Abstract
Background/Aims
There is a critical need for more non-invasive biomarkers for nephritis in patients with SLE. Recent studies in a model mouse and an adult SLE patient cohort suggest that anti-basement membrane antibody levels correlate well with lupus activity and kidney injury. The purpose of this study was to assess anti- basement membrane reactivity in pediatric SLE (pSLE) patients with or without nephritis.
Methods
Auto-antibodies to basement membrane antigens were assessed using an anti-matrigel ELISA. End-point titers were measured in pSLE patients and healthy children, as well as in autoimmune and non-immune mice, with good reproducibility. Findings were also analyzed with respect to presence or absence of nephritis, dsDNA antibodies, and other manifestations of pSLE.
Results
MRL/lpr mice developed high-titer anti-matrigel antibodies, whereas C57BL/6 mice did not. In a cohort of 21 pSLE patients and 22 pediatric controls, high-titer anti-matrigel IgG, IgM and IgA antibody levels were specific for pSLE. High-titer anti-matrigel IgG3 levels could distinguish with good sensitivity the 13 pSLE patients with a history of nephritis from the 8 non-renal pSLE patients. High-titer anti-matrigel IgG, IgA, IgM or IgG3 did not correlate with positive anti-double stranded DNA, but defined an overlapping subset of patients.
Conclusion
The addition of anti-basement membrane antibody testing to serologic testing in pSLE may help to monitor disease activity or to define important subsets of patients with risks for specific disease manifestations.
Keywords: glomerulonephritis, pediatrics, inflammation
INTRODUCTION
There has been a large effort to develop diagnostic tools for the presence of nephritis in Systemic Lupus Erythematosus (SLE)[1–4]. The need is particularly great in pediatric patients with SLE because the prevalence and severity of nephritis is greater than in adults[5]. Hypocomplementemia, as measured by CH50 is 70% sensitive and 70% specific for SLE, low C3 levels are 64% sensitive and 91% specific, and low C4 levels are 64% sensitive and 65% specific for SLE diagnosis[6]. The use of proteinuria and creatinine clearance as markers for renal disease activity is controversial. Persistent proteinuria can be caused by acute or chronic lesions, and does not necessarily reflect ongoing inflammation in the kidneys. Kidney flares can occur before renal function decline by available laboratory parameters[7]. Several scoring systems based on combinations of clinical parameters, such as SLEDAI and BILAG, have been developed and validated in clinical trials, but have not been widely used to predict either nephritis risk or response to therapy in clinical practice.
Several candidate urinary biomarkers have also been studied for the monitoring of kidney inflammation in pSLE. One study in adults and children reported that a combination of elevated urinary MCP-1, ceruloplasmin, α1-acid glycoprotein, and NGAL was predictive of a more active nephritis (AUC 0.85), whereas elevated MCP-1 and NGAL were together more predictive of chronic renal injury (AUC 0.83)[8]. A prospective pediatric study demonstrated that either urinary MCP1 or NGAL could discriminate between active renal lupus and non-renal pSLE with an AUC value 0.81 (p=0.013) and 0.76 (p=0.003) respectively[9]. These markers and others are being confirmed in larger cohorts, but additional markers will be needed to be added to these panels in order to achieve sufficient accuracy (AUC >0.9) required to help guide LN therapy.
Others have focused on subsets of auto-antibodies (Abs) that either cause nephritis or that could serve as markers for the presence of nephritis in SLE. The subset of auto-Abs that is used by most in the clinic to monitor for renal disease is that with reactivity against anti-double stranded DNA[10]. Serum titers of anti-dsDNA Abs are routinely used clinically to monitor for renal flares and response to therapy. In a meta-analysis performed by the American College of Rheumatology’s ad hoc Committee on Immunologic Testing Guidelines, assays measuring anti-dsDNA Abs predicted a diagnosis of SLE with a weighted mean sensitivity of 57%, specificity of 97% [10]. The presence of high-titer anti-dsDNA Abs predicted the presence of active renal disease in SLE patients with a weighted mean sensitivity of 86% and a specificity of 45%. Titers of anti-dsDNA Abs correlate with the degree of renal injury in SLE, but only to a limited extent[10].
