Abstract
Cerebellar degeneration is traditionally associated with ataxia. Yet, there are examples of both ataxia and dystonia occurring in individuals with cerebellar degeneration. There is also substantial evidence suggesting that cerebellar dysfunction alone may cause dystonia. The types of cerebellar defects that may cause ataxia, dystonia, or both have not been delineated. In the current study, we explored the relationship between cerebellar degeneration and dystonia using the leaner mouse mutant. Leaner mice have severe dystonia that is associated with dysfunctional and degenerating cerebellar Purkinje cells. Whereas the density of Purkinje cells was not significantly reduced in 4 week-old leaner mice, approximately 50% of the neurons were lost by 34 weeks of age. On the other hand, the dystonia and associated functional disability became significantly less severe during this same interval. In other words, dystonia improved as Purkinje cells were lost, suggesting that dysfunctional Purkinje cells, rather than Purkinje cell loss, contribute to the dystonia. These results provide evidence that distorted cerebellar function may cause dystonia and support the concept that different types of cerebellar defects can have different functional consequences.
Keywords: Dystonia, cerebellum, Purkinje cell, leaner
2. Introduction
The dystonias are a group of disorders broadly characterized by involuntary excessive muscle activity leading to abnormal twisting or repetitive movement (Albanese, Bhatia et al. 2013). There are many potential etiologies, both inherited and acquire (Fung, Jinnah et al. 2013). Traditionally, the dystonias have been attributed to dysfunction of the basal ganglia, but more recently a number of studies also have implicated the cerebellum (Neychev, Gross et al. 2011; Standaert 2011; Sadnicka, Hoffland et al. 2012; Charlesworth and Bhatia 2013; Filip, Lungu et al. 2013; Lehericy, Tijssen et al. 2013; Prudente, Hess et al. 2014).
The evidence suggesting that cerebellar dysfunction may cause dystonia has raised several important questions that have yet to be addressed. For example, what types of abnormalities in the cerebellum may cause dystonia? Cerebellar lesions, such as those caused by stroke, most often result in ataxia (Caplan 2005; Deluca, Moretto et al. 2011), though sometimes they are associated with dystonia (Rumbach, Barth et al. 1995; Alarcon, Tolosa et al. 2001; O'Rourke, O'Riordan et al. 2006; Zadro, Brinar et al. 2008; Waln and LeDoux 2010; Usmani, Bedi et al. 2011). The majority of cerebellar degenerative syndromes are also associated with ataxia. However, in several of these, ataxia may be combined with dystonia, or the dystonia may be the presenting or dominant motor abnormality (Sethi and Jankovic 2002; Kuoppamaki, Giunti et al. 2003; Wilder-Smith, Tan et al. 2003; Hagenah, Zuhlke et al. 2004; Le Ber, Clot et al. 2006; van Gaalen, Giunti et al. 2011; Jhunjhunwala, Netravathi et al. 2013). The reasons that cerebellar defects may sometimes cause ataxia or dystonia are unknown, but may be related to the type of lesion.
In the current study, we explored the relationship between cerebellar degeneration and dystonia in the leaner mouse mutant, which carries a mutation in the Cacan1a gene, encoding CaV2.1 (P/Q)-type calcium channels (Doyle, Ren et al. 1997). This gene is prominently expressed in cerebellar Purkinje cells and different mutations in humans have been associated with markedly different clinical phenotypes including spinocerebellar ataxia type 6, episodic ataxia type 2, benign torticollis of infancy, focal dystonia in adults, familial hemiplegic migraine and epilepsy (Ophoff, Terwindt et al. 1996; Zhuchenko, Bailey et al. 1997; Pujana, Corral et al. 1999; Giffin, Benton et al. 2002; Spacey, Materek et al. 2005; Roubertie, Echenne et al. 2008; Cuenca-Leon, Banchs et al. 2009; Hu, Jiang et al. 2013). Different Cacan1a mutations in rodent models cause a similarly broad array of disorders, including generalized dystonia or ataxia, paroxysmal dystonia, epilepsy and migraine (Meier and MacPike 1971; Doyle, Ren et al. 1997; van den Maagdenberg, Pietrobon et al. 2004; Raike, Jinnah et al. 2005; Tokuda, Kuramoto et al. 2007; Shirley, Rao et al. 2008). We selected the leaner mutant not because of its relationship to a variety of different disorders, but because it is known to have severe chronic dystonia (Yoon 1969; Meier and MacPike 1971; Doyle, Ren et al. 1997) that is associated with abnormal physiological activity and slow degeneration of cerebellar Purkinje neurons (Meier and MacPike 1971; Herrup and Wilczynski 1982; Heckroth and Abbott 1994; Lau, Frank et al. 2004; Walter, Alvina et al. 2006; Ovsepian and Friel 2008; Ovsepian and Friel 2012). Thus it provides an ideal tool to address questions regarding the relationships between dystonia and cerebellar dysfunction.
