Abstract
Molecular oxygen has long been recognized as a powerful radiosensitizer that enhances the cell-killing efficiency of ionizing radiation. Radiosensitization by oxygen occurs at very low concentrations with the half-maximum radiosensitization at approximately 3 mmHg. However, robust hypoxia-induced signal transduction can be induced at <15 mmHg and can elicit a wide range of cellular responses that will affect therapy response as well as malignant progression. Great strides have been made, especially since the 1990s, toward identification and characterization of the oxygen-regulated molecular pathways that affect tumor response to ionizing radiation. In this review, we will discuss the current advances in our understanding of oxygen-dependent molecular modification and cellular signal transduction and their impact on tumor response to therapy. We will specifically address mechanistic distinctions between radiobiological hypoxia (0–3 mmHg) and pathological hypoxia (3–15 mmHg). We also propose a paradigm that hypoxia increases radioresistance by maintaining the cancer stem cell phenotype.
Introduction
A solid tumor grows autonomously as an independent tissue that defies or evades normal physiological controls of the host tissue or organ. Abnormalities in angiogenesis, vascular formation, cellular compositions and tissue structures are commonly observed in solid tumors. This unique tumor microenvironment can induce adaptive responses in tumor cells that will eventually affect tumor cell sensitivity to chemotherapy and/or radiation therapy.
In addition to intrinsic (genetic and epigenetic) factors, tumor response to therapy is also heavily influenced by a plethora of extrinsic factors. Hypoxia or insufficient oxygenation is arguably the most prominent feature of tumor microenvironment and can be found in a wide range of solid tumors. Hypoxia develops as a consequence of insufficient oxygen (O2) supply to meet the demands of the metabolically active tumor cells. Several excellent review articles offer detailed discussion on the potential causes of tumor hypoxia (1–4). Data suggest that intratumoral O2 transport is often retarded due to structurally defective blood vessel formation, fluctuating red blood cell flux and limited arteriolar blood supply. It is important to note that tumor hypoxia is highly dynamic in terms of both duration and degree of hypoxia. The intermittent or transient nature of tumor hypoxia was first reported by Dr. J. Martin Brown in 1979 (5). Dewhirst and colleagues further observed that many tumor types exhibited periodically fluctuating hypoxia, which led them to define “acute” or “intermittent” hypoxia, which is now more appropriately called “cycling hypoxia” (1, 6). It is worth noting that hypoxic areas are heterogeneously distributed throughout a solid tumor proper and both chronically stable hypoxia and cycling hypoxia can be found in the same tumor.
In the classic study of human lung cancer specimens, Thomlinson and Gray (7) found that tumor cords with >200 μm in diameter inevitably developed central necrosis and the sheath of viable tumor cells surrounding the necrotic center never exceeded 180 μm. Using mathematic models, they analyzed the supply and consumption as well as regional distribution of oxygen in these tumors, which led to the prediction of tumor hypoxia. However, the presence of hypoxia in human tumors was not directly demonstrated until 1985 when Gatenby et al. measured oxygen tensions in 16 cancer patients using CT-guided pO2 needle electrodes (8). In a subsequent study examining 31 fixed lymph node metastases from squamous cell carcinoma of the head and neck, Gatenby et al. found that tumors containing >26% tumor volume with pO2 ≤ 8 mmHg responded poorly to radiotherapy (9). It should be noted however that the Clark-type pO2 electrodes used by Gatenby et al. consume large quantities of pO2 during the measurement process and therefore the reported intratumoral pO2 is likely to be lower than the actual values. Using a computerized pO2 measurement system with a programmed pattern of electrode movement through tissue to improve measurement accuracy, Vaupel et al. later found that human breast tumors contained regions of hypoxia with a significant proportion of pO2 readings between 0 and 10 mmHg compared to normal breasts (pO2 > 12.5 mmHg and median pO2 = 65 mmHg) (10). Subsequently, tumor hypoxia has been observed in many other human tumors including tumors of uterine cervix, prostate, pancreas, lung, brain, soft tissue sarcoma, melanoma, non-Hodgkin lymphoma, liver, kidney and rectum (11–13). In cancers of the uterine cervix, head and neck and breast, it has been found that the overall median tumor pO2 was 10 mmHg (11). Importantly, pO2 ≤ 10 mmHg predicts poor outcomes for these patients (14–19). It should be noted that different oxygenation parameters (i.e. pO2 values) have been used to determine the prognostic significance of tumor hypoxia by different research groups and/or in different tumor types [extensively reviewed by Vaupel et al. (11)]. The lack of a generally accepted pO2 threshold to categorize tumor hypoxia in the clinic might reflect differences in patient- or tumor-dependent response/adaptation to hypoxia or different approaches to tumor sampling by different investigators. It is also worth noting that, despite the prevailing evidence showing tumor hypoxia as an independent prognostic parameter for aggressive tumor behavior, Nordsmark et al. found no significant correlation between pO2 and response to radiotherapy from a prospective multicenter study of 120 patients with primary cervical cancer (20). Although the reason for this discrepancy remains to be determined, it is possible that these results could be affected by the type (cycling or chronic), heterogeneity and extent (hypoxic volume) of hypoxia in different studies. Nonetheless, a preponderant majority of the clinical evidence has shown that tumor hypoxia predicts poor overall survival and disease-free survival of cancer patients independently of tumor grade, nodal involvement and other commonly used prognostic factors. In this review, we discuss molecular mechanisms underlying oxygen-dependent regulation of tumor response to ionizing radiation.
Radiobiological Hypoxia Versus Pathological Hypoxia
Radiobiological Hypoxia and Oxygen Fixation Hypothesis
The potential impact of oxygen on radiosensitivity was first implicated in the 1900s by work showing that compression of skin by the applicator reduced the skin reaction to X rays [see reviews (21, 22)]. Fast forward to the 1950s, Gray and colleagues conducted a series of experiments investigating the effects of oxygen on radiation response of microbes, plants, cells and animals (7, 23–25). This body of classic work defined oxygen effects on cellular sensitivity to X-ray radiation. When X irradiated under anoxia, i.e., the absence of oxygen, mammalian cells are approximately 2.5–3 times more resistant to radiation-induced clonal cell death than the same cells irradiated at physiological or higher levels of oxygen concentration (22, 26). The ratio of X-ray doses between anoxia (pO2 = 0 mmHg) and a given pO2 that are required to kill the same numbers of clonogenic tumor cells is commonly referred to as the oxygen enhancement ratio (OER). In essence, the OER is a quantitative representation of the relative radiosensitivity of mammalian cells with radiosensitivity at anoxia (pO2 = 0 mmHg) set as 1 (22, 26). O2-dependent radiosensitivity rises sharply when pO2 increases from 0 to 10 mmHg. The half-maximum radiosensitivity occurs at approximately pO2 =; 3 mmHg (21, 27). Once pO2 rises into the physiological range (>30 mmHg) and even up to 100% pure O2, radiosensitivity increases only gradually (22, 26), suggesting that mammalian cells are by and large fully sensitized by molecular oxygen to ionizing radiation under most physiological conditions. For the purposes of discussion herein, we define the radiobiological hypoxia as pO2 <3 mmHg or 0.4% O2 where mammalian cells are the most radioresistant.
The ability of molecular oxygen as a potent radiosensitizer lies in its chemical properties as a highly reactive electrophile. The customarily accepted mechanism for O2-dependent enhancement of ionizing radiation-induced damage is known as the oxygen fixation hypothesis (OFH), developed from the work of Alexander and Charlesby (28) as well as that of Johansen and Howard-Flanders (29). As shown in Fig. 1, X rays do not directly cause significant damages to cellular macromolecules including DNA. Instead, the ionizing radiation produces hydroxyl free radicals upon encountering the abundant water molecules in biological systems. Subsequent interaction between hydroxyl free radicals and DNA strands results in the formation of DNA-derived free radicals. In the absence of O2, the DNA free radicals can be relatively easily restored through chemical reduction by sulfhydryl compounds and/or other reducing molecules. However, upon interaction with O2, the DNA-derived free radicals can be converted to peroxides that cannot be structurally restored by chemical reduction, which leads to “fixation” of DNA damages. However, the OFH has its own limitations. It does not consider lesions in DNA-associated proteins or chemical alterations in chromatin. Furthermore, work by David Ewing (30) suggests that DNA repair pathways may also affect O2-dependent radiosensitization.
