Abstract
Objective
We present two families that were identified with novel mutations in LOXHD1, as a cause of non-progressive hearing loss.
Methods
One thousand three hundred fourteen (1,314) Japanese subjects with sensorineural hearing loss from unrelated families were enrolled in the study. Targeted genomic enrichment and massively parallel sequencing of all known non-syndromic hearing loss genes were performed to identify the genetic cause of hearing loss.
Results
Two patients in one family affected with homozygous mutation; c.879+1G>A in LOXHD1, showed profound congenital hearing loss, whereas two patients in the other family with compound heterozygous mutations; c.5869G>T (p.E1957X) and c.4480C>T (p.R1494X) showed moderate to severe hearing loss.
Conclusion
Mutations in LOXHD1 are extremely rare, and these cases are the first identified in a Japanese population. The genotype-phenotype correlation in LOXHD1 is still unclear. The differences of phenotypes in each patient might be the result of the nature of the mutations, or the location at the gene, or be influenced by genetic modifier.
Keywords: Hearing loss, genetics, LOXHD1, DFNB77, massively parallel sequencing
INTRODUCTION
Sensorineural hearing loss (SNHL) associated with autosomal recessive inheritance is commonly supposed to have a phenotypic feature resulting in severe to profound hearing loss. The most common genetic cause of hearing loss is GJB2, affected with prelingual severe to profound SNHL, and most of these cases occur with non-progressive hearing loss [1]. Several studies have reported that some genetic causes associated with recessive inheritance might be naturally occurring progressive hearing loss, such as SLC26A4, CDH23 and MYO3A. The LOXHD1 gene mapped at chromosome 18q12-21 is known to be the cause of DFNB77, a progressive form of autosomal recessive non-syndromic SNHL [2]. Mutations in LOXHD1 have been extremely rare; only five reports, six pedigrees. The LOXHD1 consists of 2,211 amino acids that encodes 15 polycystin-1/lipoxygenase/alpha-toxin (PLAT) domains. Each single PLAT domain consists of about 120 amino acids, and interacts with plasma membrane. In the mouse study, Loxhd1 was expressed along the membrane of mature hair cell stereocilia. It played an important role in maintaining normal hair cell function in the cochlea [2]. SNHL, caused by the mutations in LOXHD1, had a phenotypic feature showing progressive hearing loss at mid to high frequencies during childhood [2,3]. On the other hand, non-progressive congenital profound hearing loss has been reported as well [4].
Here, we describe two families that were identified with novel mutations in LOXHD1, as a cause of progressive hearing loss using massively parallel sequencing (MPS).
SUBJECTS and METHODS
Subjects
We recruited two groups from a Japanese hearing loss population for this study. All subjects had presumed non-syndromic SNHL. For each proband, informed consent was obtained to participate in this study, which was approved by the human subjects ethical committee associated with each clinic. Clinical information and blood samples were obtained for each proband and for all consenting affected and unaffected relatives.
The first group – 1,120 Japanese samples:
One thousand one hundred twenty (1,120) Japanese hearing loss patients (ADSNHL: 266, ARSNHL: 600, unknown: 254) from 53 ENT departments nationwide participated.
The second group –194 Japanese samples:
One hundred ninety four (194) Japanese subjects from unrelated families were ascertained through 33 otolaryngology clinics in 28 prefectures across Japan.
Methods
The first group – 1,120 Japanese samples:
Amplicon Library Preparation
Amplicon libraries were prepared using an Ion AmpliSeq™ Custom Panel (Applied Biosystems, Life Technologies) for 63 genes reported to cause non-syndromic hearing loss according to the manufacturers’ instructions. The detailed protocol was described elsewhere [5]. After preparation, the amplicon libraries were diluted to 20pM and equal amounts of 6 libraries for 6 patients were pooled for one sequence reaction.
Emulsion PCR and Sequencing
Emulsion PCR and sequencing were performed according to the manufacturer’s instructions. The detailed protocol was described elsewhere [5]. MPS was performed with an Ion Torrent Personal Genome Machine (PGM) system using the Ion PGM™ 200 Sequencing Kit and Ion 318™ Chip (Life Technologies).
