Abstract
Introduction
Earlier initiation of cannabis use is associated with poorer neuropsychological functioning across several domains. Given well-documented sex differences in neuromaturation during adolescence, initiation of cannabis use during this time may affect neuropsychological functioning differently for males and females.
Method
In the current study, we examined sex differences in the relationship between age of initiated cannabis use and neuropsychological performance after controlling for amount of lifetime cannabis use in 44 male and 25 female young adult cannabis users.
Results
We found that an earlier age of initiated use was related to poorer episodic memory, especially immediate recall, in females, but not in males. On the other hand, we found that, surprisingly, an earlier age of initiated use was associated with better decision-making overall. However, exploratory analyses found sex-specific factors associated with decision-making and age of initiated use, specifically that ADHD symptoms in females may drive the relationship between an earlier age of initiated use and better decision-making. Further, an earlier age of initiated use was associated with less education, a lower IQ, and fewer years of mother’s education for females, but more lifetime cannabis use for males.
Conclusions
Taken together, our findings suggest there are sex-differences in the associations between age of initiated cannabis use and neuropsychological functioning. The current study provides preliminary evidence that males and females may have different neuropsychological vulnerabilities that place them at risk for initiating cannabis use and continued cannabis use, highlighting the importance of examining the impact of cannabis on neuropsychological functioning separately for males and females.
Keywords: cannabis, cognition, marijuana, sex differences, THC
Introduction
As cannabis is legalized in more states and the perceived risk of cannabis use decreases, the rates and frequency of adolescent and adult cannabis use is increasing (Johnston, O’Malley, Miech, Bachman, & Schulenberg, 2014; SAMSHA, 2013). Over a period of six years, the number of daily or almost daily cannabis users have nearly doubled. In 2012, an estimated 5.4 million individuals 12 years or older used cannabis daily or almost daily, while there were about 3.1 million daily or almost daily cannabis users in 2006 (SAMSHA, 2013). This use is particularly concerning, as regular cannabis use is associated with neuropsychological deficits (Meier et al., 2012; Pope, Gruber, Hudson, Huestis, & Yurgelun-Todd, 2001; Solowij et al., 2002), lower educational attainment (Horwood et al., 2010; Meier et al., 2012), and poorer health outcomes (Kalant, 2004), especially if cannabis use is initiated earlier in adolescence (Crane, Schuster, Fusar-Poli, & Gonzalez, 2013; Lisdahl, Gilbart, Wright, & Shollenbarger, 2013). However, accumulating evidence suggests there may be important sex differences on the effects of cannabis on the neuropsychological outcomes of cannabis users (Crane, Schuster, Fusar-Poli, et al., 2013; Crane, Schuster, & Gonzalez, 2013; Lisdahl & Price, 2012), which is an important area that needs further research. Furthermore, the age of onset of cannabis use has emerged as another important factor that influences the effects of cannabis on neurocognition (Battisti et al., 2010; Ehrenreich et al., 1999; Fontes et al., 2011; Gruber, Sagar, Dahlgren, Racine, & Lukas, 2012; Pope et al., 2003; Solowij et al., 2011; Solowij et al., 2012). In the current study, we expanded upon our previous findings (Crane, Schuster, & Gonzalez, 2013) of a sex-specific relationship between amount of cannabis use and neuropsychological functioning, to examine how age of initiated cannabis use may contribute to neuropsychological performance among male and female cannabis users.
Initiation of cannabis use often occurs in adolescence, a critical period of neurodevelopment when growth of the prefrontal cortex, structures in the limbic system, and myelination of white matter associational, commissural, and projectional fibers takes place (Giedd et al., 1999). Therefore, the adolescent brain may be especially vulnerable to any adverse effects of cannabis use. This effect may be particularly salient due to the high density of CB1 receptors in the prefrontal cortex and limbic areas (Mackie, 2005; Piomelli, 2003). Importantly, the endocannabinoid system plays a crucial role in neuromaturation and synaptic pruning (Viveros et al., 2012). As such, initiation of cannabis use during this time may disrupt normal neuromaturation (Bava & Tapert, 2010), and in turn, lead to impairments in neuropsychological functioning.
Indeed, several studies have found an earlier age of initiated regular cannabis use is associated with poorer cognitive functioning (Battisti et al., 2010; Ehrenreich et al., 1999; Fontes et al., 2011; Gruber, Sagar, Dahlgren, Racine, & Lukas, 2012; Pope et al., 2003; Solowij et al., 2011; Solowij et al., 2012), including poorer episodic memory (Pope et al., 2003; Solowij et al., 2012) and inhibitory control (Battisti et al., 2010; Gruber, Sagar, Dahlgren, Racine, & Lukas, 2012; Solowij et al., 2012). Many of these studies have used a median-split approach to stratify the sample by age of initiated use in order to examine differences in neuropsychological functioning, which may diminish some of the effects, as it is not clear if there is a uniform specific age (or critical period) during adolescence when cannabis use transitions from being more to less harmful or if such a critical period is the same for males and females. In addition, age of onset is often measured in different ways, with some studies reporting age of onset to be the first time an individual uses cannabis and other studies reporting age of onset to be the age of regular initiated use, which may also be defined differently across studies. Although a few recent studies have looked at age of onset continuously (Battisti et al., 2010; Hooper, Woolley, & De Bellis, 2014; Solowij et al., 2011; Solowij et al., 2012), no studies have yet examined whether there are sex differences in the relationship between age of intitated use and neuropsychological functioning and also what may be the most sensitive measure of age of onset (i.e., median split or age as a continuous variable). Further, few studies have controlled for amount of cannabis use when examining age of inititiated use, making it difficult to understand the unique influence of age of initiated use on neuropsychological functioning, as age of initiation is often confounded with amount of use (i.e., users who started earlier have consumed more cannabis than those who began later).
Recent evidence suggests endocannabinoid signaling also plays a crucial role in establishing normal sex differences in the brain (Viveros et al., 2012) and disruption of this process may also cause sex-specific cognitive deficits. Given these differences, cannabis may differentially affect males and females, especially when taking into account the age of initiation of use. Importantly, males and females have different neurodevelopmental trajectories. Females’ total brain size peaks when they are about 10–11 years old, while males’ total brain size peaks when they are about 14–15 years old (Lenroot et al., 2007). Similarly, prefrontal cortex gray matter volume seems to peak 1–2 years earlier in females than in males (Giedd et al., 1999). This evidence indicates the female brain may mature at an earlier age than the male brain. Therefore, if cannabis use is initiated in adolescence, it may affect males more than females, as males’ brains are undergoing more protracted neurodevelopment during that time. This, coupled with the fact that males often initiate their use earlier than females (Gfroerer & Epstein, 1999; Pope et al., 2003), may make cannabis’ negative impact on neurocognitive functioning even more pronounced among males. Indeed, in a recently published study with data derived from the same sample, we found that that more cannabis use was more consistently associated with poorer episodic memory performance in females than in males, but more cannabis use was associated with poorer decision-making performance for males, but not females (Crane, Schuster, & Gonzalez, 2013).
