Abstract
Background: Dietary guidelines recommend interchanging protein foods (e.g., chicken for red meat), but they may be exchanged for carbohydrate-rich foods varying in quality [glycemic load (GL)]. Whether such exchanges occur and how they influence long-term weight gain are not established.
Objective: Our objective was to determine how changes in intake of protein foods, GL, and their interrelationship influence long-term weight gain.
Design: We investigated the association between 4-y changes in consumption of protein foods, GL, and their interaction with 4-y weight change over a 16- to 24-y follow-up, adjusted for other lifestyle changes (smoking, physical activity, television watching, sleep duration), body mass index, and all dietary factors simultaneously in 3 prospective US cohorts (Nurses’ Health Study, Nurses’ Health Study II, and Health Professionals Follow-Up Study) comprising 120,784 men and women free of chronic disease or obesity at baseline.
Results: Protein foods were not interchanged with each other (intercorrelations typically <|0.05|) but with carbohydrate (negative correlation as low as −0.39). Protein foods had different relations with long-term weight gain, with positive associations for meats, chicken with skin, and regular cheese (per increased serving/d, 0.13–1.17 kg; P = 0.02 to P < 0.001); no association for milk, legumes, peanuts, or eggs (P > 0.40 for each); and relative weight loss for yogurt, peanut butter, walnuts, other nuts, chicken without skin, low-fat cheese, and seafood (−0.14 to −0.71 kg; P = 0.01 to P < 0.001). Increases in GL were independently associated with a 0.42-kg greater weight gain per 50-unit increase (P < 0.001). Significant interactions (P-interaction < 0.05) between changes in protein foods and GL were identified; for example, increased cheese intake was associated with weight gain when GL increased, with weight stability when GL did not change, and with weight loss when exchanged for GL (i.e., decrease in GL).
Conclusion: Protein foods were commonly interchanged with carbohydrate, and changes in protein foods and GL interacted to influence long-term weight gain.
Keywords: dietary change, glycemic index, glycemic load, protein, weight change
INTRODUCTION
Whereas many studies have assessed weight-loss interventions in obese subjects, less is known about primary prevention of weight gain. Just as primary prevention of cardiovascular disease and diabetes has emerged as essential complements to secondary treatments, the primary prevention of long-term weight gain, which often occurs subtly (∼1 lb or 0.45 kg/y), is a crucial strategy for reducing population adiposity and associated morbidities. Current dietary guidelines and policy measures to reduce obesity are strongly calorie focused, advising individuals to pay “attention to consuming only enough calories to meet their needs” (1) and emphasizing total calorie counts on food nutrition labels (2) and restaurant menu boards (3). Yet, growing evidence indicates that specific types of foods and drinks consumed, rather than simply their calorie content, influence long-term weight gain very differently (4).
The interactions of protein-rich and carbohydrate-rich foods could be especially relevant for long-term weight gain (4–7): protein has been considered potentially protective against obesity (8), whereas high–glycemic index (GI)5 starches, refined grains, and sugars may be especially adverse (4–6) due to the greater and more rapid rises in postprandial blood glucose and insulin they induce, which mechanistic and physiologic studies link to pathways of weight gain (9). Glycemic load (GL) considers both the GI of the food as well as the total carbohydrate content, thereby reflecting both carbohydrate quality and quantity (9), which may be relevant to long-term weight gain. GI and GL often closely correlate, although some foods with a low carbohydrate content per serving may have discordant GI and GL values (e.g., watermelon has a high GI but a low GL) (10).
Current dietary guidelines do not distinguish between different protein sources in relation to weight gain: individuals are advised to select from a variety of protein foods, including fish, poultry, lean red meat, dairy, legumes, and nuts (1). Such recommendations also include implicit and explicit guidance to interchange different protein foods (e.g., poultry or fish for red meat). Yet, the extent to which people actually substitute one protein food for another is not established. Individuals might also choose to exchange protein sources with carbohydrate, the amount and quality of which could influence weight gain.
Thus, it remains unclear how different protein foods are interchanged with each other and with carbohydrate and how these interactions relate to weight gain. To investigate these key questions, we assessed the correlations of changes in different protein foods with each other and with carbohydrate, the associations of changes in the intake of protein foods and changes in carbohydrate quality with long-term weight gain, and the interaction between protein foods and carbohydrate quality with long-term weight gain in 3 separate prospective cohorts of US men and women.
METHODS
Study design and population
We evaluated 3 separate, well-established prospective cohorts, including the Nurses’ Health Study (NHS; n = 121,701 female registered nurses from 11 US states enrolled in 1976), the Nurses’ Health Study II (NHS II; n = 116,686 younger female registered nurses from 14 states enrolled in 1989), and the Health Professionals Follow-Up Study (HPFS; n = 51,529 male health professionals from all 50 states enrolled in 1986) (4). In each cohort, participants were followed by biennial validated questionnaires concerning medical history, lifestyle, and health practices. Follow-up rates for the 3 cohorts exceed 90%. For this analysis, baseline was the first year for which detailed information was available on diet, physical activity, and smoking—1986 in the NHS and HPFS and 1991 in the NHS II. As described previously (4), we excluded participants at baseline who were already obese [BMI (in kg/m2) ≥30], aged >65 y, had missing or implausible data (energy intake <900 kcal/d or >3500 kcal/d, 9 or more blank responses on the diet questionnaire, or missing data on baseline weight), had prevalent chronic disease (cancer, cardiovascular disease, diabetes, renal disease, pulmonary disease, rheumatologic disorders, or ulcerative colitis), and were or became pregnant. In total, 120,784 generally healthy participants were included in this analysis, including 46,994 in the NHS, 47,928 in the NHS II, and 25,862 in the HPFS. To minimize reverse causation, we also censored participants during follow-up at age 65 y and 6 y before the diagnosis of cancer, cardiovascular disease, diabetes, renal disease, pulmonary disease, rheumatologic disorders, or ulcerative colitis. The study protocol was approved by the institutional review boards of Brigham and Women’s Hospital and Harvard School of Public Health.