The second group – 194 Japanese samples:
Targeted Genomic Enrichment and Massively Parallel Sequencing
Genomic DNA was assessed for quality by gel electrophoresis and spectrophotometry (Nanodrop 1000; Thermo Fisher Scientific, Waltham, MA; 260/280 ratio of 1.8-2.2) and quantity by fluorometry (Qubit 2.0 Fluorometer; Life Technologies, Carlsbad, CA). TGE of all exons of all genes implicated in non-syndromic SNHL, including non-syndromic SNHL mimics, was completed as described, targeting 89 genes as part of the OtoSCOPE® v5 platform. Libraries were prepared using a modification of the solution-based Agilent SureSelect target enrichment system (Agilent Technologies, Santa Clara, CA) [6]. Of the 194 samples, 58 samples were processed manually; the remainder was prepared robotically using the Sciclone NGS Workstation.
In brief, 3μg gDNA was randomly fragmented to an average size of 250 bp (Covaris Acoustic Solubilizer; Covaris Inc., Woburn, MA), fragment ends were repaired, A-tails were added, and sequencing adaptors were ligated before the first amplification. Solid phase reverse immobilization purifications were performed between each enzymatic reaction. Hybridization and capture with RNA baits was followed by a second amplification before pooling for sequencing. Minimal amplification was used – typically 8 cycles for the pre-hybridization PCR (range 8–10 cycles) using NEB Phusion HF Master Mix (New England BioLabs Inc, Ipswich, MA) and 14 cycles for the post-hybridization PCR (range 12–16 cycles) using Agilent Herculase II Fusion DNA Polymerase. All samples were barcoded and multiplexed before sequencing on either an Illumina MiSeq or HiSeq (Illumina Inc, San Diego, CA) in pools of 4–6 or 48, respectively, using 100-bp paired-end reads.
Base Call and Data Analysis
The first group – 1,120 Japanese samples:
The sequence data were mapped to the human genome sequence (build GRCh37/hg19) with a Torrent Mapping Alignment Program. After the sequence mapping, the DNA variant regions were piled up with Torrent Variant Caller plug-in software. After variant detection, variant effects were analyzed using the ANNOVAR software [7,8]. The missense, nonsense, insertion/deletion and splicing variants were selected from among the identified variants. Variants were further selected as less than 1% of 1) the 1,000 genome database (http://www.1000genomes.org/), 2) the 6,500 exome variants (http://evs.gs.washington.edu/EVS/), 3) the Human Genetic Variation Database (dataset for 1,208 Japanese exome variants) (http://www.genome.med.kyoti-u.ac.jp/SnpDB/index.html), and 4) the 269 in-house Japanese normal hearing controls.
To predict the pathogenicity of missense variants, the following functional prediction software was used; PhyloP (http://hgdownload.cse.ucsc.edu/goldenPath/hg18/phyloP44way/), Sorting Intolerant from Tolerant (SIFT; http://sift.jcvi.org/), Polymorphism Phenotyping (PolyPhen2; http://genetics.bwh.harvard.edu/pph2/), LRT (http://www.genetics.wustl.edu/jflab/lrt_query.html), MutationTaster (http://www.mutationtaster.org/), and GERP++ (http://mendel.stanford.edu/SidowLab/downloads/gerp/index.html).
Candidate mutations were confirmed by Sanger sequencing and the responsible mutations were identified by segregation analysis using samples from among the patients’ family members.
The second group – 194 Japanese samples:
Data were analyzed as described using a local installation of the open-source Galaxy software (http://galaxyproject.org) and the following open-source tools: BWA [9] for read mapping, Picard for duplicate removal, GATK [9] for local re-alignment and variant calling and NGSRich [11] for enrichment statistics [12]. We reported and annotated variants with custom software.
Variant Confirmation
All pathogenic variants were confirmed by Sanger sequencing and segregation analysis using exon-specific custom primers.
RESULT
We identified four patients, two families had causative mutations in LOXHD1 in 1,314 hearing loss Japanese.
