Abstract
Introduction. Inflammation is ubiquitous during sepsis and may be influenced by body mass index (BMI). We sought to evaluate if BMI was associated with serum levels of several cytokines measured at intensive care unit admission due to sepsis. Methods. 33 septic patients were included. An array of thirty-two cytokines and chemokines was measured using Milliplex technology. We assessed the association between cytokine levels and BMI by generalized additive model that also included illness severity (measured by SAPS 3 score); one model was built for each cytokine measured. Results. We found that levels of epidermal growth factor, vascular endothelial growth factor, and interleukins 4, 5, and 13 were associated with BMI in a complex, nonlinear way, independently of illness severity. Higher BMI was associated with higher levels of anti-inflammatory interleukins. Conclusion. BMI may influence host response to infection during critical illness. Larger studies should confirm these findings.
1. Introduction
Sepsis is a major cause of mortality and morbidity and its incidence is increasing [1]. Despite the major social impact of sepsis, its physiopathology is still incompletely understood [2, 3]. Inflammation is ubiquitous during the course of critically ill septic patients and may be modulated by several patient's particularities, such as age, gender, and comorbidities [3–5]. Nevertheless, the extend by which each patient characteristic impacts the inflammatory response is unclear.
Body mass index is an important prognostic factor in general population and is associated with inflammation [6, 7]. BMI may also impact the prognosis of critically ill patients. For example, it has been suggested that obesity might be associated with improved prognosis in critically ill patients (the so-called “obesity paradox”), although reasons for this are unclear [8, 9]. It has been hypothesized that changes in BMI may alter the host response to the pathogen and inflammatory response, thereby influencing the course of critical illness and therefore prognosis, but evidence available is conflicting [9]. On the other hand, underweight patients may also display higher inflammation on specific clinical conditions [10], suggesting that the interplay between BMI and inflammation may be complex and nonlinear.
We therefore performed an explanatory analysis to evaluate the association between BMI and serum levels of several cytokines measured at ICU admission. Our initial hypothesis was that BMI would be associated with serum levels of several inflammatory markers. The subset of septic patients of a unicentric cohort of critically ill patients was used for this analysis.
2. Methods
This study is a subset analysis of a previous study that evaluated the association between components of acid-base status and inflammation in critically ill patients [11]. The study was approved by local ethical committee and is part of a larger project that aims to evaluate inflammation in critically ill patients (institutional approval number 1207/99).
Methods, including sample time and cytokine analysis, have been described elsewhere [11]. In brief, blood sample for cytokine analysis was collected in the morning following ICU admission. The 87 patients included in the main original study were consecutively admitted patients during the study period that fulfilled inclusion criteria and that were admitted from Sunday afternoon until early Friday morning, as it was technically not possible to process blood samples during weekends. Cytokine analysis was performed using Milliplex technology (Merck, Genese Diagnostics, Darmstadt, Germany). Illness severity was assessed through Simplified Acute Physiology Score 3 (SAPS3 score [12]). The following cytokines were included in this analysis: epidermal growth factor (EGF); vascular endothelial growth factor (VEGF); eotaxin; fibroblast growth factor 2 (FGF2); granulocyte macrophage colony-stimulating factor (GMCSF); granulocyte colony-stimulating factor (GCSF); fractalkine; interferon alpha and gamma (IFNα and IFNγ); interleukin (IL) 1α (IL1α); IL1β; IL1 receptor antagonist (IL1RA); IL2; IL3; IL4; IL5; IL6; IL7; IL8; IL9; IL10; IL12p40; and IL12p70; IL13; IL15; IL17; monocyte chemoattractant proteins (MCP) 1 and 3 (MCP1 and MCP3); macrophage inflammatory protein (MIP) 1α (MIP1α) and macrophage inflammatory protein 1β (MIP1β); tumor necrosis factor (TNF) α (TNFα) and tumor necrosis factor β (TNFβ). C-reactive protein (CRP) levels were also evaluated as a global marker of inflammation.