Assessment of protein foods and glycemic load
In each cohort, usual dietary habits were assessed every 4 y by using validated food-frequency questionnaires (11–13) from which we calculated 4-y dietary change in total and subcategories of various meats, poultry, fish, dairy, nuts, and legumes (Supplemental Table 1). Butter, although not a significant source of protein, was included in the analysis of protein foods for completeness because it may be considered a dairy product and is of animal origin. Changes in carbohydrate amount and quality were assessed by using changes in GI and GL. The daily GI was calculated as a continuous value as the product of the carbohydrate content of each food per serving, the average daily servings of the food, and the GI, as described by Salmerón et al. (14). In a validation study of the food-frequency questionnaire, high correlations were reported for foods from which GI was calculated; furthermore, calculated and measured GIs for mixed meals have been reported to correlate well (15). GL was calculated as the daily GI times the total carbohydrate content per day of the diet. Therefore, GL accounts for both the GI and the carbohydrate amount (grams of available carbohydrate) of each food item, summed across all foods consumed (10). Examples of carbohydrate-rich foods items on the food-frequency questionnaire include white bread, muffins or biscuits, pancakes or waffles, white rice, chocolate, candy bars, cookies, brownies, cake, pies, and pretzels.
Assessment of weight change
Weight and height were collected via self-report on the questionnaire: weight was asked every 2 y, and height was collected once in 1976 for the NHS, 1991 for the NHS II, and 1986 for the HPFS. BMI was calculated as the product of weight (kg) and inverse of height (m) squared. A validation study confirmed that self-reported weights correlated closely with actual body weight (R = 0.96) (16). Weight change was calculated every 4 y.
Statistical analysis
We assessed the simultaneous association between changes in protein foods and weight changes within 4-y periods over the follow-up period of 24 y in the NHS, 16 y in the NHS II, and 24 y in the HPFS by using generalized estimating equations, with an unstructured correlation matrix, to account for within-individual repeated measures across follow-up (e.g., 6 time periods for the NHS, 4 time periods for the NHS II, and 6 time periods for the HPFS). We adjusted for age; baseline BMI in each 4-y period; changes in all protein food simultaneously (see Supplemental Table 1 for the list and definition of all protein foods); changes in intake of fruit, vegetables, fried foods consumed at home, fried foods consumed away from home, trans fats, physical activity (metabolic equivalents h/wk), smoking status (current, former, or never), and alcohol intake; and hours of television watching and sleep. Changes in dietary habits were evaluated as continuous variables censored at the 0.5th and 99.5th percentiles to minimize the influence of outliers. We used carried-forward values for missing dietary and weight values (range: 14–51%); dropping missing values from the analysis demonstrated similar findings (data not shown). Lifestyle covariates were coded as categorical variables and included in the model as indicator variables, with missing values coded by using a missing indicator category. When subcategories of foods were included in the model (e.g., low-fat and regular cheese or regular and lean hamburger), the main category (total cheese, total hamburger) was removed from the model.
Correlations of changes in protein foods with each other and with changes in carbohydrate were assessed by using Pearson correlations. The potential modifying effect of changes in GL on the relation between changes in protein foods and weight gain was evaluated in a model including indicator categories for GL, an indicator for protein change (increase or no change/decrease), and their interaction. The GL indicator categories included 1) no change in GL, 2) minimal (−10- to +10-unit change excluding 0) change in GL, 3) an increase of >10 units in GL, and 4) and decrease of <−10 units in GL. For comparison, a GL of 10 units represents about one-third of an SD of GL in our cohorts or one slice (30 g) of white bread (10). P-trend for the interaction was evaluated in a separate model by using the multiplicative term between an ordinal variable for categories of GL change (see above) and continuous change in protein food. In all analyses, no significant interactions between protein change and GL change were seen for any protein food when its intake was decreased; therefore, results from the interaction model are shown only for an increase in protein intake. Findings were evaluated both separately by cohort and pooled across cohorts by using inverse variance–weighted random-effects meta-analysis with SAS 9.2 (SAS Institute). A P value <0.05 was considered statistically significant.
RESULTS
Baseline characteristics
Weight, lifestyle, and dietary characteristics at baseline and changes during 16–24 y of follow-up are shown in Supplemental Table 2. At baseline, women in the NHS were age (mean ± SD) 48.9 ± 2.7 y; women in the NHS II, 37.7 ± 3.2 y; and men in the HPFS, 47.3 ± 2.7 y. During follow-up, women in the NHS II gained +2.1 kg (4.7 lb)/4 y, women in the NHS gained +1.1 kg (2.3 lb)/4 y, and men in the HPFS gained +0.7 kg (1.6 lb)/4 y. Among different protein foods, total dairy was the most frequently consumed (∼2 servings/d), followed by red meat (∼0.5 servings/d).
Intercorrelations between changes in protein foods and changes in carbohydrate
Across all 3 cohorts, the 4-y changes in intakes of different protein foods in the 1986–1990 (NHS and HPFS) or 1991–1995 (NHS II) time periods were uncorrelated with each other (Supplemental Table 3; see Supplemental Table 2 for baseline levels of carbohydrate intake): nearly all were >|0.10|. A few exceptions were seen between protein foods that might be commonly consumed together or substituted for one another: for example, changes in bacon and eggs were positively correlated (r = 0.24), whereas changes in low-fat milk and whole milk (r = −0.25) were inversely correlated. Intercorrelations between protein foods were similar within each 4-y period across follow-up (data not shown).