Given sex differences in neurodevelopment, the pharmacological effects of cannabis, and neuropsychological functioning, it seems that cannabis use may differentially affect males and females; however, few studies have examined the impact of this interaction on neuropsychological functioning. It is important to identify potential sex differences in neuropsychological functioning among cannabis users to better understand how important aspects of use, like age of initiation are associated with neuropsychological functioning in male and female cannabis users. Our recent findings of cannabis use being more consistently associated with poorer episodic memory for females than males, but poorer decision-making for males only (Crane, Schuster, & Gonzalez, 2013), warrants further attention with respect to the influence of age of initiation. Given that males initiate their cannabis use earlier and also undergo protracted neurodevelopment compared to females, it is possible that their poorer decision-making is due to an earlier age of initiated use disrupting prefrontal cortex development and not just from a higher lifetime exposure to cannabis. On the other hand, females’ poorer episodic memory may be due to the negative effects of exposure to cannabis use on hippocampal functioning and not necessarily due to the negative effects of cannabis on neurodevelopment. Of course, it is difficult to parse apart the individual effects of age of initiated use, duration of use, and lifetime exposure to cannabis, as these variables are generally highly correlated. However, controlling for cumulative amount of use helps us to begin disentangling the age of initiation from amount of cannabis consumed. It may be that more cannabis use leads to poorer neuropsychological functioning in females, while an earlier age of initiated use predicts worse neuropsychological functioning in males. However, to date, few studies have looked at how age of initiated use is related to neuropsychological functioning in cannabis users after controlling for amount of use and, to our knowledge, no studies have examined sex differences in these relationships or contrasted findings between using a median split or continuous variable with regards to age of initiation.
In this study, we wanted to extend our prior findings (Crane, Schuster, & Gonzalez, 2013) to better understand if age of initiated use uniquely contributes to neuropsychological performance over and above the influence of amount of cannabis use in male and female cannabis users. Thus, we examined how different indices of age of initiated use (i.e., age of first use and age of regular initiated use) were associated with episodic memory and decision-making, two domains we found to be associated with amount of cannabis use (Crane, Schuster, & Gonzalez, 2013), in the same sample after controlling for amount of cannabis use. Based on behavioral and neurodevelopmental sex-differences, we hypothesized that an earlier age of use will be associated with worse episodic memory and decision-making in male and female cannabis users. However, after controlling for lifetime cumulative amount of cannabis use, we hypothesized that an earlier age of use will no longer be associated with episodic memory performance, as amount of cannabis use may be more strongly related to episodic memory performance than age of initiated use, especially among females. On the other hand, after controlling for lifetime cumulative amount of cannabis use, and earlier age of use will be associated with poorer decision-making only among males, as poorer decision-making in males may be more strongly related to an earlier age of initiated use disrupting prefrontal cortex development than lifetime exposure to cannabis. In addition, we wanted to examine whether age of first use or age of regular initiated use was more strongly associated with neurocognitive performance. Further, we wanted to examine if results using age of initiated use as a continuous variable differed from results using a median split of age of initiated use.
Methods
Participants
Participants were cannabis users from the Chicago-metropolitan area recruited through word-of-mouth and informational fliers. All participants were 18–24 years old; had education >8 years; had estimated full-scale IQ >75; had no diagnosis of a learning disability, developmental delay, mental illness (including Attention Deficit Hyperactivity Disorder; ADHD), or neurological condition; had no significant birth complications; had no loss of consciousness >10 min; had no current use of psychotropic medication; demonstrated English fluency; had no significant recent alcohol use (AlcoMate Prestige Model AL6000; Palisades Park, NJ); had no illicit drug use other than cannabis in the past 30 days or >10× in life for each drug class (other than cannabis, alcohol, nicotine, or hallucinogens); had no recent illicit drug use other than cannabis (10-panel Drug Check Cup; Express Diagnostics, Blue Earth, Minnesota); used cannabis: >200 in life, >4× per week during peak use, and at least once in the past 45 days; reported no cannabis use on testing day; and identified cannabis as their drug of choice. The Institutional Review Board at the University of Illinois at Chicago approved the study and written informed consent was obtained. Additional details regarding the larger study, methods, and participants have been previously reported (Gonzalez et al., 2012).
Demographics, Potential Confounds, and Substance Use
Demographic information, including race/ethnicity, and family of origin information was obtained through an examiner-led questionnaire. The Wechsler Test of Adult Reading assessed premorbid full-scale IQ (Wechsler, 2001), while current and lifetime substance use were diagnosed with the Structured Clinical Interview for DSM-IV (First, Spitzer, Gibbon, & Williams, 2002). The Beck Depression Inventory-II (Beck, Brown, & Steer, 1996) and Beck Anxiety Inventory (Beck & Steer, 1990) assessed depression and anxiety symptoms, the Barratt Impulsiveness Scale-11 evaluated trait impulsivity (Patton, Stanford, & Barratt, 1995), the Marijuana Problem Scale measured negative consequences of cannabis use in the past 90 days (Stephens, Roffman, & Curtin, 2000), and the Wender-Utah Rating Scale (WURS) assessed ADHD symptoms (scores >46 indicates possible ADHD diagnosis; Ward, Wender, & Reimherr, 1993). An examiner-led semi-structured interview collected participants’ amount and frequency of alcohol, nicotine, and illicit substance use during their lifetime, the past year and the past month (Gonzalez et al., 2012), this method has been used in several previous studies (e.g., Gonzalez et al., 2004; Rippeth et al., 2004). Participants were also asked to report their age of first cannabis use and alcohol use and the age that they started using cannabis and the age that they started using alcohol at least once a week for three straight months (age of regular initiated use).
Laboratory Measures of Neurocognitive Functioning
Verbal Episodic Memory
Verbal episodic memory was assessed using the Hopkins Verbal Learning Test–Revised (HVLT-R; (Benedict, Schretlen, Groninger, & Brandt, 1998) normative-based, age corrected z-scores for immediate recall (total words recalled over three learning trials), delayed recall (total words recalled after a 20–25 minute delay), and recognition discrimination (hits minus false positives).
Decision-making
Decision-making was assessed using the Iowa Gambling Task (IGT) total net normative-based T-score (choices from advantageous decks minus disadvantageous decks; Bechara, Damasio, Damasio, & Anderson, 1994). Demographically corrected norms controlling for age and education were use. In this task, T-scores with lower values indicate poorer decision-making or a bias toward immediate versus long-term rewards (Bechara, 2007).
