Abstract
Background: National Health and Nutrition Examination Survey data show an increased risk of cardiovascular disease (CVD) mortality with an increased intake of added sugar.
Objective: We determined the dose-response effects of consuming beverages sweetened with high-fructose corn syrup (HFCS) at zero, low, medium, and high proportions of energy requirements (Ereq) on circulating lipid/lipoprotein risk factors for CVD and uric acid in adults [age: 18–40 y; body mass index (in kg/m2): 18–35].
Design: We conducted a parallel-arm, nonrandomized, double-blinded intervention study in which adults participated in 3.5 inpatient days of baseline testing at the University of California Davis Clinical and Translational Science Center’s Clinical Research Center. Participants then consumed beverages sweetened with HFCS at 0% (aspartame sweetened, n = 23), 10% (n = 18), 17.5% (n = 16), or 25% (n = 28) of Ereq during 13 outpatient days and during 3.5 inpatient days of intervention testing at the research center. We conducted 24-h serial blood collections during the baseline and intervention testing periods.
Results: Consuming beverages containing 10%, 17.5%, or 25% Ereq from HFCS produced significant linear dose-response increases of lipid/lipoprotein risk factors for CVD and uric acid: postprandial triglyceride (0%: 0 ± 4; 10%: 22 ± 8; 17.5%: 25 ± 5: 25%: 37 ± 5 mg/dL, mean of Δ ± SE, P < 0.0001 effect of HFCS-dose), fasting LDL cholesterol (0%: −1.0 ± 3.1; 10%: 7.4 ± 3.2; 17.5%: 8.2 ± 3.1; 25%: 15.9 ± 3.1 mg/dL, P < 0.0001), and 24-h mean uric acid concentrations (0%: −0.13 ± 0.07; 10%: 0.15 ± 0.06; 17.5%: 0.30 ± 0.07; 25%: 0.59 ± 0.09 mg/dL, P < 0.0001). Compared with beverages containing 0% HFCS, all 3 doses of HFCS-containing beverages increased concentrations of postprandial triglyceride, and the 2 higher doses increased fasting and/or postprandial concentrations of non–HDL cholesterol, LDL cholesterol, apolipoprotein B, apolipoprotein CIII, and uric acid.
Conclusions: Consuming beverages containing 10%, 17.5%, or 25% Ereq from HFCS produced dose-dependent increases in circulating lipid/lipoprotein risk factors for CVD and uric acid within 2 wk. These results provide mechanistic support for the epidemiologic evidence that the risk of cardiovascular mortality is positively associated with consumption of increasing amounts of added sugars. This trial was registered at clinicaltrials.gov as NCT01103921.
Keywords: apolipoprotein CIII, fructose, risk factors, sugar, uric acid
INTRODUCTION
Considerable epidemiologic evidence suggests that increased intake of added sugars, sucrose and/or high-fructose corn syrup (HFCS),7 or sugar-sweetened beverages is associated with dyslipidemia, cardiovascular disease (CVD), and metabolic syndrome (1). Recent evidence from National Health and Nutrition Examination Survey III suggests that the higher the intake of added sugar, the greater the risk (2). The adjusted HRs of CVD mortality across quintiles of percentage of daily calories consumed from added sugar (quintile 1: 0–9.59%; quintile 2: 9.6–13.09%; quintile 3: 13.1–16.69%; quintile 4: 16.7–21.29%; quintile 5: ≥21.3% of daily calories) were 1.00 (reference) for quintile 1, 1.07 (95% CI: 1.02–1.12) for quintile 2, 1.18 (95% CI: 1.06–1.31) for quintile 3, 1.38 (95% CI: 1.11–1.70) for quintile 4, and 2.03 (95% CI: 1.26–3.27) for quintile 5 (P = 0.004; n = 11,733) (2). These data suggest that the average amount of added sugar consumption in the United States, ∼13–14% of daily calories for adults 20–60 y of age (3) and 16% for children and adolescents (4), is associated with an 18% increase in CVD mortality risk (2). However, investigators of a recent dose-response study, in which ad libitum diets of men and women were supplemented with beverages containing 8%, 18%, or 30% energy requirement (Ereq) from sucrose or HFCS for 10 wk (5, 6), have reported that consumption of added sugar does not increase fasting cholesterol and LDL cholesterol (5) and that there were no differences between the 3 doses of sugar in 24-h circulating triglyceride and uric acid concentrations (6). We have conducted a dose-response study in which the ad libitum diets of young men and women were supplemented with HFCS-sweetened beverages for 2 wk. Our objective was to determine the dose-response effects of consuming of beverages providing 0%, 10%, 17.5%, or 25% Ereq from HFCS (Ereq-HFCS) on circulating concentrations of lipid/lipoprotein risk factors for CVD and uric acid.
METHODS
Participants in this study are a subgroup from an NIH-funded investigation in which a total of 187 participants assigned to 8 experimental groups were studied. A primary objective of this 5-y investigation was to compare the metabolic effects of consuming beverages containing fructose, glucose, or HFCS at 25% Ereq, and the results from the first 48 subjects to complete the study protocol in these experimental groups (n = 16/group) have been reported (7). The current article reports the results from 85 participants consuming beverages containing 0% (n = 23), 10% (n = 18), 17.5% (n = 16), and 25% (n = 28) Ereq from HFCS.
Participants, who were recruited through an Internet listing (craigslist.com) and local postings of flyers, underwent telephone and in-person interviews with medical history, complete blood count, and serum biochemistry panel to assess eligibility. Inclusion criteria included age 18–40 y and BMI 18–35 kg/m2 with a self-report of stable body weight during the prior 6 months. Exclusion criteria included diabetes (fasting glucose >125 mg/dL), evidence of renal or hepatic disease, fasting plasma triglyceride >400 mg/dL, hypertension (>140/90 mm Hg), hemoglobin <8.5 g/dL, and surgery for weight loss. Individuals who smoked, habitually ingested >2 alcoholic beverages/d, exercised >3.5 h/wk at a level more vigorous than walking, or used thyroid, lipid-lowering, glucose-lowering, antihypertensive, antidepressant, or weight loss medications were also excluded. Assignment to the experimental groups was not randomized; by design, the experimental groups were matched for sex, BMI, and concentrations of fasting triglyceride, cholesterol, HDL cholesterol, and insulin in plasma collected during the in-person interviews.
For the 5 wk before study, subjects who were scheduled for participation were asked to limit daily consumption of sugar-containing beverages to no more than one 8-oz serving of fruit juice and to discontinue consumption of any vitamin, mineral, dietary, or herbal supplements. Ninety-seven subjects were enrolled in the experimental groups consuming 0%, 10%, 17.5%, or 25% Ereq-HFCS. Five subjects withdrew or were dismissed from the study before the start of the intervention for reasons that included scheduling problems, new job, dissatisfaction with inpatient meals, family emergency, and personal anxiety. Of the 7 subjects who participated in intervention, one (0% group) withdrew due to job-related conflict, one (10% group) was lost to follow-up due to loss of contact, one (17.5% group) withdrew due to a family emergency, and 4 (25% group) did not complete the study for other reasons [one withdrew due to back pain (one fasting intervention sample was collected from this subject), one withdrew due to personal anxiety, one was dismissed for medical issues not apparent during screening, and one was dismissed for illness (e.g., flu-like symptoms)]. A sensitivity analysis was conducted, which demonstrated that the higher number of noncompleters in the 25% group did not influence the results (see Supplemental Figure 1). A total of 85 subjects completed the dose-response study. The data from one subject (17.5% group) were included in the fasting analyses but not postprandial analyses because a family emergency prevented completion of the 24-h serial blood collection during the intervention period. The sample size calculation, based on the fasting apolipoprotein B (apoB) results generated during a previous study (8), indicated that 25 subjects per group would be sufficient to detect differences between the 25% Ereq and 0% Ereq groups. This number was not achieved in all groups due to insufficient funding. The larger number of subjects in the 25% group is due to additional funding (R01 HL107256) being obtained to conduct mechanistic studies in participants from this group. The 28 participants who consumed 25% Ereq-HFCS included 16 participants whose results were previously reported (7). A comparison of these 16 subjects with the 12 additional subjects in the 25% group is provided in Supplemental Table 1 and reveals no differences in baseline parameters and outcome responses.
