Abstract
Introduction
Erythropoiesis-stimulating agents (ESAs) reduce red blood cell (RBC) transfusions in approximately 40% of patients with myelodysplastic syndrome (MDS) in clinical trials. We studied the association of timing of ESA initiation, agent (epoetin alfa, darbepoetin) and number of weeks of ESA use with response in MDS patients in routine practice.
Methods
Patients diagnosed with MDS from 2001–2005 were identified in the Surveillance Epidemiology and End Results-Medicare linked database. The study cohort consisted of patients with new-onset transfusion dependence (TD). All patients received an ESA at least once during the study period, which began the week that criteria for TD were met and continued until transfusion independence (TI). Kaplan-Meier statistics and Cox Proportional Hazard models were used to assess relationships between time to ESA initiation, agent and number of weeks of ESA use and TI attainment.
Results
Of 610 TD patients treated with ESAs, 210 (34.4%) achieved TI. Median time from ESA initiation to TI was 13 weeks. Shorter time from TD to ESA initiation and use of darbepoetin were associated with higher probability of achieving TI. The probability of achieving TI decreased beyond 8 weeks of treatment, and was very low beyond 16 weeks (8–15 weeks: HR=0.64, 16–31 weeks: HR=0.25, 32+ weeks HR=0.10).
Conclusions
In this observational, population-based study, variations in ESA administration impacted response in transfusion-dependent MDS patients, with higher response rates with early administration and use of darbepoetin, and low response likelihood in non-responders beyond 16 weeks of therapy.
Keywords: Myelodysplastic syndromes, erythropoiesis-stimulating agents, anemia, comparative effectiveness
1. INTRODUCTION
Anemia is present in at least 85% of patients with myelodysplastic syndromes (MDS) at diagnosis [1], and significantly impacts quality of life. Erythropoiesis-stimulating agents (ESAs) ameliorate anemia associated with MDS in approximately 20% of unselected patients and 40% of lower-risk patients [2, 3]. Response rates may be improved by selecting patients with low endogenous serum erythropoietin levels and low transfusion burden and by co-administering granulocyte colony-stimulating factor (G-CSF) [4–6].
While ESAs are commonly administered to MDS patients [7, 8] and a randomized phase III trial provided evidence of efficacy compared to best supportive care in lower-risk disease [9], MDS remains an unapproved indication for these agents. Most published trials of ESAs in MDS have used significantly higher doses of epoetin alfa than those used for other indications, in the range of 40,000–80,000 U/week [10, 11]. Thus response rates in routine practice may be lower than those reported in clinical trials if labeled dosing instructions for other indications are followed. The timing of ESA initiation may also affect response rates, as a shorter time interval between diagnosis and treatment with ESAs has been correlated with increased response rates [12]. Finally, the value of continuing ESAs in the absence of an early response is unclear. Treatment guidelines recommend modifying therapy in patients who have not manifested an increase in hemoglobin level or a decrease in red blood cell (RBC) transfusion requirements after ESA administration for 6 to 8 weeks [13, 14].
We used cancer registry data linked to Medicare claims to study the impact of variations in ESA administration on treatment response. Because the Surveillance Epidemiology and End Results (SEER)-Medicare database does not include data on blood counts, we limited the study population to patients receiving RBC transfusions and measured the impact of ESA therapy on cessation of transfusions.
2. METHODS
2.1 Study Population
MDS cases newly reported between 2001 and 2005 were identified from SEER data matched to Medicare enrollment and claims files [15]. Patients were required to have ≥1 claim for an ESA while transfusion-dependent (TD), as defined below. Patients were excluded if they had a history of chronic renal failure, if the diagnosis or death dates were not recorded, if they were not continuously enrolled in Medicare Parts A and B, or were enrolled in Medicare Advantage during the 12 months prior to, or any time after, diagnosis.
2.2 Study endpoints
A weekly measure of transfusion status was created based on the frequency and timing of claims for RBC transfusions over an 8-week period consisting of the current and preceding 7 weeks. Patients were categorized as TD if they had ≥ 2 weeks with a claim for RBC transfusion(s), with any two claims separated by at least two weeks. For example, a patient who received RBC transfusions during weeks 1, 2, and 4 would be considered transfusion-dependent, but a patient who received RBC transfusions only during weeks 1 and 2 would not. Patients were classified as transfusion-independent (TI) during 8-week periods in which they received no transfusions. This algorithm was repeated for every week of follow-up from MDS diagnosis. As it would be expected that anemia management would be initiated on or before acceleration of transfusion need, patients with claims for ESAs prior to TD (the look-back period) were included (Figure 1).
Figure 1. Study Design.
