisecond range, indicating that it is also likely to be directly gated by force [22].
In order to transmit force to the pore region, an elastic structure induces a conformational change, leading to pore opening. A feature of many TRP channels is a tandem array of N-terminal ankyrin repeats, the longest of which is a record-breaking set of 29 in TRPN channels [24]. This cluster of ankyrin repeats is proposed to function as a “gating spring,” enabling external force to be conveyed to the channel pore [43]. The model is supported by molecular dynamic simulations, suggesting that the extension and stiffness of the structure match those predicted by the gating spring model [44]. Using atomic force microscopy, ankyrin repeats appear to unfold under force, and this process generates a refolding force, which is consistent with the hypothesis that ankyrin repeats act as gating spring [45]. TRPA1 also includes an impressive array of 14 N-terminal ankyrin repeats [46], and amphipathic molecules that cause mechanical stress to the lipid bilayer activate this channel, indicating that it is a MSC [47]. Therefore, some TRP channels may be force-activated through a mechanical mechanism involving ankyrin repeats.
Direct mechanical gating of TRP channels via a multistep cascade
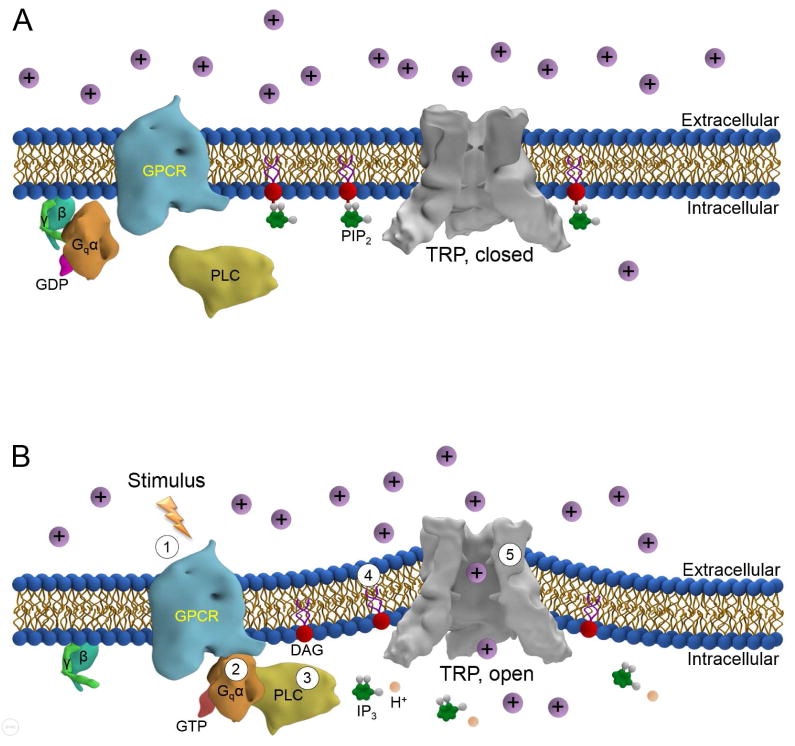
The title of this section might seem counterintuitive. How can a TRP channel be directly mechanically gated if it is coupled to a signaling pathway? In fact, there is compelling evidence from Drosophila visual transduction that a non-mechanical stimulus (light) triggers a signaling cascade that generates force on the lipid-bilayer, which then serves as the trigger that opens the stretch-activated TRP channels [48]. In fly photoreceptor cells, a single photon is captured by rhodopsin, and the signal is amplified through a pathway that employs a heterotrimeric G-protein and activation of PLC [29,49] (Fig. 2). The PLC cleaves a minor phospholipid component of the plasma membrane, phosphatidylinositol 4, 5-bisphosphate (PIP2) resulting in the production of inositol 1,4,5-trisphosphate (IP3), diacylglycerol (DAG) and a proton (H+). The removal of the bulky inositol head group from PIP2, resulting in the production of DAG, causes stress resulting in contractions of the microvilli membrane [48]. This “photomechanical response” affecting photoreceptor cells, which is detectable by light microscopy and quantifiable by atomic force microscopy, generates sufficient mechanical force to open the TRP and TRPL channels. The conclusion that light produces mechanical force is further substantiated by light-induced activation of a known MSC, gramicidin, introduced in photoreceptor cells in place of TRP and TRPL [48].
Figure 2.
