SUMMARY
The enzyme lysostaphin possesses potent anti-staphylococcal activity and represents a promising antibacterial drug candidate; however, its immunogenicity poses a barrier to clinical translation. Here, structure-based biomolecular design enabled widespread depletion of lysostaphin’s DRB1*0401 restricted T cell epitopes, and resulting deimmunized variants exhibited striking reductions in anti-drug antibody responses upon administration to humanized HLA-transgenic mice. This reduced immunogenicity translated into improved efficacy in the form of protection against repeated challenges with methicillin-resistant Staphylococcus aureus, or MRSA. In contrast, while wild type lysostaphin was efficacious against the initial MRSA infection, it failed to clear subsequent bacterial challenges that were coincident with escalating anti-drug antibody titers. These results extend the existing deimmunization literature, in which reduced immunogenicity and retained efficacy are assessed independently of each other. By correlating in vivo efficacy with longitudinal measures of anti-drug antibody development, we provide the first direct evidence that T cell epitope depletion manifests enhanced biotherapeutic efficacy.
INTRODUCTION
Biotherapeutics represent a growing share of the pharmaceuticals market (Aggarwal, 2014), and they are expected to become increasingly important as new molecules are brought online to address an ever wider array of indications. While these biological agents generally benefit from exceptional specificity and potency, they are also subject to unique risk factors that distinguish them from conventional small molecule drugs. In particular, proteins are susceptible to human immune surveillance and have a propensity to elicit anti-drug antibodies, which can compromise efficacy and even threaten patient safety. Protein immunogenicity can be caused by a wide variety of factors (Schellekens, 2002; Jawa et al., 2013), with one important driver being a protein’s origins: non-human proteins are predisposed towards immune recognition as foreign agents. Due in part to concerns over immunogenicity, a promising reservoir of drug candidates from the broader biosphere has been left largely untapped, and fully exploiting the medicinal potential of exogenous proteins will require effective strategies by which to mitigate immunogenicity risks.
The antibacterial enzyme lysostaphin (LST) is one biotherapeutic candidate whose clinical development has been stymied in part by immunogenicity concerns. LST is a peptidoglycan-degrading biocatalyst with potent and selective bactericidal activity towards the dangerous human pathogen Staphylococcus aureus (Szweda et al., 2012). It has been shown to be efficacious in a wide variety of preclinical animal models, and has proven capable of clearing human nasal carriage of S. aureus and even clearing a drug-resistant systemic infection in one patient (Stark et al., 1974). Unfortunately the bacterial origins of LST, which is derived from the S. aureus environmental competitor Staphylococcus simulans, contribute to a variety of immunogenicity related issues in mice, rats, rabbits, dogs, non-human primates, and even human subjects (Kokai-Kun, 2012).
As with many biotherapeutics, T cell epitopes are one likely driver of lysostaphin’s immunogenicity. Upon administration of LST or any foreign protein to a human subject, antigen presenting cells sample the agent from the extracellular environment and proteolytically degrade it within lysosomal compartments. A subset of peptide fragments, known as T cell epitopes, are loaded into the groove of class II major histocompatibility complex proteins (MHC II, also called HLA in humans). The MHC-bound peptides are then presented on the cell surface, where they have the opportunity to form ternary complexes with cognate receptors on the surface of CD4+ helper T cells (Trombetta et al., 2005). Simultaneous engagement of co-receptors generates an immune synapse, which initiates a signaling cascade that ultimately drives T cell activation, B cell maturation, and production of circulating IgG antibodies that bind to the offending therapeutic protein (Jawa et al., 2013). Thus, a T cell-mediated anti-drug antibody response is fundamentally driven by molecular recognition of peptide fragments derived from the therapeutic agent.
It has been well established that mutagenic deletion of a protein’s constituent epitopes can reduce immunogenic potential. In some instances, a foreign protein may contain only one or two immunodominant regions that are readily deleted with a small number of mutations (Cizeau et al., 2009; Harding et al., 2005). In other cases, proteins may contain a larger number of distributed epitopes. In such a scenario, redesigning one region might remain relatively straightforward (Warmerdam et al., 2002; Mazor et al., 2012), but aggressive deimmunization targeting multiple dispersed epitopes represents a more serious challenge requiring extensive experimental effort (Mazor et al., 2014). As a complement to experimentally driven methods, computational epitope predictors and computational modeling of the structural and functional consequences of epitope-deleting mutations can accelerate the deimmmunization process. In particular, fully integrated protein design and deimmunization tools have the capacity to rapidly identify candidate epitopes and quickly select the most promising deimmunizing mutations (Parker et al., 2010; Parker et al., 2011; Parker et al., 2013; He et al., 2012; Choi et al., 2013; King et al., 2014). Several experimental studies of designs produced by various algorithms have yielded promising results based on surrogate measures of immunogenicity (i.e., peptide-MHC II binding assays and/or ex vivo cellular immunoassays) (King et al., 2014; Osipovitch et al., 2012; Salvat et al., 2014; Salvat et al., 2015). However, the ultimate goal of protein deimmunization is the mitigation of anti-drug antibody responses so as to enhance efficacy, and assessing such an outcome is currently only possible in vivo.
Here, we sought to assess the effects of LST epitope depletion using clinically relevant metrics. Structure-based design of individual variants as well as structure-based library design accelerated the deimmunization process, and the performance of engineered variants was compare to the parental wild type enzyme in HLA-transgenic mice. Longitudinal measures of anti-drug antibody development correlated with in vivo antibacterial activity, thereby providing the first direct evidence that protein deimmunization manifests enhanced biotherapeutic efficacy.
RESULTS
Overview
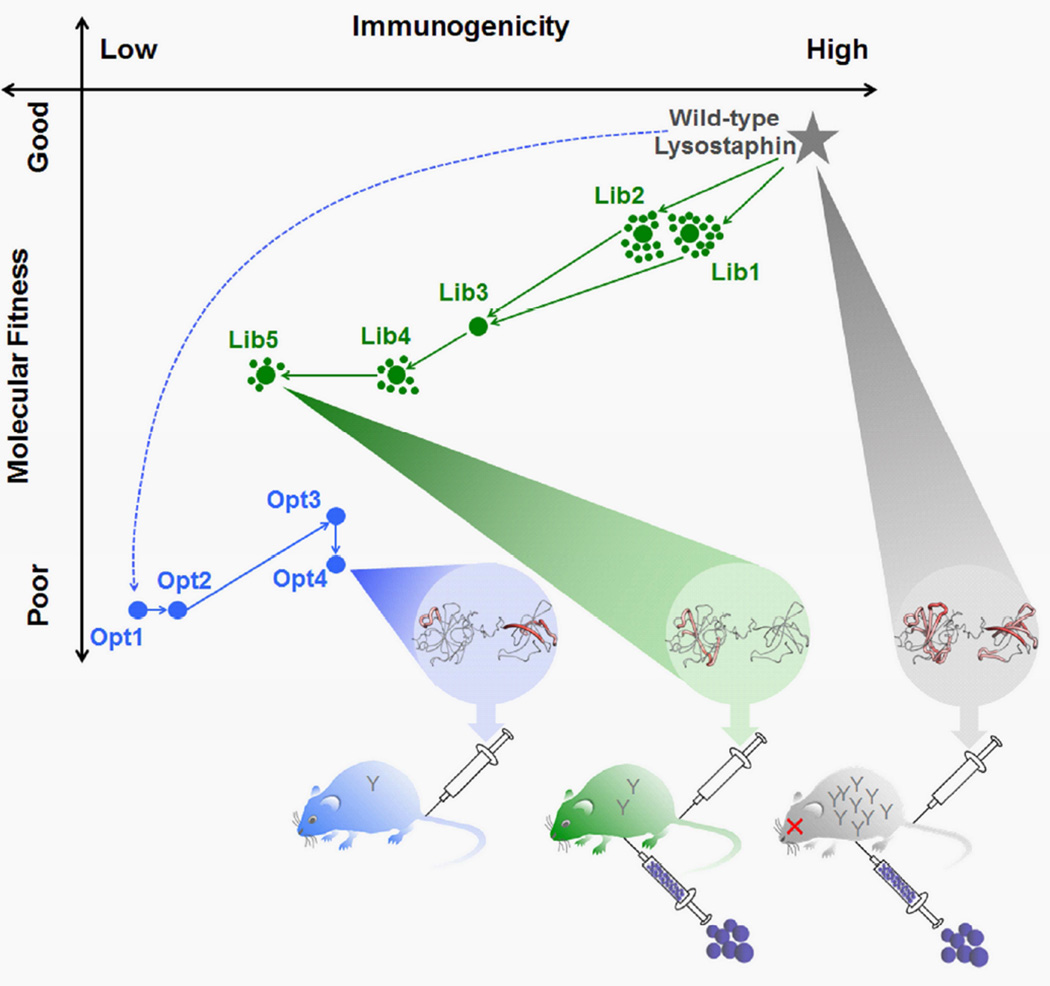
In this study, we sought to test the hypothesis that depletion of putative T cell epitopes in LST would mitigate the anti-drug antibody response and consequently enhance therapeutic efficacy. Epitope depleted variants were developed using two distinct computationally-guided strategies: structure-based design of individual deimmunized variants followed by empirical improvement (Fig. 1, blue path) and structure-based design and screening of combinatorial libraries enriched in functionally deimmunized members (Fig. 1, green path). Humanized HLA-transgenic mice were used to assess the efficiency with which each method deleted putative immunogenic epitopes and thereby prevented formation of anti-LST antibodies in vivo (Fig. 1, bottom). Subsequently, a recurrent bacteremia model was used to gauge the extent to which LST deimmunization enabled clearance of systemic S. aureus infections. This systematic comparison between deimmunized variants and their wild type counterpart provides direct experimental evidence of the clinically relevant connections between putative T cell epitopes, in vivo immunogenicity, and therapeutic efficacy.
