Abstract
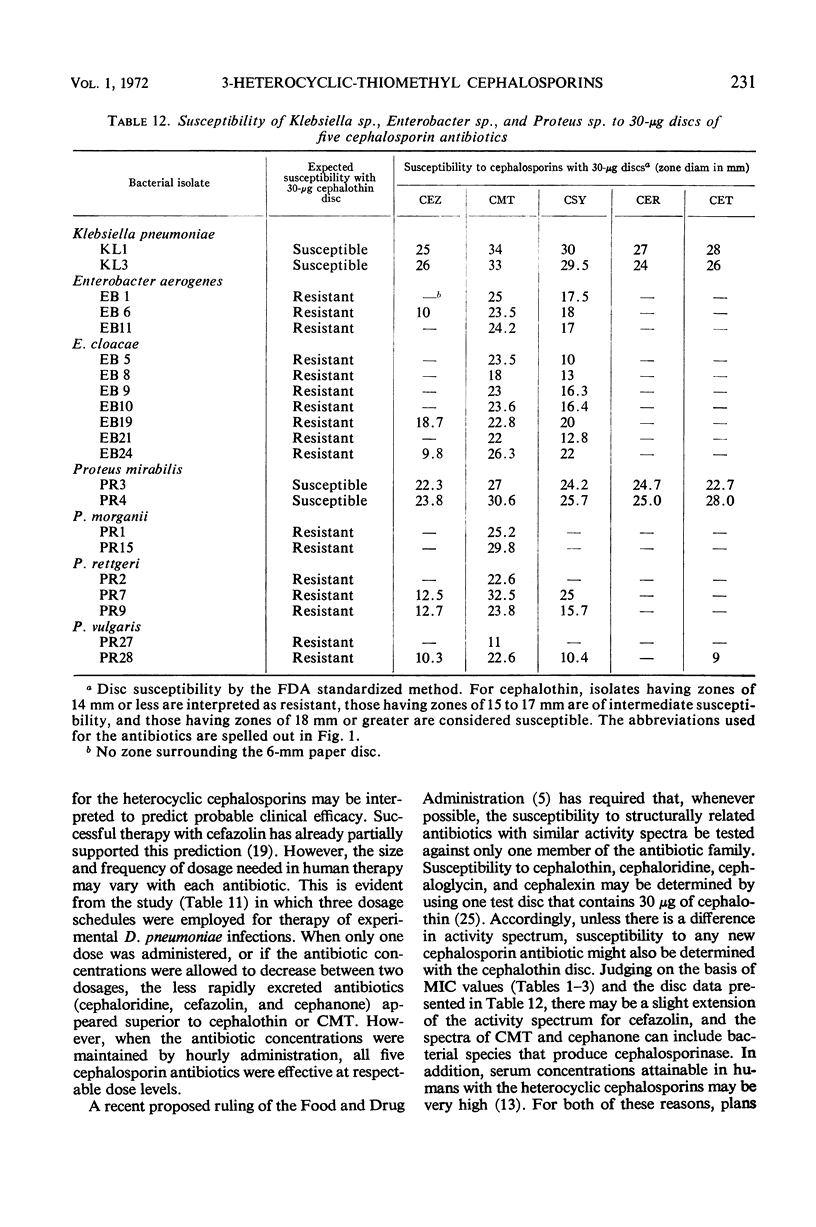
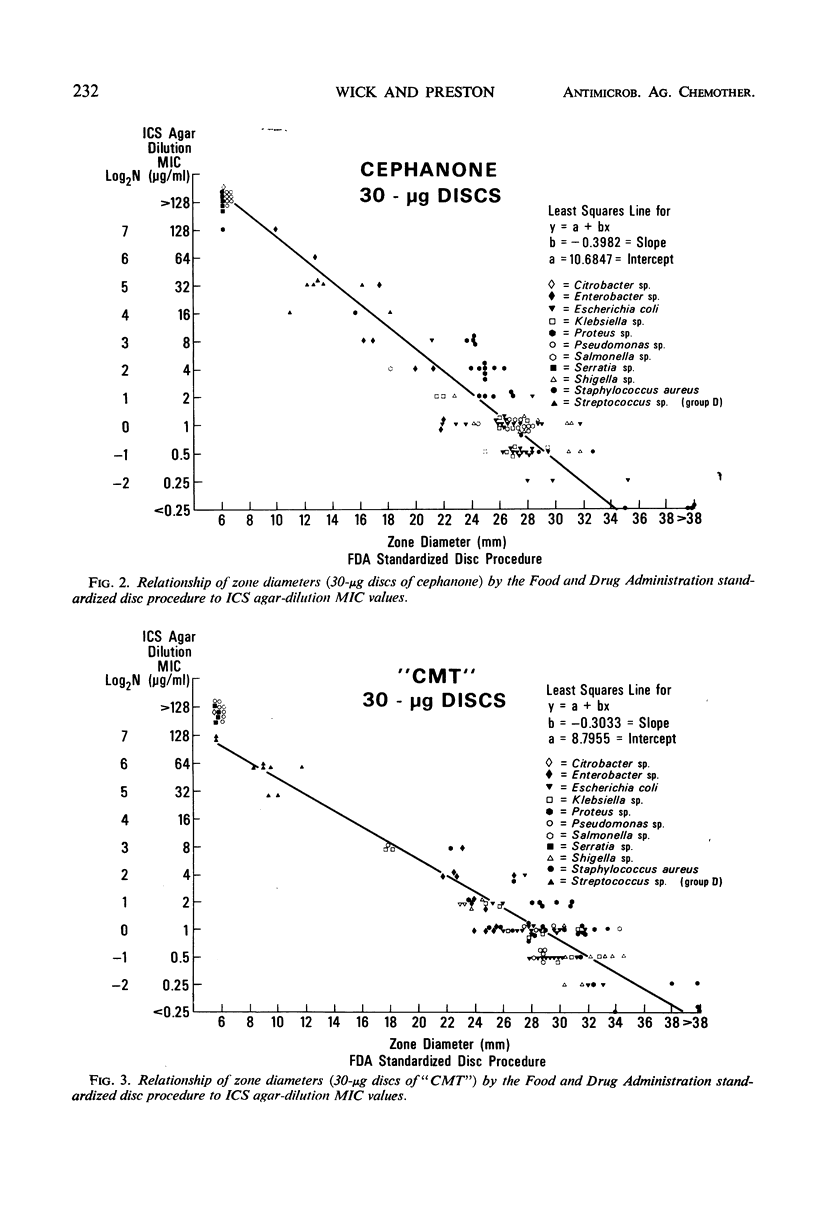
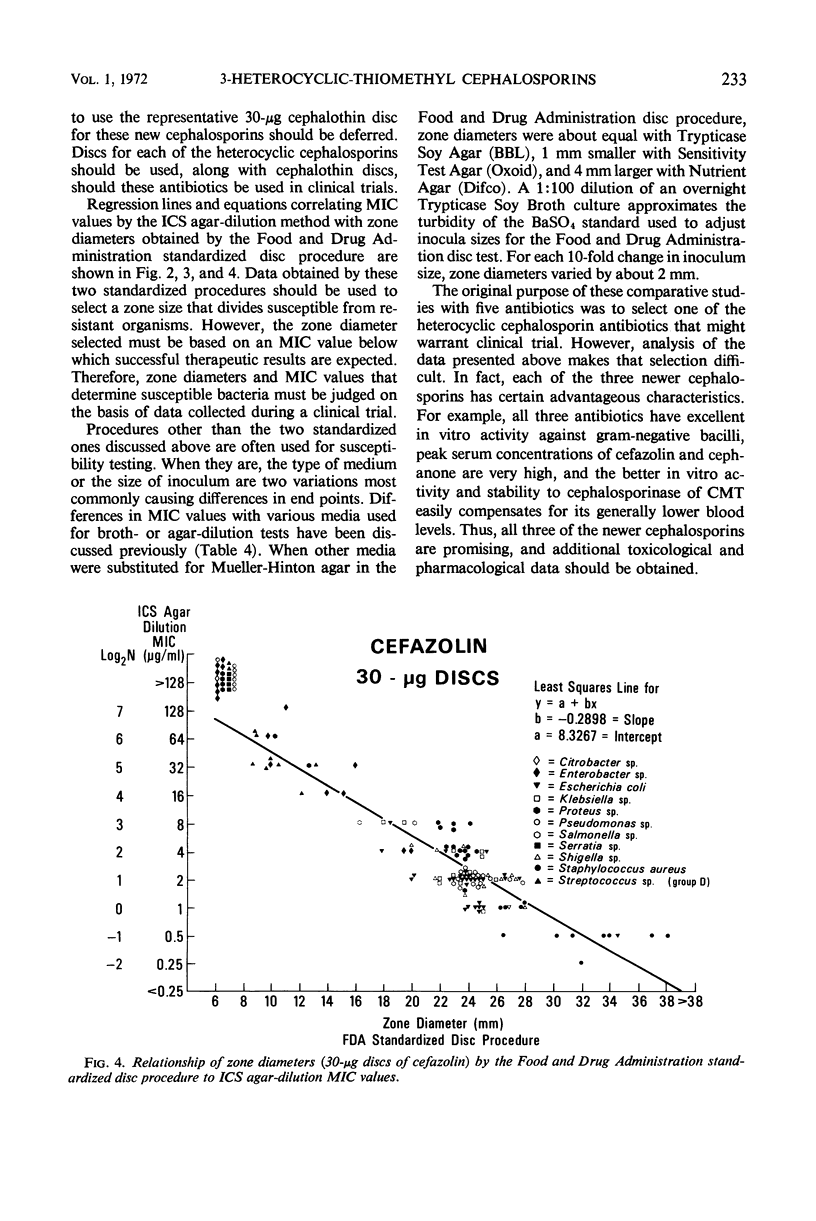
Three new cephalosporin antibiotics, prepared by substitution of heterocyclic groups on 7-aminocephalosporanic acid, possess certain desirable chemical or biological properties. All three compounds are active in vitro against a variety of gram-positive and gram-negative bacteria. Minimal inhibitory concentrations (MIC) of these bactericidal antibiotics were not significantly affected by changes in pH or NaCl content of nutrient broth, or by the use of different inoculum sizes. However, agar-dilution MIC values were generally two- to fourfold lower than the MIC values in comparable broth-dilution tests. Stability to cephalosporinase by two of the compounds extended their antibacterial spectra over cephalothin and cephaloridine to include strains of Enterobacter sp. and indole-positive Proteus sp. Binding to serum proteins of the new cephalosporins