Abstract
Objective
To describe our center's middle-term outcomes following trans-catheter creation of atrial communication (ASD) in patients on mechanical circulatory support.
Background
Trans-catheter creation of an ASD in patients on mechanical circulatory support is an adjuvant therapy to reduce left atrial pressure and associated morbidity. Data on middle term outcomes following this procedure, specifically in regards to the fate of the ASD, are limited.
Methods
Retrospective observational study of consecutive children and adults undergoing trans-catheter creation of an atrial septal communication between 1/1/2006 to 5/1/2014, reviewing their baseline characteristics, procedural details, and data from follow-up.
Results
Over the study period, 37/227 (16%) subjects undergoing veno-arterial extra-corporeal membrane oxygenation (VA-ECMO) underwent trans-catheter creation of an atrial communication. Mortality on VA-ECMO support in this subgroup was 19%, with an additional 24% transitioning to ventricular assist device. Of the 57% who survived to separation from VA-ECMO, 16/21 (76%) had residual atrial communications. 56% of these underwent closure procedures.
Conclusions
Following trans-catheter creation of ASD, a residual ASD is present in the majority of assessable survivors and represents a potential volume overload and/or right to left shunt that may need to be addressed.
Keywords: Mechanical circulatory support, heart catheterization, outcomes, extra-corporeal membrane oxygenation, congenital heart disease, heart failure
Introduction
Use of mechanical circulatory support in children (through ventricular assist devices (VAD) and extra-corporeal membrane oxygenation (ECMO)) is increasing in frequency(1). Mechanical circulatory support allows for stabilization of patients and maintenance of systemic organ perfusion while correctable causes of cardiac dysfunction are addressed and potentially provides a period for myocardial recovery. However, left ventricular (LV) dysfunction can result in elevated left-sided filling pressures, which impedes myocardial recovery and can lead to pulmonary edema and hemorrhage.
Creation of an atrial septal communication (ASD) and the resultant left-to-right shunt, can reduce left atrial (LA) and LV end-diastolic pressures, reduce wall stress and relieve pulmonary edema. This can be performed by a surgeon in the operating room, but this is accompanied by risks of morbidity and mortality associated with open heart surgery, which may be magnified in a patient receiving mechanical circulatory support. Augmentation of an atrial communication in neonates through balloon atrial septostomy as first described by William Rashkind and colleagues has been widely adopted(2). This concept has been extended, and several trans-catheter techniques have been applied to creation of atrial communications in older patients(3-5) and have been accepted as a less risky method of relieving left atrial hypertension. Our center has used trans-septal needle perforation of the inter-atrial septum and static balloon dilation to create an ASD in patients on veno-arterial ECMO (VA-ECMO) with suspected LA hypertension. Though use of static balloon dilation to create an atrial communication has been described incidentally in larger case series of trans-catheter atrial communication(5), no larger series have been described. The assumption has long been made that atrial communications created for this purpose were transient or at least without hemodynamic significance, however, the fate of such “man-made holes” is unclear. We reviewed our center's experience and outcomes with this procedure between 2006 and 2014, with the goal of determining the risk of producing persistent ASD and the consequences of those defects.
Materials and Methods
Study Population
We performed a single-center retrospective observational study. The Institutional Review Board of The Children's Hospital of Philadelphia approved the proposed study and granted a waiver of consent. Inclusion criteria were children and adults of all ages who underwent creation of an ASD (whether by balloon atrial septostomy, blade atrial septostomy, static balloon dilation, or inter-atrial stent) to reduce LA pressure while receiving VA-ECMO 1/1/2006 and 5/1/2014. Subjects who underwent trans-catheter creation of an ASD or augmentation of ASD for other indications (pulmonary hypertension with right ventricular failure, single ventricle physiology and restrictive atrial septal communications, or transposition of the great arteries with insufficient mixing) were excluded. Subjects were identified by query of cardiac catheterization laboratory database and review of institutional mechanical support records. Eligibility was confirmed through review of subject charts. Limited data about children and adults who received mechanical circulatory support without augmentation of atrial communication were recorded.