This was a parallel-arm, double-blinded diet intervention study with 3 phases: 1) a 3.5-d inpatient baseline period during which subjects resided at the University of California Davis Clinical and Translational Science Center’s Clinical Research Center (CCRC), consumed a standardized baseline diet, and participated in experimental procedures; 2) a 12-d outpatient intervention period during which subjects consumed their assigned sweetened beverages providing 0% (aspartame sweetened) or 10%, 17.5%, or 25% Ereq-HFCS along with their usual ad libitum diets; and 3) a 3.5-d inpatient intervention period during which subjects resided at the CCRC and consumed standardized diets that included the sweetened beverages, and all experimental procedures were repeated.
Inpatient diets
During days 2 and 3 of the baseline and intervention inpatient periods, subjects consumed energy-balanced meals consisting of conventional foods. Daily energy requirements were calculated by the Mifflin equation (9), with adjustment of 1.3 for activity on the days of the 24-h serial blood collections and adjustment of 1.5 for the other days. The baseline diet contained 55% Ereq mainly as low-fiber complex carbohydrate (i.e., white bread, white rice, regular pasta), 30% from fat, and 15% from protein. The meals during the inpatient intervention period included the assigned study beverages and were as identical as possible to baseline meals, except for the substitution of the sugar-sweetened beverage in place of isocaloric amounts of complex carbohydrate. The intervention meals contained 19–20 g fiber/2000 kcal fiber, and the baseline meals contained 22 g fiber/2000 kcal. The timing of inpatient meals and the energy distribution were as follows: breakfast, 0900 (25%); lunch, 1300 (35%); and dinner, 1800 (40%).
Study beverages and outpatient diet
HFCS-containing beverages were sweetened with HFCS-55 (Isosweet 5500, 55% fructose, 45% glucose; Skidmore Sales and Distributing), flavored with an unsweetened drink mix (Kool-Aid; Kraft Inc.). A fruit-flavored aspartame drink mix (Market Pantry) was used to prepare the 0% Ereq-HFCS beverages and to augment the sweetness of the 10% Ereq-HFCS beverages. Participants were blinded to their beverage assignment, as were all CCRC and study personnel who interacted with participants or analyzed samples. Voluntary feedback from participants indicated that they were unable to distinguish between beverages containing aspartame or HFCS. The amount (grams) of beverage provided was standardized among the 4 groups and based on energy requirements [calculated with the Mifflin equation (9) plus 1.5 activity adjustment]. During the 12-d outpatient phase of the study, participants were instructed to drink one serving of study beverage with each meal, to consume their usual diet, and to not consume other sugar-sweetened beverages, including fruit juice. To monitor compliance of beverage consumption (10, 11), the study beverages contained a biomarker (riboflavin) that was measured fluorimetrically in urine samples collected at the times of beverage pickup. Subjects were informed about the biomarker but were not provided information regarding its identity. Fasting urinary riboflavin concentrations (Supplemental Figure 2) following 9 d and 13 d of unmonitored beverage consumption were not different from those following one day of monitored beverage consumption at the CCRC, suggesting good and comparable compliance in all groups.
Procedures
The 24-h serial blood collections (7) were conducted on the third day of the baseline (0 wk) and intervention (2 wk) inpatient periods. Three fasting blood samples were collected at 0800, 0830, and 0900 h. Twenty-nine postprandial blood samples were collected at 30- to 60-min intervals until 0800 the following morning. Additional 6-mL blood samples were collected at the fasting time points (0800, 0830, and 0900 h) and also at the late-evening time points (2200, 2300, and 2400 h). The additional plasma from the 3 fasting samples was pooled, as was that from the 3 late-evening postprandial samples; multiple aliquots of each pooled sample were stored at −80°C. The plasma concentrations of triglyceride and uric acid were measured at all time points and calculated for mean 24-h concentration and for 24-h AUC by the trapezoidal method. The concentrations of non–HDL cholesterol, LDL cholesterol, apoB, apolipoprotein CIII (apoCIII), and apolipoprotein AI were measured during the fasting and late-evening postprandial period. These postprandial measures were conducted on samples collected or pooled from the 2200, 2300, and 2400 h time points because this was the period during which peak postprandial triglyceride concentrations were observed in our previous study (8). Lipid, lipoprotein, and uric acid concentrations were measured with a Polychem Chemistry Analyzer (PolyMedCo Inc.) with reagents from MedTest DX. The intra- and interassay CVs during the time period when these measurements were conducted (2010–2014) were as follows: triglyceride: 3.1%, 7.6% (intra-assay, interassay); total cholesterol: 2.3%, 4.4%; HDL cholesterol: 3.0%, 5.5%; direct LDL cholesterol: 2.4%, 4.7%; apoB: 3.5%, 6.9%; apoCIII: 2.0%, 6.5%; and uric acid: 1.9, 5.6%.
Ethics
The study was conducted in accordance with an experimental protocol that was approved by the UC Davis Institutional Review Board, and participants provided written informed consent.
Statistical analyses
The absolute change (Δ) at 2 wk of intervention compared with the 0-wk baseline value for each outcome was tested for a dose-response trend in a general linear model (SAS 9.3; SAS Institute), adjusted for sex, BMI, and outcome concentration [outcome] at baseline by using HFCS dose (0%, 10%, 17.5%, 25%) as a continuous variable. Departures from linearity were tested with polynomial terms by using the same model. The proportion of variance explained by each covariate was calculated as follows: (type III sum of squares/corrected total sum of squares) × 100 (Supplemental Table 2). A secondary trend-testing general linear model that also included Δbody weight as a continuous covariate was conducted. The Δ for each outcome was also analyzed in a general linear model (SAS 9.3), adjusted for sex, BMI, and [outcome] at baseline, with HFCS-group (1, 2, 3, 4) as a categorical variable. This model allowed for testing of outcomes that were significantly changed from baseline concentrations as least squares means of Δ different from zero and identified significant differences between groups by Tukey’s multiple-comparisons test. The Δnon–HDL cholesterol, LDL cholesterol, and apoB concentrations were further investigated in the adjusted general linear model that included HFCS dose (0%, 10%, 17.5%, 25%) as a continuous variable and Δpostprandial triglyceride, apoCIII, and uric acid as continuous covariates, singly and in combination. This model was also used to test for relations between Δpostprandial triglyceride, apoCIII, and uric acid. Sixteen outcomes were tested for nominal significance (P < 0.05) and notated when the effect of HFCS-dose did not obtain a level of significance corrected for 16 comparisons (P < 0.0031). Data presented in Table 1 are means ± SDs; all other data are means ± SEs.
TABLE 1.
Description of participants at baseline by HFCS content of beverage1
| HFCS 0% | HFCS 10% | HFCS 17.5% | HFCS 25% | |
| Men/women, n | 11/12 | 9/9 | 7/9 | 15/13 |
| Age, y | 25 ± 62 | 28 ± 6 | 24 ± 5 | 27 ± 7 |
| BMI, kg/m2 | 24.8 ± 3.3 | 24.9 ± 3.8 | 24.2 ± 3.3 | 24.9 ± 4.0 |
| Body fat, % | 27.1 ± 9.8 | 26.8 ± 7.4 | 26.3 ± 9.6 | 25.9 ± 9.9 |
| Energy requirement,3 kcal/d | 2354 ± 322 | 2323 ± 247 | 2326 ± 375 | 2390 ± 350 |
| Waist circumference, cm | 74.8 ± 6.2 | 75.3 ± 8.7 | 73.1 ± 8.0 | 76.3 ± 9.7 |
| Systolic blood pressure, mm Hg | 112 ± 12 | 115 ± 10 | 115 ± 8 | 117 ± 10 |
| Diastolic blood pressure, mm Hg | 69 ± 9 | 73 ± 8 | 71 ± 5 | 74 ± 9 |
| Fasting glucose, mg/dL | 91.3 ± 7.1 | 89.2 ± 8.0 | 87.2 ± 6.5 | 89.9 ± 6.5 |
| Fasting insulin, μU/mL | 12.8 ± 5.5 | 11.8 ± 3.4 | 11.7 ± 3.0 | 13.0 ± 5.2 |
| Fasting triglyceride, mg/dL | 101 ± 53 | 122 ± 70 | 97 ± 34 | 108 ± 50 |
| Fasting cholesterol, mg/dL | 149 ± 25 | 162 ± 27 | 165 ± 35 | 158 ± 34 |
| Fasting HDL cholesterol,4 mg/dL | 36 ± 7/43 ± 7 | 42 ± 11/46 ± 12 | 44 ± 7/48 ± 11 | 42 ± 8/50 ± 18 |
| HFCS provided in beverage, g/d | 0 ± 0 | 63 ± 7 | 111 ± 18 | 162 ± 24 |
Values are means ± SDs. HFCS, high-fructose corn syrup.
