Abstract
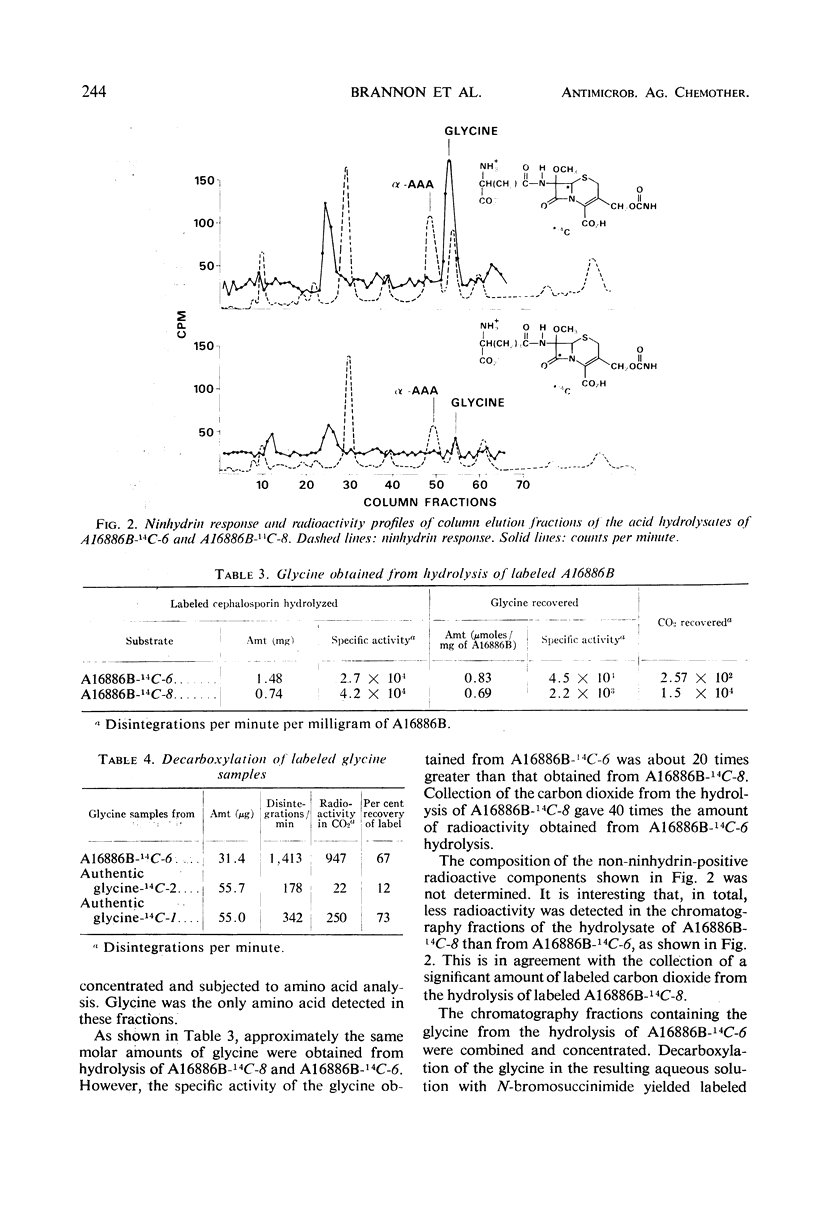
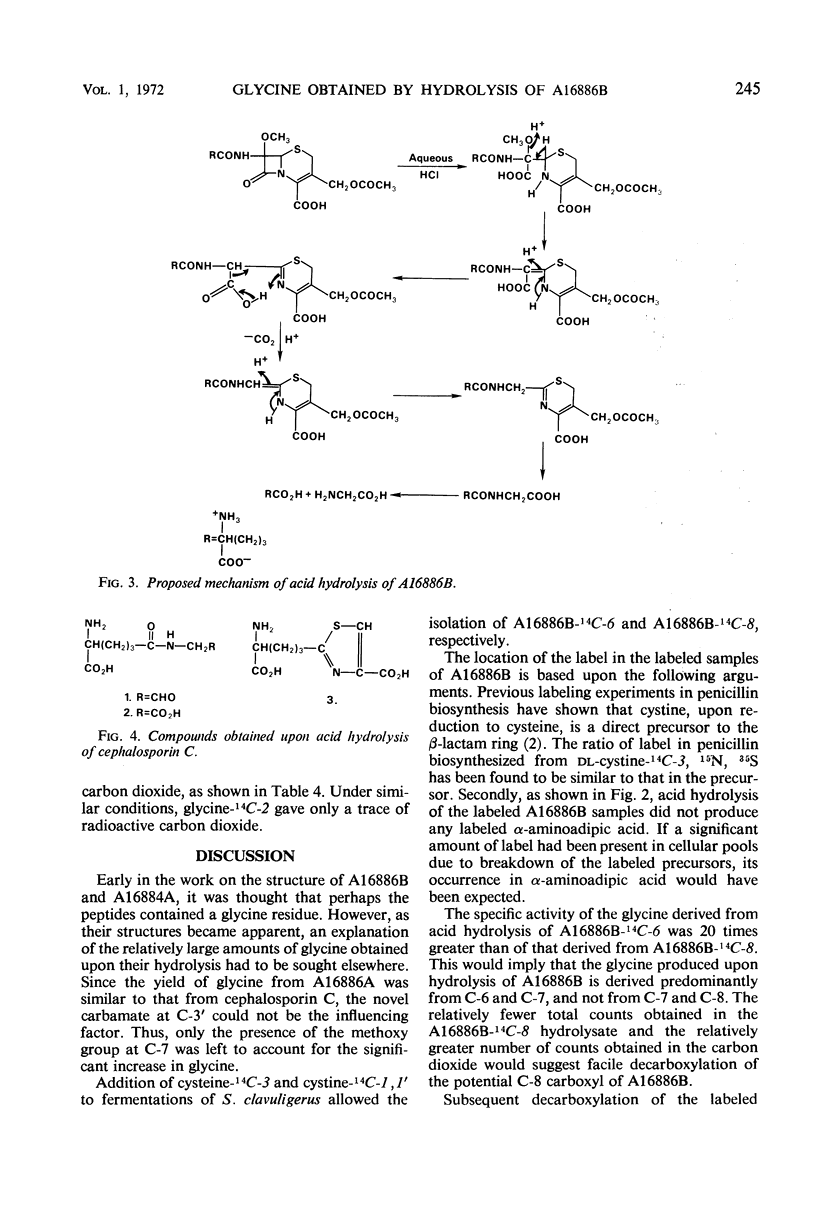
Structural analysis of two new β-lactam antibiotics, A16884A and A16886B, indicated that they, like cephalosporin C, were composed of modified valine and cysteine residues, and α-aminoadipic acid. However, acid hydrolysis of A16886B and A16884A produced three times as much glycine as did hydrolysis of cephalosporin C under the same conditions. Samples of A16886B-14C-6 and A16886B-14C-8 were prepared by the addition of cysteine-14C-3 and cystine-14C-1 to fermentations of Streptomyces clavuligerus. The specific activity of glycine obtained from hydrolysis of A16886B-14C-6 was considerably higher than that from hydrolysis of A16886B-14C-8. An explanation for the difference in amounts of glycine obtained from hydrolysis of these antibiotics is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAHAM E. P., NEWTON G. G. Synthesis of D-delta-amino-delta-carboxyvalerylglycine (a degradation product of cephalosporin N) and of DL-delta-amino-delta-carboxyvaleramide. Biochem J. 1954 Oct;58(2):266–268. doi: 10.1042/bj0580266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARNSTEIN H. R., GRANT P. T. The biosynthesis of penicillin. 