Abstract
Co-morbid use of nicotine-containing tobacco products and alcohol (ethanol) is prevalent in young adults initiating use and in alcohol dependent adults, suggesting that these drugs in combination may increase risk to develop dependence on one or both drugs. Neuroadaptations caused by repeated drug exposure are related to the development of drug dependence and vulnerability to relapse. Locomotor sensitization has been used as a behavioral measure used to detect changes in neural drug sensitivity that are thought to contribute to drug dependence and relapse. Locomotor sensitization was measured in the current studies to examine potential differences in the effects of nicotine and ethanol given alone and in combination. Baseline activity levels of DBA/2J mice were assessed on 2 days, then mice were treated for ten days with saline, nicotine (1 or 2 mg/kg of nicotine tartrate), ethanol (1 or 2 g/kg), or nicotine plus ethanol and locomotor activity was assessed every third day. On the following day, all mice were challenged with ethanol to measure the expression of sensitization. Mice treated with both nicotine and ethanol exhibited greater stimulation than predicted from the combined independent effects of these drugs, consistent with our previously published results. The combined effects of nicotine and ethanol on locomotor sensitization were dependent on the dose of ethanol and whether testing was performed after the drugs were given together, or after challenge with ethanol alone. These results suggest that nicotine and ethanol in combination can have neuroadaptive effects that differ from the independent effects of these drugs.
Keywords: Alcohol, Mice, DBA/2J, stimulation, comorbidity, activity
1. Introduction
Alcohol (ethanol) and nicotine-containing tobacco products are two of the most commonly used psychoactive substances and their excessive use remains at the top of the list of preventable causes of death (Rehm et al. 2009; Danaei et al. 2009). Epidemiological studies have consistently found that nicotine and ethanol share a high rate of co-abuse (Anthony & Echeagaray-Wagner 2000; Falk et al. 2006). Young adults were found to co-use tobacco and ethanol at high rates (Weitzman & Chen et al., 2005; Dierker et al., 2006; Nichter et al., 2010) and binge drinking has been found to be predictive of smoking status (Jiang & Ling, 2013). Further, very high rates of smoking have been reported in individuals diagnosed with ethanol use disorders (Kozlowski et al., 1986; Sobell, 2002; Marks et al., 1997) and individuals who smoke and drink were found to have greater severity of ethanol dependence (Daeppen et al., 2000) and greater difficulty quitting both drugs (Hymowitz et al., 1997; Tsoh et al., 2011). This suggests that nicotine and ethanol in combination may have profound combined effects that may contribute to the development and severity of dependence to one or both drugs.
Our previously published work found that doses of nicotine that did not have stimulant effects enhanced the acute locomotor stimulant effect of ethanol in DBA/2J mice (Gubner et al., 2013). One potential interpretation of this finding is that these drugs in combination cause enhanced activation of the mesolimbic dopamine system. Activation of the mesolimbic dopamine system has been strongly implicated in mediating drug craving and reward (Wise & Bozarth 1987) and to play a role in drug-induced locomotor stimulation. Thus, drug-induced stimulation, in part, provides a behavioral model to assess activation of this system (Wise & Bozarth 1987; Phillips & Shen, 1996). It is possible that nicotine and ethanol in combination cause greater activation of brain pathways that are involved in drug reward and neuroadaptation than caused by either drug alone. In fact, our previous data suggest greater than additive effects of the drugs in combination (Gubner et al., 2013).
In the current studies, the hypothesis that nicotine enhances the development of neuroadaptations that contribute to the development of ethanol dependence was investigated by measuring the effect of nicotine on the development of ethanol-induced behavioral sensitization, using a mouse model. Repeated exposure to drugs of abuse can cause an enhanced behavioral response (e.g. locomotor activation), such that the same dose of drug results in a greater response than that seen initially, a process called behavioral sensitization. Magnitude of sensitization provides a behavioral index of underlying neuroadaptation caused by repeated drug exposure (for reviews see Phillips et al., 2011; Steketee & Kalivas 2011). The altered neurochemical mechanisms underlying behavioral sensitization are thought to be related to the development of drug dependence and vulnerability to relapse (Pastor et al., 2008; Kalivas et al., 2005; Robinson & Berridge, 1993). Behavioral sensitization can be measured experimentally by changes in locomotor activity (Champtiaux et al., 2006). For these studies, DBA/2J mice were used because they are an inbred strain of mice that is particularly sensitive to ethanol-induced locomotor sensitization, whereas some other inbred strains, such as C57BL/6J mice, show low sensitivity to this effect (Phillips et al., 1994; Lessov et al., 2001; Meyer et al., 2005). While nicotine has been found to induce locomotor sensitization in rats, limited effects have been reported in mice (see DiFranza & Wellman for review). However, mecamylamine, a nonselective nicotinic acetylcholine receptor (nAChR) antagonist, was found to block the development and expression of ethanol-induced sensitization (Bhutada et al., 2010), indicating a role for nAChR-mediated processes. What is not known is whether nicotine in combination with ethanol enhances the development of behavioral sensitization. If this hypothesis is correct, it would suggest that these drugs in combination could increase risk for dependence by enhancing neural changes that drive compulsive drug use.
2. Materials and methods
2.1. Animals
Male and female DBA/2J mice were purchased from The Jackson Laboratory (Sacramento, CA) and group housed (2–4 per cage). All mice were allowed to acclimate for at least 2 weeks after arrival before testing began and behavioral testing began when mice were 57–71 days old. All mice were maintained in standard mouse shoebox cages (28.5 L x 17.5 W x 12 H cm) lined with Bed-o’Cobs® bedding (The Andersons, Inc., Maumee, OH, USA) and had ad libitum access to water and food (LabDiet® 5001, PMI Nutrition International LLC, St. Louis, MO, USA) that was purchased from Animal Specialties Inc. (Hubbard, OR, USA). DBA/2J mice were used in these studies because of their high sensitivity to ethanol-induced locomotor sensitization (Phillips et al., 1994; Lessov et al., 2001; Meyer et al., 2005; Meyer & Phillips 2003). All mice were experiment- and drug-naïve prior to testing, and behavioral testing was conducted during the light phase of the 12:12 h light:dark cycle (lights on at 0600 h), between 0800 and 1600 h. Data were collected in four total passes; two passes each for the 1 and 2 g/kg ethanol dose experiments.