Fig. 1. Schematic overview of molecular engineering process and in vivo outcomes.
(Top) A plot of Molecular Fitness vs. Immunogenicity is shown for individually optimized variants and selected library members. The Y-axis reflects trends in experimental performance measurements and the X-axis reflects trends in computational predictions of T cell epitope content. LSTWT has both high fitness and high immunogenicity, where the latter is shown as a structural epitope map (grey) (thick red indicates dense epitope content and thin grey indicates no epitope content). Deimmunization by design of individual optimal variants is shown in blue, where the initial design was fully depleted of DR4 epitopes, but failed to express. Subsequent designs reverted problematic mutations in a reverse engineering process, ultimately yielding the expressible, aglycosylated, but inactive variant Opt4, whose structural epitope map is shown (blue). Deimmunization by library design and screening is shown in green. Deimmunizing mutations were accumulated in an iterative process, ultimately yielding the active and deimmunized Lib5 variant, whose structural epitope map is shown (green). (Bottom) The in vivo immunogenicities of LSTWT, Opt4, and Lib5 were evaluated in transgenic DR4 mice, and trends in observed antibody titers are indicated as Y-shaped IgG cartoons in the mice. Subsequently, the efficacy of LSTWT and Lib5 were evaluated in a recurrent bacteremia model that required repeated dosing of the antibacterial enzyme. Variant Lib5 enabled repeated rescue of DR4 mice, whereas LSTWT lost efficacy due to high antibody titers. See also Table S1.
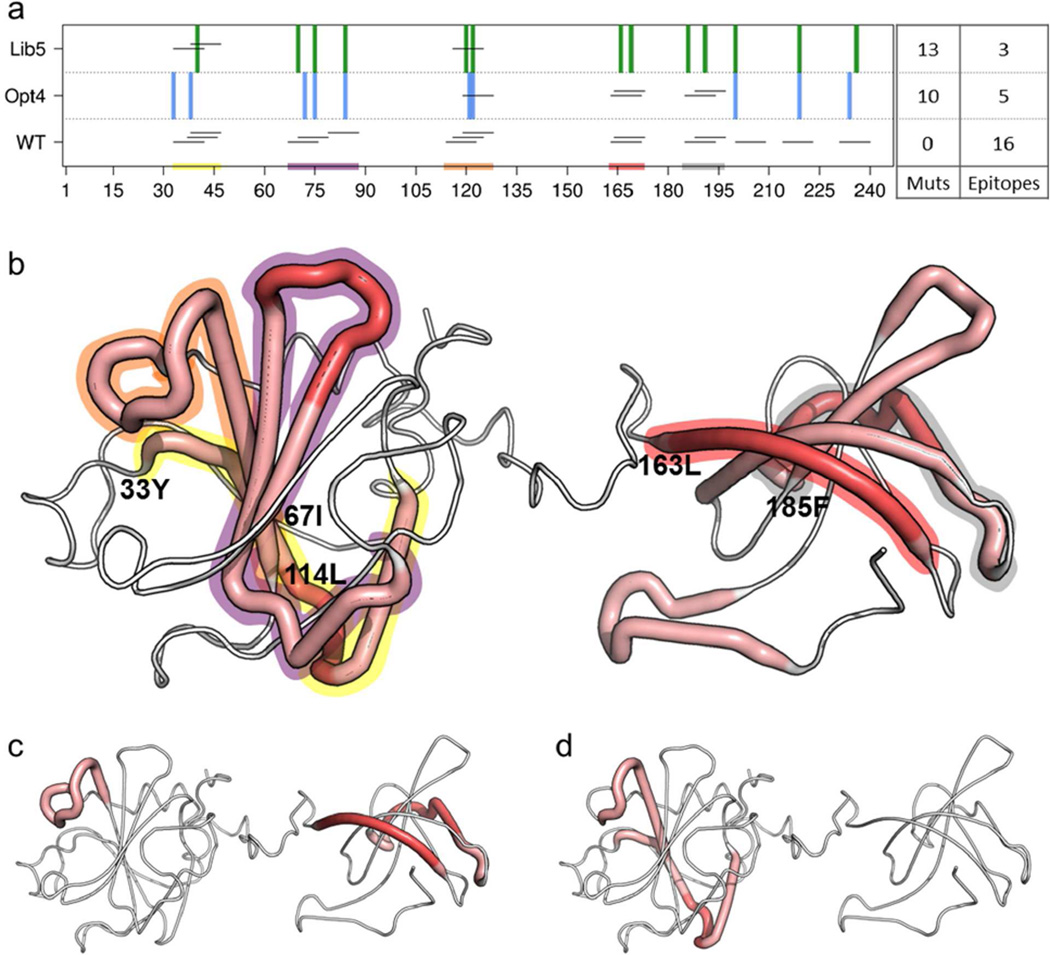
As a proof of concept, we focused on allele DRB1*0401 (hereafter DR4), which is highly prevalent in North American and European populations. At a 5% threshold (i.e. peptides among the top 5% of predicted binders), the ProPred analysis tool (Singh et al., 2001) predicted 16 DR4 restricted T cell epitopes within wild type LST (LSTWT epitope score=16). The peptide epitopes were arrayed as both overlapping clusters and isolated nonamers distributed throughout the protein’s sequence and structure (Fig. 2a,b). Interestingly, ProPred predicted more epitopes for DR4 than for any of the seven other representative DRB1 alleles: 0101, 0301, 0701, 0801, 1101, 1301, and 1501 (Fig. S1). Considering any single allele, therefore, our chosen DR4 model represented a high bar for global protein redesign.
Fig. 2. Epitope maps of LST enzymes.
- Predicted DR4 T cell epitopes are shown for variants Lib5 (top), Opt4 (middle), and LSTWT (bottom). Nonamer epitopes are shown as black horizontal lines at the corresponding position in the LST amino acid sequence (X-axis). LSTWT contains 16 putative epitopes, Opt4 contains 5, and Lib5 contains only three. Regions of overlapping epitope density in LSTWT are highlighted with colored blocks (x-axis). Positions of deimmunizing mutations in Lib5 are shown as vertical green bars (top), and those in Opt4 as vertical blue bars (middle). The mutation and epitope counts for each design are indicated at right.
- Predicted epitopes in LSTWT are mapped onto a structural model. Thick red tubes indicate dense overlapping epitopes and thin white tubes indicate absence of epitopes. High density regions from the sequence map are highlighted in the structural model with corresponding background coloring. The P1 anchor residue of the first epitope in each cluster is annotated.
- Predicted epitopes remaining in Opt4 are mapped onto a structural model.
- Predicted epitopes remaining in Lib5 are mapped onto a structural model.
See also Figure S1.