Model of direct activation of TRP channels by force generated via a signaling cascade. (A) TRP channel is closed before the stimulus. (B) TRP channel is opened following activation of a the signaling cascade: 1) a stimulus, such as light or a chemical agonist, activates the GPCR, 2) the heterotrimeric G-protein (Gq) engages the GPCR, resulting in exchange of GTP for GDP, and dissociation of the βγ from the Gqα subunit, 3) PLC is stimulated by interaction with Gqα-GTP, resulting in the hydrolysis of PIP2 and production of IP3, DAG and H+, 4) the membrane stretches due to cleavage of the head group (DAG) from PIP2, and 5) membrane-deformation activates the TRP channel, leading to influx of cations. We also propose that hydrolysis of PIP2 through engagement of an RTK and stimulation of PLCγ1, leads to stretch-activation of TRP channels (not shown).
In principle, any TRP channel that is activated through a multistep cascade that alters the concentration of membrane lipids has the potential to be a MSC. Indeed, this includes most TRP channels since hydrolysis of PIP2 and other membrane lipids is linked to the activation of TRPs that belong to the TRPC, TRPV, TRPM, TRPA, TRPP and TRPML families. Among the mammalian TRPs, the seven members of the TRPC subfamily are most homologous to Drosophila TRP and TRPL, and all can be activated through stimulation of PLC [4]. Notable examples include the TRPC6 and TRPC7 channels, which are activated in mammalian intrinsically sensitive retinal ganglion cells through a light-initiated PLC-dependent pathway [9]. In addition, TRPC1 can sense hypo-osmolarity-induced membrane stretch in Xenopus oocytes and, and the channel retains mechanosensitivity after it is reconstituted into liposomes following detergent solubilization [50]. TRPC3 might also be a MSC since in the mammalian brain it is activated by a pathway that is initiated by brain-derived nerve growth factor (BDNF), which engages a receptor tyrosine kinase (RTK), TrkB, leading to stimulation of PLCγ1, and hydrolysis of PIP2 [51]. TRPM5, which functions in mammalian sweet, bitter and umami taste, is also gated through a GPCR/Gq/PLC signaling pathway, and therefore has the potential to be a MSC [13,52].
Mechanical gating of thermoTRPs
Multiple TRP channels are gated by changes in temperature, including TRPV1 and TRPM8, which respond to heating (≥43) and cooling (<23°C), respectively [17,18,53]. How these changes are gated by changes in temperature, is unresolved. The proteins themselves may be directly heat-sensitive since they are activated by changes in temperature after reconstitution of these proteins in liposomes [54,55]. However, an alternative possibility is that “thermoTRPs” are MSCs, which respond to mechanical force through temperature-induced changes in membrane curvature (Fig. 1B).
Hydrolysis of PIP2 might also affect temperature gating of thermoTRPs. Drosophila TRPA1 is activated by increases in temperature, with a threshold of ~25–27°C [56]. However, below this threshold, TRPA1 functions in temperature discrimination in larvae, allowing the animals to avoid temperatures such as 24°C in favor of slightly lower temperatures. The contribution of TRPA1 to temperature selection below 25°C depends on a Gq, P LC signaling cascade [57]. Remarkably, this cascade also depends on rhodopsin, and this represents a light-independent role for this archetypal GPCR [58]. We suggest that temperatures above 25–27°C induce sufficient membrane tension to activate TRPA1, and hydrolysis of PLC lowers the thermal threshold below 25–27°C by contributing to the architectural changes in the plasma membrane.
Concluding remarks
We propose that most TRP channels are MSCs, and this represents one unifying theme explaining how TRPs can be activated by a diversity of stimuli. Furthermore, changes in membrane tension, which are relieved by conformational changes that lead to TRP channel gating, could account for how one stimulus could lower the threshold for activation by another. In addition, we propose that the effects of some diverse chemicals agonists on TRP channel gating may occur through effects on membrane curvature, rather than direct binding to the channel. Nevertheless, it is clear that some chemicals bind directly to TRP channels, such as reactive electrophiles that covalently bind to cysteines in TRPA1 [59]. Therefore, we are not proposing that mechanical-gating represents the only strategy for activating TRP channels. An important goal for the future is to interrogate TRP channels to determine the extent to which they are activated through membrane stretch and mechanical force, especially in contexts in which the initial stimuli are not mechanical input.
Supplementary Material
We propose most TRP channels are mechanosensitive channels.
Force is generated directly or indirectly via a signaling cascade.
Non-mechanical stimuli can induce force that gates downstream TRP channels.
