Synopsis
There is epidemiological evidence that a substantial number of adults suffer YOD. The diversity of types and syndromes makes recognition and diagnosis difficult. An algorithmic approach to interpreting clinical data, informed by clinical epidemiology, integrates data pertaining to defining syndromes, and their chronology and tempo, family history, and other neuropsychiatric features and neurological signs, to reach a preliminary diagnosis and direct diagnostic tests and their interpretation. Screening for YOD in the psychiatric context is a rational process in which vigilance is combined with careful searches for ‘red flags’ that signal a neurodegenerative etiology.
Keywords: young onset dementia, epidemiology, risk factors, differential diagnosis, screening
Introduction
Young-onset presentations of dementia are increasingly recognized as important causes of midlife morbidity and mortality. Young-onset dementia (YOD), typically defined as dementia arising before the age of 65, is also referred to as early-onset dementia (and in older times it was commonly known as presenile dementia). This age threshold, albeit socially determined and arbitrary, has proved useful for practice innovations and for research. It has, for example, provided a framework for distinguishing young-onset dementias from the more common late-life occurrences, which serves important differences in etiology, phenotypes, handicaps, psychosocial difficulties and, ultimately, clinical care. Whereas late-life dementias are, with the exception of those stemming from cerebrovascular disease, generally neurodegenerative, the YOD are more heterogeneous. Although most YOD are neurodegenerative, many cases arise from genetic, infectious, autoimmune, vascular, nutritional, and metabolic etiologies.
YOD is overshadowed in the clinical and public consciousness by the higher prevalence of dementia in later life – and the perception that dementia is a condition of the elderly. Owing to this low consciousness, and physicians’ lack of familiarity with the various conditions from which YOD arises, the diagnosis is frequently missed or late. For example, one study showed an average time to diagnosis of 4.5 years, which was 1.6 years longer than for the late onset group in that study1.
Knowledge amongst clinicians, and the public, of the clinical and demographic characteristics of YOD will facilitate recognition and accurate diagnosis, and thereby prompt and effective care. Effective care includes treatment of the neuropsychiatric facets of these disorders, and management of the psychosocial needs of the individuals and families suffering these conditions. YOD frequently manifest neuropsychiatric phenomena alongside impairments of cognitive functions, often presenting with abnormalities of affect, temperament, judgment, dispositions and self-control, perception, ideation, and subsistence behaviors (feeding, elimination, sexual expression, and sleep). The psychosocial problems arising from these conditions include conflict, financial strain (since the patient may be a primary breadwinner or the caregiver of children), emotional stress on spouses (and other relatives) who provide care, work, and parenting, and suffer social disconnection due to distraction and isolation 2. Children of individuals who suffer young-onset dementias also suffer adaptation-related stresses 3,4. Thus YOD is topical for psychiatry because of the phenotypes firstly, the psychosocial dimensions of the suffering too, and the skillset of the specialty. Psychiatrists, by virtue of their multidimensional training and professional orientation, have the tools to treat the illness, manage the problems, and direct the rehabilitation.
This paper, like others in this issue, provides an introduction to aspects of YOD that are important for psychiatric practice. Here we discuss the clinical epidemiology of the neurodegenerative forms of YOD, including the pertinence of this to clinical work. We point out the demographic and phenotypic attributes of YOD that provide clues aiding the clinical recognition of the various etiologic types. Screening and measurement are also covered. The aim is to provide psychiatrists and other mental health professionals with a basic understanding that, it is hoped, will enhance their capacity to identify the cases, treat and rehabilitate them, and advocate for policies that meet their needs.
Method
A literature search for studies of the epidemiology of young onset dementia used the PubMed and Google Scholar databases, searching for full text papers written in the English language. The search terms are: “Young Onset Dementia” or “Early Onset Dementia” in combinations with “prevalence” or “incidence” or “survival” or “mortality”. We reviewed the references of the papers we found for additional sources. We also scoured sources with which we were already familiar, particularly topical reviews and white papers.
Frequency and distribution
Estimates of the frequency of YOD are scarce; what data are available derive from catchment area or specialty clinic samples, using for case identification surveys of local clinics and hospitals, medical record review, disease registries, and passive surveillance of defined geographical regions. Direct ascertainment from the population is difficult, due to the rarity and diversity of YOD conditions, their diagnostic complexity (which demands expertise), and the public's lack of familiarity with these conditions (which makes surveys difficult to design).
The proportions of specialist clinic (or memory clinic) patients who suffer YOD are shown in Table 1. These data indicate that the frequency of YOD in specialist clinics ranges from 7.3-44% 5-11, these estimates reflecting differences between regions and centers in the clinical focus, local practices, referral base, and sampling methods. For example, the study conducted in Greece, which reports the highest frequency, received referrals mainly from academic psychiatrists and neurologists – which may have resulted in a case selection favoring atypical forms of dementia (and younger cases than the typical memory clinic).
Table 1.