Case Details
Family number 963; AH 4029, AH 4028
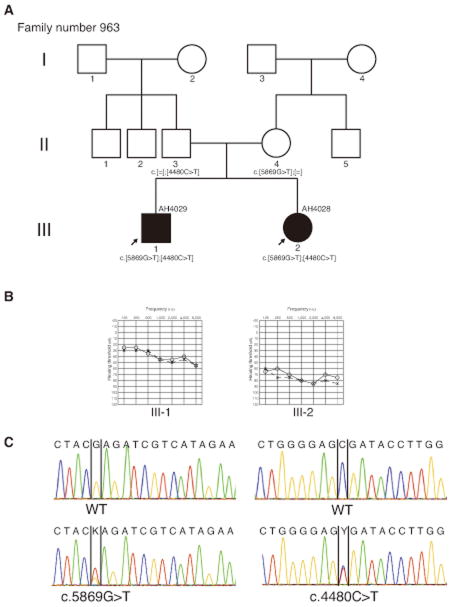
AH 4029 was a 9 year-old boy. He had not undergone a newborn hearing screening. At the age of 6, hearing loss was detected at a school check-up. He had been referred to the Iwate Medical University Hospital, Department of Otolaryngology for further examinations. His tympanic membranes were normal. An auditory brain-stem response (ABR) with click stimuli showed bilateral hearing loss that was approximately 40 dBnHL in both ears, and pure tone audiometry (PTA) showed bilateral moderate sensorineural hearing loss (Figure 1). He was promptly fitted with a left hearing aid when he was 7 years 3 months old. He showed 100% in word scores at the 50dB sound field threshold. His hearing was unchanged from 6 to 9 years old. He didn’t have any episodes of dizziness or vertigo attacks. Computed tomography (CT) findings of the middle and inner ear showed no abnormalities.
Figure 1.
Pedigree and clinical findings of family number 963. (A) Pedigree shows autosomal recessive inherited cases in this family. (B) Pure tone audiometry (PTA) shows bilateral moderate sensorineural hearing loss in AH 4029 and bilateral severe sensorineural hearing loss in AH 4028. (C) The electropherogram shows mutations in two cases. AH 4029 and AH4028 had identical compound heterozygous mutations; c.5869G>T, p.E1957X and c.4480C>T, p.R1494X. Each parent had one of the heterozygous mutations.
AH 4028 was a 3 year-old girl. She was a younger sister of AH4029, and her parents noticed that she didn’t have any intelligible words until she was 1 year 6 months old. She visited the Iwate Medical University Hospital at the same time as AH 4029 received his check-up. Her bilateral tympanic membranes were normal. A previous ABR test 2 years of age had shown bilateral severe hearing loss that was approximately 80 dBnHL in both ears, and PTA had also shown bilateral severe SNHL at the age of 3 (Figure 1). Her hearing had been unchanging from 3 to 5 years old. She started to wear a right hearing aid when she was 3 years old. CT findings of the middle and inner ear showed no abnormalities.
Family number 143; 2061, 2059
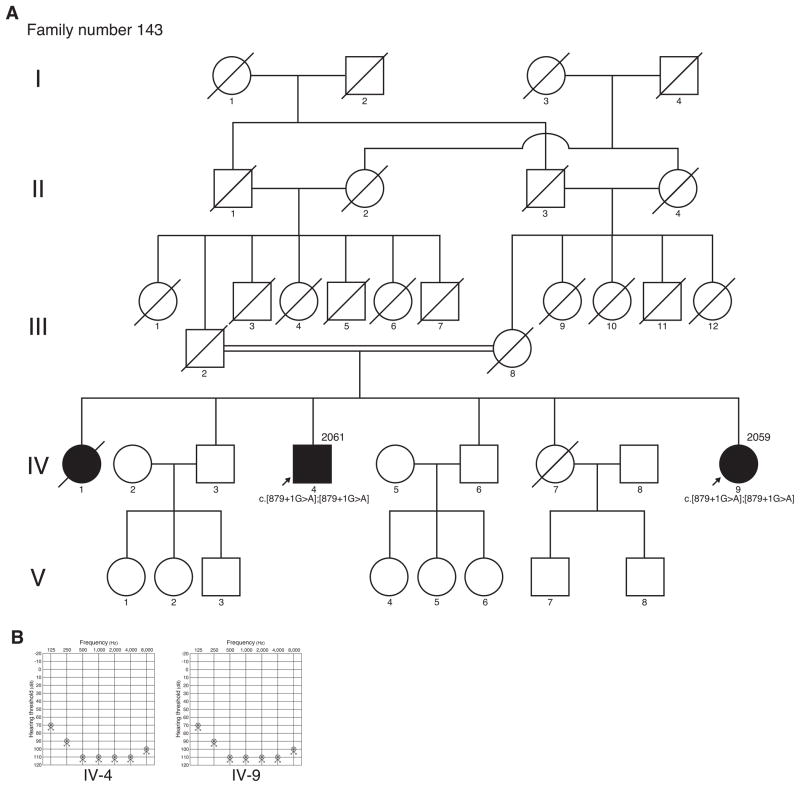
Patient 2061 was a 70 year-old male. He was born in 1929. PTA showed bilateral profound SNHL (Figure 2). In his medical examination by interview, he had congenital non-progressive hearing loss. He didn’t have any episodes of vertigo. He had not undergone CT of the temporal bone.