General Statistical Procedures
All analyses were carried out using SPSS 20.0 (IBM). Data were inspected for non-normal distribution and outliers. Square-root transformations were used for amount of cannabis and alcohol use and nonparametric procedures were used for analyses of participant characteristics with data that violated assumptions of parametric procedures. Males and females were compared on demographic, substance use, and mental health variables using t-tests or chi-square tests as appropriate. In addition, males and females were compared on general neuropsychological performance using separate analysis of variance. We conducted moderated hierarchical multiple regression analyses with centered age of first cannabis use and age of regular cannabis use entered as separate independent variables in the first block; vectors for sex (i.e., male, female) as well as with centered amount of lifetime cannabis use (in order to control for the effects of amount of use) in the second block; and their interaction in the third block as predictors. We also controlled for amount of past month alcohol use in the second block of the model, but this covariate was not significant in any model and was therefore removed from final models. The Variance Inflation Factor (VIF) for variables in each model ranged from 1.00–1.99, indicating that multicollinearity was not a significant issue. Performance on neuropsychological measures served as the separate dependent variables. Results were deemed statistically significant when p-values < .05.
Results
Demographics, Mental Health, Substance Use and Other Potential Confounds
Males and females reported minimal mental health complaints, and they did not differ on potential confounds, with the exception that males had higher alcohol consumption during the past 30 days than females (p<.04; see Table 1; also reported in Crane, Schuster, & Gonzalez, 2013). Age of first use was significantly correlated with age of regular initiated use (r= −.74, p< .01) and with cumulative lifetime amount of cannabis use (r= −.26, p< .05). Age of regular initiated use was significantly correlated with cumulative lifetime amount of cannabis use (r= −.33, p< .01).
Table 1.
Participant Characteristics
| Male CU (n=44) % or M ± SD (range) |
Female CU (n=25) % or M ± SD (range) |
p-value | |
|---|---|---|---|
| Demographics | |||
| Age | 20.75 ± 1.89 (18 – 24) | 20.72 ± 1.62 (18 – 24) | .95 |
| Estimated FSIQ | 102.11 ± 10.24 (76 – 118) | 102.80 ± 10.02 (82 – 120) | .79 |
| Years of Education | 13.34 ± 1.67 (10 – 16) | 13.64 ± 1.68 (11 – 18) | .48 |
| Ethnicity/Race | .70 | ||
| Caucasian | 43% | 36% | |
| Black | 34% | 40% | |
| Hispanic | 7% | 16% | |
| Asian | 7% | 4% | |
| Other | 9% | 4% | |
| Annual Household Income in Thousands of | 26 [9, 61] | 33 [7, 94] | .84 |
| Dollars [Md, IQR] | |||
| Mother’s Education | 14.23 ± 2.68 (7 – 18) | 14.13 ± 3.00 (5 – 20) | .89 |
| Mental Health | |||
| BDI-II Total Score [Md, IQR] | 5 [2.25, 7.75] | 5 [1.50, 10] | .81 |
| BAI Total Score [Md, IQR] | 4 [2, 9] | 5 [3, 8] | .21 |
| WURS, % of scores >46 [IQR] | 2% [15.25, 30] | 8% [9.50, 18.50] | .27 |
| BIS-11 Total Score | 59.48 ± 9.16 (41 – 82) | 59.04 ± 10.58 (37 – 79) | .86 |
| Substance Use | |||
| Current (30 day) DSM-IV SUD | |||
| Alcohol Abuse | 11% | 0% | .08 |
| Alcohol Dependence | 0% | 0% | 1.00 |
| Cannabis Abuse | 34% | 28% | .60 |
| Cannabis Dependence | 27% | 28% | .95 |
| Lifetime DSM-IV SUD | |||
| Alcohol Abuse | 25% | 16% | .38 |
| Alcohol Dependence | 2% | 4% | .68 |
| Cannabis Abuse | 41% | 44% | .80 |
| Cannabis Dependence | 34% | 28% | .60 |
| Years of cannabis use | 5.18 ± 2.44 (1 – 12) | 4.68 ± 2.14 (1 – 9) | .39 |
| Age of 1st Cannabis Use | 15.80 ± 2.12 (11 – 21) | 16.29 ± 2.35 (11 – 20) | .38 |
| Age of Regular Cannabis Use | 17.36 ± 1.98 (13 – 22) | 17.96 ± 2.32 (13 – 23) | .26 |
| Days since last cannabis use | 4.18 ± 4.05 (1 – 26) | 5.52 ± 8.45 (1 – 45) | .38 |
| % THC+ | 77% | 76% | .90 |
| MPS Total Score | 5.54 ± 3.63 (0–15) | 4.43 ± 2.89 (0–9) | .24 |
| Age of 1st Alcohol Use | 15.68 ± 2.07 (10 – 20) | 15.80 ± 1.68 (13 – 19) | .81 |
| Age of Regular Alcohol Use | 18.12 ± 1.49 (16 – 22) | 19.10 ± 1.66 (16 – 21) | .13 |
| Lifetime [Md, IQR] | |||
| Alcoholic drinks | 569.50 [189.75, 1215] | 288 [104.50, 1527.50] | .40 |
| Cigarettes | 1512.50 [19.50, 7515] | 574 [0, 3186] | .37 |
| Cannabis (grams) | 625.15 [198.50, 2219.41] | 482.40 [124.63, 1328.70] | .47 |
| Past Year [Md, IQR] | |||
| Alcoholic drinks | 132 [33, 291] | 80 [24, 210] | .33 |
| Cigarettes | 72 [0.50, 1417.50] | 48 [0, 540] | .35 |
| Cannabis (grams) | 114 [55.65, 440.63] | 90 [24, 383.40] | .42 |
| Past 30 days [Md, IQR] | |||
| Alcohol drinks | 11.50 [2.25, 20.75] | 3 [0.50, 15] | .04* |
| Cigarettes | 6 [0, 90] | 7 [0, 50] | .52 |
| Cannabis (grams) | 10.75 [5.15, 36.68] | 12 [2.38, 33.55] | .81 |
| Neuropsychological Performance | |||
| Verbal Episodic Memory | |||
| HVLT Immediate Recall (z score) | −0.81 ± 1.23 (−3.62 – 1.51) | −0.77 ± 1.45 (−3.89 – 1.24) | .90 |
| HVLT Delayed Recall (z score) | −0.83 ± 1.32 (−4.13 – 0.88) | −0.90 ± 1.26 (−2.88 – 0.88) | .83 |
| HVLT Recognition Discrimination (z score) | 0.01 ± 0.82 (−2.83 – 0.5) | 0.03 ± 0.95 (−2.86 – 0.5) | .95 |
| Decision Making | |||
| IGT Net Total (T score) | 45.59 ± 9.50 (26 – 63) | 45.60 ± 10.26 (22 – 65) | 1.00 |
Note: all values are means, standard deviations, or ranges, unless otherwise noted; CU, cannabis users; Md, Median; IQR, interquartile range; FSIQ, Full Scale IQ; BDI-2, Beck Depression Inventory-2nd Edition; BAI, Beck Anxiety Inventory; WURS, Wender-Utah Rating Scale; BIS, Barratt Impulsiveness Scale-11th version; DSM-IV SUD, Diagnostic and Statistical Manual IV substance use disorders; THC+, positive rapid urine toxicology testing; MPS, Marijuana Problem Scale.
