Summary
Passively-administered anti-tumor mAbs rapidly kill tumor targets via FcγR-mediated cytotoxicity (ADCC), a short-term process. However, anti-tumor mAb treatment can also induce a vaccinal effect, in which mAb-mediated tumor death induces a long-term anti-tumor cellular immune response. To determine how such responses are generated, we utilized a murine model of an anti-tumor vaccinal effect against a model neoantigen. We demonstrate that FcγR expression by CD11c+ antigen-presenting cells is required to generate anti-tumor T cell responses upon ADCC-mediated tumor clearance. Using FcγR-humanized mice, we demonstrate that anti-tumor huIgG1 must engage hFcγRIIIA on macrophages to mediate ADCC, but also engage hFcγRIIA, the sole hFcγR expressed by human DCs, to generate a potent vaccinal effect. Thus, while next-generation anti-tumor antibodies with enhanced binding to only hFcγRIIIA are now in clinical use, ideal anti-tumor antibodies must be optimized for both cytotoxic effects as well as hFcγRIIA engagement on DCs to stimulate long-term anti-tumor cellular immunity.
Introduction
Passive administration of anti-tumor antibodies is an important clinical tool for the management of a variety of cancers (Pincetic et al., 2014), and generally functions by targeting malignant cells through Fc-receptor for IgG (FcγR)-mediated antibody-dependent cellular cytotoxicity (ADCC) by myeloid effector cells (Clynes et al., 2000; Taylor and Lindorfer, 2008; Uchida et al., 2004) or possibly natural killer (NK) cells. Because of this FcγR-mediated mechanism of action, next-generation versions of anti-tumor mAbs that have been Fc-engineered for enhanced engagement of activating FcγRs are now being used in the clinic or are under investigation (Goede et al., 2014). However, while ADCC-mediated tumor killing is rapid and relatively short-acting, patients with some malignancies see long-term responses after cessation of antibody therapy; this has prompted the hypothesis that a vaccinal or auto-immunization effect is initiated, in which tumor targeting by a monoclonal antibody (mAb) primes the patient's immune system to generate an anti-tumor T cell memory response (Cartron et al., 2004). Thus, it has been demonstrated that cellular immune responses are generated in both mice and patients treated with anti-HER-2/neu mAb (Park et al., 2010; Taylor et al., 2007). Anti-MUC1 cellular immune responses have also been reported after the use of anti-MUC1 mAb in patients with MUC1+ tumors (de Bono et al., 2004). Evidence in lymphoma patients suggests that a vaccinal effect can be generated by anti-hCD20 mAb immunotherapy (rituximab), since a single course of treatment with mAb can result in long-lasting, durable responses (Cartron et al., 2004). In support of this, it has been reported that some patients treated with rituximab developed lymphoma-specific anti-idiotype T cell responses after mAb treatment (Hilchey et al., 2009). Recent studies in mice have also demonstrated that passive administration of anti-CD20 mAbs can initiate anti-tumor cellular immune responses (Abes et al., 2010). Therefore, while the hypothesis of a tumor-specific antibody-induced anti-tumor vaccinal effect has persisted for more than a decade, an experimentally-derived mechanistic explanation is lacking.
New technologies have enabled the identification of tumor mutational signatures, some common across multiple cancer types while others are restricted to specific malignancies (Alexandrov et al., 2013). Thus, mutation-induced, developmentally-restricted, or over-expressed tumor neoantigens are a major target of tumor-infiltrating lymphocytes in patients (Fritsch et al., 2014; Tran et al., 2014). Neoantigen-specific CD4+ and CD8+ T cells have been identified, showing that such antigens are indeed processed and presented (Gros et al., 2014; van Rooij et al., 2013). Further, new immune-checkpoint blockade therapies function in patients by amplifying neoantigen-specific responses (van Rooij et al., 2013). However, although studies analyzing antibody responses to tumor neoantigens are lacking, antibody:antigen immune complexes can stimulate cellular immunity by engaging activating FcγRs on antigen-presenting cells, such as dendritic cells (DCs), to induce DC maturation, traditional antigen presentation and cross-presentation, co-stimulatory molecule upregulation, and stimulate cellular immune responses in both mice (Kalergis and Ravetch, 2002; Rafiq et al., 2002) and humans (Boruchov et al., 2005; Dhodapkar et al., 2005). Often, antibody:antigen immune complex immunization results in more potent cross-presentation and CD4 or CD8 T cell responses than antigen immunization alone. Thus, a logical approach to boosting cellular immune responses involves passive administration of antibodies reactive with tumor antigens or tumor neoantigens. Therefore, in this current study, we utilize a tumor model expressing a model tumor neoantigen to test whether and how passive anti-tumor antibody treatment stimulates an anti-tumor vaccinal effect and cellular immune response.
Three activating FcγRs are expressed in mice (mFcγRI, mFcγRIII, and mFcγRIV) and humans (hFcγRI, hFcγRIIA and hFcγRIIIA), and a single inhibitory FcγR, FcγRIIB, is expressed in both species. The cellular outcome of IgG interactions with FcγRs is governed by the affinity of an antibody's Fc for the specific receptor and the expression pattern of those receptors on effector cells (Nimmerjahn and Ravetch, 2008). Since most effector cells co-express activation and inhibitory FcγRs, it is the ratio of the binding affinities of a specific IgG Fc to these receptors that will determine the outcome of the IgG-FcγR interaction. These binding affinities are determined by the amino acid sequences of the IgG Fc subclasses and the IgG Fc's N-linked glycan. The IgG Fc composition can dramatically influence the in vivo outcome of engaging a tumor antigen by directing the antibody-antigen immune complex or opsonized cell into either a pro- or anti-inflammatory response. For example, mouse (m)IgG2a antibodies trigger cytotoxicity by virtue of this Fc having a 2-log greater affinity for the activating mFcγRIV receptor as compared to the inhibitory mFcγRIIb receptor, while mIgG1 preferentially engages the inhibitory mFcγRIIb receptor.
Mice and humans differ regarding the specific FcγRs expressed on various APCs and the relative affinities of each IgG Fc subclass for each FcγR (Nimmerjahn and Ravetch, 2007). Thus, human (h)IgG1 Fc does not preferentially engage a single hFcγR, as occurs in mice. Two low-affinity activating FcγRs are expressed on monocytes and macrophages in both mice (mFcγRIII and mFcγRIV) and humans (hFcγRIIA and hFcγRIIIA). Murine NK cells express only mFcγRIII, while human NK cells express only hFcγRIIIA. Importantly, while mice express two low-affinity activating FcγRs, mFcγRIII and mFcγRIV, on DCs, humans only express one low-affinity activating FcγR on DCs, hFcγRIIA. Because of these species differences, we have generated FcγR-humanized mice, which express the full array of hFcγRs on a background lacking all murine FcγRs (Smith et al., 2012). Appropriate cell type-specific expression of all hFcγRs is observed in the FcγR-humanized mice, allowing the characterization of hIgG1 antibody-mediated effector function in the context of human FcγRs.
Here, using a murine lymphoma expressing a model tumor neoantigen (human CD20; hCD20), we mechanistically dissect how an anti-tumor cellular immune response is generated after passive treatment of lymphoma-bearing mice with anti-hCD20 mAb. We demonstrate that not only are activating FcγRs required for macrophage-mediated ADCC, but activating FcγR expression specifically on CD11c+ antigen-presenting cells is required for the generation of an anti-tumor T cell memory immune response and long-term survival. Because of the complexity of the FcγR system, with multiple genes expressed and regulated differentially on the diverse cells of the immune system, designing an anti-tumor antibody for optimal activity requires compatible model systems. We therefore assessed the generation of an anti-tumor vaccinal effect for a hIgG1 anti-hCD20 mAb in FcγR-humanized mice. We show that anti-tumor mAb must engage hFcγRIIIA on CLOD-sensitive macrophages to mediate immediate ADCC, as well as hFcγRIIA (the sole FcγR expressed by human DCs) in order to stimulate a long-term anti-tumor cellular immune response. Thus, while next-generation anti-tumor antibodies with enhanced binding to only hFcγRIIIA on innate effector cells are now in clinical use, our results indicate that anti-tumor antibodies with optimal long-term survival benefit must be optimized for both their short-acting cytotoxic effects through hFcγRIIIA, as well as for engagement of hFcγRIIA on DCs to stimulate long-term anti-tumor cellular immunity.