Frequency of young-onset dementia in specialized clinics
| Study/year | Location | Study population | Case ascertainment | YOD/total | % YOD |
|---|---|---|---|---|---|
| Yokota et al., 2005 | Okayama, Japan | Outpatient clinic | Referrals from generalists, neurologists, and psychiatrists | 34/464 | 7.3 |
| McMurtray et al., 2006 | Los Angeles, USA | Memory center | Medical records | 278/1683 | 30 |
| Shinagawa et al., 2007 | Ehime, Japan | Memory clinic | Referrals from generalists, geriatricians, and neuropsychiatrists | 185/861 | 27.7 |
| Nandi et al., 2008 | West Bengal, India | Specialized clinic | Medical records | 94/379 | 24.5 |
| Papageorgiou et al., 2009 | Athens, Greece | Specialized center | Referrals from neurologists and psychiatrists | 114/260 | 44 |
| Picard et al., 2011 | Amiens, Lille, and Rouen, France | Cohort of 3 memory clinics | Referrals | 811/3473 | 23.5 |
| Croisile et al., 2012 | Lyons, France | Memory clinic | Referrals | 91/746 | 12.2 |
The prevalence of all-cause YOD in communities (see Table 2). These studies, which typically identify cases in the catchment area of a specialist center or network, show prevalence rates ranging 42.3–68.2 per 100,000 12-16. Variability results from differences between case mix (see Table 3), and in the sampling methods (for example, see the differences in sample age ranges shown in Table 2). The incidence of all-cause YOD, based on the three studies conducted to date, is 11-13.4 per 100,000 17-19.
Table 2.
Frequency of young-onset dementia (all causes) in geographic populations
| PREVALENCE STUDIES | ||||||
|---|---|---|---|---|---|---|
| Study/year | Location | Study population | Case ascertainment | Age, years | Prevalence* | 95% CI |
| Harvey et al., 2003 | London, UK | Catchment area | Registry and surveillance of local practices | 35-64 | 54 | 45.1-64.1 |
| Ikejima et al., 2009 | Ibaraki, Japan | Regional network | Postal survey to clinics, hospitals, nursing facilities, and health agencies | 20-64 | 42.3 | 39.4-45.4 |
| Borroni et al., 2011 | Brescia County, Italy | Regional network of hospital-based centers | Disease registry | 45-65 | 55.1 | 47.0-63.4 |
| Renvoize et al., 2011 | Blackpool, Wyre, and Fylde, UK | Catchment area | Medical records | 45-64 | 62.8 | 48.0-82.3 |
| Withall et al., 2014 | Eastern Sydney, Australia | Catchment area | Structured questionnaire to health professionals, and medical records | 30-64 | 68.2 | 54.9-83.4 |
| INCIDENCE STUDIES | ||||||
|---|---|---|---|---|---|---|
| Incidence* | ||||||
| Mercy et al., 2008 | Cambridgeshire, UK | Catchment area | Referrals from generalists and specialists | 45-64 | 11.5 | 8.6-15.0 |
| Garre-Olmo et al., 2010 | Catalonia, Spain | Regional network of hospitals | Standardized clinical registry | 30-64 | 13.4 | 11.3-15.8 |
| Sanchez Abraham et al., 2013 | Mar del Plata, Argentina | Hospital serving large catchment area | Database of geriatric care department | <65 | 11 | 6.25-19.1 |
per 100,000 persons at risk
Table 3.
Frequency of young-onset dementia by etiologic type
| Location | N | *Age range | *Age, onset | *Age, exam | AD | FTD | VaD | HD | DLB/PDD | ARD | TBI | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ferran et al., 1996 | Liverpool, North Wales, Cheshire, and Lancashire, UK | 200 | <65 | 52.6 | 56 | 27 | 4 | 17 | NR | 2 | 12 | NR |
| Harvey et al., 2003 | London, UK | 185 | 30-65 | NR | 58.7 | 34 | 12 | 18 | 4.9 | 7.5 | 10 | EX |
| Yokota et al., 2005 | Okayama, Japan | 34 | <65 | NR | NR | 38.8 | 14.7 | 23.5 | NR | 2.9 | NR | NR |
| McMurtray et al., 2006 | Los Angeles, USA | 278 | <65 | 51.5 | 56.5 | 17 | 3 | 29 | NR | NR | 5 | 24 |
| Kelley et al., 2008 | Minnesota, USA | 235 | 17-45 | 34.7 | 36.7 | 1.7 | 13.2 | 5.9 | 7.7 | 0.4 | 0.4 | EX |
| Nandi et al., 2008 | West Bengal, India | 94 | <65 | 56.5 | NR | 33 | 27 | 20 | 4 | 4 | NR | NR |
| Ikejima et al., 2009 | Ibaraki, Japan | 617 | 20-64 | 53.4 | NR | 25.6 | 2.8 | 42.5 | NR | 6.2 | NR | 7.1 |
| Papageorgiou et al., 2009 | Athens, Greece | 114 | <65 | 55.1 | 58.7 | 27.2 | 24.6 | 6.1 | 2.6 | 4.4 | NR | 0.87 |
| Picard et al., 2011 | Amiens, Lille, and Rouen, France | 811 | <65 | 55.9 | NR | 22.3 | 79.7 | 15.9 | 3 | 5.3 | 9.4 | 3.8 |
| Renvoize et al., 2011 | Blackpool, Wyre, and Fylde, UK | 55 | <65 | NR | NR | 24.2 | 2.4 | 6.0 | 2.4 | 1.2 | 10.9 | NR |
| Withall et al., 2014 | Eastern Sydney, Australia | 141 | <65 | 55 | 56.5 | 17.7 | 11.3 | 12.8 | 5.7 | 4.9 | 18.4 | 2.1 |
Age variables are in years; means are reported for age of onset and age at exam (or at ascertainment). Age range refers to the reference range for the study. Frequencies are reported as percentages of the sample, for ease of comparison.
AD = Alzheimer disease, FTD = frontotemporal dementia, VaD = vascular dementia, HD= Huntington's disease, DLB = dementia with Lewy bodies, PDD = Parkinson disease dementia, ARD = alcohol-related dementia, TBI = traumatic brain injury.
