Abstract
Rationale
Myeloid-derived C/EBP-homologous protein (CHOP), an effector of the endoplasmic reticulum (ER) stress-induced Unfolded Protein Response, promotes macrophage apoptosis in advanced atherosclerosis, but the role of CHOP in vascular smooth muscle cells (VSMCs) in atherosclerosis is not known.
Objective
To investigate the role of CHOP in SM22α+ VSMCs in atherosclerosis.
Methods and Results
Chopfl/fl mice were generated and crossed into the Apoe−/− and SM22α-CreKI+ backgrounds. SM22α-CreKI causes deletion of floxed genes in adult SMCs. After 12 wks of Western-type diet feeding, the content of α-actin-positive cells in aortic root lesions was decreased in Chopfl/flSM22α-CreKI+Apoe−/− vs. control Chopfl/flApoe−/− mice, and aortic explant-derived VSMCs from the VSMC-CHOP-deficient mice displayed reduced proliferation. Krüppel-like factor 4 (KLF4), a key suppressor of VSMC proliferation, was increased in lesions and aortic VSMCs from Chopfl/flSM22α-CreKI+Apoe−/− mice, and silencing Klf4 in CHOP-deficient VSMCs restored proliferation. CHOP deficiency in aortic VSMCs increased KLF4 through two mechanisms mediated by the ER stress effector ATF4: transcriptional induction of Klf4 mRNA and decreased proteasomal degradation of KLF4 protein.
Conclusions
These findings in SM22α-CHOP-deficient mice imply that CHOP expression in SM22α+ VSMCs promotes cell proliferation by down-regulating KLF4. The mechanisms involve newly discovered roles of CHOP in the transcriptional and post-translational regulation of KLF4.
Keywords: Atherosclerosis, vascular smooth muscle cells, C/EBP-homologous protein, Krüppel-like factor 4, activating transcription factor 4, cell signaling, animal model cardiovascular disease, ER stress, unfolded protein response
INTRODUCTION
The inflammatory and vascular cells that populate atherosclerotic lesions are exposed to chronic endoplasmic reticulum (ER) stress, leading to persistent activation of the Unfolded Protein Response (UPR).1–3 A key UPR pathway involves activation of protein kinase RNA-like ER kinase (PERK), which phosphorylates eukaryotic initiation factor 2α (eIF2α) and thereby suppresses protein translation. However, activating transcription factor 4 (ATF4) escapes this translational inhibition and is preferentially synthesized when the PERK-eIF2α pathway is activated.4 ATF4 induces its transcriptional target C/EBP homologous protein (CHOP/Ddit3), and if CHOP expression is chronically elevated due to prolonged ER stress, apoptosis can ensue.5 CHOP also induces the phosphatase co-activator GADD34, which helps mediate the feedback dephosphorylation of eIF2α and suppression of ATF4.6 Recent causation studies using holo-CHOP knockout mice and transplantation with CHOP-deficient-bone marrow in mouse models of atherosclerosis strongly support a key role for CHOP in macrophage apoptosis and plaque necrosis in advanced atherosclerotic lesions.7–9 These findings are consistent with studies showing strongly positive correlations among CHOP expression, lesional cell death, and clinical plaque stage in human coronary artery and carotid lesions.2,10 In addition, a reverse bone marrow transplantation study in atherosclerosis-prone mice suggested that CHOP in non-bone marrow-derived lesional cells also promotes atherosclerosis.9 However, neither the identity of the non-myeloid-derived cell type(s) in which CHOP plays a role nor the mechanisms or atherosclerosis-related consequences have been elucidated. In this context, we sought to determine the role of CHOP in vascular smooth muscle cells (VSMCs) in atherosclerosis by using a new mouse model of VSMC-specific CHOP deficiency.
METHODS
An expanded Methods section, including description of statistical analyses, is available in the Online Data Supplement.
Briefly, Chop/Ddit3 floxed mice were generated as described in Results and the Online Data Supplement. The mice were then crossed onto Apoe−/− and SM22α-CreKI+ mice. All mice were on the C57BL/6J background (the strain of generation for the floxed mice and at least 10 backcrosses for the others). For atherosclerosis studies, the mice were placed on a Western diet (WD) for 12 wks. For ex-vivo cell culture studies, VSMCs were cultured from aortic explants from the mice.
RESULTS
VSMC-specific CHOP deficiency reduces the content of α-Actin-positive cells in Apoe−/− atherosclerotic lesions
Chopfl/fl mice were generated as depicted in the scheme in Online Figure IA and the Online Figure IB–D, with details provided in the Online Supplement. The mice were bred with Apoe−/− mice to generate Chopfl/flApoe−/− mice, which were further bred with SM22α-CreKI+ mice to generate VSMC-specific Chop-deficient mice (Chopfl/flSM22α-CreKI+Apoe−/−) (Online Figure IIA). Note that SM22α-CreKI+ deletes floxed genes in adult SMCs and not embryonic SMCs, thus increasing cell-type specificity.11 The expression of CHOP was evaluated in SMCs, endothelial cells (ECs), and macrophages from Chopfl/flApoe−/− and Chopfl/flSM22α-CreKI+Apoe−/− mice before and after treatment with the UPR inducer tunicamycin. Tunicamycin increased CHOP expression in all three cell types from control mice but only in ECs and macrophages from Chopfl/flSM22α-CreKI+Apoe−/− mice (Online Figure IIB). Thus, CHOP is specifically deleted in SMCs among these three atherosclerosis-relevant cell types.