Creation of atrial communication
Rashkind balloon atrial septostomy was performed using B. Braun Medical (Melsungen, Germany) or Edwards (Edwards Lifesciences LLC Irvine, CA United States of America) septostomy balloons with balloon catheter directed across a patent foramen ovale, inflated with contrast, and pulled across the inter-atrial septum with a controlled tug under biplane digital fluoroscopy and/or trans-thoracic echocardiographic guidance.
For static balloon dilation, the inter-atrial septum was crossed via trans-septal puncture, performed using Brockenbrough (Medtronic Minneapolis, MN) or pediatric trans-septal needle (Cook Medical, Bloomington, IN) under digital fluoroscopy with position in the LA confirmed with a hand injection of contrast. Pressure measurements were generally directly measured in LA prior to dilation followed by pressure pullbacks to confirm decompression of the LA. A guide-wire was advanced into one of the left pulmonary veins. Over the wire, low (0-3 atm) (Tyshak or Tyshak2 (both from B. Braun Medical, Melsungen Germany)), medium (4-8 atm) (XXL or Sterling (both from Boston Scientific Corporation Marlborough, MA)), and high (10-30 atm) (Atlas or Atlas gold (Bard Medical Covington, GA)) pressure balloon catheters were and inflated under biplane digital fluoroscopy. Serial dilations are performed with increasing balloon diameter and pressure delivered until adequate LA decompression is achieved at the discretion of the operator, using pressure gradient, lung compliance, or changes in radiographic appearance of the lung fields on fluoroscopy. Though no clinical protocol was in place over the study period, practice was uniform and followed the described standards.
Study measures
Demographic information, past medical history, echocardiograms, data from catheterization procedure, and data from clinical follow-up were extracted from the electronic medical record. The indication for trans-catheter creation of an atrial communication was extracted from the chart based on notes from the ICU attending and catheterization report. When more than one indication was listed, the more stringent or severe trumped other indications. Properties of ECMO circuit function (flow rate (ml/kg/min), circuit pressure, and venous pressure) were all extracted from flow sheets. Echocardiograms were reviewed by a single member of the study staff (MLO). The echocardiograms reviewed were comprised of 1) the first performed after creation of the atrial communication and prior to cessation of ECMO support and 2) the most recent echocardiogram following cessation of VA-ECMO support and prior to any closure procedure. The primary outcome of interest was whether the atrial communication remained patent after cessation of circulatory support and whether there were consequences of the residual ASD. These included right ventricular volume overload (measured qualitatively on echocardiogram and quantitatively by ratio of pulmonary and systemic blood flow in the subset of subjects who underwent catheterization) or right to left shunt (either by catheterization, desaturation without other cause, or echocardiography). Whether the defect underwent closure procedure was also noted. For all echocardiograms, the size of the defect was measured on two-dimensional images.
Other clinical outcomes of interest included adverse events (death, bleeding, need for operative intervention) in the process of creating the communication, hemodynamic changes following creation of the atrial communication, and outcomes following creation of the atrial communication (death, orthotopic heart transplantation, or separation from mechanical circulatory support).
Statistical Analysis
Descriptive statistics were calculated and expressed as mean + standard deviation, median (range and interquartile range (IQR)), and percent (count) as appropriate. The primary objective of this study was descriptive. Differences in individual subjects LA and RA pressures both before and after static balloon dilation were assessed using Wilcoxon sign-rank test (nonparametric test of difference between two paired observations). An exploratory analysis was performed assessing for differences in demographic and procedural characteristics of subjects with persistent atrial communication and those in whom atrial communications spontaneously closed. Sample size limited the capacity for multivariable analysis but bi-variable analysis was included to aid in hypothesis generation. A threshold for statistical significance was set at p<0.05. All analyses are performed using Stata MP v.13 (Statacorp, College Station, TX).