2.2. Drugs
Ethyl alcohol was purchased from Decon Laboratories Inc. (King of Prussia, PA, USA). Nicotine tartrate salt was purchased from Sigma Aldrich (St. Louis, MO, USA). All drugs used in the behavioral studies were prepared in physiological (0.9%) saline (Baxter Healthcare Corp., Deerfield, IL, USA) and administered as intraperitoneal (IP) injections in a volume of 20 ml/kg. Nicotine and ethanol combined doses were delivered together in a cocktail (wt/vol solution, pH 3.6 – 3.8), consistent with our previously published work (Gubner et al., 2013). Doses of nicotine are expressed as mg/kg of the tartrate salt (1 mg nicotine tartrate = 0.33 mg freebase nicotine).
2.3. Locomotor sensitization and testing procedures
Locomotor activity was measured using sixteen automated locomotor activity monitors made by AccuScan Instruments, Inc. (Columbus, OH, USA). Each monitor was equipped with 16 photocell beams located 2 cm above the 40 W x 40 L x 30 H cm clear acrylic chamber floor, with corresponding photocell detectors located on opposite sides. A computer was used to record beam breaks, which were converted into horizontal distance traveled (in centimeters) using VERSADAT version 1.8 software (AccuScan Instruments, Inc.). Each monitor was enclosed in an Environmental Control Chamber constructed from PVC/lexan (AccuScan Instruments, Inc.) and each chamber was equipped with a fan that provided ventilation and background noise, and was illuminated by a 3.3 Watt incandescent light bulb that was on during testing.
The effect of nicotine on ethanol-induced locomotor sensitization was examined using an established procedure for producing ethanol-induced sensitization in DBA/2J mice (Phillips et al., 1994; Lessov et al., 2001; Meyer et al., 2005; Meyer & Phillips 2003) across a 13-day period (see Table 1 for treatment schedule and dose groups). Days 1 and 2 were used to assess baseline locomotor activity. On these days, all mice received an IP injection of saline immediately before being placed into the locomotor activity chamber. Day 1 testing familiarized the animals with all handling and testing procedures; day 2 (habituated baseline) testing provided a measure of baseline activity collected under now familiar conditions. Over the next 10 days (acquisition phase), mice were injected with their dose group-specific treatment and locomotor activity was tested every third day (see Table 1). On day 13, all mice were challenged with ethanol to allow for a between-group assessment of sensitization. This also allowed us to determine if repeated treatment with nicotine and ethanol in combination altered the response to ethanol alone. On each of the days when locomotor activity was assessed, mice were moved into the testing room 45 minutes prior to the start of testing to acclimate to the test room environment, and mice were weighed, injected with the group-specific treatment, and immediately placed into the locomotor activity monitors for 15 min. On days when activity was not assessed, mice were weighed, injected, and returned to their home cages. Immediately after activity testing on day 13, a 20μl periorbital sinus blood sample was obtained from ethanol-treated mice with a calibrated glass micro-Hematocrit capillary tube (Fisher Scientific, city state) and used to determine blood ethanol concentration (BEC). Blood samples were processed and analyzed, using an established gas chromatography method (Boehm et al., 2000).
Table 1.
Outline of experimental groups for the nicotine + ethanol sensitization studies
| Baseline | Acquisition | Ethanol Challenge | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment Groups Day 3–12 (Day 13) | Day 1–2 | Day 3 | Day 4–5 | Day 6 | Day 7–8 | Day 9 | Day 10–11 | Day 12 | Day 13 |
| 1 g/kg ethanol groups | |||||||||
| SAL (E1) | SAL | SAL | SAL | SAL | SAL | SAL | SAL | SAL | (E1) |
| N1 (E1) | SAL | N1 | N1 | N1 | N1 | N1 | N1 | N1 | (E1) |
| N2 (E1) | SAL | N2 | N2 | N2 | N2 | N2 | N2 | N2 | (E1) |
| E1 (E1) | SAL | E1 | E1 | E1 | E1 | E1 | E1 | E1 | (E1) |
| N1+E1 (E1) | SAL | N1+E1 | N1+E1 | N1+E1 | N1+E1 | N1+E1 | N1+E1 | N1+E1 | (E1) |
| N2+E1 (E1) | SAL | N2+E1 | N2+E1 | N2+E1 | N2+E1 | N2+E1 | N2+E1 | N2+E1 | (E1) |
| 2 g/kg ethanol groups | |||||||||
| SAL (E2) | SAL | SAL | SAL | SAL | SAL | SAL | SAL | SAL | (E2) |
| N1 (E2) | SAL | N1 | N1 | N1 | N1 | N1 | N1 | N1 | (E2) |
| N2 (E2) | SAL | N2 | N2 | N2 | N2 | N2 | N2 | N2 | (E2) |
| E2 (E2) | SAL | E2 | E2 | E2 | E2 | E2 | E2 | E2 | (E2) |
| N1+E2 (E2) | SAL | N1+E2 | N1+E2 | N1+E2 | N1+E2 | N1+E2 | N1+E2 | N1+E2 | (E2) |
| N2+E2 (E2) | SAL | N2+E2 | N2+E2 | N2+E2 | N2+E2 | N2+E2 | N2+E2 | N2+E2 | (E2) |
| Activity test | Yes | Yes | No | Yes | No | Yes | No | Yes | Yes & Blood |
“E1” or “E2” = 1 or 2 g/kg ethanol. “SAL”= 0.9% Saline. “N1” or “N2” = 1 or 2 mg/kg of nicotine tartrate. Yes = activity test occurs. No = no activity test.