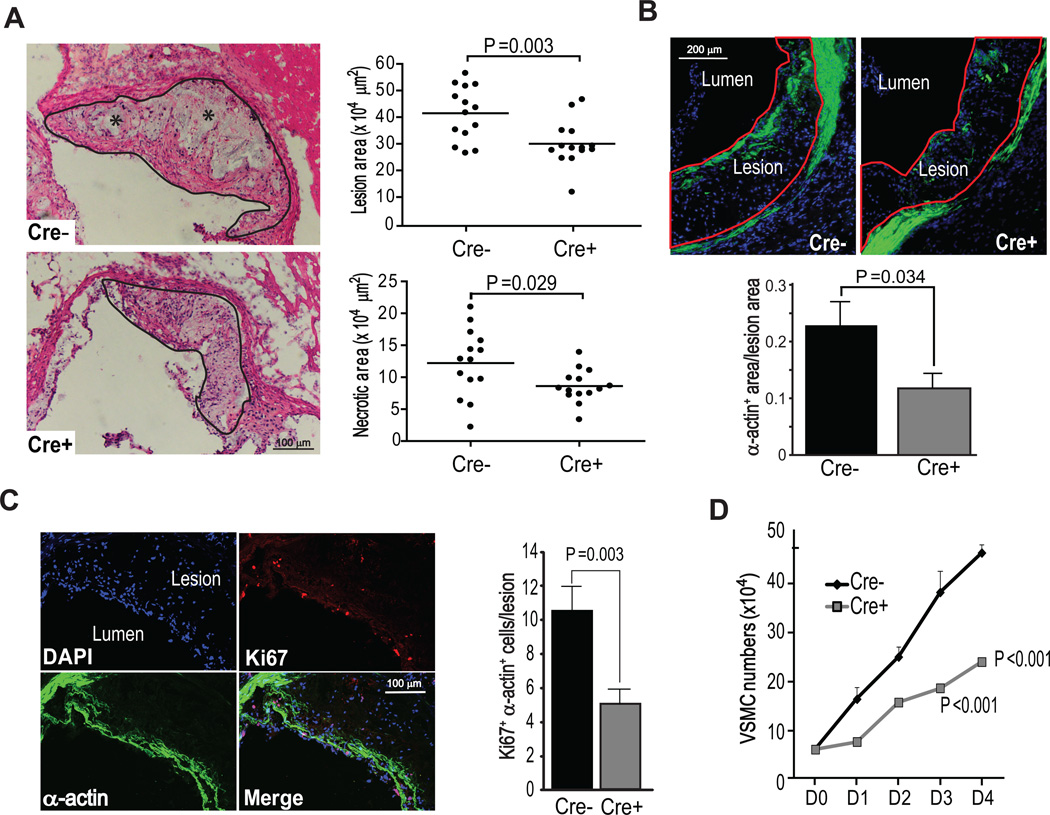
After 12 wks of WD feeding, Chopfl/flApoe−/− and Chopfl/flSM22α-CreKI+Apoe−/− mice did not differ significantly in terms of body weight, blood glucose, total plasma cholesterol, HDL cholesterol, plasma triglycerides, lipoprotein profile, or blood pressure. (Online Figure III). Thus, atherosclerotic lesions could be evaluated in the absence of major changes in systemic metabolism. Quantitative analysis of aortic root lesions revealed a ~30% reduction in lesion area (P=0.003) and a similar decrease in necrotic area (P=0.029) in Chopfl/flSM22α-CreKI+Apoe−/− vs. Chopfl/flApoe−/− mice, consistent with decreased plaque progression (Figure 1A).
Figure 1. CHOP deletion in VSMCs in Apoe−/− mice reduces the content of α-actin-positive cells in atherosclerotic lesions and suppresses VSMC proliferation.
A, Representative images and quantitative analyses of aortic root lesion area and necrotic area in Chopfl/flApoe−/− and Chopfl/flSM22α-CreKI+Apoe−/− mice (n=14). The lesions are delineated with a black line, and necrotic areas are shown by asterisks. B, Representative images of α-actin staining (green) and quantification of proportion of the α-actin-positive area in Chopfl/flApoe−/− and Chopfl/flSM22α-CreKI+Apoe−/− aortic root lesions (n=8). The lesions are delineated with a red line. C, Representative Z series projection images of Ki67 (red) and α-actin staining (green) in Chopfl/flApoe−/− lesions and quantification of Ki67/α-actin-positive VSMCs (n=8). The upper left image shows the DAPI+ cells in this projection. Scale bar, 50 µm. D, Proliferation curve of Chopfl/flApoe−/− and Chopfl/flSM22α-CreKI+Apoe−/− VSMCs cultured in 10% FBS medium 0–4 days (D0–D4) after serum-starvation (n=3).
Both lesional cell content and extracellular matrix contribute to lesion size. To determine if a decrease in one or more of these parameters contributed to the reduced lesion size in Chopfl/flSM22α-CreKI+Apoe−/− mice, aortic root sections were stained with DAPI (nuclei) to measure total cell content; immunostained for smooth muscle α-actin, F4/80, and CD11c to assess lesional content of α-actin+ VSMCs, macrophages, dendritic-like cells, respectively; and stained with Masson trichrome to assess collagen. The number of cells positive for α-actin, F4/80, and CD11c and the collagen content were all significantly decreased in Chopfl/flSM22α-CreKI+Apoe−/− lesions (Online Figure IVA–C), consistent with an overall decrease in lesion progression. However, only the number of lesional α-actin+ VSMCs was significantly lower in the VSMC-CHOP-deficient cohort when corrected for the decrease in total lesion area, whereas the decreases in F4/80+ macrophages, CD11c+ cells, extracellular matrix, and plaque necrosis were proportional to the decrease in overall lesion area (Figure 1B). One possible explanation for this finding is a hierarchical relationship in which SMC-CHOP deletion, by decreasing lesional SMCs, leads to a secondary decrease in overall lesion progression. Lesion size and SMC content were also lower in aortic arch lesions of Chopfl/flSM22α-CreKI+Apoe−/− mice compared with Chopfl/flApoe−/− mice, and en face lesion area in the descending aorta of the SMC-CHOP deficient mice was lower as well (Online Figure VA–D). In contrast, the content of aortic wall (media) SMCs in non-atherosclerotic chow-fed Chopfl/flSM22α and Chopfl/flSM22α-CreKI+ mice was similar between the two groups of mice (Online Figure VE).
