Abstract
Background
Some studies have suggested that in stroke patients, spatial inattention on the cancellation test is closely related to disorganized visual search. However, methods to assess spatial aspects of search organization on cancellation tests have not been well developed. In this study the authors design and evaluate new methods to quantify strategies of spatial exploration on the cancellation test in stroke patients who showed a broad range of spatial attentional abilities, and test whether disorganization and inattention are related.
Methods
Twenty stroke patients were videotaped while they performed a cancellation test. Several variables that reflect spatial aspects of search organization were measured through subsequent video playback. Two patients with severe neglect were excluded from further analysis to avoid constraining the spatial expression of search organization. Spearman correlations were used to assess whether severity of spatial inattention correlated with the individual search organization measures.
Results
Of the 18 remaining patients, 10 had mild-moderate spatial neglect (pathologic inattention), while the other 8 omitted at most one target (normal performance). There were no significant correlations between the number of targets omitted and any of the search organization measures.
Conclusions
Spatial inattention on cancellation due to neglect following stroke is not closely related to the organization of visual search. Instead, search disorganization during cancellation may reflect disturbance of an unspecified executive control mechanism.
The cancellation test has long been used in stroke patients to assess spatial inattention or neglect (pathologic inattention), partly because it is easy to reproduce and administer. The test presents numerous printed images (targets) on a conventionally sized sheet of paper that are to be marked once and individually with a pen or pencil; they are thus “canceled” as if they are postage stamps. Spatial inattention is measured by the amount of targets left unmarked after the patient indicates test completion. Cancellation neglect is highly correlated with other measures of neglect1,2 and has repeatedly been found to predict impaired recovery of daily living activities after stroke.2–6
In contrast, the spatial progression of cancellation by stroke patients has not been comprehensively examined. Several reports have observed that stroke patients with spatial neglect often fail to cancel in a recognizable pattern; they move from target to target erratically.7–10 This behavior may appear even if the test consists solely of random letters in regular rows,7 thus showing that experimentally attempting to induce a scanning pattern akin to normal reading may be confounded in some cases. Nonetheless, the extent of disorganization during cancellation, as well as neglect itself, can be influenced by the extent to which the targets themselves are spatially organized.8 In contrast, neurologically healthy individuals usually mark the page in an organized fashion, moving within sequential rows or columns, even when the target arrays themselves are disorganized and do not comprise strictly defined rows or columns.8,9,11 These observations have led some investigators to conclude that neglect is generally accompanied by disorderly cancellation.9 Others have even suggested that such disorganization may contribute to the severity of neglect.8 For example, patients might quit searching prematurely because of fatigue that may result from inefficient search, leading to neglect of targets.
However, other studies have not supported a close association between neglect and disorganized target marking on cancellation tests. Several reports have demonstrated columnar target marking strategies coexisting with left neglect.10,12,13 In contrast, another study11 found that stroke patients were generally disorganized during cancellation, regardless of whether they had spatial neglect. Indeed, patients without neglect were more disorganized than were patients with neglect, while healthy subjects were not disorganized.
These observations suggest that disordered search and spatial inattention during cancellation may be distinct and dissociated behaviors following stroke. However, studies of target marking sequences following stroke until now have had limited value. Either they risked observer bias from using direct observation to evaluate or record target marking sequences, without quantifying results,7,9,10,12 or they subjectively classified marking sequences without validation.8 An alternate approach used computer registration of target marking sequences and quantified organization by the number of intersections in the reconstructed cancellation path.11 However, this study used an orderly stimulus array with few targets, which may have limited the test’s sensitivity to neglect and disorganized search.
We reexamined the relationship between spatial attention and target marking patterns in stroke patients by using a primarily objective method to record target marking sequences, a large number of targets in an irregular array, and several objective methods to quantify organization. We hypothesized that if spatial inattention were closely related to search organization, measures of spatial inattention and search organization would be significantly correlated.
Participants and methods
Participants
Adult patients with stroke resulting in nondominant limb hemiparesis were consecutively enrolled in this study during their hospitalizations at several rehabilitation facilities. Patients were included only if they showed that they could perform the test comfortably on a practice cancellation page for a few targets. They were not selected for demonstrating any particular pattern during cancellation. Consent to participate was obtained in accordance with the various institutions’ research review boards.
We initially evaluated 20 stroke patients on a cancellation test (below). Because severe neglect might constrain the expression of search organization, we excluded two patients who failed to cancel at least half of the targets. The remaining 18 patients consisted of 12 men and 6 women, mean age 69.1 years, SD 11.2 years. Table 1 provides the patients’ identifying numbers and radiologic findings; patients are ranked according to the severity of inattention (number of target omissions) on the cancellation test. Most stroke patients had hemispheric lesions, except Patient 6, who had a brainstem infarct. The patients were assessed between 6 days and 8 years after stroke onset; all but three (Patients 1, 7, and 17) were within the first 3 months of stroke onset.
Table 1.