Fig. 1.
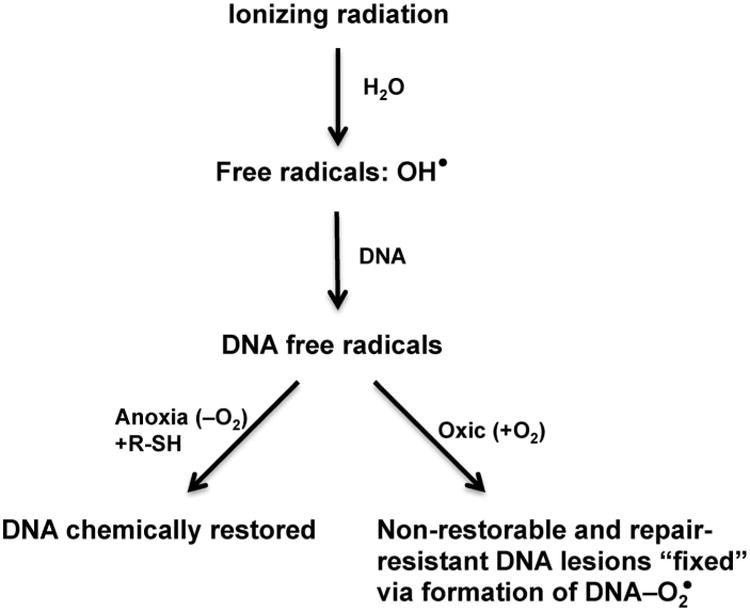
Schematic representation of the oxygen fixation hypothesis (OFH). When living cells are irradiated, X rays interact primarily with H2O to generate the hydroxyl free radicals. These highly reactive free radicals then interact with DNA macromolecules to generate initial DNA free radicals. In the absence of O2, these highly unstable DNA radicals can be chemically restored upon reduction by sulfhydryl compounds (R-SH) and/or other reducing molecules. Under oxic conditions, O2 reacts with DNA radicals and converts them into chemically nonrestorable oxidized derivatives, as if these DNA lesions are “fixed”. These “fixed” lesions are also resistant to enzyme-mediated repair.
Pathological Hypoxia and Activation of the Hypoxia-Inducible Factor Pathway
Mammalian cells respond to O2 deprivation by mobilizing multiple intracellular signal transduction pathways. Hypoxia-induced signal transduction is primarily mediated by the hypoxia-inducible factor (HIF)-1 and HIF-2 by directly binding the hypoxia-responsive enhancer element (HRE, 5′-ACGTG-3′) in hypoxia-induced genes (31, 32). These heterodimeric transcription factors consist of an O2-regulated alpha subunit (primarily HIF-1α or -2α) and the common O2-insensitive HIF-1β (33). Under most physiological conditions with pO2 ≥ 20 mmHg, HIF-α subunits are unstable due to O2-dependent hydroxylation of the two proline residues located in the O2-dependent degradation (ODD) domain (34, 35). The hydroxylated HIF-α is ubiquitinated via interaction with the von Hippel-Lindau (VHL) protein in the E3 ubiquitin ligase complex and eventually degraded by proteasomes (36, 37). Both HIF-1α or -2α proteins become stable when environmental pO2 decreases to or below 2% or 15 mmHg (38, 39). To draw contrast to the radiobiological hypoxia (pO2 = 0–3 mmHg), we will define the range of moderate hypoxia (approximately pO2 = 3–15 mmHg) as pathological hypoxia where robust HIF-α stabilization and HIF-dependent signal transduction occur. Radiosensitivity of mammalian cells is likely to be affected more strongly by hypoxia-dependent signaling mechanisms than by the OFH under such moderate or pathological hypoxia.
Hypoxia-Induced Molecular and Cellular Events Affecting Radiosensitivity
Hypoxia and Selection for Apoptosis-Resistant Clones
The distribution of hypoxia and cell proliferation in patients' tumors has been investigated using immunohistochemical approaches (40–42). Tumor hypoxia has been detected by the hypoxia marker pimonidazole hydrochloride (42) or EF5 (40, 41), both of which are derivatives of 2-nitroimidazole. Nuclear antigen Ki-67 (MIB-1) and/or proliferating cell nuclear antigen (PCNA) were used to identify proliferating cells. These clinical studies found an inverse correlation between binding of hypoxia markers and expression of the proliferation markers. Similar observations were also made in xenografts of several human tumor cell lines (43). According to the Law of Bergonié and Tribondeau (44), hypoxia is capable of reducing radiosensitivity of tumor cells by suppressing cell proliferation.
In an elegant study using mixed populations of transformed cells either proficient or deficient for the tumor suppressor gene p53, the Giaccia group showed that the p53-deficient cells out-survived their p53-proficient counterparts when treated with severe hypoxia in vitro (45). Furthermore, the p53-deficient cells also resisted hypoxia-induced apoptosis in xenografts (45). This seminal work clearly demonstrates that the hypoxic microenvironment favors the survival and expansion of apoptosis-resistant clones. The enrichment of these apoptosis-refractive cells will likely contribute to increased tumor cell survival in response to ionizing radiation.
Hypoxia and Cancer Stem Cells
Clonogenic and tumor-initiating cells are likely to be subpopulations of the cancer stem cell (CSC) repertoire. Recent work has shown that CSCs have much improved DNA repair capability, which confers CSCs with robust survival and also makes them a likely source of relapse after radiotherapy. Elevated DNA repair potentials have been observed in CSCs of several tumor types. Using the mouse mammary tumor virus (MMTV)-Wnt1 spontaneous breast tumor model, Diehn et al. have shown that the Thy1+/CD24+/Lin− CSCs develop fewer γH2AX-positive foci, an often used surrogate marker of DNA double-strand breaks, after ionizing radiation in comparison to their non-CSC counterparts (46). Interestingly, the CSC population is nearly doubled in MMTV-Wnt1 mammary tumors after in vivo X irradiation (46). Consistently, ionizing radiation dramatically increased the Lin−CD24+CD29+ population of CSCs in MCF7 human breast cancer cells (47).
The remarkable radioresistance exhibited by CSCs can potentially be attributed to increased DNA damage checkpoint signal transduction and/or expression of DNA repair genes. Using a syngeneic p53-null mouse mammary tumor model, Zhang et al. have shown that the CSC-like Lin−CD29hiCD24hi cells have increased expression of a set of genes involved in DNA damage response and repair (48). Bao et al. observed that glioma cells expressing the surface antigen CD133, a commonly used CSC marker, are capable of repairing ionizing radiation-induced DNA damage more efficiently than CD133− glioma cells (49). The enhanced repair activity in these CSC-like glioma cells is attributed to robust activation of the ATM-CHK2 and ATR-CHK1 pathways in response to ionizing radiation (49). The ATM-CHK2 and ATR-CHK1 pathways are the critical checkpoints activated in response to DNA double-strand and single-strand breaks, respectively. Consistent with this observation, DNA damage checkpoint activation was also involved in radioresistance of the CD133+/CD44+ prostate CSCs (50) and CD44+/CD24−/low breast CSCs (51).