In order to select combinations of mutations likely to preserve structural integrity while deleting predicted epitopes, it was necessary to develop a homology model of LST, as no crystal structure was available at the initiation of our studies. LST is a two-domain protein with a cell wall binding domain that targets S. aureus peptidoglycan and a catalytic domain that specifically cleaves the pentaglycine cross-links in the cell wall, causing rapid bacterial lysis and death. We separately modeled and redesigned the catalytic domain and the cell wall binding domain. Recently a crystal structure was obtained of both domains together (Sabala et al., 2014), and our models displayed TM-scores (Zhang et al., 2004) of 0.88 for the catalytic domain and 0.95 for the cell wall binding domain, indicating a high level of agreement. The primary differences between the homology model and LST crystal structure were associated with loops surrounding active sites. Consistent with observations on a homologous protein (Firczuk et al., 2005), the corresponding loop structures are highly variable (Fig. S2). Thus, the model deviations were relatively minor, and indeed the model ultimately proved useful in guiding LST redesign.
Structure-based design of individual variants
The EpiSweep algorithm (Parker et al., 2013) was used to optimize deimmunized variants, making the best trade-offs between predicted reduction in epitope content, as evaluated by ProPred, and predicted maintenance of protein stability, as evaluated by structure-based rotamer energy. Panels of 20 fully depleted designs (i.e. DR4 epitope scores=0) were generated separately for both the catalytic and cell wall binding domains. A variant combining low energy designs from each domain, Opt1 (Table 1), was selected for experimental analysis and cloned into an optimized Pichia pastoris expression system (Zhao et al., 2014). Unfortunately, this 14-mutation design failed to yield functional protein (Fig.1). Other LST redesign work had shown that single mutations could undermine otherwise stable and active deimmunized variants (Blazanovic et al., accepted), and we therefore identified detrimental mutations in the Opt1 design by systematically reverting mutations and mutation combinations. Analysis of isolated Opt1 mutations revealed that M119R singlehandedly abolished protein secretion, but reversion of this single mutation in design Opt2 (Table 1) failed to restore expression (Fig. 1). Ultimately, it was found that the three mutation combination S166E, S168K, and V193W also undermined expression, and reversion to wild type at these three sites as well as M119 generated an expressible 10-mutation variant, Opt3 (Fig. 1).
Table 1.
Lysostaphin Designs and Preliminary Performance Parameters
| Designa | Y33 | F38 | N40 | I70 | N72 | V75 | S84 | M119 | V120 | N121 | S122 | S166 | S168 | A169 | R186 | S191 | V193 | I200 | N219 | S234 | N236 | Mutation Load |
Epitope Score |
Expression Level (%WT) |
Activity (%WT) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LSTWT | 0 | 16 | 100 | 100 | |||||||||||||||||||||
| Opt1 | T | S | H | Q | Y | R | G | G | E | K | W | T | Y | D | 14 | 0 | NDc | NDc | |||||||
| Opt2 | T | S | H | Q | Y | G | G | E | K | W | T | Y | D | 13 | 1 | NDc | NDc | ||||||||
| Opt3 | T | S | H | Q | Y | G | G | T | Y | D | 10 | 5 | 20 | NDb | |||||||||||
| Opt4 | T | S | H | Q | Y | G | G | T | Y | K | 10 | 5 | 10 | 0.25 | |||||||||||
| Lib1 | K | E | D | T | 4 | 13 | 80 | 100 | |||||||||||||||||
| Lib2 | T | G | T | Y | 4 | 12 | 80 | 100 | |||||||||||||||||
| Lib3 | K | E | D | T | T | G | T | Y | 8 | 8 | 60 | 50 | |||||||||||||
| Lib4 | K | E | G | D | T | T | G | T | Y | D | 10 | 6 | 50 | 50 | |||||||||||
| Lib5 | T | K | E | G | D | T | T | G | T | A | T | Y | D | 13 | 3 | 50 | 50 | ||||||||
Sites in the catalytic domain are indicated in italics and those in the cell wall binding domain in plain text
Activity measured as minimal inhibitory concentration (MIC), reported as the % fold dilution, relative to wild type, at which the MIC was achieved.
Not Detectable
Although Opt3 achieved reasonable expression levels (Table 1), purity analysis by SDS-PAGE revealed two bands: one at the expected 25 kDa mass and a second at approximately 30 kDa. Based on prior experience with LST glycosylation in P. pastoris (Zhao et al., 2014), we suspected that a latent N-linked glycosylation sequon in the C-terminal cell wall binding domain (232-NKS-234) had been activated. We therefore exchanged the deimmunizing N236D mutation with the S234K mutation, which deleted both the C-terminal epitope and the N-linked glycosylation sequon. The resulting 10-mutation variant, Opt4, bore only 5 predicted DR4 epitopes and expressed as a single 25 kDa band, albeit with lower yields than Opt3 (Fig. 1). Although the highly engineered Opt4 variant was produced in a folded and secretion competent state, it was subsequently found to possess only a small fraction of the wild type enzyme’s antibacterial activity (Table 1).
Structure-based deimmunization via computational library design
In parallel to design of individual variants, we also pursued an alternative combinatorial approach in which computational design was used to generate LST libraries predicted to be enriched in functional, deimmunized variants. The structure-based library design method SOCoM (Verma et al., 2015) was augmented with epitope analysis in order to identify residue positions and mutations whose combinations yielded variants with low epitope scores, as evaluated by ProPred, along with good energies, as evaluated by a Cluster Expansion (Grigoryan et al., 2009; Grigoryan et al., 2006) potential trained on Rosetta (Rohl et al., 2004) models. In the initial round, the designs were based on the wild type reference, while in succeeding rounds the reference was shifted to the lead clone selected from the previous library screen.
Library A targeted nine sites in the catalytic domain, and the complementary Library B population targeted eight sites in the cell wall binding domain (Table S1). Although the deimmunized LST design space was massive, we constrained our initial libraries to fewer than 40,000 members so as to maintain some parity between library size and the screening capacity of agar plate halo formation assays. Approximately 10,000 clones were screened from both Libraries A and B, and 10 large-halo-forming colonies from each were sequenced and functionally validated. The most promising variant from Library A (clone Lib1, Fig. 1) contained four mutations in the catalytic domain and exerted antibacterial activity equivalent to LSTWT (Table 1). Likewise, the most promising variant from Library B (clone Lib2, Fig. 1) possessed four mutations in the cell wall binding domain and also had wild type antibacterial activity (Table 1).
The respective deimmunized domains of variants Lib1 and Lib2 were combined to produce variant Lib3 (Fig. 1), containing eight mutations, a 50% reduction in predicted epitope content, and retention of 50% wild type activity (Table 1). The structures of the two Lib3 domains were subsequently modeled and used as templates in another round of deimmunized library design. The resulting Library C targeted five sites in the catalytic domain and three sites in the cell wall binding domain (Table S1). Functional screening of 10,000 clones yielded variant Lib4, which deleted 10/16 DR4 epitopes (Fig. 1). Relative to its Lib3 starting template, Lib4 contained one additional mutation each in the catalytic and cell wall binding domains and retained equivalent antibacterial activity (Table 1). The domains of Lib4 were used in another iterative round of modeling and deimmunized library design, yielding Library D (Table S1), from which 10,000 clones were screened to isolate variant Lib5 (Fig. 1). This variant deleted 13/16 putative DR4 epitopes, yet retained 50% wild type expression and 50% antibacterial activity (Table 1). Compared to variant Opt4, the best enzyme from the individual protein design efforts, Lib5 exhibited both a lower predicted epitope score and higher measured functionality (Fig. 1).
Further library construction and screening efforts (Library E, Table S1) failed to identify additional functional constructs, therefore the 13-mutation Lib5 variant was designated the lead candidate for further analysis. It is interesting to note that the three putative epitopes remaining in variant Lib5 (33-YGVDFFMTI-41, 38-FMTIGTPVK-46, and 116-FQRMDNTFS-124, Fig. 2a) either encompass or are adjacent to amino acids responsible for active site Zn2+ coordination (His32, Asp36 and His115). This fact may explain the elusive nature of functional mutations within these regions; screening of diverse library populations (Table S1) identified only one functional substitution in region 33–46 and two in region 116–124 (Table 1). Together, Lib5 mutations resulted in a 10 °C reduction in thermostability relative to LSTWT (Tm=48.64±.09 vs. 59.2±0.3 °C, respectively).