Some causes are not shown here (owing to our focus, limited coverage in the papers, small numbers, and space limits): dementia associated with depression (18% of cases in Ferran et al., 1996), corticobasal degeneration, progressive supranuclear palsy, Creutzfeldt-Jakob disease, depression, cerebral tumor, multiple sclerosis, HIV infection, epilepsy, and unspecified types.
EX indicates that the condition was an exclusion criterion; NR indicates that the data pertaining to the variable was not reported
Etiology
The case mix in prevalence studies of YOD is broad, comprising Alzheimer disease (AD), frontotemporal dementia (FTD), vascular dementia (VaD), Huntington disease (HD), Parkinson disease (PDD) and dementia with Lewy bodies (DLB), alcohol-related dementia (ARD), and traumatic brain injury (TBI) 5,6,8-10,12,13,15,16,20,21. AD and FTD are the most frequent neurodegenerative causes of YOD (see Table 3 for frequencies derived from prevalence studies). In the United States, it has been estimated that 200,000 suffer young-onset AD 22 and between 12,000 and 18,000 suffer young-onset FTD 23. Cerebrovascular disease and stroke are important causes of YOD in Japan 13. Neurodegenerative causes of YOD predominate beyond age 3521, and it has been estimated that they comprise 30% of all YOD 24. TBI, ARD, and HIV-Associated Neurocognitive Disorder are mainly seen among the poor living in inner cities 6.
Risk factors
Hereditary transmission is the most established risk factor for neurodegenerative forms of YOD. Hereditary forms of AD and FTD arises arise from autosomal dominant mutations in several genetic loci (see Table 4, which also shows data for Huntington disease and prion disease). Mutations in specific genetic loci are often associated with phenotypic variants, as shown in Table 4. For example, the mutation in the C9ORF72 gene gives rises to a phenotype of FTD with amyotrophic lateral sclerosis (FTD-ALS), which is often also heralded or complicated by psychosis.
Table 4.
Genetic loci for common neurodegenerative disorders
| Gene | Locus | Clinical feature | |
|---|---|---|---|
| Alzheimer's disease | PSEN1 | 14q24 | Often resembles sporadic AD, however behavior presentation (agitation, depression, delusions and hallucinations) and motor symptoms (myoclonus, spastic paresis, parkinsonism, seizures are prominent in some cases. |
| PSEN 2 | 1q31 | Very rare (typically Volga German ancestry). Most commonly presents with amnesia. There is a high degree of phenotypic variation, 33% presenting with hallucinations and delusions, and 31% with seizures. Disease progression is slow, with rigidity, mutism and a bedridden state in the end stages. | |
| APP | 21q21 | Amnesic dementia associated with seizures commonly developing within 1-9 years after onset of dementia. Intracerebral hemorrhage is not uncommon. | |
| Frontotemporal dementia | C9ORF72 | 9p21 | Behavioral dementia (FTD) with amyotrophic lateral sclerosis |
| MAPT | 17q21 | Behavioral dementia with Parkinsonism | |
| GRN | 17q21 | Behavioral dementia, with aphasia, apraxia, parkinsonism, dystonia | |
| VCP | 9p13 | Inclusion body myopathy with osteolytic bone disease (Paget's disease) and behavioral dementia. | |
| CHMP2B | 3p11.2 | Very rare, seen in Danish kindred; behavioral dementia, parkinsonism and progressive spastic paresis are often seen. | |
| Huntington disease | Huntingtin | 4p16 | Choreoathetosis early in the course, with dystonia and akinetic rigidity in later stages. Neuropsychiatric features include executive dysfunction, depression, irritability, impulsivity, compulsive behaviors, anger and hostility, and depression. |
| Prion disease | PRNP | 20p13 | Accounts for 15% of human prion disease. Several types are recognized: fCJD, which in rare instances mimics amnesic AD, typically features myoclonic jerks, cerebellar signs, and akinetic mutism; FFI (progressive insomnia, dysautonomia, selective thalamic degeneration); GSS (a hereditary form with chronic cerebellar ataxia, pyramidal features, dysathria, oculodysmetria, and hyporeflexia, with dementia in later stages. |
PSEN=Presenilin, APP=Amyloid precursor protein, C9ORF72 = Chromosome 9 open reading frame 72, MAPT=Microtubule-associated protein tau, GRN=Progranulin, VCP= Valosin-containing protein, CHMP2B=Chromatin-modifying protein 2B, PRNP=prion protein; f CJD=familial CJD, FFI=Familial fatal insomnia, GSS= Gerstmann-Straussler-Sheinker Syndrome
Repeated concussive head traumas, typically sustained in sports such as boxing, American football, and ice hockey, are now known to cause chronic traumatic encephalopathy, a neurodegenerative dementia 25 characterized by widespread cortical and subcortical tauopathy. Low cognition, alcohol abuse, high blood pressure, stroke, depression, and neuroleptic use in youth, have been linked to YOD in midlife in one study 26. Another study has shown association between YOD and cardiovascular disease – stroke, TIA, chronic kidney disease, and hypertension 27.
These findings suggest opportunities for primary prevention 28
Survival
Life expectancy after diagnosis varies widely in YOD, according to the etiologic type, but appears to be shorter in general than for late-onset dementia 29. It has been estimated that median survival from diagnosis for YOD (derived from a study in sampling mainly AD and VaD cases) is 6 years 30. Median survival from diagnosis of FTD is about 7-13 years in clinic cohorts and 6-8 years in neuropathological series 31. Survival is much shorter in FTD-ALS, showing median survival of 27 months 32. Survival in prion disease cases is comparable to that in FTD-ALS, although some cases survive up to 2-3 years 33.