Recently, there has been renewed interest in anti-basement membrane (BM) Abs, due to new findings reported in the NZB/W F1 mouse model of lupus[4]. This model displays loss of tolerance, auto-Ab generation, and inflammatory kidney injury comparable to that seen in patients with SLE. Genetic variation in the F1 mice leads to variable production of auto-Abs of varying specificities that correspond in differing degrees of nephritis[11]. Anti-dsDNA Ab titers are not predictive of subsequent nephritis in the NZB/W F1.
However, among 69 monoclonal Abs originating from the mouse strain, there was a perfect correlation between Abs that bound to BM antigens with high affinity in vitro and those that accumulated in glomeruli and caused significant proteinuria after injection into non-immune mice[4]. An ELISA was used with matrigel as a surrogate for detecting mouse Abs that bound to BM antigens. Although anti-matrigel Ab titers have not been rigorously tested as a diagnostic tool in human SLE, there is some promising data. Multiplex analysis of circulating auto-Abs in a single center cohort of 37 adults with SLE showed a correlation between the presence of high IgG titer anti-matrigel Abs, anti-DNA Abs (ssDNA, dsDNA, chromatin), and higher total and renal SLE disease activity scores (SLEDAI scores)[12].
In order to determine whether heightened reactivity to BM antigens occurs in pediatric SLE patients, reactivity to matrigel in human plasma and serum was developed. Children with and without lupus were tested to assess whether a correlation exists between anti-BM Ab titer and a clinical diagnosis of pSLE, a history of nephritis, or reactivity to anti-dsDNA. Titers of IgG, IgM, IgA and Ig3 anti-BM Abs were measured in all samples. Relationships between ACR criteria and clinical findings were also explored.
METHODS
Matrigel (BD Biosciences) was used as a surrogate BM preparation. Matrigel is derived from an EHS sarcoma cell line and is composed primarily of laminin (55%), collagen type IV (30%), entactin (8%), and heparin sulfate proteoglycans (5%). An anti-matrigel ELISA for detection of mouse Abs was performed as previously described 4. Briefly, high protein-binding 96-well microtiter plates (Nunc Apogent) were coated with Matrigel at 500 mcg /well in cold PBS (4°C). After gelling at 4°C overnight, serum or plasma samples were added at 2-fold serial dilutions. Bound immunoglobulin from samples was detected using a biotin-conjugated goat anti-mouse IgG polyclonal Ab (Invitrogen) diluted 1:20000, followed by streptavidin–HRP (R&D systems) diluted 1:500, and TMB substrate (Pierce). Optical density was measured at 450nm (OD450) using a SpectraMax i3 plate reader (Molecular Devices). The protocol was changed for detection of anti-matrigel Abs in humans by substituting anti-human IgG polyclonal Ab (Invitrogen), also diluted at 1:20000. For anti-Matrigel IgA, IgM, and IgG3 ELISAs, the same protocol was followed, except for the use of a biotin-conjugated goat anti-human IgA diluted at 1:5000, anti-human IgM diluted at 1:2000, or anti-human Ig-G3 diluted at 1:1000 (Invitrogen). Testing of individual patient samples on separate occasions by two different experimenters (A.O. and A.S.) produced results that were highly reproducible with low inter-assay variation (CV 10–15%). End-point titers were determined as the dilution at which binding was no greater than the non-specific binding measured from normal human serum.
For anti-dsDNA Ab ELISA, plates were coated in poly-L-lysine 5 mcg/well, and then with 10mcg/mL calf thymus DNA (Sigma) pre-treated with S1 nuclease for 30 min at 37°C to remove any single stranded DNA. Wells were then blocked with 5% BSA/PBS. After washing, serial plasma dilutions were added. After a 2-h incubation at room temperature, plates were washed and horseradish peroxidase–conjugated detection Ab was added (anti-human IgG; Jackson Immunoresearch), diluted 1:20,000. TMB substrate was added, OD450 was measured, and end-point titers were calculated in comparison with pooled negative control plasma. A positive test was defined as an end-point titer > 1:40.