VSMC-CHOP deficiency decreases the proliferation of aortic explant-derived VSMCs
The lower content of α−actin+ VSMCs in the lesions of Chopfl/flSM22α-CreKI+Apoe−/− mice could be due to increased VSMC death. However, very few apoptotic VSMCs were detected in the lesions of either group, and apoptosis did not differ between the two groups of mice and showed no correlation with lesional VSMC count (Online Figure VIA–C). We therefore explored the possibility that CHOP deficiency decreases VSMC proliferation and found that the lesions of Chopfl/flSM22α-CreKI+Apoe−/− mice showed less Ki67+ VSMCs (Figure 1C), which is a marker of proliferating cells. Because Ki67 is not a dynamic marker of cell proliferation, we compared the growth curves of aortic explant-derived VSMCs from the two groups of mice using a standard protocol, namely, incubation of serum-starved VSMCs in medium containing 10% FBS. The growth rate of CHOP-deficient VSMCs was significantly lower compared with that of control VSMCs (Figure 1D). These combined data suggest that at least one mechanism for the decrease in VSMCs in the lesions of Chopfl/flSM22α-CreKI+Apoe−/− mice is decreased proliferation.
Decreased proliferation in CHOP-deficient VSMCs is causally associated with the up-regulation of Krüppel-like factor 4 (KLF4)
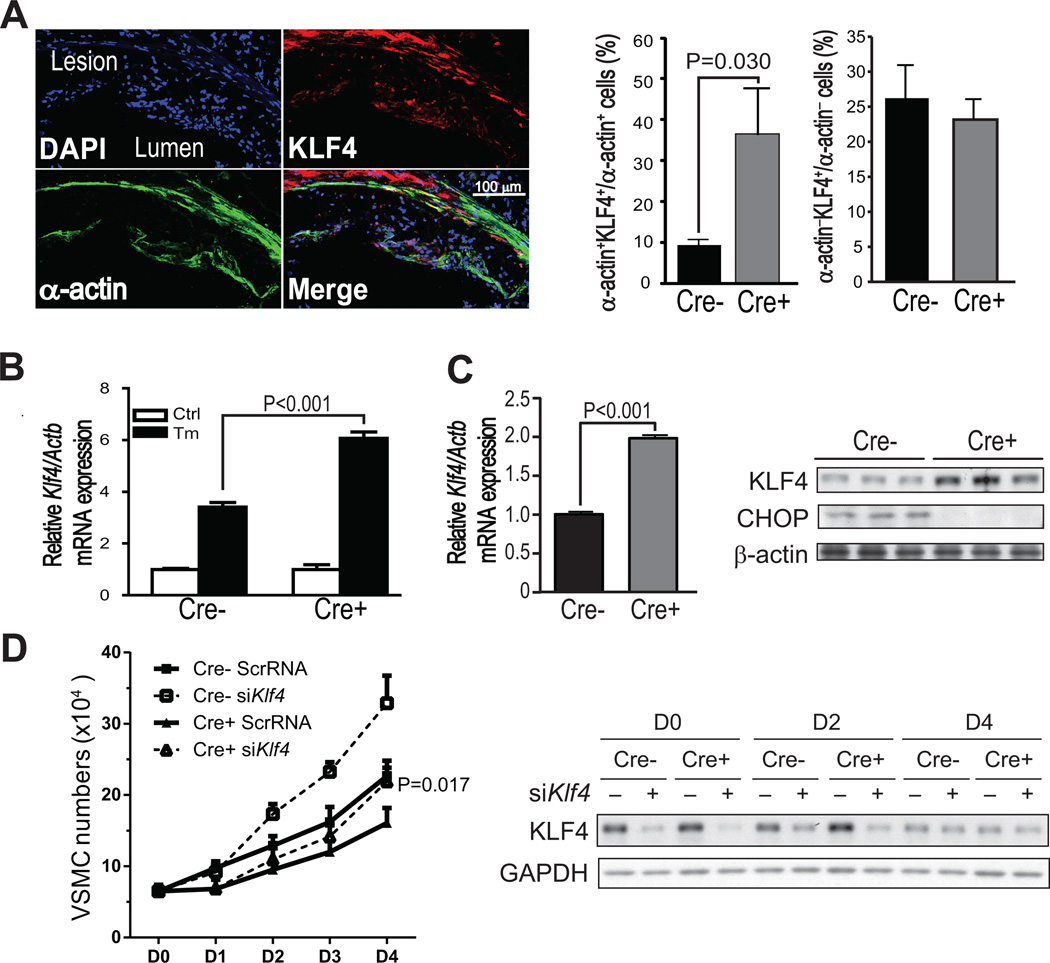
We interrogated a microarray dataset derived from tunicamycin-treated wild-type and CHOP-deficient murine embryonic fibroblasts (MEFs) for mRNAs that might be linked to VSMC proliferation.12 One of the genes increased in CHOP-deficient MEFs was Klf4. KLF4 inhibits VSMC proliferation, and mice lacking KLF4 exhibit enhanced neointimal formation in response to vascular injury.13 To explore the relevance of these findings to the atherosclerosis data described here, we assayed KLF4 and α-actin in lesions from Chopfl/flApoe−/− and Chopfl/flSM22α-CreKI+Apoe−/− mice using immunofluorescence microscopy. KLF4 is expressed in multiple cell types in atherosclerotic lesions, and so the goal was to compare KLF4 expression in α-actin+ VSMCs vs. other cells in the lesions of the two groups of mice. We found that the lesions of Chopfl/flSM22α-CreKI+Apoe−/− mice had an increased content of KLF4-positive cells in the α-actin+ cell population, whereas the number of KLF4-positive cells in the population of α-actin− cells was similar between the two groups (Figure 2A). We then asked whether ER-stressed SMCs in aortic explants have higher levels of KLF4 when CHOP is absent. We found that tunicamycin induced Klf4 mRNA in both Chopfl/flSM22α-CreKI+Apoe−/− and Chopfl/flApoe−/− aortic VSMCs, but induction was higher in the CHOP-deficient cells (Figure 2B). These data suggest that ER