Abstract
Background
Menopause is one of the most critical periods of woman’s life. With reducing of ovarian estrogen; women are more prone to psychological and physical symptoms. The present study aimed to investigate the effect of melatonin on the climacteric symptoms.
Methods
The present double blind, placebo randomized controlled clinical trial was conducted on 240 menopausal women (40 - 60 years old) referring to the gynecology clinics of Shiraz University of Medical Sciences (January - November 2012). The participants were randomly divided into two groups through sortition. Demographic characteristics, Goldberg’s general health questionnaire (GHQ), Greene Climacteric Scale and level of Follicle Stimulating Hormone (FSH) were determined for both groups before the intervention. The intervention group received one 3mg melatonin tablet each night for 3 months and the control group received the placebo in the same period. Changes of climacteric symptoms and drug complications were measured 1, 2 and 3 months after the intervention
Results
We analyzed the data of 99 postmenopausal women in the intervention group and 101 postmenopausal women in the control group. In the melatonin group, the climacteric symptoms score decreased from 35.73+11.6 to 17.09+10.22 during the 3-month study period and regardless of time, a significant difference was observed between the two groups (P<0.001). In addition, a significant difference was found between the two groups regarding various dimensions of the climacteric symptoms over time (P<0.001). No significant difference was found regarding side effects between the two groups (P= 0.135).
Conclusion
The study findings showed that using melatonin improved the climacteric symptoms.
Keywords: Climacteric symptoms, Green scale, Melatonin, Menopausal women, RCT
Introduction
Menopause is defined as the permanent cessation of menstrual cycles and is physiologically associated with the decrease in the number of follicles, cessation of the production of ovarian estrogens and progestins, and lack of the ovary’s ability to respond to the pituitary hormones (1–3). This leads to gradual increase of FSH, which is the major sign of menopause (1, 3).
By the incidence of menopause, the women are susceptible to the symptoms of lack of estrogen, such as hot flashes, osteoporosis, cardiovascular diseases, and so on(4, 5). Hot flashes are normally accompanied by fatigue, anxiety, irritability, depression, and reduced quality of sleep.
In order to diminish these problems, common treatments, such as hormone replacement therapy (HRT), are utilized. This treatment leads to vaginal bleeding, nausea, vomiting, and breast cancer (1, 4, 5). Thus, such treatments have caused the physicians as well as the patients to face difficulties in decision-making (1, 6). Melatonin , as a food supplement, is nacetyle-5-methoxytryptamine which is produced by the pineal gland (7, 8). The rhythmic secretion of melatonin which has great mediating roles in regulating sleep, body temperature, and mental states (8, 9). A pluripotent molecule acts as the chronobiological hormone and as a cytoprotective mediator. Melatonin and its metabolites are important free radical scavengers that reduce deleterious oxidant activities and contribute to the regulation of the redox state of the cells (10).
Besides, melatonin has been identified as a strong antioxidant (11). Due to the increase of oxidative stresses during menopause, the use of antioxidants could reduce the side effects of this exposure (12). It is involved in the mechanisms that regulate the ovarian aging process and lead to the beginning of menopause (8, 9). To confirm this, the study by Toffol and et al. showed that serum concentration and duration of melatonin secretion levels in post-menopausal women were lower in comparison with primenopausal women (13). Due to the its reduction during menopause, it can be expected that some of the climacteric symptoms are caused by these fluctuations (8, 9) (14). Considering the multifaceted function of melatonin in the body(15), exogenous melatonin utilization might be effective in decreasing the climacteric symptoms (16–19). The joint use of melatonin and soy do not have any effects on the relief of climacteric symptoms (20). However, melatonin can improve psychological and mood status in postmenopausal women (21). Because of the menopause can disrupt the sense of well-being and be healthy and can cause emotional and social problems for women are considered as a public health problem in female population. So therapeutic interventions with the aim of prolonging the period that women use their maximum physical, mental and social activity is essential. The main research question in our trial was whether melatonin is helpful in relief of climacteric symptoms. In addition, this study was designed due to the limitations of the studies in this field and contradictory results of those studies.
Materials and Methods
Questionnaire/ sample selection
The study population included the postmenopausal women between 40 and 60 years old referring to the selected gynecology and obstetrics clinics of Shiraz University of Medical Sciences in January - November 2012 who complained about the menopausal symptoms. A total of 680 menopausal women participated in this study after a public call in the city. All of them were evaluated regarding the inclusion criteria and some women were withdrawn from the study. The study data were gathered through their demographic, laboratory and clinical characteristics, and questionnaires including Goldberg’s general health questionnaire (before intervention), Greene Climacteric Scale (as main outcome), and the drug complications (before intervention, 1, 2, and 3 months after intervention). Goldberg’s general health questionnaire was used in order to determine the women’ general health before being enrolled into the study. This questionnaire consists of 28 items in four dimensions of depression, anxiety, somatoform symptoms, and social dysfunction containing four options of quite less than usual=0, less than usual=1, as usual=2, and more than usual=3 based on Likert scoring system. Higher scores represent lower general health. Moreover, <23 and >24 scores were given to healthy individuals and those with suspected disorders, respectively (22). The reliability and validity of this questionnaire have been confirmed in various studies and the reliability coefficient of the whole questionnaire and sub-scales of depression, anxiety, somatoform symptoms, and social dysfunction were 0.96, 0.94, 0.90, 0.89, and 0.78, respectively. This questionnaire has been used in different studies in Iran (23–26). Greene Climacteric Scale consists of 21 symptoms in 4 areas. Items evaluating psychological symp toms, somatic, vasomotor, reduction of sexual desire (27). The scoring scale was 0=nothing, 1=mild, 2=average and 3=severe; total scores above 21 were considered to represent the existence of climacteric symptoms. The individuals were required to mark the symptoms they had experienced during the last month with regard to the severity of the symptoms (28–30). Reliability and validity have been evaluated in Moghassemi’s study (r=0.8) (31).