Results
Generation of an anti-tumor vaccinal effect by passive anti-tumor antibody treatment
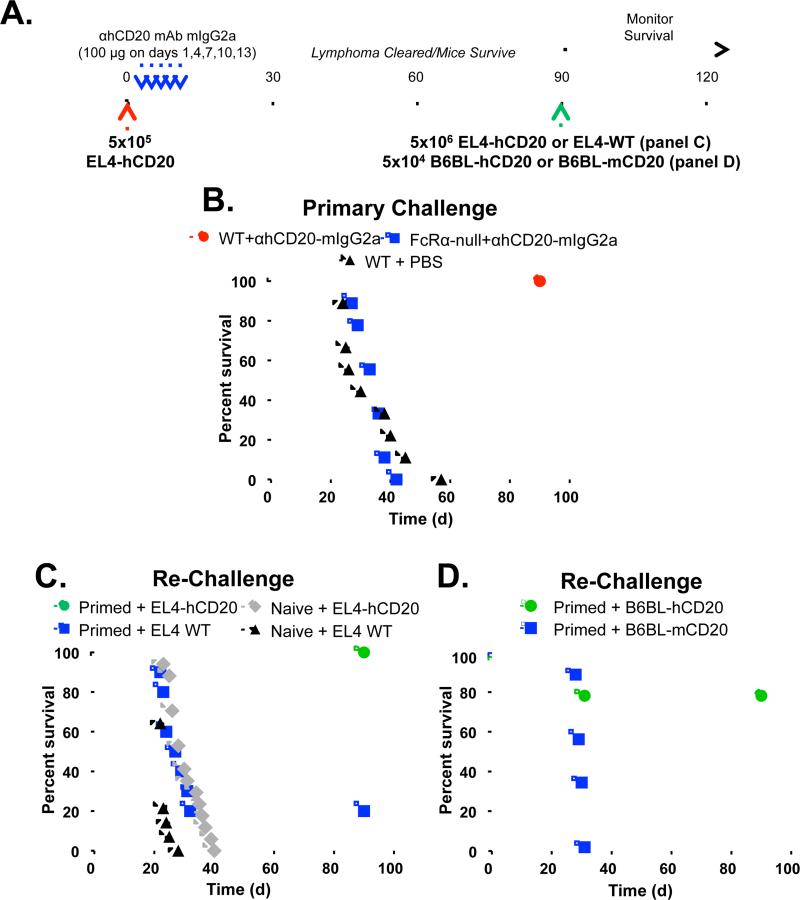
To understand how passive anti-tumor mAb treatment can induce long-term anti-tumor cellular immune responses, we adapted a murine lymphoma model (Abes et al., 2010) of an anti-neoantigen mAb-mediated vaccinal effect (Fig. 1A). Wild-type C57BL/6 mice were given syngenic EL4 lymphoma cells that express hCD20 as a tumor neoantigen (EL4-hCD20 cells), followed by mIgG2a isotype anti-hCD20 mAb. The mIgG2a isotype preferentially engages activating mFcγRs and is the most potent mouse subclass for triggering effector cells to result in cellular cytotoxicity, phagocytosis, and inflammatory responses (Nimmerjahn and Ravetch, 2005; Pincetic et al., 2014). Mice receiving lymphoma cells plus anti-hCD20 mAb clear the tumors and survive in an activating FcγR-dependent manner; wild-type mice survive the tumor challenge, while FcRα-null (Smith et al., 2012) mice (which lack all mFcγRs: mFcγRI, mFcγRIIB, mFcγRIII, and mFcγRIV) and Fcer1g−/− mice (which lack all activating mFcγRs: mFcγRI, mFcγRIII, and mFcγRIV) do not (Fig. 1B and Supplementary Fig. 1A). The activating mFcγRIV is a major contributor during this clearance of tumor cells, since only 52% of Fcgr4−/− mice survive tumor challenge after anti-hCD20 mAb treatment (p<0.001; Supplementary Fig. 1B). Non-FcγR binding DA265 mutant anti-hCD20 was also unable to clear tumors (Supplementary Fig. 1C). Thus, activating FcγRs mediate the initial ADCC clearance of tumor cells by anti-tumor mAb, as reported (Clynes et al., 2000; Uchida et al., 2004).
Figure 1.
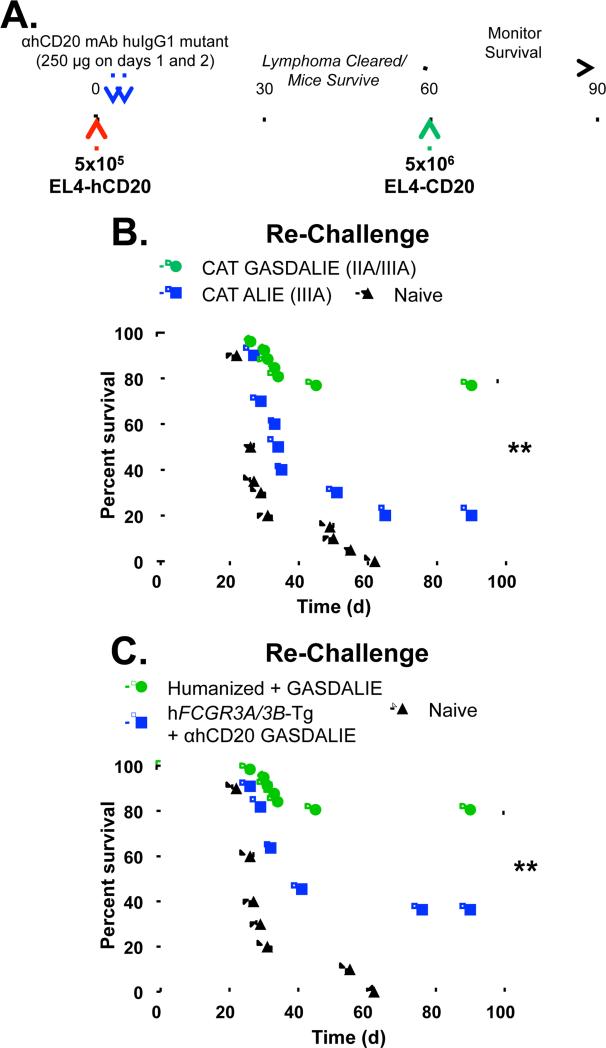
Fc-FcγR interactions are required for the clearance of lymphoma by mAb and the initiation of an anti-tumor vaccinal effect. A, Experimental protocol: Mice were injected i.v. with 5×105 EL4-hCD20 lymphoma cells on day 0 (red arrow), and received 100μg of mIgG2a isotype anti-hCD20 mAb (clone CAT13.6E12) on days 1, 4, 7, 10, and 13 (blue arrows). On day 90, surviving mice were re-challenged i.v. with 5×106 EL4-hCD20 lymphoma cells (green arrow) or EL4-WT cells, a 10-fold greater dose of tumor compared to the primary lymphoma challenge, and survival was monitored daily. Alternatively, surviving mice were re-challenged with 5×104 B6BL-hCD20 or B6BL-mCD20 cells i.v. B, Wildtype (red circles) or FcRα-null mice (blue squares) were injected with EL4-hCD20 cells and treated with IgG2a isotype anti-hCD20 mAb, with survival assessed daily (n=9-11 mice per group). C, After 90 days, surviving (primed) mice treated with mIgG2a anti-hCD20 mAb from (b) were re-challenged with EL4-hCD20 cells (green circles) or EL4-WT cells (blue squares) with survival assessed daily. For comparison, naïve mice were also challenged with EL4-hCD20 cells (gray diamonds) or EL4-WT cells (filled triangles). n=10-13 mice per group. D, Mice were primed with EL4-hCD20 lymphoma cells and mAb as in (A-B) before re-challenge on day 90 with B6BL tumor cells expressing either hCD20 (green circles) or msCD20 (blue squares). n=10 mice per group.
We next assessed whether an anti-tumor vaccinal effect was generated in mice that survived the initial EL4-hCD20 challenge via treatment with anti-hCD20 mAb. Ninety days after the initial challenge when anti-hCD20 mAb had been cleared (mIgG2a half-life = 7 days (Vieira and Rajewsky, 1988)), surviving tumor/mAb-primed mice were re-challenged with 5×106 EL4-hCD20 tumor cells, a dose that is 10-fold greater than the initial challenge, without treatment with any additional anti-hCD20 mAb. These primed mice showed 100% survival during re-challenge with EL4-hCD20 cells (Fig. 1C). By contrast, surviving tumor/mAb-primed mice re-challenged with EL4-WT cells, which do not express hCD20, showed poor survival. Similar results were seen using a different anti-hCD20 mAb, clone 2B8, which is the parental hybridoma from which rituximab was generated (Supplementary Fig. 1D-E). Thus, mice primed with EL4-hCD20 and anti-hCD20 mAb generate a memory immune response and subsequently reject re-challenge with EL4-hCD20 cells, but not EL4 WT cells.
In this model of the vaccinal effect, both CD4+ and CD8+ T cells are required after antibody treatment in order to reject the tumor re-challenge (Abes et al., 2010). Specifically, T cell depletion studies demonstrated that CD4+ T cells are required during the initial phases of antibody therapy as well as during tumor re-challenge in order to reject tumors. Using CD8-deficient mice, it was also shown that CD8+ cells are required for tumor rejection during re-challenge. By contrast, mice do not mount any detectable antibody response against hCD20 or EL4-hCD20 cells, and adoptive transfer of serum from tumor/mAb-primed mice does not protect against tumor challenge (Abes et al., 2010). In figure 1C, we show that EL4-hCD20/anti-hCD20 mAb-primed mice only reject EL4-hCD20 cells, and not wild-type EL4 cells, suggesting that the vaccinal effect is directed against hCD20, with little detectable antigen spreading. To confirm that at least a portion of the vaccinal effect cellular immune response is directed at hCD20, mice primed with EL4-hCD20 cells and anti-hCD20 mAb were re-challenged with a distinct tumor cell line, B6BL lymphoma cells, that express either cell-surface hCD20 or an irrelevant antigen (mCD20). While 100% of mice re-challenged with B6BL-mCD20 died by day 31, 80% of mice re-challenged with B6BL-hCD20 cells survived at least 90 days (Fig 1D; p=0.0001). Thus, only cells expressing hCD20 were capable of being rejected. Collectively, these experiments demonstrate that an anti-huCD20 immune response is generated after the initial FcγR-mediated clearance of tumor cells by ADCC.