In order to evaluate the association and impact of a body mass index, a generalized additive model (GAM) was created to evaluate the association of each cytokine level with BMI. SAPS3 score was added to the model in order to account for multiple possible confounders, such as illness severity and age. The main advantage of GAM is that no inference is made a priori regarding the type of link between predictors and outcome variable. Therefore, nonlinear association between variables and predictors can be evaluated through GAM. GAM output provides P value for the association between each predictor and the variable of interest, as well as a graphical plot of variable values versus smooth terms and degrees of freedom. Both SAPS3 and BMI were added as smooth terms on GAM models. The smoothing parameter estimating method was GCV (generalized cross validation) criterion. Smoothing parameters are automatically selected in order to obtain the smallest GCV possible. One model was built for each cytokine investigated. When an association between cytokine and BMI was found, we further assessed the effects of adding other relevant variables to the model, specifically, gender, pulmonary source of infection, and diabetes.
3. Results
From the original 87 patients included in the main study, 41 patients were admitted due to sepsis. Thirty-three of those patients had weight measured and were therefore elected for analysis. We considered only weight values that were collected in the ward and/or emergency department before ICU admission or a body weight measured at ICU admission using a weighting machine. Patients with estimated body weight were not included. General features of the population are shown in Table 1. Mean age was 50.6 years (SD 19.6) and 60% were males. Mean body mass index was 25.2 (SD 5.8). BMI was similar in hospital survivors and nonsurvivors (26.1 ± 6.2 versus 23.8 ± 5.1; P = 0.256). Kernel density plot for BMI stratified by hospital discharge status is shown in Figure 1. The most common sources of infection were lungs (33%), bloodstream (9%), and soft tissue (12%). Mean SAPS3 score was 60 (IQ range 48–70). Most patients (73%) required vasopressors and close to half (42%) had acute kidney injury. Other features are shown in Table 1.
Table 1.
Demographic and clinical features.
| Number of patients | 33 |
|---|---|
| Age, years, mean ± SD | 50.6 ± 19.6 |
| Male sex, n (%) | 20 (60) |
| Body mass index, kg/m2, mean ± SD | 25.2 ± 5.8 |
| Source of infection | |
| Respiratory | 11 (33) |
| Bloodstream/intravascular catheter | 9 (27) |
| Soft tissue/fasciitis | 4 (12) |
| Abdominal | 3 (11) |
| Meningitis | 2 (6) |
| Urinary tract | 1 (3) |
| Other | 3 (11) |
| Illness severity | |
| SAPS 3 score, points, median [IQ] | 60 [48–70] |
| SOFA score, points, median [IQ] | 7 [5–9] |
| Vasopressor use, n (%) | 17 (73) |
| Mechanical ventilation, n (%) | 9 (27) |
| Acute kidney injury, n (%) | 14 (42) |
| Comorbidities | |
| Hypertension, n (%) | 15 (45) |
| Diabetes, n (%) | 11 (33) |
| Chronic kidney disease, n (%) | 8 (24) |
| Neoplasia, n (%) | 1 (3) |
| Cirrhosis, n (%) | 3 (10) |
| ICU mortality, n (%) | 10 (30) |
| Hospital mortality, n (%) | 13 (40) |
Figure 1.
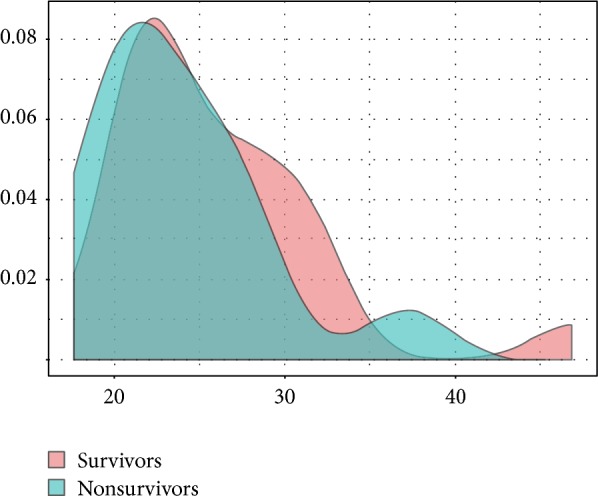
Density plot for BMI in survivors and nonsurvivors. There was no difference between groups (P = 0.256).