stress induces Klf4 expression in VSMCs but that CHOP "fine-tunes" this expression in a suppressive manner. Klf4 mRNA and KLF4 protein were also elevated in CHOP-deficient VSMCs under the conditions of the proliferation assay, which involves serum repletion after a period of serum starvation (Figure 2C). Most importantly, CHOP-deficient VSMCs subjected to siRNA-mediated silencing of KLF4 no longer showed a defect in proliferation after 4 days in culture (Figure 2D, left). Note that KL4 expression was higher in CHOP-deficient (Cre+) SMCs at day 0 and day 2, while by day 4 KL4 expression was lower in all groups and not different between the Cre− and Cre+ group (Figure 2D, right). These data suggest that the effect of KLF4 silencing on cell proliferation at day 4 was a delayed effect from events occurring earlier in the time course. These combined data suggest that at least one mechanism for the decrease in proliferation in CHOP-deficient VSMCs is an increase in KLF4.
Figure 2. KLF4 is increased in α-actin-positive cells in Chopfl/flSM22α-CreKI+Apoe−/− lesions and is a mechanism of decreased VSMC proliferation.
A, Representative Z series projection images of KLF4 staining (red) and α-actin staining (green) in Chopfl/flSM22α-CreKI+Apoe−/− lesions and quantification of KLF4+;α-actin+ cells or KLF4+;α-actin− cells in Chopfl/flApoe−/− and Chopfl/flSM22α-CreKI+Apoe−/− lesions (n=8). The upper left image shows the DAPI+ cells in this projection. Scale bar, 100 µm. B, Klf4 mRNA relative to Actb in Chopfl/flApoe−/− and Chopfl/flSM22α-CreKI+Apoe−/− VSMCs treated with 2.5 µg/ml tunicamycin for 12 h (n=3). C, Klf4 mRNA relative to Actb (n=6, graph) and immunoblot of KLF4 and CHOP protein (n=3) in Chopfl/flApoe−/− and Chopfl/flSM22α-CreKI+Apoe−/− VSMCs cultured in 10% FBS medium for 24 hours after serum starvation. β-actin was included as loading control. D, Left panel: Proliferation curve of scrambled (Scr) or Klf4 siRNA-transfected Chopfl/flApoe−/− and Chopfl/flSM22α-CreKI+Apoe−/− VSMCs cultured in 10% FBS medium 0–4 days after starvation (n=3; at day 4, the Cre+ siKlf4 value is different from the Cre+ ScrRNA value at P=0.017). Right panel: KLF4 protein expression in Scr or Klf4 siRNA-transfected Chopfl/flApoe−/− and Chopfl/flSM22α-CreKI+Apoe−/− VSMCs.
ATF4 is elevated in CHOP-deficient VSMCs and mediates increased KLF4 expression
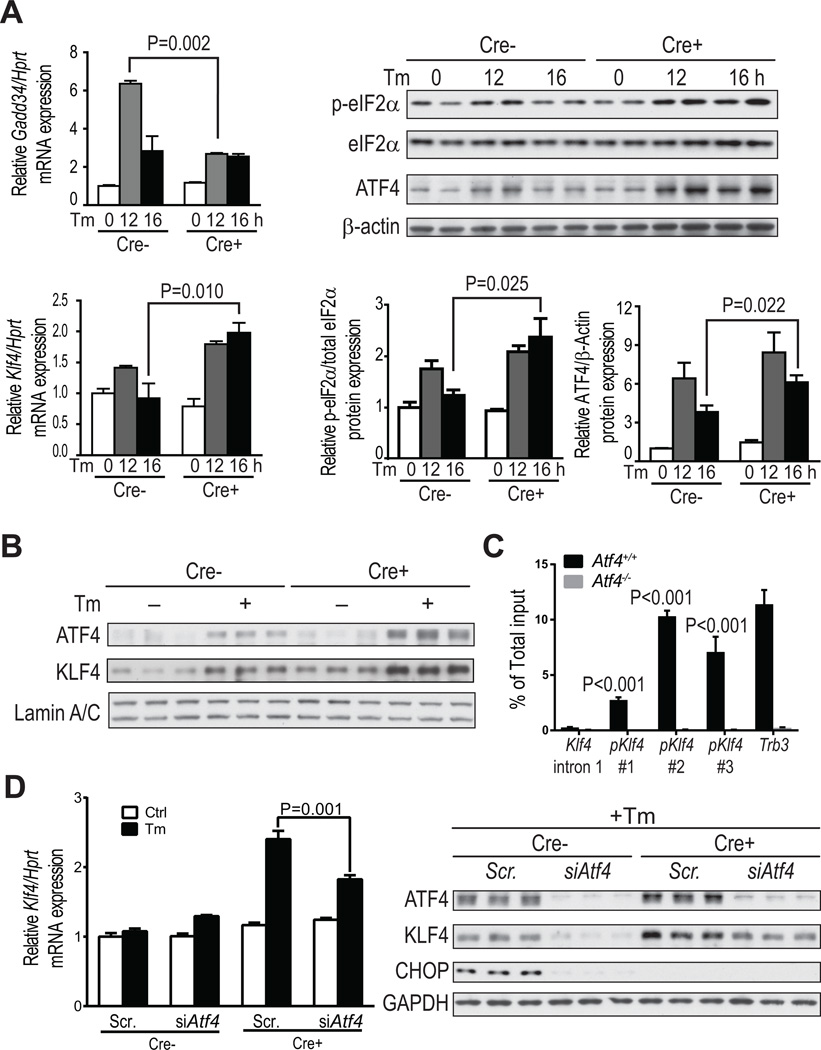