Mouse studies
Serum was obtained from 24-wk-old female MRL/lpr (age where all mice develop immune complex nephritis and >50% die from kidney failure) and age-matched female C57BL/6 mice. C57BL/6 mice are maintained in the Baylor College of Medicine (BCM) animal colony. MRL/lpr sera was a kind gift from Dr. Michael C. Braun (at BCM). All animal studies were performed after approval from BCM’s Institutional Animal Care and Use Committee.
pSLE patients
Serum samples were obtained from 21 unique pSLE patients enrolled in a longitudinal study of cognitive impairment in pSLE (June 2008–Sept 2011)[13]. Eligibility criteria included: age ≤18-yrs at the time of enrollment and ≤16-yrs at the time of diagnosis, and diagnosis of SLE according to the revised 1997 American College of Rheumatology criteria[14]. Samples from subjects with acute kidney injury due to etiologies other than autoimmune nephritis (pyelonephritis, acute tubular necrosis, or acute interstitial nephritis) were excluded.
Consent was obtained for reviewing the medical records of 10 of the 21 study subjects. Demographics and lab results were obtained from clinical encounters within 2 months of sample collection. Renal function was assessed by serum creatinine or 24-hr creatinine clearance. Estimated GFR was calculated for each patient using the Schwartz formula [15]. Lupus activity was assessed by the levels of complement components C3 and C4 and anti-ds DNA and anti-RNP Abs titers. Renal involvement was assessed in terms of proteinuria, as measured by 24-hour urine collection and/or urine protein creatinine ratio on random urine samples, and hematuria, as recorded by dipstick, urinalysis, and urinary sediment analysis. All human studies were performed in accordance with the Declaration of Helsinki after approval by BCM’s Institutional Review Board.
Healthy Controls
Control sera from 22 healthy children without renal failure were obtained from a bio-repository maintained at TCH (Table 3). Eligibility criteria included: age ≤18 years, and no known diagnosis of any form of autoimmune disease.
Statistical analysis
Statistical analyses were performed using Sigma Plot software, version 11 (www.systat.com) and SAS Software version 9.3 (Cary, NC). Continuous data are expressed as means with standard deviations or standard errors, medians with ranges, or frequencies with percentages as appropriate. Fisher’s exact was used to compare proportions between groups and the Mann-Whitney test was used to evaluate differences in continuous data between groups. General linear mixed models were used to examine the relationship between OD450 levels at varying dilutions for each patient group (normal controls, pSLE patients with nephritis, and pSLE patients without nephritis) taking into account the nature of repeated sampling for some pSLE patients. The area under the curve (AUC) of the receiver operating characteristic (ROC) curve was calculated to examine the ability of the end-point titer to distinguish between specific groups of patients, and Youden’s index was calculated to determine the cut point for defining high titer autoantibodies. In addition, the sensitivity and specificity of cutoff values for defining high and low-end point titers was calculated. Summary statistics are presented as medians with ranges due to the small sample size. Finally, a general linear mixed model was used to compare interval clinical characteristics between pSLE patients with either low or high end-point titers. A p-value <0.05 was considered statistically significant for all analyses.