Cytokine levels and results for GAM analysis are shown in Table 2 and in Figures 2, 3, and 4. As shown in Table 2, EGF, VEGF, IL4, IL5, and IL13 were associated with BMI. Illness severity, as assessed by SAPS3 score, was associated with GCSF, IL1RA, IL6, IL8, IL15, MCP1, and CRP levels. The plots for BMI versus the smoothed coefficient for its association with cytokine levels are shown in Figures 2–4. The y-axis should be interpreted as how changes in BMI affect the mean value of the y-axis (i.e, cytokine value), with positive values meaning an increase and negative values a decrease. In brief, BMI over 30 kg/m2 was associated with higher EGF and VEGF levels (Figure 2). For EGF, the lower values were found for BMI between 25 and 30 kg/m2 (overweight patients). The association between BMI and IL4, IL5, and IL13 levels had a similar pattern (Figures 3 and 4), with higher interleukin values for low (<20 kg/m2) and high (above 30 kg/m2) BMI values, with a plateau between 20 and 30 kg/m2.
Table 2.
Serum levels and results for generalized additive model including SAPS 3 score and cytokine for the 33 included patients. ∗CRP levels are shown in mg/dL.
| Cytokine | Serum levels pg/mL∗ median [IQ] |
SAPS 3 score P value |
Body mass index P value |
|---|---|---|---|
| EGF | 153 [106; 290] | 0.458 | 0.001 |
| VEGF | 263 [153; 429] | 0.197 | <0.001 |
| Eotaxin | 103 [79.23; 145] | 0.200 | 0.159 |
| FGF2 | 121 [88.62; 153] | 0.133 | 0.100 |
| Fractalkine | 255 [147; 304] | 0.711 | 0.776 |
| GCSF | 253 [108; 591] | <0.001 | 0.111 |
| GMCSF | 47.45 [27.35; 66.68] | 0.577 | 0.625 |
| IFNα | 80.74 [58.01; 99.33] | 0.716 | 0.072 |
| IFNγ | 16.52 [7.37; 32.57] | 0.957 | 0.369 |
| IL1α | 50.42 [22.01; 153] | 0.453 | 0.404 |
| IL1β | 3.60 [1.49; 6.67] | 0.119 | 0.408 |
| IL1RA | 38.52 [17.33; 65.49] | 0.013 | 0.191 |
| IL2 | 10.03 [7.17; 14.82] | 0.567 | 0.945 |
| IL3 | 1.64 [1.06; 2.92] | 0.179 | 0.494 |
| IL4 | 18.11 [4.70; 33.67] | 0.566 | <0.001 |
| IL5 | 3.92 [2.25; 9.44] | 0.222 | <0.001 |
| IL6 | 70.48 [45.76; 393] | <0.001 | 0.188 |
| IL7 | 17.93 [12.95; 30.73] | 0.212 | 0.827 |
| IL8 | 70.7 [30.49; 132] | 0.012 | 0.087 |
| IL9 | 3.32 [1.74; 5.23] | 0.428 | 0.080 |
| IL10 | 53.74 [25.11; 101] | 0.471 | 0.916 |
| IL12p40 | 46.85 [26.63; 104] | 0.150 | 0.126 |
| IL12p70 | 10.63 [6.08; 22.58] | 0.680 | 0.879 |
| IL13 | 4.22 [1.64; 13.71] | 0.649 | <0.001 |
| IL15 | 10.62 [7.39; 18.07] | 0.020 | 0.368 |
| IL17 | 8.65 [3.2; 11.24] | 0.512 | 0.971 |
| MCP1 | 874 [325; 2101] | 0.017 | 0.311 |
| MCP3 | 42.68 [24.36; 69.91] | 0.559 | 0.275 |
| MIP1α | 25.24 [18.14; 49.78] | 0.463 | 0.728 |
| MIP1β | 75.14 [48.11; 97.04] | 0.715 | 0.533 |
| TNFα | 34.15 [26.96; 86.96] | 0.759 | 0.714 |
| TNFβ | 3.85 [2.78; 8.62] | 0.905 | 0.544 |
| CRP | 83.5 [9.91; 216.2] | 0.031 | 0.954 |
Figure 2.