Inclusion and excluded criteria
Inclusion criteria: having general health based on the Goldberg’s questionnaire (<23 score), obtaining scores>21 in Greene scale, plasma FSH level>40 IU, not using vitamin supplements, soya or its products and HRT during the recent 3 months, not using sedative, antidepressants, hypnotic, and blood pressure lowering drugs, and no medical history. Excluded criteria: suffering from liver, kidney, and gastrointestinal diseases, not being able to continue using drugs for any reason, not being interested in continuing participation in the study, and showing any allergic reaction to the drug.
Randomization
For randomization in the present double-blind, placebo controlled clinical trial, numbers 1-240 was written on small slips of paper, allocation ratio was selected 1:1; each time by stirring the numbers, one of them was out of the sortation box, and was written in one of the two lists A and B, then was returned to the box. Therefore, each number had an equal chance for placement in groups. This process was continued until the 240 numbers were divided into two groups (120 participants in each group). During the study, specific intervention for each group was done based on the list. The researcher who performed the random allocation was aware of the type of intervention for each group and until the end of the intervention had no role in the study, she packed medications in the same pocket. The second researcher only delivered the drugs. Third researcher interviewed with the participants and was unaware of the type of the drugs and groups.
Procedure
During the study, 30 melatonin tablets and 30 placebo tablets were respectively given to the intervention and control group participants at the beginning of the study and at the end of each month; it was explained to them how to use the medication.
The intervention group received 3mg melatonin tablets prepared by Pourateb Company under the license of Nature Made Company, U.S. the other group of 120 participants received placebo tablets made of lactose, avicel, and magnesium stearate in the same form and color as the melatonin tablets. Each participant had to take one tablet every evening between 6-9 P.M. before sleeping. The participants were also required to return the tablets they had forgotten during the last month when receiving the new package. In addition, they were asked to sleep in completely dark rooms in order to produce endogenous melatonin. The study questionnaires were completed by the participants before and 1, 2, and 3 months after the intervention. In order to prevent sample loss during the intervention, the participants were called at the end of each week and followed up regarding how to use the medicine and the date of the next referral.
The participants and the researcher who was responsible for performing the interviews and physical as well as mental evaluations were unaware of the participants’ allocation to the study groups. In this study, we used two assistant researchers. The medications were given to the participants by the assistant researcher in based on codes A and B written on completely similar packages.
The medications were packed in completely similar packages and encoded by the other assistant researcher who was not involved in the interviews or filing process. In addition, the nature of the packages was unknown to the interviewer (to avoid the influence of her awareness on questioning and assessment) and the participants up to the end of data analysis. The researcher’s task was only completing the questionnaires and assessing the participants.
Statistical analysis
According to the results of Secreto’s study and considering the power of 90%, confidence interval of 0.95, and significance level of 0.05, 100 women were assigned to each study group. Based on the loss rate of 20% and in order to find a difference of 46.1% in the severity of the climacteric symptoms, sampling was continued until 240 women (120 in each group) volunteered for taking part in the study. Finally, data were entered into the SPSS statistical software (v. 16) and descriptive Statistics were used to summarize demographic data. The Independent t-test was used to compare the mean scores of climacteric symptoms and others clinical features and compare the adverse events in both groups at baseline (α ≤ 0.05, confidence interval of 0.95). ANOVA was used to compare the differences in mean scores of climacteric symptoms between the two groups. Sidak’s Post-hoc was used for pair comparisons. In addition, the coefficient of variation was used to compare each primary and secondary outcome in the two groups. Besides, ANCOVA was used in order to reduce the effect of the confounder variables (age, menopausal age, last menstrual period, BMI, mother’s menopause age) Significance level was considered when P<0.05.
Ethics
The Medical Research Ethics Committee of Shiraz University of Medical Sciences approved the present study (code: 90-5880). Before the beginning of the intervention and enrollment, comprehensive information about the study objectives, climacteric symptoms, melatonin, benefits and probable side effects, type of the intervention (that the participants might be assigned to either group), length of the intervention, and follow-up was provided to the participants to make up their minds for taking part in the study. Then, written informed consents were obtained from the participants and they were given two phone numbers to call in case they had any questions. They were assured of the confidentiality and anonymity of the study. (Registration code: IRCT201201041548N13)
Results
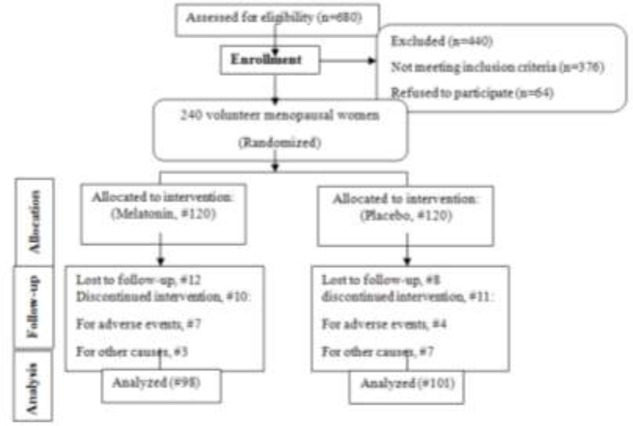
At the beginning of the study, 680 women were screened according to our inclusion criteria and 240 participants were divided into two groups among whom, 440 women were not included in the study: 376 women were excluded and 64 refused to participate.