RESULTS
Sera from autoimmune MRL/lpr mice bind to matrigel at higher titers than non-autoimmune B6 mice sera
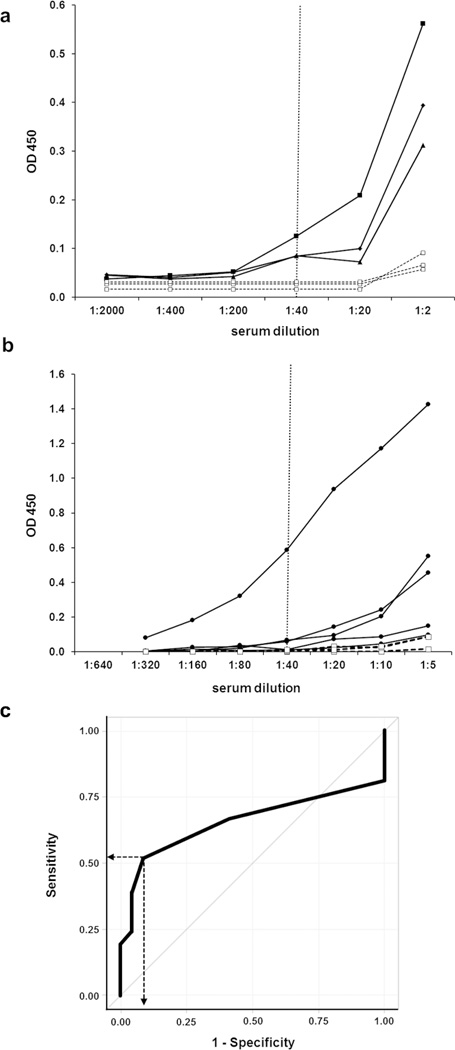
In order to confirm the previous findings of anti-BM reactivity in murine lupus models, anti-matrigel ELISA[4] was used to test sera from 6-mo female MRL/lpr and non-autoimmune C57BL/6 mice. By 6-months of age, female MRL/lpr mice all have high titer anti-dsDNA antibodies, lymphadenopathy, glomerulonephritis, and some degree of kidney failure. An increase in end-point titer in MRL/lpr mice corresponded with comparable increases in OD450 at 1:20 and 1:40 dilution of the serum samples. Results demonstrated that MRL/lpr mice had developed anti-matrigel Ab titers that were 10 to 20-fold higher than non-autoimmune mice (Fig 1a).
Fig 1. Autoimmune MRL/lpr mice and a subset of children with SLE develop high-titer anti-matrigel antibodies.
Representative ELISA results from serial dilutions of (a) sera from 3 separate 6-month old female MRL/lpr mice with nephritis (black circles) and 3 separate age-matched female non-autoimmune C57BL/6 mice (white squares), or (b) plasma from separate children with SLE (black circles) and age matched non-SLE controls (white squares). Samples were tested in triplicate and results are shown as mean OD. Samples requiring dilution greater than 1:40 to reach end–point were considered to have a high-titer anti-matrigel Abs. (c) ROC (receiver operating characteristics) curve using IgG anti-matrigel end-point titers to predict the presence of SLE in children (SLE n=21, non-SLE n=22). AUC (area under the curve) = 0.656. Dotted line represents the cut point indicated by Youden’s index, with 91% specificity and 52% sensitivity, used for Table 1.
Anti-matrigel antibody titers as predictor for pSLE
Next, the ELISA protocol was modified to detect human IgG and used to measure anti-BM reactivity in a cohort of 21 unique pediatric SLE patients and 22 age-matched, non- SLE controls. The majority of non-SLE controls had end-point titers of 1:40 or lower and this was designated as the cut-off for “high-titer” anti-matrigel Ab levels (Fig 1b). Of the 21 pSLE patients tested, 11 had end-point titers > 1:40 and were considered high-titer. Analysis of the receiver operating characteristics (ROC) curve for prediction of pSLE by presence of high-titer anti-matrigel IgG revealed an AUC of 0.66 (95% CI: 0.48, 0.84, Fig 1c), which is comparable to that of anti-dsDNA IgG and other subsets of anti-nuclear antibodies in the literature. The specificity of high-titer anti-matrigel IgG Abs for detecting pSLE was measured to be 91% with a sensitivity of 52%, using the definition of end-point titer > 1:40 (Table 1).
Table 1.