Clinical and diagnostic considerations
The diagnosis of YOD is frequently missed, because of the wide diversity of types, a predominance of non-memory and neuropsychiatric features over cognitive deficits in many, and (relative to late-onset dementia) a high frequency of syndromes that are defined by motor dysfunctions such as parkinsonism, apraxia, and ataxia.
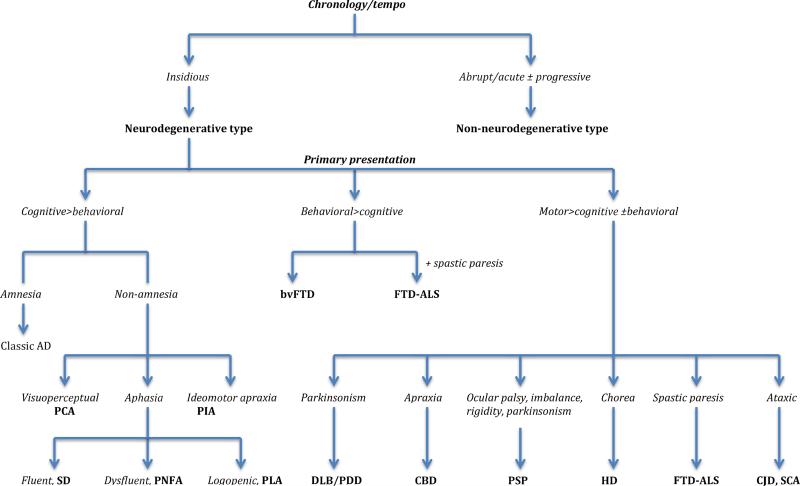
When certain diagnostic principles are followed, which derive from a clinical epidemiology that defines syndromes according to their signal phenomena, the differential diagnosis can be sharply narrowed. This entails an algorithmic approach, not as a sequence of procedures, but in the sense of a methodical consideration of the clinical data. The key data elements are the cluster of cognitive, neuropsychiatric and motor symptoms and signs defining a syndrome, their chronology and tempo, and family history (particularly of neuropsychiatric and neurologic illnesses). These data guide what diagnostic procedures are selected – psychometric measurements, serological and biochemical assays, brain imaging, and genotyping – and facilitates their interpretation. This approach is illustrated in Figure 1, which presents an algorithmic approach for using syndrome type (defined from the most salient symptoms and signs) to progressively narrow the differential diagnosis.
Figure 1.
Algorithm for discriminating the neurodegenerative types of young-onset dementia
Differential diagnosis as a function of defined syndromes
The first branch point in our algorithm (in Figure 1) involves weighing the chronology and tempo of the illness. An insidious, ingravescent course is typical of most neurodegenerative disorders, including AD, FTD, HD, and DLB, and is also true of some presentations of vascular dementia 34. An abrupt and rapid development can arise from some neurodegenerative states, such as prion disease and FTD-ALS, but is frequently the manifestation of non-neurodegenerative processes such as stroke, autoimmune encephalitides, and CNS infections. Prion and other rapidly progressing dementias are covered in another paper in this issue. As noted earlier, neurodegenerative YOD can manifest in a variety of ways, as cognitive syndromes, neuropsychiatric states, and motor syndromes that are accompanied by cognitive and/or behavioral features.
A. Syndromes that are primarily cognitive (i.e., cognitive > behavioral in Figure 1)
cognitive presentations predominate in YOD, in part because AD is the most common type. Impaired episodic memory is the typical presenting feature of AD, in young- and late-onset cases. In this sense, young-onset AD resembles the classic late-onset phenotype in its presenting features and clinical evolution. However, non-amnesic phenotypes are common in YOD – i.e., syndromes in which visuoperceptual impairments, aphasia, or ideomotor apraxias predominate 35, and those in which the syndrome is defined by neuropsychiatric states (such as abnormal conduct, affective disturbance, and psychosis), or by motor dysfunctions (such as parkinsonism, ataxia, apraxia, or paresis). Posterior cortical atrophy is one such syndrome, where the highlight is progressive visual or visuospatial impairment in absence of ophthalmological impairment. Classically, three subtypes have been recognized – a biparietal syndrome (featuring limb and oculomotor apraxia, visuospatial disturbance, simultagnosia, optic ataxia, and agraphia), an occipital syndrome (featuring alexia, apperceptive agnosia and prosopagnosia), and a visual variant (manifesting as primary visual failure and impairment of basic perceptual abilities). Logopenic progressive aphasia (PLA), an aphasia variant of AD, is more common in younger AD patients. It features impaired word retrieval (in spontaneous speech and confrontation naming), severe sentence repetition deficits, and phonological errors in spontaneous speech. Another well recognized aphasia syndrome, semantic dementia, is a variant of FTD. Semantic dementia (SD) is a syndrome of progressive loss of word and object knowledge that, in its early stages, presents with dysnomia and ‘forgetting’ of words and objects, along with regularization errors in reading and writing. In later stages, the patient's speech becomes progressively vacuous (i.e., empty), even though articulation, syntax, and grammar are not impaired. Speech production, whether spontaneous or in repetition tasks, is unimpaired in SD. Progressive non-fluent aphasia, PNFA, is another widely recognized aphasia syndrome of FTD. It is a state in which there is progressive loss of speech fluency and articulation. This manifests clinically as hesitant, effortful, halting speech, with errors of grammar. Many cases of PNFA are accompanied by speech apraxia, recognized can be recognized from the sound distortions in speech which arise from articulatory dyscontrol. Impaired comprehension of complex sentences is also observed (since a key aspect of the condition is impaired processing of the syntactical aspects of language). The patients eventually become mute. A formal classification of the aphasia syndromes has been developed 36 and widely adopted.