All variables (excepting HFCS in beverage): P > 0.05 for differences among groups at baseline (general linear model ANOVA with HFCS-group as categorical variable: SAS 9.3).
Energy requirement calculated by the Mifflin equation with 1.5 adjustment for physical activity.
Data from men/women.
RESULTS
There were no significant differences between the 4 experimental groups in anthropomorphic or metabolic parameters at baseline (Table 1).
Effect of HFCS-dose
The outcome means at 0 wk and at the end of the 2-wk intervention by group, as well as the P values for the effects of HFCS-dose, are presented in Table 2. The consumption of beverages containing 0%, 10%, 17.5%, and 25% Ereq-HFCS produced positive dose-response effects that did not deviate significantly from linear in all outcomes presented. All outcomes, except Δbody weight, fasting triglyceride, and fasting apoCIII, retained significance after correction for multiple comparisons (P < 0.0031). Supplemental Table 2 shows the variance explained by HFCS-dose, sex, BMI, [outcome] at baseline, and the complete model for each outcome. The Δ24-h mean uric acid concentration was the outcome most affected by HFCS-dose (proportion of variance: 36%; P < 0.0001) and Δfasting triglyceride was the least affected (proportion of variance: 6%; P = 0.019). When tested in the general linear model that included adjustment for the Δbody weight, the effect of HFCS-dose remained significant for all outcomes with the exception of fasting triglyceride (Supplemental Table 3).
TABLE 2.
Body weight and plasma concentrations of risk factors before and 2 wk after consuming 0%, 10%, 17.5%, or 25% Ereq as HFCS-sweetened beverages in young men and women1
| HFCS dose |
|||||
| Outcome | 0% (n = 23) | 10% (n = 18) | 17.5% (n = 16) | 25% (n = 28) | Effect of dose2 (P value) |
| Body weight, kg | |||||
| 0 wk | 71.8 ± 2.2 | 70.9 ± 2.4 | 69.9 ± 3.6 | 72.9 ± 2.7 | 0.0143 |
| 2 wk | 71.7 ± 2.2 | 70.9 ± 2.4 | 70.2 ± 3.7 | 73.7 ± 2.8 | |
| FST non–HDL cholesterol, mg/dL | |||||
| 0 wk | 110 ± 5 | 118 ± 6 | 119 ± 8 | 112 ± 6 | <0.0001 |
| 2 wk | 107 ± 5 | 126 ± 7 | 126 ± 7 | 128 ± 6 | |
| PP non–HDL cholesterol, mg/dL | |||||
| 0 wk | 101 ± 5 | 111 ± 5 | 113 ± 8 | 103 ± 5 | <0.0001 |
| 2 wk | 99 ± 5 | 120 ± 7 | 126 ± 8 | 124 ± 6 | |
| FST LDL cholesterol, mg/dL | |||||
| 0 wk | 84 ± 5 | 95 ± 5 | 93 ± 8 | 91 ± 5 | <0.0001 |
| 2 wk | 83 ± 6 | 102 ± 6 | 102 ± 6 | 107 ± 6 | |
| PP LDL cholesterol, mg/dL | |||||
| 0 wk | 81 ± 5 | 89 ± 4 | 89 ± 7 | 86 ± 5 | <0.0001 |
| 2 wk | 80 ± 4 | 99 ± 7 | 99 ± 7 | 105 ± 6 | |
| FST apoB, mg/dL | |||||
| 0 wk | 64.8 ± 3.6 | 69.0 ± 3.3 | 69.4 ± 5.6 | 69.6 ± 3.5 | 0.0002 |
| 2 wk | 65.1 ± 3.0 | 73.6 ± 3.9 | 73.2 ± 4.4 | 80.0 ± 4.1 | |
| PP apoB, mg/dL | |||||
| 0 wk | 62.0 ± 3.6 | 65.3 ± 3.1 | 66.0 ± 4.9 | 65.3 ± 3.4 | <0.0001 |
| 2 wk | 61.3 ± 3.0 | 70.1 ± 4.0 | 73.7 ± 4.7 | 77.4 ± 4.2 | |
| FST apoCIII, mg/dL | |||||
| 0 wk | 7.31 ± 0.52 | 8.63 ± 0.65 | 8.08 ± 0.48 | 8.20 ± 0.50 | 0.00543 |
| 2 wk | 7.25 ± 0.47 | 8.34 ± 0.55 | 8.55 ± 0.52 | 8.84 ± 0.52 | |
| PP apoCIII, mg/dL | |||||
| 0 wk | 6.71 ± 0.62 | 7.82 ± 0.58 | 7.48 ± 0.52 | 7.40 ± 0.46 | <0.0001 |
| 2 wk | 6.55 ± 0.51 | 8.13 ± 0.53 | 8.65 ± 0.55 | 8.48 ± 0.55 | |
| FST uric acid, mg/dL | |||||
| 0 wk | 4.57 ± 0.22 | 4.27 ± 0.29 | 4.40 ± 0.20 | 4.55 ± 0.22 | <0.0001 |
| 2 wk | 4.51 ± 0.20 | 4.42 ± 0.30 | 4.70 ± 0.23 | 5.03 ± 0.24 | |
| 24-h Mean uric acid, mg/dL | |||||
| 0 wk | 4.35 ± 0.21 | 4.10 ± 0.29 | 4.22 ± 0.21 | 4.27 ± 0.21 | <0.0001 |
| 2 wk | 4.22 ± 0.19 | 4.25 ± 0.31 | 4.56 ± 0.23 | 4.86 ± 0.24 | |
| FST triglyceride, mg/dL | |||||
| 0 wk | 101 ± 11 | 122 ± 17 | 97 ± 9 | 108 ± 9 | 0.0193 |
| 2 wk | 98 ± 10 | 114 ± 14 | 97 ± 9 | 119 ± 10 | |
| 24-h Mean triglyceride, mg/dL | |||||
| 0 wk | 109 ± 14 | 134 ± 19 | 109 ± 10 | 119 ± 10 | 0.0014 |
| 2 wk | 104 ± 12 | 135 ± 20 | 119 ± 12 | 131 ± 12 | |
| PP triglyceride, mg/dL | |||||
| 0 wk | 94 ± 14 | 125 ± 23 | 100 ± 11 | 108 ± 11 | <0.0001 |
| 2 wk | 94 ± 13 | 147 ± 25 | 125 ± 14 | 145 ± 14 | |
Values are means ± SEs. apoB, apolipoprotein B; apoCIII, apolipoprotein CIII; Ereq, energy requirement; FST, fasting; HFCS, high-fructose corn syrup; PP, postprandial; Δ, absolute change.
The absolute change (Δ from 2-wk intervention with sweetened beverage compared with 0 wk baseline) for each outcome was analyzed in a general linear model (SAS 9.3) with dose of HFCS in beverage as a continuous variable (0%, 10%, 17.5%, 25%) and adjustment for sex, BMI, and [outcome] at baseline; departures from linearity were tested with polynomial terms in the same model. All outcomes exhibited trends that did not significantly deviate from linear.
Nominally significant outcome that is not significant (P < 0.0031) when corrected for 16 comparisons.