To study how CHOP suppresses Klf4 expression, we analyzed MEF ChIP-sequencing data and found that ATF4 but not CHOP directly associates with the Klf4 promoter region.14 We therefore reasoned that CHOP might suppress Klf4 by down-regulating ATF4, which could occur through the CHOP-GADD34 negative-feedback pathway that decreases p-eIF2α and thereby decreases ATF4 translation.6 Consistent with the hypothesis, CHOP deficiency was associated with a decrease in Gadd34 mRNA after 12 h of tunicamycin treatment, and after 16 h of treatment, there were increases in p-eIF2α protein, ATF4 protein, and Klf4 mRNA (Figure 3A). Moreover, the levels of nuclear ATF4 and KLF4 in VSMCs were also elevated by CHOP deficiency (Figure 3B). To link to the atherosclerosis data, we analyzed nuclear ATF4-KLF4 co-expression and found that SMC-CHOP deficiency was associated with a higher percentage of total lesional cells that co-expressed nuclear ATF4 and KLF4 and a higher percentage that co-expressed nuclear ATF4 and α-actin (Online Figure VII).
Figure 3. ATF4 enhances KLF4 expression in ER-stressed Chopfl/flSM22α-CreKI+Apoe−/− VSMCs.
A, Chopfl/flApoe−/− and Chopfl/flSM22α-CreKI+Apoe−/− VSMCs were treated with 2.5 µg/ml tunicamycin (Tm) for 0, 12 and 16 h and then assayed for Gadd34 mRNA relative to Hprt, phosphorylated eIF2α relative to total eIF2α protein, ATF4 protein relative to β-actin (n=4), and Klf4 mRNA relative to Hprt (n=6). B, Chopfl/flSM22α-CreKI+Apoe−/− VSMCs were treated with or without Tm for 16 h and then assayed for nuclear ATF4 and KLF4 protein relative to lamin A/C (n=3). C, Atf4+/+ and Atf4−/− MEFs were subjected to ATF4 ChIP after treatment with Tm for 8 h, followed by RT-qPCR of the immunoprecipitates using three different primers targeting Klf4 promoter (#1–3). Primers targeting Klf4 intron 1 and the Trb3 promoter were used as negative and positive controls, respectively (n=3, **P<0.01 vs intron 1). D, Scr- or Atf4 siRNA-transfected Chopfl/flApoe−/− and Chopfl/flSM22α-CreKI+Apoe−/− VSMCs were treated with Tm for 16 h and then assayed for Klf4 mRNA and KLF4 protein (n=3).
We next verified by chromatin-immunoprecipitation (ChIP) analysis that ATF4 directly associates with the Klf4 promoter. Genomic fragments containing the ATF4-binding region in the Klf4 promoter were significantly enriched in tunicamycin-treated Atf4+/+ vs. Atf4−/− MEFs (Figure 3C), consistent with the conclusion that ATF4 regulates Klf4 mRNA transcription. Most importantly, silencing ATF4 significantly reduced both Klf4 mRNA and KLF4 protein in tunicamycin-treated CHOP-deficient VSMCs (Figure 3D). The protein data show that the level of KLF4 in siAtf4-treated CHOP-deficient VSMCs was restored to the level in scrambled RNA-treated WT SMCs. These combined data support the hypothesis that an increase in ATF4 in CHOP-deficient VSMCs mediates an increase in KLF4. The reason why ATF4 is higher in the face of CHOP deficiency may be due to the increase in p-eIF2α, which in turn could be explained by the decrease in CHOP-induced GADD34.
