Abstract
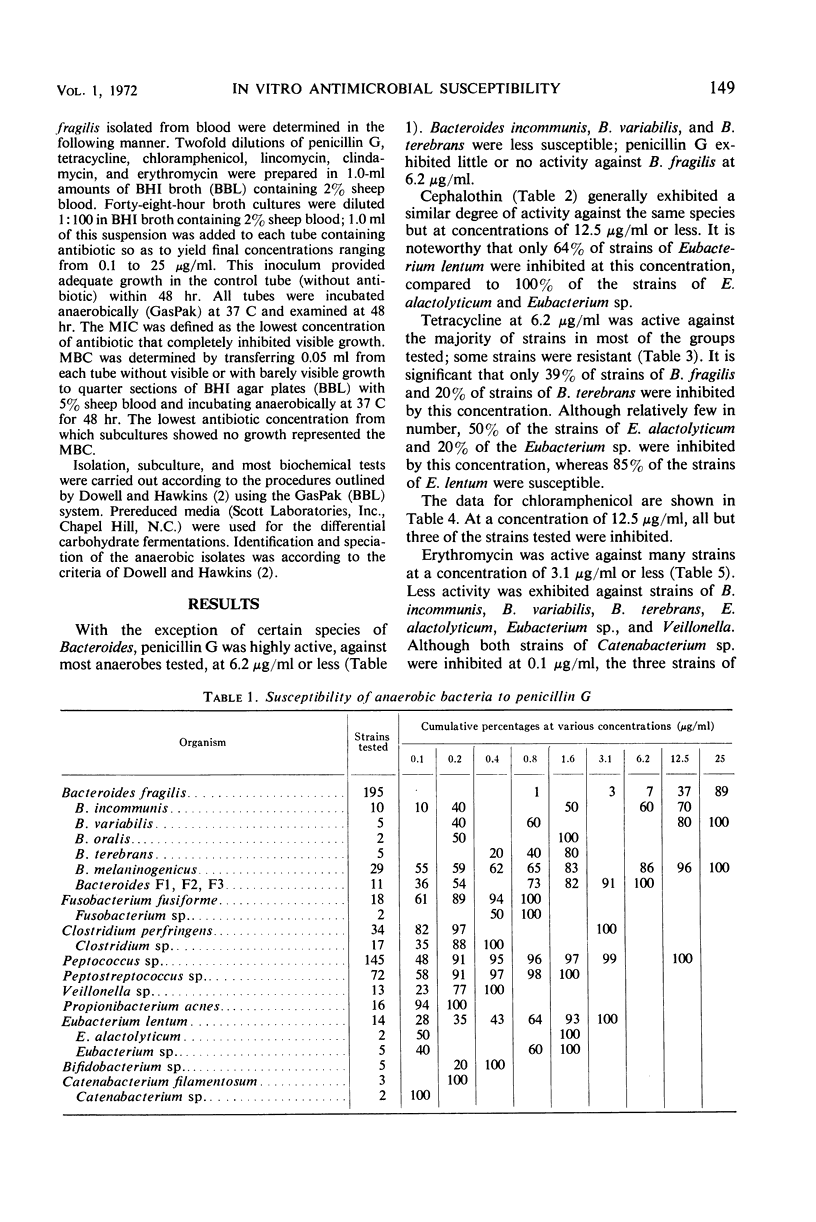
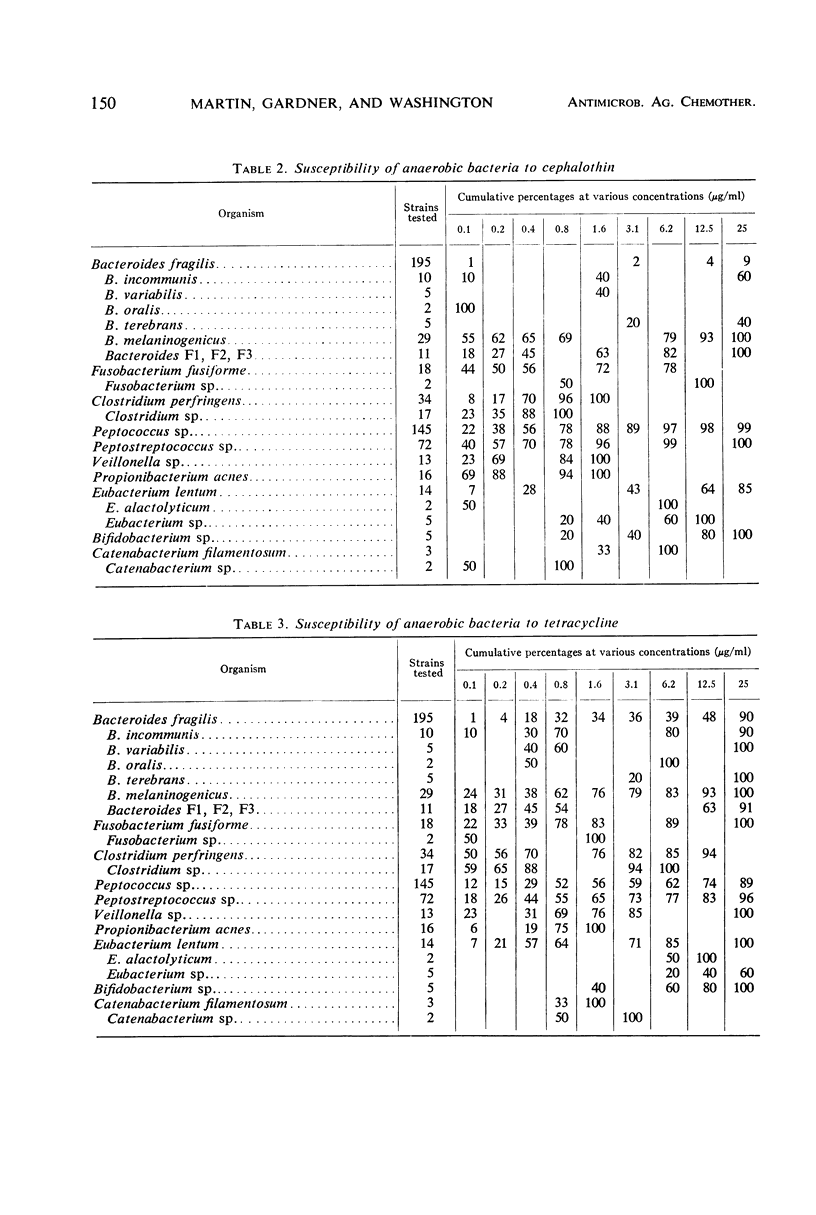
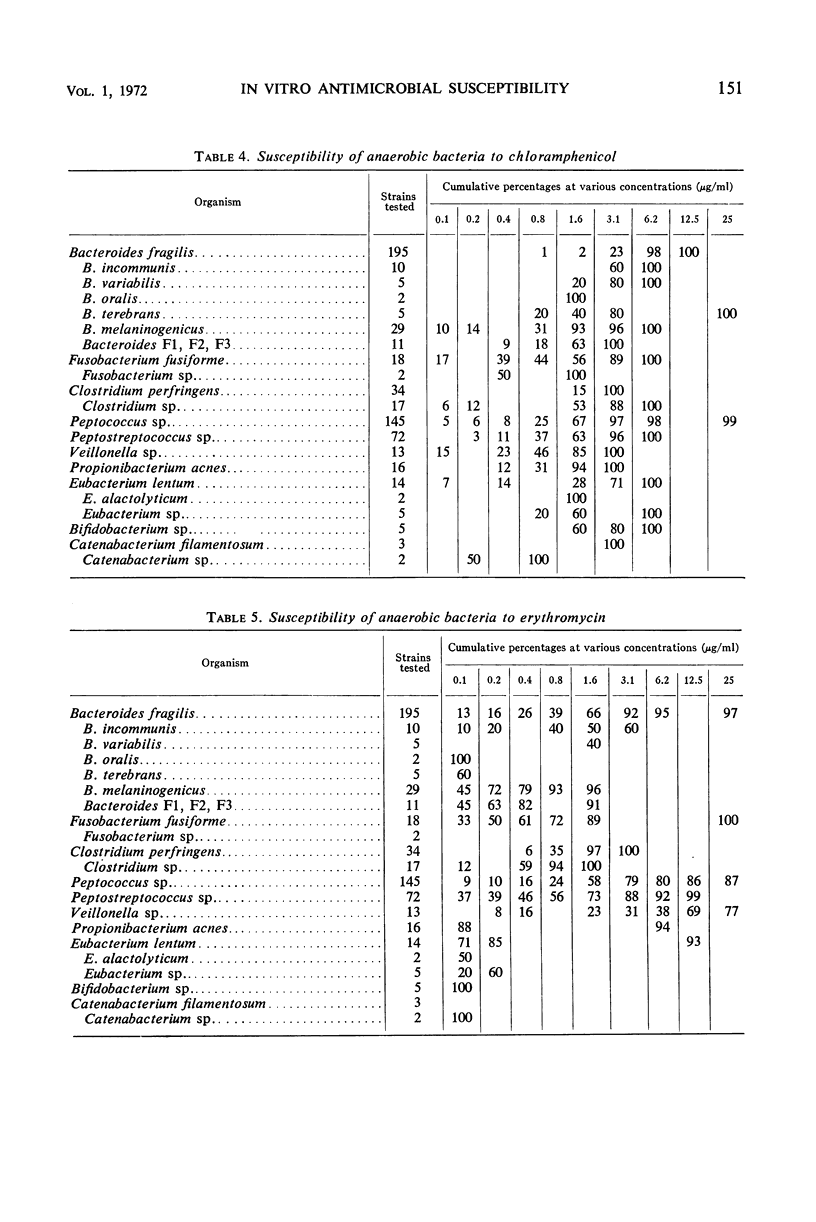
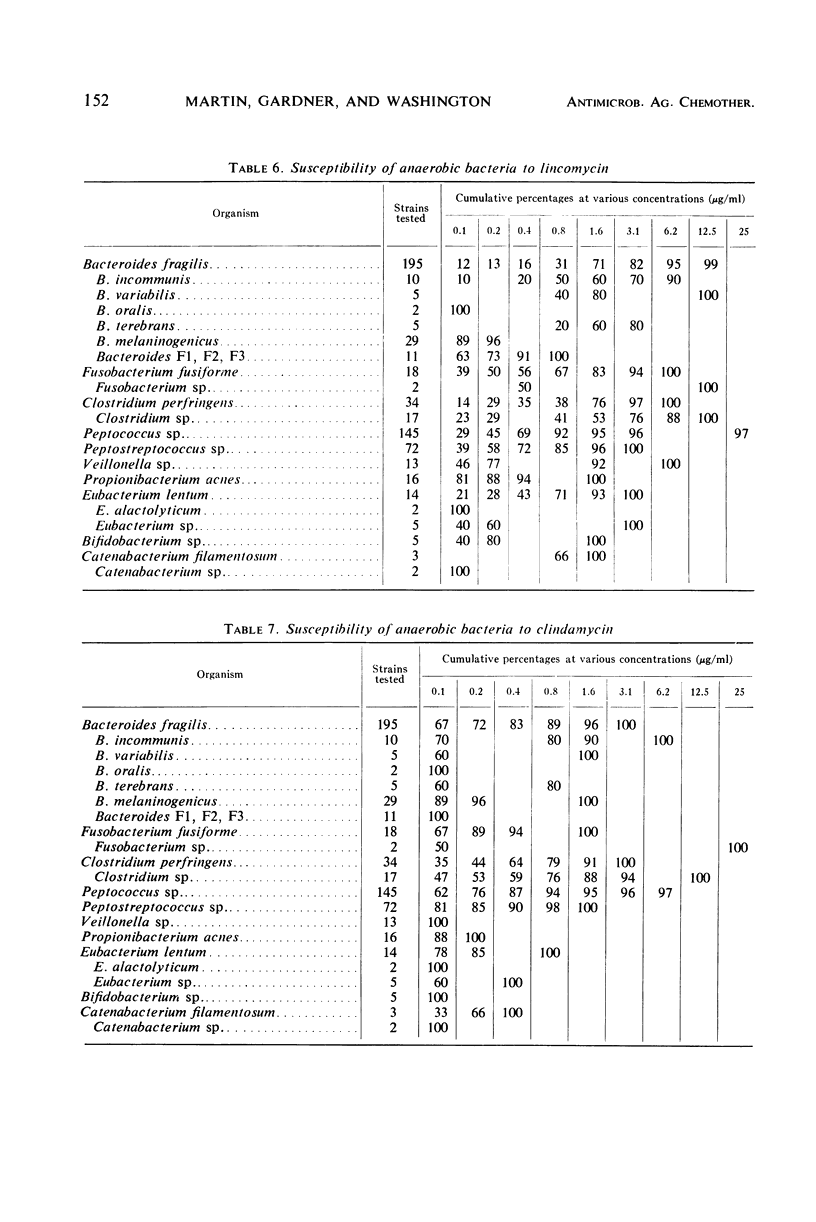
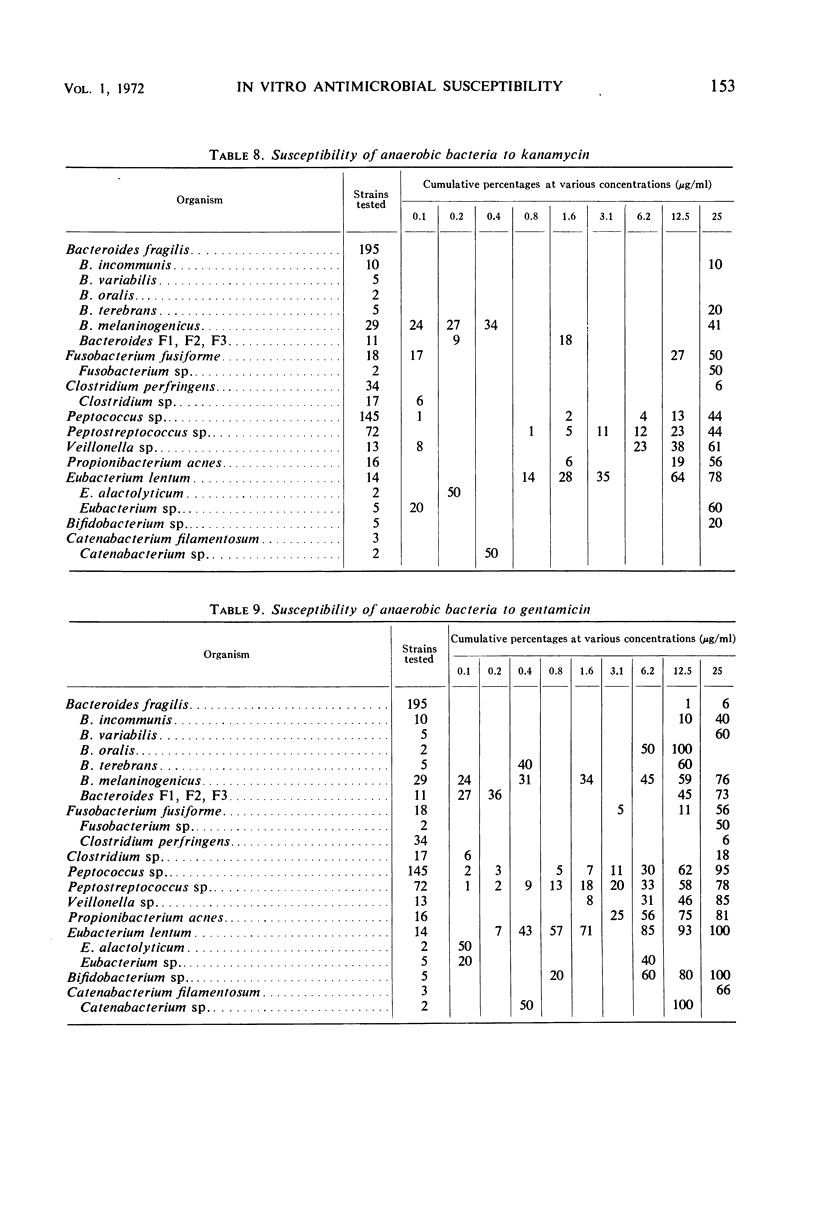
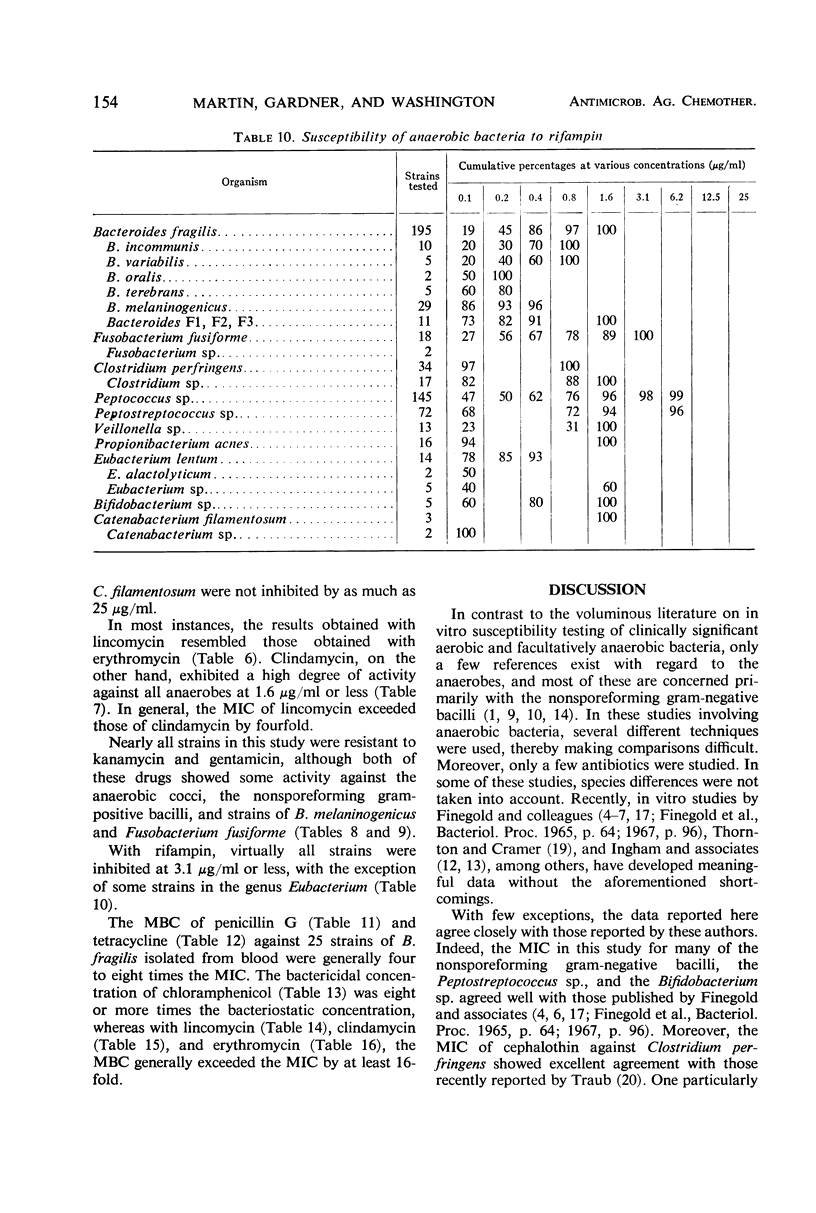
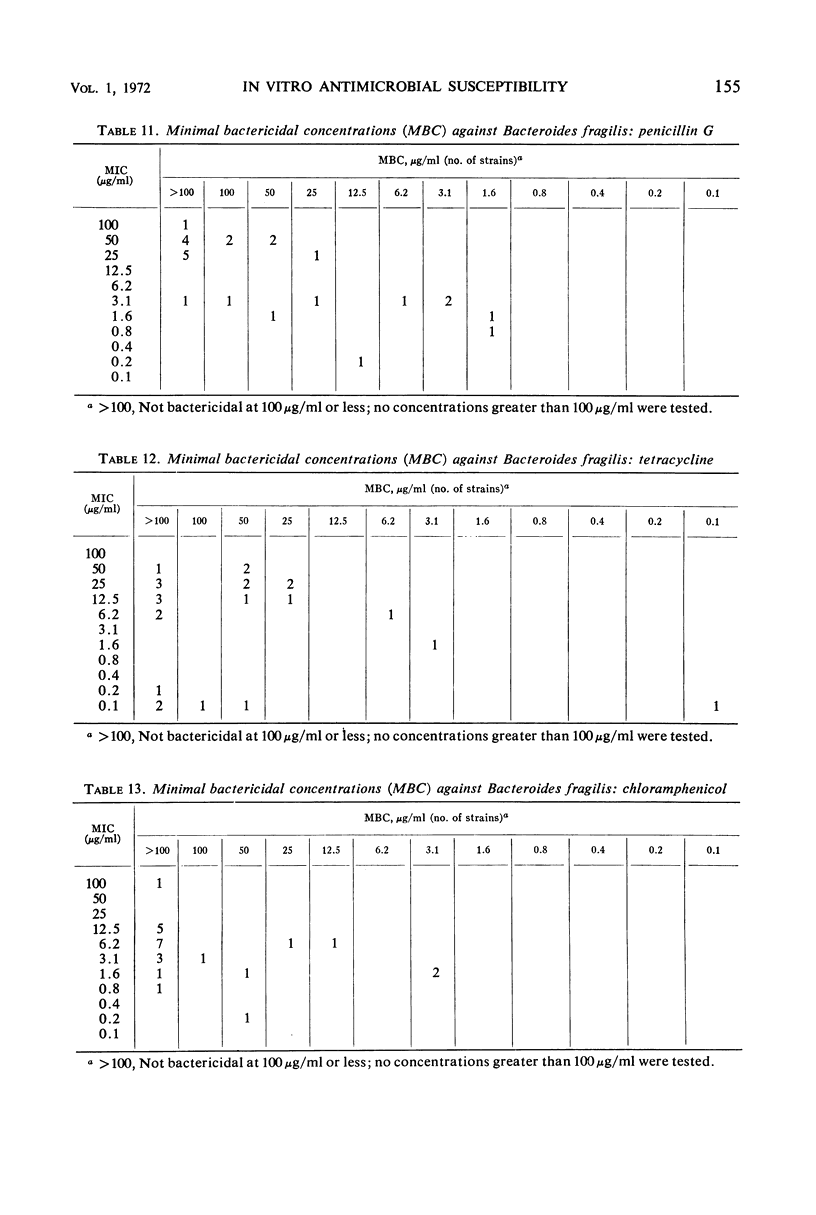
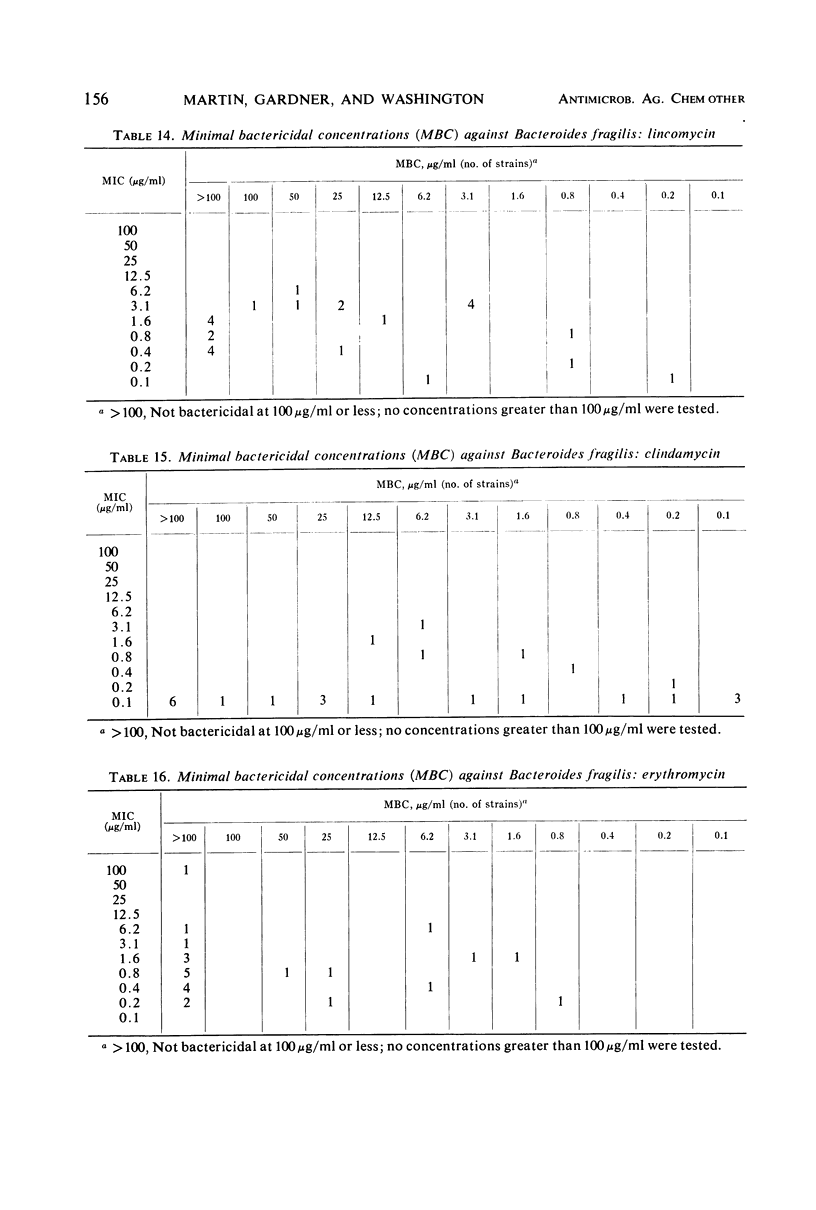
The minimal inhibitory concentrations of 601 clinical isolates of anaerobic bacteria to 10 different antimicrobial agents were determined by an agar-dilution technique. Nearly all strains were resistant to kanamycin and gentamicin, although moderate