As noted earlier, a small number of non-amnesic young-onset AD cases manifest a progressive ideomotor and limb apraxia. These have are designated progressive ideomotor apraxia.
B. Syndromes that are primarily neuropsychiatric (i.e., behavioral>cognitive)
Abnormalities of conduct define the neuropsychiatric variant of FTD (widely known as behavioral variant frontotemporal dementia, bvFTD). This bvFTD syndrome and the language syndromes discussed earlier are the canonical FTD states 37. This condition manifests as a progressive coarsening of temperament, judgment, conduct, and social skills, and derangements of volition. Formal diagnostic criteria have been developed 38. It is not uncommon for bvFTD to be accompanied by a progressive spastic paresis, in which case the condition is known as FTD-ALS. Parkinsonism can also accompany it, but the condition is primarily a derangement of behavior.
C. Syndromes defined by motor dysfunction (i.e., motor disorder > cognitive/behavioral)
these syndromes are defined by motor dysfunction – parkinsonism, motor apraxia, dyscontrol, spastic paresis, abnormalities of posture and gait, chorea, and/or ataxia. DLB and PDD overlap in their clinical and pathological features, differing primary in the order of emergence of parkinsonism and dementia, and the in symmetry of the former (lateral asymmetry is typical of Parkinson disease). In DLB, dementia precedes parkinsonism, or both states emerge simultaneously (or in very close temporal proximity), Generally in PDD, dementia does not appear in the first decade of the illness, although formal neuropsychological assessment may uncover subclinical executive dysfunction in some patients in the early stages. Dementia is a central feature of DLB. Parkinsonism is a core feature of the syndrome. Dementia arising within versus one year after parkinsonism is the convention for separating DLB from PDD 39. Rest tremor is uncommon common in DLB, whereas it is seen in the majority of PDD cases. As noted earlier, lateral asymmetry is typical of PDD. Axial tendency, and more pronounced masked facies, postural instability and gait disorder in DLB, are additional features that facilitate distinguishing the two conditions 39. DLB and PDD show fluctuations of alertness, attention and mentation, and recurrent formed visual hallucinations. Paranoia and delusions are not uncommon in these conditions. Chorea and dyskinesias are typical of adult onset HD. Chorea is the most prominent feature and appears early. Impaired involuntary movements such as incoordination, bradykinesia and rigidity are mainly seen in earlier onset HD. Executive dysfunction, behavioral rigidity, irritability, and flighty emotions are also early features 40,41, but are overshadowed by the dramatic motor phenomena.
Corticobasal degeneration (CBD) and progressive supranuclear palsy (PSP) have overlapping clinical and pathological features. CBD features asymmetric limb apraxia, akinesia, rigidity, dystonia, along with oral buccal apraxia, dysarticulation of speech (speech apraxia), and dysarthria. Executive dysfunction, which develops early, is overshadowed by the motor phenomena. Many cases develop the behavioral and language dysfunctions associated with FTD during the illness 42. PSP features oculomotor palsy, postural instability with retropulsion (i.e., a tendency to fall backwards), executive dysfunction, behavioral dyscontrol, and symmetric parkinsonian features (rigidity, tremor, hypo/bradykinesia).
Amyotrophic lateral sclerosis (ALS) commonly features cognitive impairments, and is accompanied by aphasia and FTD syndromes in a subset43. This phenomenon is now known to be linked to mutation of the C9ORF72 gene 44,45. ALS features progressive limb and/or pharyngeal spasticity, muscle wasting and fasciculations, and paresis (progressing to paralysis). Many cases manifest emotional dysfunction, characterized by involuntary or hair-trigger laughing and crying (the so called pseudobulbar affect).
Most cases of Creutzfeldt-Jakob disease (CJD) are sporadic forms (i.e., sCJD). sCJD is a rapidly progressive dementia manifesting some combination of ataxia, myoclonus, cortical blindness, motor dyscontrol, spasticity, incoordination, parkinsonism, and akinetic mutism. Several atypical forms are recognized: an ataxic CJD in which loss of coordination predominates; a visuoperceptual variant that culminates in cortical blindness; an amyotrophic variant featuring progressive spastic paresis and progressive muscle atrophy; an encephalopathic type featuring rapidly progressive dementia with myoclonus and akinetic mutism. CJD may also present with a predominance of cognitive impairments (mainly amnesia, disorientation and apraxia), or as a primary neuropsychiatric state featuring depression, anxiety, and agitation 46.
Spinocerebellar ataxias are a family of hereditary cerebellar degenerations manifesting progressive ataxia and incoordination, accompanied by dysarthria and dysphagia. They can be distinguished from ataxic CJD states by their very gradual evolution, typically spanning a few decades. Executive dysfunction, cognitive dysmetria, and affective lability can be observed seen in many cases 47. Dementia is not universal, but severe dysarthria, motor incoordination, incontinence, and dyscontrol of cognition and affect can mimic a mild dementia.