One hundred and one women in the placebo and ninety-eight in the melatonin group continued their cooperation with the researcher up to the end of the study. In melatonin group 12/120 (10%) were lost to follow-up; 10/120 (8.3%) women discontinued intervention, 7/10 (70%) for adverse events and3/10 (30%) for other causes. In placebo group 8/120(6.67%) were lost to follow-up; 11/120 (9.16%) discontinued intervention, 4/11 (36.36%) for adverse events, 7/11 (63.63%) for other causes (Fig. 1). However, the data from 240 participants were available for the primary intention-to-treat analysis.
Fig. 1.
CONSORT flow diagram of progress through stages of a randomized trial of two groups
Participants’ status at the baseline
The mean age of the participants was 53.12±4.24 years. No significant difference was found between the two groups regarding the demographic and clinical characteristics, such as age, menarche age, menopausal age, date of the last menstrual cycle, length of marriage, systolic and diastolic blood pressure and BMI,by independent t-test before the intervention (P>0.05) (Table 1). In addition, no significant difference was observed between the two groups regarding their mean climacteric symptoms score before the intervention (P: 0.356) (Table 2). ANCOVA test was used to evaluation of effect of confounder variables on the dependent variable (Green score). The test showed no significant difference between groups and dependent variable after adjustment of means, it can be concluded, the variables had no effect on the dependent variable as a confounder variable : age * group(P:0.244), menarche age * group(P:0.866), length of marriage* group(P:0.203), date of the last menstrual cycle* group(P:0.057), menopausal age* group(P:0.082), mother’s menopausal age* group(P:0.537), GHQ * group(P:0.712), BMI* group(P:0.627).
Table 1.
Comparison of demographic and clinical characteristics in two groups
| Groups | Melatonin n= 120 |
Placebo n= 120 |
|
|---|---|---|---|
| Features | Mean + SD | Mean + SD | P-value |
| Age (yr) | 52.85+4.26 | 53.39+4.22 | 0.162 |
| Length of marriage (year) | 34.15+6.71 | 34.99+6.59 | 0.164 |
| Menarche age (years old) | 13.30+1.56 | 13.60+1.35 | 0.061 |
| Last menstrual cycle (month) | 56.18+42.85 | 53.77+44.65 | 0.335 |
| Menopause age (years old) | 48.12+4.04 | 48.88+4.44 | 0.082 |
| Weight(kg) | 65.66+10.11 | 65.10+10.77 | 0.312 |
| Systolic blood pressure (mmgh) | 113.98±15.50 | 111.79±14.56 | 0.131 |
| Diastolic blood pressure (mmgh) | 72.25±9.19 | 71.38±9.03 | 0.228 |
| Body Mass Index (kg/m2) | 26.66±3.77 | 26.49±4.25 | 0.370 |
| Follicle Stimulating Hormone (FSH) | 89.85±44.70 | 88.67±40.39 | 0.423 |
Homogeneity test for demographic characteristics Based on Independent sample t- test
Table 2.
Menopausal women’s climacteric symptoms scores in the two groups over time
| Time | Melatonin group* (CI 95%) † | Placebo group (CI 95%) † |
|---|---|---|
| Before the intervention n1:120, n2:120†† | 35.22+10.50b,c,d**(33.12-37.33) | 35.68+9.93b,c,d (33.72-37.64) |
| 1 month after the intervention n1:115, n2:116 | 25.43+11.11a,c,d (23.20-27.66) | 29.33+10.35a,c,d (27.28-31.37) |
| 2 months after the intervention n1:109, n2:109 | 21.16+10.54a,b,d (19.05-23.28) | 25.81+11.10a,b (23.62-28.00) |
| 3 months after the intervention n1:98, n2:101 | 17.09+10.17a,b,c (15.05-19.13) | 24.42+11.01a,b (22.24-26.59) |
*: values are given as mean± standard deviation. Repeated Measure ANOVA test and then Sidak Post hoc showed that a significant difference was found in the melatonin group’s climacteric symptoms score 3 months after the intervention (P=0.005).
**: the two groups were similar regarding the Greene Climacteric Scale before the intervention (P>0.05)./ a: significant difference compared to before the intervention/b: significant difference compared to 1 month after the intervention/c: significant difference compared to 2 months after the intervention/d: significant difference compared to 3 months after the/†: Confedence interval 95% / ††:n1 number of women in melatonin group, n2: number of women in placebo group
Outcome of the total Green Climacteric Scale
Considering the effect of melatonin on the climacteric symptoms, time was a significant factor in change in the mean scores of climacteric symptoms (P<0.001). Regardless of time also, a significant difference was observed between the two groups concerning the mean score of climacteric symptoms (P=0.001). Besides, the results of the effect of the mutual interference of time and group revealed the effectiveness of melatonin intervention in the intervention group (P<0.001) (Table 2).
Outcome of the Green Climacteric Subscales
The study results revealed a significant difference between the two groups regarding the scores of psychological (P<0.001, F: 6.729, d.f: 2.529), somatic (P=0. 003, F: 4.419, d.f: 2.623), vasomotor (P<0.001, F: 8.145, d.f: 2.638), and sexual (P<0.001, F: 6.930, d.f: 2.632) dimensions after the intervention over time (Table 3).