Effect of HFCS-group
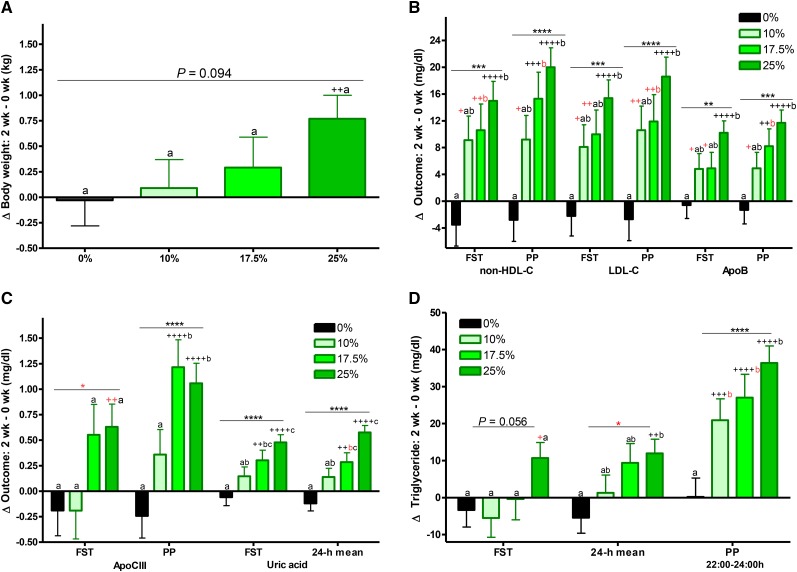
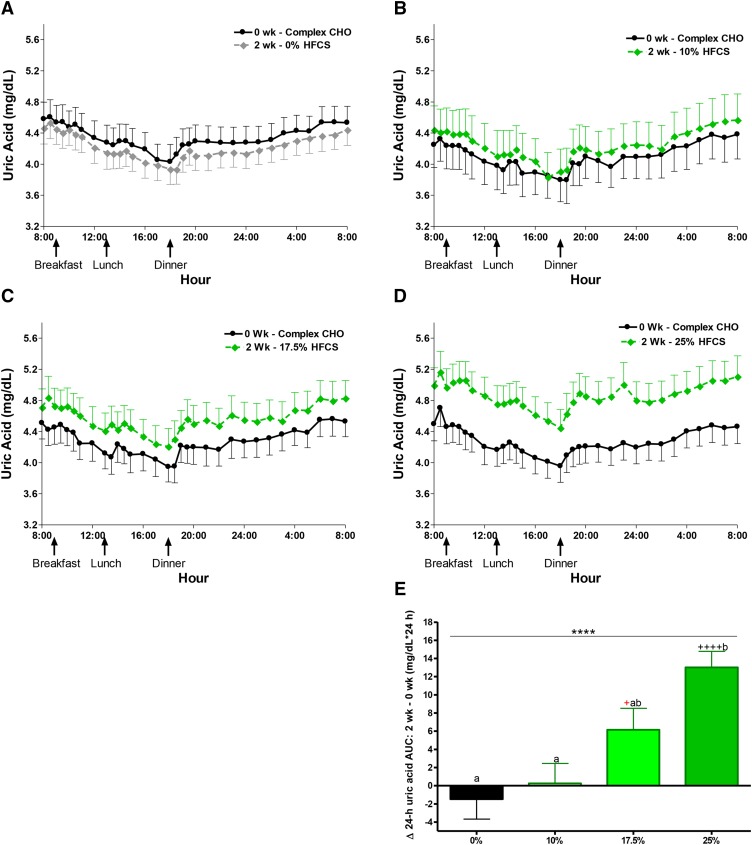
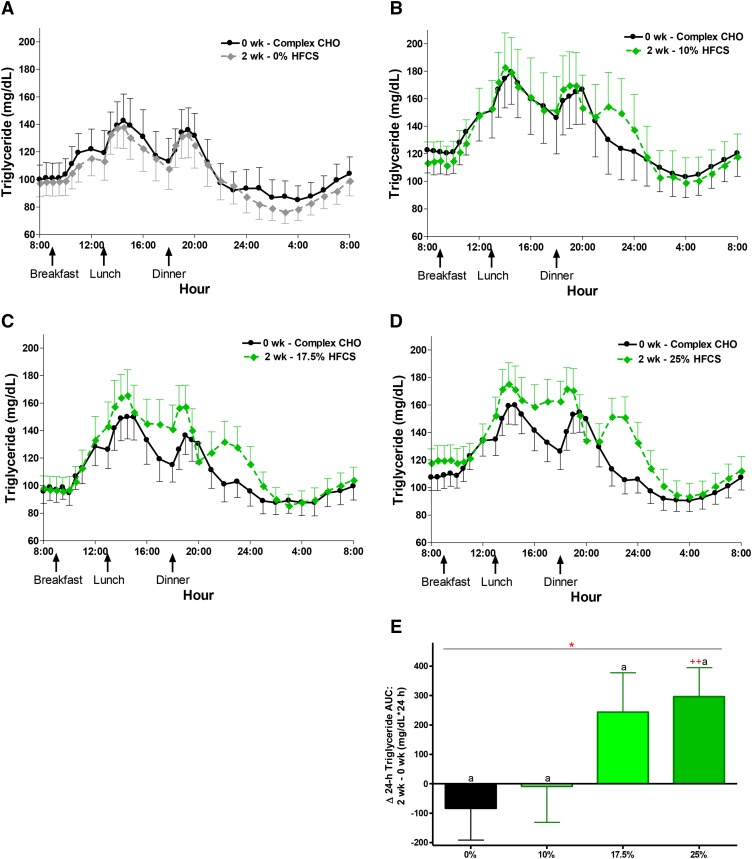
The least squares means of each Δoutcome (2 wk minus 0 wk with adjustment for sex, BMI, and [outcome] at baseline) are presented in Figure 1A–D and labeled for significant effect of HFCS-group and differences between groups and from baseline [significance notations in red indicate that difference did not retain significance after correction for multiple comparisons (P < 0.0031)]. With the exception of Δbody weight and fasting triglyceride, all outcomes presented in were significantly affected by HFCS-group. Compared with 0% Ereq-HFCS, consumption of beverages containing 25% Ereq-HFCS resulted in significantly higher concentrations of postprandial non–HDL cholesterol, LDL cholesterol, and triglyceride, as well as fasting and 24-h mean uric acid (all P < 0.0001, Tukey’s multiple-comparisons test); fasting non-HDL-C and LDL and postprandial apoB and apoCIII (all P < 0.001); fasting apoB (P < 0.01); and 24-h mean triglyceride (P < 0.05). The changes measured during consumption of 17% Ereq-HFCS were significantly higher compared with those induced by 0% Ereq-HFCS for postprandial apoCIII (P < 0.001); postprandial non–HDL cholesterol and triglycerides, as well as 24-h mean uric acid (all P < 0.01); and fasting non–HDL cholesterol and uric acid, as well as postprandial LDL cholesterol and apoB (all P < 0.05). Consumption of the 10% Ereq-HFCS beverages, which is comparable to consuming slightly more than half of a 12-oz (355 mL) can of soda (40 g sugar/can) with each of the 3 major meals, increased concentrations of postprandial triglyceride compared with 0% Ereq-HFCS beverages (P < 0.05). The increases of non–HDL cholesterol, LDL cholesterol, apoB, and 24-h mean uric acid were larger in men than in women (P = 0.0025 – P = 0.038, effect of sex; Supplemental Figure 3A–C). As shown in Figure 2A–D, consumption of HFCS resulted in increases of uric acid concentrations that were consistent throughout the 24-h collection period within each dose group. The 24-h uric acid AUC was significantly increased in the 25% group compared with both the 0% and 10% groups (Figure 2E). The differential effects of HFCS compared with complex carbohydrate on 24-h circulating triglyceride concentrations (Figure 3) were most marked during the late-evening postprandial period (Figure 1D). HFCS consumption consistently resulted in a third triglyceride peak 4–6 h after dinner, whereas consumption of 55% Ereq complex carbohydrate at baseline or along with aspartame-sweetened beverages did not (Figure 3A–D).
FIGURE 1.