Relationships between Age of Initiated Use and Neuropsychological Performance
Males and females did not differ on neuropsychological performance (Table 1; also reported in Crane, Schuster, & Gonzalez, 2013). However, males and females had poorer performance on immediate and delayed recall compared to the published normative samples for the HVLT-R (Table 1), suggesting mild memory impairments in both groups. In contrast, decision-making performance for males and females was comparable to that of healthy controls based on published normative data for the IGT.
Episodic Memory
The interaction between age of first use and sex was significant for immediate and delayed recall, after controlling for amount of use (Table 2). Follow-up of the simple slopes found a trend towards an earlier age of first use being more strongly associated with poorer immediate recall for females (β = .21, p = .06) than for males (β = −.20, p = .09). However, age of first use was not significantly associated with delayed recall (females: β = .18, p = .11; males: β = −.17, p = .14).
Table 2.
Hierarchical Moderated Regression Models for Predicting How Age of Initiated Cannabis Use and Sex Affect Neuropsychological Functioning
| Variable | Age of 1st Use | Age of Regular Use | ||||
|---|---|---|---|---|---|---|
| R2 | B | p | R2 | β | p | |
| HVLT (Immediate Recall) | ||||||
|
| ||||||
| Block 1-Age of Onset | 0.01 | 0.08 | .53 | 0.05 | 0.22 | .07 |
| Block 2-Age of Onset | 0.13 | −0.02 | .91 | 0.14 | 0.11 | .36 |
| Sex | -- | −0.03 | .83 | -- | −0.03 | .81 |
| Amount of Lifetime Cannabis Use | -- | −0.37 | .004 | -- | −0.32 | .01 |
| Block 3-Age of Onset x Sex | 0.22 | 0.38 | .01 | 0.22 | 0.38 | .01 |
|
| ||||||
| HVLT (Delayed Recall) | ||||||
|
| ||||||
| Block 1-Age of Onset | 0.01 | 0.10 | .43 | 0.05 | 0.23 | .06 |
| Block 2-Age of Onset | 0.19 | −0.01 | .92 | 0.22 | 0.09 | .44 |
| Sex | -- | −0.05 | .65 | -- | −0.08 | .49 |
| Amount of Lifetime Cannabis Use | -- | −0.44 | .001 | -- | −0.44 | .001 |
| Block 3-Age of Onset x Sex | 0.25 | 0.32 | .03 | 0.26 | 0.25 | .09 |
|
| ||||||
| IGT (Net Total) | ||||||
|
| ||||||
| Block 1-Age of Onset | 0.03 | −0.16 | .19 | 0.01 | −0.10 | .41 |
| Block 2-Age of Onset | 0.16 | −0.26 | .04 | 0.14 | −0.23 | .07 |
| Sex | -- | −0.02 | .88 | -- | 0.00 | .99 |
| Amount of Lifetime Cannabis Use | -- | −0.38 | .002 | -- | −0.38 | .003 |
| Block 3-Age of Onset x Sex | 0.16 | −0.05 | .76 | 0.17 | −0.23 | .14 |
Note. The sex variable was dummy coded, with males serving as the referent group; covariates were only included in models in which they were significant; HVLT, Hopkins Verbal Learning Task; IGT, Iowa Gambling Task; n/a, non-applicable; bold and italicized p-values are significant or trending toward significance.
The interaction between age of regular initiated use and sex, after controlling for amount of use, was significant for immediate recall and trended towards significance for delayed recall (Table 2). Follow-up of the simple slopes found that an earlier age of regular initiated use was associated with poorer immediate recall for females (β = .28, p = .01), but not for males (β = −.11, p = .35) and this effect trended towards significance for delayed recall (females: β = .20, p = .08; males: β = −.06, p = .61) (Figure 1).
Figure 1.
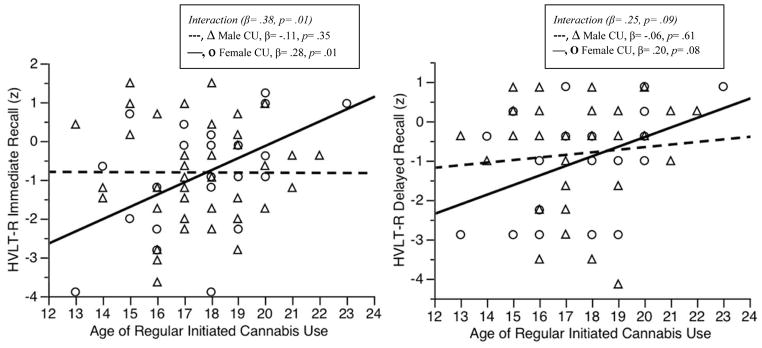
Interactions between Age of Regular Initiated Cannabis Use and Sex on Episodic Memory Performance
Decision-Making
The interaction between age of initiated use (first use or regular initiated use) and sex, after controlling for amount of use, was not significant for decision-making (Table 2), and an earlier age of initiated use (first use or regular initiated use) alone was not associated with decision-making performance. Surprisingly, after controlling for cumulative lifetime cannabis use, an earlier age of first use predicted better decision-making, regardless of sex, and this effect trended towards significance for age of regular initiated use (Table 2).
Exploratory Analyses
Age of Initiated Use and Decision-Making
To better understand our unexpected findings suggesting that an earlier age of first use and age of regular initiated use was associated with better decision-making, and the similar pattern for age of regular initiated use that trended towards significance, we performed several exploratory analyses. To understand if age of initiated use was related to decision-making in the same direction and magnitude in males and in females, we performed follow-up analyses of the simple slopes for sex differences in the relationship between age of initiated use and decision-making. Follow-up analyses of the simple slopes for sex differences with earlier age of first cannabis use did not significantly predict decision-making in females (β = −.19, p = .11) or in males (β = −.18, p = .15) (Figure 2). However, follow-up analyses of the simple slopes found that an earlier age of initiated regular cannabis use was associated with better decision-making in females (β = −.28, p = .02), but not in males (β = −.05, p = .67) (Figure 2).
Figure 2.