Ages and radiologic and experimental findings in patients
| Patient no. | Age, y | Lesion location | Omissions | Mean cancellation distance, cm | Intersections rate* | Best r | Perseverations† | Commission errors |
|---|---|---|---|---|---|---|---|---|
| 1 | 71 | Thalamus | 0 | 3.00 | 0.23 | 0.77 | 5 | 0 |
| 2 | 71 | PO | 0 | 2.23 | 0.02 | 0.97 | 2 | 0 |
| 3 | 77 | MCA | 0 | 2.96 | 0.11 | 0.48 | 0 | 0 |
| 4 | 81 | BG | 0 | 2.56 | 0.05 | 0.99 | 1 | 0 |
| 5 | 77 | P | 1 | 3.81 | 0.31 | 0.74 | 3 | 0 |
| 6 | 73 | Pons | 1 | 2.54 | 0.16 | 0.87 | 1 | 0 |
| 7 | 38 | TBG | 1 | 3.30 | 0.23 | 0.57 | 2 | 0 |
| 8 | 74 | MCA | 1 | 2.20 | 0.02 | 0.98 | 0 | 0 |
| 9 | 76 | TPO | 3 | 3.98 | 0.62 | 0.62 | 14 | 1 |
| 10 | 61 | FP | 3 | 2.70 | 0.05 | 0.95 | 0 | 2 |
| 11 | 61 | F | 3 | 2.62 | 0.04 | 0.96 | 4 | 0 |
| 12 | 79 | Thalamus | 3 | 4.64 | 0.59 | 0.48 | 3 | 0 |
| 13 | 70 | T | 4 | 3.17 | 0.18 | 0.08 | 27 | 0 |
| 14 | 58 | MCA | 4 | 2.41 | 0.08 | 0.98 | 0 | 0 |
| 15 | 73 | TP | 6 | 3.57 | 0.22 | 0.68 | 8 | 0 |
| 16 | 51 | F | 6 | 2.80 | 0.14 | 0.97 | 0 | 0 |
| 17 | 79 | TP | 11 | 2.54 | 0.34 | 0.61 | 2 | 0 |
| 18 | 73 | Thalamus | 14 | 5.01 | 0.23 | 0.86 | 1 | 0 |
| Average | 69.1 | 3.39 | 3.11 | 0.20 | 0.75 | 4.06 | 0.17 |
Intersections per number of sequentially different locations marked (see text).
Nonconsecutive perseverations only.
P = parietal; O = occipital; MCA = middle cerebral artery; BG = basal ganglia; T = temporal; F = frontal.
A control group of 21 hospitalized rehabilitation patients (9 men, 12 women, mean age 66.1 years, SD 9.3 years) without known cerebral illness was also given the cancellation test to establish cut-off scores for pathologic spatial inattention or neglect. The control group did not differ in age from the stroke patients (t = 0.8; p = 0.4).
Cancellation method
Patients were given a modified version of the widely used Star Cancellation Test (SCT).14 The SCT presents 56 small black target stars (9 mm across) against a white background, intermixed with 52 large star non-targets (14 mm across) and 23 isolated words and letters (9 mm tall, approximately 36-point font) in a minimally structured arrangement. Prior to this study, we had found that some stroke patients failed to distinguish between small and large stars despite practice trials. Therefore, we modified the SCT by replacing the large non-target stars with similar-size large triangles (figures 1 and 2). This modified SCT (mSCT) was otherwise identical to the original SCT. We have found that patients distinguish small stars from triangles with greater consistency but nonetheless still often show spatial neglect. Therefore, we retained this modification for the present study.
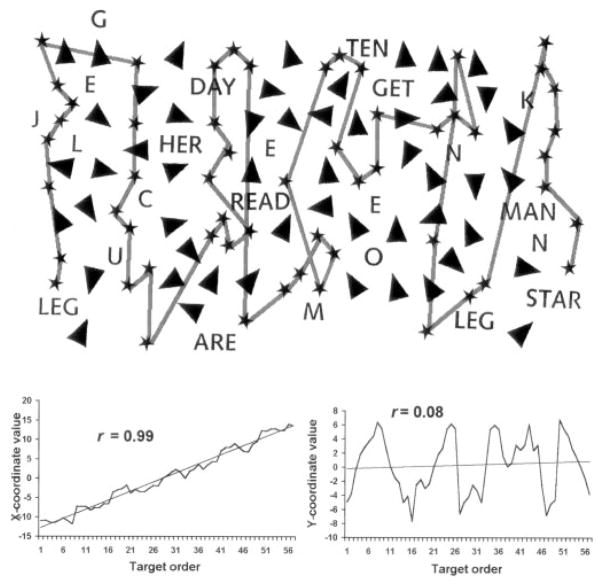
Figure 1.
Example of well-organized cancellation (Patient 4). Top: Reconstructed cancellation path against the target array. Bottom: plots of x-coordinate and y-coordinate values against target marking order, used to determine the “best r” (correlation coefficient) for this patient. Star Cancellation Test modified and reproduced with permission of Thames Valley Test Company.