2. Results
2.1 The density of Purkinje cells in the leaner mouse cerebellum decreases with age
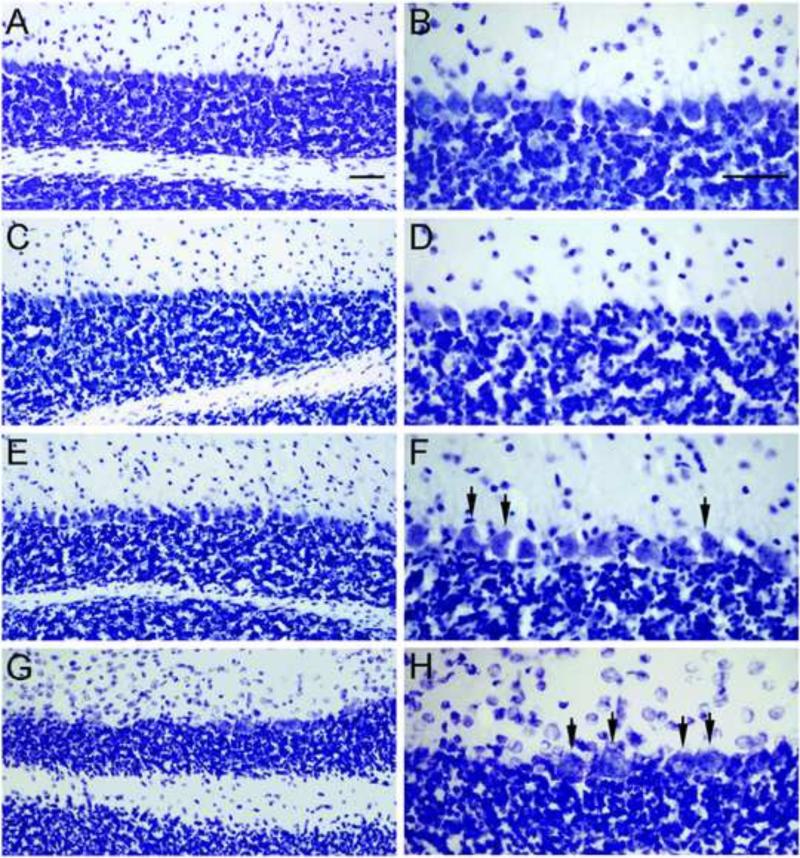
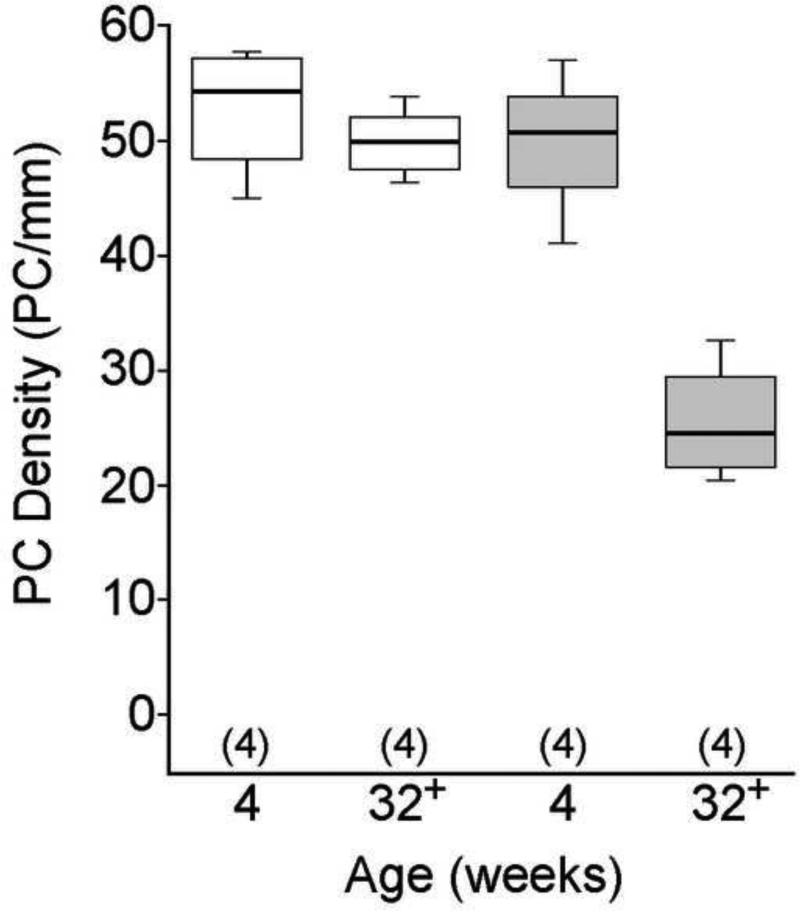
Several prior studies have revealed Purkinje cell loss in the leaner cerebellum beginning at 4 weeks of age, peaking at 8-9 weeks of age, and continuing gradually for several months thereafter (Meier and MacPike 1971; Herrup and Wilczynski 1982; Heckroth and Abbott 1994; Lau, Frank et al. 2004). Prior to their death Purkinje neurons show strikingly aberrant morphology (Rhyu, Abbott et al. 1999) and electrophysiological function (Walter, Alvina et al. 2006; Ovsepian and Friel 2008; Ovsepian and Friel 2012). Consistent with prior studies, we did not detect overt signs of Purkinje cell loss within the leaner mouse cerebellum when comparing tissue sections from 4 week-old normal control mice (Figure 1A, B) to those from age-matched leaner mice (Figure 1E, F). However, the Purkinje cell layer within the leaner mouse cerebellum appeared disorganized and frequently contained misshapen Purkinje cells (Figure 1F, arrows). At 32-36 weeks of age, leaner mice had an obvious loss of Purkinje cells (Figure 1G, H), which were often swollen and granular in appearance (Figure 1H, arrows). To quantify Purkinje cell loss, we measured the linear density of Purkinje cells in sections from leaner and control mice at both ages (n = 4 mice per group; Figure 2). As expected, the median linear density of Purkinje cells was not significantly different between 4 week-old control and leaner mice (median PC/mm = 54.3 for controls and 50.9 for leaners; Wilcoxon-Mann-Whitney U test). In contrast, the median linear density of Purkinje cells was significantly reduced in 32-36 week-old leaner mice compared to the age-matched controls (median PC/mm = for 25.5 for leaners and 49.9 for controls; p < 0.05; Wilcoxon-Mann-Whitney U test), suggesting that ~50% of leaner Purkinje cells are lost within this time span.
Figure 1. Cerebellar tissue sections from young and old control and leaner mice.
Cerebellar sections from 4 and 32-36 week old normal and leaner mice were compared to assess the degeneration of Purkinje cells with age in leaner mice. Sections from 4 week-old normal mice (A, B) and from 4 week-old leaner mice (E, F) contained similar numbers of Purkinje cells, though Purkinje cell layer within sections from 4 week-old leaner mice appeared disorganized and often contained misshapen Purkinje cells (F, arrows). No obvious abnormalities were observed in sections from 32-36 control mice (C, D), but sections from 32-36 leaner mice (G, H) contained many fewer Purkinje cells that often appeared swollen and granular (H, arrows). Scale bars = 100 μm.
Figure 2. The linear density of Purkinje cells in young and old control and leaner mice.
The linear density of Purkinje cells was measured in sections from 4 and 32-36 week-old normal control mice (white boxes) and leaner mice (gray boxes). Data are expressed in box and whisker format and sample sizes are in parentheses. Data were analyzed with the Kruskal-Wallis test. The median linear density of Purkinje cells was not significantly different between sections from 4 week-old control and 4 week-old leaner mice. Compared to that of 32-36 control mice, the median linear density of Purkinje cells was significantly reduced in sections from 32-36 week-old leaner mice (p < 0.05; Wilcoxon-Mann-Whitney U test).