Expression of mFcγRIV on CD11c+ cells is required for the generation of an anti-tumor vaccinal effect
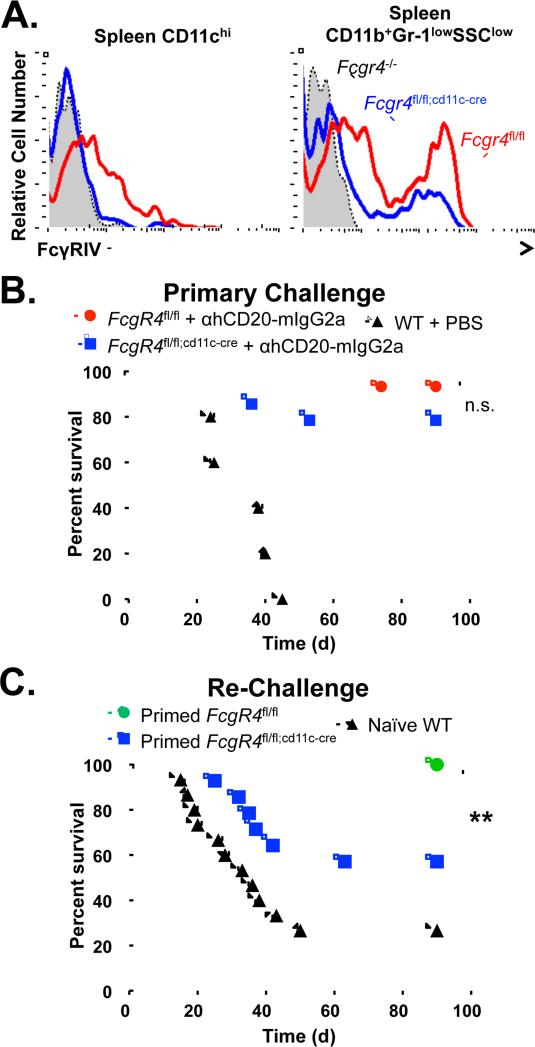
To understand the mechanistic basis for an anti-tumor vaccinal effect and to determine whether FcγR expression plays a role during this process, we utilized mice with a CD11c+ cell-specific deletion of mFcγRIV. Fcgr4fl/fl;cd11c-cre mice (Nimmerjahn et al., 2010) show a complete absence of mFcγRIV expression on spleen CD11chi DCs, but only a partial decrease in mFcγRIV expression on spleen CD11b+Gr-1loSSClo monocytes (Fig. 2A). All CD11chi cells lose mFcγRIV expression in Fcgr4fl/fl;cd11c-cre mice, while spleen CD11cint cells show partial loss of mFcγRIV and CD11c− cells show only a modest decrease in mFcγRIV (Supplementary Fig. 2A, Supplementary Table 1). The majority of CD11chiCD8+ DCs and a fraction of CD11chiCD4+ DCs express mFcγRIV, both of which lose all mFcγRIV expression in Fcgr4fl/fl;cd11c-cre mice (Supplementary Fig. 2E). While the majority of CD11cint/−CD11bint cells lose expression of mFcγRIV, only a modest reduction in mFcγRIV is seen in CD11cint/−CD11bhi cells in Fcgr4fl/fl;cd11c-cre mice (Supplementary Fig. 2B). Expression of mFcγRIV is not affected in Ly6G+ neutrophils (Supplementary Fig. 2C). Most CD11b+CD11cint/-F4/80hi macrophages lose mFcγRIV expression in Fcgr4fl/fl;cd11c-cre mice, as these cells express CD11c at intermediate levels (Supplementary Fig. 2D). Similar results demonstrating decreases in mFcγRIV expression on CD11c+ cells and lesser decreases in CD11b+ or F4/80+ cells were seen in bone marrow, peritoneal cavity, and peripheral blood from Fcgr4fl/fl;cd11c-cre mice (Supplementary Figs. 3-5, Supplementary Table 1). Therefore, because mFcγRIV expression was maintained to a sufficient degree on ADCC-mediating innate cells, both control Fcgr4fl/fl and Fcgr4fl/fl;cd11c-cre mice were able to clear primary EL4-hCD20 lymphoma cell challenge after treatment with anti-hCD20 mAb (Fig. 2B), indicating that mFcRIV expression on CD11c+ cells is not required for anti-hCD20 mAb-mediated ADCC.
Figure 2.
Expression of mFcγRIV on CD11c+ cells is required for the generation of an anti-tumor vaccinal effect. A, mFcγRIV expression levels on spleen innate cell subsets. Spleen lymphocytes were harvested from Fcgr4fl/fl (red line), Fcgr4flfl;cd11c-cre (blue line), or Fcgr4−/− (shaded line) mice and mFcγRIV expression levels on CD11c+ dendritic cells and CD11b+Gr-1lowSSClow resident monocytes was assessed. Representative flow cytometry histograms from three independent experiments are shown. B, Fcgr4fl/fl (red circles; n=15) or Fcgr4flfl;cd1c-cre (blue squares; n=14) mice were given EL4-hCD20 cells and treated with mIgG2a anti-hCD20 mAb, with survival monitored daily. C, After 90 days, surviving Fcgr4fl/fl (green circles) or Fcgr4flfl;cd1c-cre (blue squares) mice treated with mIgG2a isotype anti-hCD20 mAb from (B) were re-challenged with EL4-hCD20 cells, with survival assessed daily (n=14-16 mice per group). Significant differences between groups are indicated: **, p=0.0065; n.s., not significant
To assess the generation of the anti-hCD20 vaccinal effect in the context of CD11c+ cells lacking mFcγRIV, surviving tumor/mAb-primed Fcgr4fl/fl and Fcgr4fl/fl;cd11c-cre mice were re-challenged with EL4-hCD20 cells. Re-challenge of primed Fcgr4fl/fl mice resulted in 100% survival (Fig. 2C). By contrast, only 57% of tumor/mAb-primed Fcgr4fl/fl;cd11c-cre mice survived re-challenge with EL4-hCD20 cells (p=0.0069). It is likely that activating mFcγRIII, which is also expressed on murine DCs, compensates in the absence of mFcγRIV, thereby explaining why a modest vaccinal effect remains in Fcgr4fl/fl;cd11c-cre mice. Thus, expression of the IgG2a-preferential activating mFcγRIV on CD11c+ antigen-presenting cells is required for the generation of the anti-tumor vaccinal effect after mAb-mediated killing of tumor cells.
CD11c+ cell expression of mFcγRIV is required to generate anti-tumor cellular immunity
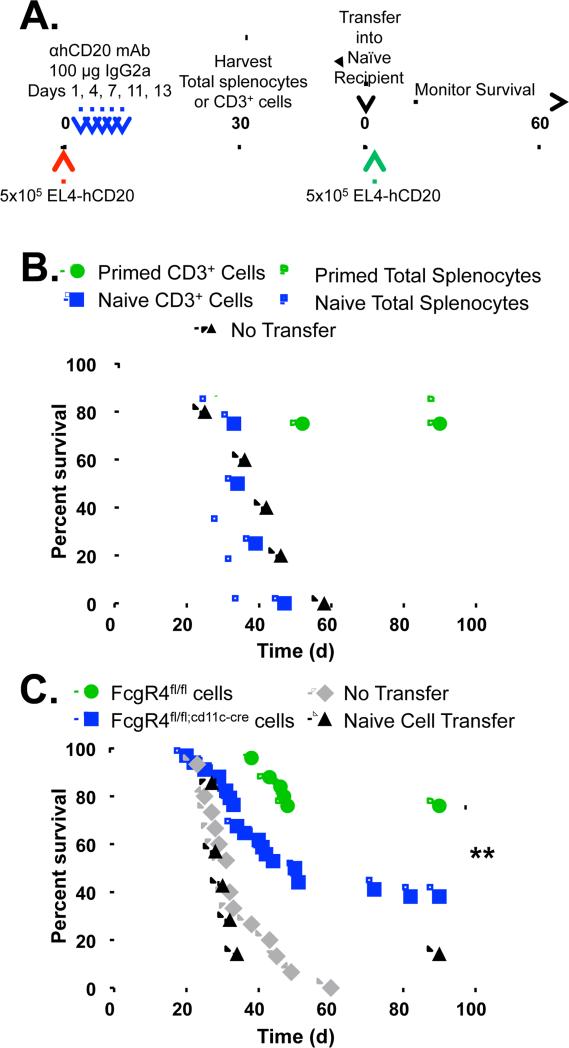
To quantify anti-tumor cellular immunity in vivo, we performed adoptive transfer experiments (Fig. 3A). Mice were given EL4-hCD20 cells and mIgG2a anti-hCD20 mAb, with spleens harvested and total splenocytes or CD3+ T cells isolated and adoptively transferred into naïve mice that were then challenged with EL4-hCD20 cells. Adoptive transfer of splenocytes or T cells from naïve mice was unable to protect against tumor growth, but transfer of splenocytes or T cells from tumor/mAb-primed mice resulted in 80% (p=0.0044) and 75% (p=0.0067) survival, respectively (Fig. 3B). Thus, tumor/mAb-primed mice generate a quantifiable anti-tumor T cell response.
Figure 3.
CD11c+ cell-specific expression of mFcγRIV is required for the generation of anti-tumor cellular immunity. A, Experimental protocol: Mice were injected i.v. with EL4-hCD20 lymphoma cells on day 0 (red arrow), and received mIgG2a anti-hCD20 mAb (blue arrows). On day 30, spleens were harvested and total splenocytes were isolated or CD3+ cells were purified. Then, 50×106 total splenocytes or 15×106 CD3+ cells were adoptively transferred into naïve mice one day before i.v. challenge with EL4-hCD20 lymphoma cells (green arrow). B, Survival was measured in naïve mice receiving CD3+ cells (green filled circles) or total splenocytes (green open circles) from tumor and mAb-primed wild-type mice, or CD3+ cells (blue filled squares) or total splenocytes (blue open squares) from naïve mice. Another group of naïve mice received no adoptive transfer (black triangles). n=4-6 mice per group. C, Survival in naïve mice receiving total splenocytes from tumor and mAb-primed Fcgr4fl/fl (green circles; n=25) or Fcgr4flfl;cd1c-cre (blue squares, n=34) mice before EL4-hCD20 cell challenge. Other groups of naïve mice received splenocytes from naïve mice (black triangles; n=7) or no adoptive transfer (gray diamonds; n=15). Significant differences between groups are indicated: **, p=0.0041.