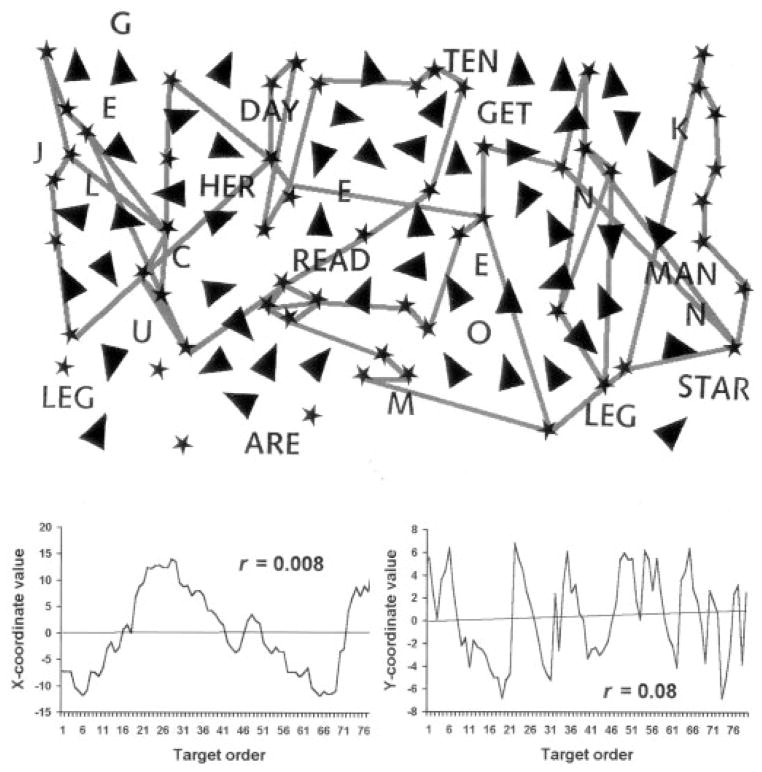
Figure 2.
Example of poorly organized cancellation (Patient 13). Conventions as shown in figure 1.
The mSCT was reproduced on white photocopy paper that was 216 mm wide by 279 mm long and secured to a horizontal table with translucent adhesive tape. The page was centered at the midsagittal plane and was approximately level with the inferior costal margin, comfortably within arm’s reach. The table was cleared of all other objects. Patients were not permitted to reposition the page, but head and trunk movements were not constrained. In contrast to the procedure used for the original SCT,14 which requires the examiner to cross out the central two targets on the test page as examples, we demonstrated cancellation on a separate practice page, to avoid possibly biasing performance from viewing additional markings.15 Thus, our test version allowed the patient to mark all 56 targets on the test page. Patients were instructed to draw an individual line through each star once and ignore all other symbols. A pen with a 3-mm black felt tip was used to mark targets. Patients were instructed to tell the examiner when they had completed crossing out all the stars. They were neither told to work quickly nor given a time limit.
Performances were recorded with a small video camera on a tripod. The camera was positioned slightly behind and above the shoulder opposite the hand that held the pen to clearly show the pen tip during cancellation while minimizing patient distraction. The magnification (zoom) was adjusted to show the closest view of the test surface that still encompassed the entire page. The examiner discreetly monitored test compliance from 1 to 2 m behind the patient (or farther away when standing in other locations) to minimize distraction. The examiner did not cue or inform patients about their progress. Patients who ceased exploring the page for about 30 seconds were asked a neutral question such as “Done?” or “Got them all?” Patients were allowed to resume canceling if they restarted exploring the page immediately. Otherwise, the test was terminated if the patient did not communicate that the test had been completed.
Cancellation measurements
Spatial inattention was quantified by the number of targets that were not marked. The target marking sequences from the patients were determined by playing the videotapes frame-by-frame with a manually operated edit control device. All test stimuli were identified by the Cartesian (x, y) coordinates of their geometric centers in reference to a consistent origin at page center. We assumed that patients treated individual targets or non-targets as whole figures and thus were not concerned with marking a specific part of a stimulus. Accordingly, when calculating spatial exploratory measures, we represented the locations of markings on targets or non-targets by the designated coordinates of the stimuli rather than by the precise points of initial pen contact. In contrast, markings that did not touch test stimuli were to be identified by the precise coordinates of initial pen contact.