2.2 Chronic generalized dystonia and motor function in leaner mice improve with age
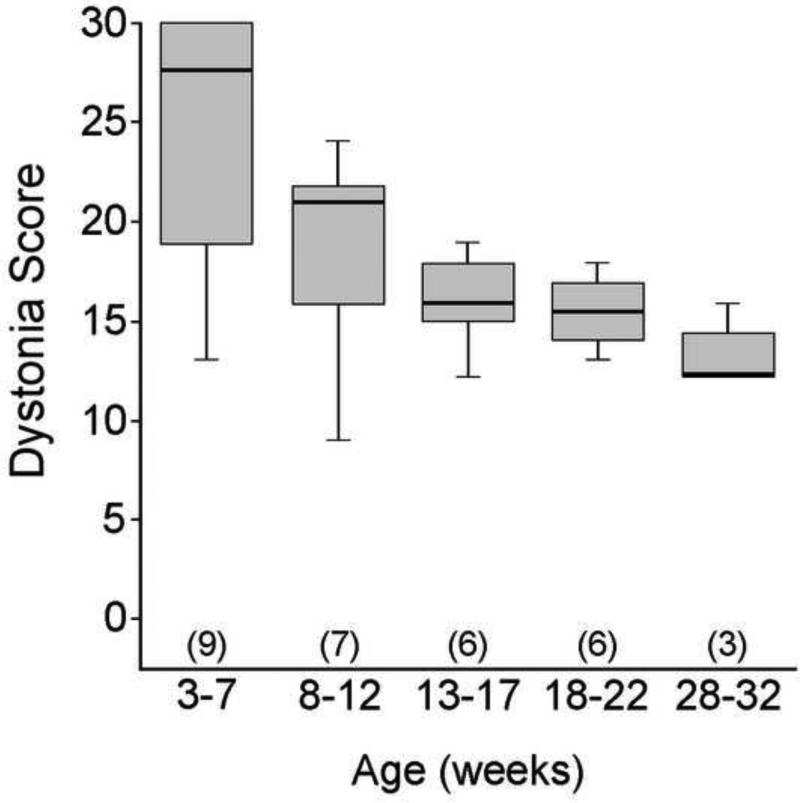
We prospectively studied motor function in leaner mice from 3-32 weeks of age. Dystonia in leaner mice is clearly identifiable in the second week of life and involves the entire body (Yoon 1969; Meier and MacPike 1971; Tsuji and Meier 1971; Raike, Jinnah et al. 2005). Dystonic movements from different body regions were recorded and used to calculate a total dystonia score (Figure 3). Median dystonia scores declined significantly with age, decreasing by >50% during the time period studied (p < 0.05; Kruskal-Wallis test).
Figure 3. The severity of dystonia in leaner mice as a function of age.
Leaner mice were assessed for the presence or absence of dystonic postures in the forelimbs, hindlimbs, trunk, neck and head from 3-32 weeks of age in 4 week groups. Data are expressed in box and whisker format and sample sizes are in parentheses. The median dystonia scores in leaner mice significantly decreased with age (p < 0.05; Kruskal-Wallis test).
Because there is no method for specifically quantifying ataxia and distinguishing it from other motor disorders in mice, a more detailed analysis was performed to assess overall motor function using several objective measures (Figure 4). Heterozygous littermates were used as controls because they do not express ataxia or dystonia (Yoon 1969), but exhibit mild motor learning and balance deficits under very specific and challenging motor tasks compared to normal mice (Katoh, Jindal et al. 2007; Alonso, Marques et al. 2008).
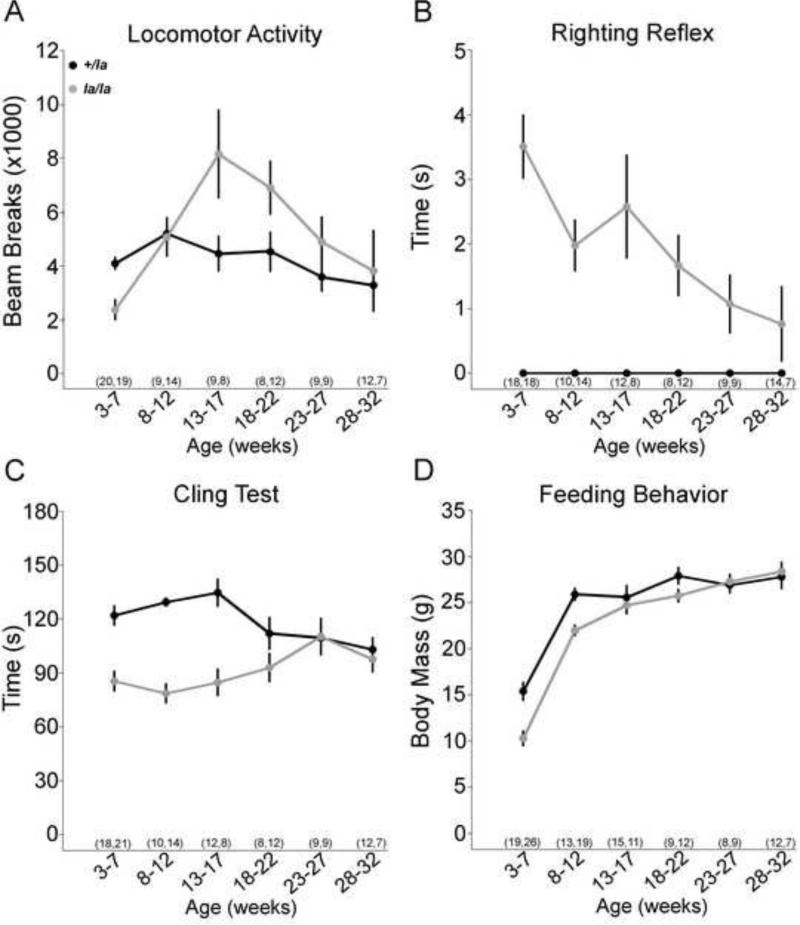
Figure 4. The motor performance of leaner mice as a function of age.
The motor behavior of leaner mice (la/la; gray circles) was further assessed from 3-32 weeks of age in 4 week groups and compared to their heterozygous littermates (+/la; black circles). Data are expressed in line graph format, with each point representing the mean, error bars representing the standard error of the mean (SEM), and the sample sizes in parentheses (+/la la/la). Photocell activity chambers were used to assess gross motor activity during the first 2 hrs of the normoactive nocturnal period (A). The mean number of beam breaks by leaner mice changed significantly with age (p < 0.001, ANOVA), but that of controls declined slightly but not significantly (p = 0.1, ANOVA). The righting reflex was used to assess the postural stability (B). The mean righting time in leaner mice from 3-27 weeks of age was significantly slower compared to that of controls (p < 0.05 for all, student's t test), but the mean righting time of leaner mice decreased significantly with age (p < 0.005, ANOVA). At 28-32 weeks of age mean righting time in leaner mice was not significantly different from controls (p = 0.2, student's t test). The mean righting time of the controls did not change significantly with age (p ANOVA). The cling test was used to assess agility and coordination (C). The mean cling time of leaner mice significantly increased with age (p < 0.05, ANOVA) whereas that of the controls exhibited a normal decline with age (p < 0.01, ANOVA). From 18-22 weeks of age onward the differences between the two groups of mice were not statistically significant (p > 0.1 for all, student's t test). Body mass was measured throughout the study to indirectly assess feeding behavior (D). The mean body mass of leaner mice increased significantly throughout the study (p < 0.001, ANOVA). The mean body mass of juvenile leaners (3-7 and 8-12 week-old mice) was significantly reduced compared to that of the controls (p < 0.001 for both, student's t test), but by young adulthood (13-17 weeks of age) the mean body mass of leaner mice was not significantly different (p < 0.001, student's t test).