Epitope depleted designs display significantly reduced immunogenicity in vivo
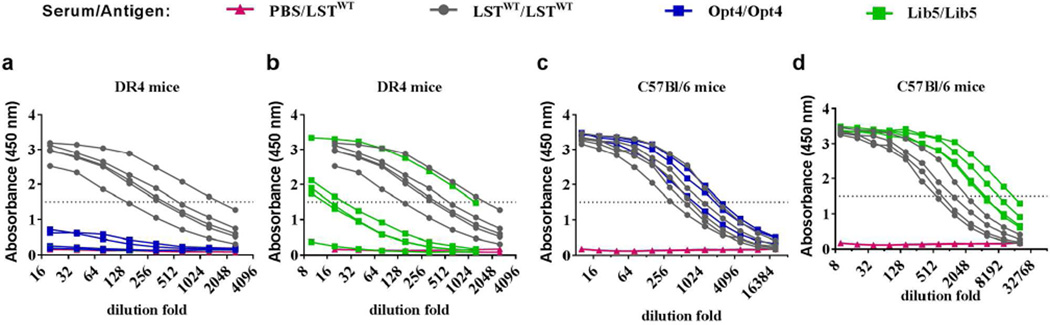
We next sought to assess the extent to which epitope depletion impacted in vivo immunogenicity. Anti-LST antibody responses were assessed in both C57Bl/6 and transgenic DR4 mice, the latter of which are null for endogenous murine MHC II but bear a chimeric MHC II receptor derived from human HLA DRB1*0401 (Ito et al., 1996). As a stringent benchmark for deimmunization, mice were immunized subcutaneously with protein in complete Freund’s adjuvant, a powerful immunostimulant. Two weeks after a single immunization with LSTWT, all DR4 mice mounted a potent anti-LST IgG antibody response, with titers between 1:150 and 1:1700 (Fig. 3a). In contrast, mice immunized with Opt4 showed a striking reduction in titers; only 2/5 mice exhibited any detectable anti-LST antibodies, and even those were substantially reduced relative to LSTWT immunized animals (Fig. 3a). Variant Lib5 also elicited a reduced antibody response; only one animal exhibited high antibody titers (1:1200), three showed significantly lower antibody titers (1:15 to 1:26), and 1 mouse exhibited near background levels of anti-LST antibodies (Fig. 3b). Importantly, both LSTWT and Opt4 were equally immunogenic in the C57Bl/6 laboratory mouse strain (Fig. 3c), while Lib5 was actually more immunogenic than LSTWT (IgG titers 1:4400 to 1:15,000 versus 1:560 to 1: 2100, respectively) (Fig. 3d). Thus, the striking reductions in Opt4 and Lib5 immunogenicity were specifically associated with disruption of molecular recognition by human DR4, as opposed to the native murine MHC II.
Fig. 3. In vivo immunogenicity of LST and deimmunized candidates, as measured by anti-LST IgG antibody ELISA following immunization in complete Freund’s adjuvant.
- Immunogenicity of LSTWT and Opt4 in humanized DR4 mice. LSTWT immunized mice exhibit high antibody titers (grey), whereas Opt4 immunized mice (blue) have titers at or near background levels seen with a PBS sham immunization (magenta).
- Immunogenicity of LSTWT and Lib5 in humanized DR4 mice. One Lib5 immunized mouse (green) exhibited antibody titers equivalent to those elicited by LSTWT (grey), with three mice having one to two orders of magnitude lower titers and a fifth mouse near PBS background levels (magenta).
- Immunogenicity of LSTWT and Opt4 in the C57Bl/6 stock mouse strain. Both enzymes are equally immunogenic.
- Immunogenicity of LSTWT and Lib5 in stock C57Bl/6 mice. Both LSTWT and Lib5 were highly immunogenic, with Lib5 representing the stronger antigen.
Together, these results demonstrate that the striking reduction in Opt4 and Lib5 immunogenicity was specific to disruption of molecular recognition by human MHC II, as designed.
It is interesting to note that although the protein design process was blinded to all but allele DR4, ProPred predictions indicated that neither Opt4 nor Lib5 contained neoepitopes for seven other representative human DRB1 alleles (Fig. S1). In fact, over and above the 11 putative DR4 epitopes deleted from Opt4, predictions suggested that 12 epitopes associated with alleles DR1, DR3, DR7, DR13, and DR15 had also been deleted. Similarly for Lib5, in addition to deletion of 13 DR4-restricted epitopes, ProPred predicted deletion of 18 additional epitopes associated with the other seven DRB1 alleles (Fig. S1).
Lysostaphin deimmunization translates into improved therapeutic efficacy
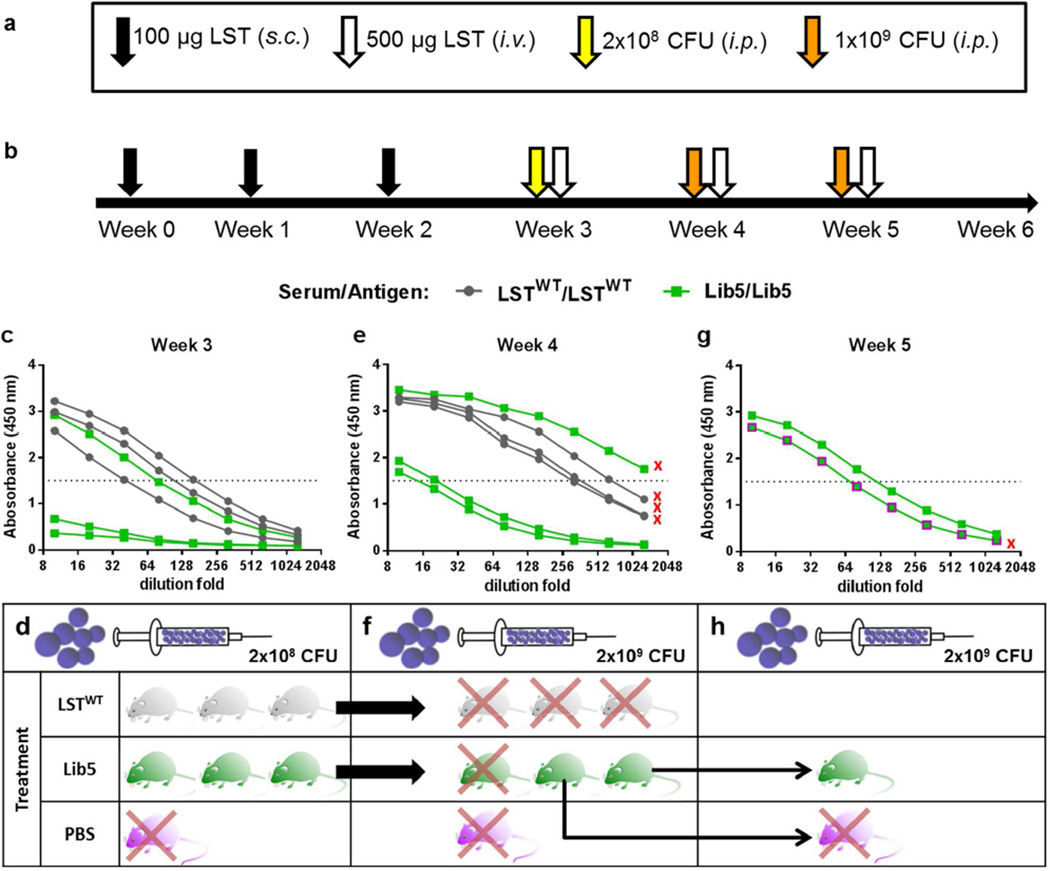
In many scenarios, therapeutic application of LST might require repeated administration to fully eradicate S. aureus infections. Therefore, to more closely mimic potential clinical applications, we monitored the immunogenicity of LSTWT and Lib5 during weekly dosing in the absence of adjuvant (Fig. 4a,b). Seven days after a third immunization, all DR4 mice receiving LSTWT had mounted a relatively strong immune response, with anti-LST titers in the 1:40 to 1:160 range (Fig. 4c). During the same timeframe, only one of three mice immunized with Lib5 developed high antibody titers (1:120), where the other two Lib5 mice exhibited titers only marginally above background.
Fig. 4. Deimmunized lysostaphin manifests therapeutic advantages in humanized DR4 mice.
- Immunization, infection, and treatment key. Routes of administration: s.c.=subcutaneous; i.v.=intravenous; i.p.=intraperitoneal. Immunizations were adjuvant-free.
- Timeline of the experimental design. Three animals per group were preimmunized with 3 weekly s.c. injections of LSTWT or Lib5 before undergoing a series of recurrent MRSA infections followed by repeated treatment with LSTWT or Lib5, respectively.
- Week three immunogenicity of LSTWT (grey) and Lib5 (green) as measured by protein specific IgG ELISA of serum. By week three, LSTWT mice exhibited high antibody titers, whereas two out of three Lib5 immunized mice exhibited low level titers.