The measurement of the spatial organization of cancellation has not been standardized. We therefore used several different methods to assess different processes in the control of spatial exploration: 1) Marking distance. The Euclidean distances between pen markings at sequentially different locations were averaged. We presumed that well-organized cancellation would involve moving to the nearest unmarked targets in succession, thereby minimizing the distances between markings. 2) Number of intersections in the reconstructed cancellation path. The cancellation paths were reconstructed from the video recordings by drawing them onto new pages. We expected that well-organized search would not likely involve re-visiting parts of the page that had already been marked. Thus, well-organized search would minimize path intersections. However, rather than simply reporting total intersections,11 we divided the total intersections by the total number of markings made at sequentially different locations. 3) Best r. We extended a technique for measuring cancellation progress that has been described by Richard M. Lazar (personal communication, 2000). Because healthy subjects typically cancel by rows or columns,8,9,11 their movement is generally either horizontal (e.g., left to right) or radial (e.g., far to near) across the page. To capture this net orthogonal movement pattern, we calculated the Pearson correlation coefficient (r) from the linear regression of the x-values of all marked locations relative to the order in which they were marked. The y-values of marked locations were analyzed in the same way. From the two linear regressions calculated for each patient, we selected the one with the higher (“best”) r-value to represent the degree to which cancellations were pursued orthogonally. For example, starting on the left side of the page and marking by columns progressively rightward would yield a higher r-value on the x-coordinate regression than on the y-coordinate regression, because the cancellation progress would be consistently horizontal (left-to-right) but inconsistently radial. In general, a highly organized approach would be reflected by a high best r. (Exceptions might occur if targets were marked in other, atypical but highly organized manners, such as marking targets in a single spiral path encompassing the whole page. This would result in a lower best r than when marking by columns or rows. However, we did not observe such exceptions.) Figures 1 and 2 illustrate examples from our patients of organized and disorganized cancellation.
The above organization measures may be affected by perseveration (repetitious marking) during cancellation, which has often been reported among stroke patients15–19 but thus far has not been addressed in studies on the spatial progression of target marking. For our study we recognized two kinds of perseveration during cancellation18,20 that could have different effects on our findings: consecutive perseveration, in which repeated markings at a particular stimulus occur without intervening markings at other stimuli, and non-consecutive perseveration, in which the repetition of a mark at a particular stimulus occurs only after marking a different stimulus. The distinction is important, because in consecutive perseveration the distance on the page between successive markings is zero, unlike in non-consecutive perseveration. Because our study assessed only the spatial (rather than temporal) patterns of cancellation progress, we measured only non-consecutive perseverations. Therefore, to calculate inter-stimulus marking distances, intersections rate, and best r, we included non-consecutive perseverations.
For each of these measures we decided a priori whether to adjust values by the number of sequentially different marked locations, based on our expectation for how variances in spatial inattention (i.e., failure to mark throughout the page) could affect a particular variable. Clearly, the sum of interstimulus marking distances would be affected by inattention to targets as well as by disorganized search. Accordingly, we calculated the average marking distance for each patient rather than the total cancellation path length. With respect to the number of path intersections, the greater the area on the page that is marked, the greater would be the potential for the reconstructed cancellation path to intersect itself, regardless of how well patients were actually organized. Consequently, the intersections total was also divided by the number of sequentially different locations marked. In contrast, best r would not be affected by the amount of spatial inattention, except for severe unilateral neglect, which was excluded from analysis in this study. Therefore, best r scores were not adjusted. Similarly, we did not expect that spatial inattention in principle would affect perseveration scores. Thus, patients with even severe inattention could still repeatedly mark a small set of stimuli.17 Accordingly, non-consecutive perseverations were not adjusted by the number of sequentially different locations marked.
Results
Table 1 provides the results from the stroke patients. The control patients omitted a mean of 0.5 targets (SD 1.0). Using the mean number of omissions plus 2 SD, we set the cut-off score for the normal number of targets omitted as 2 targets for the mSCT, which is consistent with the criterion for neglect on the original SCT.21 Ten stroke patients in our sample (Patients 9 through 18) exceeded this score and thus were judged to have neglect. To identify unilateral neglect, we used the criterion for the difference between left-sided and right-sided omissions to equal or exceed the cut-off score for nonspecific spatial neglect.22 By this standard, 7 of the 10 neglect patients (i.e., all but Patients 9, 12, and 15) had unilateral neglect, which was to the left in each case.
Two patients marked non-targets. Patient 9 marked one non-target and Patient 10 marked two non-targets. Otherwise, the patients accurately discriminated targets from non-targets, and none marked blank areas. This suggests that patients’ markings were not affected by difficulties with viewing or reaching for the targets.19
Because scores on the various measures were not normally distributed, we calculated Spearman correlations to assess the associations among spatial inattention (number of targets omitted), the various search organization measures, and non-consecutive perseverations. The results indicated that the number of targets omitted was not related to any of the other cancellation measures (table 2). In addition, the three spatial organization measures and nonconsecutive perseverations were highly intercorrelated. Distance, intersections, and perseverations were negatively correlated with best r, unlike the other significant intercorrelations. Age was not correlated with any of the measures.
Table 2.
Correlation matrix relating number of target omissions, search organization variables, nonconsecutive perseverations, and age*
| Variable | Distance
|
Intersections
|
Best r
|
Perseverations
|
Age
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | r | p | |
| Omissions | 0.26 | 0.30 | 0.36 | 0.14 | −0.14 | 0.57 | 0.08 | 0.76 | −0.19 | 0.45 |
| Distance | 0.74† | <0.001 | −0.62† | 0.006 | 0.50‡ | 0.04 | 0.12 | 0.63 | ||
| Intersections | −0.68† | 0.002 | 0.50‡ | 0.04 | 0.32 | 0.19 | ||||
| Best r | −0.53‡ | 0.03 | 0.11 | 0.67 | ||||||
| Perseverations | −0.19 | 0.46 | ||||||||
In each pair of figures, the first value is the Spearman correlation coefficient and the second is the probability (p) value.