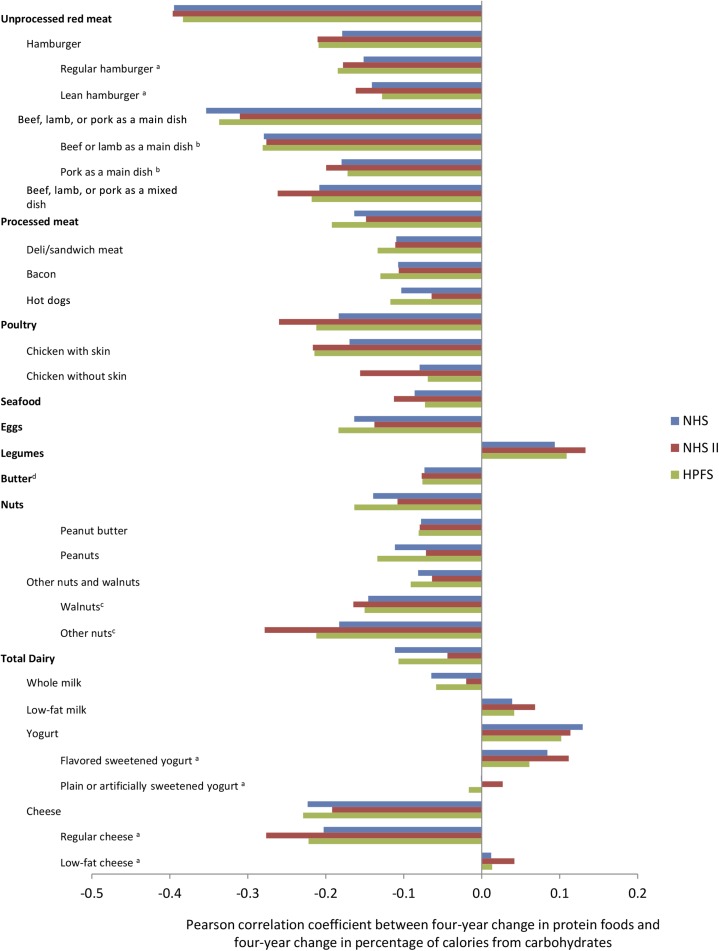
In contrast, changes in nearly every protein food were inversely correlated with changes in carbohydrate at baseline, with negative correlations ranging from −0.06 (whole milk) to −0.39 (unprocessed red meat) (Figure 1, individual cohort results; Supplemental Figure 1, pooled results). Exceptions were legumes (r = 0.10), low-fat milk (r = 0.04), yogurt (r = 0.12), flavored sweetened yogurt (r = 0.09), plain or artificially sweetened yogurt (r = 0.01), and low-fat cheese (r = 0.03), and each positively correlated with changes in carbohydrate. In other words, individuals in each cohort generally exchanged protein sources with carbohydrate-rich foods, rather than with other protein foods, and ate more carbohydrate when they increased their intake of low-fat dairy foods. These correlations remained consistent across follow-up (data not shown).
FIGURE 1.
Pearson correlation between 4-y change in protein foods and 4-y change in percentage of calories from carbohydrates. Pearson correlation between baseline change in protein food intake and change in percentage of calories from carbohydrate, adjusted for age, from 120,784 US men and women from the NHS (baseline 1986–1990), NHS II (baseline 1991–1995), and HPFS (baseline 1986–1990). aBaseline for flavored sweetened yogurt, plain or artificially flavored yogurt, regular cheese, low-fat cheese, and regular hamburger and lean hamburger was 1994–1998 for the NHS and HPFS and 1995–1999 for the NHS II based on the first appearance on the FFQ. bBaseline for beef or lamb as a main dish and pork as a main dish was 1990–1994 for the NHS and HPFS based on the first appearance on the FFQ. cBaseline for walnuts and other nuts was 1998–2002 for the NHS and HPFS and 1999–2003 for the NHS II based on the first appearance on the FFQ. dButter, although not a significant source of protein, was included in the analysis of protein foods for completeness because it may be considered a dairy product and is of animal origin. All correlations had a P value of <0.0001 except for plain or artificially sweetened yogurt for the NHS (P = 0.84) and HPFS (P = 0.02) and low-fat cheese for the NHS (P = 0.02) and HPFS (P = 0.07). FFQ, food-frequency questionnaire; HPFS, Health Professionals Follow-Up Study; NHS, Nurses’ Health Study; NHS II, Nurses’ Health Study II.