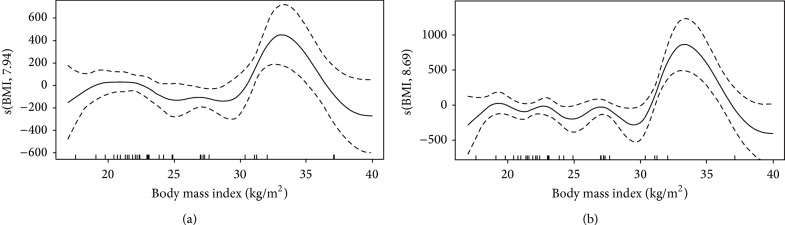
Association between EGF (a) and VEGF (b) and body mass index. Intervals are Bayesian credible intervals.
Figure 3.
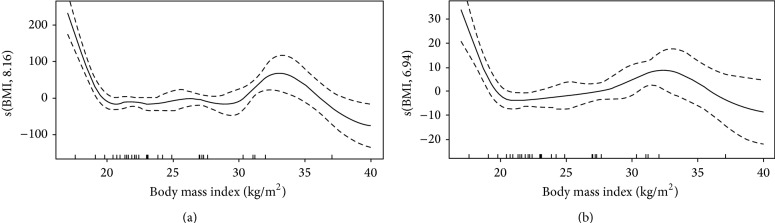
Association between IL4 (a) and IL5 (b) levels and body mass index. Intervals are Bayesian credible intervals.
Figure 4.
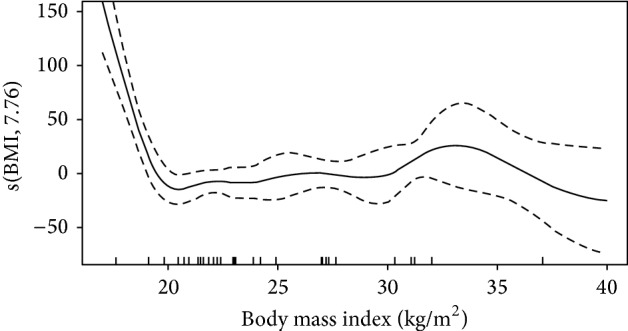
Association between IL13 levels and body mass index. Intervals are Bayesian credible intervals.
Additional models controlling for gender, pulmonary source of infection, and diabetes were built for EGF, VEGF, IL4, IL5, and IL13. Results are shown in Table 3. In brief, the effects of BMI remained even when other variables were added to the model (plots not shown). VEGF values were higher in female gender and when the lungs were the infection source. Lower IL13 values were found in diabetic patients (Table 3).
Table 3.
Results for the additional model built for EGF, VEGF, IL4, IL5, and IL13. This model included SAPS 3 score, BMI, gender, pulmonary source, and presence of diabetes. Estimates (SE) values are as follows: ∗163.75 (67.80); †150.95 (69.41); ‡−20.23 (7.00).
| Cytokine | SAPS 3 score P value |
Body mass index P values |
Female gender P value |
Presence of diabetes P value |
Pulmonary source P value |
|---|---|---|---|---|---|
| EGF | 0.386 | 0.020 | 0.620 | 0.253 | 0.066 |
| VEGF | 0.573 | <0.001 | 0.022∗ | 0.120 | 0.038† |
| IL4 | 0.673 | <0.001 | 0.314 | 0.056 | 0.782 |
| IL5 | 0.277 | <0.001 | 0.989 | 0.271 | 0.127 |
| IL13 | 0.597 | <0.001 | 0.913 | <0.001‡ | 0.122 |
4. Discussion
In this subgroup analysis, we found that EGF, VEGF, IL4, IL5, and IL13 are related to body mass index in a nonlinear way. There was a sharp increase for EGF and VEGF levels in obese patients. For interleukin values, the association followed a U shape distribution, with lower values found in the range of patients with normal weight and overweight.