Results
Study population
Between 1/1/2006 and 5/1/2014, 227 subjects were supported using VA-ECMO at our institution (Figure 1). Of these, (37/227) underwent attempted creation of an atrial communication, representing 16% of the population receiving VA-ECMO mechanical circulatory support. All of these procedures were technically successful. In terms of trans-catheter creation or augmentation of atrial communications, these cases represents 22% (37/169) of cases over the study period. Other indications for creation of an atrial communication were transposition of the great arteries with inadequate mixing (n=90), hypoplastic left heart syndrome and other single-ventricle variants with restrictive atrial communication (n=34), and pulmonary hypertension and right ventricular failure (n=7). These patients were considered qualitatively different from patients undergoing creation of an atrial communication while on mechanical circulatory support so were excluded from the remainder of the analysis.
Figure 1. Study Population.
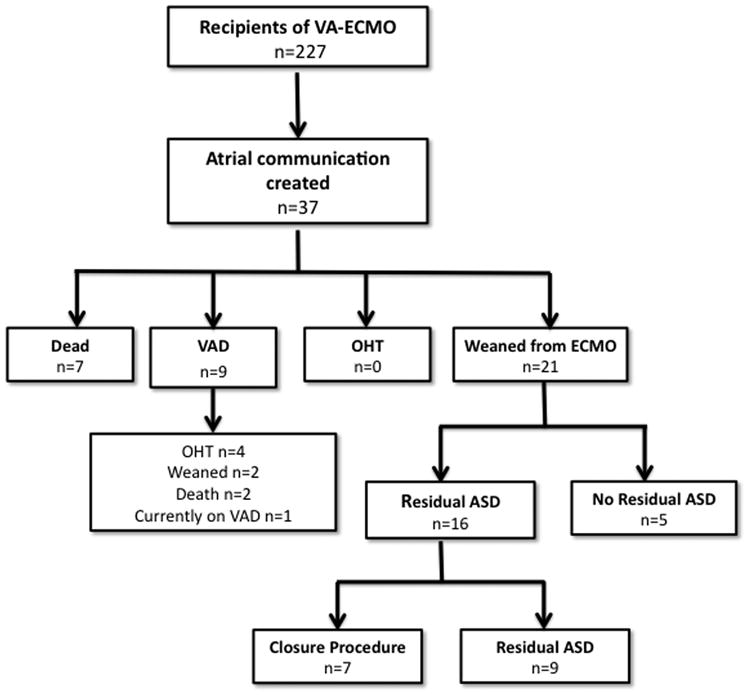
The cohort who underwent creation of an atrial communication while receiving VA-ECMO is described in Table 1. They were of median age 5 years, 62% male, and 68% white. Primary diagnoses included myocarditis (38%), congenital heart disease (27%), dilated cardiomyopathy (11%), status post heart transplant (11%), restrictive cardiomyopathy (3%), hypertrophic cardiomyopathy (3%), and other diagnoses (8%). The majority of cases (62%) underwent VA-ECMO following a cardiac arrest with a minority undergoing ECMO cannulation either semi-electively in the face of deteriorating clinical status (35%) or because of failure to separate from cardiopulmonary bypass (3%). The primary indications for creation of an atrial communication included anticipation of LA hypertension due to poor LV function in 32%, TTE evidence of LA hypertension in 30%, pulmonary edema on chest radiography in 22%, pulmonary hemorrhage in 14%, and elevated LA pressure on catheterization in the admission prior to initiation of ECMO in 3%.
Table 1. Study population.