Table 3.
Comparison of changes in the scores of climacteric symptoms dimensions in two groups
| Before the intervention | 1 month after the intervention | 2 months after the intervention | 3 months after the intervention | CV† | P-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Dimensions | Group | Mean + SD (CI 95%) n1:120, n2 :120†† |
Mean + SD (CI 95%) n1:115, n2:116 |
Mean + SD (CI 95%) n1:109, n2:109 |
Mean + SD (CI 95%) n1:98, n2:101 |
% | Time | Group | Time × group |
| Psychological | Melatonin | 18.95+6.88*b,c,d (17.57-20.33) | 13.62+7.17a,c,d (12.19-15.06) | 10.65+6.41a,b,d (9.37-11.94) | 8.51+6.38a,b,c (7.23-9.79) | 38.74 | <0.001 | 0.009 | <0.001 |
| Placebo | 19.22+6.42b,c,d (17.95-20.48) | 14.96+6.64a,c,d (13.66-16.27) | 13.25+7.08a,b,d (11.85-14.64) | 12.04+6.57a,b,c (10.75-13.33) | 21.12 | ||||
| Somatic | Melatonin | 9.58+4.23*b,c,d (8.73-10.43) | 7.09+4.03a,c,d (6.28-7.90) | 6.21+3.79a,b,d (5.45-6.97) | 4.96+3.49a,b,c (4.26-5.66) | 26.29 | <0.001 | 0.022 | 0.003 |
| Placebo | 9.62+4.03b,c,d (8.29-10.42) | 8.41+4.36a,c,d (7.55-9.27) | 7.14+4.01a,b (6.35-7.93) | 6.63+4.07a,b (5.83-7.44) | 19.54 | ||||
| Vasomotor | Melatonin | 4.13+1.79*b,c,d (3.77-4.49) | 2.83+1.82a,c,d (2.46-3.19) | 2.35+1.83a,b,d (1.98-2.71) | 1.94+1.64a,b,c (1.61-2.27) | 41.28 | <0.001 | 0.016 | <0.001 |
| Placebo | 4.11+2.33b,c,d (3.65-4.57) | 3.38+2.03a (2.98-3.78) | 3.03+1.98a (2.64-3.42) | 3.09+2.01a (2.69-3.49) | 8.25 | ||||
| Sexual | Melatonin | 2.68+0.59*b,c,d (2.57-2.80) | 2.21+0.82a,c,d (2.05-2.38) | 1.98+0.77a,b,d (1.83-2.14) | 1.68+0.89a,b,c (1.51-1.86) | 31.26 | <0.001 | 0.001 | <0.001 |
| Placebo | 2.74+0.72b,c,d (2.60-2.88) | 2.42+0.79a (2.26-2.57) | 2.26+0.86a (2.09-2.43) | 2.25+0.87a (2.08-2.42) | 12.31 |
Values are given as mean± standard deviation. Repeated Measure ANOVA test and then Sidak Post hoc
*: the two groups were similar regarding the various dimensions of Green Climacteric Scale before the intervention (P>0.05).
a: significant difference compared to before the intervention/b: significant difference compared to 1 month after the intervention/c: significant difference compared to 2 months after the intervention/d: significant difference compared to 3 months after the intervention/†: CV: Coefficient of variation/††:n1: number of women in melatonin group, n2: number of women in placebo group
As Table 4 depicts, a statistically significant difference was found between the two groups regarding the psychological subscales, including depression (P=0.001, F: 5.157, d.f: 2.549), anxiety (P=0.001, F: 5.457, d.f: 2.573). Moreover, a significant difference was observed between the two groups regarding the mean scores of depression, anxiety, vasomotor symptoms, and sexual desire since the second month after the intervention. The trend of mean score changes in the intervention group was significant over time; such a way that melatonin was most significantly effective in improvement of these four dimensions in the second and third months after the intervention. Considering the somatic symptoms, however, this difference was observed between the two groups in the first and third months of the study (P<0.05) (Table 3, , 4). In the placebo group also, a statistically significant difference was found between before the intervention and sometime points after the intervention (P<0.05); however, the changes did not follow a regular trend. For instance, using the placebo caused no significant changes in the depression symptom mean scores between the second and the third month after the intervention (P=0.146). Besides, no significant changes was found in the anxiety symptom mean scores between the first and the second month after the intervention, (P=0.331), vasomotor mean scores between the first and the second (P=0.116), the first and the third (P=0.248) as well as the second and the third month after the placebo consumption (P=0.499) and somatic mean scores between the second and the third (P=0.116) (Table 3, 4).
Table 4.