Changes in carbohydrate amount and quality, changes in protein foods, and weight gain
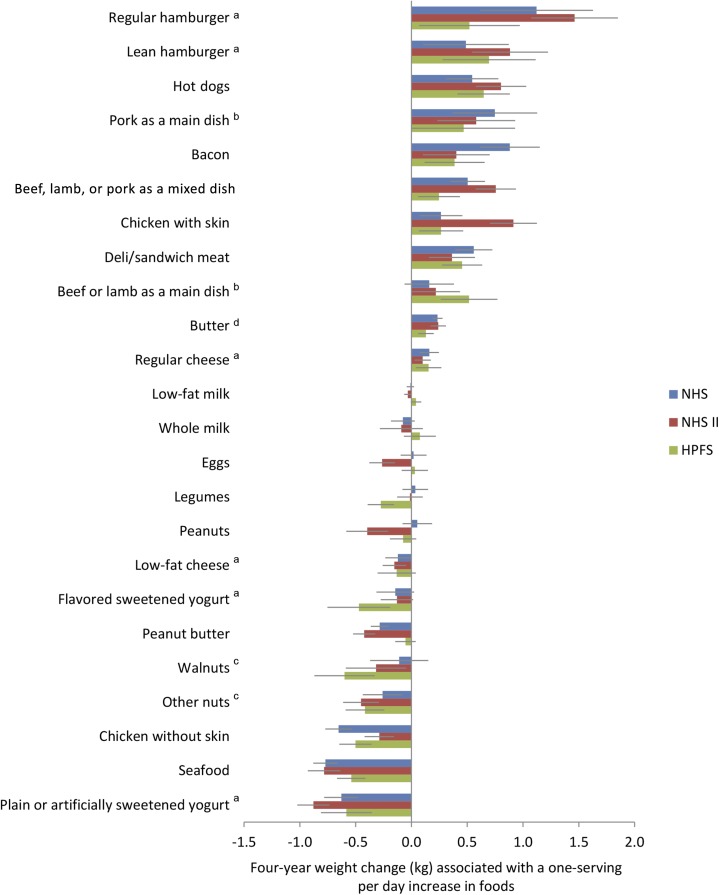
Changes in both the mean total GL and GI of the diet (multivariable adjusted, including changes in all protein foods) were positively related to weight gain (Table 1). Each 50-unit increase in GL was associated with +0.42 kg (0.93 lb); (95% CI: 0.24 kg, 0.60 kg) greater weight gain every 4 y and each 5-unit increase in GI with +0.35 kg (0.78 lb); (95% CI: 0.25 kg, 0.45 kg) greater weight gain. In contrast, changes in intakes of specific protein foods, adjusting for all protein foods simultaneously along with GL and other lifestyle characteristics, had very different associations with weight change, with highly consistent findings across the 3 cohorts (Figure 2 and Supplemental Table 4). Pooling all 3 cohorts, we found heterogeneous associations between protein foods and weight change, with increased intake of regular hamburger most strongly linked to weight gain [per increased servings/d, +1.03 kg (2.28 lb); (95% CI: 0.45 kg, 1.61 kg)] and increased intake of plain or artificially sweetened yogurt most strongly linked to relative weight loss [−0.71 kg (−1.56 lb); (95% CI: −0.90 kg, −0.52 kg)]. Because of conceivable confounding by french fry intake, we further adjusted the change in regular hamburger intake for change in french fry intake in the NHS cohort and found similar results [4-y weight gain +0.88 kg (1.94 lb); (95% CI: 0.37 kg, 1.39 kg)].
TABLE 1.
Association between changes in glycemic load or glycemic index and long-term weight change1
| Multivariable-adjusted weight change (95% CI) every 4 y (kg)2 | P value | |
| Change in glycemic load, per 50 units3 | ||
| Nurses’ Health Study | 0.41 (0.37, 0.44) | <0.001 |
| Nurses’ Health Study II | 0.59 (0.54, 0.63) | <0.001 |
| Health Professionals Follow-Up Study | 0.26 (0.22, 0.31) | <0.001 |
| Pooled | 0.42 (0.24, 0.60) | <0.001 |
| Change in glycemic index, per 5 units4 | ||
| Nurses’ Health Study | 0.31 (0.27, 0.34) | <0.001 |
| Nurses’ Health Study II | 0.45 (0.41, 0.49) | <0.001 |
| Health Professionals Follow-Up Study | 0.30 (0.24, 0.35) | <0.001 |
| Pooled | 0.35 (0.25, 0.45) | <0.001 |
Data are based on 24 y of follow-up (1986–2010) for 46,994 women in the Nurses' Health Study and 25,862 men in the Health Professionals Follow-Up Study, as well as 16 y of follow-up (1991–2007) for 47,928 women in the Nurses' Health Study II.
Mean and 95% CI of the weight changes shown are for increased glycemic load and glycemic index; decreased glycemic load/index would be associated with the inverse weight change. To convert from kilograms to pounds, multiply by 2.2. The multivariable model included age; baseline (of each 4-y period) BMI; sleep duration; changes in smoking status, physical activity, television watching, and alcohol consumption; and changes in servings/d of red meat, processed meat, poultry, seafood, eggs, legumes, butter, nuts, dairy, fruit, vegetables, fried foods consumed at home, fried foods consumed away from home, and trans fats.
For reference, the SD of glycemic load in our 3 cohorts is ∼25 units, and a slice (30 g) of white bread has a glycemic load of 10 units.
For reference, the SD of glycemic index in our 3 cohorts is ∼2 units, and a slice (30 g) of white bread has a glycemic index of ∼70 (compared with a standard dose of glucose) (10).
FIGURE 2.
Association between 4-y changes in servings of protein foods with long-term weight change. Data are based on 16–24 y of follow-up for 46,994 women in the NHS, 47,928 women in the NHS II, and 25,862 men in the HPFS. The 4-y weight changes are reported for each 1-serving/d increase in protein foods. Decreased protein food intake would be associated with the inverse weight changes. To convert kilograms to pounds, multiply by 2.2. All weight changes were adjusted for age, baseline (of each 4-y period) BMI, sleep duration, and change in smoking status, physical activity, television watching, alcohol consumption, fruit intake, vegetable intake, glycemic load, and the shown dietary factors. Because of their first appearance on the food-frequency questionnaire, data were available from a1994–2010 for the NHS and HPFS and 1995–2007 for the NHS II, b1990–2010 for the NHS and HPFS for the indicated foods, and c1998–2010 for the NHS and HPFS and 1999–2007 for the NHS II. Only subcategories of protein foods (e.g., lean hamburger and regular hamburger, not total unprocessed red meat) are shown, and protein foods have been ordered according to descending effect size. dButter, although not a significant source of protein, was included in the analysis of protein foods for completeness because it may be considered a dairy product and is of animal origin. HPFS, Health Professionals Follow-Up Study; NHS, Nurses’ Health Study; NHS II, Nurses’ Health Study II.