Correlation is significant at the 0.01 level (two-tailed).
Correlation is significant at the 0.05 level (two-tailed).
Although the search organization measures and perseverations were not significantly correlated with neglect severity, spatial neglect might still identify patients with more severely impaired search organization.8,19 The patients with neglect (Patients 9 through 18) had greater mean interstimulus marking distances than did the non-neglect patients (3.3 cm vs 2.8 cm), greater intersections rate (0.2 vs 0.1), lower best r values (0.7 vs 0.8), and more non-consecutive perseverations (5.9 vs 1.8). In contrast, the mean age did not appreciably differ between stroke patients with or without neglect (68 vs 70 years). However, differences between these groups on any of the cancellation organization measures or perseverations were not significant (ts < 1.5, ps > 0.16, two-tailed).
Our patient sample was heterogeneous with respect to lesion locations as reported from clinically ordered neuroimaging studies (see table 1). There were no consistent relations between any of the variables of interest and lesion locations. Lesion volumes or other neuroimaging values were not available.
Discussion
Our findings are consistent with previous research indicating that the spatial organization of stimulus marking is not closely related to spatial inattention on cancellation tests.11 Indeed, as shown by other studies,10,12 spatial neglect may appear despite organized search. Thus, from available evidence, neglect on cancellation tasks appears not to be strictly explained by a poorly organized strategy for visuospatial exploration. On the other hand, even though the neglect and non-neglect patients did not significantly differ on measures of search organization, the data suggested that patients with neglect are more likely to be disorganized during cancellation tests than are patients without neglect, which is consistent with other studies.8,17,19
Because our patient sample was small, our study lacked power to detect statistical significance for small-to-moderate correlations between search organization and spatial attention. For example, a small correlation of 0.36 was found between the number of omissions and intersections, and further studies using larger stroke patient samples might be warranted to examine the relationship between spatial inattention and search organization more carefully. However, one report11 observed no relationship between neglect and search disorganization in their sample of 68 right-brain injured patients, 28 of whom had neglect. The double dissociation between search disorganization and neglect that is reflected by prior investigations,10–12 combined with the modest correlations observed in our study, suggest that search disorganization and spatial inattention are mostly distinct and separate phenomena. Nonetheless, neglect may be a marker for disorganized search.
Until now, search disorganization on cancellation tests has been evaluated among brain-lesioned patients primarily in regard to whether they had spatial neglect. Although one report19 found no correlation between the number of perseverations and the number of targets omitted in patients with neglect, right-hemisphere lesioned patients with neglect (n = 28) made significantly more perseverations during cancellation than did right-hemisphere lesioned patients without neglect (n = 60). Therefore, some factor other than neglect, such as lesion volume or clinical stroke severity, may correlate with the extent of disorganized search on cancellation. Because our study was not designed to evaluate the relationship of other candidate variables to search organization, further research will be needed to identify neurologic predictors of disorganized search during cancellation.
The significant correlations among the three search organization measures and non-consecutive perseverations in this study suggest that these measures assess a related process in stroke patients. This behavior neither appears to depend on the age of the participant, nor does it seem to be a function of neglect severity. At present we are uncertain of the explanation for disorganized marking during cancellation. One possibility is that disorganized target marking, including perseveration, reflects nonspecific consequences of task novelty. For example, if a single unmarked target near the starting point of cancellation were not discovered until all of the other targets had been marked, then marking this target at the end of the test would likely increase the mean interstimulus marking distance and the number of path intersections, in contrast to leaving the target unmarked. Hence, a mostly careful patient would in effect be penalized for checking for target omissions. However, this would only minimally change the best r calculation, because this measure reflects the linear regression through all of the x- or y-coordinates of the markings at once and thus would not be sensitive to the changes in the order of one or two markings. Table 3 demonstrates the outcomes of two simulated target marking behaviors by careful patients. Case 1 represents a target marking sequence that would typify healthy individuals: minimal inter-target marking distance, no path intersections or perseverations, and consistent rightward progress across the page. Case 2 represents the discovery of an unmarked target near the starting point after all of the other targets have been marked, while otherwise applying the same rules for target marking in Case 1. All targets were marked in each case. Table 3 supports the prediction that the late discovery of an unmarked target after most other targets were marked would proportionally have the least effect on the best r.
Table 3.
Outcomes from two simulated cancellation approaches*
| Case | Distance | Intersections | Best r |
|---|---|---|---|
| 1 | 2.53 | 0 | 0.98 |
| 2 | 3.06 | 0.13 | 0.89 |
| Proportional change | 21% increase | NA | 9% decrease |
Conventions as in table 1.
NA = not applicable.