- Week three efficacy in a systemic bacteremia model. Mice were treated i.v. with LSTWT (grey), Lib5 (green), or PBS (magenta). Both enzymes rescued 100% of their respective treatment groups, whereas the PBS sham treatment did not.
- Week four immunogenicity. All mice demonstrated increased anti-LST titers relative to week three, but the two low titer Lib5 mice continued to exhibit a weaker immune response. Mice that were not rescued in subsequent efficacy studies are marked with a red “x”.
- Week four efficacy. LSTWT failed to rescue any mice, and similarly neither the high titer Lib5 mouse nor the PBS mouse survived. The two lower titer Lib5 mice were both rescued by the Lib5 treatment.
- Week five immunogenicity. The two surviving Lib5 mice again exhibited increased titers, but titers remained below that of the week four LSTWT group.
- Week five efficacy. One of the surviving mice was treated with Lib5 and survived, whereas the second mouse was given a PBS sham treatment and did not.
Using an S. aureus recurrent bacteremia model, we next evaluated the extent to which LST immunogenicity impacted in vivo efficacy. Following determination of antibody titers at week three, the above DR4 mice were infected by intraperitoneal administration of 2x108 colony forming units (CFU) of methicillin-resistant S. aureus (MRSA) strain USA400. One hour later, mice were given a 500 µg intravenous bolus of LSTWT or Lib5, respectively. Both enzymes rescued their respective groups from this initial infection, whereas a control mouse given a PBS sham treatment had to be sacrificed due to excessive morbidity (Fig. 4c).
One week later, antibody titers had increased for both the LSTWT group (1:300 to 1:650) and the Lib5 group (1 mouse >1:1000, with the remaining two between 1:15 and 1:20), but the latter continued to exhibit a lower overall trend (Fig. 4d). Mice were now infected with 109 CFU of MRSA and again treated 1 hour later with a 500 µg intravenous bolus of the respective enzyme. In this second infection cycle, where mice had developed higher antibody titers, LSTWT failed to rescue any of the three treated mice (Fig. 4d). Similarly, the single Lib5 mouse exhibiting high antibody titers succumbed to the infection, but the two lower titer Lib5 mice were rescued from the second MRSA challenge.
The following week, antibody titers in the two surviving Lib5 mice were found to have increased yet again (1:70 and 1:120, Fig. 4e), but notably they remained below the week four LSTWT titers. Following a third infection cycle with MRSA, one mouse was treated with Lib5 and survived, whereas the second mouse was given a PBS sham and succumbed to the infection (Fig. 4e). As a whole these results show that humanized DR4 mice mounted a strong immune response to LSTWT, even in the absence of adjuvant. During repeated administration, the weekly increase in anti-LSTWT antibody titers correlated with loss of efficacy. Conversely, immune responses were attenuated in two of three mice receiving the Lib5 deimmunized variant, and once again in vivo efficacy tracked with anti-LST antibody titers. Lib5 exerted potent antibacterial efficacy against as many as three consecutive challenges with MRSA.
Throughout the study, anti-LST antibody titers below 1:256 correlated with enzyme-mediated rescue from S. aureus infection, whereas titers above 256 were universally associated with failure of the antibacterial enzyme therapy. Variant Lib5’s capacity to mitigate anti-drug antibody responses therefore manifested as enhanced efficacy relative to LSTWT.
DISCUSSION
LST is a promising antibacterial drug candidate with potent bactericidal activity towards S. aureus in planktonic and biofilm culture, in metabolically active and inactive states, and towards drug-sensitive as well as drug-resistant strains. However, the enzyme’s own bacterial origins contribute to a variety of immunogenicity related issues in mice, rats, rabbits, dogs, non-human primates, and human subjects (Kokai-Kun, 2012). As with many foreign proteins, therefore, immunogenicity represents a significant barrier to LST clinical translation and widespread therapeutic application. Efforts to deimmunize LST by chemical conjugation to polyethylene glycol (PEGylation) initially showed promising results (Walsh et al., 2003), but follow on studies subsequently demonstrated that covalent coupling of LST to PEG led to complete loss of activity (Kokai-Kun, 2012). This latter observation is consistent with the inactivation of other antibacterial enzymes following PEGylation (Resch et al., 2011).
We hypothesized that LST could instead be deimmunized by mutagenic deletion of T cell epitopes, and in other studies we have shown that deletion of putative epitopes in the LST catalytic domain resulted in a large reduction of the enzyme’s immunostimulatory activity towards splenocytes from humanized mice (Blazanovic et al., accepted). Here we sought to complement and extend that work by demonstrating that more extensive protein-wide epitope depletion could suppress the anti-LST antibody response in vivo, thereby enhancing therapeutic efficacy. As a proof of concept, we first sought to fully deplete all 16 of LST’s predicted DR4-restricted epitopes. Initial efforts to simultaneously target all prospective immunogenic subsequences failed to produce a folded and functional enzyme. However, reversion of only four problematic mutations in one engineered design yielded an expressible 10-mutation variant predicted to delete 11/16 DR4 epitopes. This extensively deimmunized candidate elicited little to no anti-drug antibody response in DR4 transgenic mice, whereas the wild type enzyme proved to be highly immunogenic. Unfortunately, the variant’s low residual antibacterial activity precluded in vivo efficacy testing, and additional molecular engineering was necessary to assess the extent to which epitope depletion might enhance therapeutic performance.
Directed evolution is one powerful strategy by which to achieve molecular gain of function (Brustad et al., 2011; Goldsmith et al., 2012), and we considered the possibility that high throughput screening of combinatorial LST libraries might yield deimmunized yet active antibiotics. The single prior example of library-based protein deimmunization is that of Escherichia coli asparaginase, an important therapeutic for childhood acute lymphoblastic leukemia. As a bacterial enzyme, asparaginase suffers from prevalent anti-drug immune reactions (45–75% of human subjects), and while a PEGylated version of the enzyme is FDA-approved, it suffers from a 50% loss of activity and remains immunogenic in 5–18% of patients (Avramis et al., 2005). In pursuit of better deimmunized candidates, Cantor and colleagues combined immunoinformatic epitope prediction with combinatorial library construction and functional screening (Cantor et al., 2011). Their strategy of “T cell epitope removal using neutral drift” achieved a marked reduction in asparaginase immunogenicity in transgenic DR4 mice, but this success was reliant on ultra-high throughput screening capabilities. Specifically, their workflow included flow cytometric screening of approximately 107 clones in each of five successive libraries, from which a small fraction were found to be functional and an even smaller fraction were adventitiously deimmunized. While a similarly powerful flow cytometric screen for bacteriolytic enzymes could enable the same approach with LST (Scanlon et al., 2014), we sought here to leverage library design algorithms that might facilitate biotherapeutic deimmunization in cases where screening throughput is limited to a few thousand clones.
In order to make the best use of our screening capabilities, we computationally optimized focused libraries that were enriched in variants having good predicted energies yet low epitope content. Structure-based library design via SOCoM (Verma et al., 2015) identifies optimal sets of target positions and alternative amino acids such that resulting mutation combinations exhibit good structural energies when averaged over an entire library. By augmenting SOCoM with an epitope score evaluation and integrating it in a Pareto optimization framework (He et al., 2012), we generated libraries deemed beneficial under both criteria. This computationally-guided design strategy enabled construction of small but diverse libraries compatible with moderate throughput screening on agar medium or in microtiter plates. Four rounds of computationally-guided directed evolution produced a 13-mutation LST variant whose reduced in vivo immunogenicity translated into enhanced clearance of MRSA infections in a humanized mouse model. The literature on T cell epitope deletion offers successful examples in which reduced immunogenicity and retained in vivo efficacy are assessed independently (Cizeau et al., 2009; Mazor et al., 2014), but to the best of our knowledge, the current study is the first direct demonstration that a T cell epitope depleted variant manifests therapeutic advantages relative to the wild type counterpart.