| n | 37 |
| Age (years) | Median: 6 years (range: 3 days-17 years, IQR: 264 days-12 years) |
| Female sex % (n) | 38% (14) |
| Race % (n) | |
| White | 68% (25) |
| Black | 27% (10) |
| Asian | 3% (1) |
| Other | 3% (1) |
| Weight (kg) | Median: 18.7 kg (range: 2.2-90, IQR: 7.7-47.4) |
| Height (cm) | 112 cm (range: 47-182, IQR: 66-157) |
| Diagnosis | |
| Myocarditis | 38% (14) |
| Congenital heart disease* | 27% (10) |
| Dilated cardiomyopathy | 11% (4) |
| Status post heart transplant | 11% (4) |
| Hypertrophic cardiomyopathy | 3% (1) |
| Restrictive cardiomyopathy | 3% (1) |
| Other** | 8% (3) |
| Indication for mechanical support | |
| Elective or semi-elective | 35% (13) |
| Following cardiac arrest | 62% (23) |
| Failure to separate from CPB | 3% (1) |
| Time on ECMO prior to ASD procedure | Median: 0 (Range: 0-2, IQR: 0-1) |
| Indication for creation of atrial communication | |
| Clinical suspicion of imminent LA hypertension | 32% (12) |
| Evidence of LA hypertension on TTE | 30% (11) |
| Pulmonary edema | 22% (8) |
| Pulmonary hemorrhage | 14% (5) |
| Elevated LA pressure prior to ECMO | 3% (1) |
| Total duration of mechanical support (days) | Median: 7 (Range: 2-123, IQR: 5-10) |
Diagnoses include congenital mitral stenosis with mechanical prosthesis (n=2) and transposition of the great arteries after arterial switch operation (n=2), as well as anomalous left coronary from the pulmonary artery with bypass graft, bicuspid aortic valve with aortic insufficiency, critical coarctation of the aorta, tetralogy of Fallot, tetralogy of Fallot pulmonary atresia, and incomplete atrioventricular canal with left atrioventricular valve regurgitation
Includes multi-organ system failure due to hemophagocytic lymphohistiocytosis, myocardial infarct of unknown etiology, and transient cardiac dysfunction in a child with very long chain acyl-coenzyme A dehydrogenase deficiency
Procedural details
In four cases, Rashkind balloon atrial septostomy was performed in patients of 1 day, 3 days, 36 days, and 291 days of age. The youngest subject was a neonate with dilated cardiomyopathy. The middle two subjects were infants with transposition of the great arteries with complications following arterial switch operations. The oldest patient was an infant with myocarditis. All had patent foramen ovales, and responded to Rashkind septostomy. The remaining 33 procedures were static balloon dilations. No blade septostomies were performed. No subjects in this cohort underwent stenting of the atrial communication.
Static balloon dilation was performed as described. The procedures occurred on median day 0 of ECMO (IQR: 0-1). All but 1 procedure was performed within 24 hours of initiation of ECMO, and that procedure was performed on the second day of ECMO support. The median maximal balloon diameter was 18 mm (IQR: 14-18 range), and the median maximal pressure was 3 atm (IQR: 2-5 atm). The initial LA pressure was measured in 33/37 subjects, with median LA pressure of 18 mmHg (IQR: 13-24 mmHg). Initial RA pressure was recorded in 30/37 subjects with median RA pressure of 10 mmHg (IQR: 6-12). The gradient between LA and RA was calculable in 28/37 subjects and the median gradient was 7 mmHg (IQR: 3.5-12 mmHg). There was a significant decrease in LA pressure (median: -5 mmHg, IQR: -10 to -3 p<0.0001) and significant increase in RA pressure (median: 1 mmHg, IQR: 0-2, p=0.002). There were no changes in circuit flow rates (median 1 ml/kg/min, IQR: 0-7, p=0.07), circuit pressure (median change 10 mmHg, -11 to 30, p=0.05), or circuit venous pressure (median change -2, IQR: -8 to 4, p=0.51) to explain the changes seen in LA and RA pressure with creation of a septal communication. There were no procedural adverse events. On echocardiograms in the intensive care unit, the size of resultant holes was measurable in 36/37 subjects, with a median diameter of 4 mm (IQR: 3-5 mm), measured on two-dimensional echocardiography. The median ratio of resultant ASD to maximal balloon diameter was 0.26 (Range: 0.11-0.5, IQR: 0.22-0.31).