We therefore expect that the inclusion of a substantial number of patients with Case 2 behavior would weaken the intercorrelations among the various cancellation variables. Consequently, we suspect that few (if any) of our patients belatedly discovered one or two unmarked targets within the array of marked targets as they neared the end of the test. The penultimate discovery of more than two unmarked targets within a large marked target array would more likely reflect intrinsically aberrant search behavior, in view of the systematic cancellation approaches that are typically shown by healthy individuals.8,9,11 Our findings suggest that the best r measure may be the most useful among our several measures to characterize cancellation strategies. Comparison of cancellation measures with healthy control participants is warranted to evaluate this possibility further.
Similarly, perseverated target marking may have resulted from deliberate attempts to “touch up” the markings, or perhaps from the failure to recognize previously made markings against targets in peripheral vision,23 since a narrow black pen was used to draw through black targets. However, our patients’ markings generally extended far past the boundaries of the star targets, which would probably have helped to distinguish marked from unmarked targets. Because our patients only rarely marked non-target stimuli, it is difficult to posit a disturbance of stimulus discrimination to explain perseverated target marking. Furthermore, one study that had neglect patients use black ink to cancel red targets nonetheless noted frequent perseveration of target marking.15 We cannot exclude the possibility that at least some of our patients deliberately re-marked targets for esthetic reasons, but the failure to distinguish markings from targets appears unlikely to be the sole reason for perseverated cancellation.
Alternatively, disordered cancellation progress may reflect a kind of executive disorder. The term “executive function” describes the regulation of processes involved with attention, memory, response selection, sequencing, and other fundamental cognitive operations.24,25 Although they are sometimes considered to be synonymous with “frontal lobe” deficits, executive disorders have been shown to follow non-frontal lesions as well.25–28 Cancellation generally requires patients not only to serially attend to multiple occurrences of the same target across the page, but also to ignore non-targets as well as targets that have already been marked. Hence, careful control over diverse cognitive processes is likely required for efficient task completion.24 Disordered visuospatial search following brain injury has been suggested to result from altered regulatory functions such as impaired spatial working memory (the constant awareness of previously attended stimulus locations), deficient “inhibition of return” (the automatically delayed reorientation to previously attended locations), the loss of an abstract plan to guide spatial search, impaired disengagement of visual attention from salient stimuli, or the failure to inhibit stimulus-bound motor responses.15,17,23,29,30 Our study was not designed to evaluate these processes, and therefore we cannot comment on whether any of these disorders affected cancellation in our patients.
It should also be noted that only one of our patients with mild-moderate neglect actually omitted targets in a directionally consistent manner: Patient 13 showed distinct near left neglect (see figure 2). The other nine neglect patients’ target omissions were either discontiguous or did not form a consistent (i.e., roughly linear) boundary against the marked targets. Although such inconsistent neglect during cancellation has been demonstrated previously,31–33 the neurologic differences between such patients and those with directionally consistent cancellation neglect have not been evaluated. Directionally inconsistent cancellation neglect would appear to suggest disorganized target marking. Evaluating the target marking patterns of directionally consistent versus inconsistent cancellation neglect would help to clarify the relationship between spatial inattention and the organization of visual search in brain illness.
We excluded patients who did not mark at least half the targets to allow us to compare the target marking patterns of patients who clearly searched most of the page. Nonetheless, some studies have demonstrated marked perseveration during cancellation or visual exploration in the presence of severe unilateral neglect,17,23 although severe neglect without perseveration may also occur.13 Thus, it would certainly be feasible to evaluate patients with severe neglect with our search organization measures. In our two patients with severe neglect who were excluded from the study results above, the search organization scores differed minimally from the average values for the eight patients without neglect (mean cancellation distance = 3.13; mean intersections rate = 0.21, mean best r = 0.92). On the other hand, the patients markedly differed in the number of nonconsecutive perseverations (one had 2, the other 9). Nonetheless, these results indicate that patients with severe neglect may show marking patterns similar to stroke patients without neglect. Thus, these findings further support our conclusion that disordered search organization and neglect are distinct and different phenomena. However, further studies are needed to characterize the search behaviors of patients with severe neglect in general. We suggest that when evaluating spatial organization on cancellation, it would be important to control for the severity of neglect, which as we indicated may constrain the expression of disorganized target marking, though not of perseveration.
Clearly, more work is necessary to identify mechanisms that underlie disorganized search on cancellation tests following focal brain injury. Until now, cancellation tests have been used primarily to assess spatial inattention and cognitive processing speed. In contrast, few studies have evaluated disruptions of search organization, perhaps because methods to quantify search have not been well developed. The methods in the present study may help to develop measures of search organization that are sensitive to the effects of brain injury, thus allowing the cancellation test to have an expanded role in neurobehavioral evaluation. Development of touch-sensitive computerized registration of target marking sequences11,20,34,35 may facilitate the evaluation of search strategies on cancellation tests. Since cancellation tests already have been shown to predict functional outcomes following brain illness based on extent of target marking,2–6 as well as time used for task completion,36–38 it would be important to determine whether the evaluation of search organization during cancellation might have similar predictive value for functional recovery following brain illness.