The association between VEGF levels and sepsis has been previously reported [13]. We obtained high VEGF values, similar to other reports of VEGF levels in patients with septic shock [13]. VEGF may play an important role in both endothelial dysfunction and repair in sepsis [14]. Since endothelial dysfunction appears to be involved in the pathogenesis of organ failure [15], higher VEGF levels may be necessary to repair the endothelium after an infectious insult [14]. Our data suggests that even when illness severity is taken into account, VEGF levels tend to be higher in obese patients than in nonobese individuals, which may reflect a higher previous value in this population, as reported in [16], or an increase in the stimulus to endothelial repair in obese patients.
IL4 and IL13 are anti-inflammatory cytokine that increase in sepsis and may play important roles in immune regulation [17–19]. IL13 blockade in experimental sepsis has been shown to decrease survival and increase the expression of inflammatory cytokines [20]. Blanco-Quirós et al. suggested that in septic children lower IL13 values were associated with worst prognosis, although no association between the levels of other cytokines and IL13 was found [21]. The presence of higher values of IL4 and IL13 in obese patients may suggest that obesity is associated with a more anti-inflammatory cytokine expression during critical illness.
The association between BMI and cytokine levels was largely unaltered when gender, presence for diabetes, and pulmonary source of infection were added to the model (Table 3), suggesting that the findings are robust even when other variables are accounted for in the statistical model. The association between female gender and higher VEGF values has been suggested in animal models and may be mediated by estrogen [22, 23]. The association between pulmonary source of infection and higher VEGF values has been suggested in other scenarios, such as exacerbations of cystic fibrosis [24], hypoxia, and hantavirus infections [25]. The interaction between diabetes and IL13 levels is complex and deserves further specific studies [26].
There are several constrains in our analysis. First, and more importantly, we used a limited number of patients which limits our conclusions and the external validity of the analysis. Only six patients had a BMI above 30 kg/m2, which may limit our conclusions regarding higher BMI values. Second, we only evaluated cytokine levels at admission and we are therefore unable to evaluate if BMI would result in a different inflammation profile over time; additionally, time elapsed between ICU admission and blood sample was variable since blood was drawn in the morning after ICU arrival. Third, we have no data regarding the previous evolution of the patient before ICU precise elapsed time. Fourth, obesity may be associated with higher background values of some inflammatory mediators which could affect the results [27]. Finally, we are unable to address if changes in immune function associated with BMI have prognostic significance in critically ill patients.
5. Conclusion
BMI might influence inflammatory profile of critically ill patients admitted due to sepsis. This funding should be properly evaluated in larger series of critically ill individuals.
Acknowledgments
The authors would like to thank Dr. Francisco Aguiar for database creation and Dr. Fernanda J. Aguiar for her critical review of the paper.
Abbreviations
- BMI:
Body mass index
- EGF:
Epidermal growth factor
- FGF2:
Fibroblast growth factor 2
- GAM:
Generalized additive model
- GCV:
Generalized cross validation
- GCSF:
Granulocyte colony-stimulating factor
- GMCSF:
Granulocyte macrophage colony-stimulating factor
- IFN:
Interferon
- IL:
Interleukin
- IL1RA:
IL1 receptor antagonist (IL1RA)
- MCP:
Monocyte chemoattractant protein
- MIP:
Macrophage inflammatory protein
- SAPS3:
Simplified acute physiology state score 3
- TNF:
Tumor necrosis factor
- VEGF:
Vascular endothelial growth factor.
Conflict of Interests
The authors have no competing interests to declare.