To determine whether DC expression of mFcγRIV is required for the generation of the anti-tumor cellular immune response after passive administration of anti-tumor mAb, tumor/mAb-primed splenocytes from Fcgr4fl/fl and Fcgr4fl/fl;cd11c-cre mice were adoptively transferred into naïve mice before challenge with EL4-hCD20 cells. While 76% of mice receiving splenocytes from tumor/mAb-primed Fcgr4fl/fl mice survived EL4-hCD20 challenge, only 34% of tumor/mAb-primed Fcgr4fl/fl;cd11c-cre mice survived the challenge (p=0.0041; Fig. 3C). Thus, expression of mFcγRIV on CD11c+ antigen-presenting cells is required to mediate the generation of an anti-tumor cellular immune response after mAb-mediated clearance of tumor.
hFcγRIIIA mediates ADCC of hIgG1 mAb-targeted tumor cells in FcγR-humanized mice
Fc receptors for mouse IgG are heterogeneous, differing in their binding affinities for IgG subclasses, their expression patterns on immune cells, and signaling properties (Nimmerjahn and Ravetch, 2006; Pincetic et al., 2014). For example, NK cells in the mouse express only mFcγRIII, a low affinity activating FcγR. Macrophages and DCs express distinct combinations of both activating (mFcγRI, mFcγRIII, and mFcγRIV) and inhibitory (mFcγRIIB) receptors. Individual mouse subclasses show preferential mFcγR binding affinities, with mIgG1 preferentially engaging the inhibitory mFcγRIIB while mIgG2a engages the activating receptor mFcγRIV with a 2 log higher affinity (Nimmerjahn and Ravetch, 2008, 2011). Thus, selecting an antibody for optimal FcγR engagement requires consideration of the receptors and cell types that are to be engaged.
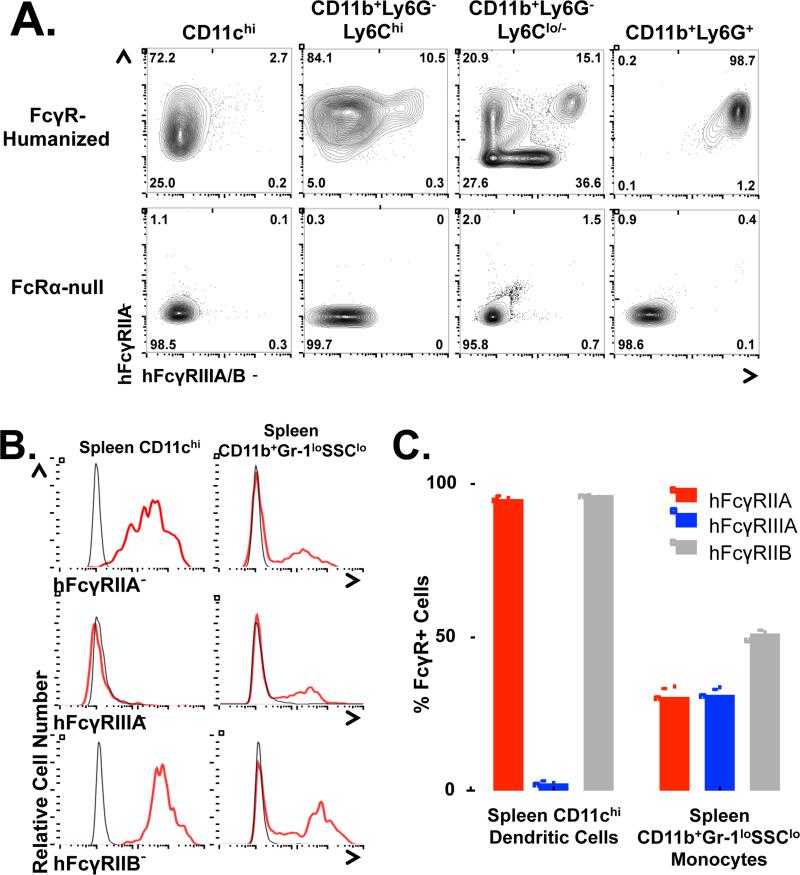
Further complicating this situation are the inter-species differences between mice and humans. Human FcγR genes, expression patterns, and affinities for the various antibody isotypes differ significantly from mice. Importantly, humans express only the low-affinity activating FcγR – hFcγRIIIA – on NK cells, the cells that are thought to primarily mediate cellular cytotoxicity in humans (Seidel et al., 2013), while antigen-presenting DCs express a single, distinct low-affinity activating FcγR, hFcγRIIA (Boruchov et al., 2005; Nimmerjahn and Ravetch, 2008). Therefore, to engineer an antibody to optimize the generation of an anti-tumor vaccinal effect initiated by a hIgG1 antibody in the context of hFcγRs, we utilized FcγR-humanized mice, which express the full array of hFcγRs on a fully immunocompetent C57BL/6 background lacking all mFcγRs (Bournazos et al., 2014a; Smith et al., 2012). FcγR-humanized mice recapitulate hFcγR expression patterns in mouse tissues; spleen CD11chi DCs express only hFcγRIIA and hFcγRIIB, but do not express hFcγRIIIA, while spleen CD11b+Ly6G−Ly6Chi and CD11b+Ly6G−Ly6Cint/- monocytes express hFcγRIIIA, hFcγRIIA and hFcγRIIB in various combinations (Fig 4, Supplementary Fig. 6A-B).
Figure 4.
Human FcγR expression on innate cells in FcγR-humanized mice. A, Representative flow cytometry dot plots show hFcγRIIA vs. hFcγRIIIA/B expression on spleen cells from FcγR-humanized or FcRα-null mice. Numbers represent the frequency of cells in the indicated gate. B, DCs and monocytes from FcγR-humanized mouse spleens (red lines) were stained for hFcγRIIA, hFcγRIIIA/B, or hFcγRIIB. Background staining by hFcγR− cells is shown (gray lines). C, Frequencies of hFcγR+ cells among spleen DCs and monocytes (n=3 per group), with frequencies generated by background staining subtracted.
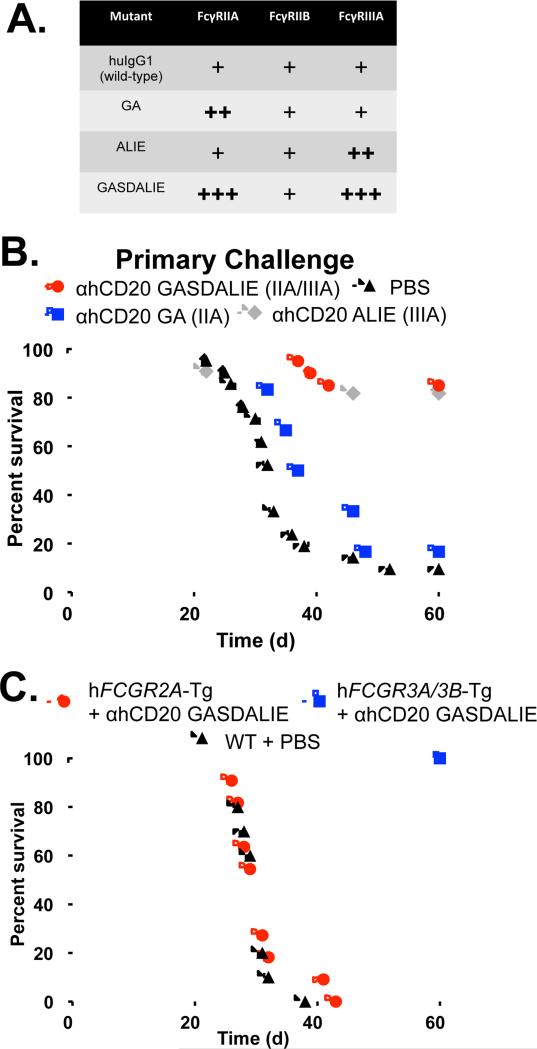
To address the relative contributions of individual hFcγRs during mAb-mediated clearance of primary tumor challenge and during the generation of an anti-tumor vaccinal effect, we generated anti-hCD20 mAb with a hIgG1 Fc backbone and introduced known point mutations (Bournazos et al., 2014b; Smith et al., 2012) that selectively enhance interactions with individual hFcγRs (Fig. 5A and Supplementary Table 2). Thus, the G236A (GA) mutant shows selectively enhanced binding to hFcγRIIA, the A330L/I332E (ALIE) mutant shows selectively enhanced binding to hFcγRIIIA, and the G236A/S239D/A330L/I332E (GASDALIE) mutant shows dramatically enhanced engagement to both hFcγRIIA and hFcγRIIIA.
Figure 5.