activity to both drugs was noted with Fusobacterium sp., anaerobic cocci, some strains of Bacteroides melaninogenicus, and nonsporeforming gram-positive bacilli. Chloramphenicol at 12.5 μg/ml inhibited all but three of the strains tested. Tetracycline at 6.25 μg/ml had high activity against all groups tested, with the exception that only 39% of strains of Bacteroides fragilis were inhibited at this concentration. Excluding certain species of Bacteroides, the majority of anaerobes were inhibited by penicillin at 3.1 μg/ml or less and by cephalothin at 12.5 μg/ml or less. Lincomycin at 6.2 μg/ml or less was active against nearly all strains. Erythromycin at a concentration of 3.1 μg/ml was active against B. fragilis; however, erythromycin was less active against the other groups. Most of the minimal inhibitory concentrations of lincomycin exceeded those of clindamycin by fourfold. Rifampin inhibited virtually all strains at 3.1 μg/ml.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodner S. J., Koenig M. G., Goodman J. S. Bacteremic Bacteroides infections. Ann Intern Med. 1970 Oct;73(4):537–544. doi: 10.7326/0003-4819-73-4-537. [DOI] [PubMed] [Google Scholar]
- Finegold S. M., Harada N. E., Miller L. G. Antibiotic susceptibility patterns as aids in classification and characterization of gram-negative anaerobic bacilli. J Bacteriol. 1967 Nov;94(5):1443–1450. doi: 10.1128/jb.94.5.1443-1450.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegold S. M., Harada N. E., Miller L. G. Lincomycin: activity against anaerobes and effect on normal human fecal flora. Antimicrob Agents Chemother (Bethesda) 1965;5:659–667. [PubMed] [Google Scholar]
- GARROD L. P. Sensitivity of four species of bacteroides to antibiotics. Br Med J. 1955 Dec 24;2(4955):1529–1531. doi: 10.1136/bmj.2.4955.