Gross ambulatory motor activity was assessed using photocell activity chambers during the first 2 hrs of the normally active nocturnal period (Figure 4A). The motor activity of leaner mice changed significantly with age (p < 0.001, ANOVA), whereas that of the littermate controls remained relative stable (p = 0.1, ANOVA). Compared to littermate controls, juvenile leaner mice had significantly reduced ambulation due to extremely stiff posturing that was associated with frequent falls and an inability to regain the upright posture. Beginning in young leaner adults, ambulation increased above littermate controls as the stiff posturing and falling gradually transitioned into mild listing and stumbling.
To specifically test the ability of leaner mice to restore upright positioning after falls, the righting reflex was evaluated, which assesses the time it takes for an animal to place all 4 paws on the floor after being placed supine. Heterozygous controls were able to restore their normal upright posture almost immediately (Figure 4B). Juvenile leaner mice struggled for several seconds to regain the upright posture, because of slow and stiff limb movements. However, leaner mice became progressively more proficient in restoring their posture with age (p < 0.005, ANOVA), becoming almost normal by 28-32 weeks of age. These results demonstrate significant improvements in postural righting with age in leaner mice.
Agility and coordination was assessed using the cling test, which measures the time to fall from a wire grid held horizontally, at 90° vertical, and then inverted upside down (Figure 4C). The mean cling time of the heterozygous controls declined with age (p < 0.01, ANOVA), as reported for normal mice (Khan and Jinnah 2002). In contrast, the mean cling time of leaner mice significantly improved with age (p < 0.05, ANOVA), so that by 18-22 weeks of age there were no significant differences between leaner mice and their littermate controls (p > 0.1 for all, student's t test). These results demonstrate that climbing and hanging skills of leaner mice also improve as a function of age.
Juvenile leaner mice suffer high mortality rates because severe dystonia limits their ability to eat and drink independently. After weaning, twice-daily gastric gavage is required to keep them alive, until approximately 8 weeks of age when they begin to eat and drink on their own. To indirectly assess volitional feeding behavior, body mass was measured at different ages. Despite gastric gavage, the mean body mass of juvenile leaner mice was ~33% lower than littermate controls (Figure 4D). With aging, mean body mass increased and was not significantly different from controls by 13-17 weeks of age, suggesting that the ability to eat and drink more normally was restored.
3. Discussion
In the current study we focused on evaluating the dystonia and overall functional disability of the leaner mouse mutant within the context of the well-documented Purkinje cell degeneration (Yoon 1969; Meier and MacPike 1971; Doyle, Ren et al. 1997). The results show that the severe dystonia and overall motor function in leaner mice both improve with age, paradoxically at the same time that Purkinje cell loss is worsening. Prior electrophysiological studies demonstrate that cerebellar Purkinje cells from juvenile leaner mice exhibit abnormal synaptic transmission and dysfunctional pacemaking (Walter, Alvina et al. 2006; Ovsepian and Friel 2008; Ovsepian and Friel 2012) at a time when dystonia and motor disability are at peak severity, but when the Purkinje cell loss was undetectable. Therefore, the results of this study provide more evidence implicating Purkinje cell dysfunction in dystonia, and are also useful for considering potential hypotheses regarding the relationships between dystonia and cerebellar function.
It may be surprising that the overall motor disability of leaner mice improves with degeneration, but the results provide support for prior proposals that different types of cerebellar defects can have different functional consequences (Prudente, Hess et al. 2014). In the cerebellum, predominantly loss of function defects such as structural lesions or degenerative cell loss may result in ataxia, while defects that predominantly distort function may instead cause dystonia. Ataxia and dystonia may instead simultaneously co-exist in the same individual when cerebellar defects combine loss of function with distorted function.
The cerebellum is a large, highly-compartmentalized structure. Degenerative neuronal loss may occur in one region simultaneous with neuronal dysfunctional in another region. Because the cerebellum has a fractured somatotopic representation (Manni and Petrosini 2004; Apps and Garwicz 2005), neuronal loss in one region may result in ataxia in some body parts whereas neuronal dysfunction in another cerebellar region may cause dystonia in other body parts. This may explain why patients with degenerative cerebellar ataxias may also have dystonia (Sethi and Jankovic 2002; Kuoppamaki, Giunti et al. 2003; Wilder-Smith, Tan et al. 2003; Hagenah, Zuhlke et al. 2004; Le Ber, Clot et al. 2006; van Gaalen, Giunti et al. 2011; Jhunjhunwala, Netravathi et al. 2013).
Ataxia and dystonia may also occur in the same individual but at different times over the course of a disorder, reflecting the temporal evolution of the underlying cerebellar defects. In some mouse models of cerebellar degeneration, the dying Purkinje neurons exhibit a lengthy period of abnormal physiological function prior to their demise (Walter, Alvina et al. 2006; Barnes, Ebner et al. 2011; Hourez, Servais et al. 2011). Because even a small region of cerebellar function is sufficient to cause dystonia (Raike, Pizoli et al. 2012), dystonia may occur early in the course of the disorder when cerebellar neurons are dysfunctional, followed by ataxia after the neurons are lost. This scenario is consistent with the evolution of the motor disorder in leaner mice, where dystonia appears to abate as Purkinje neuron loss increases. Whether this temporal pattern of changes occurs in human disorders that combine dystonia and ataxia remains to be determined.
Concepts regarding distortion versus loss of cerebellar function in dystonia have direct implications for the design of novel therapeutic strategies. Specifically, individuals determined to have abnormal cerebellar output (not loss of output) may respond to interventions designed to block this output. Several recent reports have described significant clinical benefits in some dystonia patients receiving deep brain stimulation or lesions of regions of the thalamus receiving cerebellar projections (Taira and Hori 2003; Shibata, Hirashima et al. 2005; Fukaya, Katayama et al. 2007; Goto, Shimazu et al. 2008; Kim, Jeon et al. 2008; Morishita, Foote et al. 2010; Yen, Sheehan et al. 2012; Horisawa, Taira et al. 2013). There are also older reports in the literature describing significant benefits in some dystonia patients receiving electrical stimulation of the cerebellar cortex (Fraioli, Baldassarre et al. 1980; Cooper, Upton et al. 1982). We hypothesize that the therapeutic benefits from both approaches result from preventing abnormal cerebellar signaling from reaching motor cortex, among patients where abnormal cerebellar function is the cause of dystonia. However, further work is required before applying invasive surgical interventions targeting the cerebellum or its outflow pathways in patients with dystonia. Most notably, diagnostic strategies and objective biomarkers need to be developed for identifying subtypes of dystonias with the types of cerebellar dysfunction most likely to respond.
