Abstract
The primate somatosensory neuraxis provides a highly translational model system with which to investigate adult neural plasticity. Here, we report immunohistochemical staining data for AMPA and GABAA/B receptor subunits of area 3b cortex and cuneate nucleus of adult squirrel monkeys one to five years after median and ulnar nerve transection. In Area 3B cortex, the expression of GluR1 AMPAR subunits in reorganized regions are significantly increased, while the expression of GluR2/3 AMPAR subunits are not. GABAA α1 subunit expression in the reorganized region is not significantly different from control regions. Presynaptic GABABR1a subunit expression was also not significantly different between reorganized and control regions, while postsynaptic GABABR1b subunit expression was significantly decreased. In the cuneate nucleus of the brainstem, the expression of GluR1 AMPAR subunits in reorganized regions was not significantly different, while GluR2/3 AMPAR subunit expression was significantly elevated. GABAA α1 subunit expression in the reorganized region was significantly decreased. Presynaptic GABABR1a subunit expression was not significantly different, while postsynaptic GABABR1b subunit expression was significantly decreased. When subunit expression is compared, brainstem and cortical patterns diverge over longer periods of recovery. Persistent patterns of change in the cortex are stable by 1 year. Alternatively, subunit expression in the cuneate nucleus one to five years after nerve injury is similar to that seen 1 month after a reorganizing injury. This suggests that cortical plasticity continues to change over many months as receptive field reorganization occurs, while brainstem plasticity obtains a level of stable persistence by one month.
Keywords: reorganization, peripheral nerve injury, long-term recovery, GABA, AMPA, area 3b cortex, cuneate nucleus brainstem, non-human primate
Introduction
The adult somatosensory neuraxis is capable of undergoing large shifts in topographic representations when input patterns are altered by behavior, injuries to central structures, or injuries to peripheral nerves (Buonomano and Merzenich, 1998; Navarro 2007). Large shifts in body map representations, known as somatosensory reorganization, were originally demonstrated in the adult neocortex in the early 1980’s (Merzenich et al., 1983a/b). Using a peripheral nerve injury model of sensory deprivation in non-human primates (median nerve transection), two phases of somatosensory plasticity were identified. The first was an acute phase, generally characterized as “unmasking”. During this phase, novel receptive fields are immediately expressed in the deprived cortex. The second, more protracted, phase followed over the ensuing days to weeks after injury, as neurons in the remaining regions of deprived cortex became responsive to peripheral stimulation of skin surfaces with intact innervation. This phase of reorganization required long-term potentiation because blocking NMNDA receptor activation prevented reorganization (Garraghty and Muja, 1996). At the same time, it became clear that receptor correlates of plasticity could be investigated to elucidate the mechanisms by which reorganization proceeded through its various phases.
We have continued our use of the mature non-human primate somatosensory neuraxis with the goal of further characterizing the contributions that glutamatergic and GABAergic systems make during reorganization. Our studies have provided evidence that AMPA and GABA receptors play unique roles in the adult brain’s response to sensory deprivation. In general, we find support for an early change in GABAergic circuits that are consistent with the concept of unmasking and disinhibition (Garraghty et al., 1991; 2006; Wellman et. al. 2002). This is followed by a subsequent phase of “developmental recapitulation” during which critical period like receptor expression (plasticity) is re-expressed in the deprived sensory neuraxis (Mowery and Garraghty, 2009; Mowery et al., 2011; Sarin et al., 2012). This phase could prime the cortex for the NMDA receptor-dependent stage of reorganization (Garraghty et al., 2006; Garraghty and Muja, 1996; Mowery et al., 2013; 2014 Myers et al., 2000).
Our original cortical mapping paper (Garraghty and Kaas, 1991a) demonstrated that median and ulnar nerve transection was followed by a complete reorganization of the cortical topographic map in area 3b within two months. It was further noted that a “rough topographic order” existed for the dorsum skin receptive fields that came to occupy the deprived cortical territory. The novel receptive fields of the reorganized maps were much larger than one typically finds in area 3b, and there were many multi-digit receptive fields; a rarity in normal area 3b. Later, Churchill et al., (1998) examined the consequences of longer survival durations in animals with median and ulnar nerve transection for a year or more. They reported a continued refinement of the cortical topography, with receptive fields having become much smaller. In a subsequent report, it was argued that this receptive field refinement was a cortical phenomenon, with no shrinkage of the novel subcortical receptive fields (Churchill and Garraghty, 2006). Therefore we were interested in how AMPA and GABA receptor subunit expression in the cortex and brainstem of animals with long-standing nerve injuries (years) correlates with previous electrophysiological data.
The goals of this study were to determine whether the cortex eventually returns to the state (indexed by the immuno-staining) that existed prior to the nerve injury-induce reorganization, and whether differences emerge in the patterns of immuno-staining between area 3b and the cuneate nucleus. Thus, the cortex and brainstem of adult squirrel monkeys with longstanding (1 to 5 years) nerve transections of the median and ulnar nerve were processed for immuno-histological assessment of AMPA and GABA receptors. The patterns of receptor expression in these animals agree with physiological states previously reported in the chronically reorganized brainstem and cortex (Churchill et al., 1998; 2001; Churchill and Garraghty, 2006). The cortical data show persistent changes to GluR1 AMPA and postsynaptic GABAB1b receptor subunits that might be related to the long term maintenance of deprived synapses. Furthermore, GluR2/3 AMPA, GABAA α1, and presynaptic GABAB1a receptor subunits have returned to baseline, suggesting that glutamatergic and GABAergic synaptic transmission are relatively normal. The brainstem data show that GluR2/3 is significantly elevated while GABAA α1 and postsynaptic GABAB1b are significantly decreased. GluR1 and presynaptic GABAB1a show no change. This pattern of AMPA and GABA subunits is approximately similar to that seen during early reorganization (Mowery, Kostylev, and Garraghty 2014). This suggests that the cuneate nucleus enters a persistent state of heightened reorganizational plasticity. Taken together these data demonstrate that some species of AMPA and GABA receptors remain significantly altered along the chronically reorganized neuraxis. They also suggest disparate states of plasticity between the chronically reorganized cortex and brainstem.
Results
In this study we compare the expression of AMPARs and GABARs between ipsilateral and contralateral somatosensory representations of non-human primate area 3b cortex and cuneate nucleus of the brainstem after many years of recovery from unilateral median and ulnar nerve transections. For each animal the area 3b somatosensory cortex was dissected out from the left and right hemispheres and staining intensity measurements were generated from neurons within the digit 2 representation of supragranular, granular, and infragranular layers of each cortex (Figure 1A/B/B′). The brainstem was dissected out and staining intensity measurements were generated from neurons within each of the bilateral representations of digit 2 located within the cuneate nucleus at the level of the pars rotunda (Figure 1A/C/C′; see Florence et al. 1991). Paired comparisons were used to investigate whether chronic reorganization induced significant changes to receptor expression within these regions of interest.
Figure 1.
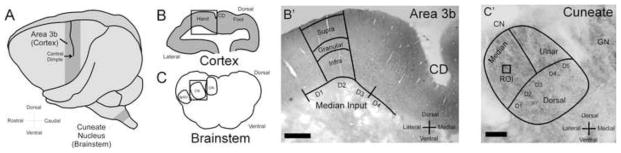
Identifying sections containing Area 3b cortex and Cuneate nucleus of the brainstem. A: Cartoon of the squirrel monkey brain identifying area 3b of the somatosensory cortex and cuneate nucleus of the brainstem, CD; central dimple. B: Cartoon of a coronal section of area 3b of the somatosensory cortex. B′: Photomicrograph of area 3b hand region. Contours indicate the regions of interest for IHC quantification. CD; central dimple; scale bar 250 μm. C: Cartoon of a coronal section of the pars rotunda of the brainstem. CN; cuneate nucleus, GN; Gracile Nucleus, SpNV: spinal nucleus. C′: Photomicrograph of the cuneate nucleus hand representation. Contours indicate the hand representation divided into its peripheral inputs. ROI; region of interest; scale bar 25 μm.