Effects of consuming beverages containing 0%, 10%, 17.5%, and 25% Ereq-HFCS. The least squares means (adjusted for sex, BMI, and [outcome] at baseline) ± SEs of (A) Δbody weight; (B) ΔFST and PP plasma non–HDL cholesterol, LDL cholesterol, and apoB concentrations; (C) ΔFST and PP plasma apoCIII concentrations and ΔFST and 24-h mean plasma uric acid concentrations; and (D) ΔFST, 24-h mean, and PP plasma triglyceride concentrations in young men and women after consuming beverages providing 0% (n = 23), 10% (n = 18), 17.5% (n = 16), or 25% (n = 28) of Ereq from HFCS for 2 wk. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, effect of HFCS group; 2-factor (HFCS group, sex) ANCOVA of Δ with adjustment for BMI and [outcome] at baseline. aΔ different from bΔ or bcΔ and abΔ different from cΔ, Tukey’s multiple-comparisons test. +P < 0.05, ++P < 0.01, +++P < 0.001, ++++P < 0.0001, least squares mean different from zero. Significance notations in red indicate that difference did not retain significance after correction for multiple comparisons (P < 0.0031). apoB, apolipoprotein B; apoCIII, apolipoprotein CIII; Ereq, energy requirement; FST, fasting; HFCS, high-fructose corn syrup; PP, postprandial; Δ, absolute change.
FIGURE 2.
The 24-h circulating uric acid concentrations during consumption of complex carbohydrate and during consumption of sweetened beverages containing 0%, 10%, 17.5%, and 25% of Ereq-HFCS. Circulating 24-h uric acid plasma concentrations during consumption of energy-balanced baseline diets containing 55% Ereq complex carbohydrate at 0 wk and during consumption of energy-balanced intervention diets containing isocaloric amounts of carbohydrate as complex carbohydrate and (A) 0% Ereq-HFCS (n = 23), (B) 10% Ereq-HFCS (n = 18), (C) 17.5% Ereq-HFCS (n = 15), or (D) 25% Ereq-HFCS (n = 28) at 2 wk. (E) The change in circulating 24-h uric acid plasma concentrations (2 wk – 0 wk) quantified for each group as Δ24-h uric acid AUC. ****P < 0.0001, effect of HFCS group; 2-factor (HFCS group, sex) ANCOVA of Δ with adjustment for BMI and [outcome] at baseline. aΔ different from bΔ, Tukey’s multiple-comparisons test. +P < 0.05, ++++P < 0.0001, least squares mean different from zero. Significance notations in red indicate that difference did not retain significance after correction for multiple comparisons (P < 0.0031). Data are means ± SEs. CHO, carbohydrate; Ereq, energy requirement; HFCS, high-fructose corn syrup; Δ, absolute change.
FIGURE 3.
The 24-h circulating triglyceride concentrations during consumption of complex carbohydrate and during consumption of sweetened beverages containing 0%, 10%, 17.5%, and 25% of Ereq-HFCS. Circulating 24-h triglyceride plasma concentrations during consumption of energy-balanced baseline diets containing 55% Ereq complex carbohydrate at 0 wk and during consumption of energy-balanced intervention diets containing isocaloric amounts of carbohydrate as complex carbohydrate and (A) 0% Ereq-HFCS (n = 23), (B) 10% Ereq-HFCS (n = 18), (C) 17.5% Ereq-HFCS (n = 15), or (D) 25% Ereq-HFCS (n = 28) at 2 wk. (E) The change in circulating 24-h triglyceride plasma concentrations (2 wk – 0 wk) quantified for each group as Δ24-h uric acid AUC. *P < 0.05, effect of HFCS group; 2-factor (HFCS group, sex) ANCOVA of Δ with adjustment for BMI and [outcome] at baseline. aΔ different from bΔ, Tukey’s multiple-comparisons test. ++P < 0.01, least squares mean different from zero. Significance notations in red indicate that difference did not retain significance after correction for multiple comparisons (P < 0.0031). Data are means ± SEs. CHO, carbohydrate; Ereq, energy requirement; HFCS, high-fructose corn syrup; Δ, absolute change.
Relations between outcomes
ΔPostprandial triglyceride, apoCIII, and uric acid concentrations were tested in the adjusted general linear regression model for their relation to each other (data not shown) and for their potential contributions to Δfasting and postprandial non–HDL cholesterol, LDL cholesterol, and apoB. The Δuric acid was not significantly related to ΔapoCIII or postprandial triglyceride (data not shown). The Δpostprandial triglyceride and Δfasting or postprandial apoCIII were highly correlated, especially Δpostprandial apoCIII and Δ24-h mean triglyceride concentrations (data not shown). The Δpostprandial apoCIII was a highly significant covariate (proportion of variance = 35%, P < 0.0001) in the adjusted model testing the effects of HFCS dose on Δ24-h mean triglyceride, and Δ24-h mean triglyceride was an almost equally significant covariate (proportion of variance = 30%, P < 0.0001) in the model testing the effects of HFCS dose on Δpostprandial apoCIII.
Despite their strong correlation, Δpostprandial triglyceride, whether indexed as the 24-h mean or the late-evening postprandial peak (or mean of postmeal peaks), was not a significant covariate in the models testing the effects of HFCS dose on Δnon–HDL cholesterol, LDL, or apoB (data not shown), whereas ΔapoCIII was highly related. In Table 3, we present the proportion of variances and P values for 4 adjusted models that tested the effects of HFCS dose, HFCS dose and Δuric acid, HFCS dose and ΔapoCIII, and HFCS dose with Δuric acid and ΔapoCIII together on the Δfasting and postprandial non–HDL cholesterol, LDL cholesterol, and apoB. Singly, Δuric acid and ΔapoCIII were significant covariates for all 6 outcomes and reduced the proportion of variance for the effect of HFCS dose by 33–77%. In combination, both Δuric acid and ΔapoCIII remained significant covariates and reduced the proportion of variance estimates for the effect of HFCS dose by 76–95%.
TABLE 3.
Relation of Δuric acid and apoCIII with the HFCS-induced increases in non–HDL cholesterol, LDL cholesterol, and apoB1
| Model 23 |
Model 34 |
Model 45 |
||||||
| Risk factor/index | Model 12: dose | Dose | Δuric acid6 | Dose | ΔapoCIII | Dose | Δuric acid6 | ΔapoCIII |
| ΔFST non–HDL cholesterol | ||||||||
| % Variance7: covariate | 15 | 4 | 8 | 8 | 138 | 2 | 6 | 118 |
| P value | <0.0001 | 0.033 | 0.0017 | 0.0016 | <0.0001 | 0.11 | 0.0025 | <0.0001 |
| % Variance: model | 29 | 38 | 43 | 49 | ||||
| ΔPP non–HDL cholesterol | ||||||||
| % Variance: covariate | 24 | 9 | 7 | 8 | 129 | 2 | 6 | 119 |
| P value | <0.0001 | 0.0007 | 0.0036 | 0.0010 | <0.0001 | 0.063 | 0.0022 | <0.0001 |
| % Variance: model | 37 | 43 | 49 | 55 | ||||
| ΔFST LDL cholesterol | ||||||||
| % Variance: covariate | 18 | 4 | 11 | 12 | 78 | 2 | 9 | 58 |
| P value | <0.0001 | 0.023 | 0.0003 | 0.0003 | 0.0051 | 0.066 | 0.0005 | 0.0080 |
| % Variance: model | 28 | 39 | 35 | 44 | ||||
| ΔPP LDL cholesterol | ||||||||
| % Variance: covariate | 20 | 7 | 6 | 12 | 88 | 4 | 5 | 68 |
| P value | <0.0001 | 0.0051 | 0.0066 | 0.0002 | 0.0026 | 0.018 | 0.0043 | 0.011 |
| % Variance: model | 35 | 38 | 39 | 44 | ||||
| ΔFST apoB | ||||||||
| % Variance: covariate | 14 | 3 | 8 | 7 | 108 | 1 | 7 | 88 |
| P value | 0.0002 | 0.050 | 0.0017 | 0.0030 | 0.0006 | 0.16 | 0.0026 | 0.0009 |
| % Variance: model | 28 | 37 | 38 | 45 | ||||
| ΔPP apoB | ||||||||
| % Variance: covariate | 20 | 5 | 10 | 7 | 89 | 1 | 9 | 79 |
| P value | <0.0001 | 0.0097 | 0.0006 | 0.0033 | 0.0026 | 0.19 | 0.0005 | 0.0019 |
| % Variance: model | 30 | 37 | 38 | 47 | ||||
apoB, apolipoprotein B; apoCIII, apolipoprotein CIII; FST, fasting; HFCS, high-fructose corn syrup; PP, postprandial; Δ, absolute change.