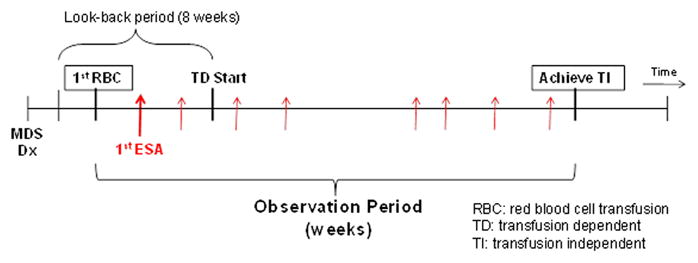
The observation period for the study began the first week in which an RBC transfusion defining TD occurred and ended at the earliest of the following: (1) achievement of TI, or censoring at (2) week 52, (3) 4 weeks prior to death, or (4) December 31, 2007. By definition, the RBC transfusion defining TD initiation occurred within the prior 8 week (look-back) period. We censored the observation period 4 weeks prior to death so that we did not incorrectly assign achievement of TI to patients for whom RBC transfusions were discontinued in anticipation of death.
The primary study endpoint was achievement of TI. The observation period for the study began the first week in which an RBC transfusion defining TD occurred and ended at the earliest of the following: (1) achievement of TI, or censoring at (2) week 52, (3) 4 weeks prior to death, or (4) December 31, 2007 (Figure 1).
2.3 ESA utilization measures and covariates
ESA utilization was calculated from weekly indicators of claims for an ESA product. Three variables were created from the weekly ESA claims measures: (1) the time from the beginning of TD to first observed ESA, (2) use of epoetin alfa, darbepoetin, or both; and (3) duration of ESA use, calculated by summing the weeks an ESA was received. We also characterized patients (yes/no) based on whether they had a claim for a serum erythropoietin determination, or any of the following while TD: G-CSF, hypomethylating agents (azacitidine or decitabine), or chemotherapy. We classified patients into lower-risk [refractory anemia, refractory anemia with ringed sideroblasts, refractory cytopenia with multilineage dysplasia or MDS with del(5q)], higher-risk (refractory anemia with excess blasts), therapy-related MDS, and MDS not otherwise specified (NOS). Demographic characteristics were abstracted from the SEER database; comorbidities were captured from diagnoses on claims.
2.4 Statistical analysis
The distribution of clinical characteristics during the study period and study covariates were compared between patients who did or did not achieve TI using Chi-square tests. Kaplan-Meier life tables and survival curves were constructed to illustrate the association between ESA utilization measures and study outcome. Differences by ESA utilization were tested for statistical significance (p<0.05) using the log rank test. Cox proportional hazards models were used to calculate hazard ratios (HR) for the study outcome, adjusting for covariates. Models examined the impact of choice of agent (epoetin alfa versus darbepoetin) and number of weeks of ESA use on the study outcome. As we did not expect a linear response between duration and likelihood of response, we grouped the ESA duration values into 8-week ranges. They included a variable to adjust for the time to initiation of ESA therapy following TD onset.
3. RESULTS
A total of 8,312 patients in the linked SEER-Medicare database had an MDS diagnosis. Among them, 2,024 had at least one TD episode. Within this group, 610 received ESAs while TD and comprise the study cohort. Patient characteristics are summarized in Table 1. The average age was 78.2 years (SD±7.78) and 56.9% were male. The majority (91.3%) were classified as “white” race. There was an approximately equal distribution of higher- and lower-risk MDS patients (25.1% and 27.9%, respectively), while 47.1% were classified as MDS-NOS or therapy-related MDS and could not be assigned a risk category. Comorbidities were common in this older population, particularly ischemic heart disease (35.7%), chronic obstructive pulmonary disease (COPD, 19.8%), and diabetes mellitus (17.5%). Among the 610 patients, 239 (47.4%) received less than 8 weeks of ESA therapy, 198 (32.5%) 8–15 weeks, 87 (14.3%) 16–31 weeks, and 36 (5.9%) more than 32 weeks until TI or censoring (Table 2). Most patients (79.5%) received epoetin alfa, while 12.1% received darbepoetin and 8.4% received both.
Table I.
Patient Characteristics (N=610)
| Characteristics | N | % |
|---|---|---|
| Age (years) | ||
| <65 | 16 | 2.6 |
| 65 – 74 | 160 | 26.2 |
| 75 – 84 | 318 | 52.1 |
| 85+ | 116 | 19.0 |
| Sex | ||
| Male | 347 | 56.9 |
| Female | 263 | 43.1 |
| Race | ||
| White | 557 | 91.3 |
| Black | 17 | 2.8 |
| Other | 36 | 5.9 |
| MDS risk category | ||
| Low-risk | 170 | 27.9 |
| High-risk | 153 | 25.1 |
| Risk not specified | 287 | 47.1 |
| Comorbidities | ||
| Congestive heart failure | 87 | 14.3 |
| Ischemic heart disease | 218 | 35.7 |
| Peripheral vascular disease | 39 | 6.4 |
| Cerebrovascular disease | 30 | 4.9 |
| Myocardial infarction | 32 | 5.3 |
| Diabetes or diabetes with complications | 107 | 17.5 |
| Chronic obstructive pulmonary disease | 121 | 19.8 |
| Rheumatologic disease | 20 | 3.3 |
| Depression or schizophrenia | 38 | 6.2 |
| Alzheimer’s disease | 30 | 4.9 |
| Ulcer | 15 | 2.5 |
Low-risk (RA, RARS, RCMD, 5q del); High-risk (RAEB); Risk not specified (Therapy-related MDS and MDS, NOS)
Table II.