AMPAR subunit expression following chronic reorganization
GluR1
Paired comparisons revealed a number of significant long term effects of reorganization on receptor expression within the cortex and brainstem. Figure 2A shows that GluR1 receptor subunit expression in the reorganized cortex is significantly elevated across all layers compared to control hemisphere [GluR1 – Mean ± SEM; soma: supragranular - ctrl 56.2 ± 2.4 vs.. exp 60.74 ± 1.4; t = 3.89, p <.05; granular - 46.2 ± 3.0 vs. exp 50.1 ± 2.5; t = 2.34, p <.05; infragranular – 45.7 ± 3.8 vs. exp 49.9 ± 3.8; t = 2.32 p < .05; neuropil: supragranular – ctrl 36.2 ± 2.3 vs. exp 41.0 ± 1.5; t = 2.39, p <.05; granular – 24.3 ± 3.2 vs. exp 27.7 ± 2.3; t = 2.04, p <.05; infragranular – 21.6 ± 3.0 vs. exp 25.2 ± 3.8; t = 2.32 p < .05]. At the same time, GluR1 subunit expression was not significantly altered in the cuneate nucleus of the brainstem [GluR1 – Mean ± SEM; ctrl 68.2 ± 1.0 vs. exp 67.8 ± 0.9, t = 0.33 p > .1]. Finally, in Figure 2B linear regression analysis indicated that there was no detectable effect of survival duration on subunit expression in either the cortex (supragranular - r2 = .024, p = 0.76; granular - r2 = .007, p = 0.86; infragranular - r2 = .009, p = 0.85) or the brainstem (r2 = .15, p = 0.43).
Figure 2.
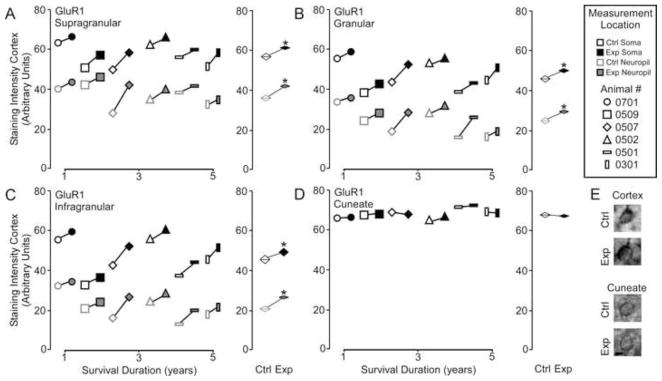
Comparisons of the pattern of expression for GluR1 receptor subunits between control and experimental regions of the chronically reorganized cortex and brainstem. A–D: Left, Comparisons showing the qualitative differences within each animal for GluR1 staining intensity between experimental and control regions of the supragranular, granular, infragranular cortex, and cuneate nucleus of the brainstem. Right, quantitative comparisons of the group average for GluR1 staining intensity between experimental and control regions of the supragranular, granular, infragranular cortex, and cuneate nucleus of the brainstem. * p < .05. E: Qualitative examples of soma staining intensity differences between chronically reorganized and control regions of the cortex and brainstem.; scale bar 2 μm.
GluR2/3
Figure 3A shows that analysis of GluR2/3 receptor subunit expression in the cortex did not reveal any effect of nerve injury [GluR2/3 – Mean ± SEM; soma: supragranular - ctrl 49.5 ± 0.8 vs.. exp 48.07 ± 0.9; t = 0.38, p >.05; granular – 35.5 ± 2.1 vs. exp 35.8 ± 1.5; t = 0.26, p >.05; infragranular – 36.0 ± 1.9 vs. exp 35.9 ± 1.2; t = 0.12 p > .05; neuropil: supragranular – ctrl 36.0 ± 2.0 vs. exp 35.4 ± 1.9; t = 1.80, p >.05; granular – 23.2 ± 3.4 vs. exp 23.5 ± 2.5; t = 0.28, p >.05; infragranular – 21.6 ± 3.1 vs. exp 21.1 ± 3.3; t = 0.36 p > .05]. Alternatively, there is a significant elevation of GluR2/3 expression in the reorganized cuneate nucleus [GluR2/3 – Mean ± SEM; ctrl 38.7 ± 0.6 vs. exp 48.9 ± 1.3, t = 6.65 p < .001]. Again linear regression analysis (Figure 3B) showed that there was no effect of survival duration on subunit expression in the cortex supragranular - r2 = .156, p = 0.43; granular - r2 = .091, p = 0.55; infragranular - r2 = .149, p = 0.44) or brainstem (r2 = .07, p = 0.59).
Figure 3.
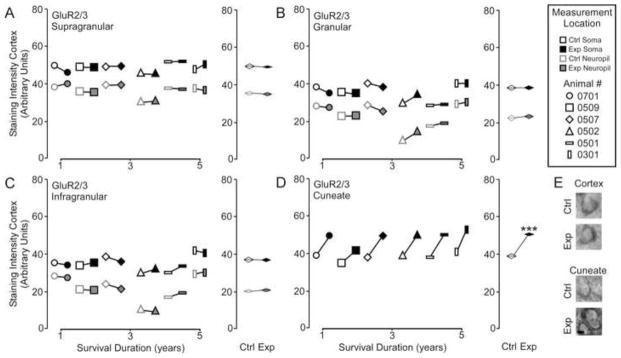
Comparisons of the pattern of expression for GluR2/3 receptor subunits between control and experimental regions of the chronically reorganized cortex and brainstem. A–D: Left, Comparisons showing the qualitative differences within each animal for GluR2/3 staining intensity between experimental and control regions of the supragranular, granular, infragranular cortex, and cuneate nucleus of the brainstem. Right, quantitative comparisons of the group average for GluR2/3 staining intensity between experimental and control regions of the supragranular, granular, infragranular cortex, and cuneate nucleus of the brainstem. * p < .05. E: Qualitative examples of soma staining intensity differences between chronically reorganized and control regions of the cortex and brainstem.; scale bar 2 μm.
GABAR subunit expression following chronic nerve transection
GABAA α1
Figure 4A illustrates that GABAA α1 receptor subunit expression in the experimental cortex was not significantly different from expression in the control cortex [GABAA α1 – Mean ± SEM; soma: supragranular - ctrl 33.6 ± 0.6 vs.. exp 32.4 ± 1.2; t = 1.31, p >.05; granular – 26.5 ± 1.7 vs. exp 25.9 ± 1.6; t = 0.99, p > .05; infragranular – 26.6 ± 2.0 vs. exp 25.8 ± 2.0; t = 1.25, p > .05; neuropil: supragranular – ctrl 21.8 ± 1.0 vs. exp 22.2 ± 0.7; t = 0.24, p > .05; granular – 15.7 ± 2.2 vs. exp 15.5 ± 2.2; t = 1.02, p >.05; infragranular – 14.0 ± 2.1 vs. exp 14.31 ± 1.9; t = 0.94 p > .05]. GABAA α1 expression, however, was significantly lowered in the reorganized cuneate nucleus [GABAA α1– Mean ± SEM; ctrl 33.2 ± 0.9 vs. exp 27.6 ± 1.0, t = 3.97 p < .01]. Linear regression analysis (Figure 4B) indicated that there was no effect of survival duration on subunit expression in either the cortex (supragranular - r2 = .143, p = 0.38; granular - r2 = .001, p = 0.97; infragranular - r2 = .455, p = 0.14) or the brainstem (r2 = .14, p = 0.46).