A rapidly growing body of literature has shown that hypoxia can play a significant role in cell fate decisions and stem cell maintenance (52, 53). Hypoxic tumor cells tend to be poorly differentiated in vivo and often show elevated expression of stem cell-associated genes (54, 55). Hypoxic tumor cells exhibit enhanced clonogenicity in vitro (56–58). Tumor cells pretreated in vitro by hypoxia also have enhanced tumorigenic potential in vivo (55). Furthermore, the metastatic potential of established xenograft tumors is increased when tumor-bearing mice are acutely exposed to atmospheric hypoxia (59, 60). Histological examinations have demonstrated that poorly differentiated primary pancreatic cancer cells show strong nuclear accumulation of HIF-1α protein (61). The CSC-like cells of neuroblastomas (62, 63) or gliomas (64) tend to have increased levels of HIF-1α and/or HIF-2α. Downregulation of HIF-1α by RNA interference results in reduced clonogenic growth of glioma CSCs (64) and chronic myeloid leukemia CSCs (65). Mathieu et al. have shown that hypoxia can activate an embryonic stem cell-like transcription program in a HIF-dependent manner (66). These results suggest that HIF-1 and/or HIF-2 facilitate the maintenance of cancer stem cells.
Indeed, the HIF pathway can induce the expression of specific genes associated with stem cell maintenance. The prominent pluripotency gene POU5F1 or Oct3/4 is capable of promoting tumorigenesis (67). Several types of human cancers have elevated levels of POU5F1 (68–71). Covello et al. have shown that HIF-2α, but not HIF-1α, regulates the transcription of POU5F1 by directly binding to its promoter/enhancer in mouse embryonic stem cells (72). However, it has yet to be determined whether hypoxia also increases POU5F1 expression in common types of tumors.
Delta-like 1 homolog (Drosophila) or DLK1, an emerging stem cell gene, is expressed primarily in embryonic tissues and immature cells (73). Overexpression of DLK1 has been observed in a wide range of tumors (74–79). DLK1 is expressed in undifferentiated neuroblastoma cells only and is required to sustain both clonogenicity and tumorigenicity (57, 80). Hypoxia strongly increases DLK1 expression mediated by direct binding of HIF-1α and HIF-2α to DLK1 promoter/enhancer (57). In addition, the DLK1-positve neuroblastoma cells are also preferentially localized in the pimonidazole-positive hypoxic region (80).
The penta-span transmembrane glycoprotein prominin-1 (CD133), a widely used CSC marker (81), is upregulated in hypoxia (1% O2)-treated human glioma cells and can promote the expansion of CSCs (82–84). Knocking down either HIF-1α (84) or HIF-2α (83) reduces the hypoxia-induced CD133 expression in glioma cells, suggesting that both HIF-1α and HIF-2α are involved in the hypoxia-induced CD133 expression. However, it is worth noting that severe hypoxia (0.1% O2) downregulates CD133 expression in gastric, colorectal and lung cancer cell lines (85). It is possible that CD133 is involved in CSC maintenance under moderate (1% O2) but not severe (0.1% O2) hypoxia. These observations suggest that stem cell pathways are differentially regulated at different levels of hypoxia or that different subpopulations of CSCs are preferentially selected at different levels of hypoxia. Collectively, these studies demonstrate the ability of hypoxia to create a favorable niche where CSCs proliferate while maintaining their undifferentiated state and sustaining self-renewal (52, 53).
Hypoxia and DNA Repair and Genomic Integrity
Hypoxia has been well recognized as a potent environmental stress on genomic integrity (86, 87). Severe hypoxia or anoxia is capable of inducing DNA over-replication (88) and point mutations (89). Interestingly, increased mutation rates were also found in tumor allografts containing large areas with 0–2 mmHg pO2 (89). Furthermore, hypoxia strongly induces fragile site formation that leads to genomic rearrangements, fusion of double minutes (DMs) and generation of homogeneously staining regions (HSRs) (90). Mechanistically, hypoxia-induced genomic instability can be attributed to diminished repair under hypoxia (91).
The homology-directed repair (HDR) is suppressed by hypoxia (92, 93). The expression of several key HDR genes including RAD51 and BRCA1 is repressed under hypoxic conditions (92, 93). Consistently, reduced expression of RAD51 protein was also observed in hypoxic regions of xenograft tumors in mice (93). Mechanistically, hypoxia increases the binding of E2F4, a transcription inhibitor of the E2F transcription factor family, to the promoter/ enhancer regions of RAD51 and BRCA1 genes while decreasing the binding by the transcription activator E2F1 (92). On the other hand, epigenetic mechanisms may also contribute to hypoxia-induced gene silencing (94). Under chronic hypoxic conditions, downregulation of RAD51 and BRCA1 protein expression can potentially be caused by hypoxia-induced selective repression of mRNA translation (95). In contrast, the expression of NBS1, a component of the MRE11A-RAD50-NBS1 (MRN) complex, is also downregulated by hypoxia, but through a HIF-1-specific mechanism (96). These observations demonstrate that expression of HDR-associated genes under hypoxia is subject to complex regulations at multiple levels including chromatin remodeling, transcription factors and protein synthesis.
The impact of hypoxia on mismatch repair (MMR) has been extensively investigated. When exposed to severe hypoxia (<0.01% O2), both human and mouse tumor cells downregulated the expression of MLH1 with minimal effects on other members of the MMR genes (97). The rate of mutagenesis induced by severe hypoxia was inversely correlated with MLH1 levels (97). MLH1 expression was also reduced in several human tumor cell lines exposed to 1% O2, and the repression was mediated by the transcription repressors DEC1 and DEC2, both of which were induced by hypoxia (98). However, epigenetic mechanisms were also implicated in hypoxia-dependent suppression of MLH1 (97). Very recently, it has been shown that the lysine-specific demethylase LSD1, a member of the histone demethylases, forms a co-repressor complex in the promoter/enhancer region of the MLH1 gene to epigenetically silence MLH1 expression under hypoxia (99). As for other members of the MMR genes, Koshiji et al. reported that expression of MSH2 and MSH6 was decreased in both normal and human tumor cells upon exposure to 1% O2 (100). Mechanistically, HIF-1α mediated the repression by displacing the transcription factor Myc from the gene promoters (100). This association between HIF-1 and reduced expression of MSH2 protein is more pronounced in human colorectal tumors with undetectable tumor suppressor p53 (100).
In addition to the HDR and MMR pathways, hypoxia can also negatively regulate the expression of genes involved in other repair pathways, including non-homologous end joining (NHEJ), nucleotide excision repair (NER) and Fanconi anemia pathway (101). Collectively, these studies clearly indicate that hypoxia has the potential to affect all the major DNA repair pathways by multiple mechanisms. These studies have also painted a complex picture of transcriptional repression mechanisms from direct action of specific transcription factors to chromatin modifications. It is likely that the actual mechanisms deployed will depend on both the cell-intrinsic properties and the severity of hypoxia.
Hypoxia-Regulated MicroRNAs
In addition to transcriptional regulation of protein-coding genes, hypoxia has been found to regulate the expression of non-coding genes. As an important class of non-coding genes, microRNAs or miRNAs are single-stranded oligoribonucleic acids consisting of approximately 22 ribonucleotides that primarily function as inhibitors of mRNA translation (102). Typically, each miRNA can have hundreds of mRNA targets. Conversely, each mRNA often contains target sites for multiple miRNAs. The expression, processing and target recognition of miRNA have been extensively reviewed in the literature. Herein, we will focus on hypoxia-regulated miRNAs and their roles in modulating radiosensitivity.
Studies during the past decade have shown that hypoxia can have a profound effect on the entire miRNA genome with both induction and suppression of miRNA expression, likely via complex mechanisms (103–105). However, with the exception of miR-210, there is little consensus among hypoxia-dependent miRNA profiles in different cell lines. These discrepancies may result from cell type-dependent genomic landscapes, different hypoxia conditions and various technology platforms of miRNA expression analysis.