CHOP deficiency also decreases proteasomal degradation of KLF4 under ER stress conditions
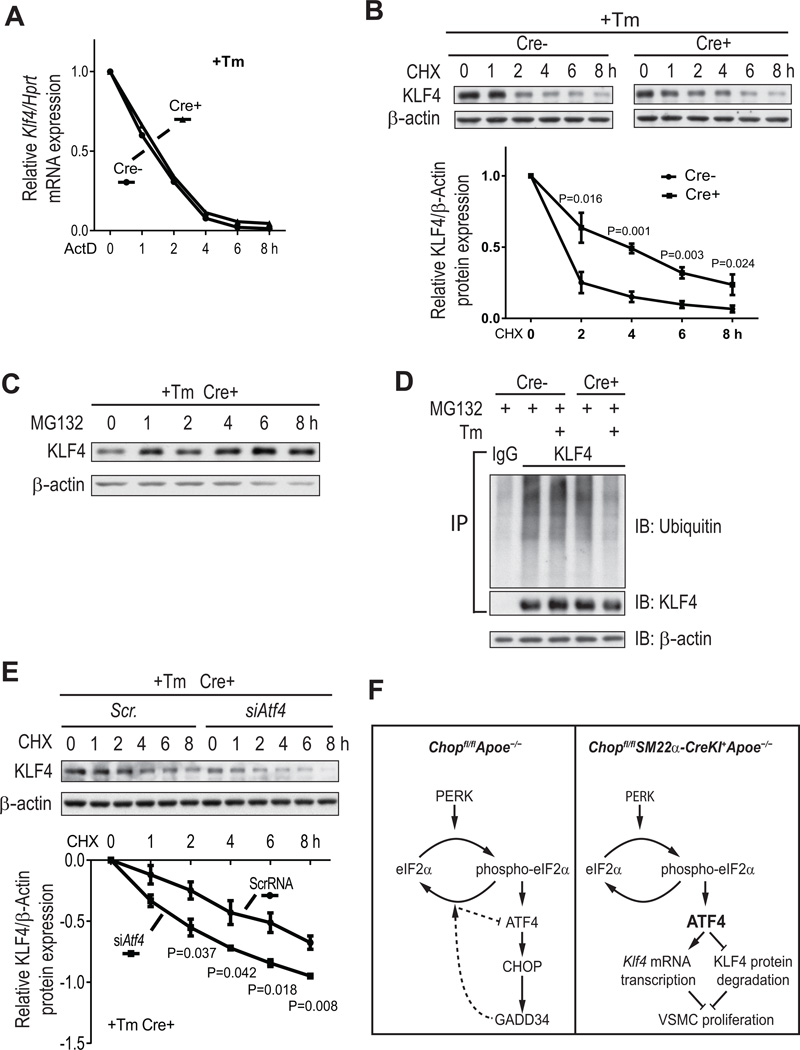
In theory, CHOP deficiency could raise KLF4 by also increasing the stability of Klf4 mRNA and/or KLF4 protein. The former possibility does not appear to be the case, as Klf4 mRNA expression following actinomycin D (ActD) treatment was not affected by CHOP deficiency in VSMCs (Figure 4A). However, KLF4 protein turnover was more rapid in WT vs. CHOP-deficient VSMCs after treatment with cycloheximide (CHX), an inhibitor of protein biosynthesis (Figure 4B). KLF4 protein is targeted for degradation by the proteasome,15 which we confirmed by showing that MG132, a proteasome inhibitor, inhibited KLF4 protein degradation in VSMCs (Figure 4C). Moreover, less ubiquitin was associated with KLF4 in tunicamycin-treated CHOP-deficient VSMCs (Figure 4D). In contrast to the situation with KLF4, the turnover of two other VSMC proteins, HIF1α and NFR2, was not altered by CHOP-deficiency, although both proteins are also targeted for degradation by the proteasome (Online Figure VIII). Most importantly, silencing ATF4 markedly increased KLF4 protein turnover in tunicamycin-treated CHOP-deficient VSMCs (Figure 4E). These data suggest that the increase in ATF4 in CHOP-deficient VSMCs raises KLF4 by two distinct mechanisms: transcriptional induction via ATF4 binding to the Klf4 promoter; and protein stabilization via suppression of KLF4 proteasomal degradation. The molecular mechanism of the latter process remains to be determined.
Figure 4. ATF4 prevents proteasomal degradation of KLF4 in VSMCs under ER stress conditions.
A, Chopfl/flApoe−/− and Chopfl/flSM22α-CreKI+Apoe−/− VSMCs were treated with 2.5 µg/ml Tm for 16 h and then with 5 µM actinomycin D (ActD) for 0, 1, 2, 4, 6, and 8 h, followed by assay of Klf4 mRNA (n=2). B, Similar to A, but the cells were treated with 10 µg/ml cycloheximide (CHX) instead of ActD and assayed for KLF4 protein. A representative immunoblot and the KLF4 degradation curve for 3 separate experiments are shown. C, Similar to B, but the cells were treated with 10 µM MG132 instead of CHX. D, VSMCs from the 2 groups of mice were treated in the absence or presence of Tm for 16 h, and then all the cells were treated for 6 h with MG132. Cell extracts were subjected to immunoprecipitation with anti-KLF4 or control IgG and then immunoblotted for ubiquitin or KLF4 (input). Also shown is a β-actin immunoblot for whole extract that was used for the KLF4 IP. E, As in B, but all the cells were Cre+, and they were transfected with either Scr- or Atf4 siRNA. A representative immunoblot and the KLF4 degradation curve for 3 separate experiments are shown. F, Working model of how CHOP deficiency increases KLF4 expression and thereby suppresses proliferation in VSMCs. Left, Under ER stress, PERK activation induces eIF2α phosphorylation and translational activation of ATF4. In control Chopfl/flApoe−/− VSMCs (left), ATF4 activation induces CHOP expression, which in turn induces Gadd34. GADD34 dephosphorylates eIF2α, leading to decreased ATF4 translation (dotted lines). Right, the translational activation of ATF4 is preserved in Chopfl/flSM22α-CreKI+Apoe−/− VSMCs due to lack of the GADD34 feedback pathway. Persistent ATF4 expression induces KLF4 by increasing its mRNA expression and reducing its proteasomal degradation, leading to inhibition of VSMC proliferation.