4. Experimental Procedures
4.1 Animals
Dystonia in leaner mice becomes apparent at 7-10 days of age. To permit earlier identification of leaner mice, we took advantage of its close linkage with oligosyndactyly (Os). Mice heterozygous for both the leaner (Cacna1a+/tg-la) and oligosyndactyly (Os+/Os) alleles were originally obtained from Jackson Laboratories (Bar Harbor, ME) on the C57BL/6J background. The leaner allele is recessive and causes severe generalized dystonia and ataxia (Yoon 1969; Meier and MacPike 1971; Raike, Jinnah et al. 2005). The Os allele is lethal in utero when homozygous, but causes fused toes when heterozygous (Magnuson and Epstein 1984; Isaacs and Abbott 1992). Male and female breeders were paired so that each carried one Cacna1a+; OsOs allele and one Cacna1atg-la; Os+ allele. This breeding scheme rarely produces Cacna1a+/+; OsOs/Os offspring, and facilitates early identification of leaner mice (Cacna1atg-la/tg-la; Os+/+) because heterozygous littermates (Cacna1a+/tg-la; Os+/Os) can be identified by their fused toes.
Without supplemental food and water, leaner homozygotes mice almost always die prior to 3 weeks of age (Yoon 1969; Meier and MacPike 1971; Tsuji and Meier 1971). Therefore, a soft mixture of food pellets and water was placed at the cage floor and floor-level water dispensers (Bioserv, Frenchtown, NJ) were used throughout the study. Beginning postnatal day 10, leaner mice were fed a liquid rodent diet (Lieber-DeCarli, Bisoerv, Frenchtown, NJ) via gastric gavage and hydrated with a sucrose solution (30% sucrose in 0.45% solution at 10 ml/kg) twice daily until ~8 weeks of age, when the mice were able to feed independently. Mice were bred and housed on a 12 hr light/dark cycle at Emory University or Johns Hopkins University vivaria. All procedures were in accordance with the Guide for the Care and Use of Laboratory Animals as adopted by the National Institutes of Health, Emory University and Johns Hopkins University.
4.2 Motor behavior
Dystonia was assessed in leaner mice from 3-32 weeks of age using a semi-quantitative motor inventory method that distinguishes dystonia from other motor problems, such as ataxia (Campbell, North et al. 1999; Fureman, Jinnah et al. 2002; Devanagondi, Egami et al. 2007). Mice were released individually into a novel cage and observed for 1 min at 10-min intervals over 60 min. Dystonia in leaner mice involves the entire body, including the limbs, trunk, neck and head (Yoon 1969; Meier and MacPike 1971; Tsuji and Meier 1971; Raike, Jinnah et al. 2005). Therefore, mice were scored for the presence or absence of dystonic postures in five body regions, including the forelimbs, hindlimbs, trunk, neck, and head, making 6 the highest possible score per body region and 30 the highest possible total score for the 60-min session. Total dystonia scores were used for analysis.
Whereas the presence or absence of dystonia in different body parts can be easily quantified using observational methods, there is no comparable observational method for quantifying the lack of body part coordination in ataxia. Therefore, the overall motor function of leaner mice was also assessed using several standard objective motor behavior tests. Gross motor activity was quantified using photocell activity chambers. Each chamber consisted of a 20 × 40 cm Plexiglass cage surrounded by a metal frame that projected a 4 × 8 grid of infrared beams 1 cm from the bottom of the cage (San Diego Instruments, San Diego, CA). Mice were individually placed into a chamber at the onset of the normally active nocturnal period. The total number of beam breaks over the first 2 hr period was recorded. Postural stability was assessed using the righting reflex. Each mouse was placed on its back in a clean cage and the time for the mouse to return to a normal position on all four limbs was recorded using a stopwatch with a maximum score of 5 sec. Each mouse was timed on 3 trials at each age examined. Agility and coordination were assessed using the cling test. Mice were habituated for 1 min on a horizontal 20 × 20 cm platform with a 6 mm grid of wire mesh surrounded by a 5 cm frame above a padded surface. The frame was then rotated 90° to vertical for 1 min, and then rotated another 90° for 1 min so that the mice were inverted. The time to fall was recorded with a maximum score of 180 sec. Mice were timed over three trials, and the average of two highest scores was used for analysis. Other motor tasks such as the Rotarod and balance beam tests could not be examined, because leaner mice are too profoundly impaired.
4.3 Purkinje cell counts
To quantify Purkinje cell loss in young and old leaner mice, the linear density of Purkinje cells was estimated in 4 and 34±2 week-old leaner and normal control mice as previously described (Raike, Pizoli et al. 2012). The cerebellum was sectioned in the coronal plane at a thickness of 30 μm using a cryostat (Leica, Wetzlar, Germany). Sections were thaw-mounted on Superfrost Plus glass slides (Fisher, Pittsburgh, PA), stained with cresyl violet and dehydrated with graded ethanols and xylenes. Stained sections were viewed under bright-field illumination using an upright BX51 light microscope (Olympus, Center Valley, PA) with a computer-controlled MAC 2000 motorized microscope stage (Ludl Inc, Hawthorne, NY). Purkinje cell layers were traced and measured at 4x magnification and Purkinje cell bodies were identified morphologically, and counted at 20x using the Stereoinvestigator/Neurolucida software package (Microbrightfield Inc, Williston, VT). The linear density of Purkinje cells was then calculated by dividing the total Purkinje cell count by total Purkinje cell layer length (PC/mm). From each animal, 3-5 random sections were analyzed to obtain 16 measurements, including 7 sections from the cerebellar vermis (one measurement each from the anterior lobe and lobules 3, 4/5, 6, 7, 8 and 9) and 9 sections from the cerebellar hemispheres (one set of bilateral measurements from the simplex and two sets of bilateral measurements from crus 1, crus 2, paramedian and copula, anterior and posterior to the primary fissure). Representative images were obtained using an Olympus DP72 digital camera.
4.4 Statistical analyses
Dystonia scores and the linear densities of Purkinje cells are expressed as median values and presented as standard box and whiskers plots where the heavy bar is the median, the bottom and top of the box represent the lower and upper quartiles, the whiskers represent the full spread of the data, and the open circles represent outliers. Body mass, gross locomotor activity, righting-reflex and cling-test data are presented as standard line graphs, with each point representing the mean and error bars representing the standard error of the mean (SEM). Dystonia scores were compared via the non-parametric Kruskal-Wallis test. Purkinje cell linear densities from leaner mice were not normally distributed, so all Purkinje cell data were analyzed using the non- parametric Wilcoxon-Mann-Whitney U test. Power analyses of Purkinje-cell linear density data from normal C57BL/6J mice (80% power; α = 0.05) indicated that a sample size of 3 animals was adequate for these studies. Body mass, gross locomotor activity, righting-reflex and cling-test data were analyzed using ANOVA with post-hoc t tests. All analyses were performed using the Statview statistical software package (SAS Institute, Cary, NC).