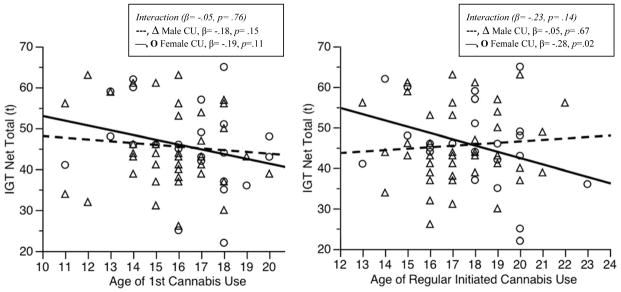
Interactions between Age of Regular Initiated Cannabis Use and Sex on Decision-Making Performance
Bivariate correlations between several theoretically relevant measures and decision-making performance for males and for females were also run to examine what variables may be contributing to an earlier age of age of initiated use and better decision-making (Table 3). Due to the fact that WURS scores had the strongest bivariate correlation with decision-making performance for females, and the results of the simple slopes analyses suggested that females may be in part driving the relationship between an earlier age of initiated use and decision-making, we controlled for WURS scores to see if ADHD symptoms influenced how age of first use related to decision-making in females. After controlling for WURS total scores and cumulative lifetime cannabis use, an earlier age of first use (β = −.29, p = .15) and an earlier age of regular use (β = −.28, p = .18) were not associated with better decision-making for females.
Table 3.
Bivariate correlations with Decision-Making Performance for Female and Male CU
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. IGT Net Total | -- | −0.26 | −0.35+ | 0.09 | −0.15 | 0.16 | 0.31 | 0.30 | 0.36+ | 0.23 | 0.44* | −0.07 | 0.02 | −0.01 |
| 2. Age of 1st Use | −0.10 | -- | 0.75* | 0.36+ | 0.51* | 0.54* | −0.17 | −0.12 | −0.23 | −0.21 | 0.06 | 0.27 | −0.12 | −0.19 |
| 3. Age of Regular Initiated Use | 0.07 | 0.72* | -- | 0.44* | 0.54* | 0.42* | −0.16 | −0.09 | −0.20 | −0.24 | −0.23 | 0.10 | −0.26 | −0.08 |
| 4. FSIQ | 0.34* | −0.08 | 0.07 | -- | 0.65* | 0.31 | 0.44* | 0.22 | 0.17 | −0.24 | −0.01 | −0.20 | −0.49* | 0.17 |
| 5. Education | 0.34* | 0.17 | 0.29+ | 0.21 | -- | 0.40+ | −0.04 | 0.10 | 0.04 | −0.34+ | −0.10 | −0.22 | −0.2 | 0.01 |
| 6. Mother’s Education | 0.09 | −0.14 | 0.09 | 0.15 | −0.03 | -- | 0.12 | 0.34 | 0.08 | 0.00 | −0.18 | 0.16 | −0.09 | −0.31 |
| 7. Current Annual Household Income | 0.03 | −0.01 | −0.04 | 0.03 | 0.11 | 0.05 | -- | 0.16 | 0.05 | 0.03 | 0.02 | −0.20 | −0.44* | 0.19 |
| 8. BDI Total | −0.13 | −0.14 | −0.03 | 0.10 | 0.01 | 0.01 | −0.34* | -- | 0.54* | 0.35+ | 0.04 | −0.11 | −0.16 | 0.36 |
| 9. BAI Total | 0.08 | 0.02 | 0.06 | 0.15 | 0.02 | −0.15 | −0.19 | 0.62* | -- | 0.25 | 0.36+ | −0.22 | −0.2 | 0.21 |
| 10. BIS Total | −0.23 | −0.15 | −0.25 | 0.03 | −0.31* | 0.07 | −0.18 | 0.27+ | 0.25 | -- | 0.47* | 0.10 | 0.13 | 0.43+ |
| 11. WURS Total | −0.16 | −0.11 | −0.12 | 0.17 | −0.35* | −0.13 | −0.26 | 0.47* | 0.32 | 0.44* | -- | −0.11 | 0.02 | 0.23 |
| 12. Amount of Cannabis Use (sq) per Year of Use | −0.31* | 0.13 | −0.03 | −0.04 | −0.49* | −0.12 | 0.04 | −0.08 | −0.03 | 0.34* | 0.37* | -- | 0.81 | −0.03 |
| 13. Amount of Lifetime Cannabis Use (sq) | −0.49* | −0.32* | −0.37* | −0.30* | −0.24 | −0.13 | 0.14 | 0.07 | 0.01 | 0.44* | 0.27+ | 0.49* | -- | −0.02 |
| 14. Marijuana Problems Scale Total | −0.13 | −0.32+ | −0.22 | 0.13 | −0.17 | −0.20 | −0.20 | 0.57* | 0.54* | 0.33+ | 0.64* | 0.35* | 0.42* | -- |
Note. Females in shaded area above dotted line and males in non-shaded area below dotted line; IGT, Iowa Gambling Task; FSIQ, Full Scale IQ; BDI-II, Beck Depression Inventory-2nd Edition; BAI, Beck Anxiety Inventory; WURS, Wender-Utah Rating Scale; and BIS, Barratt Impulsiveness Scale-11th version; sq, square root;
indicates p < .05.;
indicates p < .10.
Analyzing Age of Regular Initiated Use using a Median-Split
In order to explore if our main results would remain the same if we used a median-split analyses without controlling for amount of cumulative amount of cannabis use, which has commonly been done in several prior studies, we compared individuals who began their regular use of cannabis before the age of 16 (early-onset) to those who began their regular use of cannabis at age 16 and older (late-onset) without controlling for cumulative amount of cannabis use. Early-onset users had poorer immediate recall that late-onset users (F(3,65) = 4.45, p = .04) and the interaction between onset and sex trended toward significance (F(3,65) = 2.92, p = .09), with early-onset females performing worse than late-onset females (F(1,23) = 4.99, p = .04), while early- and late-onset males performed similarly, F(1,42) = 0.13, p = .72. A trend also emerged for delayed recall, such that early-onset users performed more poorly than late-onset users (F(3,65) = 2.93, p = .09), but there was no interaction between onset and sex, F(3,65) = 2.30, p = .13. On the other hand, there were no differences between individuals with an early-onset and those with a late-onset on decision-making performance (F(3,65) = 0.29, p = .59), nor was there an interaction between onset and sex, F(3,65) = 2.09, p = .15.
Further, using ANOVA and chi-square analyses, early-onset users were compared to late-onset on several theoretical variables. IQ, education, mother’s education, BDI total, BAI total, WURS total, or past or current cannabis abuse or dependence did not differ between groups (all p-value’s > .10). Early-onset users used more cannabis in their lifetime (F(1,67) = 11.66, p = .001) and there was a trend that they had higher total scores on the BIS (F(1,67) = 3.24, p = .08) and the Marijuana Problem Scale, F(1,54) = 3.69, p = .06), but they did not differ from late-users on amount of cannabis used per year, F(1,67) = 1.18, p = .68. Of note, when examining these relationships only among female cannabis users, early-onset female users’ mothers had less years of education than late-onset female users’ mothers (F(1,21) = 4.38, p = .049), but no other significant relationships emerged, all p-value’s > .10. When examining these relationships only among male cannabis users, early-onset male users had more lifetime cannabis use (F(1,42) = 9.53, p = .004) and had higher total scores on the BIS (F(1,42) = 4.18, p = .047) than late-onset male users, but no other significant relationships emerged, all p-value’s > .10.