Acknowledgments
Work in C.M.’s laboratory is supported by grants from the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Montell C, Jones K, Hafen E, Rubin G. Rescue of the Drosophila phototransduction mutation trp by germline transformation. Science. 1985;230:1040–1043. doi: 10.1126/science.3933112. [DOI] [PubMed] [Google Scholar]
- 2.Montell C, Rubin GM. Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron. 1989;2:1313–1323. doi: 10.1016/0896-6273(89)90069-x. [DOI] [PubMed] [Google Scholar]
- 3.Wes PD, Chevesich J, Jeromin A, Rosenberg C, Stetten G, Montell C. TRPC1, a human homolog of a Drosophila store-operated channel. Proc Natl Acad Sci USA. 1995;92:9652–9656. doi: 10.1073/pnas.92.21.9652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu LJ, Sweet TB, Clapham DE International Union of Basic and Clinical Pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol Rev. 2010;62:381–404. doi: 10.1124/pr.110.002725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gees M, Owsianik G, Nilius B, Voets T. TRP channels. Compr Physiol. 2012;2:563–608. doi: 10.1002/cphy.c110026. [DOI] [PubMed] [Google Scholar]
- 7.Montell C. Physiology, phylogeny and functions of the TRP superfamily of cation channels. Science’s STKE. 2001;2001(90):re1. doi: 10.1126/stke.2001.90.re1. [DOI] [PubMed] [Google Scholar]
- 8.Hardie RC, Minke B. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron. 1992;8:643–651. doi: 10.1016/0896-6273(92)90086-s. [DOI] [PubMed] [Google Scholar]
- 9.Xue T, Do MT, Riccio A, Jiang Z, Hsieh J, Wang HC, Merbs SL, Welsbie DS, Yoshioka T, Weissgerber P, Stolz S, Flockerzi V, Freichel M, Simon MI, Clapham DE, Yau KW. Melanopsin signalling in mammalian iris and retina. Nature. 2011;479:67–73. doi: 10.1038/nature10567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liman ER, Corey DP, Dulac C. TRP2: A candidate transduction channel for mammalian pheromone sensory signaling. Proc Natl Acad Sci USA. 1999;96:5791–5796. doi: 10.1073/pnas.96.10.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwon Y, Kim SH, Ronderos DS, Lee Y, Akitake B, Woodward OM, Guggino WB, Smith DP, Montell C. Drosophila TRPA1 channel is required to avoid the naturally occurring insect repellent citronellal. Curr Biol. 2010;20:1672–1678. doi: 10.1016/j.cub.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colbert HA, Bargmann CI. Odorant-specific adaptation pathways generate olfactory plasticity in C. elegans. Neuron. 1995;14:803–812. doi: 10.1016/0896-6273(95)90224-4. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell. 2003;112:293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
- 14.Kim SH, Lee Y, Akitake B, Woodward OM, Guggino WB, Montell C. Drosophila TRPA1 channel mediates chemical avoidance in gustatory receptor neurons. Proc Natl Acad Sci USA. 2010;107:8440–8445. doi: 10.1073/pnas.1001425107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu Y, Ulbrich MH, Li MH, Dobbins S, Zhang WK, Tong L, Isacoff EY, Yang J. Molecular mechanism of the assembly of an acid-sensing receptor ion channel complex. Nat Commun. 2012;3:1252. doi: 10.1038/ncomms2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jordt SE, Tominaga M, Julius D. Acid potentiation of the capsaicin receptor determined by a key extracellular site. Proc Natl Acad Sci USA. 2000;97:8134–8139. doi: 10.1073/pnas.100129497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 18.McKemy DD, Nenhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 19.Sun Y, Liu L, Ben-Shahar Y, Jacobs JS, Eberl DF, Welsh MJ. TRPA channels distinguish gravity sensing from hearing in Johnston’s organ. Proc Natl Acad Sci USA. 2009;106:13606–13611. doi: 10.1073/pnas.0906377106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sidi S, Friedrich RW, Nicolson T. NompC TRP channel required for vertebrate sensory hair cell mechanotransduction. Science. 2003;301:96–99. doi: 10.1126/science.1084370. [DOI] [PubMed] [Google Scholar]
- 21.Kamikouchi A, Inagaki HK, Effertz T, Hendrich O, Fiala A, Gopfert MC, Ito K. The neural basis of Drosophila gravity-sensing and hearing. Nature. 2009;458:165–171. doi: 10.1038/nature07810. [DOI] [PubMed] [Google Scholar]