High-titer anti-matrigel antibodies1 occur more commonly in SLE than non-SLE cohorts, and pSLE patients with a history of nephritis tended to have high-titer IgG3 antibodies
| Ig Isotype |
pSLE (n-21) vs. non-SLE (n=22) |
History of nephritis (n=13) vs. non-renal SLE (n=8) |
||||
|---|---|---|---|---|---|---|
| Sensitivity (%) | Specificity (%) | p – value 2 | Sensitivity (%) | Specificity (%) | p – value 2 | |
| IgG | 52 | 91 | 0.003 | 42 | 33 | 0.371 |
| IgA | 29 | 95 | 0.046 | 38 | 88 | 0.342 |
| IgM | 66 | 78 | 0.005 | 62 | 25 | 0.656 |
| IgG3 | 42 | 81 | 0.168 | 88 | 64 | 0.059 |
High reactivity defined as end-point titer > 1:40
Fisher’s exact test compared proportions of positive high-titers samples for each group
A general linear mixed model was constructed to compare OD450 measures at each dilution between pSLE patient samples and non-SLE samples. There were no statistically significant differences in OD450 measures between pSLE and non-SLE samples for any dilution (data not shown). For this reason, end-point titers were selected as the best strategy to characterize individuals with high-titer anti-matrigel Abs.
To determine if anti-matrigel IgA or IgM antibodies were better at predicting pSLE than anti-matrigel IgG, the ELISA protocol was modified and the same pSLE and non-SLE samples were tested. There are 4 different IgG isotypes, and the anti-matrigel Ab ELISA was successfully adapted for the detection of one of these subsets, IgG3. Results were normalized such that the cut-offs for high-titer Abs were also > 1:40. By Fisher’s exact test, all 3 isotypes of anti-matrigel Abs were present at high titer in a significantly greater proportion of pSLE patients than in non-SLE control children (Table 1). Anti-matrigel IgA antibodies were the most specific for discriminating between pSLE and non-SLE samples, but were the least likely isotype to be present in high titer.
Anti-matrigel antibody titers can subdivide groups of pSLE patients
Thirteen of the 21 pSLE patients tested had a history of lupus nephritis. In order to see if a high titer of anti-matrigel Abs could discriminate between pSLE patients with a history of nephritis and non-renal pSLE patients, a subset analysis was performed. General linear mixed modeling was used to compare OD measures for the IgG ELISA at each serum dilution, and we found no significant differences. There was also no statistically significant difference in end-point titers using the IgA, IgG, or IgM ELISAs (Table 1); however, the specificity of high-titer IgA anti-matrigel Abs was 88%. Patients with a history of nephritis tended to have higher IgG3 titers than non-renal pSLE patients.
In order to see if reactivity to matrigel was simply a surrogate measure of anti-dsDNA reactivity in our pSLE cohort, all samples were tested by an anti-dsDNA Ab ELISA [16]. In this cohort, high-titer dsDNA IgG Abs did not distinguish between pSLE with a history of nephritis and non-renal pSLE (specificity 50%, sensitivity 62%, p = 0.67). Results indicated that the presence of high-titer anti-matrigel IgG, IgA, IgM, or IgG3 did not correlate with the occurrence of positive anti-dsDNA IgG Abs (Table 2, p = 1.0 for IgG, IgM and IgG3 and p = 0.37 for IgA). There were 8 pSLE patients who were anti-dsDNA Ab positive and high-titer anti-matrigel IgG, 4 double positive patients with high IgG3, 6 double positive patients with high IgA, and 8 double positive patients with high-titer IgM anti-matrigel Abs. Only testing positive for both anti-dsDNA Abs and anti-matrigel IgA was significantly associated with a history of nephritis (p = 0.046).
Table 2.