Discussion
In this study we examined relationships between different indices of age of initiated use and neuropsychological functioning, namely episodic memory and decision-making, among a non-treatment-seeking, community-dwelling sample of young adult regular cannabis users who had minimal mental health problems or other drug use. We found preliminary evidence of a dissociation in how age of initiated use is related to episodic memory performance in male and female cannabis users. Specifically, we found that after controlling for amount of cannabis use, an earlier age of regular initiated use was related to poorer episodic memory, especially immediate recall, in females, but not in males. This effect trended toward significance for age of first use, indicating that age of regular initiated use may be more important than age of first use in influencing episodic memory performance in female cannabis users. On the other hand, we found that surprisingly, an earlier age of first use was associated with better decision-making overall after controlling for amount of cannabis use and a similar effect was found for age of initiated regular use that trended towards significance. This warranted further exploration and a more complex pattern of results emerged when we examined exploratory relationships among age of initiated use (first and regular initiated use) and decision-making separately for males and females.
Although the interaction terms between sex and age of initiated use were not significant, to help understand the surprising relationship between an earlier age of initiated use and better decision-making, we performed exploratory analyses and found that while there were no sex specific relationships between age of first use and decision-making, an earlier age of regular use was associated with better decision-making in females, but not in males. This evidence suggests that females may be driving this relationship. Indeed, we found sex-specific factors associated with decision-making and age of initiated use. For females, better decision-making was associated with more symptoms of ADHD, and after controlling for ADHD symptoms, an earlier age of initiated cannabis use was no longer associated with better decision-making in females. Therefore, ADHD symptoms may be more strongly related to decision-making in females than age of initiated use. Although findings on executive functioning deficits and decision-making impairment in ADHD is mixed (Castellanos, Sonuga-Barke, Milham, & Tannock, 2006), some recent evidence suggests that adolescent females with ADHD may have better decision-making than adolescent males with ADHD and adolescent healthy control females (Skogli, Andersen, Hovik, & Oie, 2014), so it is possible that we see a similar pattern in the current study: females who have higher symptoms of ADHD perform better on decision-making. On the other hand, it is important to note that other studies have found the opposite in young adults: young adult males with ADHD have better decision-making than young adult females with ADHD (Hobson, Scott, & Rubia, 2011; Toplak, Jain, & Tannock, 2005), which may reflect age or neuromaturational differences or difference in measuring decision-making (i.e, hot versus cold cognition; see (Skogli, Andersen, Hovik, & Oie, 2014). Importantly, in the current study, participants were excluded if they had a formal ADHD diagnosis (indeed only 2 female participants were above the clinical cut-off on the WURS; see Table 1), but it is possible that sub-threshold ADHD symptoms may still play an important role in decision-making for females. It is also possible that since only age of first use was significantly related to decision-making, females with better decision-making may be more likely to experiment with cannabis, but may not go on to regularly use cannabis. Of note, the beta weights in our analyses of the relationship between age of initiated use and decision-making in females with and without controlling for ADHD symptoms remain relatively similar, indicating that the non-significant relationship between age of initiated cannabis use and decision-making when controlling for ADHD symptoms may be due to power and may not reflect a true mediation effect. In addition, we also found that among females, an earlier age of use was associated with less education, a lower IQ, and fewer years of education for their mothers, so there may be other factors that were not measured in the current study that are driving this relationship. On the other hand, for males better decision-making was significantly associated with higher IQ, more education, less lifetime cannabis use, and less cannabis use per year of use, while an earlier age of use was associated with more lifetime cannabis use along with somewhat more cannabis-related problems. Taken together, our findings suggest there may be sex-differences in the reasons why males and females initiate use and then continue to use cannabis, in line with other studies (Guxens, Nebot, & Ariza, 2007; Guxens, Nebot, Ariza, & Ochoa, 2007; Pedersen, Mastekaasa, & Wichstrom, 2001; Schepis et al., 2011). For example, females with less education and females whose mothers have fewer years of education may be more vulnerable to initiate cannabis use earlier, while other factors that were not measured in the current study (e.g., behavioral dyscontrol) may increase males’ risk to initiate cannabis use earlier.
Additional analyses were conducted to better understand how results may change when using different methods for examining age of first use, as has been the case with prior studies. Analyses using a median-split analysis, as has commonly been done in several prior studies, replicated previous findings, showing that an earlier age of initiated use was associated with poorer episodic memory (Pope et al., 2003; Solowij et al., 2012). Although we found the same pattern of sex differences in episodic memory as in our analyses using a continuous measure of age of initiated use, that is, early age of initiation was associated with poorer episodic memory, especially immediate recall, in females; the interaction term between sex and age of initiated use only trended toward significance in the median-split analyses, suggesting that continuous analyses may be more sensitive to finding important sex differences. We did not find that age of initiated use was associated with decision-making performance using median-split analyses. Due to the fact that median-split analyses may diminish the power to find sex-differences, it is important that future studies consider this issue.
The current study expands upon our previous findings that there are important sex differences in how important indices of cannabis use are related to neuropsychological functioning. We previously found that more cannabis use is more consistently associated with poorer episodic memory for females than males, but poorer decision-making for males only (Crane, Schuster, & Gonzalez, 2013). Given that we controlled for amount of lifetime cannabis use in our current analyses, our findings that an earlier age of initiated use is associated with poorer episodic memory in females, adds to (but does not duplicate) our previous findings that more cannabis use is more consistently associated with poorer episodic memory for females than males. Taken together, our previous and current analyses indicate that poorer episodic memory may either be a vulnerability that places females at a higher risk for earlier initiation of cannabis use or it may be a domain that is adversely affected by cannabis use and continued use of cannabis may further add to the negative impact of an earlier age of initiated use on episodic memory in females. For example, it is possible that cannabis use disrupts estrogen-related dendritic spine maturation in the hippocampus, especially during neurodevelopment (Gillies & McArthur, 2010), which may facilitate continued use among females, leading to poorer episodic memory as use continues. It is also possible that estrogen-related learned associations with cannabis use may also contribute to females’ progression to cannabis dependence, in turn leading to even poorer episodic memory (Fattore et al., 2007). However, our current and previous analyses suggest that there may be other mechanisms involved in males’ initiation of cannabis use and it is only after continued cannabis use that poorer decision-making emerges in males. Of note, sex differences are evident among healthy, non-using adults in these domains, such that females perform better on measures of episodic memory (Kramer, Delis, & Daniel, 1988), while males perform better on measures of decision-making (Bolla, Eldreth, Matochik, & Cadet, 2004; Overman et al., 2004; Reavis & Overman, 2001). Thus, cannabis use may blunt these normal sex differences, as we found that the domains in which healthy males or females tend to have better performance are the domains most negatively impacted by cannabis use indices (e.g., age of initiated use and amount of cannabis use).