Figure 2.
Pedigree and clinical findings of family number 143. (A) Pedigree shows autosomal recessive inherited cases in this family. (B) Pure tone audiometry (PTA) shows bilateral profound sensorineural hearing loss in both cases.
Patient 2059 was a 60 year-old female. She was the younger sister of patient 2061 and born in 1939. PTA showed bilateral profound SNHL (Figure 2). In her medical examination by interview, she had congenital non-progressive hearing loss. She didn’t have any episodes of vertigo.
As shown in Figure 2, the pedigree tree showed consanguineous parental ancestry of patient 2061 and patient 2059 had profound hearing loss. They were both unmarried. We didn’t obtain any information about the symptoms of hearing loss during childhood because of World War II.
Mutation analysis
We identified compound heterozygous mutations; c.5869G>T, p.E1957X and c.4480C>T, p.R1494X in LOXHD1 gene in two patients of family number 963. We also identified homozygous splice site mutations; c.879+1G>A in LOXHD1 gene in two patients of family number 143.
DISCUSSION
We identified causative mutations with compound heterozygous p.R1494X and p.E1957X and homozygous c.879+1G>A in LOXHD1 gene. These are the first cases found in the Japanese population.
As shown in Table 1, we summarized the mutations in LOXHD1 that have been previously reported. The mutations of p.E1957X and c.879+1G>A were novel and p.R1494X had been reported in an American family [13]. LOXHD1 expresses along the plasma membrane of stereocillia. It is considered that LOXHD1 may couple the plasma membrane to underlying F-actin cytoskeleton. Although stereocilliary development was unaffected in a mouse model (samba mouse), hair cell function was disturbed and hair cell eventually degenerated [2].
Table 1.
Known mutations in LOXHD1 gene in hearing loss.
| Nucleotide change | Amino acid change | Domain | Type of mutation | Zygosity | NM number | HL on set | Type of HL | Progressiveness | Population | Refference |
|---|---|---|---|---|---|---|---|---|---|---|
| c.879+1G>A | Canonical-splice | Homozygous | NM_001145472 | Congenital | Profound | Non-progressive | Japanese | This study | ||
| c.1588C>T | p.E530X | PLAT4 | Nonsense | Homozygous | NM_144612 | Childfood | Severe-profound | Progressive | Qatar | Vozzi et al., 2014 |
| c.2008C>T | p.R670X | PLAT_repeat* | Nonsense | Homozygous | NM_144612 | Childfood | Moderate-profound | Progressive | Iranian | Grillet et al., 2009 |
| c.2863G>T | p.E955X | PLAT 7 | Nonsense | Homozygous | NM_144612 | n.a. | n.a. | n.a. | Turkey | Diaz-Horta et al., 2012 |
| c.4480C>T | p.R1494X | PLAT 11 | Nonsense | Homozygous | NM_144612 | n.a. | n.a. | n.a. | Turkey | Diaz-Horta et al., 2012 |
| c.4480C>T | p.R1494X | PLAT 11 | Nonsense | Heterozygous | NM_144612 | 40 y.o. | Severe-profound | Progressive | American | Eppsteiner et al., 2012 |
| c.4480C>T | p.R1494X | PLAT 11 | Nonsense | Heterozygous | NM_144612 | n.a. | Moderate-severe | Non-progressive | Japanese | This study |
| c.4526G>A | p.G1509E | PLAT 11 | Missense | Heterozygous | NM_144612 | 40 y.o. | Severe-profound | Progressive | American | Eppsteiner et al., 2012 |
| c.4714C>T | p.R1572X | PLAT 11 | Nonsense | Homozygous | NM_144612 | Pre-lingual | Severe-profound | Non-progressive | Ashkenazi Jews | Edvardson et al., 2011 |
| c.5869G>T | p.E1957X | PLAT 14 | Nonsense | Heterozygous | NM_144612 | n.a. | Moderate-severe | Non-progressive | Japanese | This study |
polycystin/lipoxygenase/alpha-toxin (PLAT)