Figure 4.
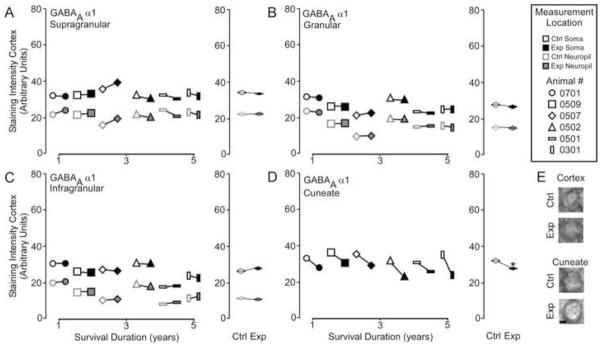
Comparisons of the pattern of expression for GABAA α1 receptor subunits between control and experimental regions of the chronically reorganized cortex and brainstem. A–D: Left, Comparisons showing the qualitative differences within each animal for GABAA α1 staining intensity between experimental and control regions of the supragranular, granular, infragranular cortex, and cuneate nucleus of the brainstem. Right, quantitative comparisons of the group average for GABAA α1 staining intensity between experimental and control regions of the supragranular, granular, infragranular cortex, and cuneate nucleus of the brainstem. * p < .05. E: Qualitative examples of soma staining intensity differences between chronically reorganized and control regions of the cortex and brainstem.; scale bar 2 μm.
GABAB1a
Figure 5A demonstrates that there was no difference in GABAB1a receptor subunit expression between the experimental cortex and the control cortex [GABAB1a – Mean ± SEM; soma: supragranular - ctrl 55.4 ± 5.4 vs.. exp 53.6 ± 4.5; t = 1.21, p > .05; granular – 46.3 ± 5.1 vs. exp 46.8 ± 5.2; t = 0.39, p >.05; infragranular – 43.9 ± 5.0 vs. exp 44.1 ± 5.2; t = 0.31 p > .05; t = 1.31, p <.05; neuropil: supragranular – ctrl 38.0 ± 1.3 vs. exp 37.0 ± 1.6; t = 0.87, p >.05; granular – 27.8 ± 1.6 vs. exp 30.2 ± 1.1; t = 1.37, p >.05; infragranular – 25.5 ± 1.1 vs. exp 27.7 ± 1.0; t = 2.09 p > .05], or within the cuneate nucleus [GABAB 1a – Mean ± SEM; ctrl 32.4 ± 0.8 vs. exp 30.7 ± 0.6, t = 1.61 p > .1]. Linear regression analysis (Figure 5B) shows that there was no effect of survival duration on subunit expression in either the cortex (supragranular - r2 = .519, p = 0.10; granular - r2 = .542, p = 0.09; infragranular - r2 = .46, p = 0.13) or the brainstem (r2 = .001, p = 0.98).
Figure 5.
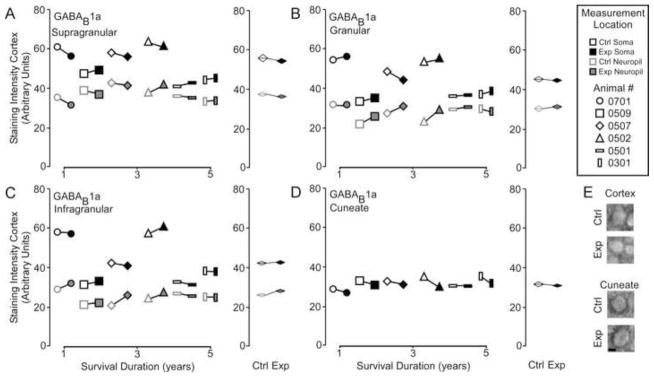
Comparisons of the pattern of expression for GABAB1a receptor subunits between control and experimental regions of the chronically reorganized cortex and brainstem. A–D: Left, Comparisons showing the qualitative differences within each animal for GABAB1a staining intensity between experimental and control regions of the supragranular, granular, infragranular cortex, and cuneate nucleus of the brainstem. Right, quantitative comparisons of the group average for GABAB1a staining intensity between experimental and control regions of the supragranular, granular, infragranular cortex, and cuneate nucleus of the brainstem. * p < .05. E: Qualitative examples of soma staining intensity differences between chronically reorganized and control regions of the cortex and brainstem.; scale bar 2 μm.
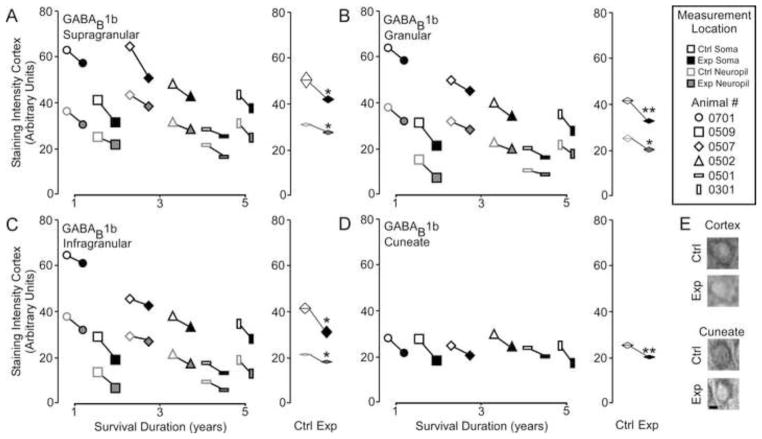
GABAB1b
Figure 6A shows that GABAB 1b receptor subunit expression was significantly lowered across all layers in the reorganized cortex [GABAB 1b – Mean ± SEM; soma: supragranular - ctrl 48.2 ± 5.2 vs. exp 42.6 ± 5.0; t = 3.67, p <.05; granular – 40.5 ± 6.1 vs. exp 34.1 ± 6.5; t = 5.8, p <.01; infragranular – 38.2 ± 6.8 vs. exp 33.5 ± 7.3; t = 3.74 p < .05; neuropil: supragranular – ctrl 31.9 ± 3.3 vs. exp 28.8 ± 3.1; t = 3.47, p <.05; granular – 23.4 ± 4.0 vs. exp 20.5 ± 4.3; t = 2.84, p <.05; infragranular – 21.5 ± 4.4 vs. exp 17.4 ± 4.6; t = 5.80 p < .01], as well as, the reorganized cuneate nucleus [GABAB 1b – Mean ± SEM; ctrl 26.3 ± 1.1 vs. exp 21.0 ± 1.3, t = 2.93 p < .01]. Linear regression analysis (Figure 6B) indicates that there was no effect of survival duration on subunit expression in either the cortex (supragranular - r2 = .441, p = 0.15; granular - r2 = .459, p = 0.13; infragranular - r2 = .674, p = 0.06) or the brainstem (r2 = .001, p = 0.93).
Figure 6.
Comparisons of the pattern of expression for GABAB1b receptor subunits between control and experimental regions of the chronically reorganized cortex and brainstem. A–D: Left, Comparisons showing the qualitative differences within each animal for GABAB1b staining intensity between experimental and control regions of the supragranular, granular, infragranular cortex, and cuneate nucleus of the brainstem. Right, quantitative comparisons of the group average for GABAB1b staining intensity between experimental and control regions of the supragranular, granular, infragranular cortex, and cuneate nucleus of the brainstem. * p < .05. E: Qualitative examples of soma staining intensity differences between chronically reorganized and control regions of the cortex and brainstem.; scale bar 2 μm.