Treatment Characteristics During Follow-up (N=610)a
| Characteristics | Study Sample
|
Stratified by TI achievement
|
||||
|---|---|---|---|---|---|---|
| No | Yes | |||||
| N=610 | N=400 | N=210 | ||||
| N | % | N | % | N | % | |
| Number of weeks of ESA administration until TI or censoringc | ||||||
| < 8 weeks | 289 | 47.4 | 184 | 46.0 | 105 | 50.0 |
| 8 – 15 weeks | 198 | 32.5 | 121 | 30.3 | 77 | 36.7 |
| 16 – 31 weeks | 87 | 14.3 | 59 | 14.8 | 28 | 13.4 |
| 32 + weeks | 36 | 5.9 | 36 | 9.0 | ||
| ESA agent typec | ||||||
| Epoetin-alfa | 485 | 79.5 | 322 | 80.5 | 163 | 77.6 |
| Darbepoetin | 74 | 12.1 | 39 | 9.8 | 35 | 16.7 |
| Both | 51 | 8.4 | 39 | 9.8 | 12 | 5.7 |
| G-CSF use | 162 | 26.6 | 113 | 28.3 | 49 | 23.3 |
| Chemotherapy use | 210 | 34.4 | 139 | 34.8 | 71 | 33.8 |
| Hypomethylating agent useb | 47 | 7.7 | 26 | 6.5 | 21 | 10.0 |
| Serum erythropoietin level measurement | 212 | 34.8 | 135 | 33.8 | 77 | 36.7 |
Abbreviations: TI-transfusion independence, G-CSF-granulocyte-colony stimulating factor, ESA-erythropoiesis-stimulating agent
Only azacitidine claims were observed in this cohort
Distribution of ESA patterns different for TI versus no TI at p<0.01
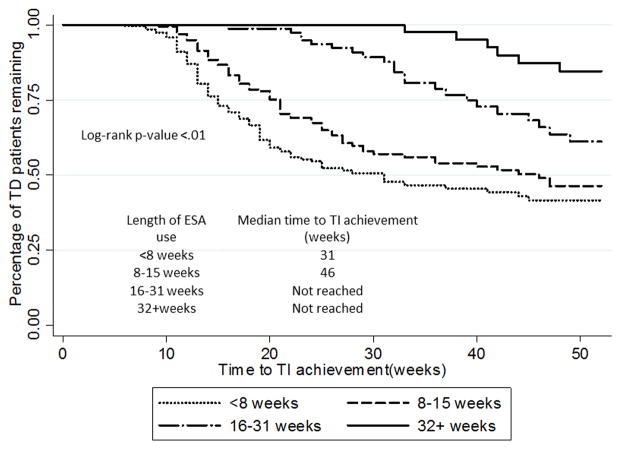
The median time from beginning of TD to first observed ESA was 4 weeks for all patients (data not shown), and there was no difference between patients receiving epoetin alfa and darbepoetin. TI was achieved in 210 (34.4%) patients, and the median time from ESA initiation to TI was 13 weeks. Among darbepoetin users, 47% (35/74) achieved TI, compared to 33.6% (163/485) of epoetin alfa users. Among the entire population, ESAs were administered an average of 11.0 (SD±10.43) weeks until TI or censoring. Among patients who achieved TI, 50% achieved TI after <8 weeks of ESA, 36.7% after 8–15 weeks and 13.4% after receiving more than 15 weeks (Table 2). Patients who achieved TI or were censored within less than 8 weeks of ESA use had the fastest median time to TI and the greatest likelihood of achieving TI in the hazard model after adjusting for age, sex, race, MDS risk category, time to ESA initiation and concurrent use of other therapies (Figure 2, Table 3). Using this group as a comparator, the HR for achieving TI was 0.64 for patients receiving 8–15 weeks, 0.25 for patients receiving 16–31 weeks, and 0.10 for patients receiving more than 31 weeks (p<0.01 for each, Table 3). Longer time from TD to initiation of ESA (HR 0.96 per week, 95% CI 0.94–0.99) predicted for lower rates of TI.
Figure 2.
Unadjusted Kaplan-Meier curve showing percentage of transfusion-dependent patients by weeks with ESA administration (N=610).
Table III.