2.4. Data analysis
All statistical analyses were performed using Statistica 12 software (StatSoft, Tulsa, OK, USA). Data were analyzed by factorial ANOVA, with repeated measures (RM-ANOVA) when appropriate. Significant interactions involving multiple factors were followed by ANOVA including fewer factors to determine the sources of interaction. Two-way interactions were interpreted using simple main effects analysis and Newman-Keuls post-hoc mean comparisons when appropriate. Male and female animals were used in all studies; sex was first included as a factor and then follow-up analyses were performed with data from the sexes combined, when sex did not interact with other factors. Effects were considered significant at an alpha level of 0.05 or less. For the sensitization studies, data were first analyzed by repeated measures ANOVA, including baseline day 2 and all other activity test day data. Significant interaction effects can be difficult to detect when a large number of groups or days are present and effects are expected in only a small number of groups or on only a single day, as is the case for the sensitization studies (see Wahlsten, 1990). Therefore, for some analyses, we used composite drug treatment (nicotine plus ethanol group) as a factor. In addition, to provide a measure of drug response, locomotor scores on days 3 and 13 were corrected for individual day 2 baseline activity scores. This provides a measure of locomotor response attributed to the drug treatment that eliminates possible influences of individual differences in baseline activity level. This method is consistent with our previous published work (Phillips et al., 1995; Kamens & Phillips 2008; Palmer et al., 2002; Gubner et al., 2013). Similarly, to detect sensitization during the acquisition phase, day 3 acute data were subtracted from final drug score data collected on day 12.
3. Results
3.1. Effects of nicotine on the development of 1 g/kg ethanol-induced locomotor sensitization
The first study examined the effects of nicotine on the development of locomotor sensitization induced by 1 g/kg ethanol. This dose of ethanol is a submaximal dose (to avoid ceiling effects) for inducing behavioral sensitization and it was hypothesized that nicotine would enhance the behavioral sensitization seen in response to 1 g/kg alone. Group size for the current study was 6–8/sex/treatment group. In general, males had greater locomotor activity scores compared to females. However, similar patterns for the drug groups were seen for both sexes and there were no significant interactions involving sex, so data were combined for males and females in subsequent analyses. There were significant time-dependent effects with the largest ethanol effects on locomotor activity seen during the first 5 min of the 15-min test. This is consistent with our previously published work (e.g., Shen et al., 1995) and corresponds with the rising phase of the blood ethanol curve (Goldstein 1983). Prior analyses have indicated that the first 5 min after ethanol treatment represents a time when purely stimulant effects of ethanol are seen that are devoid of depressant responses to ethanol (Phillips et al., 1995). In addition, this corresponds to peak nicotine levels in the mouse brain after an IP injection of 1 mg/kg nicotine (Petersen et al., 1984). Examination of the time-course data from the current study determined that the first 5-min time point best represents the drug effects seen in this study. Therefore, locomotor activity data from this time period are shown in Fig 1. A repeated measures ANOVA identified a significant day x treatment group interaction for this time period (F[25,430] = 29.85, p < 0.001).
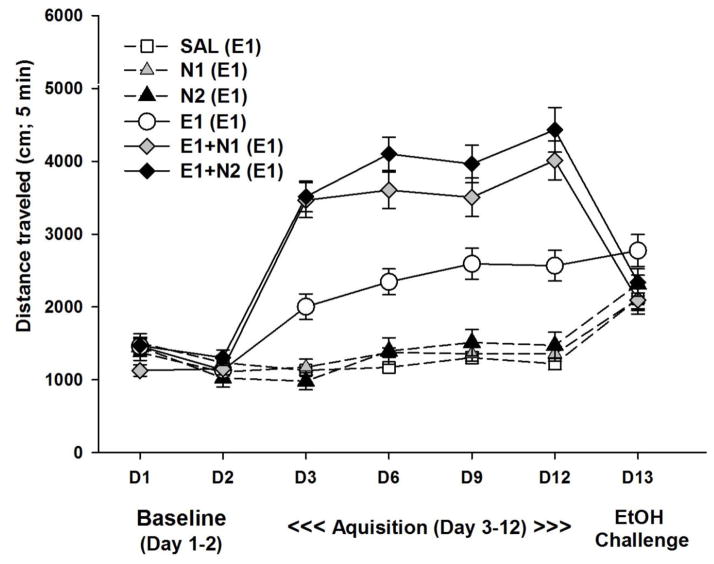
Figure 1.
Effects of nicotine on the development of locomotor sensitization to 1 g/kg ethanol. Shown is mean (± SEM) total distance traveled during the first 5 min of each 15-min locomotor activity test session. On day 1 and 2, mice received saline. On day 3 –12 (acquisition period) mice were treated with SAL, E1, N1, N2, E1+N1, or E1+N2 once daily, with locomotor activity assessed every third day. On day 13 (ethanol challenge), all mice were treated with 1 g/kg ethanol alone. Group labels show treatment during acquisition with day 13 treatment in parentheses. N1 and N2 = 1 or 2 mg/kg nicotine tartrate; E1= 1 g/kg ethanol; SAL=saline.
Data were next examined for the acute drug response, measured as day 3 locomotor response corrected for day 2 baseline (Fig. 2A). This analysis found a significant effect of treatment group (F[5,86] = 54.30, p < 0.001). Mice treated with 1 g/kg ethanol alone, but not nicotine alone (1 or 2 mg/kg nicotine tartrate), had significantly (P<0.001) larger acute drug response scores compared to the saline-treated group. Mice treated with either dose of nicotine in combination with 1 g/kg ethanol had larger acute drug response scores than both the ethanol alone treated group (p<0.001) and the saline treated group (p<0.001). Nicotine enhancement of ethanol-induced locomotor stimulation is consistent with our previous findings (Gubner et al., 2013).