Model 1: general linear model with dose (0%, 10%, 17.5%, 25%) as a continuous covariate and adjustment for sex, BMI, and [outcome] at baseline.
Model 2: model 1 plus Δuric acid as a continuous covariate.
Model 3: model 1 plus ΔapoCIII as a continuous covariate.
Model 4: model 1 plus Δuric acid and ΔapoCIII as continuous covariates.
ΔFST and Δ24-h mean uric acid yielded nearly identical results in models 2 and 4. Only FST uric acid results are presented.
% Variance: proportion of variance explained by variable or model = (type III sum of squares/corrected total sum of squares) × 100.
Results shown for ΔFST apoCIII.
Results shown for ΔPP apoCIII.
DISCUSSION
This study demonstrates for the first time that established risk factors for CVD, plasma concentrations of non–HDL cholesterol, LDL cholesterol, and apoB (12), increase in a dose-dependent manner in young adults consuming beverages providing 10%, 17.5%, or 25% Ereq from HFCS for 2 wk. The dose-dependent increases of these risk factors for CVD, which were shown to be statistically independent of body weight gain, provide mechanistic support for the recent epidemiologic findings that there is increased risk of CVD mortality with increased intake of added sugar across quintiles (2). The significant and independent correlations of both ΔapoCIII and uric acid with Δnon–HDL cholesterol, LDL cholesterol, and apoB (see Supplemental Discussion for more details) suggest the potential for 2 separate pathways by which consumption of HFCS increases these risk factors for CVD.
The increases of late-night postprandial triglyceride concentrations during consumption of all 3 doses of HFCS compared with 0% Ereq-HFCS support our previous work (7, 8) but do not support a meta-analysis concluding that fructose in isocaloric exchange for other carbohydrate does not increase postprandial triglyceride (13) (see Supplemental Discussion for details and also Supplemental Figure 4A,C). Consumption of fructose-containing sugars increases circulating triglyceride because fructokinase, which catalyzes the initial phosphorylation of dietary fructose, is not regulated by hepatic energy status (14). This results in unregulated hepatic fructose uptake (15–17), with most of the ingested fructose being metabolized in the liver and little reaching the systemic circulation (18). The excess substrate leads to increased de novo lipogenesis (8), which may increase the intrahepatic lipid supply directly (19, 20), via synthesis of fatty acids, and indirectly, by inhibiting fatty acid oxidation (21, 22). Increased intrahepatic lipid content promotes VLDL production and secretion (23, 24), leading to increased concentrations of postprandial triglyceride (25). Postprandial hypertriglyceridemia has been recently reviewed as an important risk factor for CVD (26) (see Supplemental Discussion).
This is the first report to our knowledge that consumption of HFCS, or any sugar, increases plasma concentrations of apoCIII in humans. We have, however, reported that plasma apoCIII increased in rhesus monkeys provided with fructose-sweetened beverages for 6 mo (27). The current increases of apoCIII may simply reflect the effect of HFCS to increase VLDL production, because most VLDL particles are secreted as apoCIII-containing VLDL (28). However, glucose induces apoCIII transcription in rat and human hepatocytes via a mechanism involving the transcription factor, carbohydrate response element-binding protein (29). This mechanism could be relevant to consumption of HFCS because a significant proportion of a large fructose dose is converted into glucose (30) and because HFCS contains glucose. In addition, fructose-fed rats treated with carbohydrate response element-binding protein antisense exhibited a decreased rate of hepatic triglyceride secretion (31), suggesting a role for carbohydrate response element-binding protein in the lipogenic effects of fructose feeding. Plasma apoCIII strongly predicts CVD (32) and loss-of-function mutations in the apoCIII gene are associated with lower triglyceride concentrations and reduced risk of ischemic CVD (33).
The unregulated phosphorylation of fructose to fructose-1-phosphate by fructokinase, which results in conversion of ATP to AMP and a depletion of inorganic phosphate, leads to uric acid production via the purine degradation pathway (17). It has also been reported that a high-fructose diet increases de novo purine biosynthesis in humans, and this also contributes to increased uric acid production (34). Our results regarding the proportion of variation explained by HFCS-dose suggest that these pathways are highly sensitive to the dose of fructose. These results do not support the conclusion from a meta-analysis that isocaloric exchange of fructose for other carbohydrate does not affect serum uric acid concentrations in nondiabetic subjects (35) (see Supplemental Discussion for details and Supplemental Figure 5A,C). Uric acid is also a potential mediator of metabolic disease, with most recent studies, but not all (36), showing that it is strongly associated and predictive of metabolic syndrome, fatty liver, and CVD (37–39). In one study, an HR of 1.14 for coronary heart disease events was documented for every 0.5-mg/dL increase of uric acid concentrations (40). The similar increases induced by the 25% Ereq-HFCS dose in just 2 wk [+0.5 ± 0.1 (fasting), +0.6 ± 0.1 mg/dL (24-h mean)] suggest that consumption of HFCS can have clinically relevant effects to increase circulating uric acid.
Our results do not support the recent reports that adults consuming beverages containing 8%, 18%, or 30% Ereq as sucrose or HFCS for 10 wk exhibited no differences of total cholesterol or LDL cholesterol (5) and 24-h uric acid and triglyceride AUC responses (6) between doses. The results from these studies (6) and the present study are starkly contrasting. For example, although Yu et al. (6) reported no differences in the 24-h uric acid AUC “response to the 6 different interventions at baseline or post-testing,” we report that consumption of the 25% Ereq-HFCS dose increased 24-h uric acid AUC over baseline (Figure 2E: 14.0 ± 2.2 mg/dL × 24 h, P < 0.0001, least squares mean significantly different from zero, ANCOVA) and compared with 10% Ereq-HFCS (+12.8 mg/dL × 24 h, P < 0.0001, Tukey’s multiple-comparisons test). Possible explanations for the contrasting results include the use of milk as a vehicle for the sugars, lack of a control group, the statistical analyses employed, and lack of an objective measure of compliance (see Supplemental Discussion) (6).
A strength of the current study was the presence of a biomarker in the study beverages, which provided an objective measure of compliance. Furthermore, the participants’ knowledge of the biomarker likely contributed to the excellent degree of compliance, as evidenced by the highly significant dose-response effects obtained for most of the outcomes. The 3.5-d inpatient periods during baseline and at the end of intervention, which ensured that the study results were not confounded by noncompliance or variations in diet or physical activity during the days immediately preceding the blood collection procedures, are an additional strength.
A limitation of the study was that it was not randomized, thus potentially introducing bias in the assignment of subjects to the experimental groups. Another limitation is that that the participants consumed ad libitum diets with the study beverages during the 12-d outpatient period, which precludes our drawing conclusions concerning the effects of precise amounts of sugar consumption. Also, in interventions studies lasting 3–10 wk, subjects consumed significantly more energy and gained more weight when consuming sugar-sweetened beverage/snacks compared with artificially sweetened beverages/snacks (41–43). Therefore, variations in outpatient energy intake likely explain the nominally significant dose-response effect of HFCS consumption on body weight gain. This dose-dependent body weight gain could have mediated the dose-dependent increases in the other outcomes. However, the highly significant effects of HFCS-dose in the statistical models adjusted for body weight gain suggest that the dose-dependent effects of HFCS on risk factors were largely independent of body weight gain. This suggestion is supported by reports of increases of risk factors in subjects who consumed high-sugar diets as part of energy-balanced diet protocols and, therefore, did not gain weight (44–47).
The added sugar component of the typical US diet consists of at least as much sucrose as HFCS (48); therefore, a limitation of this study is that we did not also investigate the dose-response effects of sucrose consumption. However, older and recent studies suggest that consumption of sucrose beverages also increases risk factors for CVD (19, 47, 49, 50). The duration of the 2-wk intervention could be considered a potential limitation. It also, however, indicates how quickly excess sugar consumption can initiate metabolic dysregulation. Certainly, the results from sucrose intervention studies ranging from 6 wk to 6 mo (19, 44, 47, 51) and the numerous epidemiologic studies demonstrating associations between CVD risk factors and sugar consumption (1, 2) provide evidence that the highly significant results reported here would unlikely be transient during a longer intervention period.