Acknowledgments
Supported by the Neuropsychiatric Research Institute, the Center for Aging at the University of Alabama at Birmingham, the Hartford Foundation/Southeast Center of Excellence in Geriatric Medicine, the National Institute on Aging (R03 AG 21256-01), the National Institute of Neurological Disorders and Stroke (NS 39348), and the National Center for Medical Rehabilitation Research (HD40631 and R03 HD042519-01A1).
The authors thank Nancy Waller, Cassi Fredricks, Shawn Wing Schmidt, and Joy Fairbanks for assistance in data collection.
References
- 1.Halligan PW, Marshall JC, Wade DT. Visuospatial neglect: underlying factors and test sensitivity. Lancet. 1989;ii:908–911. doi: 10.1016/s0140-6736(89)91561-4. [DOI] [PubMed] [Google Scholar]
- 2.Agrell BM, Dehlin OI, Dahlgren CJ. Neglect in elderly stroke patients: a comparison of five tests. Psychiatry Clin Neurosci. 1997;51:295–300. doi: 10.1111/j.1440-1819.1997.tb03201.x. [DOI] [PubMed] [Google Scholar]
- 3.Fullerton KJ, McSherry D, Stout RW. Albert’s test: a neglected test of perceptual neglect. Lancet. 1986;i:430–432. doi: 10.1016/s0140-6736(86)92381-0. [DOI] [PubMed] [Google Scholar]
- 4.Fullerton KJ, MacKenzie G, Stout RW. Prognostic indices in stroke. Q J Med. 1988;66:147–162. [PubMed] [Google Scholar]
- 5.Friedman PJ. The Star Cancellation Test in acute stroke. Clin Rehabil. 1992;6:23–30. [Google Scholar]
- 6.Halligan PW, Burn JP, Marshall JC, Wade DT. Visuo-spatial neglect: qualitative differences and laterality of cerebral lesion. J Neurol Neurosurg Psychiatry. 1992;55:1060–1065. doi: 10.1136/jnnp.55.11.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diller L, Weinberg J. Hemi-inattention in rehabilitation: the evolution of a rational remediation program. Adv Neurol. 1977;18:63–82. [PubMed] [Google Scholar]
- 8.Weintraub S, Mesulam MM. Visual hemispatial inattention: stimulus parameters and exploratory strategies. J Neurol Neurosurg Psychiatry. 1988;51:1481–1488. doi: 10.1136/jnnp.51.12.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gauthier L, Dehaut F, Joanette Y. The Bells Test: a quantitative and qualitative test for visual neglect. Int J Clin Neuropsychol. 1989;11:49–54. [Google Scholar]
- 10.Ishiai S, Sugishita M, Odajima N, Yaginuma M, Gono S, Kamaya T. Improvement of unilateral spatial neglect with numbering. Neurology. 1990;40:1395–1398. doi: 10.1212/wnl.40.9.1395. [DOI] [PubMed] [Google Scholar]
- 11.Donnelly N, Guest R, Fairhurst M, Potter J, Deighton A, Patel M. Developing algorithms to enhance the sensitivity of cancellation tests of visuospatial neglect. Behav Res Meth Instr Comput. 1999;31:668–673. doi: 10.3758/bf03200743. [DOI] [PubMed] [Google Scholar]
- 12.Chatterjee A, Mennemeier M, Heilman KM. Search patterns and neglect: a case study. Neuropsychologia. 1992;30:657–672. doi: 10.1016/0028-3932(92)90070-3. [DOI] [PubMed] [Google Scholar]
- 13.Werth R. Shifts and omissions in spatial reference in unilateral neglect. In: Robertson IH, Marshall JC, editors. Unilateral neglect: clinical and experimental studies. Hove, UK: Lawrence Erlbaum Associates; 1993. pp. 211–231. [Google Scholar]
- 14.Wilson B, Cockburn J, Halligan P. Behavioural Inattention Test. Bury St. Edmonds, UK: Thames Valley Test Company; 1987. [Google Scholar]
- 15.Mark VW, Kooistra CA, Heilman KM. Hemispatial neglect affected by non-neglected stimuli. Neurology. 1988;38:1207–1211. doi: 10.1212/wnl.38.8.1207. [DOI] [PubMed] [Google Scholar]
- 16.Tegnér R, Levander M. Through a looking glass. A new technique to demonstrate directional hypokinesia in unilateral neglect. Brain. 1991;114:1943–1951. doi: 10.1093/brain/114.4.1943. [DOI] [PubMed] [Google Scholar]
- 17.Na DL, Adair JC, Kang Y, Chung CS, Lee KH, Heilman KM. Motor perseverative behavior on a line cancellation task. Neurology. 1999;52:1569–1576. doi: 10.1212/wnl.52.8.1569. [DOI] [PubMed] [Google Scholar]
- 18.Manly T, Woldt K, Watson P, Warburton E. Is motor perseveration in unilateral neglect ‘driven’ by the presence of neglected left-sided stimuli? Neuropsychologia. 2002;40:1794–1803. doi: 10.1016/s0028-3932(02)00035-0. [DOI] [PubMed] [Google Scholar]