Vascular dementia (VaD) is not depicted in Figure 1; it is not neurodegenerative and often arises from overt stroke, in which case the diagnosis is straightforward. The clinical picture is variable, as it depends on the mechanism and distribution of the cerebrovascular disease underlying its development. Though VaD is common after stroke, it may follow relatively small infarcts in strategic locations, or arise from chronic cerebrovascular insufficiency 34. Thus VaD may arise as a sudden and catastrophic crippling of cognitive, mental, and motor functions following hemorrhagic stroke; as the classical stepwise decline in cognition accompanied by behavioral and motor phenomena; or as a hemiplegic or hemiparetic state with focal cognitive deficits (most commonly a non-fluent aphasia). Some cases shown an insidious progression that is difficult to distinguish from AD, FTD or DLB. The classical presentation is characterized by executive dysfunction, affective dyscontrol (typically emotional incontinence), psychomotor slowing, motor dyscontrol, parkinsonism, imbalance, and a gait impairment (small-step, apraxic or parkinsonian gait) 34,48.
Measurement and screening for case detection
Measurement is fundamental to neuropsychiatry practice, serving different goals – case detection (i.e., screening), differential diagnosis, and monitoring. At present there are no practical methods for screening for cases in the community. Proposals for screening for dementia in primary care have typically focused on late-life cases of amnesic dementia (i.e., late-onset AD), and are not likely to be practical for screening for YOD – owing to the diversity of presentations, overlap with primary affective and anxious disorders (including major depression, bipolar disorder, and obsessive compulsive disorder), and primary psychoses, and the variety of settings in which the cases present – primary care, psychiatry settings, and neurology clinics. Furthermore, bedside instruments such as the MMSE are not suitable for this population because many cases – particularly those in which neuropsychiatric phenomena define the phenotype – can attain scores matching those attained by their counterparts who either have normal cognitive function or subclinical impairments 49.
A practical approach to screening psychiatric populations for cases of neurodegenerative disease involves identifying ‘red flags’ 50 – features that serve as indicators that a clinical state may be a neurodegenerative mimic rather than a primary psychiatric state. These are depicted in Box 1. Other aspects of measurement, pertaining to differential diagnosis and monitoring of progression, are covered in the paper.
Conclusion
YOD are topical for psychiatry because of the syndromes, and their psychological and psychosocial aspects, and the skillset of the psychiatrist. Psychiatrists, have a role to play in the diagnosis of these conditions, and in their management. Understanding the clinical epidemiology of YOD can also aid the clinician in the diagnosis of these conditions. Appreciating the varied etiological distribution, the cognitive, neuropsychiatric, and motor syndromes, and the clinical genetic epidemiology, allows the physician with a high index of suspicion to apply a methodical approach to identifying potential cases, distinguishing them from primary psychiatric conditions, and making a specific diagnosis.
Box 1.
Characteristics, symptoms and signs that suggest neurodegenerative etiology of a psychiatric state
| • Historical: | • Cognitive symptoms: |
| – Later than typical age at onset | – Aphasia |
| – Puzzling “atypical” features | – Apraxia |
| – Family history of dementia, parkinsonism, or other motor disorder | – Visual complaints (other than hallucinations) |
| – Spatial disorientation | |
| – Unusual prodrome, e.g., insomnia, hyperphagia | – Incontinence |
| – Rapid evolution | |
| • Physical/motor signs | |
| – Abnormal posture and movement | |
| • Mental status: | – Frequent falls at early stage |
| – Poor insight | – Frequent (myoclonic) jerking |
| – Apathy/indifference | – Progressive motor weakness |
| – Compulsions without obsessions | – Declining motor coordination |
| – Visual hallucinations | – Left-right asymmetry |
Key points.
YOD is an important clinical and epidemiological problem that is often overshadowed in clinical and public consciousness.
Neurodegenerative diseases are the leading cause of YOD. Alzheimer disease (AD) is most common, followed closely by frontotemporal dementia and vascular dementia.
YOD may have non-amnesic, psychiatric, and neurological presentations.
An algorithmic approach interpreting clinical data, on the basis of defining syndromes, facilitates preliminary diagnosis, and guides diagnostic testing.
Screening for YOD in the psychiatric context is a rational process in which vigilance is combined with careful searches for ‘red flags’ that signal a psychiatric state is neurodegenerative.