Differential hFcγR engagement mediates tumor cytotoxicity. A, Anti-hCD20 hIgG1 mAb Fc mutants for selectively-enhanced engagement of hFcγRs. Relative binding capabilities to the indicated hFcγRs are shown, based on binding affinities from biacore experiments (Supplementary Table 2). B, hFcγRIIIA engagement mediates cytotoxic clearance of tumor cells by mAb. FcγR-humanized mice were given EL4-hCD20 cells and treated with hIgG1 mutant versions of anti-hCD20 mAb: GASDALIE mutant (enhanced engagement of hFcγRIIA and hFcγRIIIA; red circles; n=20), GA mutant (preferential hFcγRIIA engagement; blue squares; n=6), ALIE mutant (preferential hFcγRIIIA engagement; gray diamonds; n=12), or PBS (black triangles; n=17), with survival monitored daily. C. hFcγRIIIA is necessary and sufficient to mediate the immediate cytotoxic clearance of EL4-hCD20 lymphoma cells. EL4-hCD20 cells were injected into hFCGR2A-Tg mice that were given GASDALIE mutant anti-hCD20 mAb (filled red circles; n=11), hFCGR3A/B-Tg mice given GASDALIE mutant anti-hCD20 mAb (filled blue squares; n=11), or wild-type mice given PBS (filled triangles; n=10) with survival monitored daily.
To determine which hFcγRs are responsible for the initial ADCC-mediated clearance of tumor cells, FcγR-humanized mice were given EL4-hCD20 lymphoma cells, and were treated with the various Fc-engineered hIgG1 anti-hCD20 mAbs. The GA mutant anti-hCD20 mAb that selectively engages hFcγRIIA was unable to clear tumor, as 81% of mice receiving this antibody died after primary tumor challenge (Fig. 5B). By contrast, 82% and 85% of mice receiving ALIE mutant (selectively engaging hFcγRIIIA) and GASDALIE mutant (selectively enhance both hFcγRIIA and hFcγRIIIA) anti-hCD20 mAb survived the primary EL4-hCD20 tumor challenge, respectively. Further, mice expressing only hFcγRIIA (deficient for murine FcγRs) were unable to clear the primary tumor challenge after mAb treatment, while mice expressing only hFcγRIIIA and hFcγRIIIB (deficient for murine FcγRs) showed full survival when treated with GASDALIE mutant anti-hCD20 mAb (Fig. 5C). Notably, wild-type hIgG1 anti-hCD20 mAb does not protect FcγR-humanized mice or mice expressing either hFcγRIIA or hFcγRIIIA/B from EL4-hCD20 tumor challenge (Supplementary Fig. 6C-D), indicating that wild-type interactions between these hFcγRs and hIgG1 provide insufficient signaling to mediate effector functions at the doses used in this mouse model. Taken together, these results demonstrate that while hFcγRIIA is dispensable, hFcγRIIIA is both necessary and sufficient for mAb-mediated clearance of primary tumor challenge.
Clodronate liposome (CLOD)-sensitive macrophages mediate ADCC in the context of hIgG1 and the human FcγR system
In the context of the mouse FcγR system, CLOD-sensitive macrophages mediate ADCC of antibody-coated target cells (Uchida et al., 2004). NK cells are dispensable for ADCC in this context, presumably because they do not express mFcγRI or mFcγRIV, but only express the low affinity FcγR, FcγRIII, which interacts with msIgG2a antibodies with ~40-fold lower affinity than mFcγRIV (Otten et al., 2008). However, we now show that hFcγRIIIA mediates ADCC in vivo by hIgG1 antibody. Because hFcγRIIIA is expressed on both NK cells and monocytes/macrophages in FcγR-humanized mice (and humans), we determined whether CLOD-sensitive macrophages mediate ADCC of mAb-coated target cells in the context of the human FcγR system and human IgG1. CLOD decreased total numbers of splenic CD11bintF4/80hi red pulp macrophages by >90% (Supplementary Fig. 6E; p<0.0001). With the exception of CD11chi DCs (33% depletion, p=0.01), no other cellular populations analyzed were affected by CLOD treatment (Supplementary Fig. 6F).
We first confirmed that depletion of blood and spleen B220+ B cells in hCD20-Tg mice (these mice express hCD20 on mature B cells and also express the full array of murine FcγRs) by msIgG2a isotype anti-hCD20 was dependent on CLOD-sensitive macrophages. Blood and spleen B cell numbers were decreased by 97.5%-98% and 63%-78% (p<0.0001; Supplementary Fig. 7A), respectively, in mice receiving either PBS or control liposomes plus mIgG2a anti-hCD20 mAb. By contrast, no B cells were depleted in mice receiving CLOD plus mIgG2a anti-hCD20 mAb. Thus, as described (Uchida et al., 2004), ADCC of antibody-coated cells in vivo requires CLOD-sensitive macrophage populations.
We next tested the ability of hIgG1 (GASDALIE mutant) anti-hCD20 mAb to deplete hCD20+ B cells in the context of the human FcγR system in hCD20-Tg/FcγR-humanized mice (these mice express hCD20 on mature B cells and also express the full array of human FcγRs, but lack all murine FcγRs), as described above. Blood and spleen B cell numbers were decreased by 86%-90% and 81%-82% (p<0.001), respectively, in hCD20-Tg/FcγR-humanized mice receiving either PBS or control liposomes plus hIgG1 (GASDALIE) anti-hCD20 mAb. By contrast, no B cells were depleted in mice receiving CLOD plus GASDALIE anti-hCD20 mAb (Supplementary Fig. 7B). Similar results were seen in FcγR-humanized mice treated with CLOD and a depleting hIgG1 (GASDALIE) anti-mCD4 mAb (Supplementary Fig. 7C). Therefore, CLOD-sensitive macrophages are required for ADCC mediated by hIgG1 antibody in the context of the human FcγR system.
hFcγRIIA engagement by anti-tumor mAb mediates the generation of the anti-tumor vaccinal effect in FcγR-humanized mice
Finally, we assessed to what extent the anti-tumor vaccinal effect was generated in FcγR-humanized mice (Fig. 6A) treated with anti-hCD20 mAb mutants that selectively engage only hFcγRIIIA (ALIE mutant) or both hFcγRIIA and hFcγRIIIA (GASDALIE mutant). Only 20% of FcγR-humanized mice receiving ALIE mutant anti-hCD20 mAb survived re-challenge with EL4-hCD20 cells, while 77% of mice receiving GASDALIE mutant anti-hCD20 mAb survived re-challenge (p<0.0001; Fig. 6B). Further, only 36% (p=0.01) of hFcγRIIIA/B-Tg mice (which lack all murine FcγRs and express only hFcγRIIIA and hFcγRIIIB) survive EL4-hCD20 re-challenge, again demonstrating that expression of hFcγRIIA is required for optimal induction of an anti-tumor vaccinal effect. Thus, engagement of hFcγRIIA, which is the only activating hFcγR expressed by human DCs, is required for the generation of an anti-tumor vaccinal effect by passively-administered anti-tumor mAb.
Figure 6.
Selective engagement of hFcγRIIA mediates an anti-tumor vaccinal effect. A, Experimental protocol: FcγR-humanized mice were injected i.v. with 5×105 EL4-hCD20 lymphoma cells on day 0 (red arrow), and received 250 μg of hIgG1 mutant anti-hCD20 mAb on days 1 and 2 (blue arrows). On day 60, surviving mice were re-challenged i.v. with 5×106 EL4-hCD20 lymphoma cells (green arrow), and survival was monitored daily. B, Surviving tumor-primed mice that received GASDALIE hIgG1 anti-hCD20 mAb (green circles; n=28) or ALIE hIgG1 anti-hCD20 mAb (blue squares; n=10) from Fig. 5B, or naïve mice (black triangles; n=15) were re-challenged with EL4-hCD20 cells. C, Surviving tumor-primed FcγR-humanized (green circles; n=28) or hFcγRIIIA/B-Tg mice (blue squares, n=11) that received GASDALIE hIgG1 anti-hCD20 mAb from Fig. 5C, or naïve mice (black triangles; n=10) were re-challenged with EL4-hCD20 cells. Significant differences between groups are indicated: **, p<0.01.
Discussion
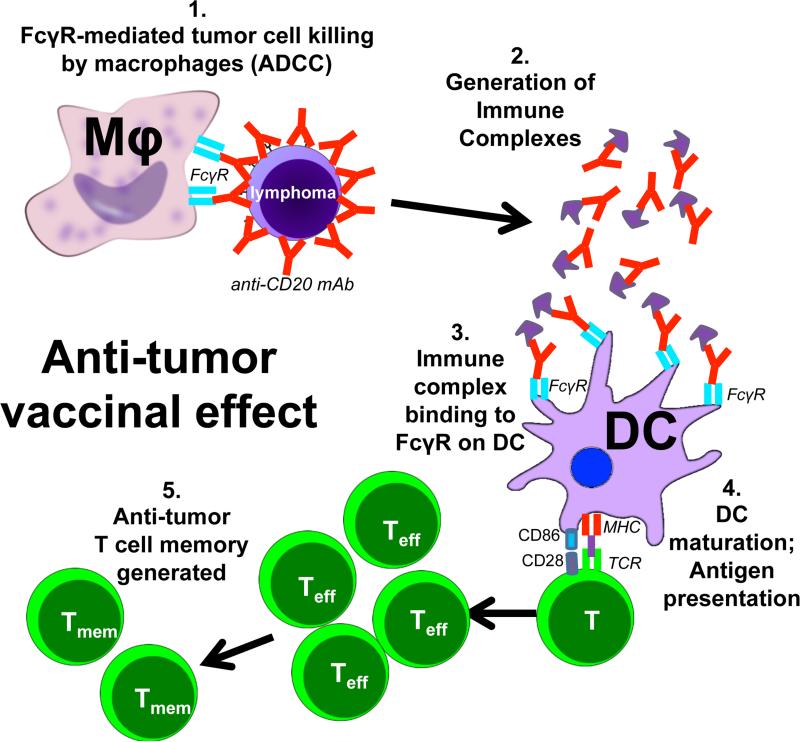
It has long been hypothesized that passive administration of anti-tumor antibodies may generate immune complexes that, upon uptake by antigen-presenting cells, stimulate anti-tumor cellular immunity. Taken together, our results now mechanistically demonstrate how passively-administered anti-tumor antibody achieves such an effect using a lymphoma cell line that expresses a model tumor neoantigen. Anti-tumor mAb opsonizes tumor cells and targets them for killing by FcγR-mediated ADCC, a process that generates antibody:tumor antigen immune complexes. These immune complexes engage activating FcγRs expressed by CD11c+ antigen-presenting cells, which results in stimulation of DC maturation and presentation of tumor antigens to T cells, thereby leading to long-term anti-tumor cellular memory formation (Fig. 7) (Boruchov et al., 2005; Dhodapkar et al., 2005; Kalergis and Ravetch, 2002; Nimmerjahn and Ravetch, 2008). In the human FcγR system, the vaccinal effect requires interactions with hFcγRIIA, the sole activating FcγR expressed by DCs. Thus, these results now mechanistically explain how passively-administered anti-tumor mAb stimulates anti-tumor cellular immune responses in vivo and suggest novel methods for augmenting such an effect.