Multivariable analysis of attainment of transfusion independencea
| Variables | Adjusted ESA Duration Model (N=610) | Adjusted ESA Agent Model (N=559)b | ||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |||
| ESA duration (weeks) | ||||||||
| <8 (ref) | ref | Ref | ref | ref | - | - | - | - |
| 8–15 | 0.63 | 0.47 | 0.86 | <.01 | - | - | - | - |
| 16–31 | 0.26 | 0.16 | 0.42 | <.01 | - | - | - | - |
| 32+ | 0.11 | 0.05 | 0.26 | <.01 | - | - | - | - |
| ESA agent | ||||||||
| Darbepoetin (ref) | - | - | - | - | ref | ref | ref | ref |
| Epoetin alfa | - | - | - | - | 0.67 | 0.46 | 0.99 | 0.04 |
| Time to ESA initiation (weeks) | 0.96 | 0.94 | 0.98 | <.01 | 0.97 | 0.95 | 1.00 | 0.05 |
| MDS risk category | ||||||||
| Lower-risk (ref) | ref | Ref | ref | ref | ref | ref | ref | ref |
| Higher-risk | 0.80 | 0.55 | 1.16 | 0.23 | 0.92 | 0.62 | 1.35 | 0.66 |
| Risk not specified | 0.66 | 0.48 | 0.91 | 0.01 | 0.83 | 0.60 | 1.15 | 0.26 |
| G-CSF use | ||||||||
| No (ref) | ref | Ref | ref | ref | ref | ref | ref | ref |
| Yes | 0.84 | 0.60 | 1.17 | 0.30 | 0.76 | 0.54 | 1.08 | 0.13 |
| Chemotherapy use | ||||||||
| No (ref) | ref | Ref | ref | ref | ref | ref | ref | ref |
| Yes | 0.78 | 0.56 | 1.09 | 0.15 | 0.74 | 0.52 | 1.05 | 0.09 |
| Hypomethylating agent usec | ||||||||
| No (ref) | ref | Ref | ref | ref | ref | ref | ref | ref |
| Yes | 1.20 | 0.71 | 2.03 | 0.50 | 1.25 | 0.72 | 2.16 | 0.43 |
| Serum erythropoietin level measurement | ||||||||
| No (ref) | ref | Ref | ref | ref | ref | ref | ref | ref |
| Yes | 0.96 | 0.71 | 1.29 | 0.77 | 0.95 | 0.70 | 1.29 | 0.74 |
| Age (years) | ||||||||
| <65 | 1.18 | 0.58 | 2.43 | 0.65 | 1.74 | 0.84 | 3.60 | 0.14 |
| 65 – 74 (ref) | ref | Ref | ref | ref | ref | ref | ref | ref |
| 75 – 84 | 1.02 | 0.74 | 1.42 | 0.89 | 1.07 | 0.77 | 1.50 | 0.68 |
| 85+ | 0.76 | 0.49 | 1.19 | 0.24 | 0.77 | 0.48 | 1.21 | 0.25 |
| Sex | ||||||||
| Male (ref) | ref | Ref | ref | ref | ref | ref | ref | ref |
| Female | 1.37 | 1.04 | 1.81 | 0.03 | 1.38 | 1.03 | 1.84 | 0.03 |
| Race | ||||||||
| White | 0.61 | 0.31 | 1.20 | 0.15 | 0.59 | 0.29 | 1.21 | 0.15 |
| Black (ref) | ref | Ref | ref | ref | ref | ref | ref | ref |
| Other | 0.59 | 0.25 | 1.39 | 0.23 | 0.66 | 0.27 | 1.62 | 0.36 |
Abbreviations: G-CSF-granulocyte-colony stimulating factor, ESA-erythropoiesis-stimulating agent
Drops beneficiaries who used both ESA agents
This reflects azacitidine claims; there were no claims for decitabine in this cohort
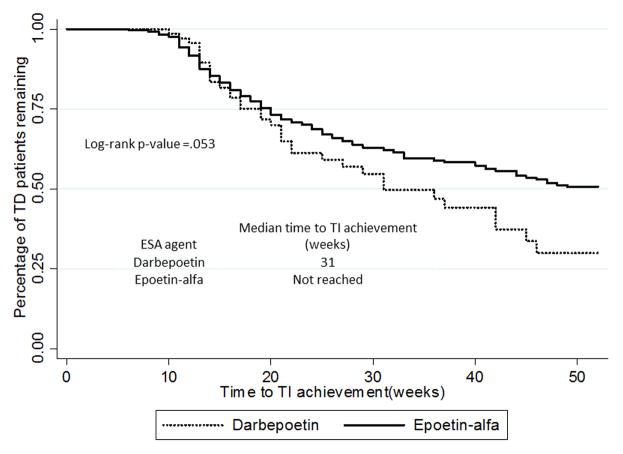
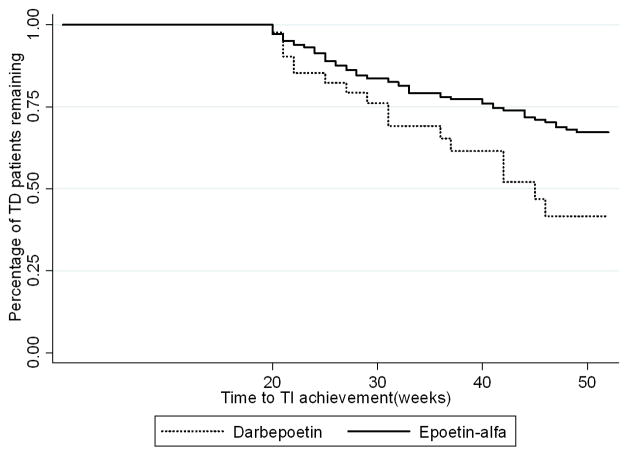
When patients who received both epoetin alfa and darbepoetin were excluded from the multivariable analysis, the results did not change substantially (Table 3). Using this model, 198 patients (35.4% of the cohort) achieved TI and use of epoetin alfa was associated with a lower likelihood of achieving TI compared to darbepoetin (HR 0.67, CI 0.46–0.99, p=0.04). The curves representing patients remaining transfusion-dependent overlap closely early on, but separate permanently at approximately 20 weeks (Figure 3). The median time from TD to TI was 31 weeks for darbepoetin, but was not reached for patients receiving epoetin alfa (log rank p=0.053). A landmark analysis of patients who remained TD, analyzed at 20 weeks, showed that darbepoetin was associated with a shorter time to TI achievement (Figure 4).
Figure 3.
Unadjusted Kaplan-Meier curve showing the percentage of transfusion-dependent patients by ESA administered (N=559).
Figure 4. Landmark analysis of patients who remained TD at 20 weeks by ESA administered.
Darbepoetin was associated with a shorter time to TI achievement.
4. DISCUSSION