Authors' Contribution
Fernando G. Zampieri helped in the conception and design of the study and collection of patient samples, performed statistical analysis, and wrote the paper. Heraldo P. de Souza helped in the design of the study and revised the paper for important intellectual content. Vanessa Jacob performed biochemical analysis and revised the paper for important intellectual content. Hermes V. Barbeiro performed biochemical analysis and revised the paper for important intellectual content. Fabiano Pinheiro da Silva helped in the conception and design of the study and helped in the paper preparation. All authors read and approved the final version of the paper. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- 1.Vincent J.-L., Sakr Y., Sprung C. L., et al. Sepsis in European intensive care units: results of the SOAP study. Critical Care Medicine. 2006;34(2):344–353. doi: 10.1097/01.ccm.0000194725.48928.3a. [DOI] [PubMed] [Google Scholar]
- 2.Namas R., Zamora R., An G., et al. Sepsis: something old, something new, and a systems view. Journal of Critical Care. 2012;27(3):314.e1–314.e11. doi: 10.1016/j.jcrc.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaffer U., Wade R. G., Gourlay T. Cytokines in the systemic inflammatory response syndrome: a review. HSR Proceedings in Intensive Care and Cardiovascular Anesthesia. 2010;2(3):161–175. [PMC free article] [PubMed] [Google Scholar]
- 4.Reade M. C., Yende S., D'Angelo G., et al. Differences in immune response may explain lower survival among older men with pneumonia. Critical Care Medicine. 2009;37(5):1655–1662. doi: 10.1097/ccm.0b013e31819da853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schröder J., Kahlke V., Staubach K.-H., Zabel P., Stüber F. Gender differences in human sepsis. Archives of Surgery. 1998;133(11):1200–1205. doi: 10.1001/archsurg.133.11.1200. [DOI] [PubMed] [Google Scholar]
- 6.Festa A., D'Agostino R., Jr., Williams K., et al. The relation of body fat mass and distribution to markers of chronic inflammation. International Journal of Obesity. 2001;25(10):1407–1415. doi: 10.1038/sj.ijo.0801792. [DOI] [PubMed] [Google Scholar]
- 7.Calle E. E., Thun M. J., Petrelli J. M., Rodriguez C., Heath C. W., Jr. Body-mass index and mortality in a prospective cohort of U.S. adults. The New England Journal of Medicine. 1999;341(15):1097–1105. doi: 10.1056/nejm199910073411501. [DOI] [PubMed] [Google Scholar]
- 8.Amundson D. E., Djurkovic S., Matwiyoff G. N. The obesity paradox. Critical Care Clinics. 2010;26(4):583–596. doi: 10.1016/j.ccc.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 9.van Eijk L. T., van der Pluijm R. W., Ramakers B. P. C., et al. Body mass index is not associated with cytokine induction during experimental human endotoxemia. Innate Immunity. 2014;20(1):61–67. doi: 10.1177/1753425913481821. [DOI] [PubMed] [Google Scholar]
- 10.Kahraman S., Yilmaz R., Akinci D., et al. U-shaped association of body mass index with inflammation and atherosclerosis in hemodialysis patients. Journal of Renal Nutrition. 2005;15(4):377–386. doi: 10.1053/j.jrn.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Zampieri F. G., Kellum J. A., Park M., et al. Relationship between acid–base status and inflammation in the critically ill. Critical Care. 2014;18(4):p. R154. doi: 10.1186/cc13993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moreno R. P., Metnitz P. G. H., Almeida E., et al. SAPS 3—from evaluation of the patient to evaluation of the intensive care unit. Part 2: development of a prognostic model for hospital mortality at ICU admission. Intensive Care Medicine. 2005;31(10):1345–1355. doi: 10.1007/s00134-005-2763-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang K.-Y., Liu K.-T., Chen Y.-C., et al. Plasma soluble vascular endothelial growth factor receptor-1 levels predict outcomes of pneumonia-related septic shock patients: a prospective observational study. Critical Care. 2011;15(1, article R11) doi: 10.1186/cc9412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang R.-Y., Liu Y.-Y., Qu H.-P., Tang Y.-Q. The angiogenic factors and their soluble receptors in sepsis: friend, foe, or both? Critical Care. 2013;17(4, article 446) doi: 10.1186/cc12857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aird W. C. The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood. 2003;101(10):3765–3777. doi: 10.1182/blood-2002-06-1887. [DOI] [PubMed] [Google Scholar]