DISCUSSION
Using a newly created mouse model of cell-specific CHOP deletion, the data in this report show that SM22α+ VSMC-targeted CHOP deficiency leads to a decrease in α-actin+ VSMC content in atherosclerotic lesions in Apoe−/− mice. The proposed mechanism involves, in part, a fine-tuning module based on the suppression of ATF4 by the CHOP-GADD34-eIF2α dephosphorylation pathway and the increase in KLF4 by ATF4 (Figure 4F). Interestingly, there are examples of CHOP-ATF4 heterodimer affecting gene transcription,16 and further work will be required to determine whether this complex plays a role in Klf4 regulation in ER-stressed SMCs. In addition, ATF4 suppresses the proteasomal degradation of KLF4 by a mechanism yet to be elucidated.
Decreased lesional SMCs in SMC-CHOP-deficient mice was associated with features of decreased lesion progression, namely, lower numbers of inflammatory cells, plaque necrosis, and collagen. How a decrease in intimal SMCs might lead to lower inflammatory cells remains to be determined. Other studies have suggested that lesional VSMCs can regulate lesion development by affecting lipid content and by retaining and promoting the survival of lesional inflammatory cells.17 Moreover, a recent study provided evidence that VSMCs can be transformed into macrophage-like cells in atherosclerotic lesions,18 but whether or not VSMC-CHOP affects this transformation process remains to be determined. Nonetheless, the data herein suggest that uncontrolled elevation of VSMC content in lesions can contribute lesion progression, while VSMC-CHOP deficiency may suppress this process by inhibiting VSMC proliferation.
The impact of the decrease in collagen content in the SMC-CHOP-deficient lesions is an interesting topic. On the one hand, this decrease could lead to thinner fibrous caps, which is a sign of advanced plaque progression.19 However, fibrous cap thickness was similar in the aortic root lesions of the two groups of mice (data not shown). On the other hand, a decrease in lesional matrix might lessen the pathological process of "arterial stiffening".20 Along these lines, it is interesting to note that silencing XBP1, a UPR effector that can have opposing actions to CHOP in the setting of prolonged ER stress, enhanced VSMC proliferation in vitro and promoted neointima formation in a murine wire injury model.21 Pending future studies to explore these and other issues, the findings in this report, together with previous studies showing the role of macrophage CHOP in advanced lesional apoptosis and plaque necrosis,7–9 help form a more complete picture of the cell-specific roles of the UPR and its effector CHOP in atherosclerosis.
Supplementary Material
Novelty and Significance.
What Is Known?
Smooth muscle cells (SMCs) in atherosclerotic lesions are the major source of collagen and elastin that forms a protective fibrous cap, but they may also contribute to inflammation and plaque progression.
A particular signaling molecule called Krϋppel-like factor 4 (KLF4) acts as a suppressor of SMC proliferation in atherosclerotic lesions.
Cells in human and murine atherosclerotic lesions are known to undergo chronic endoplasmic reticulum (ER) stress, which is probably because of their exposure to certain types of lipids that affect ER function and to factors that promote intracellular oxidative stress.
What New Information Does This Article Contribute?
Using a new mouse model in which the ER stress effector CHOP was specifically deleted in SMCs, the study shows for the first time that CHOP regulates SMC proliferation.
Deletion of CHOP in SMCs in an atherosclerosis-prone mouse model resulted in atherosclerotic lesions that had less SMCs and less plaque progression.
CHOP deficiency lowers SMC proliferation through feedback induction of the ER stress effector ATF4, which then transcriptionally induces KLF4 as well as decreases its degradation.
Cells in both human and murine atherosclerotic lesions undergo chronic ER stress, but the effect of ER stress in lesional SMCs was not known. We deleted the ER stress effector CHOP in SMCs in a mouse model of atherosclerosis and found a decrease in both lesional SMC content and overall lesion progression. CHOP deletion suppressed SMC proliferation through upregulation of a KLF4. The mechanism involved a feedback increase in ATF4, which both enhanced the transcription of Klf4 mRNA and stabilized KLF4 protein. These findings reveal a role and novel mechanism of CHOP in the progression of atherosclerotic lesions.
ACKNOWLEDGEMENTS
We thank Dr. Robert Clark, University of Michigan, for assistance in creating Chopfl/fl mice; Dr. Gary K. Owens, University of Virginia, for helpful discussions and for supplying the SM22α-CreKI mice (created by Zhang et al.11); and Dr. Michael S. Kilberg, University of Florida, for the ATF4 antibody used in the ChIP experiment.
SOURCES OF FUNDING
This work is supported by a postdoctoral fellowship from the Swedish Research Council to A.X.Z. and NIH grants HL75662 and HL57560 to I.T. and DK042394, DK088227, and HL052173 to R.J.K.
Nonstandard Abbreviations and Acronyms
- ATF4
activating transcription factor-4
- ChIP
chromatin immunoprecipitation
- CHOP
C/EBP-homologous protein
- eIF2α
eukaryotic initiation factor 2α
- ER
endoplasmic reticulum
- GADD34
growth arrest and DNA damage-inducible protein 34
- KLF4
Krüppel-like factor 4
- MEF
murine embryonic fibroblast
- PERK
protein kinase RNA-like ER kinase
- Tm
tunicamycin
- UPR
unfolded protein response
- VSMC
vascular smooth muscle cells.