Acronyms
- AD
Alzheimer Disease
- ALS
Amyotrophic Lateral Sclerosis
- ARD
Alcohol-Related Dementia
- CBD
Corticobasal Degeneration
- CJD
Creutzfeldt-Jakob Disease
- DLB
Dementia with Lewy Bodies
- FTD
Frontotemporal Dementia
- FTD-ALS
FTD with Amyotrophic Lateral Sclerosis
- HD
Huntington Disease
- PDD
Parkinson Disease
- PLA
Logopenic Progressive Aphasia
- PSP
Progressive Supranuclear Palsy
- SD
Semantic Dementia
- TBI
Traumatic Brain Injury
- VaD
Vascular Dementia
- VaD
Vascular Dementia
- YOD
Young-Onset Dementia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have nothing to disclose
Bibliography
- 1.van Vliet D, et al. Time to diagnosis in young-onset dementia as compared with late-onset dementia. Psychol Med. 2013;43:423–432. doi: 10.1017/S0033291712001122. [DOI] [PubMed] [Google Scholar]
- 2.van Vliet D, de Vugt ME, Bakker C, Koopmans RTCM, Verhey FRJ. Impact of early onset dementia on caregivers: a review. Int J Geriatr Psychiatry. 2010;25:1091–1100. doi: 10.1002/gps.2439. [DOI] [PubMed] [Google Scholar]
- 3.Rosenthal Gelman C, Greer C. Young Children in Early-Onset Alzheimer's Disease Families: Research Gaps and Emerging Service Needs. Am J Alzheimers Dis Other Demen. 2011;26:29–35. doi: 10.1177/1533317510391241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Millenaar JK, et al. The experiences and needs of children living with a parent with young onset dementia: results from the NeedYD study. Int Psychogeriatr. 2014;26:2001–2010. doi: 10.1017/S1041610213001890. [DOI] [PubMed] [Google Scholar]
- 5.Yokota O, et al. Frequency of early and late-onset dementias in a Japanese memory disorders clinic. Eur J Neurol. 2005;12:782–790. doi: 10.1111/j.1468-1331.2005.01072.x. [DOI] [PubMed] [Google Scholar]
- 6.McMurtray A, Clark D, Christine D, Mendez M. Early-onset dementia: frequency and causes compared to late-onset dementia. Dement Geriatr Cogn Disord. 2006;21:59–64. doi: 10.1159/000089546. [DOI] [PubMed] [Google Scholar]
- 7.Shinagawa S, et al. Frequency and clinical characteristics of early-onset dementia in consecutive patients in a memory clinic. Dement Geriatr Cogn Disord. 2007;24:42–47. doi: 10.1159/000102596. [DOI] [PubMed] [Google Scholar]
- 8.Nandi SP, et al. Clinical profile of young-onset dementia: A study from Eastern India. Neurology Asia. 2008;13:103–108. [Google Scholar]
- 9.Papageorgiou SG, Kontaxis T, Bonakis A, Kalfakis N, Vassilopoulos D. Frequency and causes of early-onset dementia in a tertiary referral center in Athens. Alzheimer Dis Assoc Disord. 2009;23:347–351. doi: 10.1097/WAD.0b013e31819e6b28. [DOI] [PubMed] [Google Scholar]
- 10.Picard C, Pasquier F, Martinaud O, Hannequin D, Godefroy O. Early onset dementia: characteristics in a large cohort from academic memory clinics. Alzheimer Dis Assoc Disord. 2011;25:203–205. doi: 10.1097/WAD.0b013e3182056be7. [DOI] [PubMed] [Google Scholar]
- 11.Croisile B, et al. [Diagnostic profile of young-onset dementia before 65 years. Experience of a French Memory Referral Center]. Rev. Neurol. (Paris) 2012;168:161–169. doi: 10.1016/j.neurol.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Harvey R, Skelton-Robinson M, Rossor M. The prevalence and causes of dementia in people under the age of 65 years. J Neurol Neurosurg Psychiatry. 2003;74:1206–1209. doi: 10.1136/jnnp.74.9.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ikejima C, et al. Prevalence and causes of early-onset dementia in Japan: a population-based study. Stroke. 2009;40:2709–2714. doi: 10.1161/STROKEAHA.108.542308. [DOI] [PubMed] [Google Scholar]
- 14.Borroni B, et al. Prevalence and demographic features of early-onset neurodegenerative dementia in Brescia county, Italy. Alzheimer Dis Assoc Disord. 2011;25:341–344. doi: 10.1097/WAD.0b013e3182147f80. [DOI] [PubMed] [Google Scholar]
- 15.Renvoize E, Hanson M, Dale M. Prevalence and causes of young onset dementia in an English health district. Int J Geriatr Psychiatry. 2011;26:106–107. doi: 10.1002/gps.2456. [DOI] [PubMed] [Google Scholar]
- 16.Withall A, Draper B, Seeher K, Brodaty H. The prevalence and causes of younger onset dementia in Eastern Sydney, Australia. Int Psychogeriatr. 2014;26:1955–1965. doi: 10.1017/S1041610214001835. [DOI] [PubMed] [Google Scholar]
- 17.Mercy L, Hodges JR, Dawson K, Barker RA, Brayne C. Incidence of early-onset dementias in Cambridgeshire, United Kingdom. Neurology. 2008;71:1496–1499. doi: 10.1212/01.wnl.0000334277.16896.fa. [DOI] [PubMed] [Google Scholar]
- 18.Garre-Olmo J, et al. Incidence and subtypes of early-onset dementia in a geographically defined general population. Neurology. 2010;75:1249–1255. doi: 10.1212/WNL.0b013e3181f5d4c4. [DOI] [PubMed] [Google Scholar]
- 19.Sanchez Abraham M, et al. Incidence of early-onset dementia in Mar del Plata. Neurologia. 2014 doi: 10.1016/j.nrl.2013.10.009. doi:10.1016/j.nrl.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Ferran J, et al. The early onset dementias: A study of clinical characteristics and service use. Int J Geriatr Psychiatry. 1996;11:863–869. [Google Scholar]
- 21.Kelley B, Boeve BF, Josephs K. Young-onset dementia: demographic and etiologic characteristics of 235 patients. Arch Neurol. 2008;65:1502–1508. doi: 10.1001/archneur.65.11.1502. [DOI] [PubMed] [Google Scholar]