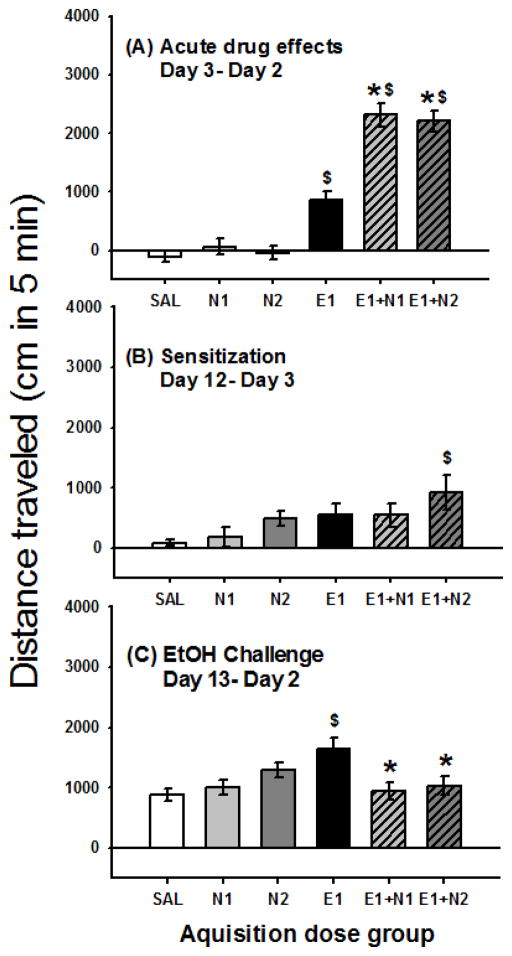
Figure 2.
Locomotor effects of nicotine and 1 g/kg ethanol. Shown is mean (± SEM) distanced traveled (cm) during the first 5 min of the 15-min activity session for (A) acute drug effect corrected for day 2 baseline (day 3- day 2); (B) sensitization during acquisition (day 12- day 3); and (C) locomotor response to the 1 g/kg ethanol challenge corrected for day 2 baseline (day 13 –day 2). Drug treatment during acquisition is shown on the x axis. N1 and N2 = 1 or 2 mg/kg nicotine tartrate; E1= 1 g/kg ethanol; SAL=saline. *: p < 0.001; for the comparison of the indicated group with the E1 group. $: p < 0.01; for the comparison of the indicated group with the SAL group.
Magnitude of sensitization during the acquisition period was measured as the change in locomotor response from the last day of acquisition minus the first time animals received drug (day 12 - day 3). These data are shown in Fig. 2B. For the day 12 - day 3 locomotor response, there was a significant effect of treatment group (F(5,86) = 2.55, p < 0.05). Only the mice treated repeatedly with 1 g/kg ethanol in combination with 2 mg/kg nicotine tartrate (E1+N2) had a significantly (P<0.05) larger sensitization score compared to the repeated saline treated group. However, the E1+N2 group was not significantly different from the E1 alone group, suggesting that this dose combination induced only a modest increase in sensitization.
On the ethanol challenge (day 13), all groups of mice were treated with 1 g/kg ethanol alone (Fig. 2C). There was a significant effect of treatment group (F[5,86] = 4.10, p < 0.01) for locomotor activity after ethanol challenge corrected for day 2 baseline activity level (day 13-day 2). Only the mice that were repeatedly treated with 1 g/kg ethanol alone had a significantly (P<0.05) larger locomotor response to ethanol challenge compared to mice repeatedly treated with saline. Interestingly, mice that received ethanol in combination with nicotine during acquisition (E1+N1 or E1+N2) had similar responses to ethanol alone, compared to mice that received saline during acquisition and were challenged with ethanol for the first time on day 13. Furthermore, both the E1+N1 and E1+N2 groups had significantly (p<0.01) lower locomotor response to the ethanol challenge compared to the group treated with E1 during acquisition. There were no significant differences between the groups for BEC on day 13; BEC was 0.62 ± 0.02 mg/ml for the group average and group means ranged from 0.59 ± 0.04 to 0.67 ± 0.04 mg/ml.
3.2. Effects of nicotine on the development of 2 g/kg ethanol-induced locomotor sensitization
Due to the limited effects seen with the 1 g/kg ethanol dose, a higher dose of ethanol (2 g/kg) that is more typically used to induce locomotor sensitization in DBA/2J mice was tested. Group size for the current study was 10–12/sex/treatment group. Similar to results from the first study, the largest ethanol effects on locomotor activity were seen during the first 5 min of the 15-min test and data analysis methods were matched to those used for the previous study examining the lower dose of ethanol. In general, males had greater locomotor activity scores compared to females. However, similar patterns were again seen for the two sexes and data for subsequent analyses were combined for males and females (Fig 3). A repeated measures ANOVA identified a significant day x treatment group interaction (F[25,580] = 85.31, p < 0.001).
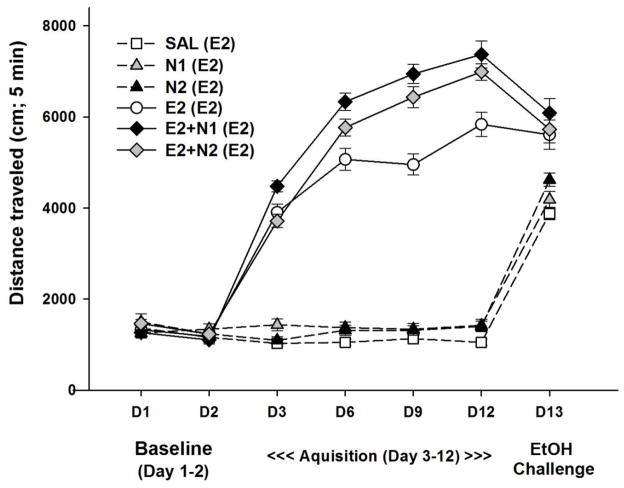
Figure 3.