Figure 7.
Model for the generation of an anti-tumor vaccinal effect. Anti-tumor mAb opsonizes tumor cells and targets them for killing by FcγR-mediated ADCC, a process that generates antibody:tumor antigen immune complexes. These immune complexes engage activating FcγRs expressed by mouse or human CD11c+ cells, which results in stimulation of DC maturation and presentation of tumor antigens to T cells, thereby leading to long-term anti-tumor cellular memory formation
It is clear that activating FcγRs expressed by antigen-presenting cells, especially DCs, are capable of capturing antibody/tumor antigen immune complexes. Upon immune complex binding, DCs undergo maturation, upregulate MHC-II and co-stimulatory molecules, and stimulate CD4 and CD8 T cells responses through traditional antigen presentation and cross-presentation (Nimmerjahn and Ravetch, 2008; Rafiq et al., 2002). DCs loaded with antibody/tumor antigen immune complexes stimulate potent T cell responses that are capable of eradicating tumors (Kalergis and Ravetch, 2002). In vitro studies have demonstrated that anti-CD20 mAb treatment of lymphoma cells stimulates DC maturation and CD8 T cell activation (Selenko et al., 2002), and a synergistic effect between vaccination with hCD20+ tumor cells and anti-hCD20 mAb treatment has been demonstrated in mice (Gadri et al., 2009). CD8+ DCs are considered to be excellent cross-presenters of cell-associated antigens (Mayer et al., 2014), and soluble immune complexes stimulate cross-presentation by DCs more potently than antigen alone (Berlyn et al., 2001; Rafiq et al., 2002). Correspondingly, a much larger fraction of CD8+ spleen CD11chi cells expresses mFcγRIV compared to CD8−CD11chi cells (60% vs. 28%, Supplementary Fig. 2E), suggesting that CD8+ DCs may play a significant role during the induction of the mAb-mediated anti-tumor vaccinal effect. Further, clinical trials have shown that combining anti-CD20 mAb treatment with administration of immunomodulatory cytokines that promote the activation of DCs or T cell responses, such as IFN-α (Kimby et al., 2008) or GMCSF (Cartron et al., 2008), synergistically increased anti-CD20 mAb efficacy, suggesting that augmenting antigen presentation and T cell responses in the context of anti-tumor mAb therapy may augment an anti-tumor vaccinal effect. Murine studies have also demonstrated a heightened vaccinal effect when administration of the pleiotropic cytokine IL-2, which activates both innate cells and T cells, is combined with anti-hCD20 mAb treatment (Abes et al., 2010). Thus, our studies now mechanistically explain how the anti-tumor cellular immune responses generated by passive antibody treatment are generated in vivo.
While healthy individuals are normally tolerized to self-antigens such as CD20 and would not develop memory T cells reactive with self-antigens, cancer-bearing patients often break tolerance and autoimmune disorders are common in these patients (Abu-Shakra et al., 2001). Thus, while tolerance to over-expressed antigens, oncoproteins, tumor suppressor proteins, differentiation antigens, or neoantigens engaged by antibodies may be broken and lead to the generation of anti-tumor memory T cells, these cells are often anergized or exhausted and unable to mount an effective cytotoxic T cell response to the tumor. Activating these T cells to become effector cells and target tumor cells is thus a goal of current immunotherapy approaches, most recently achieved by blocking inhibitory signals such as CTLA-4 (Hodi et al., 2010) and PD-1 (Brahmer et al., 2012; Topalian et al., 2012). Our data support an alternative approach in which combining anti-tumor cytotoxic antibody therapeutics with various immunotherapies that boost cellular immune responses (i.e., agonistic anti-CD40 mAb (Li and Ravetch, 2011), or antagonistic anti-CTLA-4 or anti-PD-1 mAbs) may synergistically combine with an anti-tumor mAb vaccinal effect to boost cellular memory formation. Thus, our results suggest a general mechanism by which anti-tumor antibodies can stimulate anti-tumor cellular immune responses against a variety of tumor antigens.
Significant efforts were put towards identifying the antigen-specific T cells that mediate the vaccinal effect in this study, but ex vivo re-stimulation (ELISPOT and intracellular cytokine staining) studies with tumor cell lysates, peptides, or irradiated tumor cells were not sensitive enough to detect rare tumor-specific T cells. Regardless, conclusions regarding the specificity of the anti-tumor vaccinal effect T cell response can be made. Mice primed with mAb and hCD20+ EL4 lymphoma cells rejected lymphoma re-challenge with hCD20+ EL4 cells but not wild-type EL4 cells (Fig. 1C). This result suggests that the vaccinal effect T cell response is directed at the hCD20 neoantigen and that no detectable epitope-spreading occurs in this model. We have confirmed that the T cell response is, at least in part, directed at hCD20 because mice primed with mAb and hCD20+ EL4 lymphoma rejected re-challenge with a distinct tumor cell line expressing hCD20, but not the same cell line expressing a control antigen (Fig. 1D). Whether all vaccinal effect anti-tumor T cell responses are solely directed at the mAb-targeted antigen, as in the current EL4 tumor model, or whether different tumor models or different tumor microenvironments (i.e., lymphoid vs. solid tumors) will result in different mAb-induced anti-tumor T cell responses and epitope spreading remains unclear. Whether combining passive anti-tumor mAb with checkpoint inhibitor blockade or adjuvants will result in synergistically enhanced epitope spreading and anti-neoantigen T cell responses is also unknown.
Our studies in FcγR-humanized mice with hIgG1 anti-hCD20 mAb-mediated ADCC of hCD20+ tumor cells clearly demonstrate that hFcγRIIIA is both necessary and sufficient for ADCC-mediated clearance of antibody-coated tumor cells; hFcγRIIA plays no role in this process (Fig. 5). These results correspond to findings in humans that FCGR3A polymorphisms correlate with response rates in lymphoma patients treated with anti-CD20 mAb (Cartron et al., 2002) or breast cancer patients treated with anti-Her2 mAb (Musolino et al., 2008). Importantly, FcγR signaling is required for ADCC in vivo, rather than simple cross-linking of antigen (de Haij et al., 2010). Because they solely express hFcγRIIIA, dogma has dictated that NK cells are the main mediators of ADCC in humans (Seidel et al., 2013). Further promoting the belief that NK cells are the major mediators of ADCC in humans, NK cells from human peripheral blood are routinely used during in vitro ADCC assays, which inadequately attempt to artificially re-create a complex in vivo process. Nonetheless, it has been demonstrated that CLOD-sensitive macrophages ((Uchida et al., 2004), Supplementary Fig. 7A), but not NK cells, are required for ADCC by mIgG in the context of murine FcγRs. This was partially thought to be due to the lone expression of FcγRIII on murine NK cells (Otten et al., 2008), which weakly interacts with mouse antibody Fc compared to FcγRIV. Therefore, we have now clearly determined the cellular requirements for hIgG1-triggered ADCC mediated by human FcγRs, and demonstrate that CLOD-sensitive macrophages mediate ADCC of antibody-coated target cells in vivo in the context of hIgG1 antibody and the human FcγR system (Supplementary Fig. 7B-C). This result is significant, because new therapies aimed at augmenting human NK cell activity in vivo to enhance ADCC of mAb-targeted tumor cells are currently under investigation. Further studies determining any functional differences between murine and human NK cells will shed more light on this important matter.
The results reported here highlight the importance of properly engineering antibody therapeutics to engage the appropriate FcγRs to mediate appropriate effector functions. Current efforts to augment anti-tumor antibodies have only focused on enhancing their cytotoxic effects by modulating hIgG1 Fc interactions with hFcγRIIIA to augment ADCC by innate cells, as exemplified by the next-generation glyco-engineered anti-hCD20 mAb, obinutuzumab (Goede et al., 2014). Obinutuzumab is afucosylated for augmented affinity to only hFcγRIIIA, and accordingly, extends survival by approximately one year in CLL patients when directly compared to an unmodified anti-CD20 antibody (Rituximab). However, afucosylation of obinutuzumab does not affect Fc engagement of hFcγRIIA, which is the sole activating hFcγR expressed on human antigen-presenting DCs for engagement of immune complexes and stimulation of T cell responses. Thus, our current results argue that an ideal anti-tumor therapeutic should not only optimally engage hFcγRIIIA for cytotoxic effector function, but also hFcγRIIA on DCs in order to induce long-term cellular anti-tumor immunity.