Highlights.
Types of cerebellar defects that cause dystonia, ataxia or both are not delineated
We explore the relationship between Purkinje cell loss and dystonia in leaner mice
Dystonia is severe in juvenile leaner mice when Purkinje cell loss is not significant
Dystonia and functional disability alleviate only after a ~50% loss of these neurons
Supports concept that different cerebellar defects cause different motor symptoms
Acknowledgments
This work was supported by Unites States National Institutes of Health R01 NS040470 to H.A.J., R01 NS33592 to E.J.H. and the Dystonia Medical Research Foundation to R.S.R. The authors thank Kiyoshi Egami and Doug Bernard for technical assistance.
H.A.J. has active grant support from the United States National Institutes of Health, the Bachmann-Strauss Dystonia & Parkinson's Foundation, Merz Pharmaceuticals Inc., and Ipsen Inc. H.A.J. also is principle investigator for the Dystonia Coalition, which receives the majority of its support through United States National Institutes of Health grant NS065701 from the Office of Rare Diseases Research at the National Center for Advancing Translational Sciences, and National Institutes of Neurological Disorders and Stroke. The Dystonia Coalition receives additional material or administrative support from industry sponsors (Allergan Inc. and Merz Pharmaceuticals Inc.) as well as private foundations (The American Dystonia Society, The Benign Essential Blepharospasm Foundation, Dystonia Inc., Dystonia Ireland, The Dystonia Medical Research Foundation, The European Dystonia Federation, The Foundation for Dystonia Research, The National Spasmodic Dysphonia Association and The National Spasmodic Torticollis Association). H.A.J. also serves as a consultant for Psyadon Pharmaceuticals and serves on the Scientific Advisory Boards for the Cure Dystonia Now, the Dystonia Medical Research foundation, Lesch-Nyhan Action France, the Lesch-Nyhan Syndrome Children's Research Foundation and Tyler's Hope for a Cure.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. Contributions
R.S.R and H.A.J. performed experiments. R.S.R., H.A.J. and E.J.H wrote the manuscript.
6. Conflicts of Interests
The authors have no conflicts of interests to report.
References
- Alarcon F, Tolosa E, et al. Focal limb dystonia in a patient with a cerebellar mass. Arch Neurol. 2001;58(7):1125–1127. doi: 10.1001/archneur.58.7.1125. [DOI] [PubMed] [Google Scholar]
- Albanese A, Bhatia K, et al. Phenomenology and classification of dystonia: a consensus update. Mov Disord. 2013;28(7):863–873. doi: 10.1002/mds.25475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso I, Marques JM, et al. Motor and cognitive deficits in the heterozygous leaner mouse, a Cav2.1 voltage-gated Ca2+ channel mutant. Neurobiol Aging. 2008;29(11):1733–1743. doi: 10.1016/j.neurobiolaging.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Apps R, Garwicz M. Anatomical and physiological foundations of cerebellar information processing. Nat Rev Neurosci. 2005;6(4):297–311. doi: 10.1038/nrn1646. [DOI] [PubMed] [Google Scholar]
- Barnes JA, Ebner BA, et al. Abnormalities in the climbing fiber-Purkinje cell circuitry contribute to neuronal dysfunction in ATXN1[82Q] mice. J Neurosci. 2011;31(36):12778–12789. doi: 10.1523/JNEUROSCI.2579-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DB, North JB, et al. Tottering mouse motor dysfunction is abolished on the Purkinje cell degeneration (pcd) mutant background. Exp Neurol. 1999;160:268–278. doi: 10.1006/exnr.1999.7171. [DOI] [PubMed] [Google Scholar]
- Caplan LR. Cerebellar infarcts: key features. Rev Neurol Dis. 2005;2(2):51–60. [PubMed] [Google Scholar]
- Charlesworth G, Bhatia KP. Primary and secondary dystonic syndromes: an update. Curr Opin Neurol. 2013;26(4):406–412. doi: 10.1097/WCO.0b013e3283633696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper IS, Upton AR, et al. Chronic cerebellar stimulation (CCS) and deep brain stimulation (DBS) in involuntary movement disorders. Appl Neurophysiol. 1982;45(3):209–217. doi: 10.1159/000101601. [DOI] [PubMed] [Google Scholar]
- Cuenca-Leon E, Banchs I, et al. Late-onset episodic ataxia type 2 associated with a novel loss-of-function mutation in the CACNA1A gene. J Neurol Sci. 2009;280(1-2):10–14. doi: 10.1016/j.jns.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Deluca C, Moretto G, et al. Ataxia in posterior circulation stroke: clinical-MRI correlations. J Neurol Sci. 2011;300(1-2):39–46. doi: 10.1016/j.jns.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Devanagondi R, Egami K, et al. Neuroanatomical substrates for paroxysmal dyskinesia in lethargic mice. Neurobiol Dis. 2007;3:249–257. doi: 10.1016/j.nbd.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle J, Ren X, et al. Mutations in the Cacnl1a4 calcium channel gene are associated with seizures, cerebellar degeneration, and ataxia in tottering and leaner mutant mice. Mammalian Genome. 1997;8(2):113–120. doi: 10.1007/s003359900369. [DOI] [PubMed] [Google Scholar]
- Filip P, Lungu OV, et al. Dystonia and the cerebellum: a new field of interest in movement disorders? Clin Neurophysiol. 2013;124(7):1269–1276. doi: 10.1016/j.clinph.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Fraioli B, Baldassarre L, et al. Chronic paleocerebellar stimulation in dystonia and athetosis. Report of two cases. J Neurosurg Sci. 1980;24(2):99–103. [PubMed] [Google Scholar]
- Fukaya C, Katayama Y, et al. Thalamic deep brain stimulation for writer's cramp. J Neurosurg. 2007;107(5):977–982. doi: 10.3171/JNS-07/11/0977. [DOI] [PubMed] [Google Scholar]