- 19.Rusconi ML, Maravita A, Bottini G, Vallar G. Is the intact side really intact? Perseverative responses in patients with unilateral neglect: a productive manifestation. Neuropsychologia. 2002;40:594–604. doi: 10.1016/s0028-3932(01)00160-9. [DOI] [PubMed] [Google Scholar]
- 20.Rabuffetti M, Ferrarin M, Spadone R, et al. Touch-screen system for assessing visuo-motor exploratory skills in neuropsychological disorders of spatial cognition. Med Biol Eng Comput. 2002;40:675–686. doi: 10.1007/BF02345306. [DOI] [PubMed] [Google Scholar]
- 21.Halligan PW, Cockburn J, Wilson BA. The behavioural assessment of visual neglect. Neuropsychol Rehabil. 1991;1:5–32. [Google Scholar]
- 22.Tant M, Kuks J, Kooijman AC, Cornelissen FW, Brouwer WH. Grey scales uncover similar attentional effects in homonymous hemianopia and visual hemi-neglect. Neuropsychologia. 2002;40:1474–1481. doi: 10.1016/s0028-3932(01)00197-x. [DOI] [PubMed] [Google Scholar]
- 23.Husain M, Mannan S, Hodgson T, Wojciulik E, Driver J, Kennard C. Impaired spatial working memory across saccades contributes to abnormal search in parietal neglect. Brain. 2001;124:941–952. doi: 10.1093/brain/124.5.941. [DOI] [PubMed] [Google Scholar]
- 24.Lezak MD. Neuropsychological assessment. 3. New York: Oxford University Press; 1995. [Google Scholar]
- 25.Elliott R. Executive functions and their disorders. Br Med Bull. 2003;65:49–59. doi: 10.1093/bmb/65.1.49. [DOI] [PubMed] [Google Scholar]
- 26.Teuber HL, Battersby WS, Bender MB. Performance of complex visual tasks after cerebral lesions. J Nerv Ment Dis. 1951;114:413–429. [PubMed] [Google Scholar]
- 27.Papagno C, Rizzo S, Ligori L, Lima J, Riggio A. Memory and executive functions in aneurysms of the anterior communicating artery. J Clin Exp Neuropsychol. 2003;25:24–35. doi: 10.1076/jcen.25.1.24.13629. [DOI] [PubMed] [Google Scholar]
- 28.Ruchinskas RA, Giuliano AJ. Motor perseveration in geriatric medical patients. Arch Clin Neuropsychol. 2003;18:455–461. [PubMed] [Google Scholar]
- 29.Sprenger A, Kömpf D, Heide W. Visual search in patients with left visual hemineglect. Progr Brain Res. 2002;140:395–416. doi: 10.1016/S0079-6123(02)40065-9. [DOI] [PubMed] [Google Scholar]
- 30.Vivas AB, Humphreys GW, Fuentes LJ. Inhibitory processing following damage to the parietal lobe. Neuropsychologia. 2003;41:1531–1540. doi: 10.1016/s0028-3932(03)00063-0. [DOI] [PubMed] [Google Scholar]
- 31.Mark VW, Heilman KM. Diagonal neglect on cancellation. Neuropsychologia. 1997;35:1425–1436. doi: 10.1016/s0028-3932(97)00067-5. [DOI] [PubMed] [Google Scholar]
- 32.Marshall JC, Halligan PW. Does the midsagittal plane play any privileged role in “left” neglect? Cogn Neuropsychol. 1989;6:403–422. [Google Scholar]
- 33.Small M, Cowey A, Ellis S. How lateralised is visuospatial neglect? Neuropsychologia. 1994;32:449–464. doi: 10.1016/0028-3932(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 34.Potter J, Deighton T, Patel M, Fairhurst M, Guest R, Donnelly N. Computer recording of standard tests of visual neglect in stroke patients. Clin Rehabil. 2000;14:441–446. doi: 10.1191/0269215500cr344oa. [DOI] [PubMed] [Google Scholar]
- 35.Lazar RM, Fitzsimmons BF, Marshall RS, et al. Reemergence of stroke deficits with midazolam challenge. Stroke. 2002;33:283–285. doi: 10.1161/hs0102.101222. [DOI] [PubMed] [Google Scholar]
- 36.Mazer BL, Korner-Bitensky NA, Sofer S. Predicting ability to drive after stroke. Arch Phys Med Rehabil. 1998;79:743–750. doi: 10.1016/s0003-9993(98)90350-1. [DOI] [PubMed] [Google Scholar]
- 37.Scharre DW, Kirmani JF, Davis RA, Mauger L, Kantor BS. Driving performance in Alzheimer’s disease is predicted by easily administered cognitive tasks. Neurology. 2000;54(suppl 3):A207. Abstract. [Google Scholar]
- 38.Mazer BL, Sofer S, Korner-Bitensky N, Gelinas I, Hanley J, Wood-Dauphinee S. Effectiveness of a visual attention retraining program on the driving performance of clients with stroke. Arch Phys Med Rehabil. 2003;84:541–550. doi: 10.1053/apmr.2003.50085. [DOI] [PubMed] [Google Scholar]