was intermediate between cephalothin and cephaloridine. Excellent concentrations of the antibiotics were attained in mouse blood, after subcutaneous administration of 20 mg per kg. In vitro biological characteristics of the antibiotics were verified by successful therapy of experimental mouse infections. Regression lines were calculated to show the correlation of agar-dilution MIC values with zones of inhibition by the disc testing procedure. Because each of the three new cephalosporins has certain advantageous properties over cephalothin and cephaloridine, additional toxicological and pharmacological data should be obtained for all three compounds.
Full text
PDF
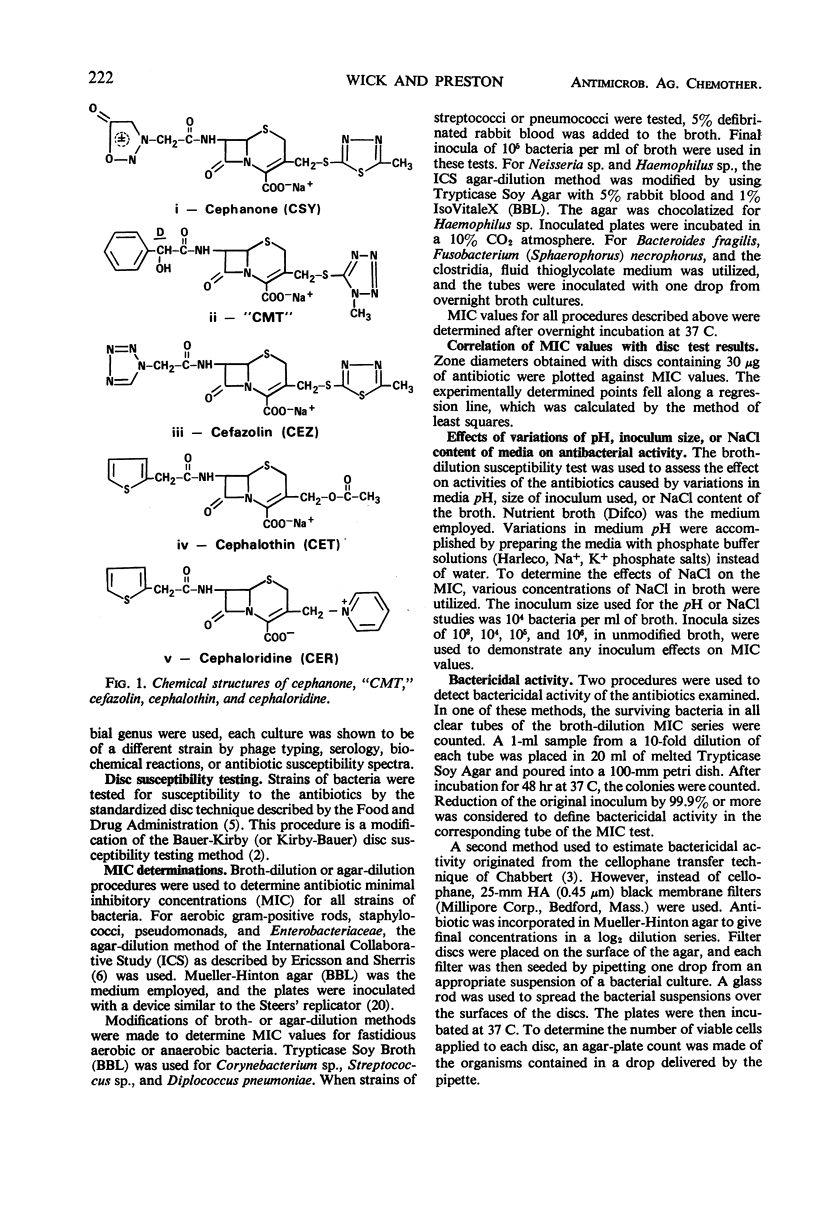

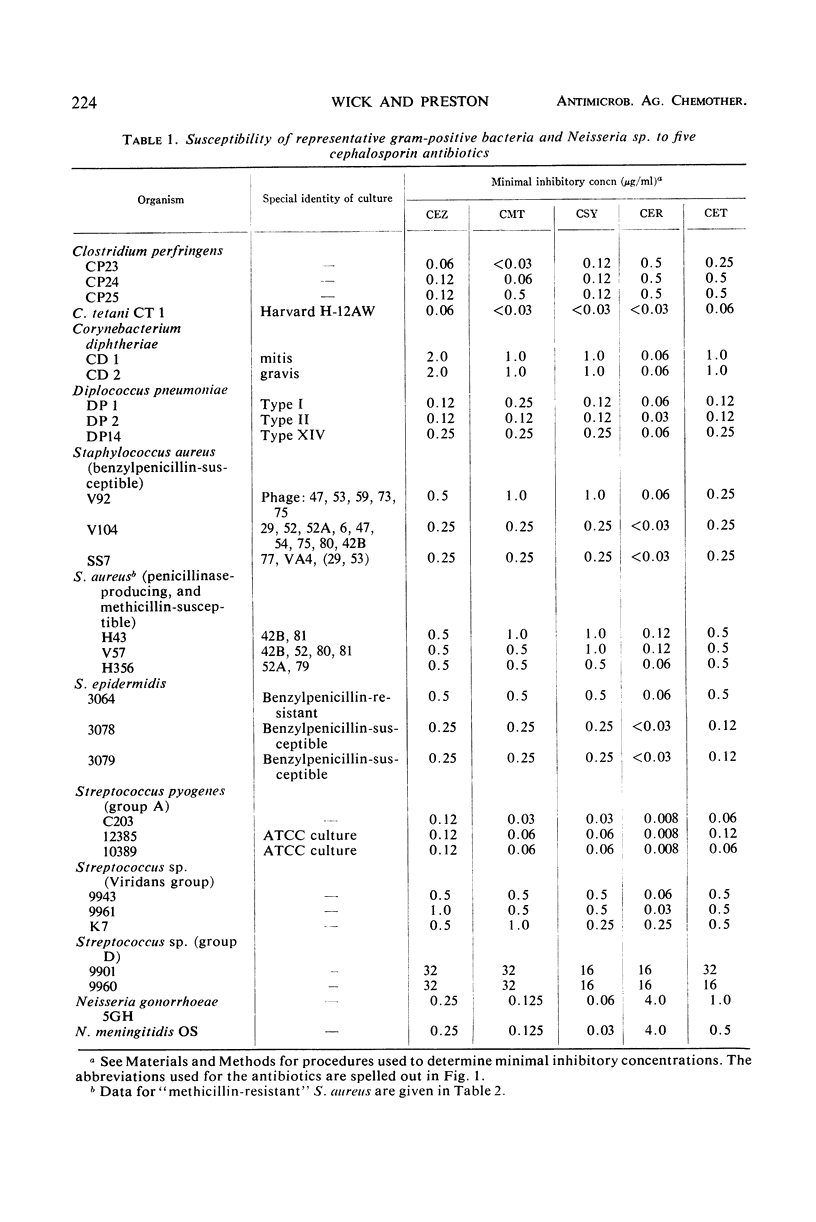
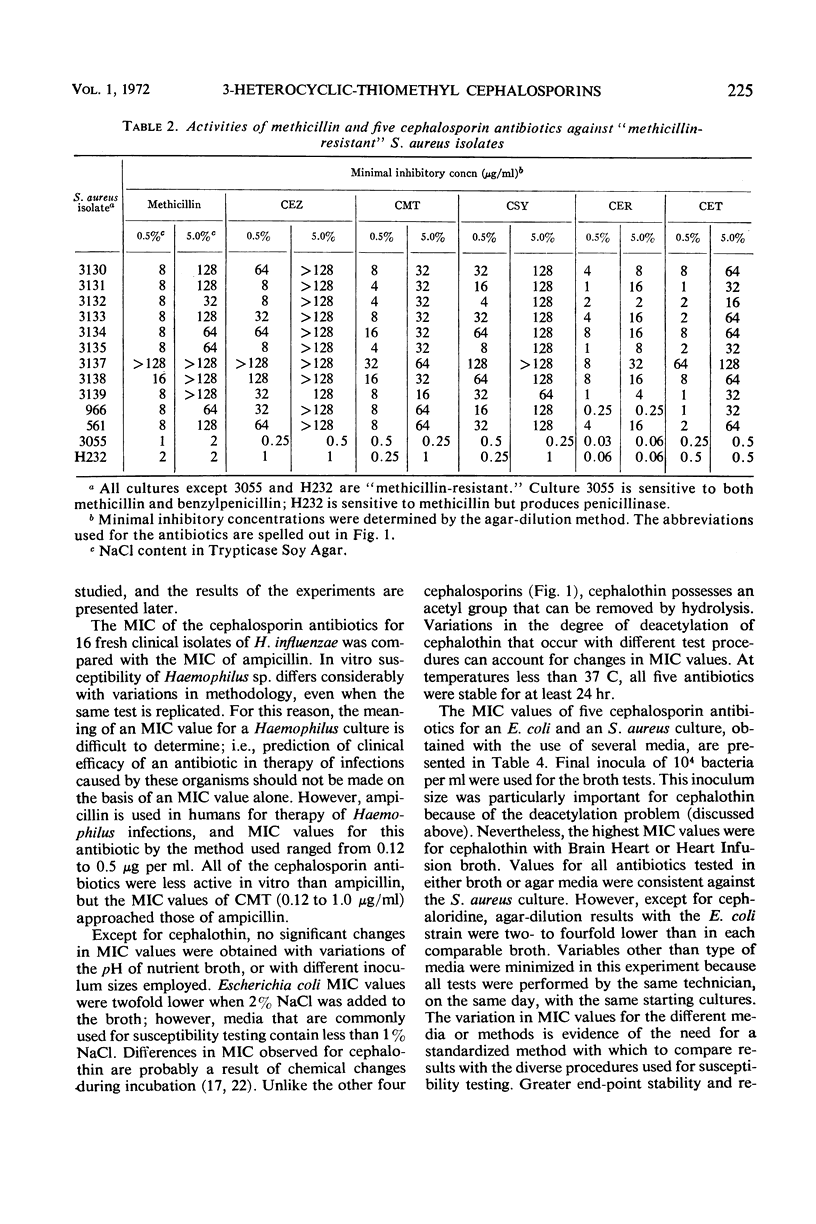
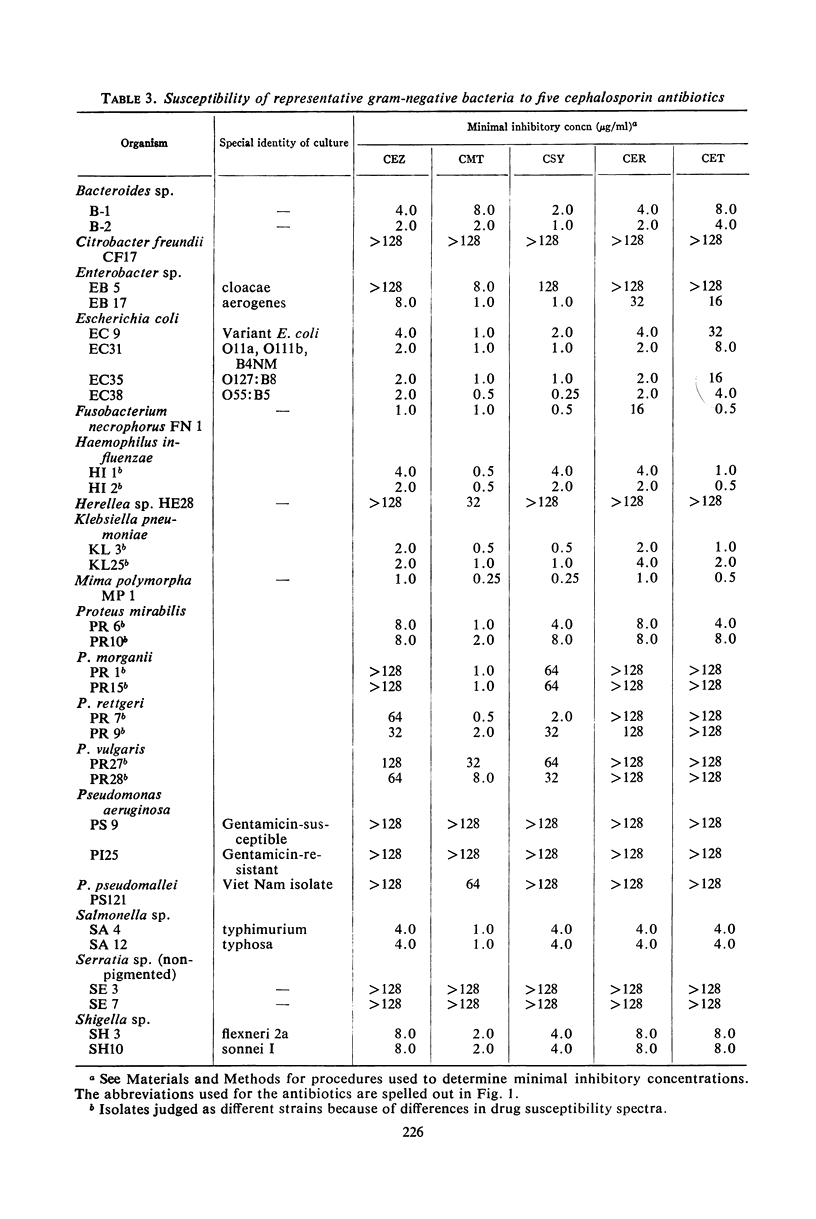

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARBER M. NATURALLY OCCURING METHICILLIN-RESISTANT STAPHYLOCOCCI. J Gen Microbiol. 1964 May;35:183–190. doi: 10.1099/00221287-35-2-183. [DOI] [PubMed] [Google Scholar]
- Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966 Apr;45(4):493–496. [PubMed] [Google Scholar]
- CHABBERT Y. Une technique nouvelle d'étude de l'action bactéricide des associations d'antibiotiques: le transfert sur cellophane. Ann Inst Pasteur (Paris) 1957 Sep;93(3):289–299. [PubMed] [Google Scholar]
- Chabbert Y. A. Behaviour of "methicillin hetero-resistant" staphylococci to cephaloridine. Postgrad Med J. 1967 Aug;43(Suppl):40–42. [PubMed] [Google Scholar]
- Ericsson H. M., Sherris J. C. Antibiotic sensitivity testing. Report of an international collaborative study. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;217(Suppl):1+–1+. [PubMed] [Google Scholar]
- FLEMING P. C., GOLDNER M., GLASS D. G. Observations on the nature, distribution, and significance of cephalosporinase. Lancet. 1963 Jun 29;1(7296):1399–1401. doi: 10.1016/s0140-6736(63)92051-8. [DOI] [PubMed] [Google Scholar]
- GODZESKI C. W., BRIER G., PAVEY D. E. Cephalothin, a new cephalosporin with a broad antibacterial spectrum. I. In vitro studies employing the gradient plate technique. Appl Microbiol. 1963 Mar;11:122–127. doi: 10.1128/am.11.2.122-127.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn M. M., Pugh C. T. Method for the detection and quantitative assay of cephaloglycin and its biologically active metabolites. Appl Microbiol. 1968 Aug;16(8):1132–1133. doi: 10.1128/am.16.8.1132-1133.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariyone K., Harada H., Kurita M., Takano T. Cefazolin, a new semisynthetic cephalosporin antibiotic. I. Synthesis and chemical properties of cefazolin. J Antibiot (Tokyo) 1970 Mar;23(3):131–136. doi: 10.7164/antibiotics.23.131. [DOI] [PubMed] [Google Scholar]
- Kind A. C., Kestle D. G., Standiford H. C., Kirby W. M. Laboratory and clinical experience with cephalexin. Antimicrob Agents Chemother (Bethesda) 1968;8:361–365. [PubMed] [Google Scholar]