Interestingly, the archetype of hypoxia-induced miRNA appears to be miR-210 (106–109). It is robustly induced by hypoxia in a wide range of cells (103). The hypoxic induction of miR-210 is transcriptionally regulated by HIF-1 (106–109) and/or HIF-2 (110). The promoter/enhancer region of the miR-210 gene contains a functional hypoxia-responsive element (HRE) that is conserved between mice and humans and is directly bound by HIF-1 (108, 111).
It has been shown that miR-210 has the potential to play an important role in the regulation of radiosensitivity by increasing radioresistance. Ectopic overexpression of miR-210 can significantly increase the clonal survival of the A549 human lung cancer cells X irradiated (0–10 Gy) in ambient air to the level of control-treated A549 cells irradiated at 1% O2 (112). The clonal survival of miR-210-expressing cells is further enhanced under hypoxia after exposure to ionizing radiation (112). On the other hand, downregulation of miR-210 expression in human hepatoma cell lines using a lentivirus-based miR-210 antisense gene results in decreased cell viability under hypoxia (1% O2) and elevated radiosensitivity in vitro (113). Because miR-210 has a multitude of targets that are involved in a wide range of cell functions including DNA repair and genomic maintenance, it remains to be determined how miR-210 regulates cellular response to ionizing radiation.
The expression of miR-21 is also significantly increased by hypoxia at 1% O2 in colon cancer cell lines (114) and in pancreatic cancer cell lines (115). The hypoxic induction of miR-21 is also mediated transcriptionally by HIF-1 (115). Antisense-mediated downregulation of miR-21 increases apoptosis and reduces proliferation of pancreatic cancer cells (115). Others have shown that miR-21 promotes radioresistance of breast cancer cells perhaps by activating the G2/M checkpoint (116). The radioresistance conferred by miR-21 may also be attributed to its involvement in the negative regulation of CDC25A phosphatase (114).
Babar et al. found that human lung cancer cell lines significantly increase miR-155 expression when exposed to severe hypoxia (117). Ectopic expression of miR-155 enhances clonogenic survival after exposure to ionizing radiation (117). It is possible that miR-155 promotes cell survival by targeting the pro-apoptosis gene FOXO3 (118) whose expression is downregulated under hypoxia (117). Another potential mechanism involves WEE1 kinase, an important G2/M-checkpoint regulator whose expression is often downregulated in malignant tumors (119). Studies have shown that miR-155 suppresses the expression of WEE1 protein (120, 121). However, the exact mechanism by which miR-155 interacts with WEE1 mRNA remains to be delineated.
Collectively, these observations suggest that the miRNA transcriptome is differentially regulated in different cell types under hypoxic conditions. Nonetheless, it can be reasonably extrapolated that different sets of miRNAs are involved in the regulation of radiosensitivity in different tumor cells exposed to different levels of hypoxia.
Summary
Undoubtedly, hypoxic cells tend to be more radioresistant than nonhypoxic cells. Nonetheless, it is also true that genes of the major repair pathways are negatively regulated by hypoxia. Thus, the unresolved question is why hypoxic cells exhibit elevated radioresistance despite the overall down-regulation of various DNA repair genes. In Fig. 2, we propose a model to reconcile these discrepancies. Central to this model is the idea that nonlethal pathological hypoxia facilitates the maintenance and expansion of CSCs. Compared to nonstem cells, CSCs may have higher basal levels of activation of the DNA damage checkpoint pathways, including the ATR-CHK1 and ATM-CHK2 pathways. The enhanced DNA damage checkpoint activities can counterbalance or compensate for the decreased levels of DNA repair genes, leading to improved efficiency of the overall DNA damage response. In addition, hypoxia-induced miRNAs such as miR-210, miR-21 and miR-155 desensitize the response of hypoxic cells to apoptosis. Several other mechanisms, including hypoxia-dependent defense against reactive oxygen species (122, 123) and hypoxia-induced autophagy (122, 124–126), may also contribute to hypoxia-dependent regulation of cellular radiosensitivity. Nevertheless, these mechanisms will likely work in synergy to increase the survival and expansion of CSCs or clonogenic tumor-initiating cells in a hypoxic microenvironment after ionizing radiation.
Fig. 2.
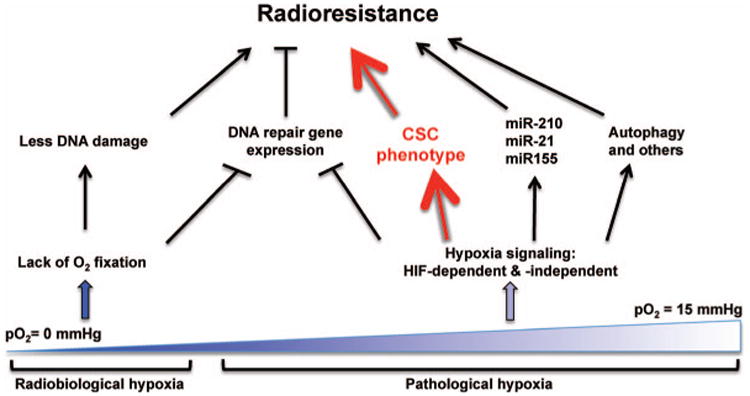
Mechanisms of radioresistance at different pO2. Under the radiobiological or severe hypoxia (pO2 < 3 mmHg), the oxygen fixation hypothesis (OFH) is likely the main mechanism of radioresistance even though expression of DNA repair genes is strongly reduced. In contrast, hypoxia-activated pathways, both HIF-dependent and independent, will be actively operative under the pathological hypoxia (pO2 = 3–15 mmHg) to elicit a multitude of molecular and cellular changes. Chief among them, the cancer stem cell (CSC) phenotype has the potential to play a prominent role in conferring radioresistance.
Acknowledgments
The authors thank members of the Yun Laboratory for insightful discussions. CL is supported by a scholarship from Xiangya Hospital, Central South University, China. ZY is supported by grants from the NIH-NCI (CA178254 and CA148996).