Effects of nicotine on the development of locomotor sensitization to 2 g/kg ethanol. Shown is mean (± SEM) total distance traveled during the first 5 min of each 15-min locomotor activity test session. On day 1 and 2, mice received saline. On day 3 –12 (acquisition period) mice were treated with SAL, E2, N1, N2, E2+N1, or E2+N2 once daily, with locomotor activity assessed every third day. On day 13 (ethanol challenge) all mice were treated with 2 g/kg ethanol alone. Group labels show treatment during acquisition with day 13 treatment in parentheses. N1 and N2 = 1 or 2 mg/kg nicotine tartrate; E2= 2 g/kg ethanol; SAL=saline.
Data were next examined for the day 3 locomotor response corrected for day 2 baseline to examine the effect of acute treatment (Fig. 4A). This analysis found a significant effect of treatment group (F[5,122] = 155.94, p < 0.001). Mice treated with 2 g/kg ethanol alone or in combination with nicotine had significantly (P<0.001) larger acute drug response scores compared to the saline-treated group. In addition, mice treated with E2+N1, but not E2+N2, had a larger acute drug response score than the ethanol alone group.
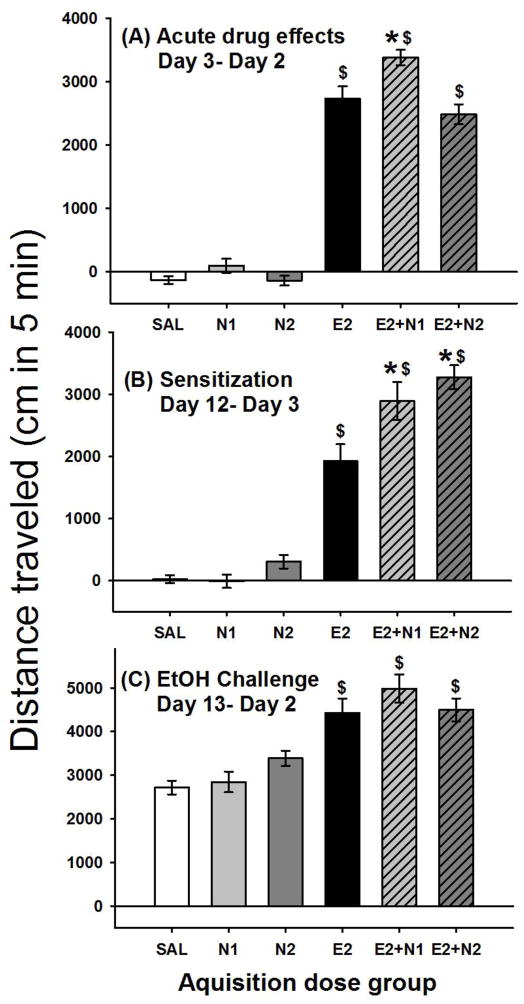
Figure 4.
Locomotor effects of nicotine and 2 g/kg ethanol. Shown is mean (± SEM) distanced traveled (cm) during the first 5 min of the 15-min activity session for (A) acute drug effect corrected for day 2 baseline (day 3- day 2); (B) sensitization during acquisition (day 12- day 3); and (C) locomotor response to the 2 g/kg ethanol challenge corrected for day 2 baseline (day 13 –day 2). Drug treatment during acquisition is shown on the x axis. N1 and N2 = 1 or 2 mg/kg nicotine tartrate; E2= 2 g/kg ethanol; SAL=saline. *: p < 0.001; for the comparison of the indicated group with the E2 group. $: p < 0.01; for the comparison of the indicated group with the SAL group.
Level of sensitization during the acquisition period was measured as the change in locomotor response from the last day of acquisition minus the first time animals received drug (day 12 - day 3); there was a significant effect of treatment group (F(5,122) = 57.09, p < 0.0001). These data are shown in Fig. 4B. All groups receiving ethanol alone or in combination with nicotine had a significantly larger sensitization score compared to the repeated saline group, indicating the development of sensitization. In addition, both groups treated with nicotine in combination with ethanol had significantly (p<0.001) larger sensitization scores compared to ethanol alone. This suggests that repeated nicotine plus ethanol resulted in a larger change in locomotor response, compared to repeated ethanol alone.
On the ethanol challenge (day 13) all groups of mice were treated with 2 g/kg ethanol alone (Fig. 4C). There was a significant effect of treatment group (F[5,122] = 13.69, p < 0.01) for locomotor activity on the ethanol challenge day corrected for day 2 baseline activity levels (day 13-day 2). All mice repeatedly treated with 2 g/kg ethanol alone or in combination with nicotine had a larger response to the ethanol challenge compared to mice receiving ethanol for the first time on day 13. This suggests that repeated exposure to 2 g/kg ethanol alone or in combination with nicotine resulted in a sensitized response to ethanol alone and is in contrast to what was seen with the 1 g/kg ethanol study. There were no significant differences between the groups for BEC on day 13; BEC was 1.75 ± 0.03 mg/ml for the group average and group means ranged from 1.69 ± 0.05 to 1.85 ± 0.05 mg/ml.
4. Discussion
The goal of the present work was to determine if nicotine enhances ethanol-induced locomotor sensitization, a model of neuroadaptation caused by repeated drug exposure that is thought to be related to the development of drug dependence and vulnerability to relapse. It was hypothesized that mice repeatedly treated with nicotine in combination with ethanol would develop greater sensitization compared to mice repeatedly treated with ethanol alone. In addition, it was hypothesized that mice treated with nicotine and ethanol in combination during acquisition would have a larger locomotor response on the ethanol challenge day compared to the group that was treated with ethanol alone. The results of the current study found that the combined effects of nicotine and ethanol on locomotor sensitization were dependent on the dose of ethanol and whether testing was performed after acquisition with the drugs in combination or with ethanol alone.