Experimental Procedures
Cell lines and mice
EL4-WT and 293T cells were obtained from ATCC (Manassas, VA) and maintained in Dulbecco's minimum essential medium (DMEM; Life Technologies, Inc., Carlsbad, CA) supplemented with 10% fetal bovine serum (Life Technologies, Inc), 100 units/ml of penicillin, and 100 g/ml of streptomycin (Life Technologies, Inc). EL4-hCD20 cells were obtained from Oliver Press (Fred Hutchinson Cancer Research Center, Seattle, WA) with permission from Josée Golay (Ospedali Riuniti di Bergamo, Bergamo, Italy) and maintained in RPMI-1640 medium (Life Technologies) supplemented with 10% fetal bovine serum, 100 units/ml of penicillin, and 100 g/ml of streptomycin. B6BL cells, a spontaneous B cell lymphoma line isolated from p53fl/flCD19-Cre+ mice on pure B6 genetic background, have been previously described (Robbiani et al., 2009), and were retrovirally transduced with constructs encoding either hCD20 or mCD20 (pVPack Vectors, Agilent Technologies, La Jolla, CA), selected to make stable cell lines, and sorted for CD20+ cells, as described previously (Li and Ravetch, 2011). C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME). Fcer1g–/– (Takai et al., 1994), Fcgr4–/– (Nimmerjahn et al., 2010), FcRα-null (Smith et al., 2012), FCGR3A/B-Tg (Li et al., 1996) (crossed to FcRα-null mice), and FCGR2A-Tg (McKenzie et al., 1999) (crossed to Fcer1g–/–Fcgr2b−/− mice) mice on the C57BL/6 genetic background have been previously described. FcγR-humanized mice, which express all hFcγRs on the FcRα-null C57BL/6 genetic background, have been described (Smith et al., 2012). Fcgr4flox mice (Nimmerjahn et al., 2010) were crossed with mice expressing cre recombinase under the control of CD11c promoter/enhancer regions (B6.Cg-Tg(Itgax-cre)1-1Reiz/J mice, Jackson Laboratories). hCD20-Tg mice were kindly provided by Dr. Andrew Chan (Genentech, San Francisco, CA), and crossed onto the FcγR-humanized background. All mice were maintained in a specific pathogen-free facility at the Rockefeller University, and all studies were approved by the Rockefeller University Institutional Animal Care and Use Committee.
Antibodies, flow cytometry, and other reagents
To generate CAT-13.6E12 and 2B8 mAb constructs, total RNA was obtained from hybridoma cells (DSMZ, Braunschweig, Germany and ATCC, Manassas, VA, respectively), and cDNA was generated by using SuperscriptIII reverse transcriptase (Life Technologies) and immunoglobulin gene-specific primers. The VH and VK genes were amplified by PCR and cloned in frame into mammalian expression vectors with mouse IgG2a, mouse IgG1, mouse DA265 mutant, mouse Kappa, huIgG1, or huKappa Fc backbones. The human G236A, A330L/I332E, and GASDALIE Fc mutants were generated by site-directed mutagenesis with PCR amplification of the entire vector, using complementary primers containing the desired point mutations, as described (Li and Ravetch, 2011). Antibodies were produced by transient transfection of 293T cells and subsequent protein G purification from culture supernatants, as described (Nimmerjahn et al., 2005). Anti-mCD4 antibody (clone GK1.5) with a hIgG1 backbone and containing GASDALIE point mutations was generated previously (Smith et al., 2012). Fluorescently-conjugated antibodies and staining procedures are listed in the Supplemental Experimental Procedures. To deplete macrophages in vivo, mice received 200 ul of clodronate liposomes or PBS liposomes i.v. through lateral tail veins (Clophosome-A, Formumax, Palo Alto, CA).
Tumor model
For the primary tumor challenge, mice were injected intravenously through lateral tail veins with 5×105 EL4-hCD20 cells in 200μl PBS on day 0. Mice then received intraperitoneal injections of 100 μg of antibody in 200 μl of PBS on days 1, 4, 7, 10, and 13. Survival was assessed daily. In some experiments, 90 days after primary tumor challenge, surviving mice were re-challenged intravenously with 5×106 EL4-hCD20 or EL4-WT cells, with survival assessed daily. In experiments in which hIgG1 antibodies or mutants were administered, FcγR-humanized mice were given 5×105 EL4-hCD20 cells in 200μl PBS on day 0, with 250 μg of antibody in 200 μl of PBS given i.p. on days 1 and 2. Surviving mice were re-challenged with 5×106 EL4-hCD20 cells on day 60. In some experiments, mice were re-challenged i.v. with 5×104 B6BL cells expressing either hCD20 or mCD20.
In adoptive transfer experiments, splenocytes from mice 30 days after primary tumor challenge were harvested and red blood cells were lysed. In some cases, CD3+ cells were negatively selected using magnetic beads (Miltenyi Biotec, San Diego, CA). Then, 50×106 total splenocytes or 15×106 CD3+ cells were adoptively transferred into naïve C57BL/6 mice. One day later, the mice were challenged with 5×105 EL4-hCD20 cells in 200μl PBS, with survival assessed daily.
Statistics
Statistical differences between survival rates were analyzed by comparing Kaplan-Meier curves using the log-rank test and GraphPad Prism Software. All other statistical differences were compared using the student's T test analysis.
Supplementary Material
Highlights.
- Tumor-bearing mice given cytotoxic anti-tumor antibody develop T cell tumor immunity.
- ADCC-mediated tumor clearance requires engagement of hFcγRIIIA on macrophages
- Selective engagement of hFc[.gamma]RIIA in DCs promotes long-term immunity against cancer
Acknowledgements
We thank Patrick Smith and Stylianos Bournazos for assistance with these studies. We are grateful to Oliver Press (Fred Hutchinson Cancer Research Center, Seattle, WA) and Josée Golay (Ospedali Riuniti di Bergamo, Bergamo, Italy) for providing EL4-hCD20 cells, and to Andrew Chan (Genentech, San Francisco, CA) for providing hCD20-Tg mice. Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number R01CA080757 to JVR. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was also supported by funding from the New York Community Trust Grants for Blood Disorder Research, Francis Florio Fund (to DJD). DJD received a postdoctoral career development fellowship from the Leukemia and Lymphoma Society of America.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
DJD and JVR designed experiments, analyzed the data, and wrote the manuscript. D.J.D. performed the experiments.
The authors declare no conflicts of interest.
References
- Abes R, Gelize E, Fridman WH, Teillaud JL. Long-lasting antitumor protection by anti-CD20 antibody through cellular immune response. Blood. 2010;116:926–934. doi: 10.1182/blood-2009-10-248609. [DOI] [PubMed] [Google Scholar]
- Abu-Shakra M, Buskila D, Ehrenfeld M, Conrad K, Shoenfeld Y. Cancer and autoimmunity: autoimmune and rheumatic features in patients with malignancies. Annals of the rheumatic diseases. 2001;60:433–441. doi: 10.1136/ard.60.5.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A, Borresen-Dale AL, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlyn KA, Schultes B, Leveugle B, Noujaim AA, Alexander RB, Mann DL. Generation of CD4(+) and CD8(+) T lymphocyte responses by dendritic cells armed with PSA/anti-PSA (antigen/antibody) complexes. Clinical immunology. 2001;101:276–283. doi: 10.1006/clim.2001.5115. [DOI] [PubMed] [Google Scholar]
- Boruchov AM, Heller G, Veri MC, Bonvini E, Ravetch JV, Young JW. Activating and inhibitory IgG Fc receptors on human DCs mediate opposing functions. The Journal of clinical investigation. 2005;115:2914–2923. doi: 10.1172/JCI24772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bournazos S, DiLillo DJ, Ravetch JV. humanized mice to study FcgammaR function. Current topics in microbiology and immunology. 2014a;382:237–248. doi: 10.1007/978-3-319-07911-0_11. [DOI] [PubMed] [Google Scholar]
- Bournazos S, Klein F, Pietzsch J, Seaman MS, Nussenzweig MC, Ravetch JV. Broadly Neutralizing Anti-HIV-1 Antibodies Require Fc Effector Functions for In Vivo Activity. Cell. 2014b;158:1243–1253. doi: 10.1016/j.cell.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. The New England journal of medicine. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, Watier H. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99:754–758. doi: 10.1182/blood.v99.3.754. [DOI] [PubMed] [Google Scholar]
- Cartron G, Watier H, Golay J, Solal-Celigny P. From the bench to the bedside: ways to improve rituximab efficacy. Blood. 2004;104:2635–2642. doi: 10.1182/blood-2004-03-1110. [DOI] [PubMed] [Google Scholar]
- Cartron G, Zhao-Yang L, Baudard M, Kanouni T, Rouille V, Quittet P, Klein B, Rossi JF. Granulocyte-macrophage colony-stimulating factor potentiates rituximab in patients with relapsed follicular lymphoma: results of a phase II study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:2725–2731. doi: 10.1200/JCO.2007.13.7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nature medicine. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- de Bono JS, Rha SY, Stephenson J, Schultes BC, Monroe P, Eckhardt GS, Hammond LA, Whiteside TL, Nicodemus CF, Cermak JM, et al. Phase I trial of a murine antibody to MUC1 in patients with metastatic cancer: evidence for the activation of humoral and cellular antitumor immunity. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2004;15:1825–1833. doi: 10.1093/annonc/mdh472. [DOI] [PubMed] [Google Scholar]
- de Haij S, Jansen JH, Boross P, Beurskens FJ, Bakema JE, Bos DL, Martens A, Verbeek JS, Parren PW, van de Winkel JG, et al. In vivo cytotoxicity of type I CD20 antibodies critically depends on Fc receptor ITAM signaling. Cancer research. 2010;70:3209–3217. doi: 10.1158/0008-5472.CAN-09-4109. [DOI] [PubMed] [Google Scholar]