Comparison of changes in the scores of psychological subscales in the two groups
| Before the intervention | 1 month after the intervention | 2 months after the intervention | 3 months after the intervention | P -value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Dimension | Group | Mean + SD (CI 95%) |
Mean + SD (CI 95%) |
Mean + SD (CI 95%) |
Mean + SD (CI 95%) |
CV | Time | Group | Time × group |
| Depression | Melatonin | 9.16±3.69*b,c,d
(8.42-9.90) |
6.40±3.91.a,c,d
(5.61-7.18) |
5.06±3.56a,b,d
(4.35-5.78) |
4.03+3.30a,b,c
(3.37-4.60) |
41.72 | <0.001 | 0.017 | 0.001 |
| Placebo | 9.23+3.65b,c,d (8.51-9.95) |
7.13+3.95a,c,d (6.35-7.91) |
6.22±3.72a,b (5.48-6.95) |
5.83+3.58a,b (5.13-6.54) |
21.81 | ||||
| Anxiety | Melatonin | 9.68+3.88*b,c,d (8.91-10.46) | 7.26+4.03a,c,d (6.45-8.06) | 5.49+3.36a,b,d (4.82-6.16) | 4.56+3.74a,b,c (3.81-5.31) | 41.83 | <0.001 | 0.007 | 0.001 |
| Placebo | 10.13+3.89b,c,d (9.36-10.90) | 7.72+3.82a,d (9.97-8.48) | 7.23+4.17a,d (6.41-8.05) | 6.44+3.83a,b,c (5.68-7.19) | 21.02 | ||||
Values are given as mean± standard deviation. Repeated Measure ANOVA test and then Sidak Post hoc/*: the two groups were similar regarding the subscales of Psychological dimension before the intervention (P>0.05)./ a: significant difference compared to before the intervention/b: significant difference compared to 1 month after the intervention/c: significant difference compared to 2 months after the intervention/d: significant difference compared to 3 months after the intervention
Adverse events
Overall, 43 participants in the two study groups complained about the side effects during the study. Most of the subjects complained about sleepiness, nausea, vomiting, and headache. No significant difference was found between the two groups regarding the complications of using the medications (P>0.05) (Table 5).
Table 5.
Frequency distribution and percentage of the complications reported by the two groups
| Complications | Melatonin | Placebo | P-value | ||
|---|---|---|---|---|---|
| Frequency | Percentage | Frequency | Percentage | ||
| Headache and vertigo | 2 | 1.67 | 2 | 1.67 | 0.689 |
| Nausea and vomiting | 5 | 4.17 | 2 | 1.67 | 0.223 |
| Sleepiness | 9 | 7.5 | 6 | 5 | 0.298 |
| Bleeding and spotting | 4 | 3.33 | 0 | 0 | 0.061 |
| Heartburn | 0 | 0 | 3 | 2.50 | 0.123 |
| Tingling of extremities | 0 | 0 | 3 | 2.50 | 0.123 |
| Feeling of fullness | 2 | 1.67 | 0 | 0 | 0.249 |
| Constipation | 1 | 0.83 | 1 | 0.83 | 0.751 |
| Diarrhea | 0 | 0 | 1 | 0.83 | 0.500 |
| Flatulence | 2 | 1.67 | 0 | 0 | 0.249 |
No significant difference was observed in the two groups regarding the complications after the intervention (P>0.05)
Acceptance of the treatment method
Furthermore, 65.31% (64) of the melatonin group participants and 34.69% (32) of the placebo group participants were satisfied with using the medication and the difference was statistically significant (P: 0.017). In addition, participants in the melatonin group were willing to continue using the medication after the study.
Discussion
The results of the present study showed that the two groups were similar regarding the demographic variables and confounding factors, which could affect the women’s climacteric symptoms. Although controlling all the variables which can cause bias in any study’s results was out of the researcher’s control, due to the homogeneity of the two groups at the beginning of the study, the statistically significant differences in the study results can be attributed to the intervention with more certainty.
Based on the results of some studies using melatonin during menopause leads to a general improvement in mood, treatment of depression, and weight reduction (32, 33). However, a limited number of studies have been conducted on using melatonin as a food supplement in the menopausal women’s food diet. This study was done to evaluate the effect of melatonin on climacteric symptoms in menopausal women. The results obtained also indicate that the supplement improves this symptom.
The study findings revealed 18.64 and 11.81 scores reduction with coefficient of variation (CV) 29.67% v.s 17.24% in the severity of the climacteric symptoms compared to before the intervention in the melatonin and placebo groups, respectively. A significant difference was observed between melatonin and the placebo regarding their improvement of the climacteric symptoms mean scores from the first to the end of the third month of the study.
The changes in the mean scores of the psychological dimension followed a regular trend in both group, a significant difference and more reduction were detected in the melatonin group in the third month of the study in comparison to the placebo group (10.44 vs. 7.18). Investigation of the results related to depression and anxiety subscales of the psychological dimension showed that melatonin had a significant decreasing trend during the study, while the placebo showed its effects up to the second month which might be due to the psychological effects of using the placebo. Thus, it can be concluded that melatonin improved the psychological symptoms in the menopausal women under study. Receiving melatonin accompanied by isoflavones improved the psychological domain of Greene’s scale. One of the possible mechanisms of melatonin in improvement of the psychological moods is inhibition of adenyl cyclase by its specific receptor, MT1, which improves sleep as well as the psychological dimension (20). Toffol et al. study showed a significant association between the mean scores of depression and anxiety with melatonin concentrations in postmenopausal women (13).
Similar to the present study, Bellipanni et al. also showed that melatonin intervention led to a significant improvement in the menopausal women’s psychological and mood status (21).
Another study showed the use of melatonin with usual dose (3 mg) has created significantly reduces in the mean scores of psychological symptoms in schizophrenic patients (34). Bustamante showed that the use of new analogues of melatonin could also improve the anxiety behaviors in mice, likely by membrane receptors MT1 and MT2 (35).