- Fung VS, Jinnah HA, et al. Assessment of patients with isolated or combined dystonia: an update on dystonia syndromes. Mov Disord. 2013;28(7):889–898. doi: 10.1002/mds.25549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fureman BE, Jinnah HA, et al. Triggers of paroxysmal dyskinesia in the calcium channel mouse mutant tottering. Pharmacol Biochem Behav. 2002;73:631–637. doi: 10.1016/s0091-3057(02)00854-7. [DOI] [PubMed] [Google Scholar]
- Giffin NJ, Benton S, et al. Benign paroxysmal torticollis of infancy: four new cases and linkage to CACNA1A mutation. Dev Med Child Neurol. 2002;44:490–493. doi: 10.1017/s0012162201002407. [DOI] [PubMed] [Google Scholar]
- Goto S, Shimazu H, et al. Thalamic Vo-complex vs pallidal deep brain stimulation for focal hand dystonia. Neurology. 2008;70(16 Pt 2):1500–1501. doi: 10.1212/01.wnl.0000310430.00743.11. [DOI] [PubMed] [Google Scholar]
- Hagenah JM, Zuhlke C, et al. Focal dystonia as a presenting sign of spinocerebellar ataxia 17. Mov Disord. 2004;19(2):217–220. doi: 10.1002/mds.10600. [DOI] [PubMed] [Google Scholar]
- Heckroth JA, Abbott LC. Purkinje cell loss from alternating sagittal zones in the cerebellum of leaner mutant mice. Brain Research. 1994;658(1-2):93–104. doi: 10.1016/s0006-8993(09)90014-2. [DOI] [PubMed] [Google Scholar]
- Herrup K, Wilczynski SL. Cerebellar cell degeneration in the leaner mutant mouse. Neuroscience. 1982;7(9):2185–2196. doi: 10.1016/0306-4522(82)90129-4. 9. [DOI] [PubMed] [Google Scholar]
- Horisawa S, Taira T, et al. Long-term improvement of musician's dystonia after stereotactic ventro-oral thalamotomy. Ann Neurol. 2013;74(5):648–654. doi: 10.1002/ana.23877. [DOI] [PubMed] [Google Scholar]
- Hourez R, Servais L, et al. Aminopyridines correct early dysfunction and delay neurodegeneration in a mouse model of spinocerebellar ataxia type 1. J Neurosci. 2011;31(33):11795–11807. doi: 10.1523/JNEUROSCI.0905-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Jiang H, et al. Identification of a novel nonsense mutation p.Tyr1957Ter of CACNA1A in a Chinese family with episodic ataxia 2. PLoS One. 2013;8(2):e56362. doi: 10.1371/journal.pone.0056362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs KR, Abbott LC. Development of the paramedian lobule of the cerebellum in wild-type and tottering mice. Dev Neurosci. 1992;14(5-6):386–393. doi: 10.1159/000111687. [DOI] [PubMed] [Google Scholar]
- Jhunjhunwala K, Netravathi M, et al. Profile of extrapyramidal manifestations in 85 patients with spinocerebellar ataxia type 1, 2 and 3. J Clin Neurosci. 2013;21(6):1002–6. doi: 10.1016/j.jocn.2013.10.021. [DOI] [PubMed] [Google Scholar]
- Katoh A, Jindal JA, et al. Motor deficits in homozygous and heterozygous p/q-type calcium channel mutants. J Neurophysiol. 2007;97(2):1280–1287. doi: 10.1152/jn.00322.2006. [DOI] [PubMed] [Google Scholar]
- Khan Z, Jinnah HA. Paroxysmal dyskinesias in the lethargic mouse mutant. J Neurosci. 2002;22(18):8193–8200. doi: 10.1523/JNEUROSCI.22-18-08193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Jeon SR, et al. Effect of thalamotomy on focal hand dystonia in a family with DYT1 mutation. Mov Disord. 2008;23(15):2251–2255. doi: 10.1002/mds.22337. [DOI] [PubMed] [Google Scholar]
- Kuoppamaki M, Giunti P, et al. Slowly progressive cerebellar ataxia and cervical dystonia: clinical presentation of a new form of spinocerebellar ataxia? Mov Disord. 2003;18(2):200–206. doi: 10.1002/mds.10308. [DOI] [PubMed] [Google Scholar]
- Lau FC, Frank TC, et al. Postnatal apoptosis in cerebellar granule cells of homozygous leaner (tg1a/tg1a) mice. Neurotox Res. 2004;6(4):267–280. doi: 10.1007/BF03033437. [DOI] [PubMed] [Google Scholar]
- Le Ber I, Clot F, et al. Predominant dystonia with marked cerebellar atrophy: a rare phenotype in familial dystonia. Neurology. 2006;67(10):1769–1773. doi: 10.1212/01.wnl.0000244484.60489.50. [DOI] [PubMed] [Google Scholar]
- Lehericy S, Tijssen MA, et al. The anatomical basis of dystonia: current view using neuroimaging. Mov Disord. 2013;28(7):944–957. doi: 10.1002/mds.25527. [DOI] [PubMed] [Google Scholar]
- Magnuson T, Epstein CJ. Oligosyndactyly: a lethal mutation in the mouse that results in mitotic arrest very early in development. Cell. 1984;38(3):823–833. doi: 10.1016/0092-8674(84)90277-0. [DOI] [PubMed] [Google Scholar]
- Manni E, Petrosini L. A century of cerebellar somatotopy: a debated representation. Nat Rev Neurosci. 2004;5(3):241–249. doi: 10.1038/nrn1347. [DOI] [PubMed] [Google Scholar]
- Meier H, MacPike AD. Three syndromes produced by two mutant genes in the mouse. Clinical, pathological, and ultrastructural bases of tottering, leaner, and heterozygous mice. J Hered. 1971;62:297–302. doi: 10.1093/oxfordjournals.jhered.a108176. [DOI] [PubMed] [Google Scholar]
- Morishita T, Foote KD, et al. Should we consider Vim thalamic deep brain stimulation for select cases of severe refractory dystonic tremor. Stereotact Funct Neurosurg. 2010;88(2):98–104. doi: 10.1159/000289354. [DOI] [PubMed] [Google Scholar]
- Neychev VK, Gross RE, et al. The functional neuroanatomy of dystonia. Neurobiol Dis. 2011;42(2):185–201. doi: 10.1016/j.nbd.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rourke K, O'Riordan S, et al. Paroxysmal torticollis and blepharospasm following bilateral cerebellar infarction. J Neurol. 2006;253(12):1644–1645. doi: 10.1007/s00415-006-0202-3. [DOI] [PubMed] [Google Scholar]
- Ophoff RA, Terwindt GM, et al. Familial hemiplegic migraine and episodic ataxia type-2 are caused by mutations in the Ca2+ channel gene CACNL1A4. Cell. 1996;87(3):543–552. doi: 10.1016/s0092-8674(00)81373-2. [DOI] [PubMed] [Google Scholar]
- Ovsepian SV, Friel DD. The leaner P/Q-type calcium channel mutation renders cerebellar Purkinje neurons hyper-excitable and eliminates Ca2+-Na+ spike bursts. Eur J Neurosci. 2008;27(1):93–103. doi: 10.1111/j.1460-9568.2007.05998.x. [DOI] [PubMed] [Google Scholar]