Discussion
In this study, we investigated the effect of long-term nerve deafferentation on AMPA and GABA subunit expression in somatosensory cortex and brainstem of six adult squirrel monkeys. In the chronically reorganized cortex we find no difference in GluR2/3, GABAA or presynaptic GABAB subunits. At the same time we find that Glur1 expression is significantly increased across all cortical layers, while GABAB1b expression is decreased across all cortical layers. Furthermore, we find a consistent pattern of increased GluR2/3, decreased GABAA α1 and decreased GABAB1b receptor subunit expression in the reorganized cuneate nucleus of these same animals. In both the cortex and brainstem, the effect of nerve injury is apparent at one year and remains stable up to 5 years after injury. Taken together these results demonstrate that chronic nerve injury induces persistent region specific changes to AMPA and GABA subunits. It is important to note that without corresponding functional data, definitively interpreting the physiological meaning of these specific patterns of subunit expression is difficult. It is well documented that bilateral physiological changes occur after unilateral nerve injuries (Calford and Tweedale, 1990, 1991; Clarey et al., 1996; Oaklander and Belzberg, 1997; Oaklander and Brown, 2004). Therefore we cannot rule out that control measurements include injury induced physiological changes to subunit expression via contralateral cortico-cortical connectivity. Furthermore subunit data can inform the possible species of AMPAR and GABAR that are changed subsequent to nerve injury, but alternative techniques would be required (e.g., autoradiography) to absolutely identify receptor subtypes. In the following paragraphs, possible interpretations of the current data set are provided.
The Pattern of Area 3b cortical AMPA and GABA receptor subunits after chronic nerve transection
Area 3b of somatosensory cortex has long been the primary region of interest in nerve injury related non-human primate studies (Yang et al., 2014; Churchill and Garraghty 2006; Dutta et al., 2014; Florence, Hackett, and Strata 2000; Merzenich et al., 1983a/b). Many of these studies used electrophysiological techniques to reveal the ongoing changes to receptive field maps that occur during reorganization induced by peripheral lesion, dorsal root injury, spinal column transection, and central lesions (for review see Xu, Wall, and Wang 2002). Varying the level and degree of injury can lead to variable outcomes across the neuraxis; especially as survival duration increases (e.g., Pons et al., 2001; Graziano and Jones 2009.). However, specific patterns of peripheral denervation that leave the dorsal roots intact produce highly consistent somatotopic changes within the brainstem, thalamus, and cortex (Churchill, Arnold, and Garraghty 2001; Garraghty and Kaas 1991; Garraghty et. al. 1995; Garraghty and Churchill 2006). Therefore it is important to note that the patterns of subunit expression reported here occur as the result of a highly controlled nerve injury that does not necessarily induce the same variability or severity of secondary physiological effects that are seen following dorsal rhizotomy, cervical transection, and central injury (e.g., dorsal root death, brainstem/thalamic/cortical axonal retraction, vacated synapse invasion)
In our previous studies we have found that nerve injury induced changes to NMDA, AMPA and GABA receptors have a specific pattern during the period where most of the topographic reorganization occurs within the area 3b primary cortex (Garraghty et al., 2006; Mowery, Walls, and Garraghty 2013). Specifically we find that during the second phase of injury induced plasticity (reorganization) there is evidence of increased GluR2/3 containing AMPA receptors, decreased GABAA α1 receptors, and decreased post synaptic GABAB receptors. These patterns represent putative receptor correlates of the excitatory and inhibitory changes that regulate the neural plasticity that drives reorganization (Benali et al., 2008; Dykes 1997; Castro-Alamancos, Donoghue, and Connors 1995). In the current study, we have demonstrated that with the exception of GABAB1b, the pattern of expression for AMPA and GABA receptor subunits in the chronically reorganized cortex is not like the pattern of newly reorganized cortex. This suggests two different states of functional plasticity between early and long-term reorganization.
To that end, the pattern of AMPA and GABA subunit expression reported here provide novel insight into the plasticity state of the brain after chronic survival durations. The return to control values for GABAA α1 corroborates our previous electrophysiological study that demonstrates a return of approximately normal receptive fields in the chronically reorganized cortex (Garraghty and Churchill 2006). GABAA receptor activation is directly correlated with normal receptive field grain and fidelity of evoked neural activity (Hickes and Dykes 1986; Alloway and Burton 1991). In addition to this, we find no difference between control and reorganized cortex for the presynaptic GABAB receptor. This receptor effectively regulates synaptic release of GABA and glutamate through autoregulation (Waldmeier, Kaupmann, and Urwyler 2008). Furthermore, the pattern of expression for the GluR2/3 containing AMPA receptor is not different between reorganized and control cortex. This receptor is highly involved in the regulation of normal postsynaptic activity (Passafaro, Piëch, and Sheng 2001; Shi et al., 2001). Taken together these results could suggest that pre and post synaptic excitatory and inhibitory transmission are relatively normal in the chronically reorganized cortex.
At the same time we find a significant increase in GluR1 and significant decrease in postsynaptic GABAB receptor subunits. We have previously interpreted decreased postsynaptic GABAB receptors as heightened plasticity through disinibition of the NMDAR receptor. This remains a possibility as reorganizational plasticity could be an ongoing feature of chronic recovery from nerve injury; however, it is interesting to note that the GABAB receptor also actively regulates the glutamatergic calcium currents gated by homomeric GluR1 AMPA receptors (Obrietan and van den Pol, 1999). We posit that the homomeric GluR1/Glur1 AMPA is the likely species leading to the significant increase in GluR1 subunit expression. The GluR1 subunit does form heterodimers with GluR2 subunits that regulate synaptic potentiation in an activity dependent manner (Hayashi et al., 2000; Shi et al., 2001); however, finding a lack of concomitant increase in the GluR2 subunit (stable GluR2/3) suggests that an increase in the GluR1/2 AMPAR species does not account for the increased expression of GluR1 subunits in this study.
In our previous papers we have interpreted increases in GluR1 subunits as evidence of an increase in homomeric AMPA receptors (Eybalin et al., 2004; Ho et al., 2007; Kumar et al., 2002). An elevated expression of these receptors have been associated with developmental plasticity (Cramer and Chopp 2000); however, they also serve a specialized function in maintaining synapses within neural networks along extinguished fear conditioning pathways (Clem and Huganir, 2010). Retaining these synapses under deprived conditions allows for the spontaneous recovery of behavior. They also contribute to the maintenance of long-term drug craving and relapse (Bellone and Lüscher, 2006; Conrad et al., 2008). The basic concept of adaptation to inactivity states that under conditions of inactivity synapses adapt by inserting these low threshold calcium gating receptors into the synaptic cleft (Thiagarajan et al., 2007). This effectively preserves the synapse under conditions of degraded neural transmission (Lindskog et al., 2010).
We would suggest that maintenance of deprived synapses involving GluR1/GluR1 AMPARs could also facilitate the retention of original somatotopic pathways. The immediate reinstatement of perception in humans (Halligan et. al., 1993; Moore and Schady, 2000; Schady, 1994), and the cortical activation of deafferented nerve representations in monkeys (Schroeder et al. 1997) clearly suggests that the original somatotopy is somehow maintained. In fact, there is well documented evidence in humans that perceptual receptive fields can differ from reorganized receptive fields (Knecht et al., 1995;1996). Philip and Frey (2011) find that humans with longstanding upper limb amputations have unimpaired movement representations for the missing hand, and suggest that they might be due to “persistent higher-level activity-independent internal representations.” Our findings offer a clue by which the ongoing persistence of these deprived somatotopic pathways could be maintained even after years of reorganization. The calcium gating GluR1/GluR1 AMPAR could contribute to the neural mechanism by which multiple perceptual/receptive field representations can be dynamically stored within the same neural network.