- 22.2014 Alzheimer's disease facts and figures. Alzheimer's & Dementia. 2014;10:e47–e92. doi: 10.1016/j.jalz.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 23.Knopman DS, Roberts RO. Estimating the Number of Persons with Frontotemporal Lobar Degeneration in the US Population. J Mol Neurosci. 2011;45:330–335. doi: 10.1007/s12031-011-9538-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuruppu DK, Matthews BR. Young-onset dementia. Semin Neurol. 2013;33:365–385. doi: 10.1055/s-0033-1359320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baugh CM, et al. Chronic traumatic encephalopathy: neurodegeneration following repetitive concussive and subconcussive brain trauma. Brain Imaging Behav. 2012;6:244–254. doi: 10.1007/s11682-012-9164-5. [DOI] [PubMed] [Google Scholar]
- 26.Nordström P, Nordström A, Eriksson M, Wahlund L-O, Gustafson Y. Risk factors in late adolescence for young-onset dementia in men: a nationwide cohort study. JAMA Intern Med. 2013;173:1612–1618. doi: 10.1001/jamainternmed.2013.9079. [DOI] [PubMed] [Google Scholar]
- 27.Heath CA, Mercer SW, Guthrie B. Vascular comorbidities in younger people with dementia: a cross-sectional population-based study of 616 245 middle-aged people in Scotland. J Neurol Neurosurg Psychiatry jnnp–2014–309033. 2014 doi: 10.1136/jnnp-2014-309033. doi:10.1136/jnnp-2014-309033. [DOI] [PubMed] [Google Scholar]
- 28.Onyike CU. In young men, various risk factors are associated with later development of young-onset dementia. Evid Based Ment Health. 2014;17:49–49. doi: 10.1136/eb-2013-101627. [DOI] [PubMed] [Google Scholar]
- 29.Koedam ELGE, et al. Early-Onset Dementia Is Associated with Higher Mortality. Dement Geriatr Cogn Disord. 2008;26:147–152. doi: 10.1159/000149585. [DOI] [PubMed] [Google Scholar]
- 30.KAY DWK, FORSTER DP, NEWENS AJ. Long-term survival, place of death, and death certification in clinically diagnosed pre-senile dementia in northern England Follow-up after 8-12 years. Br J Psychiatry. 2000;177:156–162. doi: 10.1192/bjp.177.2.156. [DOI] [PubMed] [Google Scholar]
- 31.Onyike CU. What Is the Life Expectancy in Frontotemporal Lobar Degeneration? Neuroepidemiology. 2011;37:166–167. doi: 10.1159/000333347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu WT, et al. Survival profiles of patients with frontotemporal dementia and motor neuron disease. Arch Neurol. 2009;66:1359–1364. doi: 10.1001/archneurol.2009.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Appleby BS, Lyketsos CG. Rapidly progressive dementias and the treatment of human prion diseases. Expert Opin Pharmacother. 2011;12:1–12. doi: 10.1517/14656566.2010.514903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Onyike CU. Cerebrovascular disease and dementia. Int Rev Psychiatry. 2006;18:423–431. doi: 10.1080/09540260600935421. [DOI] [PubMed] [Google Scholar]
- 35.Mendez MF. Early-onset Alzheimer's disease: nonamnestic subtypes and type 2 AD. Arch. Med. Res. 2012;43:677–685. doi: 10.1016/j.arcmed.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gorno-Tempini ML, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neary D, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 38.Rascovsky K, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McKeith IG, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 40.Rosenblatt A, Leroi I. Neuropsychiatry of Huntington's disease and other basal ganglia disorders. Psychosomatics. 2000;41:24–30. doi: 10.1016/S0033-3182(00)71170-4. [DOI] [PubMed] [Google Scholar]
- 41.Rosenblatt A. Understanding the psychiatric prodrome of Huntington disease. J Neurol Neurosurg Psychiatry. 2007;78:913. doi: 10.1136/jnnp.2006.112565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kertesz A, Blair M, McMonagle P, Munoz DG. The diagnosis and course of frontotemporal dementia. Alzheimer Dis Assoc Disord. 2007;21:155–163. doi: 10.1097/WAD.0b013e31806547eb. [DOI] [PubMed] [Google Scholar]
- 43.Bak T, Hodges JR. Cognition, language and behaviour in motor neurone disease: evidence of frontotemporal dysfunction. Dement Geriatr Cogn Disord. 1999;10(Suppl 1):29–32. doi: 10.1159/000051208. [DOI] [PubMed] [Google Scholar]
- 44.DeJesus-Hernandez M, et al. Expanded GGGGCC Hexanucleotide Repeat in Noncoding Region of C9ORF72 Causes Chromosome 9p-Linked FTD and ALS. Neuron. 2011;72:245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Renton AE, et al. A Hexanucleotide Repeat Expansion in C9ORF72 Is the Cause of Chromosome 9p21-Linked ALS-FTD. Neuron. 2011;72:257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Appleby BS, et al. Characteristics of established and proposed sporadic Creutzfeldt-Jakob disease variants. Arch Neurol. 2009;66:208–215. doi: 10.1001/archneurol.2008.533. [DOI] [PubMed] [Google Scholar]
- 47.Schmahmann JD. Disorders of the Cerebellum: Ataxia, Dysmetria of Thought, and the Cerebellar Cognitive Affective Syndrome. J Neuropsychiatry Clin Neurosci. 2004;16:367–378. doi: 10.1176/jnp.16.3.367. [DOI] [PubMed] [Google Scholar]
- 48.Ridha B, Josephs K. Young-onset dementia: a practical approach to diagnosis. Neurologist. 2006;12:2–13. doi: 10.1097/01.nrl.0000186798.86255.69. [DOI] [PubMed] [Google Scholar]
- 49.Onyike CU, et al. Estimating severity of illness and disability in frontotemporal dementia” preliminary analysis of the Dementia Disability Rating (DDR). Acta Neuropsychologia. 2011;9:141–153. [PMC free article] [PubMed] [Google Scholar]
- 50.Wylie MA, et al. Management of frontotemporal dementia in mental health and multidisciplinary settings. Int Rev Psychiatry. 2013;25:230–236. doi: 10.3109/09540261.2013.776949. [DOI] [PMC free article] [PubMed] [Google Scholar]