Presence of high titer anti-matrigel antibodies does not associate with the presence of anti-double stranded DNA antibodies in children with SLE 1
| sensitivity 67% specificity 56% |
IgG anti-dsDNA Abs | ||
|---|---|---|---|
| pos | neg | ||
|
IgG anti- matrigel Ab titer |
high | 8 | 4 |
| low | 4 | 5 | |
| sensitivity 62% specificity 50% |
IgG anti-dsDNA Abs | ||
|---|---|---|---|
| pos | neg | ||
|
IgG anti- matrigel Ab titer |
high | 8 | 5 |
| low | 4 | 4 | |
| sensitivity 75% specificity 54% |
IgG anti-dsDNA Abs | ||
|---|---|---|---|
| pos | neg | ||
|
IgG anti- matrigel Ab titer |
high | 6 | 2 |
| low | 6 | 7 | |
| sensitivity 50% specificity 45% |
IgG anti-dsDNA Abs | ||
|---|---|---|---|
| pos | neg | ||
|
IgG3 anti- matrigel Ab titer |
high | 4 | 4 |
| low | 6 | 5 | |
High reactivity defined as end-point titers > 1:40
Clinical data was available for 10 of the pSLE patients. All 10 patients were female and predominately Hispanic (80%). Six of the patients had a history of lupus nephritis diagnosed by renal biopsy, and these patients tended to be younger (median 14.5 vs 17-yo). The relationship between high-titer anti-matrigel Abs and each ACR (American College of Rheumatology) criteria for SLE was investigated (Supplementary table S1), but the presence of high-titers did not distinguish between presence or absence of any criterion. High-titer IgG and IgM anti-matrigel Abs tended to be less common in patients who at one point developed discoid rash. High-titer anti-matrigel IgA Abs were also more common in pSLE patients with a history of photosensitivity or mouth ulcers, and less common in those with history of hematologic or neurologic disorder. In contrast, high-titer anti-matrigel IgG3 Abs were more commonly detected in patients who at one point developed kidney disorder, and less common in those with history of photosensitivity or mouth ulcers.
Clinical data was available at time of collection for 17 samples from the 10 pSLE patients. Samples from patients with a history of nephritis had lower complement component C3 levels (75 vs. 98 mg/dL), but had roughly equivalent kidney function. The median serum creatinine at time of sampling for patients with nephritis was 0.6 mg/dL (range 0.5–0.6) and the median eGFR was 115 mL/1.73m2/min (range 108–128), compared to 0.6 mg/dL (range 0.5–0.6) and 110 mL/1.73m2/min (range108–121) in the non-renal pSLE subset. Three of the 4 were sampled while they had microscopic hematuria (5–10 rbc/hpf), but they had only minimal proteinuria (median 30 mg/dl), and none appeared to be experiencing a renal flare at the time samples were obtained. Relationships were explored between high-titer anti-matrigel Abs and routine clinical markers of kidney involvement (Supplemental table S2). High-titer IgG and IgA anti-matrigel Abs tended to be more common in patients who had hematuria. IgM was the only isotype of anti-matrigel Ab with any degree of correlation with reactivity to double stranded DNA and anti-matrigel IgA were significantly correlated with anti-RNP antibodies.
DISCUSSION
Using a single center historical cohort of patients with a diagnosis of pSLE, we demonstrate that measurement of anti-matrigel Ab titers by ELISA is feasible in patients as well as in mouse models of nephritis with good reproducibility. In our cohort, IgG, IgA and IgM anti-matrigel Abs all display a high specificity to diagnose SLE, and IgA anti matrigel Abs had a high specificity for identifying pSLE patients with a history of nephritis. Although high-titers in the plasma of pSLE patients could not discriminate between pSLE patients with a history of nephritis and non-renal pSLE, IgG3 anti-matrigel titers were more sensitive than IgG, IgM, or IgA, and may warrant assessment in prospective cohorts. The presence of anti-matrigel auto-Abs did not correlate with the presence of anti-dsDNA, which suggests that adding this auto-reactivity to serology panels may be of utility in monitoring disease activity in lupus.
Matrigel is a commercially available surrogate of BM, rich in extracellular matrix proteins such as laminin, collagen IV, entactin, and heparin sulfate. It is not specific for glomerular BM antigens; however, obtaining glomerular BM preparations from human kidney samples is laborious and would require fresh un-fixed kidney tissue that would be challenging to obtain. As a composite marker, matrigel appears to serve as the most readily available surrogate for glomerular BM antigens at this time.
The measurement of BM reactivity using matrigel ELISA has been previously published using one mouse model of immune complex kidney disease, the NZB/W F1 mouse[4]. Our study is important because it shows that anti-matrigel Abs also develop in a second mouse model of immune complex nephritis, the MRL/lpr model. Older female MRL/lpr mice with severe nephritis and renal failure have high titers of both anti-dsDNA Abs and anti-matrigel IgG Abs in the blood.