References
- 1.Dewhirst MW, Cao Y, Moeller B. Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nat Rev Cancer. 2008;8:425–37. doi: 10.1038/nrc2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vaupel P, Mayer A. Hypoxia in tumors: pathogenesis-related classification, characterization of hypoxia subtypes, and associated biological and clinical implications. Adv Exp Med Biol. 2014;812:19–24. doi: 10.1007/978-1-4939-0620-8_3. [DOI] [PubMed] [Google Scholar]
- 3.Bayer C, Vaupel P. Acute versus chronic hypoxia in tumors: Controversial data concerning time frames and biological consequences. Strahlenther Onkol. 2012;188:616–27. doi: 10.1007/s00066-012-0085-4. [DOI] [PubMed] [Google Scholar]
- 4.Ljungkvist AS, Bussink J, Kaanders JH, van der Kogel AJ. Dynamics of tumor hypoxia measured with bioreductive hypoxic cell markers. Radiat Res. 2007;167:127–45. doi: 10.1667/rr0719.1. [DOI] [PubMed] [Google Scholar]
- 5.Brown JM. Evidence for acutely hypoxic cells in mouse tumours, and a possible mechanism of reoxygenation. Br J Radiol. 1979;52:650–6. doi: 10.1259/0007-1285-52-620-650. [DOI] [PubMed] [Google Scholar]
- 6.Dewhirst MW. Intermittent hypoxia furthers the rationale for hypoxia-inducible factor-1 targeting. Cancer Res. 2007;67:854–5. doi: 10.1158/0008-5472.CAN-06-4744. [DOI] [PubMed] [Google Scholar]
- 7.Thomlinson RH, Gray LH. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br J Cancer. 1955;9:539–49. doi: 10.1038/bjc.1955.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gatenby RA, Coia LR, Richter MP, Katz H, Moldofsky PJ, Engstrom P, et al. Oxygen tension in human tumors: in vivo mapping using CT-guided probes. Radiology. 1985;156:211–4. doi: 10.1148/radiology.156.1.4001408. [DOI] [PubMed] [Google Scholar]
- 9.Gatenby RA, Kessler HB, Rosenblum JS, Coia LR, Moldofsky PJ, Hartz WH, et al. Oxygen distribution in squamous cell carcinoma metastases and its relationship to outcome of radiation therapy. Int J Radiat Oncol Biol Phys. 1988;14:831–8. doi: 10.1016/0360-3016(88)90002-8. [DOI] [PubMed] [Google Scholar]
- 10.Vaupel P, Schlenger K, Knoop C, Hockel M. Oxygenation of human tumors: evaluation of tissue oxygen distribution in breast cancers by computerized O2 tension measurements. Cancer Res. 1991;51:3316–22. [PubMed] [Google Scholar]
- 11.Vaupel P, Hockel M, Mayer A. Detection and characterization of tumor hypoxia using pO2 histography. Antioxid Redox Signal. 2007;9:1221–35. doi: 10.1089/ars.2007.1628. [DOI] [PubMed] [Google Scholar]
- 12.Koong AC, Mehta VK, Le QT, Fisher GA, Terris DJ, Brown JM, et al. Pancreatic tumors show high levels of hypoxia. Int J Radiat Oncol Biol Phys. 2000;48:919–22. doi: 10.1016/s0360-3016(00)00803-8. [DOI] [PubMed] [Google Scholar]
- 13.Molls M, Feldmann HJ, Fuller J. Oxygenation of locally advanced recurrent rectal cancer, soft tissue sarcoma and breast cancer. Adv Exp Med Biol. 1994;345:459–63. doi: 10.1007/978-1-4615-2468-7_61. [DOI] [PubMed] [Google Scholar]
- 14.Brizel DM, Dodge RK, Clough RW, Dewhirst MW. Oxygenation of head and neck cancer: changes during radiotherapy and impact on treatment outcome. Radiother Oncol. 1999;53:113–7. doi: 10.1016/s0167-8140(99)00102-4. [DOI] [PubMed] [Google Scholar]
- 15.Brizel DM, Scully SP, Harrelson JM, Layfield LJ, Bean JM, Prosnitz LR, et al. Tumor oxygenation predicts for the likelihood of distant metastases in human soft tissue sarcoma. Cancer Res. 1996;56:941–3. [PubMed] [Google Scholar]
- 16.Brizel DM, Sibley GS, Prosnitz LR, Scher RL, Dewhirst MW. Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 1997;38:285–9. doi: 10.1016/s0360-3016(97)00101-6. [DOI] [PubMed] [Google Scholar]
- 17.Fyles A, Milosevic M, Hedley D, Pintilie M, Levin W, Manchul L, et al. Tumor hypoxia has independent predictor impact only in patients with node-negative cervix cancer. J Clin Oncol. 2002;20:680–7. doi: 10.1200/JCO.2002.20.3.680. [DOI] [PubMed] [Google Scholar]
- 18.Knocke TH, Weitmann HD, Feldmann HJ, Selzer E, Potter R. Intratumoral pO2-measurements as predictive assay in the treatment of carcinoma of the uterine cervix. Radiother Oncol. 1999;53:99–104. doi: 10.1016/s0167-8140(99)00139-5. [DOI] [PubMed] [Google Scholar]
- 19.Nordsmark M, Bentzen SM, Rudat V, Brizel D, Lartigau E, Stadler P, et al. Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. An international multi-center study. Radiother Oncol. 2005;77:18–24. doi: 10.1016/j.radonc.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 20.Nordsmark M, Loncaster J, Aquino-Parsons C, Chou SC, Gebski V, West C, et al. The prognostic value of pimonidazole and tumour pO2 in human cervix carcinomas after radiation therapy: a prospective international multi-center study. Radiother Oncol. 2006;80:123–31. doi: 10.1016/j.radonc.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 21.Hall EJ, Giaccia AJ. Radiobiology for the radiologist. 7th. Philadelphia: Lippincott Williams & Wilkins; 2012. [Google Scholar]
- 22.Rockwell S, Dobrucki IT, Kim EY, Marrison ST, Vu VT. Hypoxia and radiation therapy: past history, ongoing research, and future promise. Curr Mol Med. 2009;9:442–58. doi: 10.2174/156652409788167087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gray LH. Oxygenation in radiotherapy. I. Radiobiological considerations. Br J Radiol. 1957;30:403–6. doi: 10.1259/0007-1285-30-356-403. [DOI] [PubMed] [Google Scholar]
- 24.Gray LH. Radiobiologic basis of oxygen as a modifying factor in radiation therapy. Am J Roentgenol Radium Ther Nucl Med. 1961;85:803–15. [PubMed] [Google Scholar]
- 25.Gray LH, Conger AD, Ebert M, Hornsey S, Scott OC. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radiol. 1953;26:638–48. doi: 10.1259/0007-1285-26-312-638. [DOI] [PubMed] [Google Scholar]
- 26.Kirkpatrick JP, Cardenas-Navia LI, Dewhirst MW. Predicting the effect of temporal variations in PO2 on tumor radiosensitivity. Int J Radiat Oncol Biol Phys. 2004;59:822–33. doi: 10.1016/j.ijrobp.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 27.Hockel M, Schlenger K, Knoop C, Vaupel P. Oxygenation of carcinomas of the uterine cervix: evaluation by computerized O2 tension measurements. Cancer Res. 1991;51:6098–102. [PubMed] [Google Scholar]
- 28.Alexander P, Charlesby A. Energy transfer in macromolecules exposed to ionizing radiations. Nature. 1954;173:578–9. doi: 10.1038/173578a0. [DOI] [PubMed] [Google Scholar]
- 29.Johansen I, Howard-Flanders P. Macromolecular repair and free radical scavenging in the protection of bacteria against X-rays. Radiat Res. 1965;24:184–200. [PubMed] [Google Scholar]
- 30.Ewing D. The oxygen fixation hypothesis: a reevaluation. Am J Clin Oncol. 1998;21:355–61. doi: 10.1097/00000421-199808000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol. 2000;88:1474–80. doi: 10.1152/jappl.2000.88.4.1474. [DOI] [PubMed] [Google Scholar]
- 32.Harris AL. Hypoxia–a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 33.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–32. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 34.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–8. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 35.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–72. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 36.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis [see comments] Nature. 1999;399:271–5. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 37.Ohh M, Park CW, Ivan M, Hoffman MA, Kim TY, Huang LE, et al. Ubiquitination of hypoxia-inducible factor requires direct binding to the b-domain of the von Hippel-Lindau protein. Nat Cell Biol. 2000;2:423–7. doi: 10.1038/35017054. [DOI] [PubMed] [Google Scholar]