In this study, affected individuals in family number 143 showed profound congenital SNHL, but affected individuals in family number 963 showed different degrees of SNHL; hearing loss in AH 4028 was about 30dB severer than in her older sibling. Vozzi et al. reported three patients, in a consanguineous family, who had early-onset progressive SNHL, which was differed in degree in spite of having the same genotype; homozygous nonsense alleles (c.1588G>T, p.E530X) [3]. On the other hand, non-progressive congenital SNHL was also reported in other homozygous nonsense alleles (c.4714C>T, p.R1572X) [4]. In samba mice, a homozygous missense mutation in Loxhd1 caused profound deafness shortly after birth. Nevertheless, homozygous nonsense mutation in Loxhd1 caused progressive hearing loss [2]. The cases in family number 143 had splice site mutations and were totally deafened by their sixties. It is possible that their hearing deteriorated in childhood, resulting in profound hearing loss at a younger age. The genotype-phenotype correlation in LOXHD1 is still unclear. The differences of phenotype in each affected individual might be the result of the nature of the mutations, the location at the gene, and affected by genetic modifier [14].
Two affected individuals in family number 963 were fitted with hearing aids, and both of them were able to benefit from them. If their hearing loss progresses in future, cochlear implant could be considered for them to acquire hearing ability. Eppsteiner et al. reported a patient with mutation in LOXHD1 was a good CI performer (HINT(90), CNC(73), combined(81.3)) [13].
Affected individuals in family number 963 and the family number 143 had no episodes of dizziness or vertigo attacks. Any vestibular dysfunction has yet to be reported. Grillet et al. described Loxhd1 expression was detected in stereocillia of vestibular hair cells. However, under immunofluorescence microscopy, the expression level in vestibular hair cells was much weaker than in cochlear hair cells [2]. Thus, LOXHD1 might not associate with the vestibular system.
Mutations in LOXHD1 had been reported that it was the responsible gene that caused late-onset Fuchs corneal dystrophy (FCD) [15]. FCD was a genetic disorder of the corneal endothelium and was the most common cause of corneal transplantation. Recently, Stehouwer et al. reported that rate of hearing disability in FCD group was higher than control group [16]. It suggested an association between FCD and hearing loss. It is important that hearing loss patients caused by LOXHD1 have an ophthalmology check-up. Furthermore, FCD cases and hearing loss should be screened LOXHD1 mutation. These phenotypic features are important for genetic counseling. Further following examination should be necessary for the relevance to hearing loss and FCD caused by LOXHD1 mutations.
In summary, we analyzed 1314 Japanese and identified four patients; three mutations in LOXHD1. It seems extremely rare, 0.30% in Japanese hearing loss patients. Candidate gene testing cannot be applicable for such a rare gene. MPS makes it possible to detect rare genes like LOXHD1.
Acknowledgments
We thank the participants of the Deafness Gene Study Consortium. We also thank Mr. Jim George and Mr. Sean Mehmet for help in preparing the manuscript.
Funding
This work was supported by NIDCD RO1s DC003544, DC002842 and DC012049 to RJHS. This study also was supported by a Health and Labour Sciences Research Grant for Research on rare and intractable diseases and Comprehensive Research on Disability Health and Welfare from the Ministry of Health, Labour and Welfare of Japan (S.U.), and by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan (S.U.).