Associations with weight gain (or weight loss) were generally similar within each broad category of protein food (e.g., among different unprocessed red meats, among different processed meats, and among different nuts). The exceptions were poultry [for chicken with skin, a 1-serving/d increase was associated with +0.48 kg (1.06 lb); (95% CI: 0.06 kg, 0.90 kg) weight gain every 4 y, whereas for chicken without skin, a 1-serving/d increase was associated with −0.48 kg (−1.06 lb); (95% CI: −0.70 kg, −0.27 kg) relative weight loss every 4 y] and different types of dairy products [as reported previously (4)]. Results were not appreciably altered when GI was used in the model in place of GL (Supplemental Table 4) or with further adjustment for intake of total dietary fiber (data not shown).
Interaction between changes in protein foods, GL, and weight change
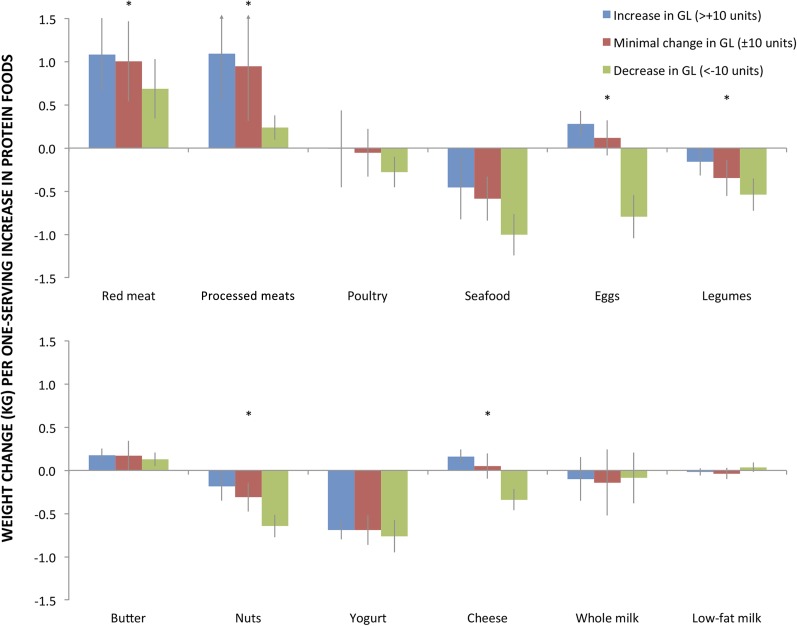
For several protein foods, associations with weight gain significantly varied (P-interaction < 0.05) depending on concurrent changes in GL (Figure 3 and Supplemental Table 5). For many protein foods positively associated with weight gain (e.g., unprocessed red meat, processed meat), a concomitant increase in GL augmented the weight gain. Conversely, when intakes of these foods were increased but GL was decreased (i.e., consistent with these foods at least partly replacing higher GL carbohydrates), the associated weight gain was significantly smaller. Conversely, for many protein foods associated with relative weight loss (e.g., nuts, seafood, plain yogurt), a concurrent increase in GL generally reduced the amount of weight loss, whereas a decrease in GL accentuated it. Notably, some foods had opposing relations with weight gain, depending on whether GL increased or decreased. A 1-serving/d increase in eggs was associated with +0.28 kg (0.61 lb); (95% CI: 0.12 kg, 0.43 kg) weight gain when GL was simultaneously increased, yet −0.79 kg (−1.75 lb); (95% CI: −1.04 kg, −0.54 kg) relative weight loss when GL was simultaneously decreased (P-interaction < 0.001). Similar findings were seen for cheese.
FIGURE 3.
Interaction between change in protein foods and change in GL in association with long-term weight change. Data are based on pooled results from 120,784 US men and women from 3 prospective cohorts with 16–24 y of follow-up. The 4-y weight gain across follow-up is shown for a 1-serving/d increase in protein food adjusted for age; baseline (of each 4-y period) BMI; sleep duration; change in smoking status, physical activity, television watching, alcohol consumption, and the shown dietary factors; change in fruit, vegetable, and trans fat intake; and indicator variables for GL change, protein change, and their interaction. The GL indicator categories included 1) no change in GL, 2) minimal change, 3) increase, and 4) decrease. For comparison, a 10-unit change in GL represents about one-third of an SD of GL change in our cohorts or one slice (30 g) of white bread (10). No significant interactions between protein food change and GL change were seen when protein food intake decreased (data not shown). Butter, although not a significant source of protein, was included in the analysis of protein foods for completeness because it may be considered a dairy product and is of animal origin. *P-trend ≤ 0.01 tested in a separate model as the multiplicative interaction term between the ordinal change in carbohydrate and the continuous change in protein food. To convert kilograms to pounds, multiply by 2.2. GL, glycemic load.
Interactions with GL were generally similar among subtypes of unprocessed red meats, processed meats, nuts, and yogurt. Some differences were seen for interactions among subtypes of poultry (with skin vs. without) and cheese (regular vs. low-fat) (Supplemental Table 5). In sensitivity analyses, we found that testing the interaction of protein foods with other metrics of carbohydrate amount and quality, including energy-adjusted GL, percentage of calories from carbohydrate, or GI, did not substantially change our results. Indeed, observed interactions for these other metrics of carbohydrate intake resulted in GL being a conservative estimate of the interaction between carbohydrate and protein, except for legumes and nuts, which showed a slightly greater interaction with GL (Supplemental Table 5).