2. The incorporation of cystine into penicillin. Biochem J. 1954 Jul;57(3):360–368. doi: 10.1042/bj0570360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAPPELLE E. W., LUCK J. M. The decarboxylation of amino acids, proteins, and peptides by N-bromosucclnimide. J Biol Chem. 1957 Nov;229(1):171–179. [PubMed] [Google Scholar]
- JEFFERY J. D., ABRAHAM E. P., NEWTON G. G. Further degradation products of cephalosporin C. Isolation and synthesis of 2-(4-amino-4-carboxybutyl)thiazole-4-carboxylic acid. Biochem J. 1960 May;75:216–223. doi: 10.1042/bj0750216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarajan R., Boeck L. D., Gorman M., Hamill R. L., Higgens C. E., Hoehn M. M., Stark W. M., Whitney J. G. Beta-lactam antibiotics from Streptomyces. J Am Chem Soc. 1971 May 5;93(9):2308–2310. doi: 10.1021/ja00738a035. [DOI] [PubMed] [Google Scholar]
- Whitney J. G., Brannon D. R., Mabe J. A., Wicker K. J. Incorporation of labeled precursors into A16886B, a novel -lactam antibiotic produced by Streptomyces clavuligerus. Antimicrob Agents Chemother. 1972 Mar;1(3):247–251. doi: 10.1128/aac.1.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]