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILLESPIE W. A., GUY J. Bacteroides in intra-abdominal sepsis: their sensitivity to antibiotics. Lancet. 1956 Jun 30;270(6931):1039–1041. doi: 10.1016/s0140-6736(56)90802-9. [DOI] [PubMed] [Google Scholar]
- Gardner M., Martin W. J. Simplified method of anaerobic incubation. Appl Microbiol. 1971 Jun;21(6):1092–1092. doi: 10.1128/am.21.6.1092-1092.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogendijk J. L. Resistance of some strains of Bacteroides to ampicillin, methicillin and cloxacillin. Antonie Van Leeuwenhoek. 1965;31(4):383–385. doi: 10.1007/BF02045917. [DOI] [PubMed] [Google Scholar]
- Ingham H. R., Selkon J. B., Codd A. A., Hale J. H. A study in vitro of the sensitivity to antibiotics of Bacteroides fragilis. J Clin Pathol. 1968 Jul;21(4):432–436. doi: 10.1136/jcp.21.4.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham H. R., Selkon J. B., Codd A. A., Hale J. H. The effect of carbon dioxide on the sensitivity of Bacteroides fragilis to certain antibiotics in vitro. J Clin Pathol. 1970 Apr;23(3):254–258. doi: 10.1136/jcp.23.3.