DISCUSSION
In these 3 large prospective cohorts of US men and women, we identified 3 key and consistent findings. First, whereas dietary guidelines emphasize exchanging different protein foods with each other (e.g., chicken for meat), we found that these foods were most frequently exchanged with carbohydrate. Second, changes in carbohydrate amount and quality (measured by GL or GI) and some protein foods were independently associated with long-term weight gain, whereas other protein foods were independently associated with relative weight loss. Third, concomitant changes in GL modified the relations of most protein foods with long-term weight gain, in some cases substantially. These findings suggest that attention to types of protein foods as well their dietary replacements, especially carbohydrate-rich foods, is crucial for long-term weight maintenance.
The importance of carbohydrate amount and quality in promoting long-term weight gain has been debated, with inconsistent epidemiologic findings for the role of GI and GL in long-term weight gain (17, 18). These prior studies evaluated only baseline GI or GL, rather than changes in intake over time. Our recent methodology work suggests that the association between prevalent, baseline intake and weight change may be biased (19). In the present work, we identified strong, consistent, and positive associations between changes in GL and GI and long-term weight gain. These results are supported by findings from weight-loss intervention trials reporting improved weight loss and weight-loss maintenance with decreased dietary GL or GI (5, 6). Furthermore, several studies have identified positive associations between foods higher in GI and GL, especially refined grains, and weight gain, as well as inverse associations between foods lower in GI and GL, such as whole grains, fruit, and vegetables, and weight loss (4, 20–23). However, not all studies have found associations between GL or GI and weight loss, which may be due to differences in the diet composition of the control/comparison group, dietary adherence/compliance of the participants, or dietary composition/definition of what constitutes a high-GI vs. low-GI diet (24–26).
Higher-protein, lower-GI diets may confer a metabolic advantage during weight-loss maintenance (6), perhaps partly based on higher resting energy expenditure (5). Compared with lower-GI meals, higher-GI meals may also induce smaller satiating effects and larger activation of reward and craving areas of the brain, leading to increased long-term overconsumption and weight gain (27). Together, these mechanistic studies support our observations that GI and GL independently promote weight gain, as well as the potential that dietary protein and GL may synergistically influence long-term weight.
In the long term, most people appear to be expanding the share of their plate for carbohydrates when protein foods are decreased or vice versa. The notable exceptions were low-fat dairy foods, for which changes in intake positively correlated with carbohydrate intake. These patterns of exchange remained consistent across the 16–24 y of follow-up, decreasing the likelihood that these results are due to participants adopting or abandoning low-fat or low-carbohydrate eating patterns. These findings provide novel and important evidence that lowering the fat content of dairy or other foods may simply lead to increased carbohydrate consumption and explain why associations with weight gain were generally not different for lower-fat vs. higher-fat versions of foods. Whether these findings in adults are generalizable to all ages should be investigated, particularly since it was recently reported that children who consume more low-fat milk have greater weight gain than those who consume whole milk (28).
The significant interactions between changes in protein foods and changes in GL also appear relevant. For foods associated with weight gain, the association was augmented when GL was also increased and blunted when GL was decreased. The inverse was true for foods associated with relative weight loss. Based on this, eating meat with white bread, white rice, or potatoes (high-GL foods) might augment its obesogenic effects, whereas eating meat in place of these starches would partly mitigate such effects. Conversely, eating seafood or yogurt with refined grains, starches, or sugars might reduce their protection against weight gain, whereas eating these foods in place of high-GI carbohydrates might accentuate the benefits. Equally interesting were findings for beans, eggs, and cheese, for which observed relations with weight gain were abolished or even reversed depending on concurrent changes in GL. Our results suggest that consuming cheese in place of refined grains, starches, or sugars could reduce weight gain, whereas eating cheese together with high-GL foods might promote long-term weight gain. Thus, rather than focusing on total calories alone, individuals should consider the types of protein foods and the concurrent quality of carbohydrate-rich foods they are choosing.
We previously reported that changes in intake of different protein foods were differentially linked to weight gain (4), and some of these findings have been confirmed by other investigators (29). However, some of our findings are incongruent with other publications (21). This may be due to methodologic differences, particularly our use of dietary change, rather than baseline dietary intake, as our exposure, which may produce a less biased association with weight change (19). The present investigation builds on and substantially extends these prior findings by evaluating several additional types and subtypes of protein foods. Subtypes of red meat and processed meat had consistent associations with weight gain, whereas subtypes of nuts had consistent associations with relative weight loss. Subtypes of poultry, chicken with skin and chicken without skin, were associated with long-term weight gain and weight loss, respectively. The potential mechanisms to explain this difference are unclear: our findings were adjusted for changes in consumption of fried foods, and changes in poultry were not strongly correlated with changes in other dietary factors. Therefore, the findings for poultry require further investigation.
Aging can alter body composition, thereby reducing the correlation between increased BMI and excess adiposity (30). Using waist circumference, rather than BMI (or body weight), in an older population would more adequately reflect adiposity (31); however, we did not have 4-y repeated measures of waist circumference in the NHS, NHS II, and HPFS cohorts. We have taken measures to reduce the bias from loss of muscle mass, however; all participants were younger than 65 y at baseline, and their data were censored once they reached age 65 or 6 y before the diagnosis of serious illness to account for preclinical disease muscle loss. Although overall consumption of greater dietary protein may be linked to preserved muscle mass in older adults (32), we saw heterogeneous associations between protein foods and weight change; that is, some protein foods were associated with weight gain, whereas others were associated with relative weight loss, suggesting that the more likely explanation is due to changes in adiposity.