Immunogenicity is now a widely recognized risk factor for therapeutic proteins, and deimmunization by T cell epitope deletion is one increasingly attractive option for dealing with this issue. Empowered by advances in experimental methodologies (Mazor et al., 2012; Cantor et al., 2011; Harding et al., 2010), epitope prediction tools (Wang et al., 2010; Bryson et al., 2010; Jawa et al., 2013), and protein design algorithms (Parker et al., 2013; Choi et al., 2013, King et al., 2014), T cell epitope deletion is being applied to a progressively larger number of therapeutic candidates. Early clinical data from epitope depleted biotherapeutics suggests that molecular engineering has had the desired effect in human patients (Morris et al., 2005; Entwistle et al., 2012), although to date there is no comparative clinical data for T cell epitope depleted and wild type versions of the same biotherapeutic. Deimmunized variants of Pseudomonas exotoxin should soon be entering clinical trials (Mazor et al., 2014), and comparison to results with the wild type immunotoxin SS1P (Kreitman et al., 2009) will provide new insights into the clinical utility of T cell epitope deletion. In the interim, the work described here provides key evidence supporting the practical utility of this protein deimmunization strategy. Using a relevant humanized mouse model, we have drawn direct connections between depletion of putative T cell epitopes, mitigation of in vivo anti-drug antibody responses, and enhancement of therapeutic efficacy. These results, and the broad applicability of the enabling computational tools, bode well for future development of clinically useful antibacterial lysins, and more generally for the prospect of engineering deimmunized biotherapeutics from a variety of non-human sources.
SIGNIFICANCE
Staphylococcus aureus is a particularly deadly pathogen responsible for nearly half of all US deaths associated with drug-resistant bacteria. Lysostaphin is a potent anti-staphylococcal enzyme with bona fide therapeutic potential, but unfortunately this promising antibacterial agent has proven to be excessively immunogenic in higher animals. Here, we employ advanced protein design tools to engineer lysostaphin variants in which immunogenic T cell epitopes have been deleted. We show that deimmunized lysostaphin is able to clear recurrent, drug-resistant, S. aureus bacteremia in mice having humanized immune systems, whereas wild type lysostaphin fails to rescue mice from repeated infections, which are coincident with escalating anti-drug antibody titers. While the tripartite linkage between T cell epitope deletion, reduction in anti-drug antibody response, and resulting enhancement of therapeutic efficacy has been a long-standing assumption motivating research in this space, the controlled head-to-head studies described here represent a milestone in biotherapeutic design and deimmunization. Namely, the results provide the first direct demonstration that depletion of putative T cell epitopes in biologics manifests enhanced therapeutic efficacy.
EXPERIMENTAL PROCEDURES
Materials
Primers were ordered with standard desalting from IDT Technologies (Coralville, IA). Restriction enzymes and Phusion DNA polymerase for molecular cloning were purchased from New England Biolabs (Ipswich, MA). All other reagents and supplies were from VWR Scientific (Philadelphia, PA), unless specifically noted.
P. pastoris expression vector pPIC9 and P. pastoris strain GS115(his4) were purchased from Invitrogen (Grand Island, NY). E. coli DH5α [F- Φ80lacZΔM15 Δ(lacZYA-argF) U169 recA1 endA1 hsdR17 (rK−, mK+) phoA supE44 λ- thi-1 gyrA96 relA1], S. aureus strain SA113, and MRSA strain USA400 were from the American Type Culture Collection (Manassas, VA).
LST homology models
Homology models for the two domains were constructed as follows. Three template structures from LytM were selected for the catalytic domain (2B0P:A, 2B44:A and 1QWY:A) (Firczuk et al., 2005; Odintsov et al., 2004) (Fig. S2). The cell wall binding domain was constructed using a single template structure (1R77:A, cell wall targeting domain structure of ALE-1, a lysostaphin homolog) (Lu et al., 2006). The domain models were constructed separately using MODELLER (Eswar et al., 2008), and a templateless region in the catalytic domain (24-PLGINGG-30) was modelled using FREAD (Choi et al., 2010). The best models were selected in terms of the DOPE statistical potential function score (Shen et al., 2006).
Preprocessing of mutation choices
For each domain, three iterations of PSI-BLAST (Altschul et al., 1997) were run against the non-redundant database to find homologs. Multiple sequence alignments (MSA) were constructed and curated to yields sets of 114 representative catalytic domain homologs and 23 representative cell wall binding domain sequences. Amino acids were identified at each position in each MSA and subsequently used as possible mutations for design.
The mutational choices were expanded for library design, since screening allows for riskier substitutions. In particular, Chou-Fasman (Chou et al., 1974) propensities were used to identify residues likely to be acceptable in the wild-type secondary structure environment, according to a stringent threshold of 1.5. In addition, since the library was constructed with degenerate oligonucleotides that can incorporate additional amino acids beyond the desired ones (inclusive of wild-type residue), these additions were allowed, as long as the desired to undesired ratio within a degenerate oligonucleotide remained above 2:3.
Structure-based design of individual variants
In order to generate epitope depleted designs, the structure-based deimmunization method EpiSweep was applied to each domain as previously described (Parker et al., 2013). OSPREY (ver. 2.0) (Chen et al., 2009; Gainza et al., 2013) was used to assess one- and two-body energy terms for possible rotamers (Lovell et al., 2000) for the mutational choices, according to the AMBER force field (Pearlman et al., 1995) and a reference energy (Lippow et al., 2007). ProPred (Singh et al., 2001) at a 5% threshold was used to assess DR4 epitope content, characterizing each 9mer as either a binder or a non-binder. A set of 20 energy optimal and near-optimal fully depleted designs were identified for each domain via the EpiSweep optimization algorithm. The most energy optimal catalytic domain design contained a mutation at S124, which was previously found to be less desirable than the alternative double mutation N121G and S122G, which remove the same epitope (Blazanovic et al., accepted). Thus, Opt1 was selected so as to possess the most energy optimal design for the cell wall binding domain and the alternative double mutation in the C-terminal portion of the catalytic domain (N121G and S122G) (Table 1).
Structure-based design of deimmunized libraries
In order to design combinatorial libraries enriched in stable, deimmunized variants, we developed a method called EpiSOCoM, which augments the SOCoM structure-based library design approach (Verma et al., 2015) with epitope analysis, and employs a sweep-based Pareto optimization algorithm (Parker et al., 2013) to simultaneously optimize both energy and epitope content. Given a set of possible positions at which to mutate and possible amino acids to incorporate at those positions (as described above in Preprocessing), along with a desired library size, EpiSOCoM selects a subset of the positions and subsets of the substitutions at those positions. It thereby specifies the construction of a library comprised of all combinations of the substitutions and corresponding wild-type residues. EpiSOCoM optimizes a library for the average energy score and the average epitope score over its constituent variants, so that in general variants will be “good”. Its Pareto optimization algorithm identifies all library designs (positions and substitutions) making undominated tradeoffs between the two scores, in that no other library design is better for both. A detailed description of the EpiSOCoM implementation is described in the Supplemental Experimental Methods.
Protein expression, purification and characterization
LST and its derivatives were secreted from P. pastoris and purified as previously described (Zhao et al., 2014). Endotoxin was removed from the protein preparation by Triton X114 extraction (Liu et al., 1997), and endotoxin levels in all samples were less than 0.1 EU/mg of protein. Protein expression levels were estimated by densitometry analysis of SDS-PAGE gels. The activities of proteins were assessed by determination of minimal inhibitory concentrations (MIC) against S. aureus strain SA113, and are reported as a normalized percentage relative to the MIC dilution determined for LSTWT (i.e. 50% activity is 2-fold higher MIC relative to wild type, and 25% activity is 4-fold higher MIC relative to wild type).
Library Construction and screening
LST libraries were constructed by splice overlapping PCR with the primers shown in Table S2. The PCR products were ligated into pPIC9 vector and transformed into DH5a. Constructs were sequence verified and transformed into P. pastoris by electroporation. Active library members were identified using a moderate throughput plate halo formation assay, as described previously (Zhao et al., 2014). Approximately 10,000 clones were screened for each round. The genes encoding the 10 variants exhibiting the largest halos were PCR amplified, subcloned back into pPIC9, sequenced, and retransformed into freshly prepared P. pastoris cells for functional validation by determination of MIC. The most deimmunized and functional variant was used as the starting point for the subsequent round of library construction and screening.
In vivo studies
The protocols for animal infection, treatment, and immunization were approved by the Institutional Animal Care and Use Committee of Dartmouth College (Hanover, NH), in accordance with the Association for the Assessment and Accreditation of Laboratory Animal Care Guidelines. All efforts were made to minimize animal suffering.
In vivo immunogenicity
A 100 µl volume of 100 µg purified wild type or variant enzyme in complete Freund’s adjuvant (CFA) was injected subcutaneously in either DR4 (N=5 per group) or C57Bl/6 (N=4 per group) mice. Thirteen days following immunization, serum was collected and anti-LST IgG antibody titers (specific to wild type or variant protein) were measured by ELISA. Reported titers were defined as the serum fold dilution yielding an absorbance of 1.5.