ESAs are an established therapeutic option for the treatment of MDS-related anemia and are widely used, despite lack of formal FDA labeling [7]. While ESAs have been shown to increase hemoglobin levels and reduce transfusion need in clinical trials, the impact of ESA use on transfusion dependence has not been examined in a large population-based cohort. Focusing on transfusion-receiving patients, because claims data do not include measures of hemoglobin, this study indicates that approximately one-third of TD patients treated with ESAs ultimately achieved TI, after receiving ESAs a median of 13 weeks from the start of the look-back period. Shorter time from onset of TD to ESA use was associated with higher rates of TI. Patients had higher rates of TI early in ESA therapy; prolonged administration in the absence of an early response was not likely to yield later responses.
Although direct comparisons across studies are difficult due to differences in patient selection criteria, the 34.4% rate of TI is similar to the rates reported in prospective clinical trials [9, 11, 16–19]. The baseline demographics in our study were similar to the expected profile of the MDS population, but we recognize that we may have captured some patients with alternative diagnoses due to the nature of the SEER-Medicare database and its reliance on physician coding. Our study included a significant proportion of patients with characteristics that predict poor response to ESAs. All of our patients were TD by definition, requiring at least 2 transfusions over an 8-week period, and heavy transfusion dependence is associated with poor response [4, 6]. Only 34.8% of patients had a baseline serum erythropoietin level measured, and thus patients were largely not selected for ESA therapy based on low erythropoietin levels; however, serum erythropoietin levels greater than 200 at presentation were relatively rare in multiple studies [9, 12, 20]. The number of patients with extremely high erythropoietin levels is therefore expected to be low in our large population study, and may partially contribute to the lack of effect on TI seen on multivariable analysis. Finally, at least 25% of patients were reported to have higher-risk disease (by WHO classification) in our study. There was a trend toward a decreased likelihood of achieving TI in these patients, consistent with prior reports [12].
The current analysis supports a strong correlation between rapidity of response and likelihood of response. TI was achieved after ESA administration for a median of 13 weeks, and only a small percentage of patients achieved TI after 31 weeks of administration. Similarly, Terpos and colleagues examined the impact of 26 weeks of EPO administration in 281 MDS patients [21], including 72 TD patients, and found that the rate of hematologic improvement-erythroid-major (termed complete erythroid response in this publication) increased from 7.8% at 12 weeks to 27.4% at 26 weeks. Although it is possible that some of our patients who eventually achieved TI first demonstrated decreased transfusion requirements within the first 8 weeks, our data, along with those of Terpos et al., suggest that continuing a trial of ESAs may sometimes be beneficial even when little or no change in hemoglobin is observed in the initial 8 to 12 weeks. This contrasts with current guidelines from the National Comprehensive Cancer Center Network (NCCN) [13] and the European Leukemia Net (ELN) [14], which recommend modifying therapy if no response is achieved within 8 weeks. However, patients who did not demonstrate a response after 16–31 weeks from the look-back period had a HR of only 0.25 for achieving TI, and it decreased further to 0.10 beyond 31 weeks. Continuing ESAs beyond these time points is unlikely to result in clinical benefit. A substantial proportion of our patients received therapy beyond 16 weeks, but this was expected, as we previously showed important gaps between ESA practice patterns and current guidelines [7]. Although the clinical data from our study are still relevant, practice patterns have likely changed over time and ESA use in MDS has decreased after the implementation of the National Coverage Determination in August 2007, restricting ESA coverage in non-MDS patients [22].