Footnotes
Declaration of Conflicting Interests
All authors have declared no competing financial interests.
References
- 1.Hilgert N, Smith R, Van Camp G. Forty-six genes causing nonsyndromic hearing impairment: which ones should be analyzed in DNA diagnostics? Mutat Res. 2009;681(2–3):189–196. doi: 10.1016/j.mrrev.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grillet N, Schwander M, Hildebrand MS, et al. Mutations in LOXHD1, an evolutionarily conserved stereociliary protein, disrupt hair cell function in mice and cause progressive hearing loss in humans. Am J Hum Genet. 2009;85(3):328–337. doi: 10.1016/j.ajhg.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vozzi D, Morgan A, Vuckovic D, et al. Hereditary hearing loss: a 96 gene targeted sequencing protocol reveals novel alleles in a series of Italian and Qatari patients. Gene. 2014;542(2):209–216. doi: 10.1016/j.gene.2014.03.033. [DOI] [PubMed] [Google Scholar]
- 4.Edvardson S, Jalas C, Shaag A, et al. A deleterious mutation in the LOXHD1 gene causes autosomal recessive hearing loss in Ashkenazi Jews. Am J Med Genet A. 2011;155A(5):1170–1172. doi: 10.1002/ajmg.a.33972. [DOI] [PubMed] [Google Scholar]
- 5.Miyagawa M, Nishio SY, Ikeda T, Fukushima K, Usami S. Massively parallel DNA sequencing successfully identifies new causative mutations in deafness genes in patients with cochlear implantation and EAS. PLoS One. 2013;8(10):e75793. doi: 10.1371/journal.pone.0075793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shearer AE, Hildebrand MS, Smith RJ. Solution-based targeted genomic enrichment for precious DNA samples. BMC Biotechnol. 2012;12(1):20. doi: 10.1186/1472-6750-12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang X, Wang K. wANNOVAR: annotating genetic variants for personal genomes via the web. J Med Genet. 2012;49(7):433–436. doi: 10.1136/jmedgenet-2012-100918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variation from high-throughput sequencing data. Nucleic Acid Res. 2010;38(16):e164. doi: 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25(14):1754–60. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKenna A, Hanna M, Banks E, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20(9):1297–303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frommolt P, Abdallah AT, Altmuller J, et al. Assessing the enrichment performance in targeted resequencing experiments. Hum Mutat. 2012;33(4):635–41. doi: 10.1002/humu.22036. [DOI] [PubMed] [Google Scholar]
- 12.Shearer AE, Black-Ziegelbein EA, Hildebrand MS, et al. Advancing genetic testing for deafness with genomic technology. J Med Genet. 2013;50(9):627–34. doi: 10.1136/jmedgenet-2013-101749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eppsteiner RW, Shearer AE, Hildebrand MS, et al. Prediction of cochlear implant performance by genetic mutation: the spiral ganglion hypothesis. Hear Res. 2012;292(1–2):51–58. doi: 10.1016/j.heares.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McHugh RK, Friedman RA. Genetics of hearing loss: Allelism and modifier genes produce a phenotypic continuum. Anat Rec A Discov Mol Cell Evol Biol. 2006;288(4):370–381. doi: 10.1002/ar.a.20297. [DOI] [PubMed] [Google Scholar]
- 15.Riazuddin SA, Parker DS, McGlumphy EJ, et al. Mutations in LOXHD1, a recessive-deafness locus, cause dominant late-onset Fuchs corneal dystrophy. Am J Hum Genet. 2012;90(3):533–539. doi: 10.1016/j.ajhg.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stehouwer M, Bijlsma WR, Van der Lelij A. Hearing disability in patients with Fuchs’ endothelial corneal dystrophy: unrecognized co-pathology? Clin Ophthalmol. 2011;5:1297–1301. doi: 10.2147/OPTH.S23091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diaz-Horta O, Duman D, Foster J, 2nd, et al. Whole-exome sequencing efficiently detects rare mutations in autosomal recessive nonsyndromic hearing loss. PLoS One. 2012;7(11):e50628. doi: 10.1371/journal.pone.0050628. [DOI] [PMC free article] [PubMed] [Google Scholar]