Outcomes
Median duration of mechanical support for the 37 patients undergoing trans-catheter creation of atrial communication was 7 days (range: 2-123, IQR: 5-10 days). Combining mortality and withdrawal of support, there was an 18% (7/37) mortality rate. An additional 24% (9/37) were transitioned from VA-ECMO to ventricular assist device. No subjects underwent cardiac transplant directly from ECMO. The remaining 57% (21/37) of subjects were weaned from ECMO-support directly. Of these, median total elapsed clinical follow-up was 1.7 years (IQR: 0.2-4.5 years) with serial trans-thoracic echocardiograms performed in 95% (20/21) of subjects. A residual atrial communication was seen in 80% (16/20) of evaluable subjects. The median size of residual communication on TTE was 5.5 mm (IQR: 4-8.8mm). On echocardiogram, 44% (7/16) had new moderate RV dilation on echocardiogram with the remainder demonstrating mild RV dilation. Catheterizations were performed in 38% (6/16) of subjects with residual ASD. Of these subjects, 2/6 had a net right to left shunt, and 4/6 had net left to right shunt with median ratio of pulmonary to systemic blood flow of 1.6:1 (range: 1.2:1-2:1).
Among the subjects with residual atrial communications, 44% (7/16) underwent procedures to close the ASD. Three had closure procedures in the operating room for closure in combination with other procedures (VAD implantation, left atrioventricular valvuloplasty, and an operation to close a residual VSD). Three subjects underwent device closure with 10mm Amplatzer septal occluder, 14mm Amplatzer septal occluder, and 30mm Helex device respectively. The final subject had a hemodynamically significant defect that was very low on the atrial septum and therefore not amenable to device closure. This patient is scheduled for operative closure.
Risk factors for persistent atrial communication
Univariate logistic regression was used to assess potential risk factors for presence of residual ASD (Table 3). None of the potential subject and procedural risk factors was significantly associated with the risk of persistent ASD. Increasing patient age, maximal balloon diameter, and initial size of defect had odds ratios that were consistent with increased risk of a persistent ASD, but were not statistically significant. Use of a high-pressure balloon was associated with a much reduced odds ratio for persistent ASD, but was not statistically significant.
Table 3. Univariate analysis of risk factors for persistent atrial communication.
| Risk Factor | Odds Ratio | 95% CI | p |
|---|---|---|---|
| Patient age (per year) | 1.2 | 0.9-1.6 | 0.12 |
| Diagnosis | |||
| Congenital heart disease | 1 | n/a | n/a |
| Myocarditis | 0.8 | 0.05-10.2 | 0.83 |
| Other | 0.5 | 0.04-7.1 | 0.61 |
| Weight (per kg) | 1.0 | 1.0-1.1 | 0.42 |
| Height (per 10 cm) | 1.2 | 0.9-1.6 | 0.18 |
| Balloon type | |||
| Low pressure | 1 | n/a | n/a |
| Medium pressure | 0.4 | 0.02-8.1 | 0.56 |
| High pressure | 0.08 | 0.003-2.6 | 0.16 |
| Maximal balloon pressure (per mmHg) | 0.8 | 0.4-1.5 | 0.49 |
| Maximal balloon diameter (per mm) | 1.2 | 0.9-1.8 | 0.25 |
| Maximal balloon diameter normalized to body surface area (per mm/sqm) | 1.0 | 0.9-1.0 | 0.48 |
| Initial size of defect (per mm) | 1.9 | 0.8-4.6 | 0.14 |
| Initial LA pressure (per mmHg) | 1.0 | 0.9-1.2 | 0.56 |
| Initial LA to RA pressure gradient (per mmHg) | 1.1 | 0.8-1.4 | 0.55 |
| Time on ECMO before creation of ASD (per day) | 0.6 | 0.11-3.2 | 0.54 |
| Total duration of ECMO (per day) | 1.1 | 0.8-1.4 | 0.70 |
Discussion
This single center case series demonstrates that trans-catheter creation of an atrial communication is technically feasible, with no technical failures or major catheterization related adverse events in 37 subjects who, by definition, are critically ill. In the subset of subjects able to separate from mechanical circulatory support however, there was significant risk of residual atrial communication and within this group a large percentage had evidence of RV volume overload or a right to left shunt. Though the sample size was too small for a definitive analysis, several possible patient and procedure level risk factors were identified.
Previous studies have detailed the use of different techniques to create atrial communications(3-5). To our knowledge no study has followed these patients beyond the incident hospitalization. Nor have any previous studies assessed surviving subjects to determine the risk of residual atrial communications.