Our current findings should be considered in the context of several important factors. First, it is important to note that in general, male and female cannabis users in this study demonstrated deficits in episodic memory, especially immediate and delayed recall, but not in decision-making, compared to their non-using counterparts in the parent project (Gonzalez et al., 2012). Further, there are limitations of the current study including a cross-sectional design that requires replication in a larger sample, and the fact that family history of substance use was not measured, a factor that may have influenced the results. The participants also had a limited age range, however, this could also be interpreted as a strength, as the sample is comprised of young adults who are still undergoing neurodevelopment, an important period to study; especially since cannabis use during this time may have more pronounced effects. Longitudinal designs will be used in ongoing and future studies to better explore mechanisms for the observed patterns of results, including the possible role of sex hormones.
In conclusion, our study expands on previously reported associations between age of initiated cannabis use and episodic memory and decision-making, using a non-treatment-seeking community sample of young adult current cannabis users with minimal mental health problems and use of other substances. We found evidence of a dissociation in how age of initiated use is related to episodic memory performance among males and females. In addition, we found that several factors seem to be related to decision-making performance and age of initiated use in a sex-specific manner, suggesting there may be sex-differences in the reasons why males and females initiate use and then continue to use cannabis. The current study provides preliminary evidence that males and females may have different neuropsychological vulnerabilities or cannabis-related neuropsychological sequelae that place them at risk for cannabis dependence and highlights the importance of examining the impact of cannabis on neuropsychological functioning separately for males and females.
Acknowledgments
This publication was funded by the National Institute on Drug Abuse (NIDA) (K23DA023560, R01DA031176, and R01DA033156, PI: RG) and (F31DA038388-01, PI: NAC); the National Institute of Mental Health (NIMH) (T32MH067631, to NAC); and the National Cancer Institute (NCI) (P01CA098262, PI: RM). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIDA, NCI or the National Institutes of Health. We thank Dr. Kathi Diviak and Dr. Mermelstein’s group for their assistance with identification and recruitment of a subset of participants.
Footnotes
The authors declare no conflicts of interest.
References
- Battisti RA, Roodenrys S, Johnstone SJ, Pesa N, Hermens DF, Solowij N. Chronic cannabis users show altered neurophysiological functioning on Stroop task conflict resolution. Psychopharmacology (Berl) 2010;212(4):613–624. doi: 10.1007/s00213-010-1988-3. [DOI] [PubMed] [Google Scholar]
- Bava S, Tapert SF. Adolescent brain development and the risk for alcohol and other drug problems. Neuropsychology Review. 2010;20(4):398–413. doi: 10.1007/s11065-010-9146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A. Iowa gambling task (IGT) professional manual. Lutz: Psychological Assessment Resources; 2007. [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50(1–3):7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Beck AT, Brown G, Steer RA. Beck Depression Inventory II Manual. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- Beck AT, Steer RA. Manual for the Beck Anxiety Inventory. San Antonio, TX: The Psychological Corporation; 1990. [Google Scholar]
- Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test Revised: Normative data and analysis of inter-form and test-retest reliability. Clinical Neuropsychologist. 1998;12(1):43–55. [Google Scholar]
- Bolla KI, Eldreth DA, Matochik JA, Cadet JL. Sex-related differences in a gambling task and its neurological correlates. Cerebral Cortex. 2004;14(11):1226–1232. doi: 10.1093/cercor/bhh083. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Sonuga-Barke EJ, Milham MP, Tannock R. Characterizing cognition in ADHD: beyond executive dysfunction. Trends in Cognitive Sciences. 2006;10(3):117–123. doi: 10.1016/j.tics.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Crane NA, Schuster RM, Fusar-Poli P, Gonzalez R. Effects of cannabis on neurocognitive functioning: recent advances, neurodevelopmental influences, and sex differences. Neuropsychology Review. 2013;23(2):117–137. doi: 10.1007/s11065-012-9222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane NA, Schuster RM, Gonzalez R. Preliminary evidence for a sex-specific relationship between amount of cannabis use and neurocognitive performance in young adult cannabis users. Journal of the International Neuropsychological Society. 2013;19(9):1009–1015. doi: 10.1017/S135561771300088X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenreich H, Rinn T, Kunert HJ, Moeller MR, Poser W, Schilling L, Hoehe MR. Specific attentional dysfunction in adults following early start of cannabis use. Psychopharmacology (Berl) 1999;142(3):295–301. doi: 10.1007/s002130050892. [DOI] [PubMed] [Google Scholar]
- Fattore L, Spano MS, Altea S, Angius F, Fadda P, Fratta W. Cannabinoid self-administration in rats: sex differences and the influence of ovarian function. British Journal of Pharmacology. 2007;152(5):795–804. doi: 10.1038/sj.bjp.0707465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Fontes MA, Bolla KI, Cunha PJ, Almeida PP, Jungerman F, Laranjeira RR, Lacerda AL. Cannabis use before age 15 and subsequent executive functioning. British Journal of Psychiatry. 2011;198(6):442–447. doi: 10.1192/bjp.bp.110.077479. [DOI] [PubMed] [Google Scholar]
- Gfroerer JC, Epstein JF. Marijuana initiates and their impact on future drug abuse treatment need. Drug and Alcohol Dependence. 1999;54(3):229–237. doi: 10.1016/s0376-8716(98)00167-7. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gillies GE, McArthur S. Estrogen actions in the brain and the basis for differential action in men and women: a case for sex-specific medicines. Pharmacological Reviews. 2010;62(2):155–198. doi: 10.1124/pr.109.002071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R, Rippeth JD, Carey CL, Heaton RK, Moore DJ, Schweinsburg BC, Grant I. Neurocognitive performance of methamphetamine users discordant for history of marijuana exposure. Drug and Alcohol Dependence. 2004;76(2):181–190. doi: 10.1016/j.drugalcdep.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Schuster RM, Mermelstein RJ, Vassileva J, Martin EM, Diviak KR. Performance of young adult cannabis users on neurocognitive measures of impulsive behavior and their relationship to symptoms of cannabis use disorders. Journal of Clinical and Experimental Neuropsychology. 2012;34(9):962–976. doi: 10.1080/13803395.2012.703642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Sagar KA, Dahlgren MK, Racine M, Lukas SE. Age of onset of marijuana use and executive function. Psychology of Addictive Behaviors. 2012;26(3):496–506. doi: 10.1037/a0026269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guxens M, Nebot M, Ariza C. Age and sex differences in factors associated with the onset of cannabis use: a cohort study. Drug and Alcohol Dependence. 2007;88(2–3):234–243. doi: 10.1016/j.drugalcdep.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Guxens M, Nebot M, Ariza C, Ochoa D. Factors associated with the onset of cannabis use: a systematic review of cohort studies. Gaceta Sanitaria. 2007;21(3):252–260. doi: 10.1157/13106811. [DOI] [PubMed] [Google Scholar]