- Dhodapkar KM, Kaufman JL, Ehlers M, Banerjee DK, Bonvini E, Koenig S, Steinman RM, Ravetch JV, Dhodapkar MV. Selective blockade of inhibitory Fcgamma receptor enables human dendritic cell maturation with IL-12p70 production and immunity to antibody-coated tumor cells. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:2910–2915. doi: 10.1073/pnas.0500014102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch EF, Hacohen N, Wu CJ. Personal neoantigen cancer vaccines: The momentum builds. Oncoimmunology. 2014;3:e29311. doi: 10.4161/onci.29311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadri Z, Kukulansky T, Bar-Or E, Haimovich J, Hollander N. Synergistic effect of dendritic cell vaccination and anti-CD20 antibody treatment in the therapy of murine lymphoma. Journal of immunotherapy. 2009;32:333–340. doi: 10.1097/CJI.0b013e31819b7c17. [DOI] [PubMed] [Google Scholar]
- Goede V, Fischer K, Busch R, Engelke A, Eichhorst B, Wendtner CM, Chagorova T, de la Serna J, Dilhuydy MS, Illmer T, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. The New England journal of medicine. 2014;370:1101–1110. doi: 10.1056/NEJMoa1313984. [DOI] [PubMed] [Google Scholar]
- Gros A, Robbins PF, Yao X, Li YF, Turcotte S, Tran E, Wunderlich JR, Mixon A, Farid S, Dudley ME, et al. PD-1 identifies the patient-specific CD8(+) tumor-reactive repertoire infiltrating human tumors. The Journal of clinical investigation. 2014;124:2246–2259. doi: 10.1172/JCI73639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilchey SP, Hyrien O, Mosmann TR, Livingstone AM, Friedberg JW, Young F, Fisher RI, Kelleher RJ, Jr., Bankert RB, Bernstein SH. Rituximab immunotherapy results in the induction of a lymphoma idiotype-specific T-cell response in patients with follicular lymphoma: support for a “vaccinal effect” of rituximab. Blood. 2009;113:3809–3812. doi: 10.1182/blood-2008-10-185280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. The New England journal of medicine. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalergis AM, Ravetch JV. Inducing tumor immunity through the selective engagement of activating Fcgamma receptors on dendritic cells. The Journal of experimental medicine. 2002;195:1653–1659. doi: 10.1084/jem.20020338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimby E, Jurlander J, Geisler C, Hagberg H, Holte H, Lehtinen T, Ostenstad B, Hansen M, Osterborg A, Linden O, et al. Long-term molecular remissions in patients with indolent lymphoma treated with rituximab as a single agent or in combination with interferon alpha-2a: a randomized phase II study from the Nordic Lymphoma Group. Leukemia & lymphoma. 2008;49:102–112. doi: 10.1080/10428190701704647. [DOI] [PubMed] [Google Scholar]
- Li F, Ravetch JV. Inhibitory Fcgamma receptor engagement drives adjuvant and anti-tumor activities of agonistic CD40 antibodies. Science. 2011;333:1030–1034. doi: 10.1126/science.1206954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Wirthmueller U, Ravetch JV. Reconstitution of human Fc gamma RIII cell type specificity in transgenic mice. The Journal of experimental medicine. 1996;183:1259–1263. doi: 10.1084/jem.183.3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer CT, Ghorbani P, Nandan A, Dudek M, Arnold-Schrauf C, Hesse C, Berod L, Stuve P, Puttur F, Merad M, et al. Selective and efficient generation of functional Batf3-dependent CD103+ dendritic cells from mouse bone marrow. Blood. 2014;124:3081–3091. doi: 10.1182/blood-2013-12-545772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie SE, Taylor SM, Malladi P, Yuhan H, Cassel DL, Chien P, Schwartz E, Schreiber AD, Surrey S, Reilly MP. The role of the human Fc receptor Fc gamma RIIA in the immune clearance of platelets: a transgenic mouse model. Journal of immunology. 1999;162:4311–4318. [PubMed] [Google Scholar]
- Musolino A, Naldi N, Bortesi B, Pezzuolo D, Capelletti M, Missale G, Laccabue D, Zerbini A, Camisa R, Bisagni G, et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:1789–1796. doi: 10.1200/JCO.2007.14.8957. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F, Bruhns P, Horiuchi K, Ravetch JV. FcgammaRIV: a novel FcR with distinct IgG subclass specificity. Immunity. 2005;23:41–51. doi: 10.1016/j.immuni.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F, Lux A, Albert H, Woigk M, Lehmann C, Dudziak D, Smith P, Ravetch JV. FcgammaRIV deletion reveals its central role for IgG2a and IgG2b activity in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:19396–19401. doi: 10.1073/pnas.1014515107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn F, Ravetch JV. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science. 2005;310:1510–1512. doi: 10.1126/science.1118948. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F, Ravetch JV. Fcgamma receptors: old friends and new family members. Immunity. 2006;24:19–28. doi: 10.1016/j.immuni.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F, Ravetch JV. Fc-receptors as regulators of immunity. Adv Immunol. 2007;96:179–204. doi: 10.1016/S0065-2776(07)96005-8. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nature reviews Immunology. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F, Ravetch JV. FcgammaRs in health and disease. Current topics in microbiology and immunology. 2011;350:105–125. doi: 10.1007/82_2010_86. [DOI] [PubMed] [Google Scholar]
- Otten MA, van der Bij GJ, Verbeek SJ, Nimmerjahn F, Ravetch JV, Beelen RH, van de Winkel JG, van Egmond M. Experimental antibody therapy of liver metastases reveals functional redundancy between Fc gammaRI and Fc gammaRIV. Journal of immunology. 2008;181:6829–6836. doi: 10.4049/jimmunol.181.10.6829. [DOI] [PubMed] [Google Scholar]
- Park S, Jiang Z, Mortenson ED, Deng L, Radkevich-Brown O, Yang X, Sattar H, Wang Y, Brown NK, Greene M, et al. The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer cell. 2010;18:160–170. doi: 10.1016/j.ccr.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincetic A, Bournazos S, DiLillo DJ, Maamary J, Wang TT, Dahan R, Fiebiger BM, Ravetch JV. Type I and type II Fc receptors regulate innate and adaptive immunity. Nature immunology. 2014;15:707–716. doi: 10.1038/ni.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafiq K, Bergtold A, Clynes R. Immune complex-mediated antigen presentation induces tumor immunity. The Journal of clinical investigation. 2002;110:71–79. doi: 10.1172/JCI15640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbiani DF, Bunting S, Feldhahn N, Bothmer A, Camps J, Deroubaix S, McBride KM, Klein IA, Stone G, Eisenreich TR, et al. AID produces DNA double-strand breaks in non-Ig genes and mature B cell lymphomas with reciprocal chromosome translocations. Molecular cell. 2009;36:631–641. doi: 10.1016/j.molcel.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel UJ, Schlegel P, Lang P. Natural killer cell mediated antibody-dependent cellular cytotoxicity in tumor immunotherapy with therapeutic antibodies. Frontiers in immunology. 2013;4:76. doi: 10.3389/fimmu.2013.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selenko N, Majdic O, Jager U, Sillaber C, Stockl J, Knapp W. Cross-priming of cytotoxic T cells promoted by apoptosis-inducing tumor cell reactive antibodies? Journal of clinical immunology. 2002;22:124–130. doi: 10.1023/a:1015463811683. [DOI] [PubMed] [Google Scholar]
- Smith P, DiLillo DJ, Bournazos S, Li F, Ravetch JV. Mouse model recapitulating human Fcgamma receptor structural and functional diversity. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:6181–6186. doi: 10.1073/pnas.1203954109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai T, Li M, Sylvestre D, Clynes R, Ravetch JV. FcR gamma chain deletion results in pleiotrophic effector cell defects. Cell. 1994;76:519–529. doi: 10.1016/0092-8674(94)90115-5. [DOI] [PubMed] [Google Scholar]
- Taylor C, Hershman D, Shah N, Suciu-Foca N, Petrylak DP, Taub R, Vahdat L, Cheng B, Pegram M, Knutson KL, et al. Augmented HER-2 specific immunity during treatment with trastuzumab and chemotherapy. Clin Cancer Res. 2007;13:5133–5143. doi: 10.1158/1078-0432.CCR-07-0507. [DOI] [PubMed] [Google Scholar]
- Taylor RP, Lindorfer MA. Immunotherapeutic mechanisms of anti-CD20 monoclonal antibodies. Current opinion in immunology. 2008;20:444–449. doi: 10.1016/j.coi.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. The New England journal of medicine. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran E, Turcotte S, Gros A, Robbins PF, Lu YC, Dudley ME, Wunderlich JR, Somerville RP, Hogan K, Hinrichs CS, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344:641–645. doi: 10.1126/science.1251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida J, Hamaguchi Y, Oliver JA, Ravetch JV, Poe JC, Haas KM, Tedder TF. The innate mononuclear phagocyte network depletes B lymphocytes through Fc receptor-dependent mechanisms during anti-CD20 antibody immunotherapy. The Journal of experimental medicine. 2004;199:1659–1669. doi: 10.1084/jem.20040119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooij N, van Buuren MM, Philips D, Velds A, Toebes M, Heemskerk B, van Dijk LJ, Behjati S, Hilkmann H, El Atmioui D, et al. Tumor exome analysis reveals neoantigen-specific T-cell reactivity in an ipilimumab-responsive melanoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:e439–442. doi: 10.1200/JCO.2012.47.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira P, Rajewsky K. The half-lives of serum immunoglobulins in adult mice. European journal of immunology. 1988;18:313–316. doi: 10.1002/eji.1830180221. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.