To our knowledge, only one prospective clinical trial, conducted by the Scandinavian MDS Group, has examined the association between time to ESA initiation and response to therapy [16]. In this phase II study, 50 evaluable patients were randomized to the combination of erythropoietin beta and 2 different schedules of G-CSF. The overall response rate was 38%, and was nearly identical in both arms. Long-term follow-up revealed that the median time from diagnosis to initiation of therapy was 6.5 months, and that this variable had no association with response to treatment, response duration, or survival. This contrasts with our findings and those of a retrospective study from the Groupe Francophone des Myélodysplasies [12] showing that earlier ESA initiation was associated with better response. Our findings may in part reflect the focus on patients initiating therapy while TD in our study, so that delay in ESA initiation increases the exposure to transfusions. Alternatively, a long delay to initiation may be associated with poor access to care, which may have independent effects on ESA administration patterns and outcomes. Finally, G-CSF was given alone for 4 weeks prior to combination therapy with erythropoietin in the delayed arm of the Scandinavian clinical trial, which may have impacted responses.
Although a much smaller percentage of patients were administered darbepoetin rather than epoetin-alfa, possibly due to physician unfamiliarity with a new product, the use of darbepoetin was associated with a greater likelihood of response in our study. We observed an apparent divergence in response to the two ESAs after 20 weeks, highlighted by the landmark analysis. A small number of darbepoetin-treated patients achieved the study endpoint after 31 weeks of exposure, while responses in the epoetin-alfa-treated patients plateaued by this point. Doses of epoetin alfa could not be assessed in our study, thus it is possible that inadequate dosing may have contributed to lower response rates and apparent inferiority to darbepoetin, as higher dosing may help to counteract intrinsic resistance of MDS erythroid precursors to epoetin alfa [23]. Based on our results and a large meta-analysis showing comparable efficacy of the two agents [5], darbepoetin is likely at least as effective as epoetin-alfa.
Myeloid growth factors, chemotherapy or hypomethylating agents did not impact the likelihood of ESA response in our study, although the number of patients receiving these therapies concurrently was low. Recent data regarding the use of lenalidomide or hypomethylating agents with ESAs suggest that erythroid response rates can be augmented by combination therapy, and this is currently the subject of prospective studies [24, 25]. Because this was an observational study based on Medicare claims data, it is difficult to identify how, when, and why these therapies were combined with ESAs. We previously showed in a similar patient population that 13.8% of MDS patients received concurrent G-CSF during the initial ESA episode. Although only 10.6% of patients with RARS (a population with lower response rates to ESAs alone) received concurrent G-CSF, the duration of use was longest in patients with this specific subtype of MDS. We were also limited to studying drugs and services that were covered under insurance in the current study. Some prescription medications currently used to treat MDS, such as lenalidomide, became available toward the end of our observation period. Additionally, the SEER-Medicare data base for the study years does not include drugs covered by Medicare Part D, and we recognize that we may have failed to observe lenalidomide use in patients who qualified for the study in 2005–2007.
Although lenalidomide has now largely supplanted ESAs in the initial management of anemia in del(5q) MDS patients, up to 50% of patients with this cytogenetic abnormality respond to ESAs [12, 26]. Current guidelines from both NCCN and the ELN consider ESAs to be a reasonable first-line treatment option in del(5q) patients, especially in patients with low endogenous erythropoietin levels and low transfusion-dependence [13, 14]. Therefore, although our data date back to 2007, our results are still applicable in the lenalidomide era. In fact, by choosing to study this earlier time period, we avoid issues associated with selection of some patients to receive other therapies prior to ESAs.
4. CONCLUSIONS
In summary, our data demonstrate that ESAs, as prescribed in the U.S. Medicare population, lead to significant hematologic benefit in approximately one-third of transfusion-dependent MDS patients. Response rates in Medicare patients outside the context of a clinical trial appear to be comparable to those previously reported in prospective studies. Our data support early initiation of ESA following onset of TD. Since we could only study TD patients due to the nature of the data available in the SEER-Medicare data base, our data do not address timing of ESA initiation prior to onset of TD. Importantly, we documented the relationship between duration of therapy and probability of response, finding that 87% of responses occurred among patients with 15 weeks or less of therapy. The absence of large numbers of conversions from non-responders to responders after 15 weeks of ESA administration may help to inform both clinical practice guidelines and policies related to reimbursement of ESA therapy. Although a cost-savings analysis was beyond the scope of our study, substantial health-care savings are possible if ESAs are no longer reimbursed in non-responders after 16 weeks.