- 38.Jiang BH, Semenza GL, Bauer C, Marti HH. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am J Physiol. 1996;271:C1172–80. doi: 10.1152/ajpcell.1996.271.4.C1172. [DOI] [PubMed] [Google Scholar]
- 39.Lin Q, Cong X, Yun Z. Differential hypoxic regulation of hypoxia-inducible factors 1α and 2α. Mol Cancer Res. 2011;9:757–65. doi: 10.1158/1541-7786.MCR-11-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Evans SM, Hahn SM, Magarelli DP, Koch CJ. Hypoxic heterogeneity in human tumors: EF5 binding, vasculature, necrosis, and proliferation. Am J Clin Oncol. 2001;24:467–72. doi: 10.1097/00000421-200110000-00011. [DOI] [PubMed] [Google Scholar]
- 41.Evans SM, Jenkins KW, Chen HI, Jenkins WT, Judy KD, Hwang WT, et al. The relationship among hypoxia, proliferation, and outcome in patients with de novo glioblastoma: a pilot study. Transl Oncol. 2010;3:160–9. doi: 10.1593/tlo.09265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kennedy AS, Raleigh JA, Perez GM, Calkins DP, Thrall DE, Novotny DB, et al. Proliferation and hypoxia in human squamous cell carcinoma of the cervix: first report of combined immunohistochemical assays. Int J Radiat Oncol Biol Phys. 1997;37:897–905. doi: 10.1016/s0360-3016(96)00539-1. [DOI] [PubMed] [Google Scholar]
- 43.Durand RE, Raleigh JA. Identification of nonproliferating but viable hypoxic tumor cells in vivo. Cancer Res. 1998;58:3547–50. [PubMed] [Google Scholar]
- 44.Bergonié J, Tribondeau L. Interpretation of some results from radiotherapy and an attempt to determine a rational treatment technique. 1906. Yale J Biol Med. 2003;76:181–2. [PMC free article] [PubMed] [Google Scholar]
- 45.Graeber TG, Osmanian C, Jacks T, Housman DE, Koch CJ, Lowe SW, et al. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature. 1996;379:88–91. doi: 10.1038/379088a0. [DOI] [PubMed] [Google Scholar]
- 46.Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780–3. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woodward WA, Chen MS, Behbod F, Alfaro MP, Buchholz TA, Rosen JM. WNT/β-catenin mediates radiation resistance of mouse mammary progenitor cells. Proc Natl Acad Sci U S A. 2007;104:618–23. doi: 10.1073/pnas.0606599104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang M, Behbod F, Atkinson RL, Landis MD, Kittrell F, Edwards D, et al. Identification of tumor-initiating cells in a p53-null mouse model of breast cancer. Cancer Res. 2008;68:4674–82. doi: 10.1158/0008-5472.CAN-07-6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–60. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 50.Wang X, Ma Z, Xiao Z, Liu H, Dou Z, Feng X, et al. Chk1 knockdown confers radiosensitization in prostate cancer stem cells. Oncol Rep. 2012;28:2247–54. doi: 10.3892/or.2012.2068. [DOI] [PubMed] [Google Scholar]
- 51.Yin H, Glass J. The phenotypic radiation resistance of CD44+/CD24(-or low) breast cancer cells is mediated through the enhanced activation of ATM signaling. PLoS One. 2011;6:e24080. doi: 10.1371/journal.pone.0024080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin Q, Yun Z. Impact of the hypoxic tumor microenvironment on the regulation of cancer stem cell characteristics. Cancer Biol Ther. 2010;9:949–56. doi: 10.4161/cbt.9.12.12347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yun Z, Lin Q. Hypoxia and regulation of cancer cell stemness. Adv Exp Med Biol. 2014;772:41–53. doi: 10.1007/978-1-4614-5915-6_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Das B, Tsuchida R, Malkin D, Koren G, Baruchel S, Yeger H. Hypoxia enhances tumor stemness by increasing the invasive and tumorigenic side population fraction. Stem Cells. 2008;26:1818–30. doi: 10.1634/stemcells.2007-0724. [DOI] [PubMed] [Google Scholar]
- 55.Jogi A, Ora I, Nilsson H, Lindeheim A, Makino Y, Poellinger L, et al. Hypoxia alters gene expression in human neuroblastoma cells toward an immature and neural crest-like phenotype. Proc Natl Acad Sci U S A. 2002;99:7021–6. doi: 10.1073/pnas.102660199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Desplat V, Faucher JL, Mahon FX, Dello Sbarba P, Praloran V, Ivanovic Z. Hypoxia modifies proliferation and differentiation of CD34(+) CML cells. Stem Cells. 2002;20:347–54. doi: 10.1634/stemcells.20-4-347. [DOI] [PubMed] [Google Scholar]
- 57.Kim Y, Lin Q, Zelterman D, Yun Z. Hypoxia-regulated delta-like 1 homologue enhances cancer cell stemness and tumorigenicity. Cancer Res. 2009;69:9271–80. doi: 10.1158/0008-5472.CAN-09-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schmaltz C, Hardenbergh PH, Wells A, Fisher DE. Regulation of proliferation-survival decisions during tumor cell hypoxia. Mol Cell Biol. 1998;18:2845–54. doi: 10.1128/mcb.18.5.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cairns RA, Hill RP. Acute hypoxia enhances spontaneous lymph node metastasis in an orthotopic murine model of human cervical carcinoma. Cancer Res. 2004;64:2054–61. doi: 10.1158/0008-5472.can-03-3196. [DOI] [PubMed] [Google Scholar]
- 60.Cairns RA, Kalliomaki T, Hill RP. Acute (cyclic) hypoxia enhances spontaneous metastasis of KHT murine tumors. Cancer Res. 2001;61:8903–8. [PubMed] [Google Scholar]
- 61.Couvelard A, O'Toole D, Turley H, Leek R, Sauvanet A, Degott C, et al. Microvascular density and hypoxia-inducible factor pathway in pancreatic endocrine tumours: negative correlation of microvascular density and VEGF expression with tumour progression. Br J Cancer. 2005;92:94–101. doi: 10.1038/sj.bjc.6602245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pietras A, Gisselsson D, Ora I, Noguera R, Beckman S, Navarro S, et al. High levels of HIF-2α highlight an immature neural crestlike neuroblastoma cell cohort located in a perivascular niche. J Pathol. 2008;214:482–8. doi: 10.1002/path.2304. [DOI] [PubMed] [Google Scholar]
- 63.Pietras A, Hansford LM, Johnsson AS, Bridges E, Sjolund J, Gisselsson D, et al. HIF-2alpha maintains an undifferentiated state in neural crest-like human neuroblastoma tumor-initiating cells. Proc Natl Acad Sci U S A. 2009;106:16805–10. doi: 10.1073/pnas.0904606106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li Z, Bao S, Wu Q, Wang H, Eyler C, Sathornsumetee S, et al. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell. 2009;15:501–13. doi: 10.1016/j.ccr.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ng KP, Manjeri A, Lee KL, Huang W, Tan SY, Chuah CT, et al. Physiologic hypoxia promotes maintenance of CML stem cells despite effective BCR-ABL1 inhibition. Blood. 2014;123:3316–26. doi: 10.1182/blood-2013-07-511907. [DOI] [PubMed] [Google Scholar]
- 66.Mathieu J, Zhou W, Xing Y, Sperber H, Ferreccio A, Agoston Z, et al. Hypoxia-inducible factors have distinct and stage-specific roles during reprogramming of human cells to pluripotency. Cell Stem Cell. 2014;14:592–605. doi: 10.1016/j.stem.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hochedlinger K, Yamada Y, Beard C, Jaenisch R. Ectopic expression of Oct-4 blocks progenitor-cell differentiation and causes dysplasia in epithelial tissues. Cell. 2005;121:465–77. doi: 10.1016/j.cell.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 68.Cheng L. Establishing a germ cell origin for metastatic tumors using OCT4 immunohistochemistry. Cancer. 2004;101:2006–10. doi: 10.1002/cncr.20566. [DOI] [PubMed] [Google Scholar]