One of the most important mediators involved in the performance of melatonin in improving the psychological symptoms is Gamma-aminobutyric acid (GABA) which affects the regulation of humans’ behavioral function. GABA reaches its maximum level during the night when melatonin secretion increases and has similar effects to clonazepam and sedatives (36). Beneficial effects of melatonin for treatment of certain insomnias are also able to improve the psychological symptoms (37). It can be stated that with improvement of sleep and mood in postmenopausal women, other health problems that are exacerbated by psychological factors are improved.
In the present study, the 4.62 point (CV: 26.29%) reduction in the melatonin group compared to the 2.99 score (CV: 19.54%) reduction in the placebo group revealed the effect of melatonin on improving the somatic symptoms. These results were in contrast to another study (20), which might be due to the larger sample size of the present study (54 vs. 120 participants) as well as the difference in the two studies’ evaluation periods (before and 3 months after the intervention in Secreto’s study vs. month by month evaluation of the present one) (20). Melatonin in comparison with pregabalin has equal effects on pain relief and severity of anxiety (38). May be melatonin reduces pain by effect on GABAergic systems and increased the release of endorphin (39–41). It is said melatonin does its function by the activation of MT2 receptors in the dorsal horn of the spinal cord (42). Other studies is needed for achieving more accurate results on the effect of melatonin on physical paining postmenopausal women.
In comparison to the placebo, melatonin intervention significantly improved the sexual desire (CV: 31.26% vs. 12.31%). The studies conducted by Babaei and Drago have also shown the improvement of sexual function in male rats after melatonin intervention (43, 44). Of course, sexual function in male and female rats depended on the dose and duration of melatonin treatment (44). Based on these results and the results of our study, 3 mg is the appropriate dose for improving sexual function in men and women. Melatonin has mutual effects on the central serotonergic system (45, 46). There is also evidence on the increasing effect of melatonin on the mammals’ sexual function through the central serotonergic system (47). Most probably, melatonin decreases the arousal threshold by moderating the activity of central 5-hydroxy tryptaminergic receptors and, consequently, improves the mammals’ sexual function (43). Of course, in case the vaginal maturation index was utilized in the present study, other mechanisms involved in sexual function improvement after melatonin intervention could have also been evaluated.
Considering the vasomotor dimension, the results of this study showed the decreasing trend following melatonin intervention over time. (2.19 vs. 1.02). These results were in contrast to another study (20). In a 24-hour cycle, increasing the melatonin level is accompanied by a decrease in the core body temperature and the cortisol level. On the other hand, when melatonin decreases, the core body temperature and the cortisol level increase. Exogenous utilization of melatonin could change the body temperature as well as the sleeping time and improve the endogenous melatonin levels (7, 8). These also support the findings of the present research. In addition, immediate vasodila-tory effects of melatonin lead to heat dissipation via skin, which may increase sleep propensity (48). In this way, the psychological and physical symptoms of menopause can be reduced.
A review of the results of the present study and those previously conducted on the issue shows a significant change in the climacteric symptoms before and after using the placebo, which might be due to the psychological effects of placebo consumption.
It should be mentioned that various methods have been used in different studies in order to improve the menopausal symptoms. For instance, Eftekhar et al. came to beneficial results through intervention with vaginal estrogen (49). Moreover, using acupuncture could decrease the depression and anxiety scores in the menopausal women; nevertheless, this method might not be accepted by all the women (50). In fact, women look for low-risk, beneficial, and non-invasive methods. Considering the effect of melatonin on various dimensions of the climacteric symptoms, it is regarded as a noninvasive method and its oral application can be highly welcomed by the women (50).
We had some limitations in our study. First, outcomes were not assessed after 3 months and must be assessed during the efficacy of interventions after treatment. Despite this, all studies have used melatonin only for a short period of time and no reliable data are available concerning the safety and efficacy of long-term melatonin use. Further studies are needed to confirm the usefulness and safety of melatonin for long-term use. Second, we did not measure the melatonin serum levels before and after the intervention.
Conclusion
Overall, the findings of the current study showed the safety of this supplement as well as the participants’ willingness to continue using melatonin 3 months after the intervention. Since the aim of treatment methods is providing the individuals’ health through the safest methods, melatonin can play a critical role in maintaining the people’s health through its multifaceted functions.
Ethical considerations
Ethical issues (Including plagiarism, Informed Consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
The present article was extracted from Nehleh Parandavar’s M.Sc. thesis in midwifery and was financially supported by the Research Vice-chancellor of Shiraz University of Medical Sciences, Shiraz, Iran (code: 90-5880). Hereby, the authors would like to thank all the individuals, particularly Mr Mehrab Sayadi (Statistics advisor), and Ms. A. Keivanshekouh at the Research Improvement Center of Shiraz University of Medical Sciences for improving the use of English in the manuscript. The authors declare that there is no conflict of interests.