Our investigation has several strengths. We comprehensively analyzed novel relations among multiple protein foods, GL and GI, and their interactions. We included 3 separate cohorts with large numbers of participants and extended follow-up, increasing statistical power and providing cross-validation of findings. Our statistical power allows us to detect small changes in weight within each 4-y period, which, although relatively small for each time period, can cumulatively lead to substantial changes in body weight over the long term. It is exactly these small changes in weight that we want to detect since they are what is typically seen in the population (i.e., weight changes of ∼1 lb or 0.45 kg/y). The repeated, validated measures of diet allowed assessment of changes in dietary intake, reducing the possibly of reverse causation (19). Our 4-y assessment periods are consistent with the physiologic time courses of weight change after a change in diet (33). Last, analyses were simultaneously adjusted for multiple lifestyle and dietary factors, reducing the influence of residual confounding.
Potential limitations should be considered. Despite adjustment for numerous relevant dietary and lifestyle factors, residual confounding remains a possibility. Yet, most dietary changes were weakly intercorrelated, reducing the likelihood of substantial confounding by diet, although confounding by general health consciousness and dietary restriction is possible. Dietary choices could reflect reverse causation—for example, an individual gaining weight might choose to decrease intake of less healthy foods or increase intake of healthier foods. Such reverse causation would cause bias in the opposite direction as our observed findings, reducing the magnitude of the true associations. Both dietary habits and weight changes are measured with some error, which could lead to underestimation of true effects or, for covariates, produce bias in unpredictable directions. We used the GI of individual foods to estimate the overall dietary GI, which some reports have suggested are not well correlated (34), although others have found good correlations between the GI of a mixed meal and the GI of the individual food components of that meal (9). Finally, results may not be generalizable to other populations; however, our findings were consistent across 3 separate cohorts, suggesting that they reflect a true biologic effect.
In conclusion, protein foods appeared to be commonly interchanged with carbohydrate, rather than each other, and concomitant changes in protein foods and carbohydrate appear to interact together to influence long-term weight gain.
Acknowledgments
The authors’ responsibilities were as follows—JDS and DM: contributed to the design of the study and drafted the manuscript; JDS, DSL, EBR, WW, FBH, and DM: contributed to the analysis and interpretation of the data; TH: developed the statistical analysis plan; JDS: prepared figures and tables; TH, DSL, EBR, WW, FBH, and DM: revised the manuscript for critically important intellectual content; and all authors: approved the final version of this manuscript for publication. JDS had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. None of the authors had any conflicts of interest to disclose. The funders of this study had no role in its design or conduct; in the collection, management, analysis, or interpretation of data; or in the preparation, review, or approval of the manuscript.
Footnotes
Abbreviations used: GI, glycemic index; GL, glycemic load; HPFS, Health Professionals Follow-Up Study; NHS, Nurses’ Health Study; NHS II, Nurses’ Health Study II.
REFERENCES
- 1.US Department of Agriculture, US Department of Health and Human Services. Dietary guidelines for Americans, 2010. 7th ed. Washington (DC): Government Printing Office, 2010. [Google Scholar]
- 2.Department of Health and Human Services, Food and Drug Administration. Food Labeling: Revision of the Nutrition and Supplement Facts Label; Proposed Rule. Federal Register March 3 2014, volume 79, number 41. [cited 2015 Mar 30.] Available from: https://www.federalregister.gov/articles/2014/03/03/2014-04387/food-labeling-revision-of-the-nutrition-and-supplement-facts-labels.
- 3.Department of Health and Human Services, Food and Drug Administration. Food Labeling: Nutrition Labeling of Standard Menu Items in Restaurants and Similar Retail Establishments; Calorie Labeling of Articles of Food in Vending Machines; Final Rule. Federal Register December 1 2014, volume 79, number 230. [cited 2015 Mar 30]. Available from: https://www.federalregister.gov/articles/2014/12/01/2014-27833/food-labeling-nutrition-labeling-of-standard-menu-items-in-restaurants-and-similar-retail-food. [PubMed]
- 4.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med 2011;364:2392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ebbeling CB, Swain JF, Feldman HA, Wong WW, Hachey DL, Garcia-Lago E, Ludwig DS. Effects of dietary composition on energy expenditure during weight-loss maintenance. JAMA 2012;307:2627–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larsen TM, Dalskov SM, van Baak M, Jebb SA, Papadaki A, Pfeiffer AF, Martinez JA, Handjieva-Darlenska T, Kunesova M, Pihlsgard M, et al. Diets with high or low protein content and glycemic index for weight-loss maintenance. N Engl J Med 2010;363:2102–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas DE, Elliott EJ, Baur L. Low glycaemic index or low glycaemic load diets for overweight and obesity. Cochrane Database Syst Rev 2007;(3):CD005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Westerterp-Plantenga MS, Lemmens SG, Westerterp KR. Dietary protein—its role in satiety, energetics, weight loss and health. Br J Nutr 2012;108(Suppl 2):S105–12. [DOI] [PubMed] [Google Scholar]
- 9.Ludwig DS. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA 2002;287:2414–23. [DOI] [PubMed] [Google Scholar]