Nicotine and ethanol in combination had greater locomotor stimulant effects compared to either drug alone. Consistent with our previously published results (Gubner et al., 2013), the largest combined effects, which were greater than additive, occurred when nicotine was combined with 1 g/kg ethanol, compared to 2 g/kg ethanol. This is likely because the 2 g/kg dose of ethanol had greater locomotor stimulant effects on its own, compared to 1 g/kg ethanol. However, the E2+N1 group had a greater acute locomotor response compared to E2 alone, so nicotine did enhance locomotor stimulation to even the 2 g/kg dose of ethanol.
As expected, there were greater sensitization scores (day 12 - day 3) for the 2 g/kg ethanol versus the 1 g/kg ethanol treatment groups. This result was expected as 2–2.5 g/kg of ethanol have been previously found to induce maximal locomotor sensitization to ethanol in DBA/2J mice (Phillips et al., 1994; Meyer et al., 2005; Meyer & Phillips 2003). Nicotine enhanced locomotor sensitization to 2 g/kg ethanol with limited effects when combined with 1 g/kg ethanol. It is possible that nicotine combined with doses of ethanol that are associated with robust sensitization enhance the development of neuroadaptations that contribute to the development of addiction.
Mice treated with either 1 or 2 g/kg ethanol during acquisition had a larger locomotor response on the ethanol challenge day (day 13) compared to mice receiving ethanol for the first time on day 13. A significant difference between treatment groups on the ethanol challenge day provides a between- groups comparison of locomotor sensitization. The results on the ethanol challenge were highly dependent on the dose of ethanol. The groups treated with 2 g/kg ethanol alone or in combination with nicotine all had similar responses to the ethanol challenge. This is in contrast to what was found during acquisition, where groups treated with nicotine and 2 g/kg ethanol had a larger change in locomotor response during the acquisition phase compared to the group treated with ethanol alone. This suggests that neuroadaptations underlying sensitization to nicotine in combination with 2 g/kg ethanol are specific to receiving these drugs in combination and not ethanol alone. These results differ from what was found for the 1 g/kg ethanol study. Mice treated with nicotine in combination with 1 g/kg ethanol during acquisition had very similar locomotor responses to the ethanol challenge (ethanol alone) compared to mice receiving 1 g/kg ethanol for the first time (saline during acquisition). In addition, the 1g/kg ethanol plus nicotine groups had lower locomotor responses compared to the group repeatedly treated with 1 g/kg ethanol alone. Thus, repeated exposure to 1 g/kg ethanol plus nicotine did not result in a sensitized response to ethanol challenge, unlike the sensitization seen in mice repeatedly treated with 1 g/kg ethanol alone. This suggests that nicotine interfered with the development of locomotor sensitization to a low dose of ethanol. One explanation for this finding is that mice experience nicotine combined with 1 g/kg ethanol differently than they experience this dose of ethanol alone. The combined effects of nicotine and ethanol were dependent on the dose of ethanol. Repeated exposure to nicotine combined with 2 g/kg but not 1 g/kg ethanol resulted in a sensitized locomotor response to ethanol alone on day 13. It is possible that the combined effects of nicotine and ethanol become more “ethanol-like” when the dose of ethanol is higher. Evidence to support this hypothesis is provided by a drug discrimination procedure in mice. In this study, nicotine was found to potentiate the salience of ethanol’s discriminative stimulus effects, for 1 g/kg ethanol, but not 2 g/kg ethanol (Ford et al., 2012). This suggests that a lower dose, but not higher dose, of ethanol combined with nicotine may have subjective effects that are perceived as being different from ethanol alone. Microdialysis studies in rats, have found greater effects on dopamine efflux in the nucleus accumbens of lower dose combinations of nicotine and ethanol versus higher dose combinations, where ceiling effects were found (Tizabi et al. 2002; 2007).
The groups of mice treated with 2 g/kg ethanol combined with nicotine had greater sensitization scores during acquisition than the group treated with ethanol alone. All groups treated with 2 g/kg ethanol alone or in combination with nicotine had similar locomotor responses when challenged with 2 g/kg alone, suggesting that nicotine did not interfere with the development of locomotor sensitization to the 2 g/kg dose of ethanol. Because nicotine enhanced the acquisition of sensitization to 2 g/kg ethanol plus nicotine, but not the response to the ethanol challenge, it suggests that the combined effects of nicotine and ethanol may be acting through different mechanisms.
Associative learning has been found to influence the expression of sensitization induced by psychostimulants. In some studies, the presence of the drug associated environment is necessary for the expression of sensitization (See McDougall et al., 2011 for review). However, in our data, if associative learning processes were influencing the expression of sensitization, a greater response to the ethanol alone challenge in the environment previously paired with nicotine plus ethanol, would have been expected. However, for the lower dose of ethanol, the opposite effect was observed, with groups treated with nicotine plus ethanol having a lower response to the ethanol challenge, compared to mice repeatedly treated with ethanol alone. One possibility is that state-dependent learning affected the expression of sensitization to the ethanol alone challenge. Nicotine was found to have the largest effects at enhancing the locomotor stimulant effects of a low dose of ethanol (1 versus 2 g/kg). It is possible that mice that had previously been treated with 1 g/kg ethanol plus nicotine experienced ethanol alone as being significantly different from the combination of nicotine and ethanol, and that this lack of similar state-dependent subjective experience did not allow for the expression of sensitization to ethanol alone. The subjective effects of nicotine plus the higher dose of ethanol may have been more similar to this dose of ethanol alone, allowing for the expression of sensitization to the ethanol alone challenge.
Overall, the current studies support the hypothesis that nicotine and ethanol in combination have enhanced neuroadaptive effects. However, the combined effects of nicotine and ethanol on locomotor sensitization were dependent on the dose of ethanol and whether testing was performed after acquisition with the drugs in combination or after treatment with ethanol alone.
Highlights.