- 69.Gidekel S, Pizov G, Bergman Y, Pikarsky E. Oct-3/4 is a dose-dependent oncogenic fate determinant. Cancer Cell. 2003;4:361–70. doi: 10.1016/s1535-6108(03)00270-8. [DOI] [PubMed] [Google Scholar]
- 70.Jones TD, Ulbright TM, Eble JN, Cheng L. OCT4: A sensitive and specific biomarker for intratubular germ cell neoplasia of the testis. Clin Cancer Res. 2004;10:8544–7. doi: 10.1158/1078-0432.CCR-04-0688. [DOI] [PubMed] [Google Scholar]
- 71.Tai MH, Chang CC, Kiupel M, Webster JD, Olson LK, Trosko JE. Oct4 expression in adult human stem cells: evidence in support of the stem cell theory of carcinogenesis. Carcinogenesis. 2005;26:495–502. doi: 10.1093/carcin/bgh321. [DOI] [PubMed] [Google Scholar]
- 72.Covello KL, Kehler J, Yu H, Gordan JD, Arsham AM, Hu CJ, et al. HIF-2α regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev. 2006;20:557–70. doi: 10.1101/gad.1399906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Floridon C, Jensen CH, Thorsen P, Nielsen O, Sunde L, Westergaard JG, et al. Does fetal antigen 1 (FA1) identify cells with regenerative, endocrine and neuroendocrine potentials? A study of FA1 in embryonic, fetal, and placental tissue and in maternal circulation. Differentiation. 2000;66:49–59. doi: 10.1046/j.1432-0436.2000.066001049.x. [DOI] [PubMed] [Google Scholar]
- 74.Jensen CH, Krogh TN, Hojrup P, Clausen PP, Skjodt K, Larsson LI, et al. Protein structure of fetal antigen 1 (FA1). A novel circulating human epidermal-growth-factor-like protein expressed in neuroendocrine tumors and its relation to the gene products of dlk and pG2. Eur J Biochem. 1994;225:83–92. doi: 10.1111/j.1432-1033.1994.00083.x. [DOI] [PubMed] [Google Scholar]
- 75.Tornehave D, Jensen CH, Teisner B, Larsson LI. FA1 immunoreactivity in endocrine tumours and during development of the human fetal pancreas; negative correlation with glucagon expression. Histochem Cell Biol. 1996;106:535–42. doi: 10.1007/BF02473268. [DOI] [PubMed] [Google Scholar]
- 76.Yin D, Xie D, Sakajiri S, Miller CW, Zhu H, Popoviciu ML, et al. DLK1: increased expression in gliomas and associated with oncogenic activities. Oncogene. 2006;25:1852–61. doi: 10.1038/sj.onc.1209219. [DOI] [PubMed] [Google Scholar]
- 77.Sakajiri S, O'Kelly J, Yin D, Miller CW, Hofmann WK, Oshimi K, et al. Dlk1 in normal and abnormal hematopoiesis. Leukemia. 2005;19:1404–10. doi: 10.1038/sj.leu.2403832. [DOI] [PubMed] [Google Scholar]
- 78.Van Limpt VA, Chan AJ, Van Sluis PG, Caron HN, Van Noesel CJ, Versteeg R. High delta-like 1 expression in a subset of neuroblastoma cell lines corresponds to a differentiated chromaffin cell type. Int J Cancer. 2003;105:61–9. doi: 10.1002/ijc.11047. [DOI] [PubMed] [Google Scholar]
- 79.Li L, Forman SJ, Bhatia R. Expression of DLK1 in hematopoietic cells results in inhibition of differentiation and proliferation. Oncogene. 2005;24:4472–6. doi: 10.1038/sj.onc.1208637. [DOI] [PubMed] [Google Scholar]
- 80.Begum A, Kim Y, Lin Q, Yun Z. DLK1, delta-like 1 homolog (Drosophila), regulates tumor cell differentiation in vivo. Cancer Lett. 2012;318:26–33. doi: 10.1016/j.canlet.2011.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–68. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 82.Griguer CE, Oliva CR, Gobin E, Marcorelles P, Benos DJ, Lancaster JR, Jr, et al. CD133 is a marker of bioenergetic stress in human glioma. PLoS One. 2008;3:e3655. doi: 10.1371/journal.pone.0003655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Seidel S, Garvalov BK, Wirta V, von Stechow L, Schanzer A, Meletis K, et al. A hypoxic niche regulates glioblastoma stem cells through hypoxia inducible factor 2α. Brain. 2010;133:983–95. doi: 10.1093/brain/awq042. [DOI] [PubMed] [Google Scholar]
- 84.Soeda A, Park M, Lee D, Mintz A, Androutsellis-Theotokis A, McKay RD, et al. Hypoxia promotes expansion of the CD133-positive glioma stem cells through activation of HIF-1α. Oncogene. 2009;28:3949–59. doi: 10.1038/onc.2009.252. [DOI] [PubMed] [Google Scholar]
- 85.Matsumoto K, Arao T, Tanaka K, Kaneda H, Kudo K, Fujita Y, et al. mTOR signal and hypoxia-inducible factor-1α regulate CD133 expression in cancer cells. Cancer Res. 2009;69:7160–4. doi: 10.1158/0008-5472.CAN-09-1289. [DOI] [PubMed] [Google Scholar]
- 86.Bristow RG, Hill RP. Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nat Rev Cancer. 2008;8:180–92. doi: 10.1038/nrc2344. [DOI] [PubMed] [Google Scholar]
- 87.Glazer PM, Hegan DC, Lu Y, Czochor J, Scanlon SE. Hypoxia and DNA repair. Yale J Biol Med. 2013;86:443–51. [PMC free article] [PubMed] [Google Scholar]
- 88.Young SD, Marshall RS, Hill RP. Hypoxia induces DNA overreplication and enhances metastatic potential of murine tumor cells. Proc Natl Acad Sci U S A. 1988;85:9533–7. doi: 10.1073/pnas.85.24.9533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Reynolds TY, Rockwell S, Glazer PM. Genetic instability induced by the tumor microenvironment. Cancer Res. 1996;56:5754–7. [PubMed] [Google Scholar]
- 90.Coquelle A, Toledo F, Stern S, Bieth A, Debatisse M. A new role for hypoxia in tumor progression: induction of fragile site triggering genomic rearrangements and formation of complex DMs and HSRs. Mol Cell. 1998;2:259–65. doi: 10.1016/s1097-2765(00)80137-9. [DOI] [PubMed] [Google Scholar]
- 91.Yuan J, Narayanan L, Rockwell S, Glazer PM. Diminished DNA repair and elevated mutagenesis in mammalian cells exposed to hypoxia and low pH. Cancer Res. 2000;60:4372–6. [PubMed] [Google Scholar]
- 92.Bindra RS, Gibson SL, Meng A, Westermark U, Jasin M, Pierce AJ, et al. Hypoxia-induced down-regulation of BRCA1 expression by E2Fs. Cancer Res. 2005;65:11597–604. doi: 10.1158/0008-5472.CAN-05-2119. [DOI] [PubMed] [Google Scholar]
- 93.Bindra RS, Schaffer PJ, Meng A, Woo J, Maseide K, Roth ME, et al. Down-regulation of Rad51 and decreased homologous recombination in hypoxic cancer cells. Mol Cell Biol. 2004;24:8504–18. doi: 10.1128/MCB.24.19.8504-8518.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lu Y, Chu A, Turker MS, Glazer PM. Hypoxia-induced epigenetic regulation and silencing of the BRCA1 promoter. Mol Cell Biol. 2011;31:3339–50. doi: 10.1128/MCB.01121-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chan N, Koritzinsky M, Zhao H, Bindra R, Glazer PM, Powell S, et al. Chronic hypoxia decreases synthesis of homologous recombination proteins to offset chemoresistance and radioresistance. Cancer Res. 2008;68:605–14. doi: 10.1158/0008-5472.CAN-07-5472. [DOI] [PubMed] [Google Scholar]
- 96.To KK, Sedelnikova OA, Samons M, Bonner WM, Huang LE. The phosphorylation status of PAS-B distinguishes HIF-1alpha from HIF-2alpha in NBS1 repression. EMBO J. 2006;25:4784–94. doi: 10.1038/sj.emboj.7601369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mihaylova VT, Bindra RS, Yuan J, Campisi D, Narayanan L, Jensen R, et al. Decreased expression of the DNA mismatch repair gene Mlh1 under hypoxic stress in mammalian cells. Mol Cell Biol. 2003;23:3265–73. doi: 10.1128/MCB.23.9.3265-3273.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nakamura H, Tanimoto K, Hiyama K, Yunokawa M, Kawamoto T, Kato Y, et al. Human mismatch repair gene, MLH1, is transcriptionally repressed by the hypoxia-inducible transcription factors, DEC1 and DEC2. Oncogene. 2008;27:4200–9. doi: 10.1038/onc.2008.58. [DOI] [PubMed] [Google Scholar]