The pattern of cuneate nucleus AMPA and GABA receptor subunits after chronic nerve transection
Corticocentric theories of synaptic plasticity and reorganization were long disputed in the field of adult somatosensory plasticity. The concept that bottom up magnification from the brainstem to the thalamus was eventually relayed to the cortex was in direct competition with theories describing the top down amplification of cortically selected peripheral inputs. Through diligent research it has been well established that bottom up and top down physiological processes drive normal function, adaptive (learning), and maladaptive (phantom pain) plasticity. The current data demonstrate that after long periods of chronic nerve transection, the brainstem and cortex differ in the pattern of AMPA and GABA receptor subunits. This suggests disparate forms of plasticity between these two regions of the neuraxis. As discussed for the cortex, the pattern of AMPA and GABA subunit distribution in the brainstem provide valuable insight into physiological states reported previously (Churchill et al., 2001; Garraghty and Churchill 2006). We also identify a very similar pattern of subunit expression observed 1 month after median nerve compression (Mowery et al., 2014). This suggests that brainstem plasticity at 1 to 5 yeas is not significantly different from the plasticity reported early after nerve injury (1 month).
Unlike cortex we see no evidence of elevated GluR1 levels. This would suggest that GluR1 containing homomeric (GluR1/Glur1) are not expressed at any greater level in the chronically reorganized brainstem. At the same time, we can not rule out a decrease in this receptor species masked by an increase in Glur1/Glur2 receptor subtypes due to the significant increase in GluR2/3 subunits. A general increase in GluR2/3 containing AMPARs is also a plausible explanation. GluR1/2 and GluR2/3 containing AMPARs are key regulators of synaptic plasticity and activity dependent long term potentiation (Malinow and Malenka, 2002; Bredt and Nicoll, 2003; Collingridge et al., 2004; Shepherd and Huganir, 2007). Finding a persistent elevation of either of these subunits in the chronically reorganized cuneate nucleus suggests that this region remains highly plastic. As the neuraxis adapts to chronic deprivation, ongoing subcortical reorganization is a possibility. This is made even more plausible by studies demonstrating that the brainstem is a major site of plasticity following nerve injury, and a source of the maladaptive plasticity that can occur over time (e.g., Kaas et al., 2008).
We also find significantly lowered GABAA α1 in the chronically reorganized cuneate nucleus. Lower inhibitory tone would provide a functional explanation for the larger receptive field grain and higher incidence of multi-source receptive fields. GABA agonist and antagonist decrease and increase receptive field grain respectively (e.g., Hicks and Dykes,1983; Alloway and Burton, 1991). This functionally manifests as neurons with receptive fields to multiple sources of peripherally evoked responses. Furthermore, lowered inhibitory tone would lead to less effective suppression of overlapping dendritic inputs and subsequently increased long-term potentiation within the neural network (Komaki et al., 2007).
Presynaptic GABAB receptors in both the reorganized cortex and brainstem are not significantly different from control regions. As previously stated these presynaptic autoreceptors gate both glutamatergic and GABAergic neural transmission (Waldmeier, Kaupmann, and Urwyler 2008). Therefore we would maintain that it is possible that neural transmission within the brainstem is normally regulated despite increased plasticity. Finally we report significantly decreased post synaptic GABAB receptors. Whereas we posit that decreased postsynaptic GABAB receptor distribution could increase the efficacy of GluR1/GluR1 calcium gating AMPARs in the cortex, here we would suggest that this phenomenon plays a more traditional role of increasing the probability of NMDAR activation. Postsynaptic GABAB receptors function to inhibit NMDAR activation (Otmakhova and Lisman 2004; Morrisett et al., 1991). Decreased expression would effectively disinhibit the LTP inducing NMDAR activation adding increased plasticity to the system.
Conclusion
In this study we have provided evidence that AMPA and GABA receptors are significantly altered in the brainstem and cortex of animals that survived peripheral nerve transection for 1–5 years. The patterns of expression between these two regions of the somatosensory neuraxis are very different. In the cortex it is possible that while neural transmission and excitatory/inhibitory tone are relatively normal, increased homomeric GluR1/GluR1 AMPA receptors contribute to the maintenance of the dynamic range of perceptual and receptive fields held within the neural networks of reorganized cortex (Knecht et al., 1995;1996). In the brainstem, the pattern of AMPA and GABA receptor subunits is very similar to that seen one month after nerve injury; a time when wide scale reorganization is likely occurring. This suggests that chronic nerve injury leads to elevated cuneate plasticity and that ongoing reorganization might be a feature of brainstem response to sensory deprivation. If dynamic somatotopy is maintained in the cortex by the GluR1/Glur1 homomeric AMPA receptors this is good news for the advancement of brain machine interface and prosthetic advances. Furthermore dampening the heightened plasticity in the brainstem (GABA agonists, NMDA blockers) might facilitate the prevention, treatment, and management of neuropathic pain/sensation.
Methods
Nerve Transection
We report data from 6 adult squirrel monkeys (Saimiri sciureus) that underwent unilateral median and ulnar nerve transections (left arm). Each animal recovered from injury for 1 to 5 years. Animals were maintained on isoflurane anesthesia (2–4%) throughout the aseptic surgery. The left ventral forearm was shaved between the wrist and elbow, and an incision was made along its midline 70 mm from the wrist. Under microscopic view, the median and ulnar nerves were located, isolated by blunt dissection, and then elevated. Nerves were transected and then the epineural sheath of the proximal stump was retracted 0.5 to 1.0 cm and the exposed nerve was avulsed. The empty epineural sheath was then re-extended, folded over the remaining nerve, and securely ligated with non-biodegradable suture. The nerve stumps were then repositioned, the skin was sutured over the incision site, and the animal was allowed to recover. All protocols were approved by the Institutional Animal Care and Use Committee (IACUC).
Immunohistochemistry
After one to five years of recovery, animals were anesthetized with isoflurane gas and transcardially perfused with cold 0.9% saline solution followed by 400 ml of 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). Following perfusion, the brain was extracted and the left and right somatosensory cortices as well as the brainstem were dissected out and post-fixed for 2 h in cold fixative (4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). Tissue was cryoprotected overnight in 30% sucrose in phosphate buffered saline (pH 7.4).
Staining
Sections from the left and right pre and post-central gyri of the cortex, or pars rotunda of the brainstem were cut in a coronal plane (40 μm) using a freezing microtome. Sections that clearly contained the central sulcus or pars rotunda were kept for immunohistochemical processing. After sectioning, all sections were washed in immuno-phosphate buffer (IPB) and incubated in blocking solution for 30 min followed by hydrogen peroxide .01% for 15 min at room temperature. Tissue sections were then incubated over-night at 4 °C in antisera containing GluR1 (1:1000 Chemicon), GluR2/3 (1:1000 Thermo Scientific), GABAA α1 (1: 1000 Thermo Scientific), GABAB1a (1:1000 Alpha Diagnostics International), or GABAB1b (1:1000 Alpha Diagnostics International). The following day tissue was washed three times for ten minutes in IPB before being incubated in goat anti-rabbit biotinylated antibodies for 1 h at room temperature. Sections were again washed three times for ten minutes in PBS, incubated in ABC solution for one hour (ABC Elite Kit, Vector), washed again three times for ten minutes in Acetate-Imidazole buffer, and incubated in Acetate-Imidazole buffer containing Nickel Sulfate, 0.5 mg/ml 3.3′-diaminobenzidine-4HCI (DAB, Vector) and 0.01% H2O2 for approximately 8 min. Sections were washed three times in PBS for ten minutes, mounted on gelatin coated glass slides and dried overnight. Once dry, the sections were dehydrated in ascending ethanols, cleared with xylenes, and cover-slipped. Positive and negative controls were generated by omitting the primary or secondary antibody. Light microscopy of tissue did not reveal the presence of any non-specific binding.