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagnoff M. F., Armstrong D. Antibiotics for bacteroides sepsis. Ann Intern Med. 1971 Aug;75(2):316–317. doi: 10.7326/0003-4819-75-2-316_2. [DOI] [PubMed] [Google Scholar]
- Keusch G. T., O'Connell C. J. The susceptibility of bacteroides to the penicillins and cephalothin. Am J Med Sci. 1966 Apr;251(4):428–432. doi: 10.1097/00000441-196604000-00007. [DOI] [PubMed] [Google Scholar]
- Martin W. J. Practical method for isolation of anerobic bacteria in the clinical laboratory. Appl Microbiol. 1971 Dec;22(6):1168–1171. doi: 10.1128/am.22.6.1168-1171.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L. G., Finegold S. M. Antibacterial sensitivity of Bifidobacterium (Lactobacillus bifidus). J Bacteriol. 1967 Jan;93(1):125–130. doi: 10.1128/jb.93.1.125-130.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton G. F., Cramer J. A. Antibiotic susceptibility of bacteroides species. Antimicrob Agents Chemother (Bethesda) 1970;10:509–513. [PubMed] [Google Scholar]
- Traub W. H. The susceptibility of Clostridium perfringens type A to cephalosporin C antibiotics in vitro. Chemotherapy. 1971;16(1):11–17. doi: 10.1159/000220710. [DOI] [PubMed] [Google Scholar]