Only a subset of pSLE patients tested positively for high-titer anti-matrigel IgG (Fig 1b). Therefore, it is not surprising that linear mixed modeling did not find significant differences between the pSLE patients and controls. The AUC was 0.66, which is comparable to values reported for IgG anti-ds DNA Abs in SLE with specificity of 0.97 and sensitivity of 0.57 [10]. The mechanism by which only a subset of pSLE patients develop this auto-Ab response is unknown, but the proportion of pSLE patients in our cohort that tested positively was similar to the proportion of adult SLE patients reported by Li et al.[12]. The 39 adults in their cohort were recruited from an internal medicine rheumatology clinic at Albert Einstein College of Medicine, mean age 39-yo (range 15–69), 46% of whom had active nephritis and 56% had positive dsDNA Abs. However only one patient was <20-yo and 74% of patients were >30-yo. Although investigators reported a clustering of anti-matrigel IgG reactivity with IgG reactivity to vimentin, laminin, heparin sulfate, and myosin, and they showed an association between this cluster of IgG auto-reactivities and SLEDAI scores >8, the actual association between anti-matrigel IgG and disease activity was not reported. Anti-matrigel IgM reactivity was also tested and correlated with polyreactivity against most of the auto-antigens tested including histones, chromatin and DNA.
Anti-matrigel Abs did not discriminate between pSLE patients with a history of nephritis and those with non-renal SLE, although the sensitivity of high-titer IgG3 anti-matrigel Abs for predicting a history of nephritis was 88% (Table 1). These results are comparable with those of anti-dsDNA Abs, which have a 41% specificity and 65% sensitivity[10]. Based on the ability of monoclonal anti-matrigel Abs to cause disease in non-autoimmune mice[4], it is possible that these Abs may be pathogenic to the kidney, like anti-MPO in ANCA-associated vasculitis. Therefore, monitoring changes in titer of anti-matrigel Abs, as is often done for anti-dsDNA Abs, may provide better prediction of an up-coming renal flare.
Limitations of this study include the small sample size and the retrospective nature of the study. From the cohort of the patients for whom we were able to obtain clinical data, none of the 4 patients with a history of nephritis had active disease during the time when samples were obtained. Only 3 patients were sampled at time of hematuria, and in most of the cases the hematuria was mild (5–10 rbc/hpf). Similarly, only 3 of the samples were collected at time of proteinuria. We were unable to develop precise ELISA protocols specific for detection of IgG1, IgG2, or IgG4 subsets of anti-matrigel Abs. Future studies are warranted in order to assess the utility of anti-matrigel Abs or dual positivity antibodies (anti-matrigel and anti-dsDNA Abs) as biomarkers predictive of disease activity and/or response to therapy. It would be interesting to assess whether high-titer serum anti-matrigel Abs correlate with more membranous or proliferative patterns of histopathology on biopsy, predominance of capillary loop immunofluorescence staining for immunoglobulin, or predominance of sub-endothelial, intramembranous, or epi-membranous deposition of immune complexes.
In summary, anti-matrigel ELISA for IgG, IgA, and IgM can be used to measure a potentially useful and likely pathogenic new autoimmune serology in pSLE. This study is, to our knowledge, the first study to detect anti-matrigel Abs in children with SLE, and the first to specifically assess isotypes of anti-matrigel Abs in any SLE cohort. Based on the findings of this study, anti-matrigel Abs should be considered as a potential serum serologic marker to be used in combination with other promising serum and urine biomarkers currently in development.
Supplementary Material
ACKNOWLEGEMENTS
The authors would like to thank clinical study coordinators Theresa Falk and Brooke Bessard and the entire staff of the Dan Duncan Institute for Clinical and Translational Research (ICTR) at BCM for their assistance in this study, and Dr. Michael Braun (BCM) for thoughtful discussions and review of the manuscript. Sources of Funding: BCM Department of Pediatrics Pilot Award Program for Translation Research, and NIH DK081663 (P.I. Wenderfer) and the Lupus Foundation of America (P.I. Eyal Muscal).