Behavioral sensitization provided a measure of drug-induced neuroadaptation.
Independent and combined effects of nicotine and ethanol on behavioral sensitization were measured.
Acute nicotine plus ethanol had synergistic locomotor stimulant effects.
Magnitude of sensitization was dependent on ethanol dose and presence of nicotine.
Nicotine and ethanol in combination have enhanced neuroadaptive effects.
Acknowledgments
We would like to thank Dr. Amy Eshleman for assisting in designing the sensitization experiments. These studies were funded by the Department of Veterans Affairs, NIH NIAAA grants P60AA010760, T32AA007468, R24AA020245 and F31AA020732, a grant from the American Psychological Association, and a Tartar Trust Fellowship. Noah Gubner was support by National Cancer Institute Grant CA-11370 during the preparation of this manuscript.
Nonstandard abbreviations
- BEC
blood ethanol concentration
- Ex
x g/kg ethanol
- Nx
x mg/kg nicotine tartrate
- nAChR
nicotinic acetylcholine receptor
- SAL
saline
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anthony JC, Echeagaray-Wagner F. Epidemiologic analysis of alcohol and tobacco use. Alcohol Res Health. 2000;24:201–208. [PMC free article] [PubMed] [Google Scholar]
- Bhutada PS, Mundhada YR, Bansod KU, Dixit PV, Umathe SN, Mundhada DR. Inhibitory influence of mecamylamine on the development and the expression of ethanol-induced locomotor sensitization in mice. Pharmacol Biochem Behav. 2010;96:266–273. doi: 10.1016/j.pbb.2010.05.015. [DOI] [PubMed] [Google Scholar]
- Boehm SL, II, Schafer GL, Phillips TJ, Browman KE, Crabbe JC. Sensitivity to ethanol-induced motor incoordination in 5-HT(1B) receptor null mutant mice is task-dependent: implications for behavioral assessment of genetically altered mice. Behav Neurosci. 2000;114:401–409. [PubMed] [Google Scholar]
- Champtiaux N, Kalivas PW, Bardo MT. Contribution of dihydro-beta-erythroidine sensitive nicotinic acetylcholine receptors in the ventral tegmental area to cocaine-induced behavioral sensitization in rats. Behav Brain Res. 2006;168:120–126. doi: 10.1016/j.bbr.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Daeppen JB, Smith TL, Danko GP, Gordon L, Landi NA, Nurnberger JI, Jr, Bucholz KK, Raimo E, Schuckit MA. Clinical correlates of cigarette smoking and nicotine dependence in alcohol-dependent men and women. The Collaborative Study Group on the Genetics of Alcoholism. Alcohol Alcohol. 2000;35:171–175. doi: 10.1093/alcalc/35.2.171. [DOI] [PubMed] [Google Scholar]
- Danaei G, Ding EL, Mozaffarian D, Taylor B, Rehm J, Murray CJ, Ezzati M. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. 2009;6:e1000058. doi: 10.1371/journal.pmed.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierker L, Lloyd-Richardson E, Stolar M, Flay B, Tiffany S, Collins L, Bailey S, Nichter M, Nichter M, Clayton R. Tobacco Etiology Research Network (TERN) (2006) The proximal association between smoking and alcohol use among first year college students. Drug Alcohol Depend. 81:1–9. doi: 10.1016/j.drugalcdep.2005.05.012. [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Wellman RJ. Sensitization to nicotine: how the animal literature might inform future human research. Nicotine Tob Res. 2007;9:9–20. doi: 10.1080/14622200601078277. [DOI] [PubMed] [Google Scholar]
- Falk DE, Yi HY, Hiller-Sturmhöfel S. An epidemiologic analysis of co-occurring alcohol and tobacco use and disorders: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Alcohol Res Health. 2006;29:162–171. [PMC free article] [PubMed] [Google Scholar]
- Ford MM, McCracken AD, Davis NL, Ryabinin AE, Grant KA. Discrimination of ethanol-nicotine drug mixtures in mice: dual interactive mechanisms of overshadowing and potentiation. Psychopharmacology. 2012;224:537–548. doi: 10.1007/s00213-012-2781-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DB. Pharmacology of Alcohol. New York: Oxford University Press; 1983. [Google Scholar]
- Gubner NR, McKinnon CS, Reed C, Phillips TJ. Accentuating effects of nicotine on ethanol response in mice with high genetic predisposition to ethanol-induced locomotor stimulation. Drug Alcohol Depend. 2013;127:108–114. doi: 10.1016/j.drugalcdep.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hymowitz N, Cummings KM, Hyland A, Lynn WR, Pechacek TF, Hartwell TD. Predictors of smoking cessation in a cohort of adult smokers followed for five years. Tob Control. 1997;6(Suppl 2):S57–62. doi: 10.1136/tc.6.suppl_2.s57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang N, Ling PM. Impact of alcohol use and bar attendance on smoking and quit attempts among young adult bar patrons. Am J Public Health. 2013;103:e53–61. doi: 10.2105/AJPH.2012.301014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Kamens HM, Phillips TJ. A role for neuronal nicotinic acetylcholine receptors in ethanol-induced stimulation, but not cocaine- or methamphetamine-induced stimulation. Psychopharmacology. 2008;196:377–387. doi: 10.1007/s00213-007-0969-7. [DOI] [PubMed] [Google Scholar]
- Kozlowski LT, Jelinek LC, Pope MA. Cigarette smoking among alcohol abusers: a continuing and neglected problem. Can J Public Health. 1986;77:205–207. [PubMed] [Google Scholar]
- Lessov CN, Palmer AA, Quick EA, Phillips TJ. Voluntary ethanol drinking in C57BL/6J and DBA/2J mice before and after sensitization to the locomotor stimulant effects of ethanol. Psychopharmacology. 2001;155:91–99. doi: 10.1007/s002130100699. [DOI] [PubMed] [Google Scholar]