Staining Intensity Quantification
Area 3b
Electrophysiological receptive field mapping was not used to verify regions of interest (ROI) due to the variables introduced via craniotomy and/or electrode penetration. However, the somatotopic representations referred to throughout this study are based on receptive field mapping observations consistently reported approximately 1 mm posterior and 1 mm anterior to the central dimple of the central sulcus (e.g., Churchill et al., 1998; Gingold et al., 1991, Garraghty et al., 1991a; 1994; Garraghty and Muja, 1995; Myers et al., 2000; Schroeder et al., 1995; 1997; Sur et al., 1982;). Within this area of cortex, the digit representations deafferented by median and ulnar nerve transection are found starting around the central dimple and extending ~ 1.5 mm lateral (see Figure 1).
For each tissue section a tracing grid was drawn for supragranular, granular, and infragranular layers and overlaid on a region of area 3b median nerve representation approximate to digit 2 (~ 1 mm lateral to the central sulcus dimple). With the tissue processing procedures used here, there are no clear histological boundaries between digits (Jain et al., 1998), so it is conceivable that the tracing grid sometimes included regions that receive thalamocortical inputs from digit 1 or 3. Layers were discernable to the trained observer based on differences in packing density (4x) and neural phenotype (40x). There are no clear markers of laminar boundary therefore luminance measurements were generated from the cell somata (~ 30 per layer) and neuropil (~ 10 per layer) contours traced within the middle of each layer.
Cuneate Nucleus
At the level of the pars rotunda within the brainstem, a bilateral representation of the hand/palm is visible following immunohistochemical staining. This makes targeting the region of interest relatively accurate without any form of electrophysiological mapping required. Therefore luminance measurements were carried out in the distal region of the median nerve representation for digit 2 (see Figure 1A/C). A 100 μm by 100 μm bounding box was placed within the region of interest at low magnification (4×) and then cell contours for cell somata (~30 per section) were traced at higher magnification (40×).
Quantification
Luminance Measurements (raw data)
Quantification of subunit staining was carried out at 1480× magnification under brightfield illumination using a microscope (Nikon Eclipse 80i; Nikon Instruments; Melville, NY, USA) and the Stereo Investigator software (MBF Bioscience; Williston, VT, USA). Within a region of interest neurons were visualized under brightfield and the soma, neuropil, or staining free region of white matter were manually traced. This created a contour around each soma, neuropil, or area of white matter. The luminance function was then used to measure the average brightness of the pixels located within the boundary of the traced contours (0 = black and 256 = white).
Subunit Staining Intensity
For each animal ~ 30 somata, and 10 neuropil measurements were generated per layer for each section/antibody. Around 30 somata measurements were generated per nucleus for each section/antibody. Around 5 white matter measurements were generated for each section. Staining intensity data were generated by quantifying the ratio of each traced soma or neuropil luminance value over the average white matter luminance value in that section. This transformed each data point into a percent darker than white matter score, which will be referred to as subunit staining intensity throughout this report.
Statistical analysis
For each animal an average staining intensity data point was calculated for the left and right cortical supragranular, granular, and infragranular layers, as well as, the left and right cuneate nucleus. This generated a total of six control and six experimental data points for area 3b cortex, as well as, one control and one experimental data point for the cuneate nuclei of each animal. Paired comparisons of control to experimental data points were used to determine whether nerve transections had significant long-term effects on receptor subunit expression. Percent difference from control measures were generated by calculating within animals the ratio of average staining intensity of a control region to its experimental region. The average of these percentage differences were used to create a difference score for each animal’s cortex and brainstem. Linear regression analysis was used to assess whether survival duration had a significant effect on subunit staining intensity.
GluR2/3, GABAA α1, and GABAB1a expression in cortex returns to baseline
GluR1 is elevated and GABAB1b is decreased in cortex
GluR2/3 is elevated, and GABAA α1 and GABAB1b are decreased in cuneate
GluR1 and GABAB1a expression in cuneate returns to baseline
Patterns of subunit expression between cortex and brainstem is different
Acknowledgments
Supported by National Institutes of Health/National Institute of Neurological Disorders and Stroke; Grant number: NS37348.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alloway KD, Burton H. Differential effects of GABA and bicuculline on rapidly- and slowly-adapting neurons in primary somatosensory cortex of primates. Exp Brain Res. 1991;85:598–610. doi: 10.1007/BF00231744. [DOI] [PubMed] [Google Scholar]
- Bellone C, Lüscher C. Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nat Neurosci. 2006;9:636–41. doi: 10.1038/nn1682. [DOI] [PubMed] [Google Scholar]
- Benali A, Weiler E, Benali Y, Dinse HR, Eysel UT. Excitation and inhibition jointly regulate cortical reorganization in adult rats. J Neurosci. 2008;28:12284–93. doi: 10.1523/JNEUROSCI.1952-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt DS, Nicoll RA. AMPA receptor trafficking at excitatory synapses. Neuron 2003. 2003;40:361–79. doi: 10.1016/s0896-6273(03)00640-8. [DOI] [PubMed] [Google Scholar]
- Buonomano DV, Merzenich MM. Cortical plasticity:from synapses to maps. Annu Rev Neurosci. 1998;21:149–86. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- Calford MB, Tweedale R. Interhemispheric transfer of plasticity in the cerebral cortex. Science. 1990;249:805–80710. doi: 10.1126/science.2389146. [DOI] [PubMed] [Google Scholar]
- Calford MB, Tweedale R. Immediate expansion of receptive fields of neurons in area 3b of macaque monkeys after digit denervation. Somatosens Mot Res. 1991;8:249–260. doi: 10.3109/08990229109144748. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Donoghue JP, Connors WB. Different forms of synaptic plasticity in somatosensory and motor areas of the neocortex. J Neurosci. 1995;15:5324–5333. doi: 10.1523/JNEUROSCI.15-07-05324.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill JD, Muja N, Myers WA, Besheer J, Garraghty PE. Somatotopic consolidation: a third phase of reorganization after peripheral nerve injury in adult squirrel monkeys. Exp Brain Res. 1998;118:189–196. doi: 10.1007/s002210050271. [DOI] [PubMed] [Google Scholar]
- Churchill JD, Arnold LL, Garraghty PE. Somatotopic reorganization in the brainstem and thalamus following peripheral nerve injury in adult primates. Brain Res. 2001;910:142–152. doi: 10.1016/s0006-8993(01)02703-2. [DOI] [PubMed] [Google Scholar]