Footnotes
DISCLOSURES
None
REFERENCES
- 1.Tabas I. The role of endoplasmic reticulum stress in the progression of atherosclerosis. Circ Res. 2010;107:839–850. doi: 10.1161/CIRCRESAHA.110.224766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Myoishi M, Hao H, Minamino T, Watanabe K, Nishihira K, Hatakeyama K, Asada Y, Okada K, Ishibashi-Ueda H, Gabbiani G, Bochaton-Piallat ML, Mochizuki N, Kitakaze M. Increased endoplasmic reticulum stress in atherosclerotic plaques associated with acute coronary syndrome. Circulation. 2007;116:1226–1233. doi: 10.1161/CIRCULATIONAHA.106.682054. [DOI] [PubMed] [Google Scholar]
- 3.Zhou J, Lhotak S, Hilditch BA, Austin RC. Activation of the unfolded protein response occurs at all stages of atherosclerotic lesion development in apolipoprotein E-deficient mice. Circulation. 2005;111:1814–1821. doi: 10.1161/01.CIR.0000160864.31351.C1. [DOI] [PubMed] [Google Scholar]
- 4.Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6:1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 5.Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13:184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Novoa I, Zeng H, Harding HP, Ron D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J Cell Biol. 2001;153:1011–1022. doi: 10.1083/jcb.153.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thorp E, Li G, Seimon TA, Kuriakose G, Ron D, Tabas I. Reduced apoptosis and plaque necrosis in advanced atherosclerotic lesions of Apoe−/− and Ldlr−/− mice lacking CHOP. Cell Metabolism. 2009;9:474–481. doi: 10.1016/j.cmet.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsukano H, Gotoh T, Endo M, Miyata K, Tazume H, Kadomatsu T, Yano M, Iwawaki T, Kohno K, Araki K, Mizuta H, Oike Y. The endoplasmic reticulum stress-C/EBP homologous protein pathway-mediated apoptosis in macrophages contributes to the instability of atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2010;30:1925–1932. doi: 10.1161/ATVBAHA.110.206094. [DOI] [PubMed] [Google Scholar]
- 9.Gao J, Ishigaki Y, Yamada T, Kondo K, Yamaguchi S, Imai J, Uno K, Hasegawa Y, Sawada S, Ishihara H, Oyadomari S, Mori M, Oka Y, Katagiri H. Involvement of endoplasmic stress protein C/EBP homologous protein in arteriosclerosis acceleration with augmented biological stress responses. Circulation. 2011;124:830–839. doi: 10.1161/CIRCULATIONAHA.110.014050. [DOI] [PubMed] [Google Scholar]
- 10.Dorweiler B, Grechowa I, Wallrath A, Vahl CF, Horke S. Activation of the proapoptotic unfolded protein response in plaques of the human carotid artery. Eur J Vasc Endovasc Surg. 2014;48:248–257. doi: 10.1016/j.ejvs.2014.06.038. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J, Zhong W, Cui T, Yang M, Hu X, Xu K, Xie C, Xue C, Gibbons GH, Liu C, Li L, Chen YE. Generation of an adult smooth muscle cell-targeted Cre recombinase mouse model. Arterioscler Thromb Vasc Biol. 2006;26:e23–e24. doi: 10.1161/01.ATV.0000202661.61837.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, Harding HP, Ron D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18:3066–3077. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshida T, Kaestner KH, Owens GK. Conditional deletion of Kruppel-like factor 4 delays downregulation of smooth muscle cell differentiation markers but accelerates neointimal formation following vascular injury. Circ Res. 2008;102:1548–1557. doi: 10.1161/CIRCRESAHA.108.176974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han J, Back SH, Hur J, Lin YH, Gildersleeve R, Shan J, Yuan CL, Krokowski D, Wang S, Hatzoglou M, Kilberg MS, Sartor MA, Kaufman RJ. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol. 2013;15:481–490. doi: 10.1038/ncb2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gamper AM, Qiao X, Kim J, Zhang L, DeSimone MC, Rathmell WK, Wan Y. Regulation of KLF4 turnover reveals an unexpected tissue-specific role of pVHL in tumorigenesis. Mol Cell. 2012;45:233–243. doi: 10.1016/j.molcel.2011.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Su N, Kilberg MS. C/EBP homology protein (CHOP) interacts with activating transcription factor 4 (ATF4) and negatively regulates the stress-dependent induction of the asparagine synthetase gene. J Biol Chem. 2008;283:35106–35117. doi: 10.1074/jbc.M806874200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doran AC, Meller N, McNamara CA. Role of smooth muscle cells in the initiation and early progression of atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:812–819. doi: 10.1161/ATVBAHA.107.159327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feil S, Fehrenbacher B, Lukowski R, Essmann F, Schulze-Osthoff K, Schaller M, Feil R. Transdifferentiation of vascular smooth muscle cells to macrophage-like cells during atherogenesis. Circ Res. 2014;115:662–667. doi: 10.1161/CIRCRESAHA.115.304634. [DOI] [PubMed] [Google Scholar]
- 19.Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circ Res. 2014;114:1852–1866. doi: 10.1161/CIRCRESAHA.114.302721. [DOI] [PubMed] [Google Scholar]
- 20.Kothapalli D, Liu SL, Bae YH, Monslow J, Xu T, Hawthorne EA, Byfield FJ, Castagnino P, Rao S, Rader DJ, Pure E, Phillips MC, Lund-Katz S, Janmey PA, Assoian RK. Cardiovascular protection by ApoE and ApoE-HDL linked to suppression of ECM gene expression and arterial stiffening. Cell Rep. 2012;2:1259–1271. doi: 10.1016/j.celrep.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishimura S, Furuhashi M, Mita T, Fuseya T, Watanabe Y, Hoshina K, Kokubu N, Inoue K, Yoshida H, Miura T. Reduction of endoplasmic reticulum stress inhibits neointima formation after vascular injury. Sci Rep. 2014;4:6943. doi: 10.1038/srep06943. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.