Highlights.
ESAs led to transfusion independence in 34.4% of MDS patients.
Response rates were higher with early initiation and with use of darbepoetin.
Administration to non-responders beyond 16 weeks was unlikely produce responses.
Acknowledgments
Funding Sources:
Funding was provided by NIH/NCI RC1 CA145831-01(Davidoff, PI).
Footnotes
Disclaimer: A portion of this research was undertaken while AJD was employed by the University of Maryland. The opinions expressed are the author’s own and do not reflect the view of the Agency for Healthcare Research and Quality, the Department of Health and Human Services, or the United States government.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Steensma DP, Bennett JM. The myelodysplastic syndromes: diagnosis and treatment. Mayo Clin Proc. 2006;81:104–30. doi: 10.4065/81.1.104. [DOI] [PubMed] [Google Scholar]
- 2.Hellstrom-Lindberg E. Efficacy of erythropoietin in the myelodysplastic syndromes: a meta-analysis of 205 patients from 17 studies. Br J Haematol. 1995;89:67–71. doi: 10.1111/j.1365-2141.1995.tb08909.x. [DOI] [PubMed] [Google Scholar]
- 3.Golshayan AR, Jin T, Maciejewski J, Fu AZ, Bershadsky B, Kattan MW, et al. Efficacy of growth factors compared to other therapies for low-risk myelodysplastic syndromes. Br J Haematol. 2007;137:125–32. doi: 10.1111/j.1365-2141.2007.06546.x. [DOI] [PubMed] [Google Scholar]
- 4.Hellstrom-Lindberg E, Gulbrandsen N, Lindberg G, Ahlgren T, Dahl IM, Dybedal I, et al. A validated decision model for treating the anaemia of myelodysplastic syndromes with erythropoietin + granulocyte colony-stimulating factor: significant effects on quality of life. Br J Haematol. 2003;120:1037–46. doi: 10.1046/j.1365-2141.2003.04153.x. [DOI] [PubMed] [Google Scholar]
- 5.Moyo V, Lefebvre P, Duh MS, Yektashenas B, Mundle S. Erythropoiesis-stimulating agents in the treatment of anemia in myelodysplastic syndromes: a meta-analysis. Ann Hematol. 2008;87:527–36. doi: 10.1007/s00277-008-0450-7. [DOI] [PubMed] [Google Scholar]
- 6.Hellstrom-Lindberg E, Negrin R, Stein R, Krantz S, Lindberg G, Vardiman J, et al. Erythroid response to treatment with G-CSF plus erythropoietin for the anaemia of patients with myelodysplastic syndromes: proposal for a predictive model. Br J Haematol. 1997;99:344–51. doi: 10.1046/j.1365-2141.1997.4013211.x. [DOI] [PubMed] [Google Scholar]
- 7.Davidoff AJ, Weiss SR, Baer MR, Ke X, Hendrick F, Zeidan A, et al. Patterns of erythropoiesis-stimulating agent use among Medicare beneficiaries with myelodysplastic syndromes and consistency with clinical guidelines. Leuk Res. 2013;37:675–80. doi: 10.1016/j.leukres.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sekeres MA, Schoonen WM, Kantarjian H, List A, Fryzek J, Paquette R, et al. Characteristics of US patients with myelodysplastic syndromes: results of six cross-sectional physician surveys. JNCI Journal of the National Cancer Institute. 2008;100:1542–51. doi: 10.1093/jnci/djn349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenberg PL, Sun Z, Miller KB, Bennett JM, Tallman MS, Dewald G, et al. Treatment of myelodysplastic syndrome patients with erythropoietin with or without granulocyte colony-stimulating factor: results of a prospective randomized phase 3 trial by the Eastern Cooperative Oncology Group (E1996) Blood. 2009;114:2393–400. doi: 10.1182/blood-2009-03-211797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steensma DP. Hematopoietic growth factors in myelodysplastic syndromes. Semin Oncol. 2011;38:635–47. doi: 10.1053/j.seminoncol.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 11.Musto P, Falcone A, Sanpaolo G, Bodenizza C, La Sala A, Perla G, et al. Efficacy of a single, weekly dose of recombinant erythropoietin in myelodysplastic syndromes. Br J Haematol. 2003;122:269–71. doi: 10.1046/j.1365-2141.2003.04435.x. [DOI] [PubMed] [Google Scholar]
- 12.Park S, Grabar S, Kelaidi C, Beyne-Rauzy O, Picard F, Bardet V, et al. Predictive factors of response and survival in myelodysplastic syndrome treated with erythropoietin and G-CSF: the GFM experience. Blood. 2008;111:574–82. doi: 10.1182/blood-2007-06-096370. [DOI] [PubMed] [Google Scholar]