In vivo efficacy
Prior to bacterial challenge, the immune systems of DR4 mice (N=3 per group) were primed 3-times with weekly subcutaneous injections of 100 µg LSTWT or Lib5 variant in sterile phosphate buffered saline (PBS: 2.7 mM KCl, 1.5 mM KH2PO4, 8.9 mM Na2HPO4, 136.9 mM NaCl, pH 7.4) with no adjuvant. Anti-LST antibody titers were determined as described above. For the first cycle of infection and treatment, mice were challenged with intraperitoneal administration of 2x108 CFU S. aureus strain USA400 in a 3% suspension of porcine mucin, and one hour later were treated by intravenous tail vein administration of 500 µg of LSTWT or Lib5 variant in sterile PBS. Mice that were rescued by the enzyme treatment underwent similar infection and treatment cycles at weekly intervals, where follow-on bacterial challenges contained 1x109 CFU USA400.
Supplementary Material
Highlights.
Lysostaphin is a potent yet immunogenic anti-staphylococcal enzyme
Novel algorithms generated highly functional and deimmunized biotherapeutic libraries
A T cell epitope depleted lysostaphin evaded immune recognition in humanized mice
T cell epitope depletion mitigated anti-drug antibodies and improved efficacy in vivo
ACKNOWLEDGMENTS
This work was supported by the National Institute of Allergy and Infectious Diseases 1R21AI098122 and the National Institute of General Medical Sciences R01-GM-098977. HZ was supported in part by funds from the Dartmouth Cystic Fibrosis Research Development Program. We also gratefully acknowledge computational resources provided by NSF grant CNS-1205521 and the technical support of the Dartmouth Transgenic and Genetic Construct Shared Resource (P30 CA023108) and Immunology COBRE Core C (P20 RR15639).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aggarwal RS. What's fueling the biotech engine-2012 to 2013. Nat. Biotechnol. 2014;32:32–39. doi: 10.1038/nbt.2794. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avramis VI, Panosyan EH. Pharmacokinetic/pharmacodynamic relationships of asparaginase formulations: the past, the present and recommendations for the future. Clin. Pharmacokinet. 2005;44:367–393. doi: 10.2165/00003088-200544040-00003. [DOI] [PubMed] [Google Scholar]
- Blazanovic K, Zhao H, Choi Y, Li W, Salvat RS, Osipovitch DC, Fields J, Moise L, Berwin BL, Fiering SN, Bailey-Kellogg C, Griswold KE. Structure-based Redesign of Lysostaphin Yields Potent Anti-Staphylococcal Enzymes that Evade Immune Cell Surveillance. Mol. Ther. Meth. Clin. Dev. 2015 doi: 10.1038/mtm.2015.21. Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brustad EM, Arnold FH. Optimizing non-natural protein function with directed evolution. Curr. Opin. Chem. Biol. 2011;15:201–210. doi: 10.1016/j.cbpa.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryson CJ, Jones TD, Baker MP. Prediction of immunogenicity of therapeutic proteins: validity of computational tools. BioDrugs. 2010;24:1–8. doi: 10.2165/11318560-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Cantor JR, Yoo TH, Dixit A, Iverson BL, Forsthuber TG, Georgiou G. Therapeutic enzyme deimmunization by combinatorial T-cell epitope removal using neutral drift. Proc. Natl. Acad. Sci. USA. 2011;108:1272–1277. doi: 10.1073/pnas.1014739108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Georgiev I, Anderson AC, Donald BR. Computational structure-based redesign of enzyme activity. Proc. Natl. Acad. Sci. USA. 2009;106:3764–3769. doi: 10.1073/pnas.0900266106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y, Griswold KE, Bailey-Kellogg C. Structure-based redesign of proteins for minimal T-cell epitope content. J. Comput. Chem. 2013;34:879–891. doi: 10.1002/jcc.23213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y, Deane CM. FREAD revisited: Accurate loop structure prediction using a database search algorithm. Proteins. 2010;78:1431–1440. doi: 10.1002/prot.22658. [DOI] [PubMed] [Google Scholar]
- Chou PY, Fasman GD. Prediction of protein conformation. Biochemistry. 1974;13:222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Cizeau J, Grenkow DM, Brown JG, Entwistle J, MacDonald GC. Engineering and biological characterization of VB6-845, an anti-EpCAM immunotoxin containing a T-cell epitope-depleted variant of the plant toxin bouganin. J. Immunother. 2009;32:574–584. doi: 10.1097/CJI.0b013e3181a6981c. [DOI] [PubMed] [Google Scholar]
- Entwistle J, Brown JG, Chooniedass S, Cizeau J, MacDonald GC. Preclinical evaluation of VB6-845: an anti-EpCAM immunotoxin with reduced immunogenic potential. Cancer Biother. Radiopharm. 2012;27:582–592. doi: 10.1089/cbr.2012.1200.271. [DOI] [PubMed] [Google Scholar]
- Eswar N, Eramian D, Webb B, Shen MY, Sali A. Protein structure modeling with MODELLER. Methods Mol. Biol. 2008;426:145–159. doi: 10.1007/978-1-60327-058-8_8. [DOI] [PubMed] [Google Scholar]
- Firczuk M, Mucha A, Bochtler M. Crystal structures of active LytM. J. Mol. Biol. 2005;354:578–590. doi: 10.1016/j.jmb.2005.09.082. [DOI] [PubMed] [Google Scholar]
- Gainza P, Roberts KE, Georgiev I, Lilien RH, Keedy DA, Chen CY, Reza F, Anderson AC, Richardson DC, Richardson JS, Donald BR. OSPREY: protein design with ensembles, flexibility, and provable algorithms. Methods Enzymol. 2013;523:87–107. doi: 10.1016/B978-0-12-394292-0.00005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith M, Tawfik DS. Directed enzyme evolution: beyond the low-hanging fruit. Curr. Opin. Struct. Biol. 2012;22:406–412. doi: 10.1016/j.sbi.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Grigoryan G, Reinke AW, Keating AE. Design of protein-interaction specificity gives selective bZIP-binding peptides. Nature. 2009;458:859–864. doi: 10.1038/nature07885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoryan G, Zhou F, Lustig SR, Ceder G, Morgan D, Keating AE. Ultra-fast evaluation of protein energies directly from sequence. PLoS Comput. Biol. 2006;2:e63. doi: 10.1371/journal.pcbi.0020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding FA, Liu AD, Stickler M, Razo OJ, Chin R, Faravashi N, Viola W, Graycar T, Yeung VP, Aehle W, et al. A beta-lactamase with reduced immunogenicity for the targeted delivery of chemotherapeutics using antibody-directed enzyme prodrug therapy. Mol. Cancer Ther. 2005;4:1791–1800. doi: 10.1158/1535-7163.MCT-05-0189. [DOI] [PubMed] [Google Scholar]
- Harding FA, Stickler MM, Razo J, DuBridge RB. The immunogenicity of humanized and fully human antibodies: residual immunogenicity resides in the CDR regions. MAbs. 2010;2:256–265. doi: 10.4161/mabs.2.3.11641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Friedman AM, Bailey-Kellogg C. A divide-and-conquer approach to determine the Pareto frontier for optimization of protein engineering experiments. Proteins. 2012;80:790–806. doi: 10.1002/prot.23237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Bian HJ, Molina M, Han J, Magram J, Saar E, Belunis C, Bolin DR, Arceo R, Campbell R, et al. HLA-DR4-IE chimeric class II transgenic, murine class II-deficient mice are susceptible to experimental allergic encephalomyelitis. J. Exp. Med. 1996;183:2635–2644. doi: 10.1084/jem.183.6.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawa V, Cousens LP, Awwad M, Wakshull E, Kropshofer H, De Groot AS. T-cell dependent immunogenicity of protein therapeutics: Preclinical assessment and mitigation. Clin. Immunol. 2013;149:534–555. doi: 10.1016/j.clim.2013.09.006. [DOI] [PubMed] [Google Scholar]
- King C, Garza EN, Mazor R, Linehan JL, Pastan I, Pepper M, Baker D. Removing T-cell epitopes with computational protein design. Proc. Natl. Acad. Sci. USA. 2014;111:8577–8582. doi: 10.1073/pnas.1321126111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokai-Kun JF. Tegos G and Mylonakis E CABI. Vol. 22. Wallingford, Oxfordshire; 2012. Lysostaphin: a Silver Bullet for Staph. In Antimicrobial Drug Discovery; pp. 147–165. [Google Scholar]