- 22.Yan Z, Zhang W, He Y, Gorczyca D, Xiang Y, Cheng LE, Meltzer S, Jan LY, Jan YN. Drosophila NOMPC is a mechanotransduction channel subunit for gentle-touch sensation. Nature. 2013;493:221–225. doi: 10.1038/nature11685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li W, Kang L, Piggott BJ, Feng Z, Xu XZ. The neural circuits and sensory channels mediating harsh touch sensation in Caenorhabditis elegans. Nat Commun. 2011;2:315. doi: 10.1038/ncomms1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walker RG, Willingham AT, Zuker CS. A Drosophila mechanosensory transduction channel. Science. 2000;287:2229–2234. doi: 10.1126/science.287.5461.2229. [DOI] [PubMed] [Google Scholar]
- 25.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 26.Mochizuki T, Wu G, Hayashi T, Xenophontos SL, Veldhuisen B, Saris JJ, Reynolds DM, Cai Y, Gabow PA, Pierides A, Kimberling WJ, Breuning MH, Deltas CC, Peters DJ, Somlo S. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science. 1996;272:1339–1342. doi: 10.1126/science.272.5266.1339. [DOI] [PubMed] [Google Scholar]
- 27.T.E.P.K.D. Consortium. The polycystic kidney disease 1 gene encodes a 14 kb transcript and lies within a duplicated region on chromosome 16. Cell. 1994;77:881–894. doi: 10.1016/0092-8674(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 28.T.I.P.K.D. Consortium. Polycystic kidney disease: the complete structure of the PKD1 gene and its protein. Cell. 1995;81:289–298. doi: 10.1016/0092-8674(95)90339-9. [DOI] [PubMed] [Google Scholar]
- 29.Montell C. Drosophila visual transduction. Trends Neurosci. 2012;35:356–363. doi: 10.1016/j.tins.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lucas RJ. Mammalian inner retinal photoreception. Curr Biol. 2013;23:R125–133. doi: 10.1016/j.cub.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 31.Niemeyer BA, Suzuki E, Scott K, Jalink K, Zuker CS. The Drosophila light-activated conductance is composed of the two channels TRP and TRPL. Cell. 1996;85:651–659. doi: 10.1016/s0092-8674(00)81232-5. [DOI] [PubMed] [Google Scholar]
- 32.Bloomquist BT, Shortridge RD, Schneuwly S, Perdew M, Montell C, Steller H, Rubin G, Pak WL. Isolation of a putative phospholipase C gene of Drosophila, norpA, and its role in phototransduction. Cell. 1988;54:723–733. doi: 10.1016/s0092-8674(88)80017-5. [DOI] [PubMed] [Google Scholar]
- 33.Julius D. TRP channels and pain. Annu Rev Cell Dev Biol. 2013;29:355–384. doi: 10.1146/annurev-cellbio-101011-155833. [DOI] [PubMed] [Google Scholar]
- 34.Eijkelkamp N, Quick K, Wood JN. Transient receptor potential channels and mechanosensation. Annu Rev Neurosci. 2013;36:519–546. doi: 10.1146/annurev-neuro-062012-170412. [DOI] [PubMed] [Google Scholar]
- 35.Everaerts W, Gees M, Alpizar YA, Farre R, Leten C, Apetrei A, Dewachter I, van Leuven F, Vennekens R, De Ridder D, Nilius B, Voets T, Talavera K. The capsaicin receptor TRPV1 is a crucial mediator of the noxious effects of mustard oil. Curr Biol. 2011;21:316–321. doi: 10.1016/j.cub.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 36.Roberts JC, Davis JB, Benham CD. [3H]Resiniferatoxin autoradiography in the CNS of wild-type and TRPV1 null mice defines TRPV1 (VR-1) protein distribution. Brain Res. 2004;995:176–183. doi: 10.1016/j.brainres.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 37.Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, Julius D, Hogestatt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]
- 38.Cao E, Liao M, Cheng Y, Julius D. TRPV1 structures in distinct conformations reveal activation mechanisms. Nature. 2013;504:113–118. doi: 10.1038/nature12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liao M, Cao E, Julius D, Cheng Y. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature. 2013;504:107–112. doi: 10.1038/nature12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ranganathan R, Harris GL, Stevens CF, Zuker CS. A Drosophila mutant defective in extracellular calcium-dependent photoreceptor deactivation and desensitization. Nature. 1991;354:230–232. doi: 10.1038/354230a0. [DOI] [PubMed] [Google Scholar]