In conclusion, this study demonstrates that consumption of beverages providing 10%, 17.5%, or 25% Ereq from HFCS results in dose-dependent increases of established risk factors for CVD within 2 wk in young men and women. Concentrations of non–HDL cholesterol, LDL cholesterol, apoB, uric acid, postprandial apoCIII, and postprandial triglyceride increased as the dose of HFCS consumed increased, and they were significant compared with consumption of 0% Ereq-HFCS beverages for most outcomes (12 of 16) in the 25% Ereq-HFCS group, for half (8 of 16) of the outcomes in the 17% Ereq-HFCS group, and for postprandial triglyceride in the 10% Ereq-HFCS group. These results provide a plausible mechanistic link to the results of the recent report that there is increased risk of CVD mortality with increased intake of added sugar across quintiles (2). Both studies suggest that humans are sensitive to the adverse effects of sustained sugar consumption at a relatively wide range of intake and highlight the need for carefully controlled diet intervention studies to determine prudent amounts of added sugar consumption.
Acknowledgments
We thank James Graham (UC Davis) for excellent technical support, Tissa Kappagoda (UC Davis) for physician support, and Janet Peerson (UC Davis) for expert guidance on the statistical analyses. We also thank the nursing staff at the CCRC for their dedicated nursing support.
The authors’ responsibilities were as follows—KLS, AAB, NLK, and PJH: designed the research; KLS, VM, AAB, VL, HDL, MVN, and GXC: conducted the research; KLS: analyzed data; KLS, AAB, NLK, and PJH: wrote the manuscript; KLS: had primary responsibility for the final content; and all authors: read and approved the final manuscript. None of the authors declared a conflict of interest.
Footnotes
Abbreviations used: apoB, apolipoprotein B; apoCIII, apolipoprotein CIII; CCRC, University of California Davis Clinical and Translational Science Center’s Clinical Research Center; CVD, cardiovascular disease; Ereq, energy requirement; HFCS, high-fructose corn syrup; Δ, absolute change.
REFERENCES
- 1.Richelsen B. Sugar-sweetened beverages and cardio-metabolic disease risks. Curr Opin Clin Nutr Metab Care 2013;16:478–84. [DOI] [PubMed] [Google Scholar]
- 2.Yang Q, Zhang Z, Gregg EW, Flanders WD, Merritt R, Hu FB. Added sugar intake and cardiovascular diseases mortality among US adults. JAMA Intern Med 2014;174:516–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ervin RB, Ogden CL. Consumption of added sugars among U.S. adults, 2005–2010. NCHS Data Brief 2013;(122):1–8. [PubMed] [Google Scholar]
- 4.Ervin RB, Kit BK, Carroll MD, Ogden CL. Consumption of added sugar among U.S. children and adolescents, 2005–2008. NCHS Data Brief 2012;(87):1–8. [PubMed] [Google Scholar]
- 5.Bravo S, Lowndes J, Sinnett S, Yu Z, Rippe J. Consumption of sucrose and high-fructose corn syrup does not increase liver fat or ectopic fat deposition in muscles. Appl Physiol Nutr Metab 2013;38:681–8. [DOI] [PubMed] [Google Scholar]
- 6.Yu Z, Lowndes J, Rippe J. High-fructose corn syrup and sucrose have equivalent effects on energy-regulating hormones at normal human consumption levels. Nutr Res 2013;33:1043–52. [DOI] [PubMed] [Google Scholar]
- 7.Stanhope KL, Bremer AA, Medici V, Nakajima K, Ito Y, Nakano T, Chen G, Fong TH, Lee V, Menorca RI, et al. . Consumption of fructose and high fructose corn syrup increase postprandial triglycerides, LDL-cholesterol, and apolipoprotein-B in young men and women. J Clin Endocrinol Metab 2011;96:E1596–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, Hatcher B, Cox CL, Dyachenko A, Zhang W, et al. . Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest 2009;119:1322–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr 1990;51:241–7. [DOI] [PubMed] [Google Scholar]
- 10.Switzer BR, Stark AH, Atwood JR, Ritenbaugh C, Travis RG, Wu HM. Development of a urinary riboflavin adherence marker for a wheat bran fiber community intervention trial. Cancer Epidemiol Biomarkers Prev 1997;6:439–42. [PubMed] [Google Scholar]
- 11.Ramanujam VM, Anderson KE, Grady JJ, Nayeem F, Lu LJ. Riboflavin as an oral tracer for monitoring compliance in clinical research. Open Biomarkers J 2011;2011:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, Wood AM, Lewington S, Sattar N, Packard CJ, et al. . Major lipids, apolipoproteins, and risk of vascular disease. JAMA 2009;302:1993–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang DD, Sievenpiper JL, de Souza RJ, Cozma AI, Chiavaroli L, Ha V, Mirrahimi A, Carleton AJ, Di Buono M, Jenkins AL, et al. . Effect of fructose on postprandial triglycerides: a systematic review and meta-analysis of controlled feeding trials. Atherosclerosis 2014;232:125–33. [DOI] [PubMed] [Google Scholar]
- 14.Hommes FA. Inborn errors of fructose metabolism. Am J Clin Nutr 1993;58(Suppl):788S–95S. [DOI] [PubMed] [Google Scholar]
- 15.Havel PJ. Dietary fructose: implications for dysregulation of energy homeostasis and lipid/carbohydrate metabolism. Nutr Rev 2005;63:133–57. [DOI] [PubMed] [Google Scholar]
- 16.Ishimoto T, Lanaspa MA, Le MT, Garcia GE, Diggle CP, Maclean PS, Jackman MR, Asipu A, Roncal-Jimenez CA, Kosugi T, et al. . Opposing effects of fructokinase C and A isoforms on fructose-induced metabolic syndrome in mice. Proc Natl Acad Sci USA 2012;109:4320–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mayes PA. Intermediary metabolism of fructose. Am J Clin Nutr 1993;58(Suppl):754S–65S. [DOI] [PubMed] [Google Scholar]
- 18.Teff KL, Grudziak J, Townsend RR, Dunn TN, Grant RW, Adams SH, Keim NL, Cummings BP, Stanhope KL, Havel PJ. Endocrine and metabolic effects of consuming fructose- and glucose-sweetened beverages with meals in obese men and women: influence of insulin resistance on plasma triglyceride responses. J Clin Endocrinol Metab 2009;94:1562–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maersk M, Belza A, Stodkilde-Jorgensen H, Ringgaard S, Chabanova E, Thomsen H, Pedersen SB, Astrup A, Richelsen B. Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: a 6-mo randomized intervention study. Am J Clin Nutr 2012;95:283–9. [DOI] [PubMed] [Google Scholar]
- 20.Sevastianova K, Santos A, Kotronen A, Hakkarainen A, Makkonen J, Silander K, Peltonen M, Romeo S, Lundbom J, Lundbom N, et al. . Effect of short-term carbohydrate overfeeding and long-term weight loss on liver fat in overweight humans. Am J Clin Nutr 2012;96:727–34. [DOI] [PubMed] [Google Scholar]
- 21.Cox CL, Stanhope KL, Schwarz JM, Graham JL, Hatcher B, Griffen SC, Bremer AA, Berglund L, McGahan JP, Havel PJ, et al. . Consumption of fructose-sweetened beverages for 10 weeks reduces net fat oxidation and energy expenditure in overweight/obese men and women. Eur J Clin Nutr 2012;66:201–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGarry JD. Malonyl-CoA and carnitine palmitoyltransferase I: an expanding partnership. Biochem Soc Trans 1995;23:481–5. [DOI] [PubMed] [Google Scholar]
- 23.Adiels M, Taskinen MR, Packard C, Caslake MJ, Soro-Paavonen A, Westerbacka J, Vehkavaara S, Hakkinen A, Olofsson SO, Yki-Jarvinen H, et al. . Overproduction of large VLDL particles is driven by increased liver fat content in man. Diabetologia 2006;49:755–65. [DOI] [PubMed] [Google Scholar]