- 10.Atkinson FS, Foster-Powell K, Brand-Miller JC. International tables of glycemic index and glycemic load values: 2008. Diabetes Care 2008;31:2281–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feskanich D, Marshall J, Rimm EB, Litin LB, Willett WC. Simulated validation of a brief food frequency questionnaire. Ann Epidemiol 1994;4:181–7. [DOI] [PubMed] [Google Scholar]
- 12.Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, Rosner B, Willett WC. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol 1989;18:858–67. [DOI] [PubMed] [Google Scholar]
- 13.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 1985;122:51–65. [DOI] [PubMed] [Google Scholar]
- 14.Salmerón J, Manson JE, Stampfer MJ, Colditz GA, Wing AL, Willett WC. Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. JAMA 1997;277:472–7. [DOI] [PubMed] [Google Scholar]
- 15.Chew I, Brand JC, Thorburn AW, Truswell AS. Application of glycemic index to mixed meals. Am J Clin Nutr 1988;47:53–6. [DOI] [PubMed] [Google Scholar]
- 16.Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology 1990;1:466–73. [DOI] [PubMed] [Google Scholar]
- 17.Du H, van der A DL, van Bakel MM, Slimani N, Forouhi NG, Wareham NJ, Halkjaer J, Tjonneland A, Jakobsen MU, Overvad K, et al. Dietary glycaemic index, glycaemic load and subsequent changes of weight and waist circumference in European men and women. Int J Obes (Lond) 2009;33:1280–8. [DOI] [PubMed] [Google Scholar]
- 18.Hare-Bruun H, Flint A, Heitmann BL. Glycemic index and glycemic load in relation to changes in body weight, body fat distribution, and body composition in adult Danes. Am J Clin Nutr 2006;84:871–9, quiz 952–3. [DOI] [PubMed] [Google Scholar]
- 19.Smith JD, Hou T, Hu FB, Rimm EB, Spiegelman D, Willett WC, Mozaffarian D. A comparison of different methods to evaluate diet, physical activity, and long-term weight gain in three prospective cohort studies. Circulation 2013;127:AP091 (abstr.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koh-Banerjee P, Rimm EB. Whole grain consumption and weight gain: a review of the epidemiological evidence, potential mechanisms and opportunities for future research. Proc Nutr Soc 2003;62:25–9. [DOI] [PubMed] [Google Scholar]
- 21.Fogelholm M, Anderssen S, Gunnarsdottir I, Lahti-Koski M.. Dietary macronutrients and food consumption as determinants of long-term weight change in adult populations: a systematic literature review. Food Nutr Res 2012;56 (Epub 2012 Aug 13; DOI: 10.3402/fnr.v56i0.19103). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koh-Banerjee P, Franz M, Sampson L, Liu S, Jacobs DR Jr, Spiegelman D, Willett W, Rimm E. Changes in whole-grain, bran, and cereal fiber consumption in relation to 8-y weight gain among men. Am J Clin Nutr 2004;80:1237–45. [DOI] [PubMed] [Google Scholar]
- 23.Liu S, Willett WC, Manson JE, Hu FB, Rosner B, Colditz G. Relation between changes in intakes of dietary fiber and grain products and changes in weight and development of obesity among middle-aged women. Am J Clin Nutr 2003;78:920–7. [DOI] [PubMed] [Google Scholar]
- 24.Esfahani A, Wong JM, Mirrahimi A, Villa CR, Kendall CW. The application of the glycemic index and glycemic load in weight loss: a review of the clinical evidence. IUBMB Life 2011;63:7–13. [DOI] [PubMed] [Google Scholar]
- 25.Juanola-Falgarona M, Salas-Salvado J, Ibarrola-Jurado N, Rabassa-Soler A, Diaz-Lopez A, Guasch-Ferre M, Hernandez-Alonso P, Balanza R, Bullo M. Effect of the glycemic index of the diet on weight loss, modulation of satiety, inflammation, and other metabolic risk factors: a randomized controlled trial. Am J Clin Nutr 2014;100:27–35. [DOI] [PubMed] [Google Scholar]
- 26.Aller EE, Larsen TM, Claus H, Lindroos AK, Kafatos A, Pfeiffer A, Martinez JA, Handjieva-Darlenska T, Kunesova M, Stender S, et al. Weight loss maintenance in overweight subjects on ad libitum diets with high or low protein content and glycemic index: the DIOGENES trial 12-month results. Int J Obes (Lond) 2014;38:1511–7. [DOI] [PubMed] [Google Scholar]
- 27.Lennerz BS, Alsop DC, Holsen LM, Stern E, Rojas R, Ebbeling CB, Goldstein JM, Ludwig DS. Effects of dietary glycemic index on brain regions related to reward and craving in men. Am J Clin Nutr 2013;98:641–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scharf RJ, Demmer RT, DeBoer MD. Longitudinal evaluation of milk type consumed and weight status in preschoolers. Arch Dis Child 2013;98:335–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang H, Troy LM, Rogers GT, Fox CS, McKeown NM, Meigs JB, Jacques PF. Longitudinal association between dairy consumption and changes of body weight and waist circumference: the Framingham Heart Study. Int J Obes (Lond) 2014;38:299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Villareal DT, Apovian CM, Kushner RF, Klein S. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Am J Clin Nutr 2005;82:923–34. [DOI] [PubMed] [Google Scholar]
- 31.Janssen I, Katzmarzyk PT, Ross R. Waist circumference and not body mass index explains obesity-related health risk. Am J Clin Nutr 2004;79:379–84. [DOI] [PubMed] [Google Scholar]
- 32.Houston DK, Nicklas BJ, Ding J, Harris TB, Tylavsky FA, Newman AB, Lee JS, Sahyoun NR, Visser M, Kritchevsky SB, et al. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the Health, Aging, and Body Composition (Health ABC) Study. Am J Clin Nutr 2008;87:150–5. [DOI] [PubMed] [Google Scholar]
- 33.Hall KD, Sacks G, Chandramohan D, Chow CC, Wang YC, Gortmaker SL, Swinburn BA. Quantification of the effect of energy imbalance on bodyweight. Lancet 2011;378:826–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flint A, Moller BK, Raben A, Pedersen D, Tetens I, Holst JJ, Astrup A. The use of glycaemic index tables to predict glycaemic index of composite breakfast meals. Br J Nutr 2004;91:979–89. [DOI] [PubMed] [Google Scholar]