- 13.Greenberg PL, Attar E, Bennett JM, Bloomfield CD, Borate U, De Castro CM, et al. NCCN clinical practice guidelines in oncology: myelodysplastic syndromes, version 2.2014. 2014 doi: 10.6004/jnccn.2011.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malcovati L, Hellstrom-Lindberg E, Bowen D, Ades L, Cermak J, Del Canizo C, et al. Diagnosis and treatment of primary myelodysplastic syndromes in adults: recommendations from the European LeukemiaNet. Blood. 2013;122:2943–64. doi: 10.1182/blood-2013-03-492884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Cancer Institute. The Surveillance, Epidemiology, and End Results (SEER) Medicare Linked Database [Google Scholar]
- 16.Hellstrom-Lindberg E, Ahlgren T, Beguin Y, Carlsson M, Carneskog J, Dahl IM, et al. Treatment of anemia in myelodysplastic syndromes with granulocyte colony-stimulating factor plus erythropoietin: results from a randomized phase II study and long-term follow-up of 71 patients. Blood. 1998;92:68–75. [PubMed] [Google Scholar]
- 17.Gotlib J, Lavori P, Quesada S, Stein RS, Shahnia S, Greenberg PL. A Phase II intra-patient dose-escalation trial of weight-based darbepoetin alfa with or without granulocyte-colony stimulating factor in myelodysplastic syndromes. Am J Hematol. 2009;84:15–20. doi: 10.1002/ajh.21316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stasi R, Abruzzese E, Lanzetta G, Terzoli E, Amadori S. Darbepoetin alfa for the treatment of anemic patients with low- and intermediate-1-risk myelodysplastic syndromes. Ann Oncol. 2005;16:1921–7. doi: 10.1093/annonc/mdi400. [DOI] [PubMed] [Google Scholar]
- 19.Mannone L, Gardin C, Quarre MC, Bernard JF, Vassilieff D, Ades L, et al. High-dose darbepoetin alpha in the treatment of anaemia of lower risk myelodysplastic syndrome results of a phase II study. Br J Haematol. 2006;133:513–9. doi: 10.1111/j.1365-2141.2006.06070.x. [DOI] [PubMed] [Google Scholar]
- 20.Gabrilove J, Paquette R, Lyons RM, Mushtaq C, Sekeres MA, Tomita D, et al. Phase 2, single-arm trial to evaluate the effectiveness of darbepoetin alfa for correcting anaemia in patients with myelodysplastic syndromes. Br J Haematol. 2008;142:379–93. doi: 10.1111/j.1365-2141.2008.07181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terpos E, Mougiou A, Kouraklis A, Chatzivassili A, Michalis E, Giannakoulas N, et al. Prolonged administration of erythropoietin increases erythroid response rate in myelodysplastic syndromes: a phase II trial in 281 patients. Br J Haematol. 2002;118:174–80. doi: 10.1046/j.1365-2141.2002.03583.x. [DOI] [PubMed] [Google Scholar]
- 22.Hendrick F, Davidoff AJ, Zeidan AM, Gore SD, Baer MR. Effect of erythropoiesis-stimulating agent policy decisions on off-label use in myelodysplastic syndromes. Medicare Medicaid Res Rev. 2014;4 doi: 10.5600/mmrr.004.04.a02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoefsloot LH, van Amelsvoort MP, Broeders LC, van der Plas DC, van Lom K, Hoogerbrugge H, et al. Erythropoietin-induced activation of STAT5 is impair ed in the myelodysplastic syndrome. Blood. 1997;89:1690–700. [PubMed] [Google Scholar]
- 24.Komrokji RS, Lancet JE, Swern AS, Chen N, Paleveda J, Lush R, et al. Combined treatment with lenalidomide and epoetin alfa in lower-risk patients with myelodysplastic syndrome. Blood. 2012;120:3419–24. doi: 10.1182/blood-2012-03-415661. [DOI] [PubMed] [Google Scholar]
- 25.Itzykson R, Thepot S, Beyne-Rauzy O, Ame S, Isnard F, Dreyfus F, et al. Does addition of erythropoiesis stimulating agents improve the outcome of higher-risk myelodysplastic syndromes treated with azacitidine? Leuk Res. 2012;36:397–400. doi: 10.1016/j.leukres.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 26.Kelaidi C, Park S, Brechignac S, Mannone L, Vey N, Dombret H, et al. Treatment of myelodysplastic syndromes with 5q deletion before the lenalidomide era; the GFM experience with EPO and thalidomide. Leukemia Research. 2008;32:1049–53. doi: 10.1016/j.leukres.2007.11.037. [DOI] [PubMed] [Google Scholar]