References
- Speroff L, Fritz MA (2005). Clinical gynecologic endocrinology and infertility. 7th ed, Lippincott Williams & Wilkins; Philadelphia, pp.260–320. [Google Scholar]
- Berek J, Novak E (2007). Menopause. In: Berek &Novak’s gynecology. Eds, Shifren JL, Schiff I. 14th ed, Lippincott Williams & Wilkins; Philadelphia, pp. 1324–1343. [Google Scholar]
- Ryan KJ, Berkowitz RS, Barbieri RL, Dunaif A (1997). Menopause. In: Kistner’s Gynecology and Women’s Health. Eds, Walsh Bw, Ginsburg ES, 7th ed. St. Louis, Mo: Mosby, pp. 540–669. [Google Scholar]
- Kojori MD, Safavi Sh, Saeed Fatami N, Mohammadi R, Naisani Samani L (2009). Women ‘s health in life cycle. 1st ed. Nordanesh; Tehran, pp. 123–146. [Google Scholar]
- Golchin M, Kamal A (2005). Women’s Health. Nasle Farda; Tehran, pp.116–121. [Google Scholar]
- Cutler WB, Genovese-Stone E (1998). Wellness in women after 40 years of age: The role of sex hormones and pheromones. Dis Mon, 44(9): 421–546. [DOI] [PubMed] [Google Scholar]
- Hall JE, Guyton AC (2011). Textbook of medical physiology. 12th ed. Elsevier/Saunders; Philadelphia, pp. 996–1026. [Google Scholar]
- Jameson JL, De groot LJ (2010). Endocrinology adult and pediatric. 6th ed. Elsevier/Saunders; Philadelphia, pp.214–554. [Google Scholar]
- Ganong WF (2010). Ganong’s review of medical physiology. 23th ed. Appletone & Lange; California: pp. 897–937. [Google Scholar]
- Tan DX, Manchester LC, Terron MP, Flores LJ, Reiter RJ (2007). One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species? J Pineal Res, 42: 28–42. [DOI] [PubMed] [Google Scholar]
- Pandi-Perumal SR, Srinivasan V, Maestroni GJM, Cardinali DP, Poeggeler B, Hardeland R (2006). Melatonin Nature’s most versatile biological signal? FEBSJ, 273: 2813–2838. [DOI] [PubMed] [Google Scholar]
- Žitnanová I, Rakovan M, Paduchová Z, et al. (2011). Oxidative stress in women with perimenopausal symptoms. Menopause, 18(11): 1249–1255. [DOI] [PubMed] [Google Scholar]
- Toffol E, Kalleinen N, Haukka J, Vakkuri O, Partonen T, Polo-Kantola P(2014). Melatonin in perimenopausal and postmenopausal women: associations with mood, sleep, climacteric symptoms, and quality of life. Menopause, 21(5): 493–500. [DOI] [PubMed] [Google Scholar]
- Díaz BL, Llaneza PC (2008). Endocrine regulation of the course of menopause by oral melatonin: first case report. Menopause, 15(2): 388–392. [DOI] [PubMed] [Google Scholar]
- Katzung BG, Masters SB, Trevor AJ (2009). Basic &Clinical Pharmacology. 11th ed. McGraw-Hill Medical; New York, pp. 240–375. [Google Scholar]
- Dollins AB, Zhdanova IV, Wurtman RJ, Lynch HJ, Deng MH(1994). Effect of inducing nocturnal serum melatonin concentrations in daytime on sleep, mood, body temperature and performance. Proc Natl Acad Sci, 91: 1824–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery D, Lenz M, Landis C (1998). Guidelines for prescribing melatonin. Ann Med, 30: 122–130. [DOI] [PubMed] [Google Scholar]
- Brzezinski A (1998). Melatonin replacement therapy for postmenopausal women: is it justified? Menopause, 5: 60–64. [PubMed] [Google Scholar]
- Sack RL, Lewy AJ, Hughes RJ (1998). Use of melatonin for sleep and circadian rhythm disorders. Ann Med, 30: 115–121. [DOI] [PubMed] [Google Scholar]
- Secreto G, Chiechi LM, Amadori A, et al. (2004). Soy isoflavones and melatonin for the relief of climacteric symptoms: a multicenter, double-blind, randomized study. Maturitas, 47(1): 11–20 [DOI] [PubMed] [Google Scholar]
- Bellipanni G, Bianchi P, Pierpaoli W, Bulian D, Ilyia E (2001). Effects of melatonin in perimenopausal and menopausal women: a randomized and placebo controlled study. Exp Gerontol, 36(2): 297–310. [DOI] [PubMed] [Google Scholar]
- Goldberg D, William P (1988). A user’s guide to the general health questionnaire. Windsor, UK: NFER - Nelson. [Google Scholar]
- Noorbala A, Yazdi M, Yasemi A (2001). Investigation of psychological health status in above 15-year-old individuals in Iran in. Hakim Research Journal, 1: 1–10. [Google Scholar]
- Asgari A, Bahmani B (2006). National standardization and evaluation of the psychometric features of the general health questionnaire for students of Iran’s universities of medical sciences. Articles of the third seminar on the mental health of Tehran students: Iran University of Science and Technology, 62: 1. [Google Scholar]
- Palahang H, Nasr M, Barahani M, Shah Mohammadi D (1996). Investigation of the epidemiology of psychological disorders in Kashan, Iran. Journal of Thought and Behavior, 4: 19–27. [Google Scholar]
- Ehsanmanesh M (2001). Epidemiology of psychological disorders in Iran; a review of the studies conducted on the issue. Journal of Thought and Behavior, 6(4): 54–69. [Google Scholar]
- Greene G (1999). Constructing a standard climacteric scale. Maturitas, 29: 25–31. [DOI] [PubMed] [Google Scholar]