Footnotes
Conflicts of Interest: None of the authors have anything to declare
REFERENCES
- 1.Woodroffe AJ, Border WA, Theofilopoulos AN, Gotze O, Glassock RJ, Dixon FJ, Wilson CB. Detection of circulating immune complexes in patients with glomerulonephritis. Kidney Int. 1977;12:268–278. doi: 10.1038/ki.1977.111. [DOI] [PubMed] [Google Scholar]
- 2.Couser WG, Steinmuller DR, Stilmant MM, Salant DJ, Lowenstein LM. Experimental glomerulonephritis in the isolated perfused rat kidney. J Clin Invest. 1978;62:1275–1287. doi: 10.1172/JCI109248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yung S, Chan TM. Anti-DNA antibodies in the pathogenesis of lupus nephritis--the emerging mechanisms. Autoimmunity reviews. 2008;7:317–321. doi: 10.1016/j.autrev.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Krishnan MR, Wang C, Marion TN. Anti-DNA autoantibodies initiate experimental lupus nephritis by binding directly to the glomerular basement membrane in mice. Kidney Int. 2012;82:184–192. doi: 10.1038/ki.2011.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barsalou J, Levy DM, Silverman ED. An update on childhood-onset systemic lupus erythematosus. Current opinion in rheumatology. 2013;25:616–622. doi: 10.1097/BOR.0b013e328363e868. [DOI] [PubMed] [Google Scholar]
- 6.Sturfelt G, Truedsson L. Complement and its breakdown products in SLE. Rheumatology (Oxford, England) 2005;44:1227–1232. doi: 10.1093/rheumatology/keh719. [DOI] [PubMed] [Google Scholar]
- 7.Mok CC. Biomarkers for lupus nephritis: a critical appraisal. Journal of biomedicine & biotechnology (2010) 2010:638413. doi: 10.1155/2010/638413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunner HI, Bennett MR, Mina R, Suzuki M, Petri M, Kiani AN, Pendl J, Witte D, Ying J, Rovin BH, Devarajan P. Association of noninvasively measured renal protein biomarkers with histologic features of lupus nephritis. Arthritis & Rheumatism. 2012;64:2687–2697. doi: 10.1002/art.34426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watson L, Beresford M. Urine biomarkers in juvenile-onset SLE nephritis. Pediatric Nephrology. 2013;28:363–374. doi: 10.1007/s00467-012-2184-y. [DOI] [PubMed] [Google Scholar]
- 10.Kavanaugh AF, Solomon DH. Guidelines for immunologic laboratory testing in the rheumatic diseases: anti-DNA antibody tests. Arthritis Rheum. 2002;47:546–555. doi: 10.1002/art.10558. [DOI] [PubMed] [Google Scholar]
- 11.Marion TN, Lawton AR, 3rd, Kearney JF, Briles DE. Anti-DNA autoantibodies in (NZB X NZW)F1 mice are clonally heterogeneous, but the majority share a common idiotype. J Immunol. 1982;128:668–674. [PubMed] [Google Scholar]
- 12.Li QZ, Xie C, Wu T, Mackay M, Aranow C, Putterman C, Mohan C. Identification of autoantibody clusters that best predict lupus disease activity using glomerular proteome arrays. J Clin Invest. 2005;115:3428–3439. doi: 10.1172/JCI23587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muscal E, Bloom DR, Hunter JV, Myones BL. Neurocognitive deficits and neuroimaging abnormalities are prevalent in children with lupus: clinical and research experiences at a US pediatric institution. Lupus. 2010;19:268–279. doi: 10.1177/0961203309352092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wenderfer SE, Ke B, Hollmann TJ, Wetsel RA, Lan HY, Braun MC. C5a receptor deficiency attenuates T cell function and renal disease in MRL/lpr mice. J Am Soc Nephrol. 2005;16:3572–3582. doi: 10.1681/ASN.2005040373. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.