- Hobson CW, Scott S, Rubia K. Investigation of cool and hot executive function in ODD/CD independently of ADHD. Journal of Child Psychology and Psychiatry. 2011;52(10):1035–1043. doi: 10.1111/j.1469-7610.2011.02454.x. [DOI] [PubMed] [Google Scholar]
- Hooper SR, Woolley D, De Bellis MD. Intellectual, neurocognitive, and academic achievement in abstinent adolescents with cannabis use disorder. Psychopharmacology (Berl) 2014;231(8):1467–1477. doi: 10.1007/s00213-014-3463-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwood LJ, Fergusson DM, Hayatbakhsh MR, Najman JM, Coffey C, Patton GC, Hutchinson DM. Cannabis use and educational achievement: findings from three Australasian cohort studies. Drug and Alcohol Dependence. 2010;110(3):247–253. doi: 10.1016/j.drugalcdep.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Miech RA, Bachman JG, Schulenberg JE. Monitoring the Future national results on adolescent drug use: Overview of key findings, 2013. Ann Arbor: Institute for Social Research, The University of Michigan; 2014. p. 90. [Google Scholar]
- Kalant H. Adverse effects of cannabis on health: an update of the literature since 1996. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2004;28(5):849–863. doi: 10.1016/j.pnpbp.2004.05.027. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Delis DC, Daniel M. Sex-Differences in Verbal-Learning. Journal of Clinical Psychology. 1988;44(6):907–915. [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Giedd JN. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36(4):1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisdahl KM, Gilbart ER, Wright NE, Shollenbarger S. Dare to delay? The impacts of adolescent alcohol and marijuana use onset on cognition, brain structure, and function. Frontiers in Psychiatry. 2013;4:53. doi: 10.3389/fpsyt.2013.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisdahl KM, Price JS. Increased marijuana use and gender predict poorer cognitive functioning in adolescents and emerging adults. Journal of the International Neuropsychological Society. 2012;18(4):678–688. doi: 10.1017/S1355617712000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie K. Distribution of cannabinoid receptors in the central and peripheral nervous system. Handbook of Experimental Pharmacology. 2005;168:299–325. doi: 10.1007/3-540-26573-2_10. [DOI] [PubMed] [Google Scholar]
- Meier MH, Caspi A, Ambler A, Harrington H, Houts R, Keefe RS, Moffitt TE. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proceedings of the Natlional Academy of Sciences. 2012;109(40):E2657–2664. doi: 10.1073/pnas.1206820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overman WH, Frassrand K, Ansel S, Trawalter S, Bies B, Redmond A. Performance on the IOWA card task by adolescents and adults. Neuropsychologia. 2004;42(13):1838–1851. doi: 10.1016/j.neuropsychologia.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. Journal of Clinical Psychology. 1995;51(6):768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Pedersen W, Mastekaasa A, Wichstrom L. Conduct problems and early cannabis initiation: a longitudinal study of gender differences. Addiction. 2001;96(3):415–431. doi: 10.1046/j.1360-0443.2001.9634156.x. [DOI] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signalling. Nature Reviews Neuroscience. 2003;4(11):873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D. Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug and Alcohol Dependence. 2003;69(3):303–310. doi: 10.1016/s0376-8716(02)00334-4. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D. Neuropsychological performance in long-term cannabis users. Archives of General Psychiatry. 2001;58(10):909–915. doi: 10.1001/archpsyc.58.10.909. [DOI] [PubMed] [Google Scholar]
- Reavis R, Overman WH. Adult sex differences on a decision-making task previously shown to depend on the orbital prefrontal cortex. Behavioral Neuroscience. 2001;115(1):196–206. doi: 10.1037/0735-7044.115.1.196. [DOI] [PubMed] [Google Scholar]
- Rippeth JD, Heaton RK, Carey CL, Marcotte TD, Moore DJ, Gonzalez R, Grant I. Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. Journal of International Neuropsychological Society. 2004;10(1):1–14. doi: 10.1017/S1355617704101021. [DOI] [PubMed] [Google Scholar]
- SAMSHA. Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings. 2013 NSDUH Series H-46, HHS Publication No. (SMA) 13–4795 2013. [Google Scholar]
- Schepis TS, Desai RA, Cavallo DA, Smith AE, McFetridge A, Liss TB, Krishnan-Sarin S. Gender differences in adolescent marijuana use and associated psychosocial characteristics. Journal of Addiction Medicine. 2011;5(1):65–73. doi: 10.1097/ADM.0b013e3181d8dc62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skogli EW, Andersen PN, Hovik KT, Oie M. Development of Hot and Cold Executive Function in Boys and Girls With ADHD: A 2-Year Longitudinal Study. Journal of Attention Disorders. doi: 10.1177/1087054714524984. in press. [DOI] [PubMed] [Google Scholar]
- Solowij N, Jones KA, Rozman ME, Davis SM, Ciarrochi J, Heaven PC, Yucel M. Verbal learning and memory in adolescent cannabis users, alcohol users and non-users. Psychopharmacology (Berl) 2011;216(1):131–144. doi: 10.1007/s00213-011-2203-x. [DOI] [PubMed] [Google Scholar]
- Solowij N, Jones KA, Rozman ME, Davis SM, Ciarrochi J, Heaven PC, Yucel M. Reflection impulsivity in adolescent cannabis users: a comparison with alcohol-using and non-substance-using adolescents. Psychopharmacology (Berl) 2012;219(2):575–586. doi: 10.1007/s00213-011-2486-y. [DOI] [PubMed] [Google Scholar]
- Solowij N, Stephens RS, Roffman RA, Babor T, Kadden R, Miller M, Vendetti J. Cognitive functioning of long-term heavy cannabis users seeking treatment. JAMA. 2002;287(9):1123–1131. doi: 10.1001/jama.287.9.1123. [DOI] [PubMed] [Google Scholar]
- Stephens RS, Roffman RA, Curtin L. Comparison of extended versus brief treatments for marijuana use. Journal of Consulting and Clinical Psychology. 2000;68(5):898–908. [PubMed] [Google Scholar]
- Toplak ME, Jain U, Tannock R. Executive and motivational prcoesses in adolescents with Attention-Deficit-Hyperactivity-Disorder (ADHD) Behavioral and Brain Functions. 2005;1(1):Article 8. doi: 10.1186/1744-9081-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viveros MP, Llorente R, Suarez J, Llorente-Berzal A, Lopez-Gallardo M, de Fonseca FR. The endocannabinoid system in critical neurodevelopmental periods: sex differences and neuropsychiatric implications. Journal of Psychopharmacology. 2012;26(1):164–176. doi: 10.1177/0269881111408956. [DOI] [PubMed] [Google Scholar]
- Ward MF, Wender PH, Reimherr FW. The Wender Utah Rating Scale: an aid in the retrospective diagnosis of childhood attention deficit hyperactivity disorder. American Journal of Psychiatry. 1993;150(6):885–890. doi: 10.1176/ajp.150.6.885. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler test of adult reading. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]