- Siarra B, Hidalgo L, Chedraui P (2005). Measuring climacteric symptoms in an Ecuadorian Population with the Greene climacteric scale. Maturitas, 15: 236–245. [DOI] [PubMed] [Google Scholar]
- Hakimi S, Mohammad Alizadeh Charandabi C, Del azar A, et al. (2006). Probabilistic effect of Trigonella foenum - graceum on hot flash in postmenopause women. Herbal med J, 19: 9–14. [Google Scholar]
- Van de Weijer P, Barentsen R (2002). Isoflavonesfrom red clover (Promensil) significantly reduces menopausal hot flush symptoms compared withplacebo. Maturitas, 42: 187–193. [DOI] [PubMed] [Google Scholar]
- Moghassemi S, Ziaei S, Hidari Z (2011). Comparative effect of the conventional hormone replacement therapy and tibolone on sexual performance in postmenopausal women. Arak Medical University Journal, 14(54): 104–113. [Google Scholar]
- Wakatsuki A, Okatani Y, Ikenoue N, Kaneda C, Fukaya T(2001). Effects of short-term melatonin administration on lipoprotein metabolism in normolipidemic postmenopausal women. Maturitas, 38(2): 171–177. [DOI] [PubMed] [Google Scholar]
- Sanchez-Mateos S, Alonso-Gonzalez C, Gonzalez A, et al. (2007). Melatonin and estradiol effects on food intake, body weight, and leptin in ovariectomized rats. Maturitas, 58: 91–101. [DOI] [PubMed] [Google Scholar]
- Modabberian A, Heidari P, Soleimani R, Sobhani A, AtrkarRoshan Z, Taslimi Sh, et al. (2014). Melatonin for prevention of metabolic side effects of olanzapine in patients with first episode schizophrenia: Randomized double-blind placebo-controlled study. J Psychi Res, 53: 133–140. [DOI] [PubMed] [Google Scholar]
- Bustamante-García R, Lira-Rocha A.S, Espejo-González O, Gómez-Martínez A.E, Picazo O (2014). Anxiolytic-like effects of a new 1-N substituted analog of melatonin in pinealectomized rats. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 51: 133–139. [DOI] [PubMed] [Google Scholar]
- Rohr UD, Herold J (2002). Melatonin deficiencies in women. Maturitas, 41, (Suppl 1): 85–104. [DOI] [PubMed] [Google Scholar]
- Turek FW, Gillette MU (2004). Melatonin, sleep, and circadian rhythms: rationale for development of specific melatonin agonists. Sleep Medicine, 5(6): 523–532. [DOI] [PubMed] [Google Scholar]
- Nasr DA, Abdellatif AA (2014). Efficacy of pre-operativemelatonin versus pregabalin on perioperative anxiety and postoperative pain in gynecological surgeries. Egyptian Journal of Anaesthesia, 30(1): 89–93. [Google Scholar]
- Golombek DA, Escolar E, Burin LJ, De Brito-Sanchez MG, Cardinali DP(1991). Time-dependent melatonin analgesia in mice:inhibition by opiate or benzodiazepine antagonism. Eur J Pharmacol, 194: 25–30. [DOI] [PubMed] [Google Scholar]
- Golombek DA, Martini M, Cardinali DP (1993). Melatonin as an anxiolytic in rats: time dependence and interaction with the central GABAergic system. Eur J Pharmacol, 237: 231–236. [DOI] [PubMed] [Google Scholar]
- Yu CX, Zhu B, Xu SF, Cao XD, Wu GC (2000). The analgesic effects of peripheral and central administration of melatonin in rats. Eur J Pharmacol, 403: 49–53. [DOI] [PubMed] [Google Scholar]
- Yu CX, Zhu CB, Xu SF, Cao XD, Wu GC (2000). Selective MT2 melatonin receptor antagonist blocks melatonin-induced antinociception in rats. Neurosci Lett, 282: 161–164. [DOI] [PubMed] [Google Scholar]
- Babaei F, Heidari R, Ilkhanipour M, Azizi S (2009). Effect of Melatonin on SexualBehavior in Male Diabetic Rats. Iran J Endocrinol Metabol, 11(2): 199–207. [Google Scholar]
- Drago F, Busa L, Benelli A, Bertolini A (1999). Acute low doses of melatonin stimulate rat sex behavior: the role of serotonin neurotransmission. Eur J Pharmacol, 385: 1–6. [DOI] [PubMed] [Google Scholar]
- Dugovic C, Leysen JE, Wauquier A (1989). Melatonin modulates the sensitivity of 5-hydroxytriptamine-2 receptor-mediated sleep – wakefulness regulation of the rat. Neurosci Lett, 104: 320–325. [DOI] [PubMed] [Google Scholar]
- Govitrapong P, Prapapanich V, Ebadi M (1991). Identification of serotonin 5-HT2 receptors in bovine pineal gland. J Pineal Res, 11(3–4): 182–187. [DOI] [PubMed] [Google Scholar]
- Brotto LA, Gorzalka BB (2000). Melatonin enhances sexual behavior in the male rat. Physiol Behav, 68: 483–486. [DOI] [PubMed] [Google Scholar]
- Krauchi K, Cajochen C, Pache M, Flammer J, Wirz-Justice A (2006). Effects of melatonin in relation to sleepiness. Chronobiol Int, 23: 475–484. [DOI] [PubMed] [Google Scholar]
- Aftekhar T, Akhoondzadeh S, Ghanbari Z, Iranshahr R, Haghollahi F (2009). Effect of vaginal estrogen on post menopausal mood & sleep disturbance and sexual satisfaction. Tehran University Medical Journal, 67(2): 118–124. [Google Scholar]
- Venzke L, Calvert JF Jr, Gilbertson B (2010). A randomized trial of acupuncture for vasomotor symptoms in post-menopausal women. Complementary Therapies in Medicine, 18: 59–66. [DOI] [PubMed] [Google Scholar]