- Churchill JD, Tharp JA, Wellman CL, Sengelaub DR, Garraghty PE. Morphological correlates of injury-induced reorganization in primate somatosensory cortex. BMC Neurosci. 2004;5:43. doi: 10.1186/1471-2202-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill JD1, Garraghty PE. The influence of post-nerve injury survival duration on receptive field size: location, location, location. Neurosci Lett. 2006;405:10–13. doi: 10.1016/j.neulet.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Clarey JC, Tweedale R, Calford MB. Interhemispheric modulation of somatosensory receptive fields: evidence for plasticity in primary somatosensory cortex. Cereb Cortex. 1996;6:196–206. doi: 10.1093/cercor/6.2.196. [DOI] [PubMed] [Google Scholar]
- Clem RL, Huganir RL. Calcium-permeable AMPA receptor dynamics mediate fear memory erasure. Science. 2010;330:1108–12. doi: 10.1126/science.1195298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge GL, Isaac JT, Wang YT. Receptor trafficking and synaptic plasticity. Nat Rev Neurosci. 2004;5:952–62. doi: 10.1038/nrn1556. [DOI] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–21. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer SC, Chopp M. Recovery recapitulates ontogeny. Trends Neurosci. 2000;23:265–271. doi: 10.1016/s0166-2236(00)01562-9. [DOI] [PubMed] [Google Scholar]
- Dutta A, Kambi N, Raghunathan P, Khushu S, Jain N. Large-scale reorganization of the somatosensory cortex of adult macaque monkeys revealed by fMRI. Brain Struct Funct. 2014;219:1305–20. doi: 10.1007/s00429-013-0569-8. [DOI] [PubMed] [Google Scholar]
- Dykes RW. Mechanisms controlling neuronal plasticity in somatosensory cortex. Can J Physiol Pharmacol. 1997;75:535–45. [PubMed] [Google Scholar]
- Eybalin M, Caicedo A, Renard N, Ruel J, Puel JL. Transient Ca2+-permeable AMPA receptors in postnatal rat primary auditory neurons. Eur J Neurosci. 2004;20:2981–2989. doi: 10.1111/j.1460-9568.2004.03772.x. [DOI] [PubMed] [Google Scholar]
- Florence SL, Wall JT, Kaas JH. Central projections from the skin of the hand in squirrel monkeys. J Comp Neurol. 1991;311:563–578. doi: 10.1002/cne.903110410. [DOI] [PubMed] [Google Scholar]
- Florence SL, Hackett TA, Strata F. Thalamic and cortical contributions to neural plasticity after limb amputation. J Neurophysiol. 2000;83:3154–9. doi: 10.1152/jn.2000.83.5.3154. [DOI] [PubMed] [Google Scholar]
- Froemke RC, Merzenich MM, Schreiner CE. A synaptic memory trace for cortical receptive field plasticity. Nature 2007. 2007;450:425–9. doi: 10.1038/nature06289. [DOI] [PubMed] [Google Scholar]
- Garraghty PE, Pons TP, Sur M, Kaas JH. The arbors of axons terminating in middle cortical layers of somatosensory area 3b in owl monkeys. Somatosensory and Motor Research. 1989;6:401–411. doi: 10.3109/08990228909144683. [DOI] [PubMed] [Google Scholar]
- Garraghty PE, Kaas JH. Large-scale functional reorganization in adult monkey cortex after peripheral nerve injury. Proc Natl Acad Sci U S A. 1991a;88:6976–6980. doi: 10.1073/pnas.88.16.6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garraghty PE, Kaas JH. Functional reorganization in adult monkey thalamus after peripheral nerve injury. Neuro Reports. 1991b;2:747–750. doi: 10.1097/00001756-199112000-00004. [DOI] [PubMed] [Google Scholar]
- Garraghty PE, LaChica EA, Kaas JH. Injury-induced reorganization of somatosensory cortex is accompanied by reductions in GABA staining. Somatosens Mot Res. 1991;8:347–354. doi: 10.3109/08990229109144757. [DOI] [PubMed] [Google Scholar]
- Garraghty PE, Muja N. Possible use-dependent changes in adult primate somatosensory cortex. Brain Res. 1995;686:119–21. doi: 10.1016/0006-8993(95)00506-l. [DOI] [PubMed] [Google Scholar]
- Garraghty PE, Muja N. NMDA receptors and plasticity in adult primate somatosensory cortex. J Comp Neurol. 1996;367:319–326. doi: 10.1002/(SICI)1096-9861(19960401)367:2<319::AID-CNE12>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Garraghty PE, Sur M. Morphology of single intracellularly stained axons terminating in area 3b of macaque monkeys. J Comp Neurol. 1990;294:583–593. doi: 10.1002/cne.902940406. [DOI] [PubMed] [Google Scholar]
- Garraghty PE, Hanes DP, Florence SL, Kaas JH. Pattern of peripheral deafferentation predicts reorganizational limits in adult primate somatosensory cortex. Somatosens Mot Res. 1994;11:109–117. doi: 10.3109/08990229409028864. [DOI] [PubMed] [Google Scholar]
- Garraghty PE, Arnold LL, Wellman CL, Mowery TM. Receptor autoradiographic correlates of deafferentation-induced reorganization in adult primate somatosensory cortex. J Comp Neurol. 2006;497:636–645. doi: 10.1002/cne.21018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingold SI, Greenspan JD, Apkarian AV. Anatomic evidence of nociceptive inputs to primary somatosensory cortex: relationship between spinothalamic terminals and thalamocortical cells in squirrel monkeys. J Comp Neurol. 1991;308:467–90. doi: 10.1002/cne.903080312. [DOI] [PubMed] [Google Scholar]
- Graziano A, Jones EG. Early withdrawal of axons from higher centers in response to peripheral somatosensory denervation. J Neurosci. 2009;29:3738–48. doi: 10.1523/JNEUROSCI.5388-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halligan PW, Marshall JC, Wade DT, Davey J, Morrison D. Thumb in cheek? Sensory reorganization and perceptual plasticity after limb amputation. Neuroreport. 1993;4:233–6. doi: 10.1097/00001756-199303000-00001. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–7. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- Hicks TP, Dykes RW. Receptive field size for certain neurons in primary somatosensory cortex is determined by GABA-mediated intracortical inhibition. Brain Res. 1983;274:160–4. doi: 10.1016/0006-8993(83)90533-4. [DOI] [PubMed] [Google Scholar]
- Ho MT, Pelkey KA, Topolnik L, Petralia RS, Takamiya K, Xia J, Huganir RL, Lacaille JC, McBain CJ. Developmental expression of Ca2+-permeable AMPA receptors underlies depolarization-induced long-term depression at mossy fiber CA3 pyramid synapses. J Neurosci. 2007;27:11651–11662. doi: 10.1523/JNEUROSCI.2671-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain N, Catania KC, Kaas JH. A histologically visible representation of the fingers and palm in primate area 3b and its immutability following long-term deafferentations. Cereb Cortex. 1998;8:227–36. doi: 10.1093/cercor/8.3.227. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Qi HX, Burish MJ, Gharbawie OA, Onifer SM, Massey JM. Cortical and subcortical plasticity in the brains of humans, primates, and rats after damage to sensory afferents in the dorsal columns of the spinal cord. Exp Neurol. 2008;209(2):407–16. doi: 10.1016/j.expneurol.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht S, Henningsen H, Elbert T, Flor H, Höhling C, Pantev C, Birbaumer N, Taub E. Cortical reorganization in human amputees and mislocalization of painful stimuli to the phantom limb. Neurosci Lett. 1995;201:262–4. doi: 10.1016/0304-3940(95)12186-2. [DOI] [PubMed] [Google Scholar]
- Knecht S, Henningsen H, Elbert T, Flor H, Höhling C, Pantev C, Taub E. Reorganizational and perceptional changes after amputation. Brain. 1996;119:1213–9. doi: 10.1093/brain/119.4.1213. [DOI] [PubMed] [Google Scholar]