- Mine Y., Nishida M., Goto S., Kuwahara S. Cefazolin, a new semisynthetic cephalosporin antibiotic. IV. Antigenicity of cefazolin and its cross reactivity with benzylpenicillin, ampicillin and cephaloridine. J Antibiot (Tokyo) 1970 Apr;23(4):195–203. [PubMed] [Google Scholar]
- Neter E., Olson T. A., Press E., Reese E. M., Roark K. T., Rosenstock I. M., Steele J. H., Mattison B. F., Baker A. G., Bierman P. Committee Reports: Committee on Evaluation and Standards: REPORT OF THE CHAIRMAN, 1967. Am J Public Health Nations Health. 1968 Feb;58(2):365–366. doi: 10.2105/ajph.58.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida M., Matsubara T., Murakawa T., Mine Y., Yokota Y., Goto S., Kuwahara S. Cefazolin, a new semisynthetic cephalosporin antibiotic. 3. Absorption, excretion and tissue distribution in parenteral administration. J Antibiot (Tokyo) 1970 Apr;23(4):184–194. [PubMed] [Google Scholar]
- Nishida M., Matsubara T., Murakawa T., Mine Y., Yokota Y., Kuwahara S., Goto S. In vitro and in vivo evaluation of cefazolin, a new cephalosporin C derivative. Antimicrob Agents Chemother (Bethesda) 1969;9:236–243. [PubMed] [Google Scholar]
- Ronald A. R., Turck M. Factors influencing in vitro susceptibility to the cephalosporins and clinical trial of an oral cephalosporin, cephaloglycin. Antimicrob Agents Chemother (Bethesda) 1966;6:82–87. [PubMed] [Google Scholar]
- Sherris J. C., Rashad A. L., Lighthart G. A. Laboratory determination of antibiotic susceptibility to ampicillin and cephalothin. Ann N Y Acad Sci. 1967 Sep 27;145(2):248–267. doi: 10.1111/j.1749-6632.1967.tb50223.x. [DOI] [PubMed] [Google Scholar]
- Shibata K., Fujii M. Clinical studies of cefazolin in the surgical field. Antimicrob Agents Chemother (Bethesda) 1970;10:467–472. [PubMed] [Google Scholar]
- Stroy S. A., Preston D. A. Specific assay of aminoglycosidic- or polymyxin-type antibiotics present in human sera in combination with cephalosporins. Appl Microbiol. 1971 Jun;21(6):1002–1006. doi: 10.1128/am.21.6.1002-1006.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WICK W. E., BONIECE W. S. IN VITRO AND IN VIVO LABORATORY EVALUATION OF CEPHALOGLYCIN AND CEPHALORIDINE. Appl Microbiol. 1965 Mar;13:248–253. doi: 10.1128/am.13.2.248-253.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WICK W. E., HOLMES D. H., BONIECE W. S. Antipenicillinase serum: use in treatment of resistant staphylococcal infections in laboratory animals. Antibiot Chemother (Northfield) 1960 Feb;10:71–77. [PubMed] [Google Scholar]
- Wick W. E. Influence of antibiotic stability on the results of in vitro testing procedures. J Bacteriol. 1964 May;87(5):1162–1170. doi: 10.1128/jb.87.5.1162-1170.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick W. E., Wright W. E., Kuder H. V. Cephaloglycin and its biologically active metabolite desacetylcephaloglycin. Appl Microbiol. 1971 Mar;21(3):426–434. doi: 10.1128/am.21.3.426-434.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]