The results of this case series demonstrate that the procedure is technically feasible across a range of ages and sizes. In addition, the procedure was overwhelmingly (97%) performed within 24 hours of initiation of ECMO cannulation, suggesting that freshly cannulated patients can safely be taken to the catheterization laboratory for this procedure. Because of uniform practice in referring patients with suspected LA hypertension for the creation of an atrial hole, there is not a convenient cohort of subjects with whom to compare outcomes. However, it is clear there was effective reduction in LA pressure and anecdotally improvement in radiographic appearance of the chest, suggesting that the desired hemodynamic effect was achieved.
In this series, 44% of residual ASD's demonstrated either a Qp:Qs>1.5 and moderate or worse RV dilation and 13% were a source of right to left shunt. This was despite the fact that defects were relatively small. In patients with the potential for persistent cardiac dysfunction, this volume load may have important clinical ramifications, emphasizing the importance of following these defects closely and potentially having a reduced threshold for intervention. As noted earlier, no subject or procedural factors were significantly associated with risk of persistent atrial communication. However, several factors had p-values in univariate analysis that were <0.2 which some authors recommend in bivariable screening(6), which is especially important in a relatively small series. Increasing patient age and height, as well as larger initial defect size were associated with higher risk of persistent atrial communication, while use of a high pressure balloon reduced the risk of persistent communication compared with subjects in whom a low pressure balloon was used. Given that our practice is to start with a low-pressure balloon, the use of a high-pressure balloon is more likely an indication of a thickened and tough inter-atrial septum than a procedure-level risk factor.
In this series, it is also clear that defect size can be dramatically different while the patient is on VA-ECMO and after separation, with the TTE measurement of initial defect size often under-estimating the size when measured off ECMO. Though there is not sufficient data to recommend a specific frequency of clinical and echocardiographic evaluation, we conclude that including assessment of the atrial septum during routine echocardiography in the group at risk is prudent. Tracking indices of RV dilation and considering the role that the residual ASD plays also may benefit patients. It is also heartening to note that, generally, these defects are centrally located. Only one in our experience was in a location that was not amenable to device closure. Device closure of the remainder of defects was uncomplicated.
There are several limitations to this study. The study was a retrospective case series and selection of subjects was based on clinical judgment. With relatively uniform practice there is no comparator group of patients with suspected LA hypertension who did not undergo creation of an atrial communication, limiting inference. In regards to risk factors for persistent atrial communication, inference is limited by the relatively small study population. In addition, in looking at procedural risk factors, the uniform practice at our institution over the study period suggests that factors are more indicative of the patient and their anatomy rather than actual differences in procedure. The small number of subjects also increases the risk of type II error.
Conclusion
Creation of an atrial communication in subjects with ventricular dysfunction is technically feasible with excellent rates of technical success and safety. However, in patients who separate from support without heart transplant, there is significant risk of persistent residual atrial communication that can represent a significant right-sided volume load or a right to left shunt. These residual lesions can and should be addressed either in the operating room during other operations or in isolation via trans-catheter device closure.
Table 2. Procedural details.