- Ovsepian SV, Friel DD. Enhanced synaptic inhibition disrupts the efferent code of cerebellar Purkinje neurons in leaner Cav2.1 Ca 2+ channel mutant mice. Cerebellum. 2012;11(3):666–680. doi: 10.1007/s12311-010-0210-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudente CN, Hess EJ, et al. Dystonia as a network disorder: What is the role of the cerebellum? Neuroscience. 2014;260:23–35. doi: 10.1016/j.neuroscience.2013.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujana MA, Corral J, et al. Spinocerebellar ataxias in Spanish patients: genetic analysis of familial and sporadic cases. The Ataxia Study Group. Hum Genet. 1999;104(6):516–522. doi: 10.1007/s004390050997. [DOI] [PubMed] [Google Scholar]
- Raike RS, Jinnah HA, et al. Animal models of generalized dystonia. NeuroRx. 2005;2(3):504–512. doi: 10.1602/neurorx.2.3.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raike RS, Pizoli CE, et al. Limited regional cerebellar dysfunction induces focal dystonia in mice. Neurobiol Dis. 2012;49C:200–210. doi: 10.1016/j.nbd.2012.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhyu IJ, Abbott LC, et al. An ultrastructural study of granule cell/Purkinje cell synapses in tottering (tg/tg), leaner (tg(la)/tg(la)) and compound heterozygous tottering/leaner (tg/tg(la)) mice. Neuroscience. 1999;90:7–28. doi: 10.1016/s0306-4522(98)00518-1. [DOI] [PubMed] [Google Scholar]
- Roubertie A, Echenne B, et al. Benign paroxysmal tonic upgaze, benign paroxysmal torticollis, episodic ataxia and CACNA1A mutation in a family. J Neurol. 2008;255(10):1600–1602. doi: 10.1007/s00415-008-0982-8. [DOI] [PubMed] [Google Scholar]
- Rumbach L, Barth P, et al. Hemidystonia consequent upon ipsilateral vertebral artery occlusion and cerebellar infarction. Mov Disord. 1995;10(4):522–525. doi: 10.1002/mds.870100424. [DOI] [PubMed] [Google Scholar]
- Sadnicka A, Hoffland BS, et al. The cerebellum in dystonia -help or hindrance? Clin Neurophysiol. 2012;123(1):65–70. doi: 10.1016/j.clinph.2011.04.027. [DOI] [PubMed] [Google Scholar]
- Sethi KD, Jankovic J. Dystonia in spinocerebellar ataxia type 6. Mov Disord. 2002;17:150–153. doi: 10.1002/mds.1252. [DOI] [PubMed] [Google Scholar]
- Shibata T, Hirashima Y, et al. Stereotactic Voa-Vop complex thalamotomy for writer's cramp. Eur Neurol. 2005;53(1):38–39. doi: 10.1159/000084262. [DOI] [PubMed] [Google Scholar]
- Shirley TL, Rao LM, et al. Paroxysmal dyskinesias in mice. Mov Disord. 2008;23(2):259–264. doi: 10.1002/mds.21829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spacey SD, Materek LA, et al. Two novel CACNA1A gene mutations associated with episodic ataxia type 2 and interictal dystonia. Arch Neurol. 2005;62(2):314–316. doi: 10.1001/archneur.62.2.314. [DOI] [PubMed] [Google Scholar]
- Standaert DG. Update on the pathology of dystonia. Neurobiol Dis. 2011;42(2):148–151. doi: 10.1016/j.nbd.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taira T, Hori T. Stereotactic ventrooralis thalamotomy for task-specific focal hand dystonia (writer's cramp). Stereotact Funct Neurosurg. 2003;80(1-4):88–91. doi: 10.1159/000075165. [DOI] [PubMed] [Google Scholar]
- Tokuda S, Kuramoto T, et al. The ataxic groggy rat has a missense mutation in the P/Q-type voltage-gated Ca2+ channel alpha1A subunit gene and exhibits absence seizures. Brain Res. 2007;1133(1):168–177. doi: 10.1016/j.brainres.2006.10.086. [DOI] [PubMed] [Google Scholar]
- Tsuji S, Meier H. Evidence for allelism of leaner and tottering in the mouse. Genet Res. 1971;17:83–88. doi: 10.1017/s0016672300012040. [DOI] [PubMed] [Google Scholar]
- Usmani N, Bedi GS, et al. Late onset of cervical dystonia in a 39-year-old patient following cerebellar hemorrhage. J Neurol. 2011;258(1):149–151. doi: 10.1007/s00415-010-5685-2. [DOI] [PubMed] [Google Scholar]
- van den Maagdenberg AM, Pietrobon D, et al. A Cacna1a knockin migraine mouse model with increased susceptibility to cortical spreading depression. Neuron. 2004;41(5):701–710. doi: 10.1016/s0896-6273(04)00085-6. [DOI] [PubMed] [Google Scholar]
- van Gaalen J, Giunti P, et al. Movement disorders in spinocerebellar ataxias. Mov Disord. 2011;26(5):792–800. doi: 10.1002/mds.23584. [DOI] [PubMed] [Google Scholar]
- Waln O, LeDoux MS. Delayed-onset oromandibular dystonia after a cerebellar hemorrhagic stroke. Parkinsonism Relat Disord. 2010;16(9):623–625. doi: 10.1016/j.parkreldis.2010.07.010. [DOI] [PubMed] [Google Scholar]
- Walter JT, Alvina K, et al. Decreases in the precision of Purkinje cell pacemaking cause cerebellar dysfunction and ataxia. Nat Neurosci. 2006;9(3):389–397. doi: 10.1038/nn1648. [DOI] [PubMed] [Google Scholar]
- Wilder-Smith E, Tan EK, et al. Spinocerebellar ataxia type 3 presenting as an L DOPA responsive dystonia phenotype in a Chinese family. J Neurol Sci. 2003;213(1-2):25–28. doi: 10.1016/s0022-510x(03)00129-1. [DOI] [PubMed] [Google Scholar]
- Yen CM, Sheehan J, et al. Successful treatment of cervical dystonia induced by basal ganglion venous angioma with gamma knife thalamotomy. J Clin Neurosci. 2012;19(3):470–471. doi: 10.1016/j.jocn.2011.04.036. [DOI] [PubMed] [Google Scholar]
- Yoon CH. Disturbances in the developmental pathways leading to a neurological disorder of genetic origin, “leaner”, in mice. Dev Biol. 1969;20:158–181. doi: 10.1016/0012-1606(69)90011-6. [DOI] [PubMed] [Google Scholar]
- Zadro I, Brinar VV, et al. Cervical dystonia due to cerebellar stroke. Mov Disord. 2008;23(6):919–920. doi: 10.1002/mds.21981. [DOI] [PubMed] [Google Scholar]
- Zhuchenko O, Bailey J, et al. Autosomal dominant cerebellar ataxia (SCA6) associated with small polyglutamine expansions in the alpha 1A-voltage-dependent calcium channel. Nat Genet. 1997;15(1):62–69. doi: 10.1038/ng0197-62. [DOI] [PubMed] [Google Scholar]