- 24.Hudgins LC, Parker TS, Levine DM, Hellerstein MK. A dual sugar challenge test for lipogenic sensitivity to dietary fructose. J Clin Endocrinol Metab 2011;96:861–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adiels M, Olofsson SO, Taskinen MR, Boren J. Overproduction of very low-density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Arterioscler Thromb Vasc Biol 2008;28:1225–36. [DOI] [PubMed] [Google Scholar]
- 26.Borén J, Matikainen N, Adiels M, Taskinen MR. Postprandial hypertriglyceridemia as a coronary risk factor. Clin Chim Acta 2014;431:131–42. [DOI] [PubMed] [Google Scholar]
- 27.Bremer AA, Stanhope KL, Graham JL, Cummings BP, Ampah SB, Saville BR, Havel PJ. Fish oil supplementation ameliorates fructose-induced hypertriglyceridemia and insulin resistance in adult male rhesus macaques. J Nutr 2014;144:5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng C, Khoo C, Ikewaki K, Sacks FM. Rapid turnover of apolipoprotein C-III–containing triglyceride-rich lipoproteins contributing to the formation of LDL subfractions. J Lipid Res 2007;48:1190–203. [DOI] [PubMed] [Google Scholar]
- 29.Caron S, Verrijken A, Mertens I, Samanez CH, Mautino G, Haas JT, Duran-Sandoval D, Prawitt J, Francque S, Vallez E, et al. . Transcriptional activation of apolipoprotein CIII expression by glucose may contribute to diabetic dyslipidemia. Arterioscler Thromb Vasc Biol 2011;31:513–9. [DOI] [PubMed] [Google Scholar]
- 30.Tran C, Jacot-Descombes D, Lecoultre V, Fielding BA, Carrel G, Le KA, Schneiter P, Bortolotti M, Frayn KN, Tappy L. Sex differences in lipid and glucose kinetics after ingestion of an acute oral fructose load. Br J Nutr 2010;104:1139–47. [DOI] [PubMed] [Google Scholar]
- 31.Erion DM, Popov V, Hsiao JJ, Vatner D, Mitchell K, Yonemitsu S, Nagai Y, Kahn M, Gillum MP, Dong J, et al. . The role of the carbohydrate response element-binding protein in male fructose-fed rats. Endocrinology 2013;154:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng C, Updates on apolipoprotein CIII: fulfilling promise as a therapeutic target for hypertriglyceridemia and cardiovascular disease. Curr Opin Lipidol 2014;25(1):35–9. DOI: 10.1097/MOL.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 33.Jørgensen AB, Frikke-Schmidt R, Nordestgaard BG, Tybjaerg-Hansen A. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N Engl J Med 2014;371:32–41. [DOI] [PubMed] [Google Scholar]
- 34.Emmerson BT. Effect of oral fructose on urate production. Ann Rheum Dis 1974;33:276–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang DD, Sievenpiper JL, de Souza RJ, Chiavaroli L, Ha V, Cozma AI, Mirrahimi A, Yu ME, Carleton AJ, Di Buono M, et al. . The effects of fructose intake on serum uric acid vary among controlled dietary trials. J Nutr 2012;142:916–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zalawadiya SK, Veeranna V, Mallikethi-Reddy S, Bavishi C, Lunagaria A, Kottam A, Afonso L. Uric acid and cardiovascular disease risk reclassification: findings from NHANES III. Eur J Prev Cardiol 2015;22:513–8. [DOI] [PubMed] [Google Scholar]
- 37.Billiet L, Doaty S, Katz JD, Velasquez MT. Review of hyperuricemia as new marker for metabolic syndrome. ISRN Rheumatol 2014;2014:852954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cai W, Wu X, Zhang B, Miao L, Sun YP, Zou Y, Yao H. Serum uric acid levels and non-alcoholic fatty liver disease in Uyghur and Han ethnic groups in northwestern China. Arq Bras Endocrinol Metabol 2013;57:617–22. [DOI] [PubMed] [Google Scholar]
- 39.Viazzi F, Garneri D, Leoncini G, Gonnella A, Muiesan ML, Ambrosioni E, Costa FV, Leonetti G, Pessina AC, Trimarco B, et al. . Serum uric acid and its relationship with metabolic syndrome and cardiovascular risk profile in patients with hypertension: Insights from the I-DEMAND study. Nutr Metab Cardiovasc Dis 2014;24:921–7. [DOI] [PubMed] [Google Scholar]
- 40.Athyros VG, Elisaf M, Papageorgiou AA, Symeonidis AN, Pehlivanidis AN, Bouloukos VI, Milionis HJ, Mikhailidis DP, Group GSC. Effect of statins versus untreated dyslipidemia on serum uric acid levels in patients with coronary heart disease: a subgroup analysis of the GREek Atorvastatin and Coronary-heart-disease Evaluation (GREACE) study. Am J Kidney Dis 2004;43:589–99. [DOI] [PubMed] [Google Scholar]
- 41.Raben A, Vasilaras TH, Moller AC, Astrup A. Sucrose compared with artificial sweeteners: different effects on ad libitum food intake and body weight after 10 wk of supplementation in overweight subjects. Am J Clin Nutr 2002;76:721–9. [DOI] [PubMed] [Google Scholar]
- 42.Reid M, Hammersley R, Hill AJ, Skidmore P. Long-term dietary compensation for added sugar: effects of supplementary sucrose drinks over a 4-week period. Br J Nutr 2007;97:193–203. [DOI] [PubMed] [Google Scholar]
- 43.Tordoff MG, Alleva AM. Effect of drinking soda sweetened with aspartame or high-fructose corn syrup on food intake and body weight. Am J Clin Nutr 1990;51:963–9. [DOI] [PubMed] [Google Scholar]
- 44.Black RN, Spence M, McMahon RO, Cuskelly GJ, Ennis CN, McCance DR, Young IS, Bell PM, Hunter SJ. Effect of eucaloric high- and low-sucrose diets with identical macronutrient profile on insulin resistance and vascular risk: a randomized controlled trial. Diabetes 2006;55:3566–72. [DOI] [PubMed] [Google Scholar]
- 45.Lewis AS, McCourt HJ, Ennis CN, Bell PM, Courtney CH, McKinley MC, Young IS, Hunter SJ. Comparison of 5% versus 15% sucrose intakes as part of a eucaloric diet in overweight and obese subjects: effects on insulin sensitivity, glucose metabolism, vascular compliance, body composition and lipid profile. A randomised controlled trial. Metabolism 2013;62:694–702. [DOI] [PubMed] [Google Scholar]
- 46.Reiser S, Bickard MC, Hallfrisch J. Michaelis OEt, Prather ES. Blood lipids and their distribution in lipoproteins in hyperinsulinemic subjects fed three different levels of sucrose. J Nutr 1981;111:1045–57. [DOI] [PubMed] [Google Scholar]
- 47.Reiser S, Hallfrisch J, Michaelis OEt, Lazar FL, Martin RE, Prather ES. Isocaloric exchange of dietary starch and sucrose in humans: I. Effects on levels of fasting blood lipids. Am J Clin Nutr 1979;32:1659–69. [DOI] [PubMed] [Google Scholar]
- 48.Carden TJ, Carr TP. Food availability of glucose and fat, but not fructose, increased in the U.S. between 1970 and 2009: analysis of the USDA food availability data system. Nutr J 2013;12:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aeberli I, Hochuli M, Gerber PA, Sze L, Murer SB, Tappy L, Spinas GA, Berneis K. Moderate amounts of fructose consumption impair insulin sensitivity in healthy young men: a randomized controlled trial. Diabetes Care 2013;36:150–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marckmann P, Raben A, Astrup A. Ad libitum intake of low-fat diets rich in either starchy foods or sucrose: effects on blood lipids, factor VII coagulant activity, and fibrinogen. Metabolism 2000;49:731–5. [DOI] [PubMed] [Google Scholar]
- 51.Raben A, Moller BK, Flint A, Vasilaris TH, Christina Moller A, Juul Holst J, Astrup A. Increased postprandial glycaemia, insulinemia, and lipidemia after 10 weeks’ sucrose-rich diet compared to an artificially sweetened diet: a randomised controlled trial. Food Nutr Res 2011 Jul 20 (Epub ahead of print; DOI: 10.3402/fnr.v55i0.5961). [DOI] [PMC free article] [PubMed] [Google Scholar]