- 16.Elfving B., Buttenschøn H. N., Foldager L., et al. Depression and BMI influences the serum vascular endothelial growth factor level. The International Journal of Neuropsychopharmacology. 2014;17(9):1409–1417. doi: 10.1017/S1461145714000273. [DOI] [PubMed] [Google Scholar]
- 17.Ng P. C., Li K., Wong R. P., et al. Proinflammatory and anti-inflammatory cytokine responses in preterm infants with systemic infections. Archives of Disease in Childhood. Fetal and Neonatal Edition. 2003;88(3):F209–F213. doi: 10.1136/fn.88.3.f209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Socha L. A., Gowardman J., Silva D., Correcha M., Petrosky N. Elevation in interleukin 13 levels in patients diagnosed with systemic inflammatory response syndrome. Intensive Care Medicine. 2006;32(2):244–250. doi: 10.1007/s00134-005-0020-6. [DOI] [PubMed] [Google Scholar]
- 19.Collighan N., Giannoudis P. V., Kourgeraki O., Perry S. L., Guillou P. J., Bellamy M. C. Interleukin 13 and inflammatory markers in human sepsis. British Journal of Surgery. 2004;91(6):762–768. doi: 10.1002/bjs.4521. [DOI] [PubMed] [Google Scholar]
- 20.Matsukawa A., Hogaboam C. M., Lukacs N. W., et al. Expression and contribution of endogenous IL-13 in an experimental model of sepsis. Journal of Immunology. 2000;164(5):2738–2744. doi: 10.4049/jimmunol.164.5.2738. [DOI] [PubMed] [Google Scholar]
- 21.Blanco-Quirós A., Casado-Flores J., Adrados J. A. G., Moro M. N., Antón J. A., Sanz E. A. Interleukin-13 is involved in the survival of children with sepsis. Acta Paediatrica. 2005;94(12):1828–1831. doi: 10.1080/08035250500275162. [DOI] [PubMed] [Google Scholar]
- 22.Crisostomo P. R., Wang M., Herring C. M., et al. Gender differences in injury induced mesenchymal stem cell apoptosis and VEGF, TNF, IL-6 expression: role of the 55 kDa TNF receptor (TNFR1) Journal of Molecular and Cellular Cardiology. 2007;42(1):142–149. doi: 10.1016/j.yjmcc.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang D.-H., Yu E. S., Yoon K.-I., Johnson R. The impact of gender on progression of renal disease: potential role of estrogen-mediated vascular endothelial growth factor regulation and vascular protection. American Journal of Pathology. 2004;164(2):679–688. doi: 10.1016/s0002-9440(10)63155-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McColley S. A., Stellmach V., Boas S. R., Jain M., Crawford S. E. Serum vascular endothelial growth factor is elevated in cystic fibrosis and decreases with treatment of acute pulmonary exacerbation. American Journal of Respiratory and Critical Care Medicine. 2000;161(6):1877–1880. doi: 10.1164/ajrccm.161.6.9905022. [DOI] [PubMed] [Google Scholar]
- 25.Gavrilovskaya I., Gorbunova E., Koster F., MacKow E. Elevated vegf levels in pulmonary edema fluid and pbmcs from patients with acute hantavirus pulmonary syndrome. Advances in Virology. 2012;2012:8. doi: 10.1155/2012/674360.674360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson C. Diabetes: IL-13 and glucose homeostasis. Nature Reviews Endocrinology. 2013;9(3):134–134. doi: 10.1038/nrendo.2013.10. [DOI] [PubMed] [Google Scholar]
- 27.Siervo M., Ruggiero D., Sorice R., et al. Body mass index is directly associated with biomarkers of angiogenesis and inflammation in children and adolescents. Nutrition. 2012;28(3):262–266. doi: 10.1016/j.nut.2011.06.007. [DOI] [PubMed] [Google Scholar]