| Hemodynamics prior to creation of atrial communication | |
| Mean LA pressure (mmHg) (n=33) | 18 (IQR: 13-24) |
| Mean RA pressure (mmHg) (n=30) | 10 (IQR: 6-12) |
| Gradient between LA and RA (mmHg) (n=28) | 7 (IQR: 3.5-12) |
| Hemodynamics following creation of atrial communication | |
| Mean LA pressure post-procedure (mmHg) (n=35) | 12 (IQR: 8-20) |
| Mean RA pressure post-procedure (mmHg) (n=35) | 11 (IQR: 8-20) |
| Gradient between LA and RA post-procedure (mmHg) (n=35) | 0 (IQR: 0-1) |
| Change in mean LA pressure (mmHg) (n=31) | -5 (IQR: -10 to -3)* |
| Change in mean RA pressure (mmHg) (n=29) | 1 (IQR: 0 to 2)** |
| Change in LA:RA gradient (n=33) | -7 (IQR: -12 to -3)*** |
| ECMO circuit prior to creation of atrial communication | |
| Flow rate (mL/min/kg) (n=36) | 76 (IQR: 57 to 108) |
| Circuit pressure (mmHg) (n=36) | 172 (IQR: 145 to 225) |
| Circuit venous pressure (mmHg) (n=36) | -41 (IQR: -61 to -26) |
| ECMO circuit following creation of atrial communication | |
| Flow rate (mL/min/kg) (n=37) | 84 (IQR: 62 to 102) |
| Circuit pressure (mmHg) (n=36) | 187 (IQR: 150 to 242) |
| Circuit venous pressure (mmHg) (n=37) | -42 (IQR: -62 to -23) |
| Change in Flow rate (mL/min/kg) (n=36) | 1 (IQR: 0-7)**** |
| Change in circuit pressure (mmHg) (n=36) | 10 (-11 to 30)***** |
| Change in circuit venous pressure (mmHg) (n=36) | -2 (-8 to 4)****** |
| Maximal balloon diameter (mm) (n=33) | 18 (IQR: 14-18) |
| Maximal balloon pressure (atm) (n=33) | 3 (IQR: 2-5, range: 1-12) |
| Balloon type used (n=33) | |
| Low pressure balloon (1-3 atm) | 68% (21) |
| Medium pressure balloon (4-8 atm) | 23% (9) |
| High pressure balloon (10-30 atm) | 10% (3) |
| Size of resultant atrial communication (mm) (n=36) | 4 (IQR: 3-5) |
| Ratio of resultant ASD to maximal balloon diameter (n=33) | 0.26 (IQR: 0.22-0.31) |
| Procedural adverse event % (n) | 0% (0) |
p<0.0001
p=0.002
p<0.0001
p=0.07
p=0.05
p=0.51
All p-values are for Wilcoxon sign-rank test
Acknowledgments
Funding sources: Dr. O'Byrne receives support from the NIH [T32 HL007915] and Entelligence Young Investigator grant. The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health. The supporting agencies had no role in the design, conduct, interpretation, or decision to publish the data in this manuscript.
Footnotes
Disclosures: None
The authors have no other conflicts to disclose.
References
- 1.Morales DLS, Zafar F, Rossano JW, et al. Use of ventricular assist devices in children across the United States: analysis of 7.5 million pediatric hospitalizations. The Annals of Thoracic Surgery. 2010;90:1313–8. doi: 10.1016/j.athoracsur.2010.04.107. – discussion 1318–9. [DOI] [PubMed] [Google Scholar]
- 2.Rashkind WJ, Miller WW. Transposition of the Great Arteries Results of Palliation by Balloon Atrioseptostomy in Thirty-one Infants. Circulation. 1968;38:453–462. doi: 10.1161/01.cir.38.3.453. [DOI] [PubMed] [Google Scholar]
- 3.Seib PM, Faulkner SC, Erickson CC, et al. Blade and Balloon Atrial Septostomy for Left Heart Decompression in Patients With Severe Ventricular Dysfunction on Extracorporeal Membrane Oxygenation. Cathet Cardiovasc Intervent. 1999;46:179–186. doi: 10.1002/(SICI)1522-726X(199902)46:2<179::AID-CCD13>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 4.Aiyagari RM, Rocchini AP, Remenapp RT, Graziano JN. Decompression of the left atrium during extracorporeal membrane oxygenation using a transseptal cannula incorporated into the circuit. Critical Care Medicine. 2006;34:2603–2606. doi: 10.1097/01.CCM.0000239113.02836.F1. [DOI] [PubMed] [Google Scholar]
- 5.Veldtman GR, Norgård G, Wåhlander H, et al. Creation and enlargement of atrial defects in congenital heart disease. Pediatr Cardiol. 2005;26:162–168. doi: 10.1007/s00246-004-0953-5. [DOI] [PubMed] [Google Scholar]
- 6.Sun GW, Shook TL, Kay GL. Inappropriate use of bivariable analysis to screen risk factors for use in multivariable analysis. Journal of Clinical Epidemiology. 1996;49:907–916. doi: 10.1016/0895-4356(96)00025-x. [DOI] [PubMed] [Google Scholar]