- Marks JL, Hill EM, Pomerleau CS, Mudd SA, Blow FC. Nicotine dependence and withdrawal in alcoholic and nonalcoholic ever-smokers. J Subst Abuse Treat. 1997;14:521–527. doi: 10.1016/s0740-5472(97)00049-4. [DOI] [PubMed] [Google Scholar]
- McDougall SA, Pothier AG, Der-Ghazarian T, Herbert MS, Kozanian OO, Castellanos KA, Flores AT. Importance of associative learning processes for one-trial behavioral sensitization of preweanling rats. Behav Pharmacol. 2011;22:693–702. doi: 10.1097/FBP.0b013e32834affb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer PJ, Palmer AA, McKinnon CS, Phillips TJ. Behavioral sensitization to ethanol is modulated by environmental conditions, but is not associated with cross-sensitization to allopregnanolone or pentobarbital in DBA/2J mice. Neuroscience. 2005;131:263–273. doi: 10.1016/j.neuroscience.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Meyer PJ, Phillips TJ. Bivalent effects of MK-801 on ethanol-induced sensitization do not parallel its effects on ethanol-induced tolerance. Behav Neurosci. 2003;117:641–649. doi: 10.1037/0735-7044.117.3.641. [DOI] [PubMed] [Google Scholar]
- Nichter M, Carkoglu A, Lloyd-Richardson E Tobacco Etiology Research Network (TERN) Smoking and drinking among college students: “it’s a package deal”. Drug Alcohol Depend. 2010;106:16–20. doi: 10.1016/j.drugalcdep.2009.07.025. [DOI] [PubMed] [Google Scholar]
- Palmer AA, McKinnon CS, Bergstrom HC, Phillips TJ. Locomotor activity responses to ethanol, other alcohols, and GABA-A acting compounds in forward- and reverse-selected FAST and SLOW mouse lines. Behav Neurosci. 2002;116:958–967. doi: 10.1037//0735-7044.116.6.958. [DOI] [PubMed] [Google Scholar]
- Pastor R, McKinnon CS, Scibelli AC, Burkhart-Kasch S, Reed C, Ryabinin AE, Coste SC, Stenzel-Poore MP, Phillips TJ. Corticotropin-releasing factor-1 receptor involvement in behavioral neuroadaptation to ethanol: a urocortin1-independent mechanism. Proc Natl Acad Sci U S A. 2008;105:9070–9075. doi: 10.1073/pnas.0710181105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen DR, Norris KJ, Thompson JA. A comparative study of the disposition of nicotine and its metabolites in three inbred strains of mice. Drug Metab Dispos. 1984;12:725–731. [PubMed] [Google Scholar]
- Phillips TJ, Dickinson S, Burkhart-Kasch S. Behavioral sensitization to drug stimulant effects in C57BL/6J and DBA/2J inbred mice. Behav Neurosci. 1994;108:789–803. doi: 10.1037//0735-7044.108.4.789. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Huson M, Gwiazdon C, Burkhart-Kasch S, Shen EH. Effects of acute and repeated ethanol exposures on the locomotor activity of BXD recombinant inbred mice. Alcohol Clin Exp Res. 1995;19:269–278. doi: 10.1111/j.1530-0277.1995.tb01502.x. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Shen EH. Neurochemical bases of locomotion and ethanol stimulant effects. Int Rev Neurobiol. 1996;39:243–282. doi: 10.1016/s0074-7742(08)60669-8. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Pastor R, Scibelli AC, Reed C, Tarragon E. Behavioral sensitization to addictive drugs: Clinical relevance and methodological aspects. In: Raber J, editor. Animal Models of Behavioral Analysis. Humana Press; New York: 2011. pp. 267–305. [Google Scholar]
- Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223–2233. doi: 10.1016/S0140-6736(09)60746-7. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Shen EH, Harland RD, Crabbe JC, Phillips TJ. Bidirectional selective breeding for ethanol effects on locomotor activity: characterization of FAST and SLOW mice through selection generation 35. Alcohol Clin Exp Res. 1995;19:1234–1245. doi: 10.1111/j.1530-0277.1995.tb01606.x. [DOI] [PubMed] [Google Scholar]
- Sobell MB. Alcohol and tobacco: clinical and treatment issues. Alcohol Clin Exp Res. 2002;26:1954–1955. doi: 10.1097/01.ALC.0000041008.52475.C5. [DOI] [PubMed] [Google Scholar]
- Steketee JD, Kalivas PW. Drug wanting: behavioral sensitization and relapse to drug-seeking behavior. Pharmacol Rev. 2011;63:348–365. doi: 10.1124/pr.109.001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizabi Y, Copeland RL, Jr, Louis VA, Taylor RE. Effects of combined systemic alcohol and central nicotine administration into ventral tegmental area on dopamine release in the nucleus accumbens. Alcohol Clin Exp Res. 2002;26:394–399. [PubMed] [Google Scholar]
- Tizabi Y, Bai L, Copeland RL, Jr, Taylor RE. Combined effects of systemic alcohol and nicotine on dopamine release in the nucleus accumbens shell. Alcohol Alcohol. 2007;42:413–416. doi: 10.1093/alcalc/agm057. [DOI] [PubMed] [Google Scholar]
- Tsoh JY, Chi FW, Mertens JR, Weisner CM. Stopping smoking during first year of substance use treatment predicted 9-year alcohol and drug treatment outcomes. Drug Alcohol Depend. 2011;114:110–108. doi: 10.1016/j.drugalcdep.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlsten D. Insensitivity of the analysis of variance to heredity-environment interaction. Behavioral and Brain Sciences. 1990;13:109–120. [Google Scholar]
- Weitzman ER, Chen YY. The co-occurrence of smoking and drinking among young adults in college: national survey results from the United States. Drug Alcohol Depend. 2005;80:377–386. doi: 10.1016/j.drugalcdep.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–492. [PubMed] [Google Scholar]