- 99.Lu Y, Wajapeyee N, Turker MS, Glazer PM. Silencing of the DNA mismatch repair gene MLH1 Induced by hypoxic stress in a pathway dependent on the histone demethylase LSD1. Cell Rep. 2014;8:501–13. doi: 10.1016/j.celrep.2014.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Koshiji M, To KK, Hammer S, Kumamoto K, Harris AL, Modrich P, et al. HIF-1α induces genetic instability by transcriptionally downregulating MutSalpha expression. Mol Cell. 2005;17:793–803. doi: 10.1016/j.molcel.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 101.Luoto KR, Kumareswaran R, Bristow RG. Tumor hypoxia as a driving force in genetic instability. Genome Integr. 2013;4:5. doi: 10.1186/2041-9414-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–31. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 103.Gee HE, Ivan C, Calin GA, Ivan M. HypoxamiRs and cancer: from biology to targeted therapy. Antioxid Redox Signal. 2014;21:1220–38. doi: 10.1089/ars.2013.5639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kulshreshtha R, Ferracin M, Negrini M, Calin GA, Davuluri RV, Ivan M. Regulation of microRNA expression: the hypoxic component. Cell Cycle. 2007;6:1426–31. [PubMed] [Google Scholar]
- 105.Voellenkle C, Rooij J, Guffanti A, Brini E, Fasanaro P, Isaia E, et al. Deep-sequencing of endothelial cells exposed to hypoxia reveals the complexity of known and novel microRNAs. RNA. 2012;18:472–84. doi: 10.1261/rna.027615.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Camps C, Buffa FM, Colella S, Moore J, Sotiriou C, Sheldon H, et al. hsa-miR-210 is induced by hypoxia and is an independent prognostic factor in breast cancer. Clin Cancer Res. 2008;14:1340–8. doi: 10.1158/1078-0432.CCR-07-1755. [DOI] [PubMed] [Google Scholar]
- 107.Crosby ME, Kulshreshtha R, Ivan M, Glazer PM. MicroRNA regulation of DNA repair gene expression in hypoxic stress. Cancer Res. 2009;69:1221–9. doi: 10.1158/0008-5472.CAN-08-2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Huang X, Ding L, Bennewith KL, Tong RT, Welford SM, Ang KK, et al. Hypoxia-inducible mir-210 regulates normoxic gene expression involved in tumor initiation. Mol Cell. 2009;35:856–67. doi: 10.1016/j.molcel.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim HW, Haider HK, Jiang S, Ashraf M. Ischemic preconditioning augments survival of stem cells via miR-210 expression by targeting caspase-8-associated protein 2. J Biol Chem. 2009;284:33161–8. doi: 10.1074/jbc.M109.020925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang Z, Sun H, Dai H, Walsh RM, Imakura M, Schelter J, et al. MicroRNA miR-210 modulates cellular response to hypoxia through the MYC antagonist MNT. Cell Cycle. 2009;8:2756–68. doi: 10.4161/cc.8.17.9387. [DOI] [PubMed] [Google Scholar]
- 111.Cicchillitti L, Di Stefano V, Isaia E, Crimaldi L, Fasanaro P, Ambrosino V, et al. Hypoxia-inducible factor 1-alpha induces miR-210 in normoxic differentiating myoblasts. J Biol Chem. 2012;287:44761–71. doi: 10.1074/jbc.M112.421255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Grosso S, Doyen J, Parks SK, Bertero T, Paye A, Cardinaud B, et al. MiR-210 promotes a hypoxic phenotype and increases radioresistance in human lung cancer cell lines. Cell Death Dis. 2013;4:e544. doi: 10.1038/cddis.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yang W, Sun T, Cao J, Liu F, Tian Y, Zhu W. Downregulation of miR-210 expression inhibits proliferation, induces apoptosis and enhances radiosensitivity in hypoxic human hepatoma cells in vitro. Exp Cell Res. 2012;318:944–54. doi: 10.1016/j.yexcr.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 114.de Oliveira PE, Zhang L, Wang Z, Lazo JS. Hypoxia-mediated regulation of Cdc25A phosphatase by p21 and miR-21. Cell Cycle. 2009;8:3157–64. doi: 10.4161/cc.8.19.9704. [DOI] [PubMed] [Google Scholar]
- 115.Mace TA, Collins AL, Wojcik SE, Croce CM, Lesinski GB, Bloomston M. Hypoxia induces the overexpression of micro-RNA-21 in pancreatic cancer cells. J Surg Res. 2013;184:855–60. doi: 10.1016/j.jss.2013.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Anastasov N, Hofig I, Vasconcellos IG, Rappl K, Braselmann H, Ludyga N, et al. Radiation resistance due to high expression of miR-21 and G2/M checkpoint arrest in breast cancer cells. Radiat Oncol. 2012;7:206. doi: 10.1186/1748-717X-7-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Babar IA, Czochor J, Steinmetz A, Weidhaas JB, Glazer PM, Slack FJ. Inhibition of hypoxia-induced miR-155 radiosensitizes hypoxic lung cancer cells. Cancer Biol Ther. 2011;12:908–14. doi: 10.4161/cbt.12.10.17681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kong W, He L, Coppola M, Guo J, Esposito NN, Coppola D, et al. MicroRNA-155 regulates cell survival, growth, and chemo-sensitivity by targeting FOXO3a in breast cancer. J Biol Chem. 2010;285:17869–79. doi: 10.1074/jbc.M110.101055. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 119.Bhattacharya A, Schmitz U, Wolkenhauer O, Schonherr M, Raatz Y, Kunz M. Regulation of cell cycle checkpoint kinase WEE1 by miR-195 in malignant melanoma. Oncogene. 2013;32:3175–83. doi: 10.1038/onc.2012.324. [DOI] [PubMed] [Google Scholar]
- 120.Butz H, Liko I, Czirjak S, Igaz P, Khan MM, Zivkovic V, et al. Down-regulation of Wee1 kinase by a specific subset of microRNA in human sporadic pituitary adenomas. J Clin Endocrinol Metab. 2010;95:E181–91. doi: 10.1210/jc.2010-0581. [DOI] [PubMed] [Google Scholar]
- 121.Pouliot LM, Chen YC, Bai J, Guha R, Martin SE, Gottesman MM, et al. Cisplatin sensitivity mediated by WEE1 and CHK1 is mediated by miR-155 and the miR-15 family. Cancer Res. 2012;72:5945–55. doi: 10.1158/0008-5472.CAN-12-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Koritzinsky M, Wouters BG. The roles of reactive oxygen species and autophagy in mediating the tolerance of tumor cells to cycling hypoxia. Semin Radiat Oncol. 2013;23:252–61. doi: 10.1016/j.semradonc.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 123.Zhao T, Zhu Y, Morinibu A, Kobayashi M, Shinomiya K, Itasaka S, et al. HIF-1-mediated metabolic reprogramming reduces ROS levels and facilitates the metastatic colonization of cancers in lungs. Sci Rep. 2014;4:3793. doi: 10.1038/srep03793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dey S, Tameire F, Koumenis C. PERK-ing up autophagy during MYC-induced tumorigenesis. Autophagy. 2013;9:612–4. doi: 10.4161/auto.23486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.He WS, Dai XF, Jin M, Liu CW, Rent JH. Hypoxia-induced autophagy confers resistance of breast cancer cells to ionizing radiation. Oncol Res. 2012;20:251–8. doi: 10.3727/096504013x13589503483012. [DOI] [PubMed] [Google Scholar]
- 126.Papandreou I, Lim AL, Laderoute K, Denko NC. Hypoxia signals autophagy in tumor cells via AMPK activity, independent of HIF-1, BNIP3, and BNIP3L. Cell Death Differ. 2008;15:1572–81. doi: 10.1038/cdd.2008.84. [DOI] [PubMed] [Google Scholar]