- 41.Hardie RC. Voltage-sensitive potassium channels in Drosophila photoreceptors. J Neurosci. 1991;11:3079–3095. doi: 10.1523/JNEUROSCI.11-10-03079.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kang L, Gao J, Schafer WR, Xie Z, Xu XZ. C. elegans TRP family protein TRP-4 is a pore-forming subunit of a native mechanotransduction channel. Neuron. 2010;67:381–391. doi: 10.1016/j.neuron.2010.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Howard J, Bechstedt S. Hypothesis: a helix of ankyrin repeats of the NOMPC-TRP ion channel is the gating spring of mechanoreceptors. Curr Biol. 2004;14:R224–R226. doi: 10.1016/j.cub.2004.02.050. [DOI] [PubMed] [Google Scholar]
- 44.Sotomayor M, Corey DP, Schulten K. In search of the hair-cell gating spring elastic properties of ankyrin and cadherin repeats. Structure. 2005;13:669–682. doi: 10.1016/j.str.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 45.Li L, Wetzel S, Pluckthun A, Fernandez JM. Stepwise unfolding of ankyrin repeats in a single protein revealed by atomic force microscopy. Biophys J. 2006;90:L30–32. doi: 10.1529/biophysj.105.078436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, Is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 47.Hill K, Schaefer M. TRPA1 is differentially modulated by the amphipathic molecules trinitrophenol and chlorpromazine. J Biol Chem. 2007;282:7145–7153. doi: 10.1074/jbc.M609600200. [DOI] [PubMed] [Google Scholar]
- 48.Hardie RC, Franze K. Photomechanical responses in Drosophila photoreceptors. Science. 2012;338:260–263. doi: 10.1126/science.1222376. [DOI] [PubMed] [Google Scholar]
- 49.Hardie RC. Photosensitive TRPs. Handb Exp Pharmacol. 2014;223:795–826. doi: 10.1007/978-3-319-05161-1_4. [DOI] [PubMed] [Google Scholar]
- 50.Maroto R, Raso A, Wood TG, Kurosky A, Martinac B, Hamill OP. TRPC1 forms the stretch-activated cation channel in vertebrate cells. Nat Cell Biol. 2005;7:179–185. doi: 10.1038/ncb1218. [DOI] [PubMed] [Google Scholar]
- 51.Li HS, Xu XZ, Montell C. Activation of a TRPC3-dependent cation current channel through the neurotrophin BDNF. Neuron. 1999;24:261–273. doi: 10.1016/s0896-6273(00)80838-7. [DOI] [PubMed] [Google Scholar]
- 52.Pérez CA, Huang L, Rong M, Kozak JA, Preuss AK, Zhang H, Max M, Margolskee RF. A transient receptor potential channel expressed in taste receptor cells. Nat Neurosci. 2002;5:1169–1176. doi: 10.1038/nn952. [DOI] [PubMed] [Google Scholar]
- 53.Peier A, Moqrich A, Hergarden A, Reeve A, Andersson D, Story G, Earley T, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 54.Cao E, Cordero-Morales JF, Liu B, Qin F, Julius D. TRPV1 channels are intrinsically heat sensitive and negatively regulated by phosphoinositide lipids. Neuron. 2013;77:667–679. doi: 10.1016/j.neuron.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zakharian E, Cao C, Rohacs T. Gating of transient receptor potential melastatin 8 (TRPM8) channels activated by cold and chemical agonists in planar lipid bilayers. J Neurosci. 2010;30:12526–12534. doi: 10.1523/JNEUROSCI.3189-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Viswanath V, Story GM, Peier AM, Petrus MJ, Lee VM, Hwang SW, Patapoutian A, Jegla T. Opposite thermosensor in fruitfly and mouse. Nature. 2003;423:822–823. doi: 10.1038/423822a. [DOI] [PubMed] [Google Scholar]
- 57.Kwon Y, Shim HS, Wang X, Montell C. Control of thermotactic behavior via coupling of a TRP channel to a phospholipase C signaling cascade. Nat Neurosci. 2008;11:871–873. doi: 10.1038/nn.2170. [DOI] [PubMed] [Google Scholar]
- 58.Shen WL, Kwon Y, Adegbola AA, Luo J, Chess A, Montell C. Function of rhodopsin in temperature discrimination in Drosophila. Science. 2011;331:1333–1336. doi: 10.1126/science.1198904. [DOI] [PubMed] [Google Scholar]
- 59.Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, Patapoutian A. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007;445:541–545. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.