- Komaki A, Shahidi S, Lashgari R, Haghparast A, Malakouti SM, Noorbakhsh SM. Effects of GABAergic inhibition on neocortical long-term potentiation in the chronically prepared rat. Neurosci Lett. 2007;422:181–6. doi: 10.1016/j.neulet.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Kumar SS, Bacci A, Kharazia V, Huguenard JR. A developmental switch of AMPA receptor subunits in neocortical pyramidal neurons. J Neurosci. 2002;22:3005–3015. doi: 10.1523/JNEUROSCI.22-08-03005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindskog M, Li L, Groth RD, Poburko D, Thiagarajan TC, Han X, Tsien RW. Postsynaptic GluA1 enables acute retrograde enhancement of presynaptic function to coordinate adaptation to synaptic inactivity. Proc Natl Acad Sci U S A. 2010;107:21806–11. doi: 10.1073/pnas.1016399107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–26. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Kaas JH, Wall JT, Nelson RJ, Sur M, Felleman DJ. Topographic reorganization of somatosensory cortical areas 3b and 1 in adult monkeys following restricted deafferentation. Neuroscience. 1983a;8:33–55. doi: 10.1016/0306-4522(83)90024-6. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Kaas JH, Wall JT, Sur M, Nelson RJ, Felleman DJ. Progression of change following median nerve section in the cortical representation of the hand in areas 3b and 1 in adult owl and squirrel monkeys. Neuroscience. 1983b;10:639–665. doi: 10.1016/0306-4522(83)90208-7. [DOI] [PubMed] [Google Scholar]
- Moore CE, Schady W. Investigation of the functional correlates of reorganization within the human somatosensory cortex. Brain. 2000;123:1883–1895. doi: 10.1093/brain/123.9.1883. [DOI] [PubMed] [Google Scholar]
- Mowery TM, Garraghty PE. Nerve-injury induced changes to GluR1 and GluR2/3 sub-unit expression in area 3b of adult squirrel monkeys: Developmental recapitulation? Front Syst Neurosci. 2009;3:1. doi: 10.3389/neuro.06.001.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowery TM, Sarin RM, Elliott KS, Garraghty EP. Nerve injury-induced changes in GABA(A) and GABA(B) sub-unit expression in area 3b and cuneate nucleus of adult squirrel monkeys: further evidence of developmental recapitulation. Brain Res. 2011;1415:63–75. doi: 10.1016/j.brainres.2011.07.066. [DOI] [PubMed] [Google Scholar]
- Mowery TM, Walls SM, Garraghty PE. AMPA and GABA(A/B) receptor subunit expression in the cortex of adult squirrel monkeys during peripheral nerve regeneration. Brain Res. 2013;1520:80–94. doi: 10.1016/j.brainres.2013.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowery TM, Kostylev PV, Garraghty PE. AMPA and GABA(A/B) receptor subunit expression in the cuneate nucleus of adult squirrel monkeys during peripheral nerve regeneration. Neurosci Lett. 2014;559:141–6. doi: 10.1016/j.neulet.2013.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrisett RA, Mott DD, Lewis DV, Swartzwelder HS, Wilson WA. GABAB-receptor-mediated inhibition of the N-methyl-D-aspartate component of synaptic transmission in the rat hippocampus. J Neurosci. 1991;11:203–9. doi: 10.1523/JNEUROSCI.11-01-00203.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers WA, Churchill JD, Muja N, Garraghty PE. Role of NMDA receptors in adult primate cortical somatosensory plasticity. J Comp Neurol. 2000;418:373–382. [PubMed] [Google Scholar]
- Navarro X, Vivó M, Valero-Cabré A. Neural plasticity after peripheral nerve injury and regeneration. Prog Neurobiol. 2007;82:163–201. doi: 10.1016/j.pneurobio.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Oaklander AL, Belzberg AJ. Unilateral nerve injury down-regulates mRNA for Na+ channel SCN10A bilaterally in rat dorsal root ganlia. Mol Brain Res. 1997;52:162–16510. doi: 10.1016/s0169-328x(97)00239-8. [DOI] [PubMed] [Google Scholar]
- Oaklander AL, Brown JM. Unilateral nerve injury produces bilateral loss of distal innervation. Ann Neurol. 2004;55:639–64410. doi: 10.1002/ana.20048. [DOI] [PubMed] [Google Scholar]
- Obrietan K, van den Pol AN. GABAB receptor-mediated regulation of glutamate-activated calcium transients in hypothalamic and cortical neuron development. J Neurophysiol 1999. 1999;82:94–102. doi: 10.1152/jn.1999.82.1.94. [DOI] [PubMed] [Google Scholar]
- Otmakhova NA, Lisman JE. Contribution of Ih and GABAB to synaptically induced afterhyperpolarizations in CA1: a brake on the NMDA response. J Neurophysiol. 2004;92:2027–39. doi: 10.1152/jn.00427.2004. [DOI] [PubMed] [Google Scholar]
- Passafaro M1, Piëch V, Sheng M. Subunit-specific temporal and spatial patterns of AMPA receptor exocytosis in hippocampal neurons. Nat Neurosci. 2001;4:917–26. doi: 10.1038/nn0901-917. [DOI] [PubMed] [Google Scholar]
- Philip BA, Frey SH. Preserved grip selection planning in chronic unilateral upper extremity amputees. Exp Brain Res. 2011;214:437–52. doi: 10.1007/s00221-011-2842-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarin RM, Mowery TM, Garraghty PE. AMPA receptor subunit expression in the cuneate nucleus of adult squirrel monkeys after peripheral nerve injury. Neurosci Lett. 2012;516:193–6. doi: 10.1016/j.neulet.2012.03.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schady W, Braune S, Watson S, Torebjörk HE, Schmidt R. Responsiveness of the somatosensory system after nerve injury and amputation in the human hand. Ann Neurol. 1994;36:68–75. doi: 10.1002/ana.410360114. [DOI] [PubMed] [Google Scholar]
- Schroeder CE, Seto S, Arezzo JC, Garraghty PE. Electrophysiological evidence for overlapping dominant and latent inputs to somatosensory cortex in squirrel monkeys. J Neurophysiol. 1995;74:722–732. doi: 10.1152/jn.1995.74.2.722. [DOI] [PubMed] [Google Scholar]
- Schroeder CE, Seto S, Garraghty PE. Emergence of radial nerve dominance in “median nerve cortex” after median nerve transection in an adult squirrel monkey. J Neurophysiol. 1997;77:522–526. doi: 10.1152/jn.1997.77.1.522. [DOI] [PubMed] [Google Scholar]
- Shepherd JD1, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol. 2007;23:613–43. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- Shi S, Hayashi Y, Esteban JA, Malinow R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell. 2001;105:331–43. doi: 10.1016/s0092-8674(01)00321-x. [DOI] [PubMed] [Google Scholar]
- Sur M, Merzenich MM, Kaas JH. Magnification, receptive-field area, and “hypercolumn” size in areas 3b and 1 of somatosensory cortex in owl monkeys. J Neurophysiol. 1980;44:295–311. doi: 10.1152/jn.1980.44.2.295. [DOI] [PubMed] [Google Scholar]
- Sur M, Nelson RJ, Kaas JH. Representations of the body surface in cortical areas 3b and 1 of squirrel monkeys: comparisons with other primates. J Comp Neurol. 1982;211:177–192. doi: 10.1002/cne.902110207. [DOI] [PubMed] [Google Scholar]
- Thiagarajan TC, Lindskog M, Tsien RW. Adaptation to synaptic inactivity in hippocampal neurons. Neuron. 2005;47:725–737. doi: 10.1016/j.neuron.2005.06.037. [DOI] [PubMed] [Google Scholar]
- Waldmeier PC, Kaupmann K, Urwyler S. Roles of GABAB receptor subtypes in presynaptic auto- and heteroreceptor function regulating GABA and glutamate release. J Neural Transm. 2008;115:1401–11. doi: 10.1007/s00702-008-0095-7. [DOI] [PubMed] [Google Scholar]
- Wall JT, Xu J, Wang X. Human brain plasticity: an emerging view of the multiple substrates and mechanisms that cause cortical changes and related sensory dysfunctions after injuries of sensory inputs from the body. Brain Res Rev. 2002;39:181–215. doi: 10.1016/s0165-0173(02)00192-3. [DOI] [PubMed] [Google Scholar]
- Wellman CL, Arnold LL, Garman EE, Garraghty PE. Acute reductions in GABAA receptor binding in layer IV of adult primate somatosensory cortex after peripheral nerve injury. Brain Res. 2002;954:68–72. doi: 10.1016/s0006-8993(02)03343-7. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Shortland P, Reynolds M, Ridings J, Doubell T, Coggeshall RE. Reorganization of central terminals of myelinated primary afferents in the rat dorsal horn following peripheral axotomy. J Comp Neurol. 1995;360:121–34. doi: 10.1002/cne.903600109. [DOI] [PubMed] [Google Scholar]