- Kreitman RJ, Hassan R, Fitzgerald DJ, Pastan I. Phase I trial of continuous infusion anti-mesothelin recombinant immunotoxin SS1P. Clin. Cancer Res. 2009;15:5274–5279. doi: 10.1158/1078-0432.CCR-09-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippow SM, Tidor B. Progress in computational protein design. Curr. Opin. Biotechnol. 2007;18:305–311. doi: 10.1016/j.copbio.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Tobias R, McClure S, Styba G, Shi Q, Jackowski G. Removal of endotoxin from recombinant protein preparations. Clin. Biochem. 1997;30:455–463. doi: 10.1016/s0009-9120(97)00049-0. [DOI] [PubMed] [Google Scholar]
- Lovell SC, Word JM, Richardson JS, Richardson DC. The penultimate rotamer library. Proteins. 2000;40:389–408. [PubMed] [Google Scholar]
- Lu JZ, Fujiwara T, Komatsuzawa H, Sugai M, Sakon J. Cell wall-targeting domain of glycylglycine endopeptidase distinguishes among peptidoglycan cross-bridges. J. Biol. Chem. 2006;281:549–558. doi: 10.1074/jbc.M509691200. [DOI] [PubMed] [Google Scholar]
- Mazor R, Vassall AN, Eberle JA, Beers R, Weldon JE, Venzon DJ, Tsang KY, Benhar I, Pastan I. Identification and elimination of an immunodominant T-cell epitope in recombinant immunotoxins based on Pseudomonas exotoxin A. Proc. Natl. Acad. Sci. USA. 2012;109:E3597–E3603. doi: 10.1073/pnas.1218138109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazor R, Eberle JA, Hu X, Vassall AN, Onda M, Beers R, Lee EC, Kreitman RJ, Lee B, Baker D, et al. Recombinant immunotoxin for cancer treatment with low immunogenicity by identification and silencing of human T-cell epitopes. Proc. Natl. Acad. Sci. USA. 2014;111:8571–8576. doi: 10.1073/pnas.1405153111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MJ, Divgi CR, Pandit-Taskar N, Batraki M, Warren N, Nacca A, Smith-Jones P, Schwartz L, Kelly WK, Slovin S, et al. Pilot trial of unlabeled and indium-111-labeled anti-prostate-specific membrane antigen antibody J591 for castrate metastatic prostate cancer. Clin. Cancer Res. 2005;11:7454–7461. doi: 10.1158/1078-0432.CCR-05-0826. [DOI] [PubMed] [Google Scholar]
- Odintsov SG, Sabala I, Marcyjaniak M, Bochtler M. Latent LytM at 1.3A resolution. J. Mol. Biol. 2004;335:775–785. doi: 10.1016/j.jmb.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Osipovitch DC, Parker AS, Makokha CD, Desrosiers J, Kett WC, Moise L, Bailey-Kellogg C, Griswold KE. Design and analysis of immune-evading enzymes for ADEPT therapy. Protein Eng. Des. Sel. 2012;25:613–623. doi: 10.1093/protein/gzs044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker AS, Zheng W, Griswold KE, Bailey-Kellogg C. Optimization algorithms for functional deimmunization of therapeutic proteins. BMC Bioinformatics. 2010;11:180. doi: 10.1186/1471-2105-11-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker AS, Griswold KE, Bailey-Kellogg C. Optimization of therapeutic proteins to delete T-cell epitopes while maintaining beneficial residue interactions. J. Bioinform. Comput. Biol. 2011;9:207–229. doi: 10.1142/s0219720011005471. [DOI] [PubMed] [Google Scholar]
- Parker AS, Choi Y, Griswold KE, Bailey-Kellogg C. Structure-guided deimmunization of therapeutic proteins. J. Comput. Biol. 2013;20:152–165. doi: 10.1089/cmb.2012.0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlmana DA, Case DA, Caldwell JW, Ross WS, Cheatham TE, III, DeBolt S, Ferguson D, Seibel G, Kollman P. Amber, a Package of Computer-Programs for Applying Molecular Mechanics, Normal-Mode Analysis, Molecular-Dynamics and Free-Energy Calculations to Simulate the Structural and Energetic Properties of Molecules. Comput. Phys. Commun. 1995;91:1–41. [Google Scholar]
- Resch G, Moreillon P, Fischetti VA. PEGylating a bacteriophage endolysin inhibits its bactericidal activity. AMB Express. 2011;1:29. doi: 10.1186/2191-0855-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohl CA, Strauss CE, Misura KM, Baker D. Protein structure prediction using Rosetta. Methods Enzymol. 2004;383:66–93. doi: 10.1016/S0076-6879(04)83004-0. [DOI] [PubMed] [Google Scholar]
- Sabala I, Jagielska E, Bardelang PT, Czapinska H, Dahms SO, Sharpe JA, James R, Than ME, Thomas NR, Bochtler M. Crystal structure of the antimicrobial peptidase lysostaphin from Staphylococcus simulans. FEBS J. 2014;281:4112–4122. doi: 10.1111/febs.12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvat R, Moise L, Bailey-Kellogg C, Griswold KE. A high throughput MHC II binding assay for quantitative analysis of peptide epitopes. J. Vis. Exp. 2014:85. doi: 10.3791/51308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvat R, Bailey-Kellogg C, Griswold KE. Protein deimmunization via structure-based design enables efficient epitope deletion at high mutational loads. Biotechnol. Bioeng. 2015 doi: 10.1002/bit.25554. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlon TC, Dostal SM, Griswold KE. A high-throughput screen for antibiotic drug discovery. Biotechnol. Bioeng. 2014;111:232–243. doi: 10.1002/bit.25019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellekens H. Immunogenicity of therapeutic proteins: clinical implications and future prospects. Clin. Ther. 2002;24:1720–1740. doi: 10.1016/s0149-2918(02)80075-3. [DOI] [PubMed] [Google Scholar]
- Shen MY, Sali A. Statistical potential for assessment and prediction of protein structures. Protein Sci. 2006;15:2507–2524. doi: 10.1110/ps.062416606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H, Raghava GP. ProPred: prediction of HLA-DR binding sites. Bioinformatics. 2001;17:1236–1237. doi: 10.1093/bioinformatics/17.12.1236. [DOI] [PubMed] [Google Scholar]
- Stark FR, Thornsva C, Flannery EP, Artenste MS. “Systemic lysostaphin in man--apparent antimicrobial activity in a neutropenic patient”. New England Journal of Medicine. 1974;291:239–241. doi: 10.1056/NEJM197408012910507. [DOI] [PubMed] [Google Scholar]
- Szweda P, Schielmann M, Kotlowski R, Gorczyca G, Zalewska M, Milewski S. Peptidoglycan hydrolases-potential weapons against Staphylococcus aureus . Appl. Microbiol. Biotechnol. 2012;96:1157–1174. doi: 10.1007/s00253-012-4484-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo . Annu. Rev. Immunol. 2005;23:975–1028. doi: 10.1146/annurev.immunol.22.012703.104538. [DOI] [PubMed] [Google Scholar]
- Verma D, Grigoryan G, Bailey-Kellogg C. Structure-based Design of Combinatorial Mutagenesis Libraries. Protein Sci. 2015 doi: 10.1002/pro.2642. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh S, Shah A, Mond J. Improved pharmacokinetics and reduced antibody reactivity of lysostaphin conjugated to polyethylene glycol. Antimicrob. Agents Chemother. 2003;47:554–558. doi: 10.1128/AAC.47.2.554-558.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Sidney J, Kim Y, Sette A, Lund O, Nielsen M, Peters B. Peptide binding predictions for HLA DR, DP and DQ molecules. BMC Bioinformatics. 2010;11:568. doi: 10.1186/1471-2105-11-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warmerdam PA, Plaisance S, Vanderlick K, Vandervoort P, Brepoels K, Collen D, De Maeyer M. Elimination of a human T-cell region in staphylokinase by T-cell screening and computer modeling. Thromb. Haemost. 2002;87:666–673. [PubMed] [Google Scholar]
- Zhang Y, Skolnick J. Scoring function for automated assessment of protein structure template quality. Proteins. 2004;57:702–710. doi: 10.1002/prot.20264. [DOI] [PubMed] [Google Scholar]
- Zhao H, Blazanovic K, Choi Y, Bailey-Kellogg C, Griswold KE. Gene and protein sequence optimization for high-level production of fully active and aglycosylated lysostaphin in Pichia pastoris . Appl. Environ. Microbiol. 2014;80:2746–2753. doi: 10.1128/AEM.03914-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.